- Department of Emergency Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Background: A protective or ultra-protective tidal volume strategy is widely applied to patients with acute respiratory distress syndrome (ARDS). The use of very low tidal volume has the potential to further redece ventilation-induced lung injury (VILI) comparde with a “normal” lung protective management. Plus, cardiogenic pulmonary edema (CPE) caused by hydrostatic mechanisms in patients with cardiogenic shock has similar respiratory mechanics to those found in patients with ARDS. And no consensus exists on mechanical ventilation parameter settings in patients with VA-ECMO. The study aimed to investigate the impact of an ultra-protective tidal volume strategy on the 28-day ventilator-free day (VFD) number in VA-ECMO–supported patients with refractory cardiogenic shock, including cardiac arrest.
Methods: The Ultra-ECMO trial is a randomized controlled, open-label, single-center prospective superiority trial. At the onset of ECMO initiation, we will divide patients randomly into an intervention group and a control group in a 1:1 ratio. The control group will adopt protective ventilation settings [initial tidal volume: 6 ml/kg of predicted body weight (PBW)] for ventilation, and the intervention group will adopt ultra-protective ventilation settings (initial tidal volume: 4 ml/kg of PBW) for ventilation. The procedure is expected to last 72 h, after which the ventilator settings will be at the intensivists' discretion. The primary outcome is the VFD number at 28 days after inclusion. The secondary outcomes will include respiratory mechanics; analgesic/sedation dosage; lung ultrasound score; interleukin-6, interleukin-8, and monocyte chemotactic protein-1 levels in broncho-alveolar lavage fluid at the moment of enrollment (T0), 24, 48, and 72 h (T1, T2, and T3, respectively) after ECMO initiation; total time (in days) required for ECMO weaning; length of stay in the intensive care unit; total cost of hospitalization; amounts of resuscitative fluids; and in-hospital mortality.
Discussion: VA-ECMO–treated patients without ARDS possess abnormal lung function. CPE, thoracic compliance reduction, and poor pulmonary blood perfusion are frequently present, and these patients can more easily progress to ARDS. It seems that targeting the protective tidal volume can lower adverse outcome incidence rates, even in patients without ARDS. This trial seeks to answer the question of whether adopting an ultra-protective tidal volume strategy can lead to superior primary and secondary outcomes compared to adopting a protective tidal volume strategy in patients treated by VA-ECMO. The Ultra-ECMO trial will provide an innovative mechanical ventilation strategy for VA-ECMO–supported patients for improving treatment outcomes at biological and potentially clinical levels.
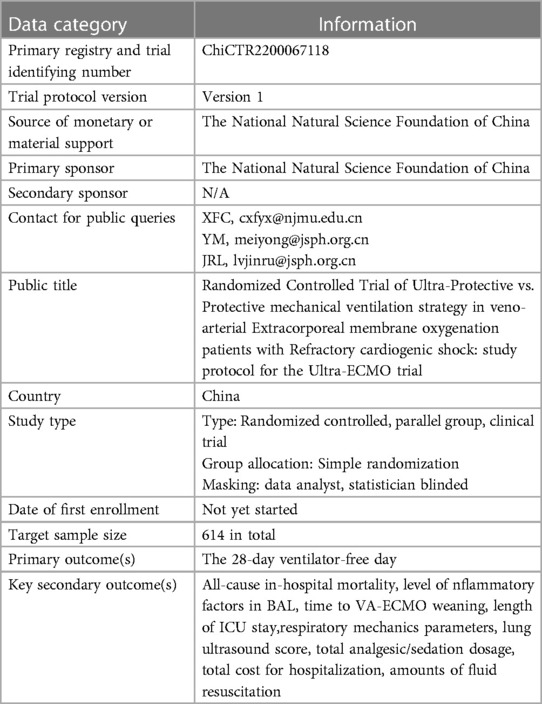
Clinical Trial Registration: ChiCTR2200067118.
