- 1Department of Stomatology, The Second Affiliated Hospital of Shandong First Medical University, Tai'an, China
- 2Department of Pharmacy, The Second Affiliated Hospital of Shandong First Medical University, Tai'an, China
- 3Department of Cardiology, The Second Affiliated Hospital of Shandong First Medical University, Tai'an, China
Background: Data about real-world effects of combined therapy with sacubitril/valsartan plus dapagliflozin in patients affected by heart failure (HF) with reduced ejection fraction (HFrEF) has not been widely reported. In this article, the benefits of dapagliflozin and sacubitril/valsartan respect to improvements of cardiac function in patients with HFrEF would be investigated.
Methods: HF patients prescribed sacubitril/valsartan between January 2020 and January 2022 in a tertiary teaching hospital were selected using the Computerized Patient Record System. Patients were divided into two groups according to whether they were taking dapagliflozin. Clinical parameters at baseline and during follow-up were retrospectively collected and analyzed.
Results: Total of 136 consecutive patients were recruited for this study. 72 patients treated with sacubitril/valsartan and dapagliflozin were assigned to Group A, and another 64 patients receiving sacubitril/valsartan monotherapy were assigned to Group B. After treatment with sacubitril/valsartan plus dapagliflozin for a median follow-up period of 189 days (IQR, 180–276), significant improvements of cardiac function were achieved in Group A. Median N-terminal pro-B-type natriuretic peptide (NT-proBNP) level was significantly decreased from 2585 pg/ml (1014–3702.5) to 1260.5 pg/ml (439.8–2214.3) (P < 0.001). Mean left ventricular ejection fraction (LVEF) improved from 34.7 ± 4.6% to 39.2 ± 7.5% (P < 0.001). Mean daily dose of loop diuretics decreased from 37.1 ± 17.3 mg/day to 25.9 ± 18.5 mg/day (P < 0.001). Regarding safety, both systolic blood pressure (P = 0.002) and diastolic blood pressure (P = 0.002) significantly decreased. For patients in Group B, significant improvements in mean LVEF (P < 0.001), decreases in mean daily dose of loop diuretics (P = 0.001) and reductions in diastolic blood pressure (P = 0.023) were observed. Strikingly, both median Δ NT-proBNP (P = 0.04) and median Δ LAD (P = 0.006) in Group A were more pronounced in comparison with those seen in Group B.
Conclusions: The combined use of sacubitril/valsartan and dapagliflozin was associated with improved cardiac function in patents with HFrEF, and led to greater reductions in LAD and NT-proBNP levels compared to sacubitril/valsartan monotherapy. These findings suggest that the combination therapy may offer more potent cardiovascular benefits.
Introduction
Heart failure (HF) is a global major public health problem, with frequent re-hospitalizations, high mortality rates, and poor quality of life (1–3). Neurohumoral antagonists including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), beta-blockers and mineralocorticoid receptor antagonists (MRAs) represent the cornerstones of modern HF therapy and have decreased the mortality and re-hospitalization rates of HF patients. However, clinical prognosis in patients with HF remains unsatisfactory (4). Therefore, novel drugs were required to improve the outcome of these patients.
As a first-in-class angiotensin receptor-neprilysin inhibitor (ARNI), sacubitril/valsartan brought new option for the treatment of HF (5, 6). The clinical trials and real-world studies have established its long-lasting efficacy in reducing the combined risk of death from cardiovascular causes or hospital admission for HF and improving several clinical, hemodynamic, and echocardiographic parameters (7–12). Additionally, dapagliflozin, sodium-glucose co-transporter-2 (SGLT-2) inhibitor, has been shown to reduce the composite of cardiovascular death or worsening HF in patients with heart failure with reduced ejection fraction (HFrEF) in the DAPA-HF (Dapagliflozin And Prevention of Adverse outcomes in Heart Failure) trial (13). Based on treatment benefits observed in the pivotal trials, both sacubitril/valsartan and dapagliflozin received a Class I indication in most international clinical practice guidelines (14, 15). Previous studies of sacubitril/valsartan or dapagliflozin had few patients taking both drugs simultaneously. As a result, it was hard to evaluate the potential incremental value of combined treatment with dapagliflozin plus sacubitril/valsartan compared to merely one drug. Based on the discovery of the lack of a treatment interaction between baseline sacubitril/valsartan use and randomized dapagliflozin therapy in DAPA-HF trial, Solomon et al. estimated indirectly that benefit of dapagliflozin plus sacubitril/valsartan would be additive (16). However, direct evidence was still required for clinical decision making. To bridge this research gap, the efficacy and safety of combined therapy with dapagliflozin and sacubitril/valsartan compared to sacubitril/valsartan monotherapy in patients with HFrEF would be investigated in this article.
