- 1Department of Cardiology, Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 2Department of Orthopedics, Affiliated Zhongshan Hospital of Dalian University, Dalian, China
Background: Framingham risk score (FRS) is an effective tool for evaluating the 10-year risk of cardiovascular diseases. However, the sensitivity of FRS for anthracycline-induced cardiotoxicity is unclear. This meta-analysis aims to evaluate the correlation between risk factors (hypertension, hyperlipidemia, diabetes, smoking, and obesity) in FRS and anthracycline-induced cardiotoxicity in breast cancer.
Methods: We searched PubMed, EMBASE, and Cochrane Library for studies published from inception to January 2022 which reported cardiotoxicity due to anthracycline. Cardiotoxicity defined as any cardiac events were used as the primary endpoint. A total of 33 studies involving 55,708 breast cancer patients treated with anthracyclines were included in this meta-analysis.
Results: At least one risk factor was identified at baseline for the 55,708 breast cancer patients treated with anthracycline. Hypertension [I2 = 45%, Fixed, RR (95% CI) = 1.40 (1.22, 1.60), p < 0.00001], hyperlipidemia [I2 = 0%, Fixed, RR (95% CI): 1.35 (1.12, 1.62), p = 0.002], diabetes [I2 = 0%, Fixed, RR (95% CI): 1.29 (1.05, 1.57), p = 0.01], and obesity [I2 = 0%, Fixed, RR (95% CI): 1.32 (1.05, 1.67), p = 0.02] were associated with increased risks of cardiac events. In addition, smoking was also associated with reduced left ventricular ejection fraction (LVEF) during anthracycline chemotherapy [I2 = 0%, Fixed, OR (95% CI): 1.91 (1.24, 2.95), p = 0.003] in studies that recorded only the odds ratio (OR).
Conclusion: Hypertension, hyperlipidemia, diabetes, smoking, and obesity are associated with increased risks of anthracycline-induced cardiotoxicity. Therefore, corresponding measures should be used to manage cardiovascular risk factors in breast cancer during and after anthracycline treatment.
1. Background
Breast cancer is the most common cancer worldwide, which affects women most frequently in both developed and underdeveloped regions (1). Anthracyclines are widely used in the treatment of breast cancer (2), but anthracycline-induced cardiotoxicity is the main reason for their limited use in the clinical setting. Previous studies reported that the incidence of doxorubicin (DOX)-induced heart failure varies among individuals with different physical constitutions and is closely associated with the cumulative dose of anthracyclines and age of patients (3, 4).
The mechanisms of anthracycline-induced cardiotoxicity are complex and involve various processes of injury such as oxidative stress, inflammation (5), mitochondrial damage (6), endoplasmic reticulum stress (7), disrupted calcium homeostasis, cell apoptosis (8), fibrosis (5), and dysregulated autophagy (9, 10). In particular, reactive oxygen species (ROS) production plays an important role in anthracycline-induced cardiotoxicity. Several studies demonstrated that DOX can increase ROS production by cardiocytes via the nicotinamide adenine dinucleotide (NADH) dehydrogenase (complex I) pathway of the mitochondrial electron transport chain (11), increased mitochondrial iron level (12), down-regulation of sirtuin-3 (SIRT3), and decreased SOD2 production (13), ultimately resulting in cardiocyte apoptosis and increased autophagy (14).
Anthracycline-induced cardiotoxicity can be classified as acute cardiotoxicity (immediately after drug injection), early onset chronic progressive cardiotoxicity (during or within 1 year after treatment), and late-onset chronic progressive cardiotoxicity (at least 1 year after treatment). The most common manifestation of anthracycline-induced cardiotoxicity is left ventricular dysfunction (LVDF) and development of overt heart failure (15).
Echocardiography is the predominant tool for diagnosing cardiotoxicity and is required for evaluating left ventricular ejection fraction (LVEF) before, during and after treatment, especially in patients with cardiovascular risk factors (smoking, hypertension, diabetes, hyperlipidemia, and obesity) (16).
The Framingham risk score (FRS) is an effective tool for evaluating the risk of cardiovascular disease. This tool divides patients into the high-, medium-, and low-risk groups and estimates the 10-year risk of cardiovascular disease based on age and risk factors for cardiovascular disease. The risk factors included in the FRS are diabetes, hyperlipidemia, hypertension, smoking, and obesity (17). A guideline has suggested that the use of cardiovascular risk assessments to estimate the probability of future cardiovascular events may be beneficial for patients with risk factors.
In this study, we examined the effect of risk factors in the FRS on anthracycline-induced cardiotoxicity to determine the importance of cardiovascular risk factor assessment in anthracycline treatment of breast cancer patients.
2. Materials and methods
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and was registered on PROSPERO (#CRD42022299098) (18).
2.1. Search strategy
Relevant studies in any language were searched in PubMed, Embase, and Cochrane Library from inception to January 2022 using the keywords [(anthracyclines or DOX or epirubicin) and (cardiac toxicity or cardiotoxicity or heart failure) and (breast or breast cancer)]. Case reports, reviews, guidelines, editorials, and letters were excluded.
2.2. Study selection
Two reviewers (JH and XJF) assessed the titles, abstracts, and full texts of the identified studies to determine whether the studies examined the associations between cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, obesity, smoking, and obesity) and anthracycline-induced cardiotoxicity. We included cross-sectional and cohort studies as well as both population-based and hospital-based case-control studies in the systematic review. Eligible studies were identified if they met the following inclusion criteria: (1) adult participants ≥18 years of age and (2) all patients were treated with anthracycline. Studies were mainly excluded for the following reasons: (1) inappropriate study type, including reviews, editorials, and case report; (2) incomplete LVEF data; and (3) animal studies.
2.3. Data extraction
The extracted data included study environment, cohort description, incidence of cardiotoxicity, and cardiac event descriptions. Reduced LVEF was the primary endpoint. Abnormal electrocardiogram, duke activity status index (DASI) score, and congestive heart failure (CHF) were also included as endpoints in this meta-analysis.
2.4. Statistical analysis
The effect of five cardiovascular risk factors (hypertension, hyperlipidemia, diabetes, smoking, and obesity) on cardiotoxicity in breast cancer patients undergoing anthracycline treatment was separately pooled in this meta-analysis. Pooled results with an I2 < 50% were analyzed using the fixed effects model, while those with an I2 > 50% were analyzed by the random effects model. Subgroup analysis was also performed based on the clinical endpoint of each study. Sensitivity analysis was conducted on all studies with I2 > 50% to explore the source of heterogeneity and the effect of heterogeneity on the stability of the combined estimated value. Statistical analyses were performed on Review Manager 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration) and STATA 16.0. In addition, publication bias was assessed using over 10 outcomes in the included studies by a funnel plot and Egger’s test.
3. Results
All statistical analysis results and literature quality evaluation (Supplementary Figure 34) are presented in two types of forest plots in the Supplementary Data, and only a summary of the results is shown in this manuscript (Tables 1, 2, 3).
