- 1Department of Cardiac Surgery, Shengjing Hospital of China Medical University, Shenyang, China
- 2Department of Ultrasound, Shengjing Hospital of China Medical University, Shenyang, China
Background: Patients usually suffer acute pain after cardiac surgery. Numerous regional anesthetic techniques have been used for those patients under general anesthesia. The most effective regional anesthetic technique was still unclear.
Methods: Five databases were searched, including PubMed, MEDLINE, Embase, ClinicalTrials.gov, and Cochrane Library. The efficiency outcomes were pain scores, cumulative morphine consumption, and the need for rescue analgesia in this Bayesian analysis. Postoperative nausea, vomiting and pruritus were safety outcomes. Functional outcomes included the time to tracheal extubation, ICU stay, hospital stay, and mortality.
Results: This meta-analysis included 65 randomized controlled trials involving 5,013 patients. Eight regional anesthetic techniques were involved, including thoracic epidural analgesia (TEA), erector spinae plane block, and transversus thoracic muscle plane block. Compared to controls (who have not received regional anesthetic techniques), TEA reduced the pain scores at 6, 12, 24 and 48 h both at rest and cough, decreased the rate of need for rescue analgesia (OR = 0.10, 95% CI: 0.016–0.55), shortened the time to tracheal extubation (MD = −181.55, 95% CI: −243.05 to −121.33) and the duration of hospital stay (MD = −0.73, 95% CI: −1.22 to −0.24). Erector spinae plane block reduced the pain score 6 h at rest and the risk of pruritus, shortened the duration of ICU stay compared to controls. Transversus thoracic muscle plane block reduced the pain scores 6 and 12 h at rest compared to controls. The cumulative morphine consumption of each technique was similar at 24, 48 h. Other outcomes were also similar among these regional anesthetic techniques.
Conclusions: TEA seems the most effective regional postoperative anesthesia for patients after cardiac surgery by reducing the pain scores and decreasing the rate of need for rescue analgesia.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, ID: CRD42021276645
1. Introduction
According to the latest cardiac surgery market report, approximately 900,000 cardiac surgeries are performed each year in the USA. Patients usually suffer acute pain after coronary artery bypass graft or valve surgery: 78% of patients experienced pain during coughing, while 49% of patients experienced pain at rest (1). It is imperative that we should take this pain seriously and treat promptly. Because inadequately controlled acute pain was associated with chronic pain and persistent postoperative pain (2). Meantime, increasing morbidity and mortality were associated with pain following cardiac surgery (3).
Although intravenous opioid is the first-line postoperative analgesic, opioid-related adverse effects, such as postoperative nausea and vomiting (PONV), pruritus, ventilator-associated pneumonia, can lead to other problems including prolonged intubation, and higher mortality (4). Numerous regional anesthetic techniques, such as thoracic epidural analgesia (TEA), paravertebral block (PVB), erector spinae plane block (ESPB), serratus anterior plane block (SAPB), pectoral nerve block (PECS), parasternal intercostal nerve block (PINB), transversus thoracic muscle plane block (TTMPB) and pecto-intercostal fascial block (PIFB) were introduced as a part of multimodal post-cardiac surgery analgesia, attempting to reduce the cumulative postoperative opiate consumption and pain scores. Few randomized controlled trials (RCTs) have compared these eight techniques. It remains uncertain which is the best technique for postoperative analgesia for cardiac surgery patients.
Network meta-analysis (NMA) pools evidence from a large number of comparisons and patients to compare several interventions, allowing for indirect comparisons and ranking. Therefore, we conducted this NMA with the aim to compare regional anesthetic techniques efficacy and safety for pain relief after cardiac surgery.
2. Materials and methods
The pre-registered protocol was implemented in the PROSPERO database (CRD42021276645). This paper was reported in accordance with PRISMA guideline.
2.1. Search strategy
On September 2, 2022, two investigators searched PubMed, MEDLINE, Embase, ClinicalTrials.gov, and Cochrane Library for relevant studies with the words “cardiac surgery/cardiac surgical procedures” and (“thoracic epidural analgesia”, or “paravertebral block”, or “erector spinae plane block”, or “serratus anterior plane block”, or “pectoral nerve block”, or “parasternal intercostal nerve block”, or “transversus thoracic muscle plane block”, or “pecto-intercostal fascial block”). Additionally, we read the references of articles in search for literature that met the criteria.
2.2. Study selection and data exclusion
Original studies were eligible if the following criteria were met: (i) RCT study in English; and (ii) assessed the efficacy and safety of regional postoperative anesthetic techniques after cardiac surgery under general anesthesia. Original studies were ineligible if the following criteria were met: (i) studies involving combination blocks (i.e., ESPB combined with PECS) (5); (ii) participants were children or animal.
The first author, year of publication, country, surgery type, anesthesia technique, groups and number of participants in each group, drug and dose for regional anesthetics, block timing, postoperative analgesia, and outcomes were extracted in the involved eligible studies. When the data could not be gathered from tables and full text, GetData Graph Digitizer (v 2.26) was used to obtain the numerical data from figures (6).
2.3. Outcomes
Efficiency outcomes were pain scores, the cumulative morphine consumption, and the need for rescue analgesia. Pain scores in the involved studies were converted to a standardized 0–100-point value (where 0 = no pain and 100 = worst pain imaginable) for statistics (6, 7). Five time points (2–4, 6, 12, 24, and 48 h after postoperative tracheal extubation) were selected. Any opiate medications other than intravenous morphine were converted to equivalents of morphine as our previous study (6). PONV and pruritus were safety outcomes. Functional outcomes included the time to tracheal extubation (min), intensive care uinit (ICU) stay (h), hospital stay (days), and mortality (in hospital) as the previous study (8).
2.4. Statistical analysis
Cochrane Collaboration's tool was used to evaluate the quality of involved studies. Mean difference (MD) and 95% confidence interval (CI) were used to report the cumulative morphine consumption, pain scores, the time to tracheal extubation, ICU stay, and hospital stay. Odds ratios (ORs) were used to report the risk of PONV, pruritus, mortality, and the need for rescue analgesia. In this Bayesian NMA, random-effects and consistency models were used to analyze data (four chains, 50,000 iterations, 20,000 per chain). Inconsistency was assessed by the node-splitting method with Bayesian P-value. The surface under the cumulative ranking curve (SUCRA) was calculated and ranked. Begg's and Egger's tests were performed to evaluate publication bias. All analyses were conducted using the “gemtc” package of R version 4.0.2 (R Foundation, Vienna, Austria).