Materials and methods
Study population
HF patients receiving therapy with sacubitril/valsartan between January 2020 and January 2022 in a tertiary teaching hospital were selected using the Computerized Patient Record System (CPRS). Patients were divided into two groups according to whether they were taking dapagliflozin at baseline. Patients were included if they were at least 18 years of age, had New York Heart Association (NYHA) functional classes II to IV, and LVEF ≤ 40% by echocardiography. The exclusion criteria were as follows: (1) patients lost to any follow-up, (2) sacubitril/valsartan and/or dapagliflozin discontinued at follow-up, (3) HF primarily resulting from right ventricular failure, pericardial disease, or congenital heart disease, and (4) patients with malignant tumors. This study was in accordance with the Declaration of Helsinki and approved by the ethics committee of the hospital.
Dosage and follow-up interval
At baseline, first dose of sacubitril/valsartan was decided by physicians according to clinical conditions. If tolerated during follow-up, patients should be titrated to the maximum tolerated dose. While, the initial dose of dapagliflozin should be the target dose (10 mg daily) or the maximally tolerated dose. The follow-up interval for assessment of blood pressure, NYHA functional class, laboratory tests, and echocardiography could not be pre-specified in the present retrospective observational study. However, to evaluate the effectiveness of the medical therapy (the combination of sacubitril/valsartan and dapagliflozin vs. sacubitril/valsartan monotherapy), we investigated the above variables at the time closest to 6 months after the initiation of medical treatment. As a result, these variables were evaluated on a median of 189 (IQR 180-276) days after the initiation of treatment.
Study parameters and data collection
Clinical characteristics, including age, gender, smoker, alcohol drinking, prior hospitalization for HF, duration of HF, HF aetiology, mean dose of sacubitril/valsartan, mean dose of dapagliflozin, comorbidities, and drugs, were recorded for every patients at baseline. Meanwhile, blood pressure, NYHA functional class, laboratory tests, echocardiography and loop diuretics dose in furosemide equivalents (furosemide 20 mg = torsemide 10 mg) aimed to evaluate efficacy and safety of therapeutic drugs should be collected at baseline and during follow-up.
Statistical analyses
Quantitative variables were presented as mean ± standard deviation if normally distributed or as median and interquartile range if not normally distributed. Normality was checked by the Kolmogorov-Smirnov test. Categorical data were expressed as numbers and percentages. Continuous data were compared with the Student's t-test or the Mann-Whitney U test, and categorical data were compared with χ2 test. Statistical significance was set at a two-tailed p-value < 0.05. Statistics were performed using the SPSS Statistics 26.0 software (Chicago, IL, USA).
Results
General information and baseline characteristics
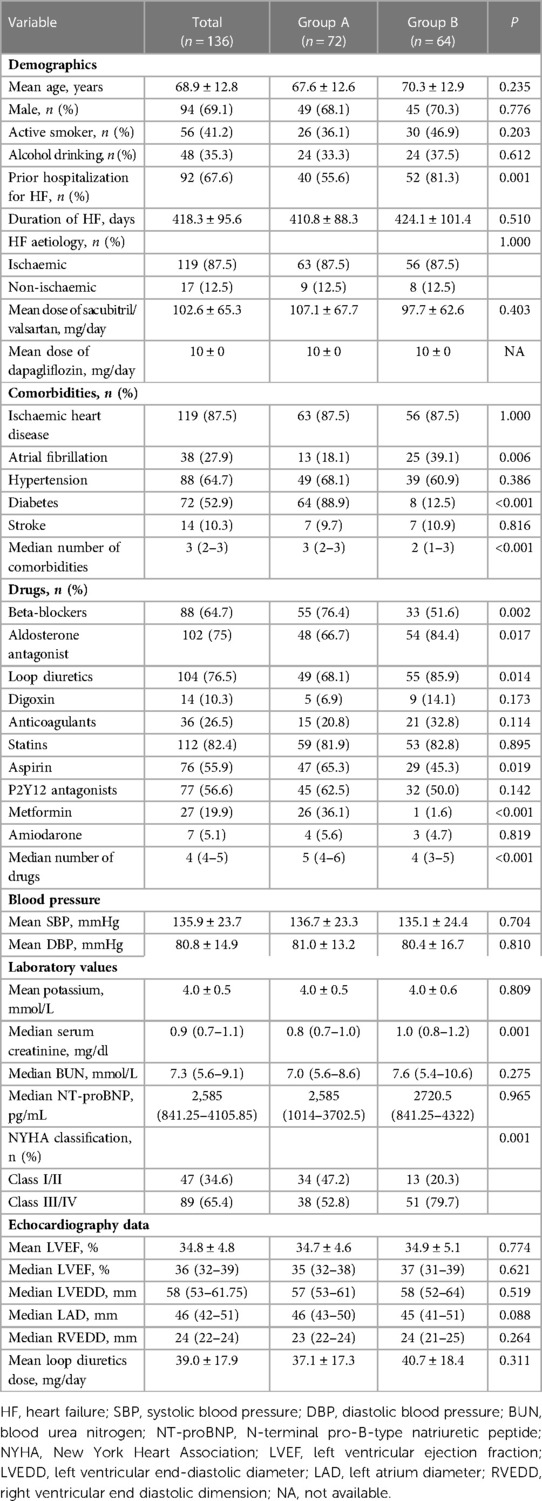
