
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 02 February 2023
Sec. Cardiovascular Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1095678
This article is part of the Research Topic Data Driven and Model Based Computational Futures in Cardiovascular Practice View all 9 articles
 Krish Chaudhuri1,2*
Krish Chaudhuri1,2* Alexander Pletzer3
Alexander Pletzer3 Steve W. F. R. Waqanivavalagi1,2
Steve W. F. R. Waqanivavalagi1,2 Paget Milsom2
Paget Milsom2 Nicolas P. Smith1,4
Nicolas P. Smith1,4Objectives: Flow competition between coronary artery bypass grafts (CABG) and native coronary arteries is a significant problem affecting arterial graft patency. The objectives of this study were to compare the predictive hemodynamic flow resulting from various total arterial grafting configurations and to evaluate whether the use of computational fluid dynamics (CFD) models capable of predicting flow can assist surgeons to make better decisions for individual patients by avoiding poorly functioning grafts.
Methods: Sixteen cardiac surgeons declared their preferred CABG configuration using bilateral internal mammary and radial arteries for each of 5 patients who had differing degrees of severe triple vessel coronary disease. Surgeons selected both a preferred 'aortic' strategy, with at least one graft arising from the ascending aorta, and a preferred “anaortic” strategy which could be performed as a “no-aortic touch” operation. CT coronary angiograms of the 5 patients were coupled to CFD models using a novel flow solver “COMCAB.” Twelve different CABG configurations were compared for each patient of which 4 were “aortic” and 8 were “anaortic.” Surgeons then selected their preferred grafting configurations after being shown predictive hemodynamic metrics including functional assessment of stenoses (instantaneous wave-free ratio; fractional flow reserve), transit time flowmetry graft parameters (mean graft flow; pulsatility index) and myocardial perfusion.
Results: A total of 87.5% (7/8) of “anaortic” configurations compared to 25% (1/4) of “aortic” configurations led to unsatisfactory grafts in at least 1 of the 5 patients (P = 0.038). The use of the computational models led to a significant decrease in the selection of unsatisfactory grafting configurations when surgeons employed “anaortic” (21.25% (17/80) vs. 1.25% (1/80), P < 0.001) but not “aortic” techniques (5% (4/80) vs. 0% (0/80), P = 0.64). Similarly, there was an increase in the selection of ideal configurations for “anaortic” (6.25% (5/80) vs. 28.75% (23/80), P < 0.001) but not “aortic” techniques (65% (52/80) vs. 61.25% (49/80), P = 0.74). Furthermore, surgeons who planned to use more than one unique “anaortic” configuration across all 5 patients increased (12.5% (2/16) vs. 87.5% (14/16), P<0.001).
Conclusions: “COMCAB” is a promising tool to improve personalized surgical planning particularly for CABG configurations involving composite or sequential grafts which are used more frequently in anaortic operations.
Total arterial grafting (TAG), which involves the exclusive use of arterial conduits such as bilateral internal mammary arteries (BIMA) and radial arteries (RA) for coronary artery bypass grafting (CABG), has been associated with improved long-term clinical outcomes (1). Total arterial, anaortic, off-pump coronary artery bypass grafting (OPCABG) is an operative approach that avoids aortic manipulation with no graft attached to the ascending aorta and thus this strategy has been endorsed for its reduction in stroke risk and other complications associated with cardiopulmonary bypass (2). However, anaortic grafting configurations typically require more composite and sequential grafts rather than separate grafts.
Composite BIMA grafting to left coronary targets with <70% diameter stenosis leads to a high rate of graft occlusion or constriction due to competitive flow (3). For a radial artery graft anastomosed to the right coronary artery (RCA) territory, the percent diameter stenosis should be at least 80–90% because the radial artery is more prone to spasm (4, 5). Functional assessment of coronary stenoses using fractional flow reserve (FFR) < 0.80 or instantaneous wave-free ratio (iFR) < 0.90 (6), and functional assessment of bypass grafts using transit-time flowmetry (TTFM) with mean graft flow (MGF) ≥ 15 ml/min and pulsatility index (PI) < 5 can avoid situations of competitive flow leading to poor graft patency following arterial grafting (7).
There is no consensus among surgeons regarding the optimal TAG configuration using BIMA for an individual patient and it has even been argued that configuration is not important (8). However, others have maintained that graft configuration is significant (9) and that suboptimal judgement in the arrangement of grafts can lead to steal of flow between grafts and native coronary arteries particularly when composite or sequential grafts are used with unbalanced native coronary stenoses (10). In this study, the importance of graft configuration was investigated using predictive patient-specific hemodynamic computational modeling and the impact of such predictive information on surgical planning was evaluated.
Institutional ethics review was obtained to conduct this study. Five patient cases for inclusion in the study were identified by screening patients who had undergone both a CT coronary angiogram (CTCA) and invasive coronary angiogram. All patients were required to have severe triple-vessel coronary artery disease with >90% diameter stenosis in the RCA territory and >75% stenosis in the left anterior descending (LAD) and circumflex (CIRC) territories such that surgeons would not deliberately avoid using a RA graft on a distal target due to concerns of competitive flow. Patients 1 and 2 both had a ramus intermedius artery (Figures 1A, B), Patients 3 and 4 had additional less severe stenoses (Figures 1C, D), and Patient 5 had an in-stent restenosis in the LAD (Figure 1E). The differing degrees of severity and distribution of stenoses evident from the coronary tree diagrams of the five patients are summarized in Table 1.