1. Introduction
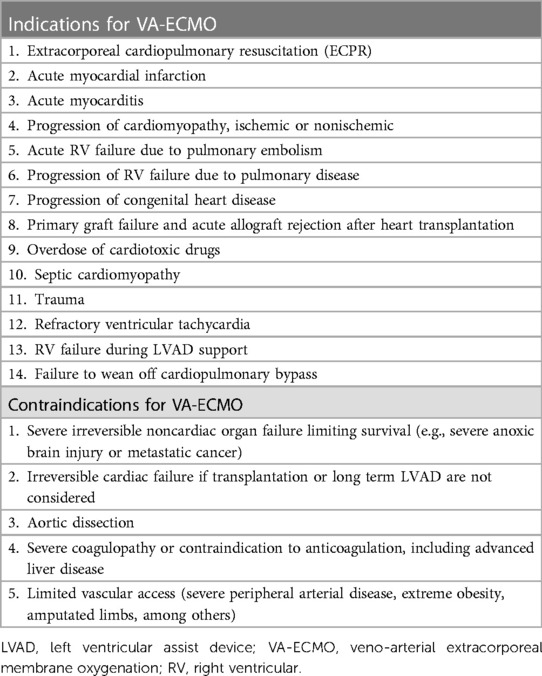
Refractory cardiogenic shock (RCS), including cardiac arrest (CA), is characterized by severe circulation failure accompanied by cardiogenic pulmonary edema (CPE) and often necessitates invasive mechanical ventilation (IMV) (1). In the most severe cases of RCS, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is a viable therapy used to maintain a certain amount of cardiac output (2). Although advances in VA-ECMO technology have significantly improved the clinical outcomes of patients suffering from RCS or CA in the past decades (3), there is a paucity of high-grade scientific evidence suggesting the optimal ventilator settings during VA-ECMO treatment. The Extracorporeal Life Support Organization has proposed a ventilation strategy with a positive end-expiratory pressure (PEEP) of ≥10 cmH2O and low minute ventilation without offering further specific details (4). In a recent observational study of 256 out-of-hospital CA (OHCA) patients, a decrease of 1 ml/kg PBW in the tidal volume (VT) during the first 48 h of intensive care unit (ICU) admission after OHCA was associated with a 61% increase in adjusted favorable neurocognitive outcomes, suggesting that a lower VT in the early phase of ICU admission may influence patient prognosis (5). However, a similar relationship has not been found among in-hospital CA patients (6).
To date, studies have advocated for the application of an ultra-protective VT strategy in patients with ARDS being treated by veno-veno ECMO (VV-ECMO) (7–9). Of note, CPE caused by hydrostatic mechanisms in patients with cardiogenic shock may have similar respiratory mechanics to those seen in patients with ARDS, although the pathophysiology is different. Similarly, IMV during VA-ECMO with ultra-protective VT may attenuate ventilator-induced lung injury due to the increased strain/stress and mechanical power transmitted by the ventilator. Thus, we hypothesized that adopting an ultra-protective VT strategy during the early phase of VA-ECMO may be a credible option to facilitate IMV weaning and even reduce in-hospital mortality. The results of this randomized controlled trial may subsequently add a novel and viable treatment solution to routine clinical practice involving VA-ECMO patients.
2. Methods and analysis
2.1. Trial design
Ultra-ECMO trial is a single-center, prospective clinical trial that will enroll 2 parallel groups of patients in a 1:1 ratio. It is designed to evaluate the 28-day number of ventilator-free days (VFDs) following an early, short-course application (72 h) of an ultra-protective VT vs. protective VT strategy in patients on VA-ECMO (Table 1) (1). Table 2 overviews the trial registration information. This trial protocol conforms to the Consolidated Standards of Reporting Trials (CONSORT) statements (10, 11) and has obtained the approval of the Research Ethics Committee at the First Affiliated Hospital of Nanjing Medical University. The legal guardians of eligible patients will sign the informed consent following the Declaration of Helsinki of 1964 (revised in 2013) (12).
2.2. Trial objectives
This trial has the main objective of assessing the potential benefit of an ultra-protective VT strategy relative to a protective VT strategy on the 28-day VFD number (13) (Table 3).
