
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 July 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1078038
This article is part of the Research Topic Advances in Imaging of Pediatric Heart Diseases View all 28 articles
 Ting Gong1,2,†
Ting Gong1,2,† Feiyan Zhang2,3,†
Feiyan Zhang2,3,† Lingxin Feng2
Lingxin Feng2 Xu Zhu2
Xu Zhu2 Dan Deng4
Dan Deng4 Tingting Ran2
Tingting Ran2 Liling Li2
Liling Li2 Li Kong2
Li Kong2 Liqun Sun5*
Liqun Sun5* Xiaojuan Ji2,6*
Xiaojuan Ji2,6*
Background: Coarctation of the aorta (CoA) is a common congenital cardiovascular malformation, and improvements in the diagnostic process for surgical decision-making are important. We sought to compare the diagnostic accuracy of transthoracic echocardiography (TTE) with computed tomographic angiography (CTA) to diagnose CoA.
Methods: We retrospectively reviewed 197 cases of CoA diagnosed by TTE and CTA and confirmed at surgery from July 2009 to August 2019.
Results: The surgical findings confirmed that 19 patients (9.6%) had isolated CoA and 178 (90.4%) had CoA combined with other congenital cardiovascular malformations. The diagnostic accuracy of CoA by CTA was significantly higher than that of TTE (χ2 = 6.52, p = 0.01). In contrast, the diagnostic accuracy of TTE for associated cardiovascular malformations of CoA was significantly higher than that of CTA (χ2 = 15.36, p < 0.0001). Infants and young children had more preductal type of CoA, and PDA was the most frequent cardiovascular lesion associated with CoA. The pressure gradient was significantly decreased after the first operation, similar at 6 months, 1 year, and 3 years follow-ups by TTE.
Conclusions: CTA is more accurate as a clinical tool for diagnosing CoA; however, TTE with color Doppler can better identify associated congenital cardiovascular malformations. Therefore, combining TTE and CTA would benefit clinical evaluation and management in patients suspected of CoA. TTE was valuable for post-operation follow-up and clinical management.
Coarctation of the aorta (CoA) is a cardiovascular malformation commonly described as the narrowing of the aortic isthmus between the left subclavian artery and ductus arteriosus, which has been reported to take three forms: preductal, ductal, and postductal CoA (1). CoA accounts for approximately 5%–7% of all congenital heart defects (2) with an incidence of 2.5–4 per 10,000 live births (3) and is approximately 4 times more frequent in males than females (4). CoA can be presented as a solitary defect but has been associated with other cardiac malformations such as bicuspid aortic valve, transposition of the great arteries, ventricular septal defect (VSD), and patent ductus arteriosus (PDA) (5). Despite the progress made in fetal diagnosis and treatment, prenatal screening for CoA is a diagnostic challenge. It is the most commonly missed diagnosis of fetal congenital heart disease (CHD), with less than one-third of cases detected (6, 7). More importantly, 60%–80% of newborns with CoA are not diagnosed before hospital discharge (8–11), and one study found that 27% of undiagnosed patients with CoA died at a median age of 17 days (12). Furthermore, recent studies have shown that in the presurgical era the median survival age was 31 years (13, 14).
Newborns are usually asymptomatic right after birth because of the PDA. Some infants complained manifestation of clinical symptoms: cardiogenic shock, absent/feeble femoral pulse, delayed capillary refill, feeding problems, decreased responsiveness, metabolic acidosis, mesenteric ischemia, myocardial depression, etc. (14). Unrepaired CoA leads to premature coronary artery disease, ventricular dysfunction, aortic aneurysm/dissection, and cerebral vascular disease by the third or fourth decade of life (7, 15, 16). However, those who have got surgical or transcatheter intervention, the natural history of the disease has significantly changed with most patients making it to adulthood (14). There is a need to improve diagnosis and early intervention to prevent unrepaired CoA complications. Postnatally, transthoracic echocardiography (TTE) is the gold standard imaging modality for preliminary and diagnostic screening for clinical suspicion of CoA, which involves 2D echo for structural evaluation and color Doppler for the direction of blood flow (6).