After applying both the inclusion and exclusion criteria, a total of 136 consecutive patients (mean age 68.9 ± 12.8 years, 69.1% male) were selected for this study. Baseline characteristics of enrolled patients are summarized in Table 1. For comparison between the two treatment strategies, 72 patients treated with sacubitril/valsartan combined with dapagliflozin were assigned to Group A, and another 64 patients receiving sacubitril/valsartan without dapagliflozin were assigned to Group B.
As shown in Table 1, compared with patients in Group B, patients receiving combination treatment (Group A) were less likely to have a prior hospitalization for HF, had better baseline NYHA functional class, higher number of comorbidities and higher number of drugs, were less likely to have a history of atrial fibrillation and more likely to have a history of diabetes, had lower serum creatinine, and were more often treated with beta-blockers, aspirin, and metformin and less likely to have received aldosterone antagonist and loop diuretics. Other variables, including age, gender, active smoker, alcohol drinking, duration of HF, HF aetiology, maintained dose of sacubitril/valsartan, history of ischaemic heart disease, hypertension, or stroke, use of digoxin, anticoagulants, statins, P2Y12 antagonists, and amiodarone, systolic blood pressure (SBP), diastolic blood pressure (DBP), laboratory tests, echocardiography data, and loop diuretics dose were similar between Group A and Group B.
Intra-group comparisons of clinical parameters from baseline to follow-up
In Group A, after treatment with sacubitril/valsartan plus dapagliflozin for a median follow-up period of 189 days (IQR, 180–276), median N-terminal pro-B-type natriuretic peptide (NT-proBNP) level was significantly decreased from 2585 pg/ml (1014–3702.5) to 1260.5 pg/ml (439.8–2214.3) (P < 0.001) (Figure 1A). The proportion of patients in NYHA Class III/IV decreased slightly from 52.8% to 51.4% (P = 0.868) (Figure 1B). Moreover, noticeable improvements in a series of echocardiographic parameters were also observed during follow-up. Mean left ventricular ejection fraction (LVEF) improved from 34.7 ± 4.6% to 39.2 ± 7.5% (P < 0.001) (Figure 1C). Median left ventricular end-diastolic diameter (LVEDD) decreased from 57 mm (IQR, 53–61) to 56 mm (IQR, 50.3–60) (P = 0.042). Median left atrium diameter (LAD) decreased from 46 mm (IQR, 43–50) to 44.5 mm (IQR, 40–48) (P = 0.003). The mean daily dose of loop diuretics in furosemide equivalents decreased from 37.1 ± 17.3 mg/day to 25.9 ± 18.5 mg/day (P < 0.001) (Figure 1I). Regarding safety, both SBP (from 136.7 ± 23.3 mmHg to 128.3 ± 21.2 mmHg, P = 0.002) and DBP (from 81 ± 13.2 mmHg to 75.4 ± 14.3 mmHg, P = 0.002) significantly reduced after combined treatment (Figures 1D,E). Median serum creatinine level (Figure 1F) and median blood urea nitrogen (BUN) level (Figure 1G) did not change obviously during follow-up, but mean potassium (Figure 1H) decreased distinctly. Additionally, hypotension (SBP < 100 mmHg) occurred only in 1 patients during the treatment period.