Meanwhile, the secondary objectives of this study include the following:
1. To examine whether ultra-protective VT can decrease the all-cause in-hospital mortality rate
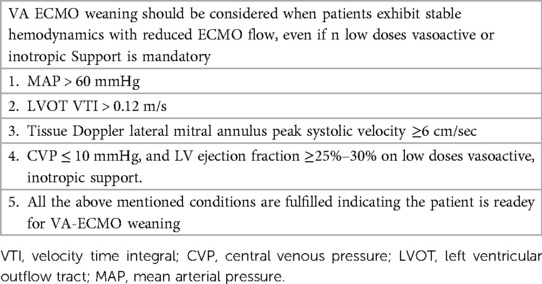
2. To examine whether ultra-protective VT can reduce the time for successful VA-ECMO weaning (Table 4)
3. To examine whether ultra-protective VT is associated with a shorter ICU length of stay
4. To examine whether ultra-protective VT is associated with a reduction in interleukin-6 (IL-6), interleukin-8 (IL-8), and monocyte chemotactic protein-1 (MCP-1) levels in broncho-alveolar lavage fluid (BALF)
5. To examine whether ultra-protective VT affects respiratory parameters (e.g., total PEEP, plateau pressure [Pplat], driving pressure [DP], airway resistance [R], static compliance [Cstat], mean airway pressure [Pmean], esophageal pressure [Pes], transpulmonary pressure [Ptp], and mechanical power [MP]), based on a daily assessment from inclusion to closeout (14)
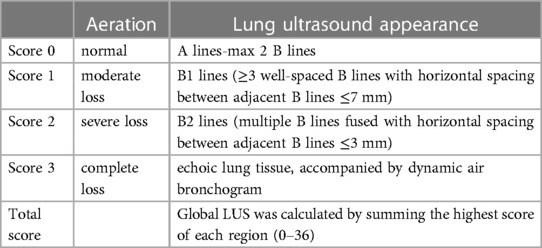
6. To examine whether the ultra-protective VT strategy decreases the lung ultrasound score (LUS) (Figure 1 and Table 5) when assessed daily from inclusion to closeout (15, 16)
7. To examine whether ultra-protective VT can lead to an increased analgesic/sedation dosage during the intervention period after inclusion
8. To examine whether ultra-protective VT predicts a decrease in the total hospitalization cost
9. To examine whether ultra-protective VT is associated with a reduced demand for fluid resuscitation
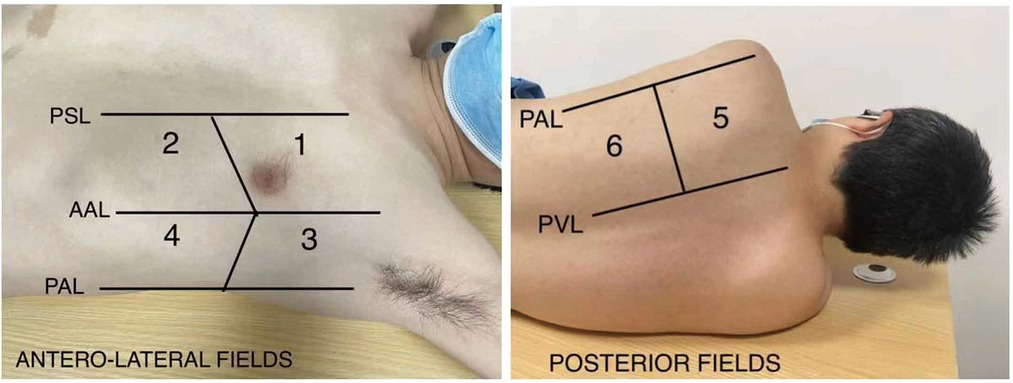
Figure 1. Lung ultrasound regions. PSL, parsternal line; AAL, anterior axillary line; PAL, posterior axillary line; PVL, paravertebral line.
2.3. Trial setting
We will conduct the Ultra-ECMO trial in the Department of Emergency Medicine at the First Affiliated Hospital of Nanjing Medical University, which is capable of initiating ECMO therapy for nearly 100 critically ill patients annually.
2.4. Inclusion and exclusion criteria
Patients ≥18 years of age receiving VA-ECMO therapy, such as extracorporeal cardiopulmonary resuscitation, will be eligible for inclusion in the Ultra-ECMO trial. Additional key inclusion criteria include (1) IMV onset of >72 h, (2) no pregnancy, (3) ECMO duration of >72 h, (4) RCS or CA with a cardiac origin, (5) no enrollment in another clinical trial, (6) a consent form signed by the guardian has been provided, and (7) a description of CPE on high-resolution chest computed tomography images has been given by two radiologists with ≥5 years of diagnostic experience who are unaware of the clinical diagnosis.
Meanwhile, the exclusion criteria for patient enrollment are as follows: (1) trauma; (2) written informed consent has not been provided by close relatives; (3) the patient has contraindications for fiberoptic bronchoscopy examination; (4) lung ultrasound images are blurry; (5) pneumothorax or massive pleural effusion; (6) ECMO is being used as a bridge for heart transplantation; and (7) the patient developed adverse events that complicate weaning from IMV (e.g., intracranial hemorrhage, thromboembolism, severe infection).