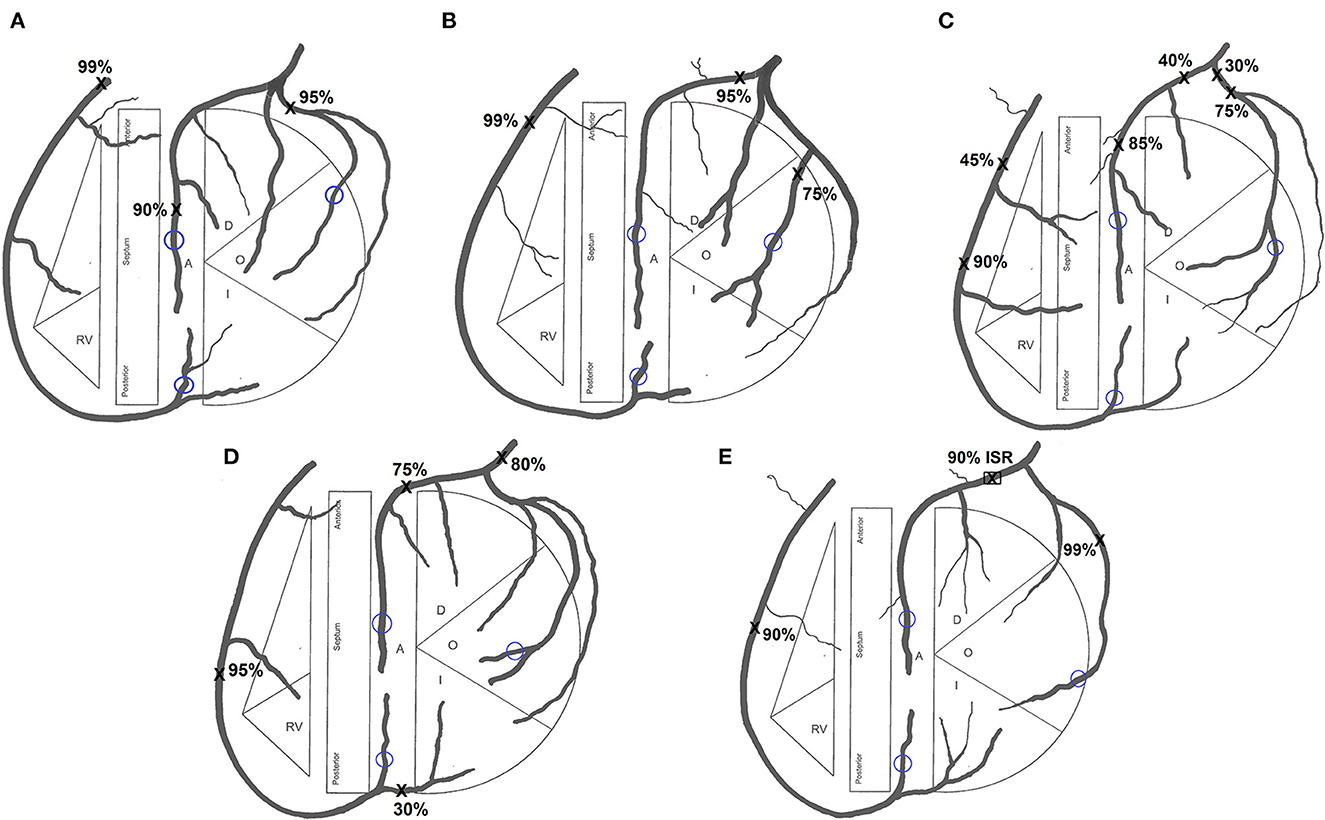
Figure 1. Coronary tree diagram for (A) Patient 1, (B) Patient 2, (C) Patient 3, (D) Patient 4 and (E) Patient 5. Sites of stenoses marked X. Sites of grafting encircled blue. RV, right ventricle; A, anterior; O, obtuse marginal; I, intermediate; ISR, in-stent restenosis.
Twelve grafting configurations utilizing BIMA and RA, used by surgeons internationally, were examined for each of the 5 patient-specific diseased coronary circulations. Four of the grafting configurations involved aorto-coronary grafts (“aortic” configurations). One configuration using BIMA exclusively, recycles the free RIMA for use to the RCA by anastomosing it from the aorta while using a LIMA/RIMA composite graft for the left-sided vessels (11) (configuration A) (Figure 2A). Other configurations have three separate inflows with separate in situ use of both internal mammary arteries (IMA) and the RA off the aorta. The RIMA can be used in situ via the transverse sinus to the CIRC branches (12) (configuration B) (Figure 2B), in situ to the distal RCA or proximal posterior descending artery (PDA) (13) (configuration C) (Figure 2C), or, if used for the LAD, then the LIMA is anastomosed to the CIRC branches (14) (configuration D) (Figure 2D).

Figure 2. Total arterial grafting configurations. (A) Configuration A: in situ LIMA to LAD; free RIMA Y (off LIMA) to OM; free RIMA (off aorta) to PDA. (B) Configuration B: in situ LIMA to LAD; in situ RIMA (via transverse sinus) to OM; RA (off aorta) to PDA. (C) Configuration C: in situ LIMA to LAD; RA (off aorta) to OM; in situ RIMA to PDA. (D) Configuration D: in situ RIMA to LAD; in situ LIMA to OM; RA (off aorta) to PDA. (E) Configuration E: in situ LIMA to LAD; RA Y (off LIMA) to OM; in situ RIMA to PDA. (F) Configuration F: in situ LIMA to LAD; in situ RIMA to OM; RA Y (off RIMA) to PDA. (G) Configuration G: in situ LIMA to LAD; free RIMA Y (off LIMA) to OM; RA Y (off RIMA) to PDA. (H) Configuration H: in situ LIMA to LAD; free RIMA Y (off LIMA) to OM sequential to PDA. (I) Configuration I: in situ LIMA to LAD; in situ RIMA to PDA with RA I (extends RIMA) sequential to OM. (J) Configuration J: in situ LIMA to LAD; in situ RIMA to OM with RA I (extends RIMA) sequential to PDA. (K) Configuration K: in situ LIMA to OM; free RIMA Y (off LIMA) to LAD with RA I (extends RIMA) sequential to PDA. (L) Configuration L: in situ LIMA to LAD; in situ RIMA to OM; RA J (off LAD) to PDA.
The other eight grafting configurations involved no proximal graft anastomosis to the aorta (“anaortic” configurations). A composite Y-graft can be constructed using the RA off the in situ LIMA to LAD and if the in situ RIMA cannot reach the RCA target then it is lengthened by a small I-graft with the remaining RA (15) (configuration E) (Figure 2E). An alternative configuration involves the RIMA in the Y-composite graft which spares the LIMA to be used as an individual graft (16) (configuration F) (Figure 2F). Certain surgeons perform a double-Y graft to revascularize all three territories and this is based on a single inflow (17) (configuration G) (Figure 2G). Another composite approach used by surgeons, based on a single inflow requiring use of the BIMA only, is a configuration that uses the free RIMA as a sequential graft (11, 18) (configuration H) (Figure 2H).
The appeal in using both IMA as in situ grafts, has led surgeons to using the RA as an I-graft to lengthen the RIMA and configure it as a sequential graft to anastomose the CIRC and RCA territories. The in situ RIMA-RA sequential can be used in an anticlockwise orientation where the first anastomosis is to the RCA and the final anastomosis is to the CIRC branches (19) (configuration I) (Figure 2I). The orientation of the RIMA-RA sequential in the clockwise version is such that it is first brought through the transverse sinus to anastomose to the CIRC branches and then terminates on the RCA (20) (configuration J) (Figure 2J). Another configuration uses an I-graft constructed with the RIMA and RA but uses it as a free Y- graft from the LIMA to CIRC (21) (configuration K) (Figure 2K). Finally, an uncommon “bail-out” configuration involves the use of a jump graft where the radial artery conduit is anastomosed from the distal LAD to the PDA (22) (configuration L) (Figure 2L).