3.1. Study selection and baseline characteristics
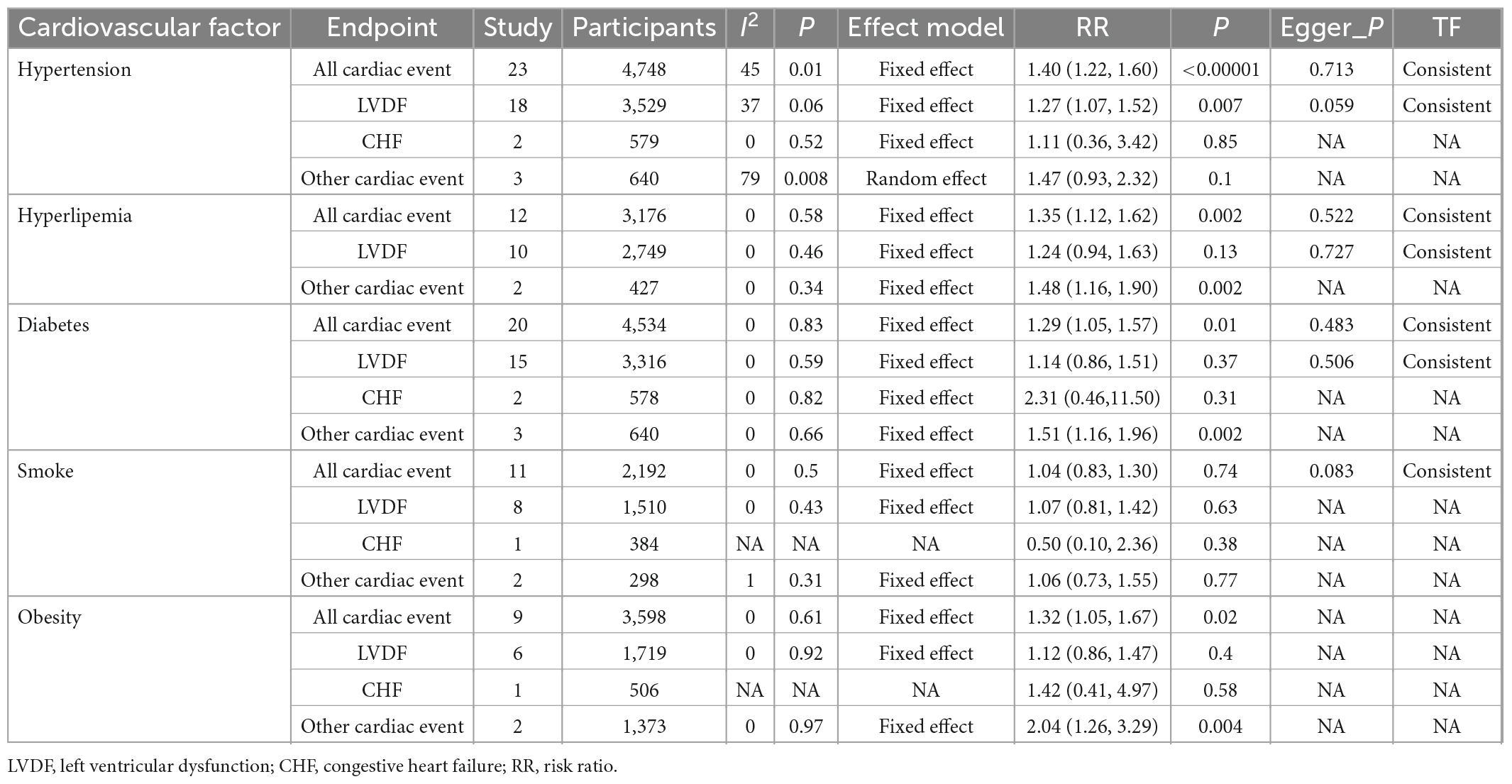
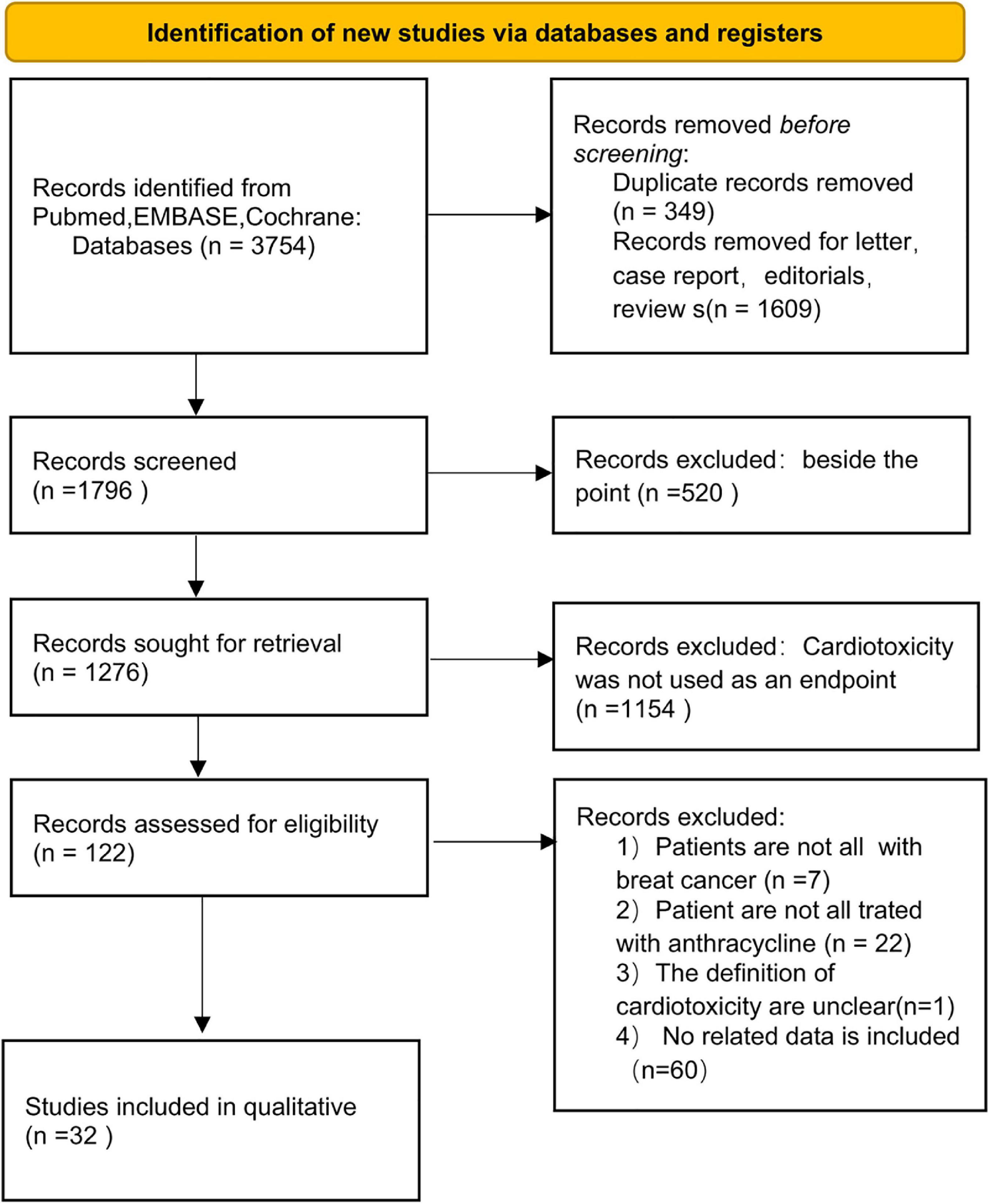
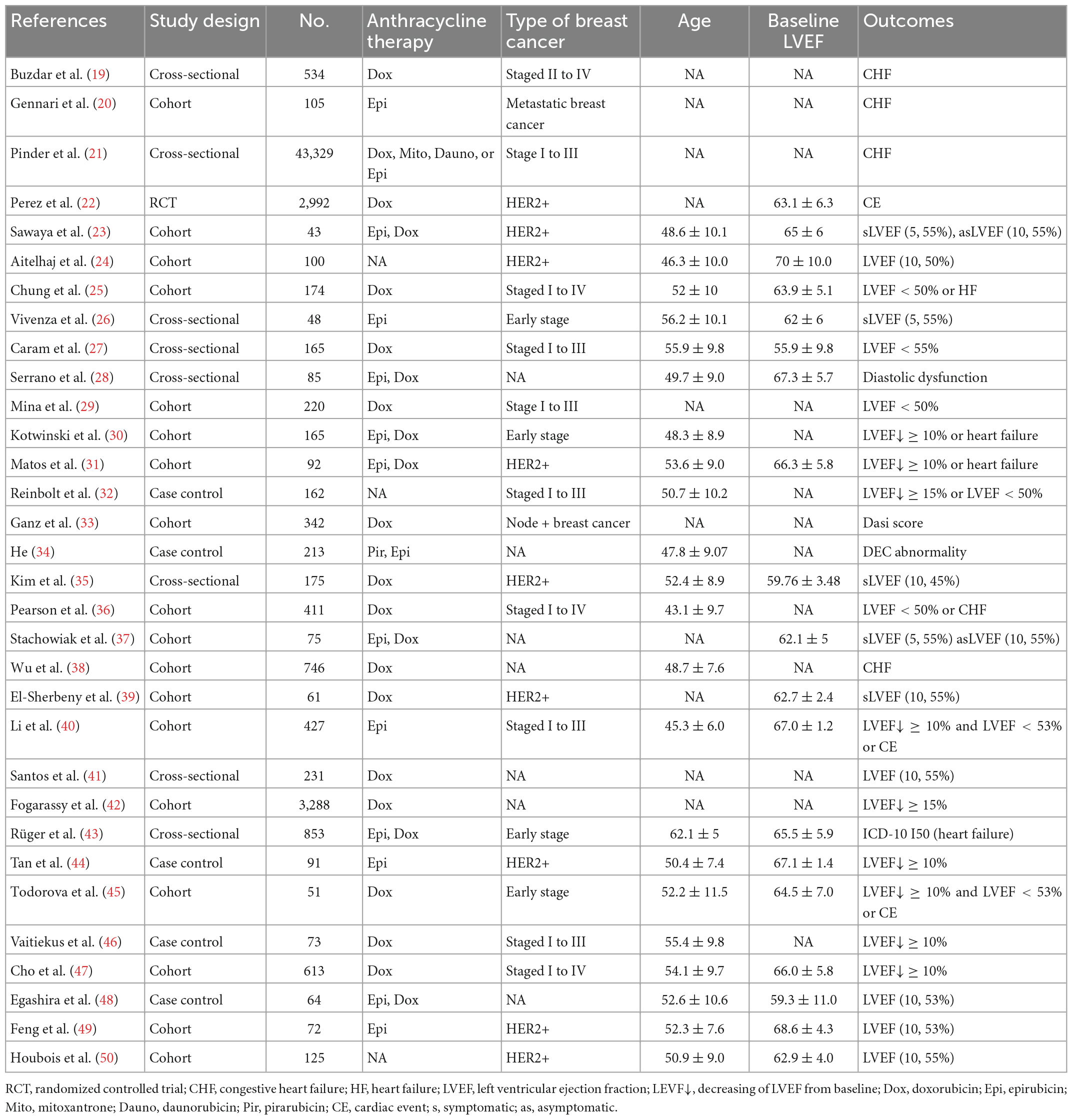
The PRISMA flow diagram of study selection is shown in Figure 1. A total of 340 articles were identified during our preliminary search, and a final total of 32 eligible studies were included for meta-analysis. Detailed data including patient population, primary endpoint, and number of patients, were collected from the 32 studies (19–50). In addition, all patients in the included studies were breast cancer patients undergoing anthracycline treatment. Analysis of various risk factors showed that 30 studies [5 only recorded odds ratio (OR) and 2 recorded hazards ratio (HR)] reported hypertension, 18 studies (5 only recorded OR and 1 recorded) reported hyperlipidemia, 28 studies (6 recorded OR and 2 recorded HR) reported diabetes, 16 studies (5 included OR) reported smoking, and 12 studies (3 included OR) reported obesity (Table 4).
3.2. Incidence of cardiotoxicity
Cardiotoxicity was observed in 11,516 of the 55,492 patients included in this meta-analysis (Rate: 0.20; 95% CI: 0.15–0.24) with high heterogeneity (I2 = 99%). The result remained unchanged after sensitivity analysis (Supplementary Figure 1).