2.5. Recruitment, randomization, and blinding
The Ultra-ECMO trial will include patients who meet the abovementioned eligibility criteria. Formal information about the trial and an official copy of the informed consent form will be sent to their guardians. We will assign participants to an intervention arm or a control arm in a random manner, with those in the former group receiving ultra-protective VT and those in the latter receiving 72 h of protective VT. Guardians will also be informed that they are allowed to drop out of the trial at any time.
The randomization list will be computer-generated according to a simple randomization principle using a ratio of 1:1 by the Clinical Research Board of the School of Public Health of Nanjing Medical University. Participants will be assigned to each group by an independent trial assistant after verification of patient eligibility. Once group allocation has been completed, local investigators will register the record, then reveal the group allocation to care providers, who will deliver the intervention.
Trial assistants responsible for group allocation will be blinded to both groups of patients, but blinding of care providers is impracticable as ventilatory settings and respiratory mechanics measurements are part of routine daily clinical practice. The outcome assessors, data analysts and statisticians will not know the group allocation.
2.6. Interventions
At the onset of ECMO initiation, all participants will be screened by therapists. Once the patient meets the inclusion criteria and is finally enrolled, the therapists will provide the selected intervention. We will assign patients in the intervention group to receive ultra-protective VT after randomization (initial settings: VT, 4 ml/kg of PBW; PEEP, 10 cmH2O; respiratory rate, 10 min−1; Vt may be decreased stepwise by 0.5 ml/kg PBW if the plateau pressure is >30 cmH2O) (17).
Patients in the control group will be managed using a protective VT strategy after randomization (initial settings: VT, 6 ml/kg of PBW; PEEP, 10 cmH2O; respiratory rate, 10 min−1; Vt may be decreased stepwise by 0.5 ml/kg of PBW if the plateau pressure is >30 cmH2O).
We will set the sweeping gas flow of ECMO, targeting a PaCO2 range of 35–45 mmHg, and the FiO2 value of the ventilator may be increased if PaO2 in the right upper limb decreases to <60 mmHg during the course of Vt and PEEP adjustments.
Furthermore, a hybrid veno-arterio-venous ECMO mode will be considered if differential oxygenation happens (18). Differential oxygenation refers to the lower body being perfused by ECMO-oxygenated blood while the upper body (coronaries, right arm, brain) receives hypoxemic blood pumped by the native heart. This pathophysiological phenomenon often results from the recovery of native left ventricular function and simultaneous severe lung failure. If optimized ventilation fails to solve this issue, it will be necessary to employ veno-arterio-venous ECMO in consideration of the potential for hypoxic damage to the brain and heart (19, 20).
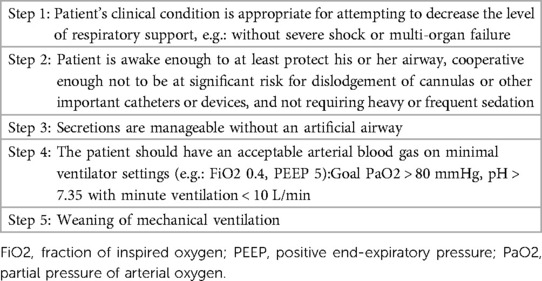
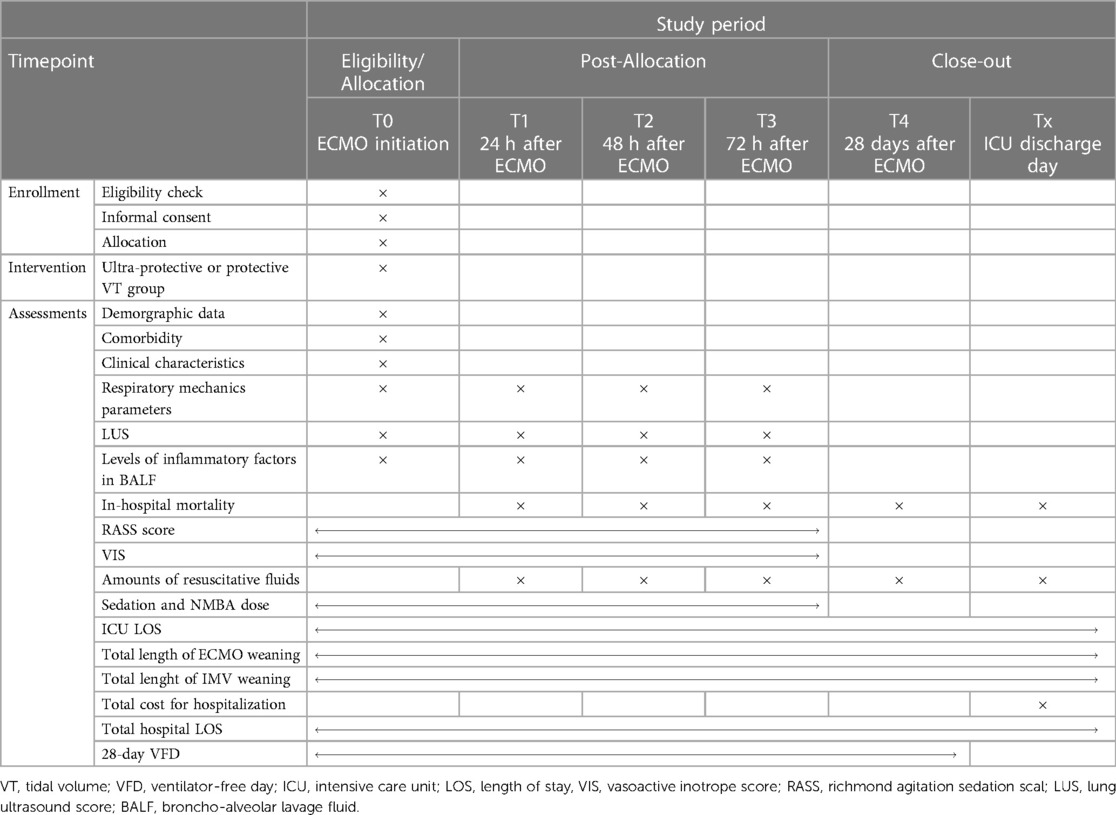