Different modalities could be used to assess CoA, including chest radiography, transthoracic echocardiography, computed tomography (CT) and computed tomographic angiography (CTA), cardiac magnetic resonance imaging (CMR), and catheter angiography (6). However, advanced modalities such as CMR and CTA confirm a suspected vascular diagnosis. Primary imaging of TTE modality for suspected CoA has given its availability, safety, and capacity to provide hemodynamic parameters. TTE can assess cardiac function and associated cardiac and valvular abnormalities. TTE is limited in evaluating extracardiac structures and collateral circulation due to poor acoustic window and operator dependence (6). It is one of the first-line imaging modalities used in the evaluation of cardiac function and disease owing to its low cost, portability, widespread availability, and lack of ionizing radiation. It is important for CoA to be diagnosed and repaired early due to its high mortality rate and the fact that even those with repaired CoA are now no longer considered benign conditions and need continuous follow-up (17). This retrospective study aimed to assess the diagnostic accuracy of TTE with CTA for the diagnosis of CoA, and the clinical management and surgical outcomes.
We collected the patients suspected of CoA and excluded ineligible patients (Figure 1). A total of 197 children assessed by preoperative TTE and CTA with a diagnosis of CoA confirmed at the surgery between July 2009 and August 2019 were included in this retrospective study. Overall, each patient had a preoperative diagnostic TTE and CTA and a postoperative TTE. The patients with incomplete clinical data were excluded from the research. This retrospective study was approved by the research ethics committee of the Children's Hospital of Chongqing Medical University, China.
The patients were examined by TTE using a Philips IE33, GE Vivid I, and GE E9 color Doppler ultrasonic instrument with a 1–8 MHz transducer. The children less than 3 years old were orally or rectally given chloral hydrate sedative at a dose of 0.5 ml/kg. The patients underwent detailed echocardiography according to published guidelines (18). The apical four-chamber view, left ventricular (LV) long-axis view, large artery short-axis view, and suprasternal view were assessed to confirm the structures and connections of the atria, ventricles, and aorta. The suprasternal view was used to assess the aortic arch and its branches. In addition, the descending aorta was assessed to determine the location and extent of the stenosis. The location and the diameter of the narrowest part at the coarctation site were evaluated. The segment of the aortic arch was measured from the inner edge to the inner edge on two-dimensional images during systole. In addition, the maximum blood flow velocity and pressure gradient by color Doppler at the coarctation site were recorded.
Before the CT examination, the guardians of the patients provided informed consent for the potential adverse reactions to contrast medium and exposure to radiation. The images were acquired with Lightspeed VCT 64-slice spiral CT (GE Healthcare, USA) and Brilliance ICT 256-slice spiral CT (Philips, Netherlands) by using the following parameters: tube voltage, 90–120 kV; automatic tube current, 60–100 mAs; pitch, 0.984; slice thickness, 5.0 mm; and slice interval, 5.0 mm. The patients were scanned in the supine position from the neck to the diaphragm. A contrast agent (Omnipaque, GE Healthcare, USA) was injected during the scan into the scalp vein or elbow vein of the patient at 300–350 mg/ml at 2–5 ml/s, dosage of 2.0 ml/kg. The children under 5 years old were sedated with contrast agent (0.5 ml/kg). The post-processing methods in the CT scanner included multiplanar reconstruction and three-dimensional volume rendering. All images were reconstructed with 1.25 mm slice thickness with a 0.625 mm slice interval. Multi-directional and multi-angle reconstruction images were used to evaluate the heart structure and origin of the blood vessels.
All cases in this cohort underwent end-to-end anastomosis, end-to-side anastomosis, extended end-to-end anastomosis, extended end-to-side anastomosis, pulmonary artery patch, pericardial patch, GORETEX patch or aortic release depending on the different type of CoA and associated cardiac malformations, the aortic arch, innominate artery, left common carotid artery, left subclavian artery, and descending aorta were completely released to prevent recoarctation. A patient follow-up was required until the first discharge and checked if any patient underwent the second operation. Transcoarctation systolic pressure gradient higher than 20 mmHg was considered a recoarctation. The postoperative pressure gradient was followed up at post-operation before discharge, 6 months, 1 year, and 3 years by TTE.
Graphpad 9.0 (Prism, USA) was used for the data analysis. Quantitative data with normal distributions were expressed as mean ± standard deviation (SD). The Chi-squared test (χ2) was used to compare the diagnostic accuracy rate of TTE and CTA, and the rates in preductal, ductal, and postductal groups. The Mann–Whitney test, paired t-test, and ANOVA were used in groups. A p-value of <0.05 was considered statistically significant.