3.2.1. Hypertension
We analyzed 23 studies involving 4,748 patients (Supplementary Figure 2) and confirmed that hypertension was a risk factor for cardiotoxicity [I2 = 45%, Fixed, RR (95% CI): 1.40 (1.22, 1.60), p < 0.00001].
Subgroup analysis using hypertension as the clinical endpoint showed that 18 studies involving 3,529 patients used LVDF as the endpoint [I2 = 37%, Fixed, RR (95% CI): 1.27 (1.07, 1.52), p = 0.007] (Supplementary Figure 3) and 2 studies involving 579 patients [I2 = 0%, Fixed, RR (95% CI): 1.11 (0.36, 3.42), p = 0.85] used CHF as the endpoint (Supplementary Figure 4).
There were three studies involving 640 patients [I2 = 79%, Random, RR (95% CI): 1.47 (0.93, 2.32), p = 0.1] that used other cardiac events as the clinical endpoints (Supplementary Figure 5). Sensitive analysis revealed that the Ganz et al. (33) study was the main source of heterogeneity and was hence removed [I2 = 0%, Fixed, RR (95% CI): 1.17 (0.87, 1.56), p = 0.3]. The reason for heterogeneity may be attributed to the large difference in clinical endpoint between this study and other studies (described as unable to reduce heterogeneity in the sensitivity analysis).
There were six studies involving 526 HER2+ patients [I2 = 0%, Fixed, RR (95% CI): 1.14 (0.77, 1.68), p = 0.52; Supplementary Figure 34].