At the moment of enrollment (T0), investigators will take charge of assessing and collecting pre-defined respiratory mechanics parameters; IL-6, IL-8, and MCP-1 levels; LUS; and other trial data as baseline information. The same procedure will be performed repeatedly at 24, 48, and 72 h (T1, T2, and T3, respectively) after ECMO initiation (Figure 2). After 72 h, the mechanical ventilation strategy in both groups will be changed at the intensivists' discretion.

Figure 2. Flowchart of the study design. ECMO, extracorporeal membrane oxygenation; VT, tidal volume; PBW, predicted body weight; CA, cardiac arrest; PEEP, positive end-expiratory pressure; CPE, cardiogenic pulmonary edema; ICU, intensive Care Unit; LUS, lung ultrasound score; VFD, ventilator-free days.
Patients in both groups will receive deep sedation (i.e., targeting a Richmond Agitation Sedation Scale score of −3 or −4 points) during the intervention period. Although deep sedation will be adopted, it is still recommended to administer a neuromuscular blocking agent specific to the situation of patient–ventilator asynchrony.
In case adverse events occur due to the intervention, patients will be managed by the attending physicians at their discretion but still remain in the allocation group to support later data analysis.
2.7. Data collection and management
The total duration of ECMO treatment, total time (in days) on IMV, ICU length of stay, analgesic/sedation dosage, all-cause hospital mortality, levels of inflammatory factors in BALF, LUS, amounts of fluid infusion, and total hospitalization cost will be calculated using patient medical records (Table 6).
We will not consider missing values for primary and secondary outcomes during hospitalization. However, missing values could appear specific to patients who survive to hospital discharge. If so, we will contact patients or their next of kin at day 28 to assess the pre-defined outcomes.
Clinical data will include but are not limited to general characteristics (age, sex, weight, height), comorbidities, original disease, the Acute Physiology and Chronic Health Evaluation II score, partial pressure of arterial oxygen/inspired oxygen fraction, PEEP, VT (in ml/kg of PBW), respiratory rate (in breaths/min), DP, Pplat, Pes, Ptp, Pmean (in cm of water), Cstat (in ml/cmH2O), R (in cmH2O/L/s), arterial blood PaO2 and PaCO2 values (in mmHg), and mechanical power (in J/min).
We will also monitor the clinical conditions as well as the ECMO running status and collect any records.
Delegated team members will take charge of inputting data in the electronic case report form, and experienced clinical research associates will take charge of the data monitoring. Team members will store research data under each participant's study identification code, which will include the first letter of the first and family names of the participant, together with their inclusion number. Only the research team will have the right to possess the key to the identification code list during the study. Detailed information regarding the identification of patients will not be available in any resulting publications.