Patient-specific 1D-0D computational fluid dynamics models were created for each of the 5 patients' theoretical non-diseased coronary circulation, their stenotic circulation and their 12 grafted circulations and these 70 networks were solved using the novel software “COMCAB” created by the authors for this purpose. This involved a manual segmentation of the native coronary artery geometry from the CTCA for each patient and the mapping of each vessel onto the 1D domain. Thereafter, coronary artery side branches were added to account for a physiological loss in pressure from proximal to distal along the coronary arteries as such branches were not visualized on the CTCA. Terminal vessels were coupled to 0D lumped parameter models. Lumped parameter stenoses were added to create the stenotic network for each patient and 1D grafts added to create the different grafting network topologies for the grafted circulations. The networks were solved for blood flow and pressure using the Richtmyer two-step Lax-Wendroff 1D numerical method (23, 24) with prescription of appropriate boundary conditions. The chosen meshgrid size, Δx, was set at Δx ≈ 0.1cm and the timestep, Δt, was chosen to be the largest value possible whilst ensuring stability of the numerical scheme with Δt ≈ 2.95 × 10−5. The methodology for the creation of the CFD models has previously been described in detail in the literature (25). The vessel segment data and vessel segment relations used for all network simulations for the five patients are provided in Supplementary material 1–5. The computational model inputs included a generic aortic root pressure waveform of 120/77 mmHg at 65 beats per minute (cardiac period 0.917 s), a generic left ventricular (LV) pressure waveform, and cardiac output (CO) of 5 L/min. Approximately 4.5% of CO was assigned to myocardial blood flow with this flow distributed to the three main coronary territories: LAD, CIRC, and RCA, as dictated by distribution of vessels on the CTCA. Total arterial compliance was estimated at 1.15 cm3/mmHg from the aortic pressure waveform and a 3-element Windkessel resistance-capacitance-resistance (RCR) model was applied at the outlets of the terminal vessels to represent the distal microcirculation (25). iFR was chosen as the metric for functional stenosis severity as it is measured at rest like TTFM graft measurements, not at hyperemia which is used for FFR. However, as iFR is a recent concept an equivalent FFR was also calculated: FFR = 0.68 × iFR + 0.18 (26).
Creation of the predictive models took ~6 h for each patient. Running all simulations using high performance computing on Xeon Broadwell CPUs (2.1 GHz) with embarrassing parallelisation, took up to 75 min. MGF and PI were calculated at the distal end of each graft (Figure 3). Myocardial territory perfusion was calculated for the theoretical non-diseased circulations and the diseased circulations along with the improvement in myocardial perfusion following restoration of blood flow by the grafted coronary circulations. Validation of the computational models' predictions was performed by comparing the calculated iFR, MGF and PI with in vivo measurements available in the literature.
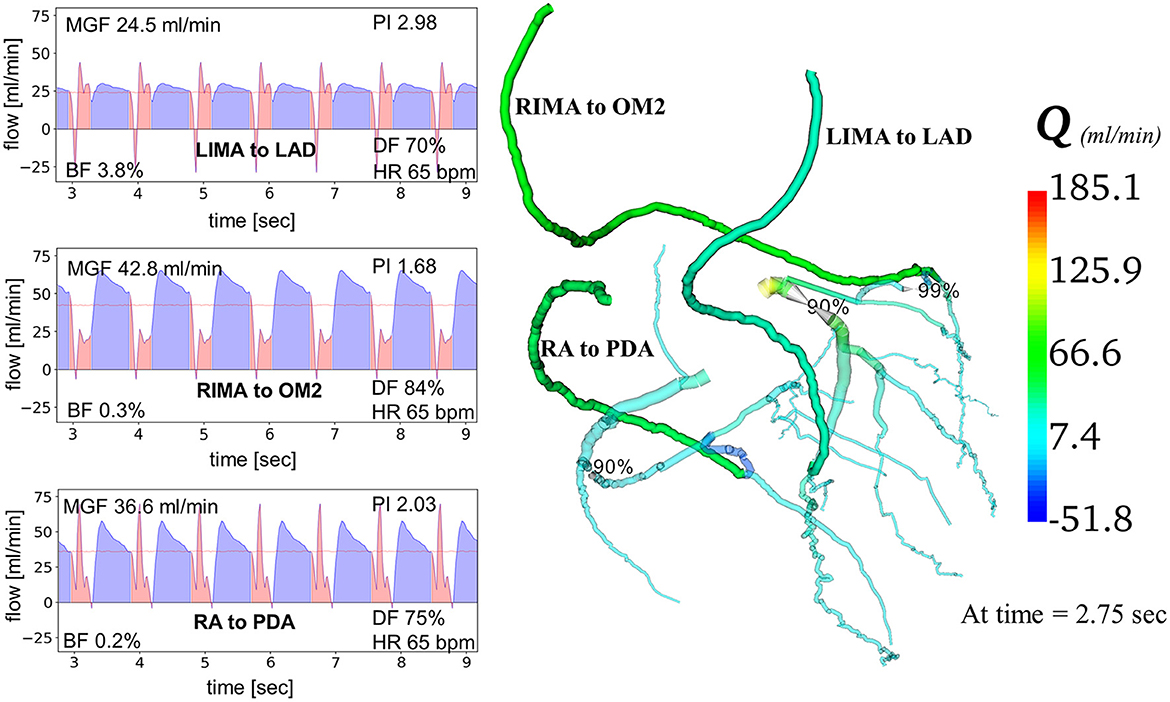
Figure 3. Computational modeling of coronary artery bypass grafts using “COMCAB” software. An example is shown of how the computational model displays predictive graft performance data measured at the distal end of the grafts from grafting configuration B for Patient 5. Q, flow; LIMA, left internal mammary artery; RIMA, right internal mammary artery; RA, radial artery; LAD, left anterior descending artery; OM2, second obtuse marginal artery; PDA, posterior descending artery; MGF, mean graft flow; PI, pulsatility index; BF, backward flow; DF, diastolic filling; HR, heart rate.