There were 12 studies involving 2,720 patients [I2 = 46%, Fixed, RR (95% CI): 1.77 (1.47, 2.13), p < 0.00001] that used DOX (Supplementary Figure 35).
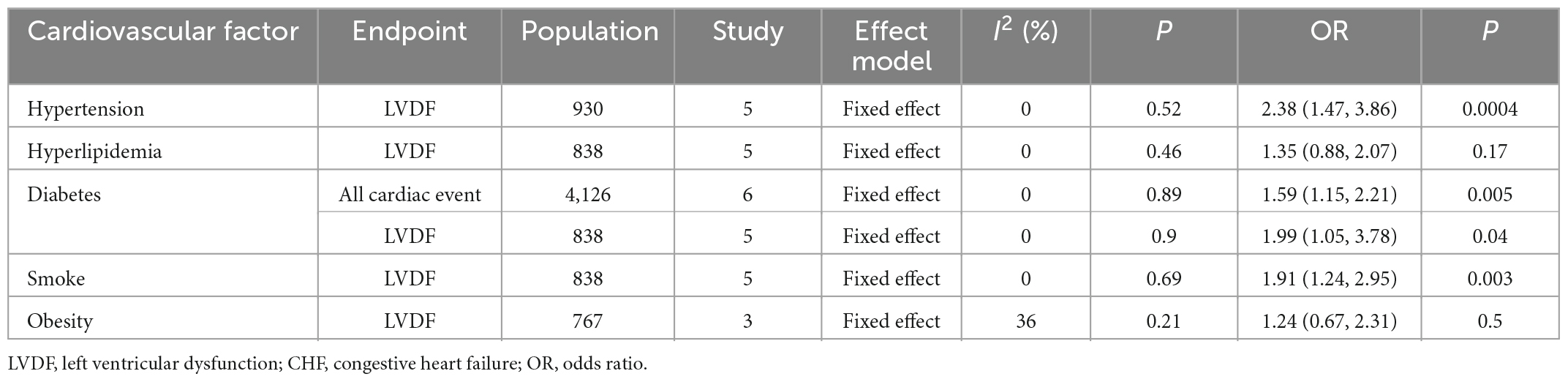
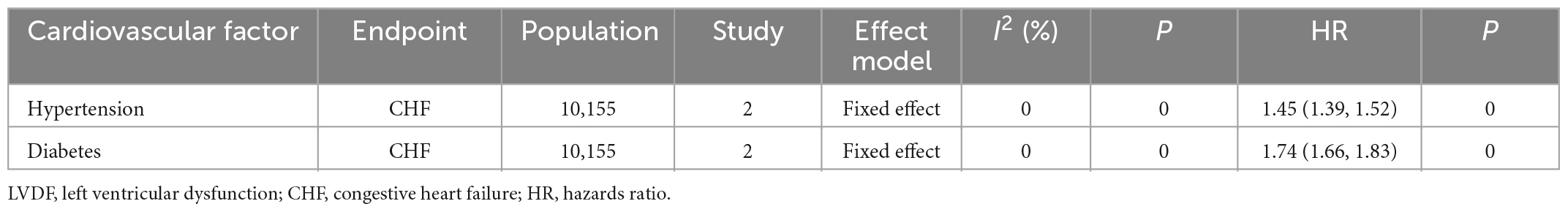
Of the studies that recorded only OR and HR, five studies [pooled: I2 = 0%, Fixed, OR (95% CI): 2.38 (1.47, 3.86), p = 0.0004; Supplementary Figure 6] and two studies [pooled: I2 = 0%, Fixed, HR (95% CI): 1.45 (1.39, 1.52), p = 0; Supplementary Figure 7] indicated that hypertension was a risk factor for cardiotoxicity, respectively.
3.2.2. Hyperlipidemia
Analysis of 12 studies involving 3,176 patients (Supplementary Figure 8) showed that hyperlipidemia was a risk factor for cardiotoxicity [I2 = 0%, Fixed, RR (95% CI): 1.35 (1.12, 1.62), p = 0.002]. LVDF was the endpoint in 10 studies (Supplementary Figure 9) involving 2,749 subjects [I2 = 0%, Fixed, RR (95% CI): 1.24 (0.94, 1.63), p = 0.13], and other cardiac events were the endpoints in 2 studies (Supplementary Figure 10) involving 427 subjects [I2 = 0%, Fixed, RR (95% CI): 1.48 (1.16, 1.90), p = 0.002].
There were three studies involving 229 HER2+ patients [I2 = 45%, Fixed, RR (95% CI): 1.44 (0.89, 2.35), p = 0.14; Supplementary Figure 36].
There were six studies involving 1,864 patients [I2 = 0%, Fixed, RR (95% CI): 1.37 (1.09, 1.72), p = 0.007] that used DOX (Supplementary Figure 37).
Of the studies that recorded OR (Supplementary Figure 11), included five studies [pooled: I2 = 0%, Fixed, OR (95% CI): 1.35 (0.88, 2.07), p = 0.17].
3.2.3. Diabetes
Analysis of 20 studies (Supplementary Figure 12) involving 4,534 patients indicated that diabetes was a risk factor for cardiotoxicity [I2 = 0%, Fixed, RR (95% CI): 1.29 (1.05, 1.57), p = 0.01].
Subgroup analysis using diabetes as the clinical endpoint showed that 15 studies (Supplementary Figure 13) involving 3,316 subjects [I2 = 0%, Fixed, RR (95% CI): 1.14 (0.86, 1.51), p = 0.37] used LVDF as the endpoint, 2 studies (Supplementary Figure 14) involving 578 subjects [I2 = 0%, Fixed, RR (95% CI): 2.31 (0.46, 11.50), p = 0.31] used CHF as the endpoint, and 3 studies (Supplementary Figure 15) involving 640 patients [I2 = 0%, Fixed, RR (95% CI): 1.51 (1.16, 1.96), p = 0.002] used other cardiac events (abnormal ECG, diastolic dysfunction, and all cardiac event) as the endpoints. The main source of heterogeneity was the difference in clinical endpoints.
There were four studies involving 329 HER2+ patients [I2 = 0%, Fixed, RR (95% CI): 1.39 (0.81, 2.39), p = 0.23; Supplementary Figure 38].
There were 10 studies involving 2,729 patients [I2 = 0%, Fixed, RR (95% CI): 1.22 (0.93, 1.61), p = 0.15] that used DOX (Supplementary Figure 39).
Of the studies that recorded only OR (Supplementary Figure 16), six confirmed that diabetes was a risk factor for cardiotoxicity [pooled OR: I2 = 0%, Fixed, OR (95% CI): 1.59 (1.15, 2.21), p = 0.005]. Of these six studies, five used LVDF as the endpoint (Supplementary Figure 17) and confirmed that diabetes was a risk factor for cardiotoxicity [pooled OR: I2 = 0%, Fixed, OR (95% CI): 1.99 (1.05, 3.78), p = 0.04]. Among the studies that recorded HR, two (Supplementary Figure 18) indicated that diabetes was a risk factor for cardiotoxicity [pooled HR: I2 = 0 %, Fixed, HR (95% CI): 1.74 (1.66, 1.83), p = 0].