2.8. Sample size calculation
PASS 11 was used for sample size calculation, where a sample size of 292 patients per group would achieve 80% power to detect a difference between the two groups, providing the means were 7.1 and 9.2 with group standard deviations of 8.8 and 9.3 and with a significance level α of 0.05 using a two-sided two-sample t-test. Accounting for an anticipated dropout rate of 5%, 614 patients were required for the study.
2.9. Statistical methods
2.9.1. Descriptive analysis
We will describe the demographic and clinical characteristics and compare them between the groups. All data will be given in the form of mean ± standard deviation values or medians with interquartile ranges specific to continuous variables or in the form of percentages specific to categorical variables. Student's t test and the Mann–Whitney U test will be used for comparing quantitative characteristics specific to normally and non-normally distributed variables, respectively. The χ2 test or Fisher's exact test will be used for comparing qualitative characteristics.
2.9.2. Primary outcome analysis
The primary outcome is the 28-day VFD number, which will be determined by the intention-to-treat analysis (ITT) (21), regardless of whether the allocated ventilation strategy was effectively applied or not. The primary outcome will be reported in each group in the form of means with standard deviation values or medians with interquartile ranges. Student's t test or the Mann–Whitney U test will support related comparisons of the 2 groups while considering their distribution characteristics.
2.9.3. Secondary outcomes analysis
The intention-to-treat analysis will help in assessing the results of the secondary outcomes. Qualitative secondary outcomes, such as the in-hospital mortality rate in each group, will be reported, and the χ2 test or Fisher's exact test will be used for the comparison. Quantitative secondary outcomes, such as LUS, will be described in each group in the form of medians with standard deviation values or medians with interquartile ranges, considering the distribution shape, and Student's t test or the Mann–Whitney U test will support related comparisons between the 2 groups. Stata version 16 (StataCorp LLC, College Station, TX, USA) will assist in carrying out all analyses. A bilateral p-value of <0.5 will be used to indicate statistical significance.
Performing an interim analysis will be mandatory in order to check whether there are any inappropriate operations related to data collection or management.
3. Discussion
In the current guidelines for ARDS, a protective ventilation strategy of limiting VT to 4–6 ml/kg of PBW is recommended, and this approach can reduce the mortality by up to 9% as proved in a randomized clinical trial (22).
Reduced VT is the mainstay of ventilatory management in ARDS, where reductions in driving pressures have been linked to a lower risk of death (23). Several authors have proposed the use of ultra-low tidal volume strategies with VTs of 4 ml/kg of PBW, which can be trialed in patients suffering from ARDS with VV-ECMO or ECCO2R-ECMO (8, 19). Tidal hyperinflation and cyclic recruitment/de-recruitment are important mechanisms leading to ventilator-induced lung injury in patients with ARDS. The mechanical ventilation strategy of ultra-protective VT or protective VT could decrease the transpulmonary driving pressure, stress, and driving pressure, thus alleviating biotrauma, volutrauma, or shearing injury. In past decades, protective mechanical ventilation strategies were extended to patients without ARDS. Fuiter et al. reported that the use of a lung-protective ventilation strategy (6.4 ± 0.8 ml/kg of PBW) in patients with intermediate and high risk levels undergoing major abdominal surgery led to enhanced clinical outcomes as well as less health care use when compared to non-protective mechanical ventilation (11.1 ± 1.1 ml/kg of PBW) (24). Neto et al. also found that ventilation with low VT (<7 ml/kg of PBW vs. ≥7 ml/kg of PBW) led to a lower risk of developing pulmonary complications in patients without ARDS (25). However, similar recommendations are unavailable in patients with refractory cardiogenic shock or CA receiving IMV, and the optimal ventilation strategy in ECMO patients is insufficiently defined (26).
Prior investigation has found that mechanical ventilation with lower VTs (odds ratio, 1.61; 95% confidence interval 1.13–2.28 per a 1-ml/kg of PBW decrease in VT; p = 0.008) following OHCA is associated with better neurologic outcomes (5). However, a similar conclusion has not been drawn in in-hospital CA patients (6). In short, it isn't yet confirmed whether ultra-protective VT is also feasible in the specific subject.