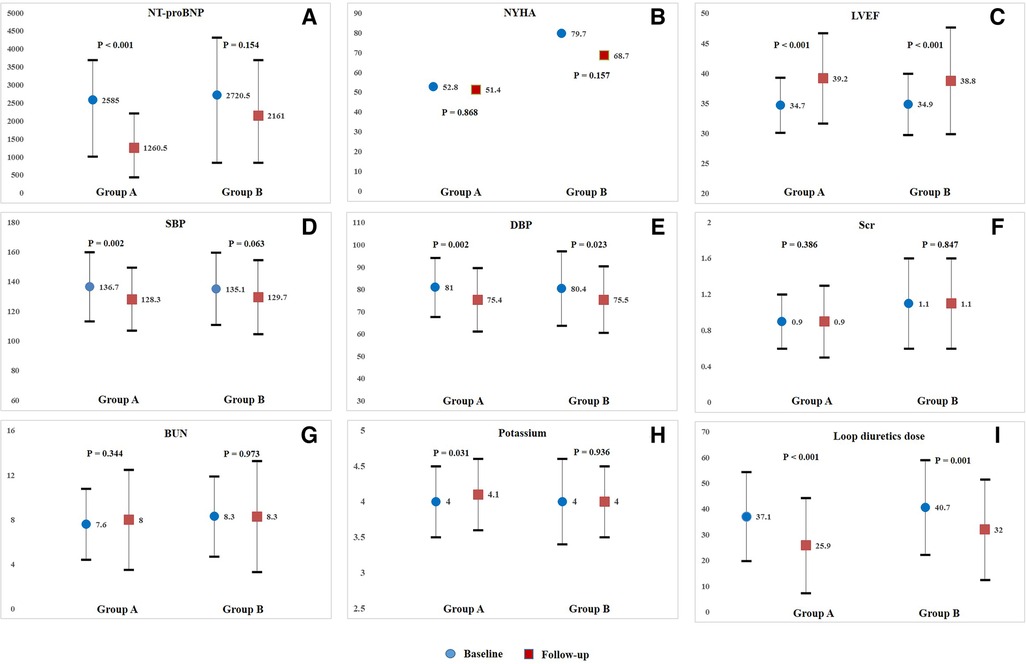
Figure 1. Intra-group comparisons of NT-proBNP(A), NYHA class (B), LVEF(C), SBP(D), DBP(E), Scr(F), BUN(G), potassium(H), and loop diuretics dose(I), from baseline to follow-up in Group A or Group B. NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; DBP, diastolic blood pressure; Scr, serum creatinine; BUN, blood urea nitrogen.
For patients in Group B, only mean LVEF improved significantly from 34.9 ± 5.1% to 38.8 ± 8.9% (P < 0.001) (Figure 1C), but no obvious improvements in other echocardiographic parameters were observed at follow-up. Additionally, mean daily dose of loop diuretics also decreased significantly from 40.7 ± 18.4 mg/day to 32.0 ± 19.5 mg/day (P = 0.001) (Figure 1I). Both median NT-proBNP level (P = 0.154) and the proportion of patients in NYHA Class III/IV decreased slightly (P = 0.157) (Figures 1A,B). Regarding safety, only DBP significantly reduced from 80.4 ± 16.7 mmHg to 75.5 ± 14.9 mmHg (P = 0.023) (Figure 1E). No obvious changes were observed in SBP (Figure 1D), median serum creatinine level (Figure 1F), median BUN level (Figure 1G), and mean potassium level (Figure 1H) from baseline to follow-up. Over the entire treatment period, hypotension occurred in 5 patients, and no drug withdrawal occurred in those patients. Intra-group comparisons of clinical parameters from baseline to follow-up in Group A or Group B were illustrated in Figure 1 and Supplementary Table S1.
Comparisons of changes in clinical parameters from baseline to follow-up between group A and group B
Table 2 illustrates comparative analysis of changes in clinical parameters from baseline to follow-up between Group A and Group B. Strikingly, both median Δ NT-proBNP (P = 0.04) and median Δ LAD (P = 0.006) in Group A were more pronounced in comparison with those seen in Group B. Changes in other clinical parameters in Group A were also obvious compared with Group B, but there were no statistically significant differences.