Grafting configurations were classified as unsatisfactory, satisfactory, or ideal, based on the graft performance indices. An unsatisfactory grafting configuration was defined as having either MGF < 15 ml/min or PI > 5 in any graft. A satisfactory grafting configuration was defined as MGF ≥ 15 ml/min in all grafts with 3 < PI < 5 for a graft to a LV target and an ideal grafting configuration was defined as MGF ≥ 15 ml/min and PI < 3 in all grafts (27).
Sixteen cardiac surgeons were recruited for participation in the study from five centers. The power calculation assumed that ~50% of the selected grafting configurations would be deemed unsatisfactory with standard surgeon decision-making but close to 0% after the provision of computer modeling predictions. Thus, the study would require a sample size of 16 to achieve two-tailed statistical significance with an alpha level of 0.05 and a power of 0.8. No dropouts were expected due to the administration of the survey in one sitting. Surgeon experience was noted by recording the volume of CABG operations performed in their career and current practice of total arterial revascularisation using BIMA.
The coronary tree diagrams, as representations of each patient's diseased coronary circulation, were presented to the surgeons (Figure 1). Surgeons were informed that the only available conduits would be the LIMA, RIMA and one RA with no contraindications for their use. All patients had an overall preserved LV function with all distal grafting target sites being suitable for grafting. Conduit lengths were adequate for an in situ RIMA to reach the obtuse marginal (OM) target and for an in situ RIMA to reach a distal RCA/PDA.
Each of the surgeons selected one preferred “aortic” configuration and one preferred “anaortic” configuration for each of the five patients (standard decision-making). They were then asked to indicate their overall grafting preference if there were no constraints on their choice such as a porcelain aorta. The surgeons were shown the 12 grafting configurations used by surgeons internationally and asked to rank their top five selections as well as indicate any configurations they would not use. They were then presented with the predictive hemodynamic graft flow information from the computer model and asked for their preferred selections before, and after, the provision of regional myocardial perfusion data (computer-informed decision-making) (Tables 2–6).
The outcomes of interest were the selected number of unsatisfactory graft configurations (primary outcome measure), the number of ideal graft configurations, and the number of unique grafting configurations among the five patients for both “aortic” and “anaortic” configurations. Additional exploratory measures included the number of configuration rankings that changed with additional regional myocardial perfusion data and the number of configurations that individual surgeons indicated they would not initially use but later would use after viewing the computational models' hemodynamic predictions.
Differences in outcome measures were compared as continuous variables for flows with paired t-tests and as proportions for discrete variables with chi-squared tests. If parametric assumptions were not satisfied, then the Mann–Whitney U-test for independent comparisons was performed or the Wilcoxon signed rank-test for dependent comparisons. For the primary outcome measure, McNemar's test was performed to determine the influence of the computational model in changing individual surgeons' selection of unsatisfactory grafting configurations. Outcomes were considered statistically significant for P < 0.05 and the statistical tests were two-tailed with power 0.8 to test hypotheses. A learning gain attributable the computational model was set at 30% of surgeons improving their unsatisfactory decisions, which is considered effective for an educational intervention (28).
Seven out of the 9 stenoses (78%) in this study that were between 71 and 90% diameter stenosis were functionally significant (Figure 4A). This proportion correlated with the findings of a large clinical study in which 80% of such lesions were found to be functionally significant (29). The MGF and PI predicted by the computational models compared well with available in vivo TTFM measurements from other clinical studies, noting that the measurement of PI depends on location along the graft and is graft-specific (Table 7). Furthermore, the correlation between MGF and iFR (R = −0.455) (Figure 4B) as well as PI and iFR (R = 0.522) for 15 separate arterial grafts in this study (Figure 4C) was consistent with an in vivo clinical study involving 25 arterial grafts where the correlation coefficients were R = −0.460 and 0.563, respectively (30).
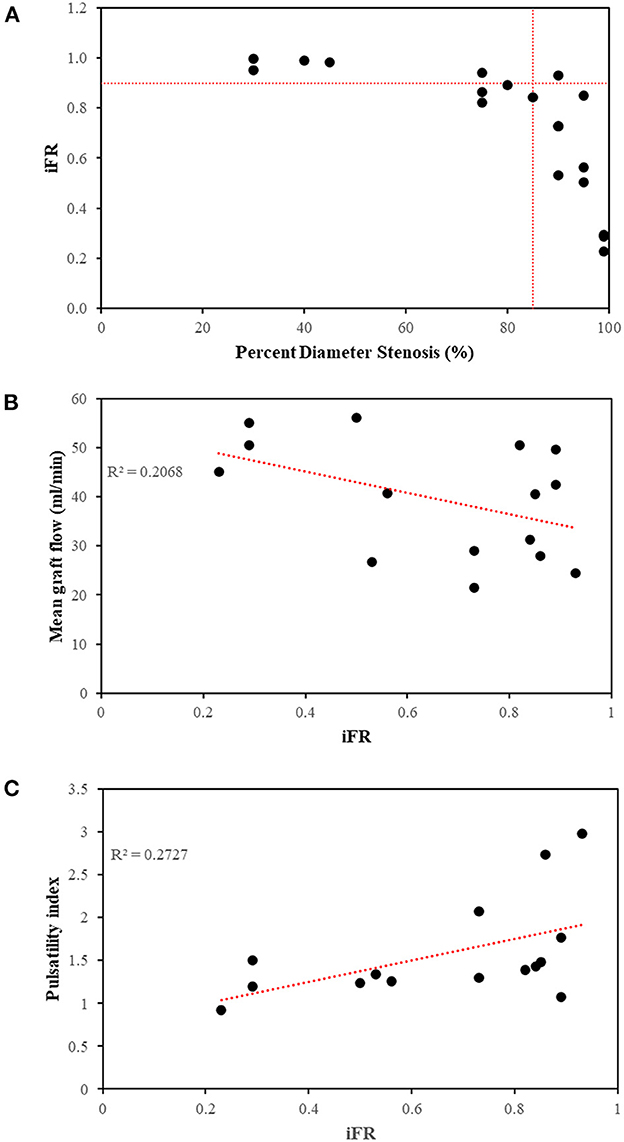
Figure 4. Relationships between metrics of functional stenoses and grafts. (A) The majority of stenoses >85% diameter were functionally significant at rest (iFR < 0.90). Two stenoses between 75 and 80% were also functionally significant on account of their increased lesion length. (B) Correlation between mean graft flow and iFR (R = −0.455) and (C) correlation between pulsatility index and iFR (R = 0.522) using the 15 arterial grafts across 5 patients using separate arterial grafts from configuration C, as an example. iFR, instantaneous wave-free ratio; R2, coefficient of determination.