In the aspect of pathophysiologic mechanism, ARDS is promoted by an aggressive inflammatory reaction when there is a pulmonary or extrapulmonary assault related to altered alveolar epithelial cells or vascular endothelial cell functions. Comparatively, CPE is a type of pure hydrostatic edema attributable to cardiovascular failure. Irrespective of the pathogenesis, patients with CPE may have similar respiratory mechanics to those found in patients with ARDS (27).
In terms of radiological manifestations, the classical morphological description of an ARDS lung features an decreased amount of sufficiently inflated alveoli and increased amount of poorly inflated alveoli that move from a non-dependent region to a dependent region—namely, the normally aerated lung region, higher-density regions possessing recognizable vessels, and higher-density regions without vessels or bronchi. Analogous to ARDS patients, we also found that some patients with CPE show ARDS-mimic radiologic characteristics (namely, gravity-dependent distribution) (Figure 3).
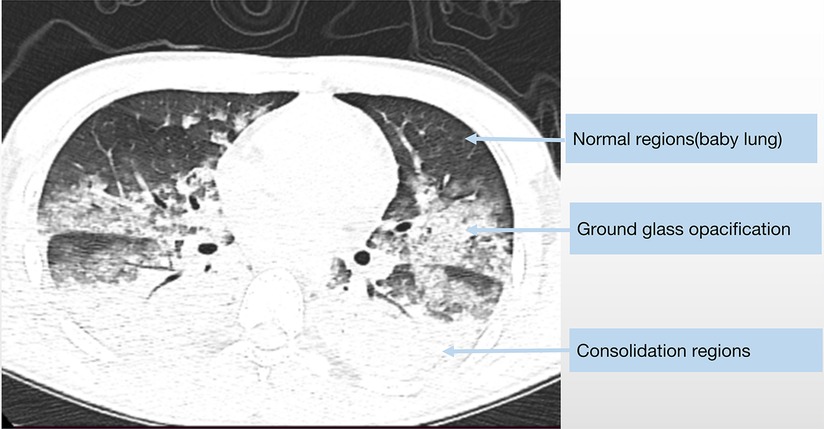
Figure 3. The chest CT scan of a 19-year-old male diagnosed with fulminant myocarditis show similar radiologic characteristics as classic ARDS.
Taken together, we reasonably hypothesize that an ultra-protective VT strategy may contribute to internal pathophysiological changes resembling those associated with an ARDS MV strategy, meanwhile be conducive to external clinical outcomes (earlier weaning of IMV, decreased LUS) in patients receiving VA-ECMO.All in all, the Ultra-ECMO trial aims to answer the question of whether early application of an ultra-protective VT strategy could facilitate weaning of IMV in patients receiving VA-ECMO.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee at the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XC, YM, JL and WL: took charge of conceiving trial. WL, CC and DH: were responsible for preparing and drafting the study protocol, as well as planning the statistical analysis. XC and YM: critically revised the protocol, trained medical staff, and coordinated the trial. XC and YM: took charge of the eligibility screening for the trial enrollment. DH, FS, WL, GZ, and YD: are the clinical members of ECMO team, taking charge of medically caring ECMO supported patients and collecting data on a daily basis. ZZ: managed the research data and communicated with the patient/guardian. All authors contributed to the article and approved the submitted version.
Funding
This work was completed with the support of the National Natural Science Foundation of China (grant no. 82072159).