3. Results
3.1. Baseline characteristics of included studies
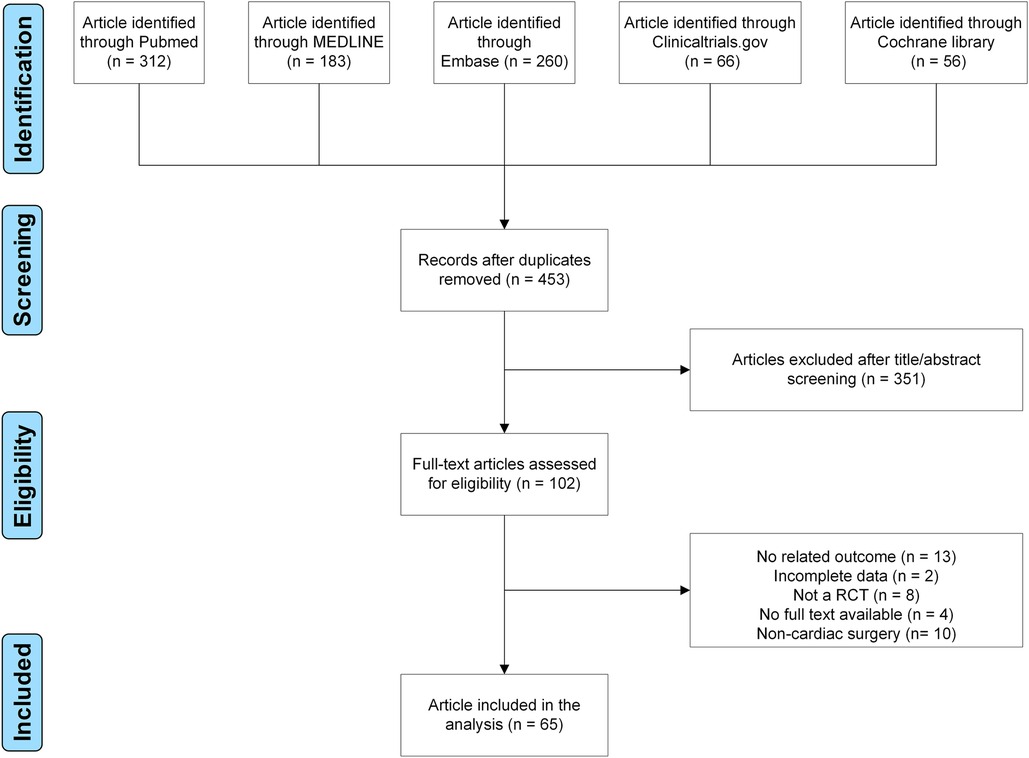
Finally, 65 RCTs were included using our search strategy (Supplementary Table S1 and Figure 1) (9–73). Assessment of bias risk is demonstrated (Supplementary Figures S1,S2). In the period from 1987 to 2021, 65 RCTs were carried out, involving 5,013 participants (Supplementary Table S2). Eight techniques were evaluated, including ESPB, PECS, PIFB, PINB, PVB, SAPB, TEA, and TTMPB (Supplementary Figure S3). 72.3% (47/65) studies involved coronary artery bypass graft; 23.1% (15/65) involved mixed surgery; 4.6% (3/65) involved valve surgery. In total, sixty-four studies were two-arm, and only one study was three-arm and published in 2020. Drugs, dose, block timing, postoperative analgesia, and outcomes are also shown in Supplementary Table S2.
3.2. Efficiency outcomes
There was no difference in pain scores at 2–4 h both at rest and cough among these regional anesthetic techniques. Pain scores at 6, 12, 24, 48 h both at rest and cough were lower for TEA than for controls (Figure 2). Pain scores at 6, 12 h at rest were lower for TTMPB than for controls. Pain scores at 6 h at rest were lower for ESPB and PECS than for controls. Pain score at 12 h at rest was lower for PIFB than for controls. Pain scores were similar among PINB, PVB, SAPB, and controls. Pairwise comparisons are shown in Supplementary Tables S3–S12.
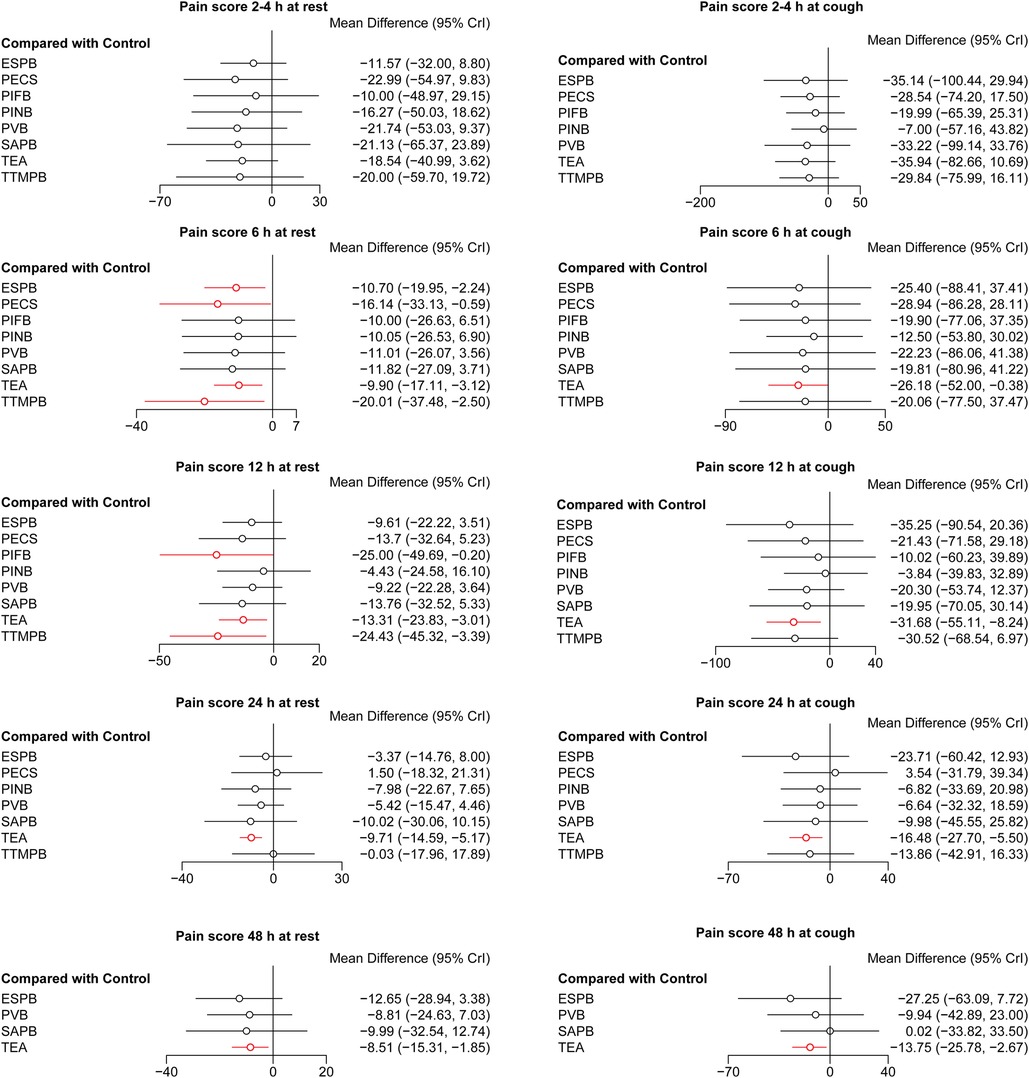
Figure 2. Forest plots of pain scores. The results with a p-value <0.05 are marked in red. ESPB, erector spinae plane block; PECS, pectoral nerve block; PIFB, pecto-intercostal fascial block; PINB, parasternal intercostal nerve block; PVB, paravertebral block; SAPB, serratus anterior plane block; TEA, thoracic epidural analgesia; TTMPB, transversus thoracic muscle plane block.
There was no difference in the cumulative morphine consumption at 24, 48 h among these regional anesthetic techniques (Figure 3). SAPB and TEA reduced the rate of need for rescue analgesia compared with controls (OR = 9.68 × 10−25, 95% CI: 2.33 × 10−74–0.078; OR = 0.10, 95% CI: 0.016–0.55, respectively, Figure 4). Pairwise comparisons are shown in Supplementary Tables S13–S15.
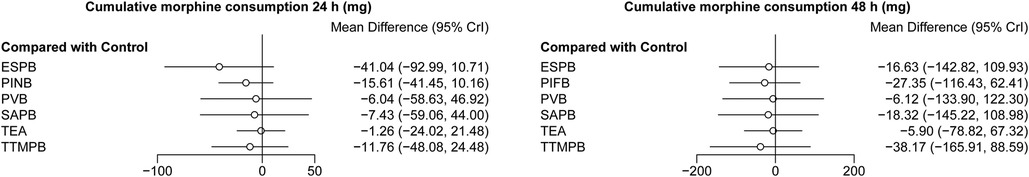
Figure 3. Forest plots of cumulative morphine consumption at 24 and 48 h. The results with a p-value <0.05 are marked in red. ESPB, erector spinae plane block; PIFB, pecto-intercostal fascial block; PINB, parasternal intercostal nerve block; PVB, paravertebral block; SAPB, serratus anterior plane block; TEA, thoracic epidural analgesia; TTMPB, transversus thoracic muscle plane block.