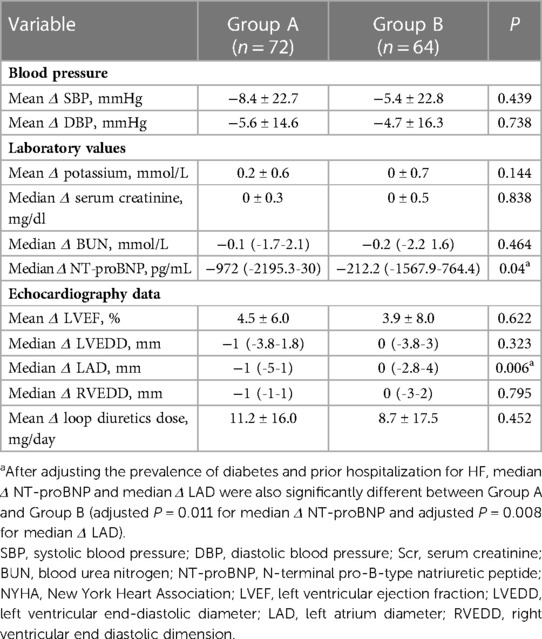
Table 2. Comparisons of changes in clinical parameters from baseline to follow-up between group A and group B.
Discussion
In this article, our data supported the evidence that combined therapy with sacubitril/valsartan plus dapagliflozin could effectively improve cardiac function and was well tolerated in Chinese patients with HFrEF. Furthermore, we showed that combined therapy with sacubitril/valsartan plus dapagliflozin led to greater reductions in LAD and NT-proBNP levels compared to sacubitril/valsartan monotherapy.
As novelty in HF therapy, sacubitril/valsartan and dapagliflozin, which reduced cardiovascular mortality and morbidity in randomized controlled trials, had emerged as evidence-based therapies for HF (7, 13, 17–20). 2021 ESC guideline on HF gave a Class I recommendation for the use of sacubitril/valsartan and dapagliflozin in HFrEF patients, and required that two drugs should be initiated simultaneously and up-titrated rapidly (21). Worth noting, patients in real-world clinical practice had many comorbidities, which might influenced therapeutic regimen (11, 12). In the present study, patients received combined treatment were more likely to have a history of diabetes mellitus compared with those taking sacubitril/valsartan monotherapy. Consistently, in a recently published study, the proportion of patients who were comorbid with diabetes mellitus in the sacubitril/valsartan plus dapagliflozin group was significantly higher than that in sacubitril/valsartan group (74.1% vs. 51.9%, P = 0.001) (22). This gives us a hint that patients with HFrEF and concomitant diabetes mellitus are more likely to be treated with sacubitril/valsartan plus dapagliflozin. Additionally, in February 2021, dapagliflozin was approved to treat HF in China. Therefore, the use of dapagliflozin for managing HF had been limited to diabetic patients before approval, which might result in difference in the prevalence of diabetes mellitus between Group A and Group B.
Another thing to be noted is that the mean maximum tolerated dose of sacubitril/valsartan achieved in Group A (107.1 ± 67.7 mg) or Group B (97.7 ± 62.6 mg) is lower than that achieved in PARADIGM-HF trial (7). Indeed, low dose of sacubitril/valsartan is very common in real-world clinical setting due to several factors (symptomatic hypotension, hyperkalemia, renal dysfunction and worsening heart failure), which is a clear difference from landmark trial (23–27). In a prospective observational cohort study, even under therapy with low-dose sacubitril/valsartan (135.9 ± 75.5 mg), significant decrease in NT-proBNP concentration (from 2,495 pg/ml to 943 pg/ml, P < 0.001) and prominent increase in the LVEF (from 35.6% ± 10% to 47.% ± 14.2%, P < 0.001) were observed (28). Another real-world study also confirmed that low-dose sacubitril/valsartan (122.5 ± 55.2 mg) significantly reduced NT-proBNP (from 3,003 pg/mL to 2,039 pg/mL, P = 0.010), improved NYHA classification (P < 0.001), and induced beneficial cardiac reverse remodeling (LVEF increased from 31 ± 6% to 38 ± 10%, P < 0.001) (11). Additionally, sacubitril/valsartan showed well tolerability, and fewer patients discontinued sacubitril/valsartan due to hypotension or abnormal laboratory values (11, 28). Consistently, patients receiving the low dosage of sacubitril/valsartan monotherapy (Group B) in this article also achieved prominent increase in LVEF (from 34.9 ± 5.1% to 38.8 ± 8.9%, P < 0.001). However, improvement in the NYHA class (P = 0.157) and reduction in NT-proBNP (P = 0.154) concentration was not significant, which could be explained by that daily dose of sacubitril/valsartan in this study was lower than that in the previous real-world studies (11, 26, 28). Fortunately, in patients treated with sacubitril/valsartan and dapagliflozin (Group A), significant decrease in median NT-proBNP level (from 2,585 pg/ml to 1260.5 pg/ml, P < 0.001), as well as pronounced improvements in left cardiac remodeling measurements including LVEF (P < 0.001), LAD (P = 0.003) and LVEDD (P = 0.042) were observed. Of note, daily dose of sacubitril/valsartan in Group A was as low as that in Group B.