Of the 197 children assessed, there were 129 (65.5%) males and 68 (34.5%) females, with a median age of 4.1 months (1 day–14.5 years) and a median weight of 2.5–44 kg. Among these cases, 174 (88.3%) children were under 3 years old. The symptoms that prompted a clinical investigation for these patients included dizziness, headache, hypertension, recurrent respiratory infection, shortness of breath, feeding difficulty, developmental delays, and abnormal blood pressure gradient between the upper and lower extremities.
The two-dimensional images captured from TTE showed luminal narrowing of the aorta. Despite the different types of CoA (preductal, ductal, or postductal), a pre- and post-stenotic dilatation often developed. The color Doppler images showed an increased systolic blood flow velocity with multicolored flow signal, and a blood pressure gradient of >20 mmHg. The blood flow velocity was decreased at the distal end of the stenosis. In addition, left ventricular hypertrophy, thickened interventricular septum and left posterior ventricular wall, and decreased left ventricular systolic function were observed (Figure 2). The postoperative median inner diameter and systolic pressure gradient of the preductal, ductal, and postductal CoA were all significantly higher than the preoperative measurements (p < 0.0001) (Table 1). However, there were no significant differences between the three different types of CoA in terms of the internal diameter of the aorta and pressure gradient at the pre- and post-surgical TTE evaluation (p > 0.05).
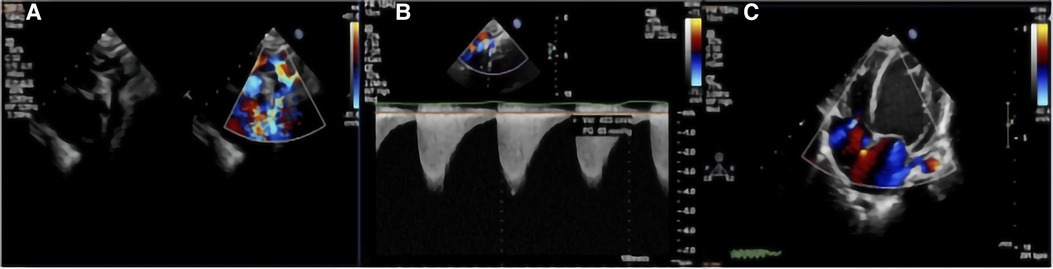
Figure 2. TTE with color Doppler flow imaging showed the location of the CoA and increased blood flow velocity: (A) indicate the location of the CoA and multicolored flow signal; and (B) increased blood flow velocity with a high-pressure gradient, which was estimated by a continuous-wave Doppler. (C) Left ventricular hypertrophy.
A three-dimensional reconstruction from multiple two-dimensional CTA acquisitions established the opportunity to evaluate aortic coarctation vessel diameter, location and degree of stenosis (Figure 3), and associated vascular malformations. CTA was superior to TTE in detecting aortic arch hypoplasia and delineating great vessel branching. The reconstructed three-dimensional CTA images showed the ascending aorta, brachiocephalic artery, left common carotid artery, left subclavian artery, descending aorta, coarctation sites, and collateral circulation. In short, CTA comprehensively showed the diameter of the aortic coarctation, the morphology and anatomy of the aortic arch and the heart, and the state and number of collateral circulations before surgery.