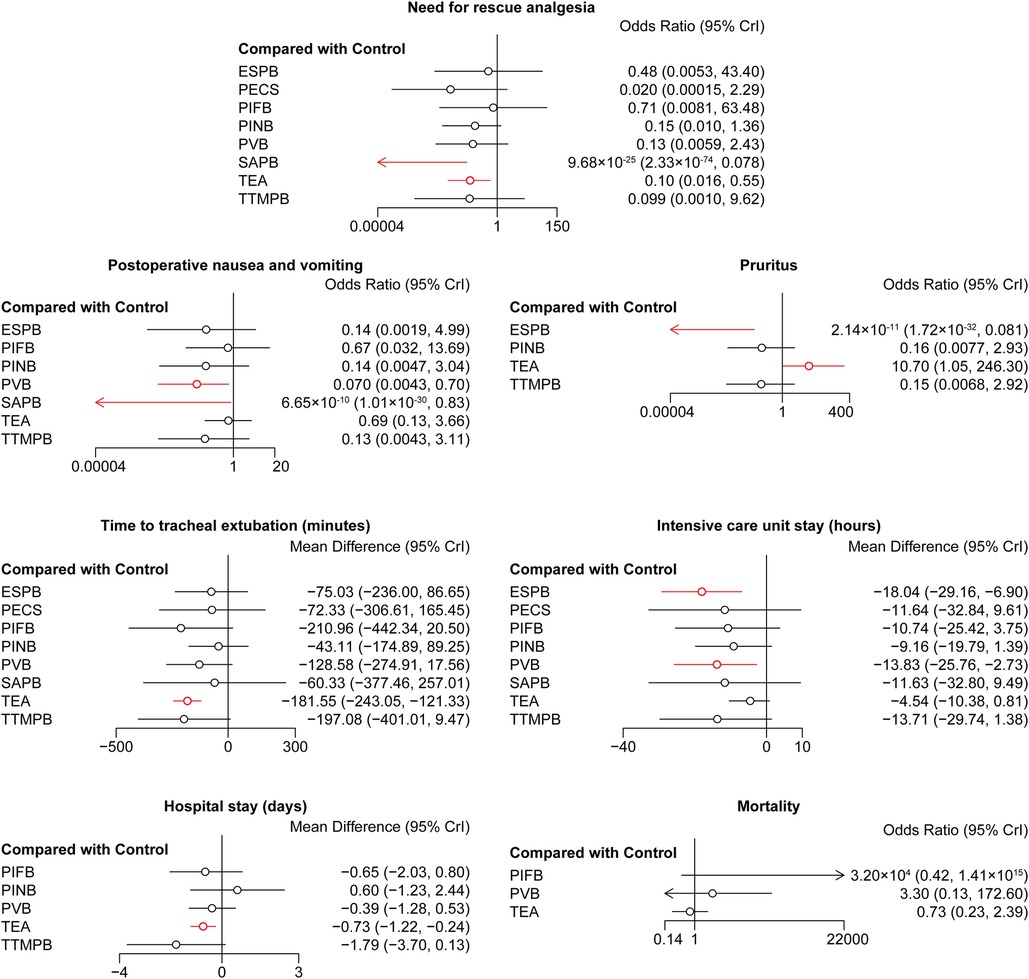
Figure 4. Forest plots of other outcomes. The results with a p-value <0.05 are marked in red. ESPB, erector spinae plane block; PECS, pectoral nerve block; PIFB, pecto-intercostal fascial block; PINB, parasternal intercostal nerve block; PVB, paravertebral block; SAPB, serratus anterior plane block; TEA, thoracic epidural analgesia; TTMPB, transversus thoracic muscle plane block.
3.3. Safety outcomes
SAPB and PVB reduced the risk of PONV compared with the control group (OR = 6.65 × 10−10, 95% CI: 1.01 × 10−30–0.83; OR = 0.070, 95% CI: 0.0043–0.70, respectively, Figure 4). ESPB reduced the risk of pruritus compared with controls (OR = 2.14 × 10−11, 95% CI: 1.72 × 10−32–0.081, Figure 4). TEA increased the risk of pruritus compared with the control group (OR = 10.70, 95% CI: 1.05–246.30, Figure 4). Pairwise comparisons are shown in Supplementary Tables S16,S17.
3.4. Functional outcomes
TEA shortened the time to tracheal extubation compared with controls (MD = −181.55, 95% CI: −243.05 to −121.33, Figure 4). ESPB and PVB shortened the ICU stay compared with controls (MD = −18.04, 95% CI: −29.16 to −6.90; MD = −13.83, 95% CI: −25.76 to −2.73, respectively, Figure 4). TEA shortened the hospital stay compared with controls (MD = −0.73, 95% CI: −1.22 to −0.24, Figure 4). There was no difference in the mortality among these regional anesthetic techniques. Pairwise comparisons are shown in Supplementary Tables S18–S21.
3.5. Inconsistency, ranking, certainty of evidence, and publication bias
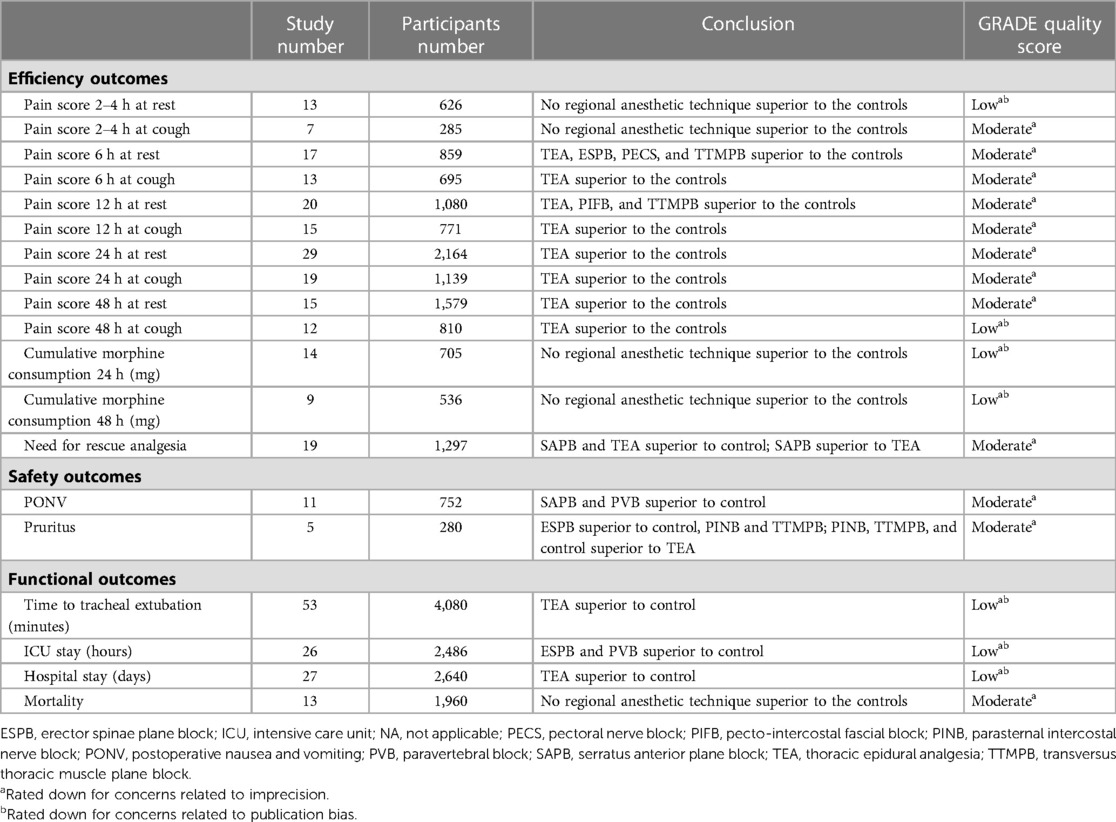
There was a significance level of P > 0.05 for all cases, indicating inconsistencies were not sufficient to influence the conclusions of our NMA (Supplementary Figures S4,S5). The ranks of each outcome are shown in Figure 5. The certainty of evidence is shown in Table 1. The assessment of publication bias is revealed in Supplementary Table S22.