3.2.4. Smoking
Analysis of 11 studies (Supplementary Figure 19) involving 2,192 patients [I2 = 0%, Fixed, RR (95% CI): 1.04 (0.83, 1.30), p = 0.74].
Subgroup analysis using smoking as the clinical endpoint showed that eight studies (Supplementary Figure 20) involving 1,510 subjects [I2 = 0%, Fixed, RR (95% CI): 1.07 (0.81, 1.42), p = 0.63] used LVDF as the endpoint, and two studies (Supplementary Figure 21) involving 298 subjects [I2 = 1%, Fixed, RR (95% CI): 1.06 (0.73, 1.55), p = 0.77] used other cardiac events (abnormal ECG, diastolic dysfunction, and all cardiac event) as the endpoints.
There were three studies involving 229 HER2+ patients [I2 = 0%, Fixed, RR (95% CI): 1.39 (0.86, 2.25), p = 0.18; Supplementary Figure 40].
There were five studies involving 1,490 patients [I2 = 39%, Fixed, RR (95% CI): 0.96 (0.61, 1.51), p = 0.85] that used DOX (Supplementary Figure 41).
Five studies (Supplementary Figure 22) with OR data indicated that smoking was a risk factor for cardiotoxicity [pooled OR: I2 = 0%, Fixed, OR (95% CI): 1.91 (1.24, 2.95), p = 0.003].
3.2.5. Obesity
Analysis of nine studies (Supplementary Figure 23) involving 3,598 patients demonstrated that obesity is a risk factor for cardiotoxicity [I2 = 0%, Fixed, RR (95% CI): 1.32 (1.05, 1.67), p = 0.02].
Subgroup analysis using obesity as the clinical endpoint showed that six studies (Supplementary Figure 24) involving 1,719 subjects [I2 = 0%, Fixed, RR (95% CI): 1.12 (0.86, 1.47), p = 0.4] used LVDF as the endpoint, and two studies (Supplementary Figure 25) involving 1,373 subjects [I2 = 0%, Fixed, RR (95% CI): 2.04 (1.26, 3.29), p = 0.004] used other cardiac events as the endpoints.
There were five studies involving 2,634 patients [I2 = 0%, Fixed, RR (95% CI):1.38 (0.92, 2.07), p = 0.12] that used DOX (Supplementary Figure 42).
Three studies with OR data (Supplementary Figure 26) [pooled OR: I2 = 36%, Fixed, OR (95% CI): 1.24 (0.67, 2.31), p = 0.5].
3.2.6. Publication bias
Funnel plots were generated for cardiovascular risk factor with >10 included studies, including hypertension (23 studies), hyperlipidemia (12 studies), diabetes (20 studies), and smoking (11 studies), and all of the plots were symmetrical (Supplementary Figures 27–30).
Among the studies that used LVDF as endpoint, funnel plots for hypertension (18 studies), hyperlipidemia (10 studies), and diabetes (15 studies) were also symmetrical (Supplementary Figures 31–33).
Among the studies that used DOX, funnel plots for hypertension (12 studies) and diabetes (10 studies) were symmetrical (Supplementary Figures 43, 44).
4. Discussion
This meta-analysis examined the correlation between five cardiovascular risk factors in the FRS and anthracycline-induced cardiotoxicity in breast cancer patients, and fond that hypertension, hyperlipidemia, diabetes, smoking, and obesity were significantly associated with cardiotoxicity. Most included studies used decreased LVEF as the clinical endpoint. Subgroup analysis of studies with large heterogeneity in hypertension and smoking showed that abnormal ECG, diastolic dysfunction, and DASI score (indicators for cardiotoxicity) were the main sources of heterogeneity. Therefore, these clinical endpoints can be further examined in subsequent studies to improve the detection rate of cardiotoxicity. The pooled incidence of cardiac event (mean: 0.20; 95% CI: 0.15–0.24) in this study was similar to a recent meta-analysis (51) but with significant heterogeneity, which was possibly related to the differences in chemotherapy regimen, follow-up time and types of breast cancer.
Our meta-analysis revealed that cardiotoxicity was more easily induced by anthracyclines in breast cancer patients with hypertension, and this phenomenon can be caused by multiple factors. The synergistic effects of excess ROS production, DOX, and hypertension on the renin-angiotensin system (RAS) may be an important potential mechanism. Several studies demonstrated that DOX-induced cardiotoxicity was more severe in animals with hypertension than in normal animals (52, 53). Furthermore, a recent study showed that DOX and angiotensin-II (ANGII) exert synergistic effect in adolescent mice, and exposure to DOX can increase ANGII-induced cardiac remodeling (54). The putative mechanism underpinning this observation may be the changes in RAS induced by hypertension and DOX, which synergistically exacerbate cardiac remodeling (55). Nicotinamide adenine dinucleotide phosphate (NADPH) produced during ventricular hypertrophy in hypertension patients is the main source of ROS (56, 57). Prior study showed that ROS production is increased in the heart tissues of mice with spontaneous hypertension (58), which suggests that the increase in ROS during anthracycline treatment may result in cardiac injury in hypertension patients.
This study also demonstrated that cardiotoxicity is more easily induced by anthracyclines in patients with hyperlipidemia, which may be attributed to oxidative overload caused by hyperlipidemia. Jia et al. (59) reported that palmitate exposure in H9C2 cardiomyocytes led to increased cardiomyocyte apoptosis as a result of oxidative stress caused by ROS produced during lipid peroxidation. Zbinden et al. (60) showed that rats on high-fat diet (HFD) had more severe cardiotoxicity following intraperitoneal DOX injection than rats on low-FD, and this may be attributed to oxidative stress caused by ROS generation during lipid peroxidation and the production of excess ceramide (61).
We also identified diabetes as a risk factor for anthracycline-induced cardiotoxicity in breast cancer patients. However, inflammatory damages to the heart caused by diabetes may also contribute to the development of cardiotoxicity. It was shown that diabetic rats had decreased plasma and renal clearance of DOX and increased cardiotoxicity than normal rats (62). Similarly, another study demonstrated that diabetic mice had a higher risk of cardiotoxicity after DOX injection than normal mice (63). Given that diabetic patients already have up-regulated inflammation-associated protein expression in the heart, increased oxidative stress (59) can synergize with anthracyclines to exacerbate cardiac injury. However, since direct evidence linking these factors and anthracycline-induced cardiotoxicity is lacking, further investigation is warranted.
We showed that smoking is a risk factor for anthracycline-induced cardiotoxicity in breast cancer patients, possibly due to the compounds generated in smoke exposure. A previous study demonstrated that smoking plays an important role in anthracycline-induced cardiotoxicity. The authors found that exposure of anthracycline-treated cardiomyocytes to cigarette smoke led to increased concentrations of two compounds related to cardiac atrophy (64). Likewise, our meta-analysis revealed that studies that examined smoking and only included OR confirmed an association between smoking and anthracycline-induced cardiotoxicity.