For Patient 1, configurations G and H had MGF < 15 ml/min in the composite LIMA to LAD graft mainly due to lower flows down this limb where the LIMA was the single inflow source for grafts to all three targets (Table 2). For Patient 2, configurations A, F, G, I and K had MGF < 15 ml/min and higher PI because composite grafts to the OM1 were subject to steal of flow, which was exacerbated by flow competition from a native OM1 75% stenosis with iFR 0.86 (Table 3). For Patients 3 and 4 only configuration L was unsatisfactory due to 90% and 95% stenosis, respectively, in the RCA that compromised the MGF and PI in the RA to PDA jump grafts (Tables 4, 5). For Patient 5, configurations A, E, G, and H were unsatisfactory with MGF < 15 ml/min in the LIMA to LAD graft due to a steal of blood flow down the other limb of the composite Y-graft, which was accentuated by the native competitive flow in the LAD due to a stenosis of 90% with an iFR 0.93 (Table 6). For Patient 5, configuration L was unsatisfactory due to a reversal of flow in the RA to PDA jump graft from a 90% RCA stenosis combined with limited blood flow supplied by the upstream LIMA to LAD (Table 6).
A total of 87.5% (7/8) of “anaortic” configurations, compared with 25% (1/4) of “aortic” configurations, led to unsatisfactory grafts in at least one of the five patients (P = 0.038) (Figure 5). Composite “aortic” configuration A led to unsatisfactory grafting configurations in Patients 2 and 5. The other three “aortic” grafting configurations with three separate grafts and inflows (configurations B, C, and D) never led to an unsatisfactory grafting configuration [0% (0/15)]. “Anaortic” configurations with two inflows (LIMA and RIMA) (configurations E, F, I, J, L) led to unsatisfactory configurations in 80% of patients (4/5), 24% of the time (6/25). However, configuration J was satisfactory in all patients. Configurations with one inflow (configurations G, H, K) led to unsatisfactory configurations in all patients [100% (5/5)], 40% of the time (6/15).

Figure 5. Unsatisfactory, satisfactory and ideal patient-specific grafting configurations. Out of 60 grafting configurations studied amongst the 5 patients, 14 were deemed unsatisfactory, 9 satisfactory and 37 ideal based on MGF and PI of individual grafts. U, unsatisfactory; S, satisfactory; I, ideal.
There was a wide range of cardiac surgical experience among the participating surgeons (Table 8). The most common “aortic” grafting strategy selected with standard decision-making was configuration C (62.5%), whereas the most common “anaortic” strategy was configuration E (71.25%) and no surgeons in this study chose configurations G or L with very few selecting configurations I or K (Table 9). As few surgeons selected configuration A [15% (12/80)] which was the only “aortic” configuration that led to unsatisfactory grafting in two patients, standard decision-making led to very few unsatisfactory “aortic” grafting configurations [5% (4/80)] (Table 9, Figure 5). However, as most surgeons selected “anaortic” configuration E [71.25% (57/80)] which led to unsatisfactory grafting in one patient as well as “anaortic” configuration H [13.75% (11/80)] with unsatisfactory grafting in two patients the rate of unsatisfactory “anaortic” selections with standard decision-making was higher [21.25% (17/80)] (Table 9, Figure 5). An “aortic” configuration was preferred over an “anaortic” configuration, 77.5% (62/80) of the time. Half (8/16) of the surgeons chose at least one “anaortic” strategy among the five patients in preference to an “aortic” configuration.
As standard surgeon decision-making led to the selection of fewer unsatisfactory “aortic” grafting configurations compared with “anaortic” configurations, the integration of the computational model-generated predictions by the cardiac surgeons led to a significant decrease in the selection of unsatisfactory grafting configurations for “anaortic” [21.25% (17/80) vs. 1.25% (1/80), P < 0.001] but not “aortic” techniques [5% (4/80) vs. 0% (0/80), P = 0.641] (Table 9). Similarly, there was an increase in the selection of ideal configurations for “anaortic” [6.25% (5/80) vs. 28.75% (23/80), P < 0.001] but not “aortic” techniques [65% (52/80) vs. 61.25% (49/80), P = 0.743] (Table 9).
For the primary outcome measure, the number of surgeons that changed from choosing at least one unsatisfactory grafting configuration to having no unsatisfactory grafting configurations was significant for “anaortic” configurations (13/16 = 81.25%, P < 0.001) but not for “aortic” configurations (2/16 = 12.5%, P = 0.480) (Table 10). The computational model predictions also led surgeons to make more patient-specific grafting selections for the “anaortic” configurations. The number of surgeons that changed from choosing no unique grafting configurations to having at least one unique grafting configuration across the five patients was significant (12/16 = 75%, P = 0.002) (Table 10). Half (8/16) of computer-informed surgeons used a configuration that they earlier stated they would not use and 56.25% (9/16) changed the order of their ranking preferences with the addition of myocardial perfusion data.
After the degree of functional coronary stenosis, graft configuration has the most significant influence on graft and native coronary artery flows (10). Despite the tendency of surgeons for using a “one-size fits all” approach, hemodynamic predictions from this study suggest that surgeons need to tailor composite and sequential grafting configurations for each individual patient. Anaortic configurations based on a smaller number of separate inflows are more prone to steal of flow and competitive flow affecting upstream segments (17).
“Anaortic” configuration J, with its sequential arrangement, was favorable for Patients 1, 2, 3, and 4 as the RCA stenoses were equal to or greater than the CIRC stenoses (31). In Patient 5, although the OM vessel stenosis was at 99% and the RCA at 90% (iFR 0.73), the flows in the graft segment to PDA were still adequate at 15.29 ml/min. A higher iFR for the RCA stenosis in this patient could compromise configuration J as being universally satisfactory. This observation highlights that the complexity of determining unsatisfactory grafting configurations is potentially beyond the capabilities of using simple heuristics and may require the quantitative predictive value of computational modeling to determine the complex interplay between graft and host vessels and distal run-off.
Standard surgeon decision-making culminated in four composite Y-graft “aortic” selections [5% (4/80)] and 17 “anaortic” selections [21.25% (17/80)] which, on the basis of predictive hemodynamics, could lead to unsatisfactory grafts with poor patency. This result reveals a significant clinical opportunity for improvement in graft selection and function for both surgeons and patients given that options exist to avoid these configurations. In a clinical study of 120 patients undergoing complex composite grafting procedures using BIMA, the graft patency at a mean follow-up of 29.9 ± 33.1 months for arterial grafts was between 80 and 98.7% (32). Although grafts may appear to be patent initially, those with poor flow have been found to be occluded within 1 year (33, 34).