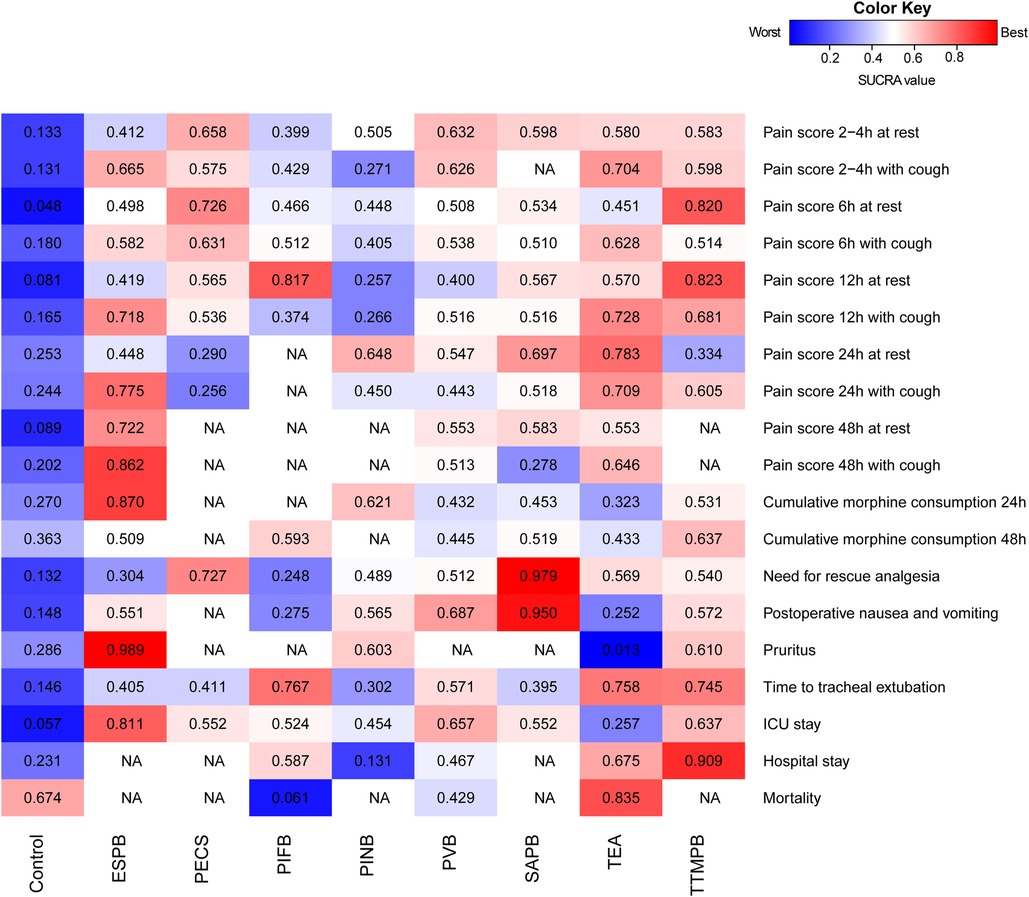
Figure 5. Heat maps of regional anesthetic techniques studied in adult patient with cardiac surgery under general anesthesia for 19 outcomes. Each box is colored according to the SUCRA value of the corresponding anesthetic technique and outcome. Uncolored boxes labeled NA mean that outcome was not involved in the underlying treatment. ESPB, erector spinae plane block; ICU, intensive care unit; NA, not applicable; PECS, pectoral nerve block; PIFB, pecto-intercostal fascial block; PINB, parasternal intercostal nerve block; PVB, paravertebral block; SAPB, serratus anterior plane block; SUCRA, surface under the cumulative ranking curve; TEA, thoracic epidural analgesia; TTMPB, transversus thoracic muscle plane block.
4. Discussion
This paper is the first comprehensive NMA assessing the efficacy and safety of regional anesthetic techniques after cardiac surgery. Though none of the regional anesthetic techniques could reduce the cumulative morphine consumption compared with controls, our results revealed that TEA, ESPB, and TTMPB reduced pain scores at different time points. TEA also reduced the rate of need for rescue analgesia, the time to tracheal extubation, and duration of hospital stay. ESPB could reduce the risk of pruritus and shorten the duration of ICU stay.
Pain is the most severe during the first 24 h following cardiac surgery, and it gradually decreases (74). Ineffective postoperative pain management may cause immunosuppression, infections, and less effective wound healing (75). For most people, their first exposure to opioids was during surgeries (76). Regional anesthetic techniques may play a role in decreasing or sparing opioid exposure when opioid dependence, opioid overdose, and community overuse and misuse are becoming incisive social issues. However, currently there is no consensus on the best regional anesthetic technique. Our results suggested TEA may be the most effective regional anesthetic technique for cardiac surgery patients under general anesthesia.
In 1954, TEA was first introduced in cardiac surgery (77). This regional anesthetic technique blocks thoracic spinal cord (T1–T5). Our NMA has once again confirmed that TEA provided superior analgesia compared with controls by reducing the pain score and the rate of need for rescue analgesia (78, 79). TEA could shorten the time to tracheal extubation, which made a step further from the goal of early mobilization. Previous study revealed mobilization reduced the risk of postoperative pulmonary thromboembolism and other complications (80). TEA was recommended as Class IIb treatment according to the guideline in 2017 (81). Meantime, a previous meta-analysis revealed TEA reduced the risk of perioperative myocardial infarction, respiratory depression, and atrial fibrillation/flutter for cardiac surgery patients (78). Our results showed a similar mortality between TEA and controls, which was consistent with the previous study (78). Whereas, a study with transapical transcatheter aortic valve implantation revealed TEA provided superior analgesia following and decreased one-year mortality (82). The changes in long-term mortality need to be further studied. As the pain was relieved, some adverse effects began to appear. The most frequently discussed complication was epidural hematoma (83). Because of the low incidence rate (incidence of 1:3,552–12,000), none of our involved study reported this complication when performing TEA (83, 84). In our NMA, we found TEA increased the risk of pruritus which is an opioid-related side effect. The cardiac anesthesiologists should pay attention to these two adverse effects when performed TEA.
It is likely that PVB is the most commonly administered paraxial nerve block (85). PONV in cardiac surgery affects 20%–67% of all patients, increases adrenergic stimulation, limits mobility and oral intake, and is distressing to the patient (86). 18.4% of patients who used opioids under cardiac surgery suffered pruritus (87). In our NMA, we found PVB reduced the risk of PONV. Similar results were reported by two meta-analyses, suggesting PVB had reduced the incidence of PONV as postcardiothoracic surgery analgesia when compared with TEA (88, 89). ESPB, as an ultrasound-guided PVB variant, was first described in 2016 (90). ESPB blocked dorsal and ventral rami of spinal nerve roots as same as PVB (85, 91). ESPB not only had a greater analgesic benefit in the first six hours after tracheal extubation, but also reduced the risk of pruritus in our analysis. However, due to limited numbers of trials, the efficacy and safety profile of ESPB require further investigation.