Furthermore, both median Δ NT-proBNP (unadjusted P = 0.04 and adjusted P = 0.011) and median Δ LAD (unadjusted P = 0.006 and adjusted P = 0.008) in Group A were more remarkable in comparison with those seen in Group B. This discovery revealed potential incremental value of treatment with both sacubitril/valsartan and dapagliflozin. In a retrospective observational study, long-term cardiac mortality rates in the sacubitril/valsartan plus dapagliflozin group (7.4%) were significantly lower than that in the sacubitril/valsartan monotherapy group (19.5%) (P = 0.01) (22). In another study conducted in diabetic patients with HFrEF, combination of ARNI and SGLT2 inhibitors could improve the clinical course of HFrEF in patients compared to ARNI monotherapy (29). Patients treated with combination of ARNI and SGLT2 inhibitors exhibited a lower risk of hospitalization for HF or cardiovascular mortality (P = 0.04) compared to those treated with ARNI only. Additionally, patients treated with combination of ARNI and SGLT2 inhibitors tended to show higher LVEF than those treated with ARNI only throughout the follow-up period. However, these differences were not statistically significant, which might attenuate incremental value of combined treatment with ARNI plus SGLT2 inhibitors on echocardiographic parameters compared to merely ARNI (29). Notably, in a study reported by Hwang et al., HF patients treated with SGLT2 inhibitors showed a significant decrease in LVEDD (P < 0.001) and improvement in LVEF (P < 0.001) (30). Therefore, further studies are required to investigate the effective mechanism of action of SGLT2 inhibitors when it is added to ARNI treatment regimens. In a subgroup analysis of the DAPA-HF trial, Solomon et al. discovered indirectly that the use of sacubitril/valsartan and dapagliflozin together could further lower morbidity and mortality in patients with HFrEF without compromising safety (16). The results in these studies provided evidence that the clinical benefits of treatment with both sacubitril/valsartan and dapagliflozin might be greater than sacubitril/valsartan monotherapy.
It is worth noting that differences in the prevalence of diabetes and incidence of prior hospitalization for HF between Group A and Group B at baseline in the study might impact incremental value of combined treatment with dapagliflozin plus sacubitril/valsartan compared to merely sacubitril/valsartan. As a common co-morbidity in patients suffering from HF, diabetes mellitus is a well-established risk factor for worse outcome in HF, and is associated with increased hospitalization and mortality rates in chronic HF (31). Diabetes can contribute to HF development and progression in multiple ways including metabolic and functional alterations, hyperglycemia-induced structural abnormalities, microvascular dysfunction, cardiac autonomic neuropathy, and neurohormonal abnormalities (32–34). Compared with nondiabetics, diabetics seem to have higher BNP levels (35) and depressed systolic function (36). Therefore, conclusion that the clinical benefits of treatment with both sacubitril/valsartan and dapagliflozin might be greater than sacubitril/valsartan monotherapy should be treated with caution due to differences in prevalence of diabetes between Group A and Group B at baseline. Hospitalization for HF represents a destabilizing event in the clinical trajectory of patients with HF (37). It should be stated that the incidence of prior hospitalization for HF was significantly higher in Group B compared to Group A at baseline in the study. This difference might suggest that patients in Group B had more advanced or prolonged HF, potentially attenuating the benefits of medical therapy. However, as another factor associated with poor outcome in HF (38), duration of HF was similar between Group A and Group B in the present study. This gave us a hint that patients in each group might have similar progression of HF. In the future, large-sample and multicenter studies are required to explore the effect of incidence of prior hospitalization for HF on the benefits of medical therapy.
Several limitations in the retrospective study should be mentioned. First, overall number of patients recruited in the current study was relatively small. Second, echocardiography data was evaluated by 2D-echocardiographic assessment in our study, which was not as accurate as 3D-echocardiography. Third, the maintenance dosage of sacubitril/valsartan was relatively low. Therefore, the optimal dosage of sacubitril/valsartan should be explored in the future. Scheduled drug-escalation programs which might be helpful to achieve higher daily dose of sacubitril/valsartan were required to establish for patients with HFrEF. Fourth, indicators of congestion, including central venous pressure and pulmonary capillary wedge pressure, were not measured in patients. Therefore, clinical data on the benefit of congestion could not be provided in the present study.