Finally, our study demonstrated that obesity also increased the risk of anthracycline-induced cardiotoxicity in breast cancer patients, and patients with smaller body mass index (BMI) had lower risk of anthracycline-induced cardiotoxicity. A previous clinical study reported that obesity was associated with adverse outcome in node-positive breast cancer patients (65). In addition, rats with HFD-induced obesity were more susceptible to adriamycin-induced cardiotoxicity (66). The possible mechanism by which obesity increases cardiotoxicity is the down-regulation of adiponectin and omentin in obese patients (67–69), and calorie restriction and exercise have been shown to effectively decrease cardiac injury in these patients (70, 71).
Compared with previous studies, our meta-analysis included more studies and evaluated five cardiovascular risk factors. However, there are still several limitations. First, we could not examine the relationship between gender and anthracyclines due to the small number of male breast cancer patients. Second, despite the definition of cardiotoxicity in relevant guidelines, cardiotoxicity was still not defined consistently in some studies. Third, the chemotherapy regimen in most studies involved other chemotherapeutic agents, which impeded us from evaluating the correlation between a single anthracycline drug and cardiotoxicity in breast cancer patients. Last, since the risk factors analyzed in this study were dichotomous and not continuous variables, the relationship between the severity of risk factors and cardiotoxicity could not be analyzed.
In sum, our findings suggest that cardiovascular risk factors in breast cancer patients should be adequately assessed before anthracycline chemotherapy to evaluate the risk of cardiotoxicity in these patients. In addition, the cardiovascular system of breast cancer patients should also be closely monitored during anthracycline treatment.
5. Conclusion
Five cardiovascular risk factors from the FRS are highly associated with anthracycline-induced cardiotoxicity. Therefore, active management of the primary disease and maintenance of a good lifestyle can lower the risk of cardiotoxicity.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements.
Author contributions
HJ: conceptualization, methodology, software, writing – original draft, data curation, and visualization. JX: investigation, writing – original draft, and writing – reviewing and editing. ZS: methodology, software, and writing – original draft. LW: conceptualization, supervision, project administration, and funding acquisition. All authors contributed to the study conception, design, read, and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1101585/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Gradishar W, Anderson B, Abraham J, Aft R, Agnese D, Allison K, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2020) 18:452–78.
3. Von Hoff D, Layard M, Basa P, Davis H Jr, Von Hoff A, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. (1979) 91:710–7. doi: 10.7326/0003-4819-91-5-710
4. Jensen B, Skovsgaard T, Nielsen S. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. (2002) 13:699–709. doi: 10.1093/annonc/mdf132
5. Narikawa M, Umemura M, Tanaka R, Hikichi M, Nagasako A, Fujita T, et al. Doxorubicin induces trans-differentiation and MMP1 expression in cardiac fibroblasts via cell death-independent pathways. PLoS One. (2019) 14:e0221940.
6. Oliveira P, Wallace K. Depletion of adenine nucleotide translocator protein in heart mitochondria from doxorubicin-treated rats–relevance for mitochondrial dysfunction. Toxicology. (2006) 220:160–8. doi: 10.1016/j.tox.2005.12.009
7. Hu J, Wu Q, Wang Z, Hong J, Chen R, Li B, et al. Inhibition of CACNA1H attenuates doxorubicin-induced acute cardiotoxicity by affecting endoplasmic reticulum stress. Biomed Pharmacother. (2019) 120:109475. doi: 10.1016/j.biopha.2019.109475
8. Childs A, Phaneuf S, Dirks A, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. (2002) 62:4592–8.
9. Xu Z, Li C, Liu Q, Li P, Yang H. Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci. (2018) 19:3658. doi: 10.3390/ijms19113658
10. Wang A, Zhang J, Xiao M, Wang S, Wang B, Guo Y, et al. Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways. Cell Mol Life Sci. (2021) 78:3105–25. doi: 10.1007/s00018-020-03729-y
11. Davies K, Doroshow J. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. (1986) 261:3060–7. doi: 10.1016/S0021-9258(17)35746-0
12. Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad S, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. (2014) 124:617–30. doi: 10.1172/JCI72931
13. Cheung K, Cole L, Xiang B, Chen K, Ma X, Myal Y, et al. Sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. J Biol Chem. (2015) 290:10981–93. doi: 10.1074/jbc.M114.607960
14. Wang X, Wang X, Chen H, Wu D, Chen J, Wang X, et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol. (2014) 88:334–50. doi: 10.1016/j.bcp.2014.01.040
15. Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri M, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. (2012) 23(Suppl. 7):vii155–66. doi: 10.1093/annonc/mds293
16. Baldassarre L, Yang E, Cheng R, DeCara J, Dent S, Liu J, et al. Cardiovascular care of the oncology patient during COVID-19: an expert consensus document from the ACC cardio-oncology and imaging councils. J Natl Cancer Inst. (2021) 113:513–22. doi: 10.1093/jnci/djaa177
17. D’Agostino R Sr, Grundy S, Sullivan L, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. (2001) 286:180–7. doi: 10.1001/jama.286.2.180
18. Jin H, Jiang H. Cardiovascular Risk Factors Increase the Incidence of Anthracyclines Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. London: NIHR (2022).