PINB, TTMPB, and PIFB complement the anteromedial chest wall by providing anesthesia confined to the parasternal region. According to the nuance of the injection position, they were divided into two categories: regional anesthetics was injected between the internal intercostal and pectoralis major muscles in PIFB and PINB; regional anesthetics was deeply injected between the internal intercostal and transverse thoracic muscles in TTMPB (3, 91, 92). All these three techniques anesthetized the anterior branches of the intercostal nerves. PINB, also known as parasternal block, was first described for cardiac surgery in 2005 (30). When PINB was performed, regional anesthetics was usually given for five interspaces bilaterally, over the periosteum, and/or around the mediastinal tubes. Under ultrasound guidance, the patients with PIFB received regional anesthetics bilaterally at the target sites for breast surgery since 2014 (93). TTMPB became an analgesic block for cardiac surgery soon afterward it was described as an adjunct of PECS during breast surgery in 2015 (94). Because of the similarities of PINB, TTMPB, and PIFB mentioned above, their anesthetic efficiency and safety were also similar in our analysis. Some researchers believed that PINB and PIFB were the same blocks (95). Some authors promoted PIFB because of easily identify with ultrasound (91). Although pain scores at 6, 12 h at rest were lower for TTMPB, evidence for cardiac surgery patients is extremely limited.
On the anterolateral chest wall, a new method named “PECS” was reported to anesthetize the medial-lateral pectoral nerves, long thoracic nerve, and thoracodorsal nerve since 2011 (96). In the similar region, SAPB was established which could block the anterior and lateral cutaneous branches of the intercostal nerves. Our results revealed SAPB reduced the rate of need for rescue analgesia compared with TEA, and PECS had lower pain score at 6 h at rest compared with controls. As PECS and SAPB don't cause sympathectomy and can be performed in patients on anticoagulants, they are the options for patients with contraindications for PVB or TEA (97).
4.1. Limitation
First, the results regarding to the emerging techniques, including TTMPB, PIFB, ESPB, PECS, and SAPB, need to be confirmed in more RCTs. Several ongoing trials with regional anesthetic techniques for cardiac surgery were shown in Supplementary Table S23. The results of mentioned techniques may be affected by publication bias, suggesting that future meta-analyses may draw different conclusions on some outcomes we analyzed. Second, surgical techniques, perioperative care protocols, local anesthetics, and postoperative adjunctive analgesia may be underlying confounders that were not adjustable. Meanwhile, not all of the RCTs clearly stated that multimodal postoperative analgesia was utilized. Regional anesthesia modalities should be regarded as complementary rather than an alternative to a multimodal analgesic strategy (8, 98). Third, not all time points of pain score or cumulative morphine consumption were assessed in each involved study. Fourth, wound infusion was not involved in our analysis because wound infusion was not recommended in cardiac surgery in the guideline of European Association of Cardio-Thoracic Surgery (81). Fifth, some safety outcomes (hematoma, wound infection, sedation, and urinary retention) and cardiac functional outcomes (myocardial infarction, arrhythmia, and supraventricular tachycardia) were limited and excluded in this NMA. Fourth, all blocks in the involved studies were performed before operation or after operation. None of previous studies reported there was potential differences between these groups.
5. Conclusions
TEA seems the most effective regional postoperative anesthesia for patients after cardiac surgery by reducing pain scores and decreasing the rate of need for rescue analgesia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors were fully involved in the study and has contributed significantly to the submitted work, in terms of conception and design of the study, analysis and interpretation of the results, and critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1078756/full#supplementary-material.
References
1. Lahtinen P, Kokki H, Hynynen M. Pain after cardiac surgery: a prospective cohort study of 1-year incidence and intensity. Anesthesiology. (2006) 105:794–800. doi: 10.1097/00000542-200610000-00026
2. Echeverria-Villalobos M, Stoicea N, Todeschini AB, Fiorda-Diaz J, Uribe AA, Weaver T, et al. Enhanced recovery after surgery (ERAS): a perspective review of postoperative pain management under ERAS pathways and its role on opioid crisis in the United States. Clin J Pain. (2020) 36:219–26. doi: 10.1097/AJP.0000000000000792
3. Caruso TJ, Lawrence K, Tsui BCH. Regional anesthesia for cardiac surgery. Curr Opin Anaesthesiol. (2019) 32:674–82. doi: 10.1097/ACO.0000000000000769
4. Liu J, Zhang S, Chen J, Mao Y, Shao X, Li Y, et al. Risk factors for ventilator-associated events: a prospective cohort study. Am J Infect Control. (2019) 47:744–9. doi: 10.1016/j.ajic.2018.09.032
5. Gaweda B, Borys M, Belina B, Bak J, Czuczwar M, Woloszczuk-Gebicka B, et al. Postoperative pain treatment with erector spinae plane block and pectoralis nerve blocks in patients undergoing mitral/tricuspid valve repair - a randomized controlled trial. BMC Anesthesiol. (2020) 20:51. doi: 10.1186/s12871-020-00961-8
6. Wang J, Zhao G, Song G, Liu J. The efficacy and safety of local anesthetic techniques for postoperative analgesia after cesarean section: a Bayesian network meta-analysis of randomized controlled trials. J Pain Res. (2021) 14:1559–72. doi: 10.2147/JPR.S313972
7. Mercier F, Claret L, Prins K, Bruno R. A model-based meta-analysis to compare efficacy and tolerability of tramadol and tapentadol for the treatment of chronic non-malignant pain. Pain Ther. (2014) 3:31–44. doi: 10.1007/s40122-014-0023-5
8. Wong HY, Pilling R, Young BWM, Owolabi AA, Onwochei DN, Desai N. Comparison of local and regional anesthesia modalities in breast surgery: a systematic review and network meta-analysis. J Clin Anesth. (2021) 72:110274. doi: 10.1016/j.jclinane.2021.110274
9. El-Baz N, Goldin M. Continuous epidural infusion of morphine for pain relief after cardiac operations. J Thorac Cardiovasc Surg. (1987) 93:878–83. doi: 10.1016/S0022-5223(19)37048-5