Conclusion
In patients with HFrEF, treatment with the combination of sacubitril/valsartan and dapagliflozin was associated with improved cardiac function, and resulted in greater reductions in LAD and NT-proBNP levels compared to sacubitril/valsartan monotherapy. These data would expand the combined use of sacubitril/valsartan and dapagliflozin as a daily routine in clinical practice if supported by more high-quality, large-sample, multicenter studies in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Second Affiliated Hospital of Shandong First Medical University Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CWW designed the study; GJ, ZXZ and LYM performed the study; DHQ and LYL analyzed the data; JJ wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Tai'an Science and Technology Innovation Development Project (No. 2020NS226) and Academic promotion programme of Shandong First Medical University (No. 2019QL017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fcvm.2023.1097066/full#supplementary-material
References
1. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. (2014) 1(1):4–25. doi: 10.1002/ehf2.12005
2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. (2014) 63(12):1123–33. doi: 10.1016/j.jacc.2013.11.053
3. Braunwald E. The war against heart failure: the lancet lecture. Lancet (London, England). (2015) 385(9970):812–24. doi: 10.1016/S0140-6736(14)61889-4
4. Zhang R, Sun X, Li Y, He W, Zhu H, Liu B, et al. The efficacy and safety of sacubitril/valsartan in heart failure patients: a review. J Cardiovasc Pharmacol Ther. (2022) 27:10742484211058681. doi: 10.1177/10742484211058681
5. Campbell DJ. Long-term neprilysin inhibition—implications for ARNIs. Nature reviews. Cardiology. (2017) 14(3):171–86. doi: 10.1038/nrcardio.2016.200
6. Sauer AJ, Cole R, Jensen BC, Pal J, Sharma N, Yehya A, et al. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev. (2019) 24(2):167–76. doi: 10.1007/s10741-018-9757-1
7. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371(11):993–1004. doi: 10.1056/NEJMoa1409077
8. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-Neprilysin inhibition in acute decompensated heart failure. N Engl J Med. (2019) 380(6):539–48. doi: 10.1056/NEJMoa1812851
9. Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. (2019) 21(8):998–1007. doi: 10.1002/ejhf.1498
10. Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J. (2017) 38(15):1132–43. doi: 10.1093/eurheartj/ehw570
11. Chen W, Liu Y, Li Y, Dang H. Sacubitril/valsartan improves cardiac function in Chinese patients with heart failure: a real-world study. ESC Heart Fail. (2021) 8(5):3783–90. doi: 10.1002/ehf2.13491
12. Armentaro G, D'Arrigo G, Magurno M, Toscani AF, Condoleo V, Miceli S, et al. Impact of sacubitril/valsartan on clinical and echocardiographic parameters in heart failure patients with reduced ejection fraction: data from a real life 2-year follow-up study. Front Pharmacol. (2021) 12:733475. doi: 10.3389/fphar.2021.733475
13. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
14. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
15. Writing Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. (2022) 28(5):e1–e167. doi: 10.1016/j.cardfail.2022.02.010
16. Solomon SD, Jhund PS, Claggett BL, Dewan P, Køber L, Kosiborod MN, et al. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA-HF trial. JACC Heart Fail. (2020) 8(10):811–8. doi: 10.1016/j.jchf.2020.04.008
17. Solomon SD, Vaduganathan M, Claggett BL, Packer M, Zile M, Swedberg K, et al. Sacubitril/valsartan across the Spectrum of ejection fraction in heart failure. Circulation. (2020) 141(5):352–61. doi: 10.1161/CIRCULATIONAHA.119.044586
18. Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, et al. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail. (2019) 7(6):457–65. doi: 10.1016/j.jchf.2019.02.015
19. Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, et al. Efficacy and safety of dapagliflozin in heart failure with mildly reduced or preserved ejection fraction according to age: the DELIVER trial. Circ Heart Fail. (2022) 15(10):e010080. doi: 10.1161/CIRCHEARTFAILURE.122.010080
20. Butt JH, Jhund PS, Belohlávek J, de Boer RA, Chiang CE, Desai AS, et al. Efficacy and safety of dapagliflozin according to frailty in patients with heart failure: a prespecified analysis of the DELIVER trial. Circulation. (2022) 146(16):1210–24. doi: 10.1161/CIRCULATIONAHA.122.061754
21. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. doi: 10.1002/ejhf.2333
22. Karabulut U, Keskin K, Karabulut D, Yiğit E, Yiğit Z. Effect of sacubitril/valsartan combined with dapagliflozin on long-term cardiac mortality in heart failure with reduced ejection fraction. Angiology. (2022) 73(4):350–6. doi: 10.1177/00033197211047329
23. Kido K, Bianco C, Caccamo M, Fang W, Sokos G. Evaluating sacubitril/valsartan dose dependence on clinical outcomes in patients with heart failure with reduced ejection fraction. Ann Pharmacother. (2021) 55(9):1069–75. doi: 10.1177/1060028020983522
24. Pandey AC, Jer D, Kuo RS, Yoo DH, Christophy A, Mohan RC, et al. Novel doses of sacubitril/valsartan in patients unable to tolerate traditional therapy: effects on N-terminal pro B-type natriuretic peptide levels. Clin Cardiol. (2021) 44(1):85–90. doi: 10.1002/clc.23509
25. Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril-valsartan in a real-world heart failure population: a community-based single-centre study. ESC heart Fail. (2018) 5(2):337–43. doi: 10.1002/ehf2.12251
26. De Vecchis R, Ariano C, Di Biase G, Noutsias M. In HFREF patients, sacubitril/valsartan, given at relatively low doses, does not lead to increased mortality or hospitalization: a retrospective cohort study. Herz. (2019) 44(7):651–8. doi: 10.1007/s00059-018-4690-6
27. Ekici B, Yaman M, Küçük M, Dereli S, Yenerçağ M, Yiğit Z, et al. Angiotensin receptor neprilysin inhibitor for patients with heart failure and reduced ejection fraction: real-world experience from Turkey (ARNi-TR). Turk Kardiyoloji Dernegi Arsivi. (2021) 49(5):357–67. doi: 10.5543/tkda.2021.63099
28. Hu J, Wu Y, Zhou X, Wang X, Jiang W, Huo J, et al. Beneficial effects of sacubitril/valsartan at low doses in an Asian real-world heart failure population. J Cardiovasc Pharmacol. (2020) 76(4):445–51. doi: 10.1097/FJC.0000000000000873
29. Kim HM, Hwang IC, Choi W, Yoon YE, Cho GY. Combined effects of ARNI and SGLT2 inhibitors in diabetic patients with heart failure with reduced ejection fraction. Sci Rep. (2021) 11(1):22342. doi: 10.1038/s41598-021-01759-5
30. Hwang IC, Cho GY, Yoon YE, Park JJ, Park JB, Lee SP, et al. Different effects of SGLT2 inhibitors according to the presence and types of heart failure in type 2 diabetic patients. Cardiovasc Diabetol. (2020) 19(1):69. doi: 10.1186/s12933-020-01042-3
31. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes Mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American heart association. Circulation. (2016) 134(23):e535–78. doi: 10.1161/CIR.0000000000000450
32. Cas A Dei, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. (2015) 3(2):136–45. doi: 10.1016/j.jchf.2014.08.004
33. Bauters C, Lamblin N, Mc Fadden EP, Van Belle E, Millaire A, de Groote P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol. (2003) 2:1. doi: 10.1186/1475-2840-2-1
34. Kasznicki J, Drzewoski J. Heart failure in the diabetic population—pathophysiology, diagnosis and management. Arch Med Sci. (2014) 10(3):546–56. doi: 10.5114/aoms.2014.43748
35. Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. (2004) 27(8):1929–35. doi: 10.2337/diacare.27.8.1929
36. Julián MT, Alonso N, Lupón J, Gavidia-Bovadilla G, Ferrer E, de Antonio M, et al. Long-term LVEF trajectories in patients with type 2 diabetes and heart failure: diabetic cardiomyopathy may underlie functional decline. Cardiovasc Diabetol. (2020) 19(1):38. doi: 10.1186/s12933-020-01011-w
37. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. (2007) 116(13):1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906
Keywords: heart failure, sacubitril/valsartan, dapagliflozin, combined therapy, effect
Citation: Jiang J, Gao J, Zhang X, Li Y, Dang H, Liu Y and Chen W (2023) Combined treatment with sacubitril/valsartan plus dapagliflozin in patients affected by heart failure with reduced ejection fraction. Front. Cardiovasc. Med. 10:1097066. doi: 10.3389/fcvm.2023.1097066
Received: 13 November 2022; Accepted: 7 March 2023;
Published: 22 March 2023.
Edited by:
Nicola Riccardo Pugliese, University of Pisa, ItalyReviewed by:
Pietro Mazzeo, University of Foggia, ItalyIn-Chang Hwang, Seoul National University Bundang Hospital, Republic of Korea
© 2023 Chen, Jiang, Gao, Zhang, Li, Dang, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Chen d2VuLTg2MDUyMUAxNjMuY29t
Specialty Section: This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
 Juan Jiang1
Juan Jiang1 Wenwen Chen
Wenwen Chen