The computational model was effective in reducing the proportion of surgeons choosing unsatisfactory “anaortic” configurations from 87.5 to 6.25% (P < 0.001), far exceeding the learning change set a priori at 30% (28). One surgeon continued to pursue an unsatisfactory grafting configuration for one patient which may be explained by cognitive biases when evaluating computational models (35). The computational model influenced 50% of surgeons to select grafting configurations that they stated they would not usually use and led to more patient-specific tailoring of “anaortic” configurations, rather than a “one-size fits all” approach. This change in decision-making is understandable as the biophysics of the coronary circulation is complex, often difficult to completely measure and highly patient-specific. Thus, the standard decision-making for a CABG configuration is made in an environment of significant uncertainty (36). This study demonstrates that, when faced with uncertainty, the additional quantified flow information provided by predictive computational modeling will change surgical planning especially for configurations involving composite grafts.
The provision of additional myocardial perfusion data led 56.25% (9/16) of surgeons to change their graft configuration rankings. Indeed, surgeons tend to be poor at predicting qualitative effects on perfusion post CABG (37) and are not accustomed to assimilating data providing quantification of myocardial perfusion.
There were a number of limitations in this study relating to how surgeons were engaged. Using only five patients did allow 12 grafting configurations to be interrogated on each patient. While relevant predictive hemodynamic data were presented to all 16 surgeons, other TTFM parameters which “COMCAB” is capable of measuring such as diastolic filling percentage and backward flow were not provided, to avoid information overload. This would not affect results as this study addressed situations of flow competition rather than technical anastomotic errors and hence providing MGF and PI were considered sufficient (38). Despite this, the study still typically took up to 45 min for each surgeon to complete. As this virtual surgical planning study omitted the real-life execution of CABG, the authenticity of surgeon responses may also be questioned. Only four out of 16 surgeons performed more than 25 BIMA operations per year and their rate of BIMA use was 53.39% (35.48–85%). For the other 12 surgeons, the BIMA use was only 14.45% (0–25%). This variability is consistent with 20% TAG utilization in Europe, up to 80% in some Australian centers (39) and 3% overall BIMA usage on multi-institutional analysis (40). A further subgroup analysis is planned as an extension to this study to investigate the effect of surgeon experience on engagement with information provided by the computational modeling. Future studies could also be streamlined by including more surgeons who perform anaortic total arterial OPCABG revascularisation using BIMA or even CABG involving more composite and sequential grafts.
The limitations arising from computational modeling have been described previously (25). With more invasive clinical patient data available, the idealized generic parameters of aortic root pressure, LV pressure, heart rate, CO, total arterial compliance and systemic arterial branch dimensions could be made more patient-specific. Despite this, the differences in patient-specific coronary artery disease resulted in differing outcomes from different grafting configurations between patients. The 7 h duration required to create the computer models included manual processing and therefore further automation of methods would be beneficial. Although hemodynamic predictions generally agreed with in vivo clinical data from other studies, further validation of the computational models could also be performed in the same patient data set by measuring in vivo pre-operative and post-operative iFR, intraoperative TTFM, as well as post-operative myocardial perfusion imaging.
The present study investigated 12 different TAG configurations using BIMA and RA for severe triple vessel coronary disease for each of five patients and is therefore the most comprehensive study of its kind in the literature, modeling 60 virtual grafting configurations (41–43). The computational methodology developed has the potential to investigate a wide range of other CABG configurations and hemodynamic scenarios. It is uncertain whether composite grafts can sustain adequate perfusion at both rest and hyperemia (44, 45). Future models could also account for variations in flow and incorporate the effects of cardiopulmonary bypass, anesthesia, exercise, hyperemia, coronary autoregulation and increased graft diameters over time. They could also investigate patients with saphenous vein grafts, poorer ejection fractions, or those with LV hypertrophy and microvascular coronary disease where the use of anaortic configurations with composite Y-grafts has been clinically cautioned (44).
Patient-specific computational modeling provides important predictive hemodynamic flow information that cardiac surgeons can incorporate into their decision-making when planning graft configurations for individual patients. Surgeons take heed of computer model predictions as their current practice involves significant uncertainty regarding native coronary and bypass graft flows achieved following CABG. Based on these results, “COMCAB” has the potential to be a promising clinical decision-support software tool for personalized surgical planning for CABG configurations. This is particularly important in avoiding situations of flow competition affecting bypass graft patency arising from the use of composite and sequential grafts such as those used more frequently in “anaortic” grafting techniques.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Health and Disability Ethics Committee (HDEC) New Zealand (15/STH/245 and 29/03/2016). The patients/participants provided their written informed consent to participate in this study.
KC and NS conceptualized the computational modeling. AP improved the code efficiency and visualization of the computational models. KC, PM, and NS conceptualized the study involving the cardiac surgeons. KC, AP, SW, PM, and NS edited the manuscript for intellectual content. All authors gave approval for the final version of the manuscript to be published.
KC was funded by the Royal Australasian College of Surgeons' (RACS) Surgeon Scientist Scholarship (165165, 1st January 2017 to 1st January 2020).
The authors acknowledge the use of New Zealand eScience Infrastructure (NeSI) high performance computing facilities, consulting support and training services. New Zealand's national facilities are provided by NeSI and funded jointly by NeSI's collaborator institutions and through the Ministry of Business, Innovation and Employment's Research Infrastructure programme.
AP was employed by New Zealand eScience Infrastructure (NeSI).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1095678/full#supplementary-material
Data Sheet 1 to 5 are each an excel file for each of the 5 patients containing 15 worksheets with segmented coronary geometry as well as vessel segment data and relations for non-diseased, stenotic, and 12 grafting configurations A to L.