10. Rein KA, Stenseth R, Myhre HO, Levang OW, Krogstad A. The influence of thoracic epidural analgesia on transcapillary fluid balance in subcutaneous tissue. A study in patients undergoing aortocoronary bypass surgery. Acta Anaesthesiol Scand. (1989) 33:79–83. doi: 10.1111/j.1399-6576.1989.tb02865.x
11. Liem TH, Hasenbos MA, Booij LH, Gielen MJ. Coronary artery bypass grafting using two different anesthetic techniques: part 2: postoperative outcome. J Cardiothorac Vasc Anesth. (1992) 6:156–61. doi: 10.1016/1053-0770(92)90190-I
12. Stenseth R, Bjella L, Berg EM, Christensen O, Levang OW, Gisvold SE. Effects of thoracic epidural analgesia on pulmonary function after coronary artery bypass surgery. Eur J Cardiothorac Surg. (1996) 10:859–65; discussion 866. doi: 10.1016/S1010-7940(96)80311-3
13. Fawcett WJ, Edwards RE, Quinn AC, MacDonald IA, Hall GM. Thoracic epidural analgesia started after cardiopulmonary bypass. Adrenergic, cardiovascular and respiratory sequelae. Anaesthesia. (1997) 52:294–9. doi: 10.1111/j.1365-2044.1997.80-az0088.x
14. Brix-Christensen V, Tonnesen E, Sorensen IJ, Bilfinger TV, Sanchez RG, Stefano GB. Effects of anaesthesia based on high versus low doses of opioids on the cytokine and acute-phase protein responses in patients undergoing cardiac surgery. Acta Anaesthesiol Scand. (1998) 42:63–70. doi: 10.1111/j.1399-6576.1998.tb05082.x
15. Loick HM, Schmidt C, Van Aken H, Junker R, Erren M, Berendes E, et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesth Analg. (1999) 88:701–9. doi: 10.1097/00000539-199904000-00001
16. Tenling A, Joachimsson PO, Tyden H, Wegenius G, Hedenstierna G. Thoracic epidural anesthesia as an adjunct to general anesthesia for cardiac surgery: effects on ventilation-perfusion relationships. J Cardiothorac Vasc Anesth. (1999) 13:258–64. doi: 10.1016/S1053-0770(99)90260-4
17. Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. (2001) 15:288–92. doi: 10.1053/jcan.2001.23271
18. Jidéus L, Joachimsson PO, Stridsberg M, Ericson M, Tyden H, Nilsson L, et al. Thoracic epidural anesthesia does not influence the occurrence of postoperative sustained atrial fibrillation. Ann Thorac Surg. (2001) 72:65–71. doi: 10.1016/S0003-4975(01)02631-5
19. Scott NB, Turfrey DJ, Ray DA, Nzewi O, Sutcliffe NP, Lal AB, et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. (2001) 93:528–35. doi: 10.1097/00000539-200109000-00003
20. Bach F, Grundmann U, Bauer M, Buchinger H, Soltesz S, Graeter T, et al. Modulation of the inflammatory response to cardiopulmonary bypass by dopexamine and epidural anesthesia. Acta Anaesthesiol Scand. (2002) 46:1227–35. doi: 10.1034/j.1399-6576.2002.461010.x
21. de Vries AJ, Mariani MA, van der Maaten JM, Loef BG, Lip H. To ventilate or not after minimally invasive direct coronary artery bypass surgery: the role of epidural anesthesia. J Cardiothorac Vasc Anesth. (2002) 16:21–6. doi: 10.1053/jcan.2002.29645
22. Fillinger MP, Yeager MP, Dodds TM, Fillinger MF, Whalen PK, Glass DD. Epidural anesthesia and analgesia: effects on recovery from cardiac surgery. J Cardiothorac Vasc Anesth. (2002) 16:15–20. doi: 10.1053/jcan.2002.29639
23. Priestley MC, Cope L, Halliwell R, Gibson P, Chard RB, Skinner M, et al. Thoracic epidural anesthesia for cardiac surgery: the effects on tracheal intubation time and length of hospital stay. Anesth Analg. (2002) 94:275–82. doi: 10.1213/00000539-200202000-00009
24. Berendes E, Schmidt C, Van Aken H, Hartlage MG, Wirtz S, Reinecke H, et al. Reversible cardiac sympathectomy by high thoracic epidural anesthesia improves regional left ventricular function in patients undergoing coronary artery bypass grafting: a randomized trial. Arch Surg. (2003) 138:1283–90; discussion 1291. doi: 10.1001/archsurg.138.12.1283
25. Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg. (2003) 75:93–100. doi: 10.1016/S0003-4975(02)04074-2
26. Volk T, Dopfmer UR, Schmutzler M, Rimpau S, Schnitzler H, Konertz W, et al. Stress induced IL-10 does not seem to be essential for early monocyte deactivation following cardiac surgery. Cytokine. (2003) 24:237–43. doi: 10.1016/S1043-4666(03)00090-5
27. Kendall JB, Russell GN, Scawn ND, Akrofi M, Cowan CM, Fox MA. A prospective, randomised, single-blind pilot study to determine the effect of anaesthetic technique on troponin T release after off-pump coronary artery surgery. Anaesthesia. (2004) 59:545–9. doi: 10.1111/j.1365-2044.2004.03713.x
28. Nygård E, Sorensen LH, Hviid LB, Pedersen FM, Ravn J, Thomassen L, et al. Effects of amiodarone and thoracic epidural analgesia on atrial fibrillation after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. (2004) 18:709–14. doi: 10.1053/j.jvca.2004.08.006
29. Barrington MJ, Kluger R, Watson R, Scott DA, Harris KJ. Epidural anesthesia for coronary artery bypass surgery compared with general anesthesia alone does not reduce biochemical markers of myocardial damage. Anesth Analg. (2005) 100:921–8. doi: 10.1213/01.ANE.0000146437.88485.47
30. McDonald SB, Jacobsohn E, Kopacz DJ, Desphande S, Helman JD, Salinas F, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. (2005) 100:25–32. doi: 10.1213/01.ANE.0000139652.84897.BD
31. Hansdottir V, Philip J, Olsen MF, Eduard C, Houltz E, Ricksten SE. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology. (2006) 104:142–51. doi: 10.1097/00000542-200601000-00020
32. Bakhtiary F, Therapidis P, Dzemali O, Ak K, Ackermann H, Meininger D, et al. Impact of high thoracic epidural anesthesia on incidence of perioperative atrial fibrillation in off-pump coronary bypass grafting: a prospective randomized study. J Thorac Cardiovasc Surg. (2007) 134:460–4. doi: 10.1016/j.jtcvs.2007.03.043
33. Barr AM, Tutungi E, Almeida AA. Parasternal intercostal block with ropivacaine for pain management after cardiac surgery: a double-blind, randomized, controlled trial. J Cardiothorac Vasc Anesth. (2007) 21:547–53. doi: 10.1053/j.jvca.2006.09.003
34. Kiliçkan L, Yumuk Z, Bayindir O. The effect of combined preinduction thoracic epidural anaesthesia and glucocorticoid administration on perioperative interleukin-10 levels and hyperglycemia. A randomized controlled trial. J Cardiovasc Surg. (2008) 49:87–93.
35. Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann Card Anaesth. (2008) 11:91–6. doi: 10.4103/0971-9784.41576
36. Palomero Rodríguez MA, Suarez Gonzalo L, Villar Alvarez F, Varela Crespo C, Moreno Gomez Limon I, Criado Jimenez A. Thoracic epidural anesthesia decreases C-reactive protein levels in patients undergoing elective coronary artery bypass graft surgery with cardiopulmonary bypass. Minerva Anestesiol. (2008) 74:619–26.