19. Buzdar A, Marcus C, Smith T, Blumenschein G. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. (1985) 55:2761–5. doi: 10.1002/1097-0142(19850615)55:12<2761::AID-CNCR2820551206>3.0.CO;2-P
20. Gennari A, Salvadori B, Donati S, Bengala C, Orlandini C, Danesi R, et al. Cardiotoxicity of epirubicin/paclitaxel-containing regimens: role of cardiac risk factors. J Clin Oncol. (1999) 17:3596–602. doi: 10.1200/JCO.1999.17.11.3596
21. Pinder M, Duan Z, Goodwin J, Hortobagyi G, Giordano S. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. (2007) 25:3808–15. doi: 10.1200/JCO.2006.10.4976
22. Perez E, Suman V, Davidson N, Sledge G, Kaufman P, Hudis C, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. (2008) 26:1231–8. doi: 10.1200/JCO.2007.13.5467
23. Sawaya H, Sebag I, Plana J, Januzzi J, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. (2011) 107:1375–80. doi: 10.1016/j.amjcard.2011.01.006
24. Aitelhaj M, Lkhouyaali S, Rais G, Mohtaram A, Raissouni S, Ghissassi B, et al. Cardiac safety of the adjuvant trastuzumab in a Moroccan population: observational monocentric study of about 100 patients. BMC Res Notes. (2013) 6:339. doi: 10.1186/1756-0500-6-339
25. Chung W, Yi J, Jin J, Choi Y, Park C, Park W, et al. Early cardiac function monitoring for detection of subclinical doxorubicin cardiotoxicity in young adult patients with breast cancer. J Breast Cancer. (2013) 16:178–83. doi: 10.4048/jbc.2013.16.2.178
26. Vivenza D, Feola M, Garrone O, Monteverde M, Merlano M, Lo Nigro C. Role of the renin-angiotensin-aldosterone system and the glutathione S-transferase mu, pi and theta gene polymorphisms in cardiotoxicity after anthracycline chemotherapy for breast carcinoma. Int J Biol Markers. (2013) 28:e336–47. doi: 10.5301/JBM.5000041
27. Caram M, Guo C, Leja M, Smerage J, Henry N, Giacherio D, et al. Doxorubicin-induced cardiac dysfunction in unselected patients with a history of early-stage breast cancer. Breast Cancer Res Treat. (2015) 152:163–72. doi: 10.1007/s10549-015-3454-8
28. Serrano J, González I, Del Castillo S, Muñiz J, Morales L, Moreno F, et al. Diastolic dysfunction following anthracycline-based chemotherapy in breast cancer patients: incidence and predictors. Oncologist. (2015) 20:864–72. doi: 10.1634/theoncologist.2014-0500
29. Mina A, Rafei H, Khalil M, Hassoun Y, Nasser Z, Tfayli A. Role of baseline echocardiography prior to initiation of anthracycline-based chemotherapy in breast cancer patients. BMC Cancer. (2015) 15:10. doi: 10.1186/s12885-014-1004-0
30. Kotwinski P, Smith G, Cooper J, Sanders J, Ma L, Teis A, et al. Body surface area and baseline blood pressure predict subclinical anthracycline cardiotoxicity in women treated for early breast cancer. PLoS One. (2016) 11:e0165262. doi: 10.1371/journal.pone.0165262
31. Matos E, Jug B, Blagus R, Zakotnik B. A prospective cohort study on cardiotoxicity of adjuvant trastuzumab therapy in breast cancer patients. Arq Bras Cardiol. (2016) 107:40–7. doi: 10.5935/abc.20160084
32. Reinbolt R, Patel R, Pan X, Timmers C, Pilarski R, Shapiro C, et al. Risk factors for anthracycline-associated cardiotoxicity. Support Care Cancer. (2016) 24:2173–80. doi: 10.1007/s00520-015-3008-y
33. Ganz P, Romond E, Cecchini R, Rastogi P, Geyer C Jr, Swain S, et al. Long-term follow-up of cardiac function and quality of life for patients in NSABP protocol B-31/NRG oncology: a randomized trial comparing the safety and efficacy of doxorubicin and cyclophosphamide (AC) followed by paclitaxel with AC followed by paclitaxel and trastuzumab in patients with node-positive breast cancer with tumors overexpressing human epidermal growth factor receptor 2. J Clin Oncol. (2017) 35:3942–8. doi: 10.1200/JCO.2017.74.1165
34. He F. An analysis of incidence and risk factor of acute cardiactoxicity associated with anthracyclines use in patients with breast cancer. Chin Pharmaceut J. (2017) 24:1089–92.
35. Kim I, Lee J, Youn H, Song B, Chae B. Cardioprotective effect of dexrazoxane in patients with HER2-positive breast cancer who receive anthracycline based adjuvant chemotherapy followed by trastuzumab. J Breast Cancer. (2017) 20:82–90. doi: 10.4048/jbc.2017.20.1.82
36. Pearson E, Nair A, Daoud Y, Blum J. The incidence of cardiomyopathy in BRCA1 and BRCA2 mutation carriers after anthracycline-based adjuvant chemotherapy. Breast Cancer Res Treat. (2017) 162:59–67. doi: 10.1007/s10549-016-4101-8
37. Stachowiak P, Wojtarowicz A, Milchert-Leszczyńska M, Safranow K, Falco M, Kaliszczak R, et al. The paradox of the first cycle of chemotherapy-transient improvement of contractility and diastolic function after the first cycle of anthracycline-based chemotherapy: a prospective clinical trial. Oncotarget. (2017) 8:96442–52. doi: 10.18632/oncotarget.21279
38. Wu S, Tam M, Vega R, Perez C, Gerber N. Effect of breast irradiation on cardiac disease in women enrolled in BCIRG-001 at 10-year follow-up. Int J Radiat Oncol Biol Phys. (2017) 99:541–8. doi: 10.1016/j.ijrobp.2017.06.018
39. El-Sherbeny W, Sabry N, Sharbay R. Prediction of trastuzumab-induced cardiotoxicity in breast cancer patients receiving anthracycline-based chemotherapy. J Echocardiogr. (2019) 17:76–83. doi: 10.1007/s12574-018-0394-4
40. Li H, Hu B, Guo Z, Jiang X, Su X, Zhang X. Correlation of UGT2B7 polymorphism with cardiotoxicity in breast cancer patients undergoing epirubicin/cyclophosphamide-docetaxel adjuvant chemotherapy. Yonsei Med J. (2019) 60:30–7. doi: 10.3349/ymj.2019.60.1.30
41. Santos, D, Tettamanti M, Chacón C, Nadal J, Costanzo V, Nervo A. Cardiotoxicity alerts during treatment with trastuzumab in breast cancer at four-year follow-up. Rev Argent Cardiol. (2019) 87:105–10.
42. Fogarassy G, Fogarassyné Vathy Á, Kováts T, Hornyák L, Kenessey I, Veress G, et al. [Analysing the risk factors of doxorubicin-associated heart failure by a retrospective study of integrated, nation-wide databases]. Orv Hetil. (2020) 161:1094–102. doi: 10.1556/650.2020.31739
43. Rüger A, Schneeweiss A, Seiler S, Tesch H, van Mackelenbergh M, Marmé F, et al. Cardiotoxicity and cardiovascular biomarkers in patients with breast cancer: data from the GeparOcto-GBG 84 trial. J Am Heart Assoc. (2020) 9:e018143. doi: 10.1161/JAHA.120.018143
44. Tan L, Su X, Li X, Li H, Hu B. Correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab adjuvant chemotherapy. Int J Clin Exp Pathol. (2020) 13:286–94.
45. Todorova V, Hsu P, Wei J, Lopez-Candales A, Chen J, Su L, et al. Biomarkers of inflammation, hypercoagulability and endothelial injury predict early asymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. Am J Cancer Res. (2020) 10:2933–45.