1. Rayol SC, Eynde JVd, Cavalcanti LRP, Escorel Neto AC, Rad AA, Amabile A, et al. Total arterial coronary bypass graft surgery is associated with better long-term survival in patients with multivessel coronary artery disease: a systematic review with meta-analysis. Braz J Cardiovasc Surg. (2021) 36:78–85. doi: 10.21470/1678-9741-2020-0653
2. Ramponi F, Seco M, Brereton RJL, Gaudino MF, Puskas JD, Calafiore AM, et al. Toward stroke-free coronary surgery: the role of the anaortic off-pump bypass technique. J Card Surg. (2021) 36:1499–510. doi: 10.1111/jocs.15372
3. Pevni D, Hertz I, Medalion B, Kramer A, Paz Y, Uretzky G, et al. Angiographic evidence for reduced graft patency due to competitive flow in composite arterial T-grafts. J Thorac Cardiovasc Surg. (2007) 133:1220–5. doi: 10.1016/j.jtcvs.2006.07.060
4. Tatoulis J, Buxton BF, Fuller JA. Patencies of 2,127 arterial to coronary conduits over 15 years. Ann Thorac Surg. (2004) 77:93–101. doi: 10.1016/S0003-4975(03)01331-6
5. Gaudino M, Tondi P, Benedetto U, Milazzo V, Flore R, Glieca F, et al. Radial artery as a coronary artery bypass conduit: 20-year results. J Am Coll Cardiol. (2016) 68:603–10. doi: 10.1016/j.jacc.2016.05.062
6. Sen S, Asrress KN, Nijjer S, Petraco R, Malik IS, Foale RA, et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration: results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study). J Am Coll Cardiol. (2013) 61:1409–20. doi: 10.1016/j.amjcard.2013.01.134
7. Kieser TM, Rose S, Kowalewski R, Belenkie I. Transit-time flow predicts outcomes in coronary artery bypass graft patients: a series of 1000 consecutive arterial grafts. Eur J Cardio Thor Surg. (2010) 38:155–62. doi: 10.1016/j.ejcts.2010.01.026
8. Magruder JT, Young A, Grimm JC, Conte JV, Shah AS, Mandal K, et al. Bilateral internal thoracic artery grafting: does graft configuration affect outcome? J Thorac Cardiovasc Surg. (2016) 152:120–7. doi: 10.1016/j.jtcvs.2016.03.022
9. Kalavrouziotis D, Mohammadi S. “How to BIMA?” is in fact the question. J Thorac Cardiovasc Surg. (2021) 161:e31. doi: 10.1016/j.jtcvs.2020.06.029
10. Glineur D, Hanet C. Competitive flow in coronary bypass surgery: is it a problem? Curr Opin Cardiol. (2012) 27:620–8. doi: 10.1097/HCO.0b013e3283583000
11. Paterson HS, Naidoo R, Byth K, Chen C, Denniss AR. Full myocardial revascularization with bilateral internal mammary artery Y grafts. Ann Cardiothor Surg. (2013) 2:444. doi: 10.3978/j.issn.2225-319X.2013.07.07
12. Lev-Ran O, Matsa M, Ishay Y, Shabtai A, Vodonos A, Sahar G. Retroaortic right internal thoracic artery grafting of circumflex artery targets. Asian Cardiovasc Thor Ann. (2015) 23:543–51. doi: 10.1177/0218492315573360
13. Parsa CJ, Daneshmand MA, Gaca JG, Rankin JS. Arterial bypass grafting of the coronary circulation. HSR Proc Intensive Care Cardiovasc Anesth. (2011) 3:227.
14. Hayward PA, Hare DL, Gordon I, Matalanis G, Buxton BF. Which arterial conduit? Radial artery versus free right internal thoracic artery: six-year clinical results of a randomized controlled trial. Ann Thorac Surg. (2007) 84:493–7. doi: 10.1016/j.athoracsur.2007.03.053
15. Puskas JD, Yanagawa B, Taggart DP. Off-pump, multiple arterial grafting with minimal aortic manipulation: is it for everyone? J Thorac Cardiovasc Surg. (2016) 151:4–6. doi: 10.1016/j.jtcvs.2015.09.116
16. Prapas SN, Pangiotopoulos IA, Leivaditis VN, Katsavrias KP, Prapa VS, Linardakis IN, et al. The π-circuit technique in coronary surgery: analysis of 1359 consecutive cases. Open J Cardiovasc Surg. (2019) 11:1179065219871948. doi: 10.1177/1179065219871948
17. Mannacio V, Cirillo P, Mannacio L, Antignano A, Mottola M, Vosa C. Multiple composite grafts (k, π or double-Y) in coronary artery surgery: a choice or a necessity? Interact Cardiovasc Thorac Surg. (2015) 20:60–6. doi: 10.1093/icvts/ivu338
18. Al-Attar N, Nataf P. Exclusive internal mammary artery bypass for complete myocardial revascularisation. European Cardiovascular Disease. 2:1–3. doi: 10.15420/ecr.2006.0.2.1k
19. Nakajima H, Kobayashi J, Funatsu T, Shimahara Y, Kawamura M, Kawamura A, et al. Predictive factors for the intermediate-term patency of arterial grafts in aorta no-touch off-pump coronary revascularization. Eur J Cardio Thorac Surg. (2007) 32:711–7. doi: 10.1016/j.ejcts.2007.07.025
20. Ramponi F, Seco M, Edelman JB, Sherrah AG, Bannon PG, Brereton RJL, et al. Dual inflow, total-arterial, anaortic, off-pump coronary artery bypass grafting: how to do it. Ann Cardiothorac Surg. (2018) 7:552. doi: 10.21037/acs.2018.06.17
21. Raja SG. Composite arterial grafting. Expert Rev Cardiovasc Ther. (2006) 4:523–33. doi: 10.1586/14779072.4.4.523
22. Taggart DP. How I deploy arterial grafts. Ann Cardiothorac Surg. (2018) 7:690. doi: 10.21037/acs.2018.09.06
23. Richtmyer RD. A Survey of Difference Methods for Non-Steady Fluid Dynamics. Boulder: National Center for Atmospheric Research (1963).