37. Tenenbein PK, Debrouwere R, Maguire D, Duke PC, Muirhead B, Enns J, et al. Thoracic epidural analgesia improves pulmonary function in patients undergoing cardiac surgery. Can J Anaesth. (2008) 55:344–50. doi: 10.1007/BF03021489
38. Lenkutis T, Benetis R, Sirvinskas E, Raliene L, Judickaite L. Effects of epidural anesthesia on intrathoracic blood volume and extravascular lung water during on-pump cardiac surgery. Perfusion. (2009) 24:243–8. doi: 10.1177/0267659109348724
39. Mehta Y, Vats M, Sharma M, Arora R, Trehan N. Thoracic epidural analgesia for off-pump coronary artery bypass surgery in patients with chronic obstructive pulmonary disease. Ann Card Anaesth. (2010) 13:224–30. doi: 10.4103/0971-9784.69062
40. Sharma M, Mehta Y, Sawhney R, Vats M, Trehan N. Thoracic epidural analgesia in obese patients with body mass index of more than 30 kg/m2 for off pump coronary artery bypass surgery. Ann Card Anaesth. (2010) 13:28–33. doi: 10.4103/0971-9784.58831
41. Caputo M, Alwair H, Rogers CA, Pike K, Cohen A, Monk C, et al. Thoracic epidural anesthesia improves early outcomes in patients undergoing off-pump coronary artery bypass surgery: a prospective, randomized, controlled trial. Anesthesiology. (2011) 114:380–90. doi: 10.1097/ALN.0b013e318201f571
42. Kirov MY, Eremeev AV, Smetkin AA, Bjertnaes LJ. Epidural anesthesia and postoperative analgesia with ropivacaine and fentanyl in off-pump coronary artery bypass grafting: a randomized, controlled study. BMC Anesthesiol. (2011) 11:17. doi: 10.1186/1471-2253-11-17
43. Svircevic V, Nierich AP, Moons KG, Diephuis JC, Ennema JJ, Brandon Bravo Bruinsma GJ, et al. Thoracic epidural anesthesia for cardiac surgery: a randomized trial. Anesthesiology. (2011) 114:262–70. doi: 10.1097/ALN.0b013e318201d2de
44. El-Morsy GZ, El-Deeb A. The outcome of thoracic epidural anesthesia in elderly patients undergoing coronary artery bypass graft surgery. Saudi J Anaesth. (2012) 6:16–21. doi: 10.4103/1658-354X.93048
45. Nielsen DV, Bhavsar R, Greisen J, Ryhammer PK, Sloth E, Jakobsen CJ. High thoracic epidural analgesia in cardiac surgery. Part 2–high thoracic epidural analgesia does not reduce time in or improve quality of recovery in the intensive care unit. J Cardiothorac Vasc Anesth. (2012) 26:1048–54. doi: 10.1053/j.jvca.2012.05.008
46. Gurses E, Berk D, Sungurtekin H, Mete A, Serin S. Effects of high thoracic epidural anesthesia on mixed venous oxygen saturation in coronary artery bypass grafting surgery. Med Sci Monit: Int Med J Exp Clin Res Med. (2013) 19:222–9. doi: 10.12659/MSM.883861
47. Onan B, Onan IS, Kilickan L, Sanisoglu I. Effects of epidural anesthesia on acute and chronic pain after coronary artery bypass grafting. J Card Surg. (2013) 28:248–53. doi: 10.1111/jocs.12086
48. Neuburger PJ, Ngai JY, Chacon MM, Luria B, Manrique-Espinel AM, Kline RP, et al. A prospective randomized study of paravertebral blockade in patients undergoing robotic mitral valve repair. J Cardiothorac Vasc Anesth. (2015) 29:930–6. doi: 10.1053/j.jvca.2014.10.010
49. Zawar BP, Mehta Y, Juneja R, Arora D, Raizada A, Trehan N. Nonanalgesic benefits of combined thoracic epidural analgesia with general anesthesia in high risk elderly off pump coronary artery bypass patients. Ann Card Anaesth. (2015) 18:385–91. doi: 10.4103/0971-9784.159810
50. Doğan Bakı E, Kavrut Ozturk N, Ayoğlu RU, Emmiler M, Karslı B, Uzel H. Effects of parasternal block on acute and chronic pain in patients undergoing coronary artery surgery. Semin Cardiothorac Vasc Anesth. (2016) 20:205–12. doi: 10.1177/1089253215576756
51. Ozturk NK, Baki ED, Kavakli AS, Sahin AS, Ayoglu RU, Karaveli A, et al. Comparison of transcutaneous electrical nerve stimulation and parasternal block for postoperative pain management after cardiac surgery. Pain Res Manag. (2016) 2016:4261949. doi: 10.1155/2016/4261949
52. Lockwood GG, Cabreros L, Banach D, Punjabi PP. Continuous bilateral thoracic paravertebral blockade for analgesia after cardiac surgery: a randomised, controlled trial. Perfusion. (2017) 32:591–7. doi: 10.1177/0267659117715507
53. Zhan Y, Chen G, Huang J, Hou B, Liu W, Chen S. Effect of intercostal nerve block combined with general anesthesia on the stress response in patients undergoing minimally invasive mitral valve surgery. Exp Ther Med. (2017) 14:3259–64. doi: 10.3892/etm.2017.4868
54. Kumar KN, Kalyane RN, Singh NG, Nagaraja PS, Krishna M, Babu B, et al. Efficacy of bilateral pectoralis nerve block for ultrafast tracking and postoperative pain management in cardiac surgery. Ann Card Anaesth. (2018) 21:333–8. doi: 10.4103/aca.ACA_15_18
55. Nagaraja PS, Ragavendran S, Singh NG, Asai O, Bhavya G, Manjunath N, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. (2018) 21:323–7. doi: 10.4103/aca.ACA_16_18
56. Obersztyn M, Trejnowska E, Nadziakiewicz P, Knapik P. Evaluation of thoracic epidural analgesia in patients undergoing coronary artery bypass surgery - a prospective randomized trial. Kardiochir TorakoChirurgia Pol. (2018) 15:72–8. doi: 10.5114/kitp.2018.76471
57. Venkataswamy M, Ramakrishna PS, Nagaraja PS, Singh NG, Adoni PJ. Efficacy of bilateral continuous paravertebral block for off pump coronary artery bypass surgery. J Cardiovasc Dis Res. (2018) 9:59–62. doi: 10.5530/jcdr.2018.2.15
58. Fujii S, Roche M, Jones PM, Vissa D, Bainbridge D, Zhou JR. Transversus thoracis muscle plane block in cardiac surgery: a pilot feasibility study. Reg Anesth Pain Med. (2019) 44:556–60. doi: 10.1136/rapm-2018-100178
59. Krishna SN, Chauhan S, Bhoi D, Kaushal B, Hasija S, Sangdup T, et al. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: a randomized controlled trial. J Cardiothorac Vasc Anesth. (2019) 33:368–75. doi: 10.1053/j.jvca.2018.05.050
60. Lee CY, Robinson DA, Johnson CA, Zhang Y, Wong J, Joshi DJ, et al. A randomized controlled trial of liposomal bupivacaine parasternal intercostal block for sternotomy. Ann Thorac Surg. (2019) 107:128–34. doi: 10.1016/j.athoracsur.2018.06.081
61. Sun L, Li Q, Wang Q, Ma F, Han W, Wang M. Bilateral thoracic paravertebral block combined with general anesthesia vs. General anesthesia for patients undergoing off-pump coronary artery bypass grafting: a feasibility study. BMC Anesthesiol. (2019) 19:101. doi: 10.1186/s12871-019-0768-9
62. Aydin ME, Ahiskalioglu A, Ates I, Tor IH, Borulu F, Erguney OD, et al. Efficacy of ultrasound-guided transversus thoracic muscle plane block on postoperative opioid consumption after cardiac surgery: a prospective, randomized, double-blind study. J Cardiothorac Vasc Anesth. (2020) 34:2996–3003. doi: 10.1053/j.jvca.2020.06.044
63. El Shora HA, El Beleehy AA, Abdelwahab AA, Ali GA, Omran TE, Hassan EA, et al. Bilateral paravertebral block versus thoracic epidural analgesia for pain control post-cardiac surgery: a randomized controlled trial. Thorac Cardiovasc Surg. (2020) 68:410–6. doi: 10.1055/s-0038-1668496
64. Gautam S, Pande S, Agarwal A, Agarwal SK, Rastogi A, Shamshery C, et al. Evaluation of serratus anterior plane block for pain relief in patients undergoing MIDCAB surgery. Innov: Technol Tech Cardiothorac Vasc Surg. (2020) 15:148–54. doi: 10.1177/1556984520908962
65. Magoon R, Kaushal B, Chauhan S, Bhoi D, Bisoi A, Khan M. A randomised controlled comparison of serratus anterior plane, pectoral nerves and intercostal nerve block for post-thoracotomy analgesia in adult cardiac surgery. Indian J Anaesth. (2020) 64:1018–24. doi: 10.4103/ija.IJA_327_20