Acknowledgments
We greatly appreciate LM for her contribution to group allocation and YZ for his contribution to study design and data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol. (2019) 6:698–716. doi: 10.1016/j.jacc.2018.11.038
2. Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. (2016) 5:889–96. doi: 10.1007/s00134-016-4273-z
3. de Chambrun MP, Bréchot N, Combes A. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock: indications, mode of operation, and current evidence. Curr Opin Crit Care. (2019) 4:397–402. doi: 10.1097/MCC.0000000000000627
4. Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. (2021) 3:221–8. doi: 10.1097/MAT.0000000000001344
5. Beitler JR, Ghafouri TB, Jinadasa SP, Mueller A, Hsu L, Anderson RJ, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med. (2017) 9:1198–206. doi: 10.1164/rccm.201609-1771OC
6. Moskowitz A, Grossestreuer AV, Berg KM, Patel PV, Ganley S, Casasola Medrano M, et al. The association between tidal volume and neurological outcome following in-hospital cardiac arrest. Resuscitation. (2018) 124:106–11. doi: 10.1016/j.resuscitation.2017.12.031
7. Giraud R, Banfi C, Assouline B, De Charrière A, Cecconi M, Bendjelid K. The use of extracorporeal CO2 removal in acute respiratory failure. Ann Intensive Care. (2021) 1:43. doi: 10.1186/s13613-021-00824-6
8. Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. An international multicenter prospective cohort. Am J Respir Crit Care Med. (2019) 8:1002–12. doi: 10.1164/rccm.201806-1094OC
9. Combes A, Fanelli V, Pham T, Ranieri VM, European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective lung ventilation withExtracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) investigators. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. (2019) 5:592–600. doi: 10.1007/s00134-019-05567-4
10. Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. Br Med J. (2008) 337:a2390. doi: 10.1136/bmj.a2390
11. Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT Statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med. (2017) 167:40–7. doi: 10.7326/M17-0046
12. Association WM. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
13. Schmidt G, Girard T, Kress J, Morris P, Ouellette D, Alhazzani W, et al. Official executive summary of an American thoracic society/American college of chest physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. Am J Respir Crit Care Med. (2017) 195:115–9. doi: 10.1164/rccm.201610-2076ST
14. Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. (2016) 10:1567–75. doi: 10.1007/s00134-016-4505-2
15. Sun Z, Zhang Z, Liu J, Song Y, Qiao S, Duan Y, et al. Lung ultrasound score as a predictor of mortality in patients with COVID-19. Front Cardiovasc Med. (2021) 8:633539. doi: 10.3389/fcvm.2021.633539
16. Mongodi S, De Luca D, Colombo A, Stella A, Santangelo E, Corradi F, et al. Quantitative lung ultrasound: technical aspects and clinical applications. Anesthesiology. (2021) 6:949–65. doi: 10.1097/ALN.0000000000003757
17. Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. (2019) 1:69. doi: 10.1186/s13613-019-0540-9
18. Brogan T, Lequier L, Lorusso R, MacLaren G, Peek G. The ELSO Red Book. 5th edition, ELSO Eds, Ann Arbor, MI: ELSO (2017).
19. Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenationin adult cardiac patients. ASAIO J. (2021) 8:827–44. doi: 10.1097/MAT.0000000000001510
20. Brasseur A, Scolletta S, Lorusso R, Taccone FS. Hybrid extracorporeal membrane oxygenation. J Thorac Dis. (2018) 10:S707–15. doi: 10.21037/jtd.2018.03.84
21. Kamper S. Per-protocol, intention-to-treat, and complier average causal effects analyses in randomized controlled trials: linking evidence to practice. J Orthop Sports Phys Ther. (2021) 51:314–5. doi: 10.2519/jospt.2021.0701
22. Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Arthur Wheele, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342(18):1301–8. doi: 10.1056/NEJM200005043421801
23. Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. (2013) 5:847–56. doi: 10.1007/s00134-012-2787-6
24. Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. (2013) 5:428–37. doi: 10.1056/NEJMoa1301082
25. Neto AS, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. (2015) 10:2155–63. doi: 10.1097/CCM.0000000000001189
26. Gattinoni L, Tonetti T, Quintel M. How best to set the ventilator on extracorporeal membrane lung oxygenation. Curr Opin Crit Care. (2017) 1:66–72. doi: 10.1097/MCC.0000000000000376
Keywords: cardiogenic pulmonary edema (CPE), ventilator-free days, protective ventilation, ultra-protective ventilation, veno-arterial extracorporeal membrane oxygenation (VA ECMO)
Citation: Li W, Chen C, Hu D, Sun F, Zhang G, Zhang Z, Dong Y, Lv J, Mei Y and Chen X (2023) Randomized controlled trial of ultra-protective vs. protective ventilation strategy in veno-arterial extracorporeal membrane oxygenation patients with refractory cardiogenic shock: a study protocol for the ultra-ECMO trial. Front. Cardiovasc. Med. 10:1092653. doi: 10.3389/fcvm.2023.1092653
Received: 8 November 2022; Accepted: 30 March 2023;
Published: 5 May 2023.
Edited by:
Jan D. Reinhardt, Sichuan University, China© 2023 Li, Chen, Hu, Sun, Zhang, Zhang, Dong, Lv, Mei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinru Lv bHZqaW5ydUBqc3BoLm9yZy5jbg== Yong Mei bWVpeW9uZ0Bqc3BoLm9yZy5jbg== Xufeng Chen Y3hmeXhAbmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
 Wei Li
Wei Li Chen Chen†
Chen Chen† Yanbin Dong
Yanbin Dong Xufeng Chen
Xufeng Chen