24. LeVeque RJ, Leveque RJ. Numerical Methods for Conservation Laws. Birkhauser: Springer (1992). doi: 10.1007/978-3-0348-8629-1
25. Chaudhuri K, Pletzer A, Smith NP. A predictive patient-specific computational model of coronary artery bypass grafts for potential use by cardiac surgeons to guide selection of graft configurations. Front Cardiovasc Med. (2022) 9:953109. doi: 10.3389/fcvm.2022.953109
26. Matsuo H, Kawase Y, Kawamura I. FFR and iFR Similarities, Differences, and Clinical Implication. Ann Nuclear Cardiol. (2017) 3:53–60. doi: 10.17996/anc.17-00036
27. Ohmes LB, Di Franco A, Di Giammarco G, Rosati CM, Lau C, Girardi LN, et al. Techniques for intraoperative graft assessment in coronary artery bypass surgery. J Thorac Dis. (2017) 9:S327. doi: 10.21037/jtd.2017.03.77
28. Hake RR. Interactive-engagement versus traditional methods: a six-thousand-student survey of mechanics test data for introductory physics courses. Am J Phys. (1998) 66:64–74. doi: 10.1119/1.18809
29. Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study: fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. (2010) 55:2816–21. doi: 10.1016/j.jacc.2009.11.096
30. Tolegenuly A, Ordiene R, Mamedov A, Unikas R, Benetis R. Correlation between preoperative coronary artery stenosis severity measured by instantaneous wave-free ratio and intraoperative transit time flow measurement of attached grafts. Medicina. (2020) 56:714. doi: 10.3390/medicina56120714
31. Nakajima H, Kobayashi J, Toda K, Fujita T, Shimahara Y, Kasahara Y, et al. Determinants for successful sequential radial artery grafting to the left circumflex and right coronary arteries. Interact Cardiovasc Thor Surg. (2011) 12:125–9. doi: 10.1510/icvts.2010.247122
32. Shih BC, Chung S, Kim H, Chang HW, Kim DJ, Lim C, et al. Outcomes and patency of complex configurations of composite grafts using bilateral internal thoracic arteries. Korean J Thor Cardiovasc Surg. (2020) 53:64. doi: 10.5090/kjtcs.2020.53.2.64
33. Parvaiz I, Lund JT, Kelbæk H. The arterial sling operation: one-year follow-up. Ann Thorac Surg. (2005) 80:1375–80. doi: 10.1016/j.athoracsur.2005.03.054
34. Mohsin I, Namburu L, Sadiq Z, Newberry B, Ahmed HM. Competitive flow: closure of internal thoracic artery graft after successful coronary artery bypass graft surgery. CJC Open. (2021) 3:1406–9. doi: 10.1016/j.cjco.2021.06.017
35. Sargent RG. Verification and validation of simulation models. J Simul. (2013) 7:12–24. doi: 10.1057/jos.2012.20
36. Ove Hansson S. Decision making under great uncertainty. Philos Soc Sci. (1996) 26:369–86. doi: 10.1177/004839319602600304
37. Eckardt R, Kjeldsen BJ, Haghfelt T, Grupe P, Johansen A, Andersen LI, et al. Angiography-based prediction of outcome after coronary artery bypass surgery versus changes in myocardial perfusion scintigraphy. Interact Cardiovasc Thorac Surg. (2011) 13:505–10. doi: 10.1510/icvts.2011.274068
38. Di Giammarco G, Rabozzi R. Can transit-time flow measurement improve graft patency and clinical outcome in patients undergoing coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. (2010) 11:635–40. doi: 10.1510/icvts.2010.235663
39. Raja SG. Total arterial coronary grafting: outcomes, concerns and controversies. Vessel Plus. (2019) 3:23. doi: 10.20517/2574-1209.2019.05
40. LaPar DJ, Crosby IK, Rich JB, Quader MA, Speir AM, Kern JA, et al. Bilateral internal mammary artery use for coronary artery bypass grafting remains underutilized: a propensity-matched multi-institution analysis. Ann Thorac Surg. (2015) 100:8–15. doi: 10.1016/j.athoracsur.2015.02.088
41. Koyama S, Itatani K, Yamamoto T, Miyazaki S, Kitamura T, Taketani T, et al. Optimal bypass graft design for left anterior descending and diagonal territory in multivessel coronary disease. Interact Cardiovasc Thorac Surg. (2014) 19:406–13. doi: 10.1093/icvts/ivu182
42. Rezaeimoghaddam M, Oguz GN, Ates MS, Bozkaya TA, Piskin S, Lashkarinia SS, et al. Patient-specific hemodynamics of new coronary artery bypass configurations. Cardiovasc Eng Technol. (2020) 42:663–663. doi: 10.1101/2020.08.22.20179804
43. Ballarin F, Faggiano E, Manzoni A, Quarteroni A, Rozza G, Ippolito S, et al. Numerical modeling of hemodynamics scenarios of patient-specific coronary artery bypass grafts. Biomech Model Mechanobiol. (2017) 16:1373–99. doi: 10.1007/s10237-017-0893-7
44. Mannacio V, De Vita A, Antignano A, Mottola M, Di Tommaso L, Graniero A, et al. Y grafts with the left internal mammary artery and radial artery. Mid-term functional and angiographic results. Cohort study. Int J Surg. (2014) 12:952–7. doi: 10.1016/j.ijsu.2014.07.008
45. Sakaguchi G, Tadamura E, Ohnaka M, Tambara K, Nishimura K, Komeda M. Composite arterial Y graft has less coronary flow reserve than independent grafts. Ann Thorac Surg. (2002) 74:493–6. doi: 10.1016/S0003-4975(02)03729-3
46. Onorati F, Rubino AS, Cristodoro L, Scalas C, Nucera S, Santini F, et al. In vivo functional flowmetric behavior of the radial artery graft: is the composite Y-graft configuration advantageous over conventional aorta–coronary bypass? J Thorac Cardiovasc Surg. (2010) 140:292, 297.e2. doi: 10.1016/j.jtcvs.2009.10.028
47. Han Z, Zhang G, Chen S, Liu G, Chen Y. Application of bilateral internal mammary artery with different configurations in coronary artery bypass grafting. J Cardiothorac Surg. (2021) 16:1–6. doi: 10.1186/s13019-020-01380-z
48. Zhang G, Zhao Z, Chen Y, Chen S, Liu G. Use of the right internal mammary artery and the great saphenous vein for left anterior descending artery revascularization in patients whose left internal mammal artery cannot be used: a study based on transit-time flow measurement. J Cardiothorac Surg. (2020) 15:1–7. doi: 10.1186/s13019-020-01172-5
Keywords: coronary artery bypass and grafting, total arterial grafting, graft configuration, computational fluid dynamics modeling, instantaneous wave-free ratio (iFR), transit-time flowmetry (TTFM), surgeon decision-making, surgical planning
Citation: Chaudhuri K, Pletzer A, Waqanivavalagi SWFR, Milsom P and Smith NP (2023) Personalized surgical planning for coronary bypass graft configurations using patient-specific computational modeling to avoid flow competition in arterial grafts. Front. Cardiovasc. Med. 10:1095678. doi: 10.3389/fcvm.2023.1095678
Received: 11 November 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
Marco Manca, SCImPULSE Foundation, NetherlandsReviewed by:
Stephen Gent, South Dakota State University, United StatesCopyright © 2023 Chaudhuri, Pletzer, Waqanivavalagi, Milsom and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krish Chaudhuri,  a2NoYTYzNkBhdWNrbGFuZHVuaS5hYy5ueg==
a2NoYTYzNkBhdWNrbGFuZHVuaS5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.