66. Vilvanathan S, Saravanababu MS, Sreedhar R, Gadhinglajkar SV, Dash PK, Sukesan S. Ultrasound-guided modified parasternal intercostal nerve block: role of preemptive analgesic adjunct for mitigating poststernotomy pain. Anesth Essays Res. (2020) 14:300–4. doi: 10.4103/aer.AER_32_20
67. Athar M, Parveen S, Yadav M, Siddiqui OA, Nasreen F, Ali S, et al. A randomized double-blind controlled trial to assess the efficacy of ultrasound-guided erector spinae plane block in cardiac surgery. J Cardiothorac Vasc Anesth. (2021) 35:3574–80. doi: 10.1053/j.jvca.2021.03.009
68. Bloc S, Perot BP, Gibert H, Law Koune JD, Burg Y, Leclerc D, et al. Efficacy of parasternal block to decrease intraoperative opioid use in coronary artery bypass surgery via sternotomy: a randomized controlled trial. Reg Anesth Pain Med. (2021) 46:671–8. doi: 10.1136/rapm-2020-102207
69. Khera T, Murugappan KR, Leibowitz A, Bareli N, Shankar P, Gilleland S, et al. Ultrasound-guided pecto-intercostal fascial block for postoperative pain management in cardiac surgery: a prospective, randomized, placebo-controlled trial. J Cardiothorac Vasc Anesth. (2021) 35:896–903. doi: 10.1053/j.jvca.2020.07.058
70. Kumar AK, Chauhan S, Bhoi D, Kaushal B. Pectointercostal fascial block (PIFB) as a novel technique for postoperative pain management in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. (2021) 35:116–22. doi: 10.1053/j.jvca.2020.07.074
71. Wasfy SF, Kamhawy GA, Omar AH, Abd El Aziz HF. Bilateral continuous erector spinae block versus multimodal intravenous analgesia in coronary bypass surgery. A randomized trial. Egypt J Anaesth. (2021) 37:152–8. doi: 10.1080/11101849.2021.1904548
72. Zhang Y, Gong H, Zhan B, Chen S. Effects of bilateral pecto-intercostal fascial block for perioperative pain management in patients undergoing open cardiac surgery: a prospective randomized study. BMC Anesthesiol. (2021) 21:175. doi: 10.1186/s12871-021-01391-w
73. Zhang Y, Li X, Chen S. Bilateral transversus thoracis muscle plane block provides effective analgesia and enhances recovery after open cardiac surgery. J Card Surg. (2021) 36:2818–23. doi: 10.1111/jocs.15666
74. Bjornnes AK, Rustoen T, Lie I, Watt-Watson J, Leegaard M. Pain characteristics and analgesic intake before and following cardiac surgery. Eur J Cardiovasc Nurs. (2016) 15:47–54. doi: 10.1177/1474515114550441
75. Zubrzycki M, Liebold A, Skrabal C, Reinelt H, Ziegler M, Perdas E, et al. Assessment and pathophysiology of pain in cardiac surgery. J Pain Res. (2018) 11:1599–611. doi: 10.2147/JPR.S162067
76. McCoul ED, Barnett ML, Brenner MJ. Reducing opioid prescribing and consumption after surgery-keeping the lock on pandora’s box. JAMA Otolaryngol Head Neck Surg. (2021) 147:819–21. doi: 10.1001/jamaoto.2021.1846
77. Clowes GH Jr, Neville WE, Hopkins A, Anzola J, Simeone FA. Factors contributing to success or failure in the use of a pump oxygenator for complete by-pass of the heart and lung, experimental and clinical. Surgery. (1954) 36:557–79.13195976
78. Guay J, Kopp S. Epidural analgesia for adults undergoing cardiac surgery with or without cardiopulmonary bypass. Cochrane Database Syst Rev. (2019) 3:CD006715. doi: 10.1002/14651858.CD006715.pub3
79. Zhang S, Wu X, Guo H, Ma L. Thoracic epidural anesthesia improves outcomes in patients undergoing cardiac surgery: meta-analysis of randomized controlled trials. Eur J Med Res. (2015) 20:25. doi: 10.1186/s40001-015-0091-y
80. Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. (2012) 23:5–13. doi: 10.1097/01823246-201223010-00002
81. Sousa-Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. (2018) 53:5–33. doi: 10.1093/ejcts/ezx314
82. Amat-Santos IJ, Dumont E, Villeneuve J, Doyle D, Rheault M, Lavigne D, et al. Effect of thoracic epidural analgesia on clinical outcomes following transapical transcatheter aortic valve implantation. Heart. (2012) 98:1583–90. doi: 10.1136/heartjnl-2012-302185
83. Landoni G, Isella F, Greco M, Zangrillo A, Royse CF. Benefits and risks of epidural analgesia in cardiac surgery. Br J Anaesth. (2015) 115:25–32. doi: 10.1093/bja/aev201
84. Bracco D, Hemmerling T. Epidural analgesia in cardiac surgery: an updated risk assessment. Heart Surg Forum. (2007) 10:E334–7. doi: 10.1532/HSF98.20071077
85. Liu H, Emelife PI, Prabhakar A, Moll V, Kendrick JB, Parr AT, et al. Regional anesthesia considerations for cardiac surgery. Best Pract Res Clin Anaesthesiol. (2019) 33:387–406. doi: 10.1016/j.bpa.2019.07.008
86. Mace L. An audit of post-operative nausea and vomiting, following cardiac surgery: scope of the problem. Nurs Crit Care. (2003) 8:187–96. doi: 10.1046/j.1362-1017.2003.00029.x
87. Allen KB, Brovman EY, Chhatriwalla AK, Greco KJ, Rao N, Kumar A, et al. Opioid-related adverse events: incidence and impact in patients undergoing cardiac surgery. Semin Cardiothorac Vasc Anesth. (2020) 24:219–26. doi: 10.1177/1089253219888658
88. Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. (2016) 2:CD009121. doi: 10.1002/14651858.CD009121.pub2
89. Scarfe AJ, Schuhmann-Hingel S, Duncan JK, Ma N, Atukorale YN, Cameron AL. Continuous paravertebral block for post-cardiothoracic surgery analgesia: a systematic review and meta-analysis. Eur J Cardiothorac Surg. (2016) 50:1010–8. doi: 10.1093/ejcts/ezw168
90. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. (2016) 41:621–7. doi: 10.1097/AAP.0000000000000451
91. Balan C, Bubenek-Turconi SI, Tomescu DR, Valeanu L. Ultrasound-guided regional anesthesia-current strategies for enhanced recovery after cardiac surgery. Medicina. (2021) 57:312. doi: 10.3390/medicina57040312
92. Smith LM, Barrington MJ, M. St Vincent's Hospital. Ultrasound-guided blocks for cardiovascular surgery: which block for which patient? Curr Opin Anaesthesiol. (2020) 33:64–70. doi: 10.1097/ACO.0000000000000818
93. de la Torre PA, Garcia PD, Alvarez SL, Miguel FJ, Perez MF. A novel ultrasound-guided block: a promising alternative for breast analgesia. Aesthet Surg J. (2014) 34:198–200. doi: 10.1177/1090820X13515902
94. Ueshima H, Kitamura A. Blocking of multiple anterior branches of intercostal nerves (Th2-6) using a transversus thoracic muscle plane block. Reg Anesth Pain Med. (2015) 40:388. doi: 10.1097/AAP.0000000000000245
95. Piraccini E, Calli M, Corso RM, Maitan S. Pectointercostal fascial block (PIFB) and parasternal block (PSB): two names for the same block? J Clin Anesth. (2019) 58:130. doi: 10.1016/j.jclinane.2019.07.010
96. Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. (2011) 66:847–8. doi: 10.1111/j.1365-2044.2011.06838.x
97. Yu S, Valencia MB, Roques V, Aljure OD. Regional analgesia for minimally invasive cardiac surgery. J Card Surg. (2019) 34:1289–96. doi: 10.1111/jocs.14177
Keywords: cardiac surgery, postoperative pain, regional anesthesia, meta-analysis, network
Citation: Zhou K, Li D and Song G (2023) Comparison of regional anesthetic techniques for postoperative analgesia after adult cardiac surgery: bayesian network meta-analysis. Front. Cardiovasc. Med. 10:1078756. doi: 10.3389/fcvm.2023.1078756
Received: 24 October 2022; Accepted: 3 May 2023;
Published: 22 May 2023.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Sudhakar Subramani, The University of Iowa, United StatesHimani Bhatt, Icahn School of Medicine at Mount Sinai, United States
© 2023 Zhou, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Song c29uZ2c4NEAxNjMuY29t
 Ke Zhou1
Ke Zhou1 Guang Song
Guang Song