46. Vaitiekus D, Muckiene G, Vaitiekiene A, Maciuliene D, Vaiciuliene D, Ambrazeviciute G, et al. Impact of arterial hypertension on doxorubicin-based chemotherapy-induced subclinical cardiac damage in breast cancer patients. Cardiovasc Toxicol. (2020) 20:321–7. doi: 10.1007/s12012-019-09556-3
47. Cho H, Lee S, Sim S, Park I, Lee K, Kwak M, et al. Cumulative incidence of chemotherapy-induced cardiotoxicity during a 2-year follow-up period in breast cancer patients. Breast Cancer Res Treat. (2020) 182:333–43. doi: 10.1007/s10549-020-05703-5
48. Egashira K, Sueta D, Tomiguchi M, Kidoh M, Oda S, Usuku H, et al. Cardiac computed tomography-derived extracellular volume fraction in late anthracycline-induced cardiotoxicity. Int J Cardiol Heart Vasc. (2021) 34:100797. doi: 10.1016/j.ijcha.2021.100797
49. Feng Q, Ren Y, Hou A, Guo J, Mao Z, Liu S, et al. MicroRNA-130a increases and predicts cardiotoxicity during adjuvant chemotherapy in human epidermal growth factor receptor-2-positive breast cancer. J Breast Cancer. (2021) 24:153–63. doi: 10.4048/jbc.2021.24.e15
50. Houbois C, Nolan M, Somerset E, Shalmon T, Esmaeilzadeh M, Lamacie M, et al. Serial cardiovascular magnetic resonance strain measurements to identify cardiotoxicity in breast cancer: comparison with echocardiography. JACC Cardiovasc Imaging. (2021) 14:962–74. doi: 10.1016/j.jcmg.2020.09.039
51. Guenancia C, Lefebvre A, Cardinale D, Yu A, Ladoire S, Ghiringhelli F, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol. (2016) 34:3157–65. doi: 10.1200/JCO.2016.67.4846
52. Kala P, Bartušková H, Pit’ha J, Vaòourková Z, Kikerlová S, Jíchová Š, et al. Deleterious effects of hyperactivity of the renin-angiotensin system and hypertension on the course of chemotherapy-induced heart failure after doxorubicin administration: a study in ren-2 transgenic rat. Int J Mol Sci. (2020) 21:9337. doi: 10.3390/ijms21249337
53. Sharkey L, Radin M, Heller L, Rogers L, Tobias A, Matise I, et al. Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension-heart failure (SHHF), spontaneously hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol Appl Pharmacol. (2013) 273:47–57. doi: 10.1016/j.taap.2013.08.012
54. Agostinucci K, Grant M, Seelig D, Yücel D, van Berlo J, Bartolomucci A, et al. Divergent cardiac effects of angiotensin II and isoproterenol following juvenile exposure to doxorubicin. Front Cardiovasc Med. (2022) 9:742193. doi: 10.3389/fcvm.2022.742193
55. Sobczuk P, Czerwińska M, Kleibert M, Cudnoch-Jêdrzejewska A. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system-from molecular mechanisms to therapeutic applications. Heart Fail Rev. (2022) 27:295–319. doi: 10.1007/s10741-020-09977-1
56. Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. (2000) 35:580–6. doi: 10.1161/01.HYP.35.2.580
57. Li J, Gall N, Grieve D, Chen M, Shah A. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. (2002) 40:477–84. doi: 10.1161/01.HYP.0000032031.30374.32
58. Chen Y, Li S, Guo Y, Yu H, Bao Y, Xin X, et al. Astaxanthin attenuates hypertensive vascular remodeling by protecting vascular smooth muscle cells from oxidative stress-induced mitochondrial dysfunction. Oxid Med Cell Longev. (2020) 2020:4629189. doi: 10.1155/2020/4629189
59. Jia W, Bai T, Zeng J, Niu Z, Fan D, Xu X, et al. Combined administration of metformin and atorvastatin attenuates diabetic cardiomyopathy by inhibiting inflammation, apoptosis, and oxidative stress in type 2 diabetic mice. Front Cell Dev Biol. (2021) 9:634900. doi: 10.3389/fcell.2021.634900
60. Zbinden G, Brändle E, Pfister M. Modification of adriamycin toxicity in rats fed a high fat diet. Agents Actions. (1977) 7:163–70. doi: 10.1007/BF01964915
61. Butler T, Ashford D, Seymour A. Western diet increases cardiac ceramide content in healthy and hypertrophied hearts. Nutr Metab Cardiovasc Dis. (2017) 27:991–8. doi: 10.1016/j.numecd.2017.08.007
62. Al-Shabanah O, El-Kashef H, Badary O, Al-Bekairi A, Elmazar M. Effect of streptozotocin-induced hyperglycaemia on intravenous pharmacokinetics and acute cardiotoxicity of doxorubicin in rats. Pharmacol Res. (2000) 41:31–7. doi: 10.1006/phrs.1999.0568
63. Aluganti Narasimhulu C, Singla D. Doxorubicin-induced apoptosis enhances monocyte infiltration and adverse cardiac remodeling in diabetic animals. Can J Physiol Pharmacol. (2022) 100:441–52. doi: 10.1139/cjpp-2021-0596
64. Nishiyama K, Numaga-Tomita T, Fujimoto Y, Tanaka T, Toyama C, Nishimura A, et al. Ibudilast attenuates doxorubicin-induced cytotoxicity by suppressing formation of TRPC3 channel and NADPH oxidase 2 protein complexes. Br J Pharmacol. (2019) 176:3723–38. doi: 10.1111/bph.14777
65. de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown J, Vicente M, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. (2010) 119:145–53. doi: 10.1007/s10549-009-0512-0
66. Mitra M, Donthamsetty S, White B, Mehendale H. High fat diet-fed obese rats are highly sensitive to doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol. (2008) 231:413–22. doi: 10.1016/j.taap.2008.05.006
67. Maruyama S, Shibata R, Ohashi K, Ohashi T, Daida H, Walsh K, et al. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J Biol Chem. (2011) 286:32790–800. doi: 10.1074/jbc.M111.245985
68. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. (1999) 257:79–83. doi: 10.1006/bbrc.1999.0255
69. Lage R, Cebro-Márquez M, Rodríguez-Mañero M, González-Juanatey J, Moscoso I. Omentin protects H9c2 cells against docetaxel cardiotoxicity. PLoS One. (2019) 14:e0212782. doi: 10.1371/journal.pone.0212782
70. Kirkham A, Paterson D, Prado C, Mackey J, Courneya K, Pituskin E, et al. Rationale and design of the caloric restriction and exercise protection from anthracycline toxic effects (CREATE) study: a 3-arm parallel group phase II randomized controlled trial in early breast cancer. BMC Cancer. (2018) 18:864. doi: 10.1186/s12885-018-4778-7
Keywords: Framingham risk score, breast cancer, anthracycline-induced cardiotoxicity, cardiovascular risk factors, anthracycline
Citation: Jin H, Xu J, Sui Z and Wang L (2023) Risk factors from Framingham risk score for anthracyclines cardiotoxicity in breast cancer: A systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1101585. doi: 10.3389/fcvm.2023.1101585
Received: 18 November 2022; Accepted: 05 January 2023;
Published: 19 January 2023.
Edited by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Yan Luo, University of South China, ChinaDongliang Du, Moffitt Cancer Center, United States
Yi Liao, Moffitt Cancer Center, United States
Copyright © 2023 Jin, Xu, Sui and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Sui,  c3VpemhlbmcxMTIzQDE2My5jb20=; Lili Wang,
c3VpemhlbmcxMTIzQDE2My5jb20=; Lili Wang,  d2xsX2RtdUBzb2h1LmNvbQ==
d2xsX2RtdUBzb2h1LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Hao Jin1†
Hao Jin1† Zheng Sui
Zheng Sui