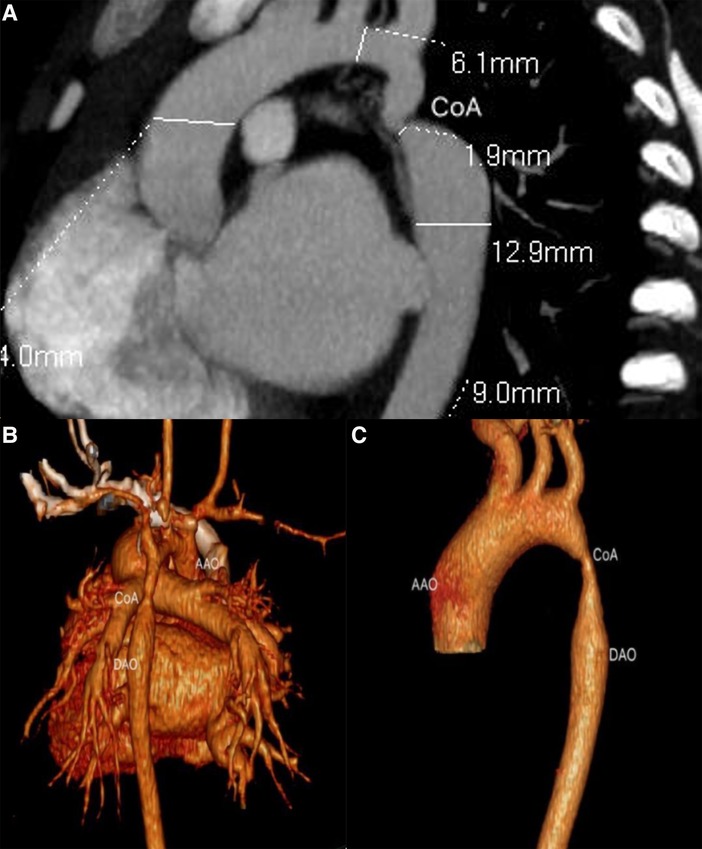
Figure 3. Ductal CoA by CTA. (A) The anatomical measurements by CTA before cardiac surgery; (B,C) sagittal multiplanar reconstruction showed volume rendering images with CoA.
The diagnostic accuracy of TTE and CTA were compared against the diagnosis of CoA confirmed at surgery. Of the 197 cases assessed, 184 patients (93.4%) were diagnosed correctly by TTE, nine (4.6%) were missed, and four cases (2.0%) were misdiagnosed as interrupted aortic arch (IAA) type B. In comparison, 194 children (98.5%) were diagnosed correctly by CTA, 2 cases (1.0%) were misdiagnosed as IAA, and 1 (0.5%) was missed. The diagnostic accuracy of CTA was significantly higher than TTE for CoA (χ2 = 6.52, p = 0.01) (Table 2). One patient with a preductal CoA was misdiagnosed in both TTE and CTA examinations. There were 468 associated cardiovascular malformations confirmed at surgery (Table 3). Among them, 434 were correctly detected by TTE. At the same time, 34 were misdiagnosed, with 4 cases misdiagnosed as other malformations: 1 patent foramen ovale (PFO), 1 atrial septal aneurysm, 1 collateral, and 1 vascular malformation (pulmonary artery origin); and 30 cases misdiagnosed from being undetected: 12 hypoplastic aortic arch (HAA), 6 collateral circulation, 3 PDA, 2 persistent left superior vena cava (PLSVC), 2 ventricular septal defect (VSD), 1 atrial septal defect (ASD), 1 aortic valve hypoplasia, 1 PFO, 1 aortic angulation, and 1 total anomalous pulmonary venous connection (TAPVC).
In comparison, 396 associated cardiovascular malformations were correctly detected by CTA, and 72 were misdiagnosed, with 11 cases misdiagnosed as other malformations: 5 PFO, 3 PDA, 1 ASD, 1 aortic arch dysplasia, and 1 vagus subclavian artery. The undetected cases include 15 PFO, 12 VSD, 10 valvular malformations, 7 collaterals, 1 PDA, 2 TAPVC, 3 vascular malformations, 2 HAA, 1 aortic angulation, 1 endocardial elastofibrosis (EFE), 1 PLSVC, 1 ASD, 1 atrial septal aneurysms, 1 double-chambered left ventricle (DCLV), 1 double-chambered right ventricles (DCRV), and 1 double outlet right ventricle (DORV). The diagnostic accuracy of TTE and CTA for associated cardiovascular defects was 92.7% (434/468) and 84.6% (396/468), respectively, where the diagnostic accuracy of TTE was significantly higher than CTA for CoA (χ2 = 15.36, p < 0.0001) (Table 2).
Based on the surgical findings, three types of CoA were identified: the preductal type (n = 93, 47.2%), the ductal type (n = 86, 43.7%), and the postductal type (n = 18, 9.1%) (Table 4). The surgical findings confirmed that 19 patients (9.6%) had isolated CoA and 178 (90.4%) had CoA combined with other congenital cardiovascular malformations (Table 3). The top five congenital cardiovascular malformations combined with CoA were PDA (25.0%), VSD (24.8%), ASD (22.2%), PFO (7.1%), and HAA (4.7%). The surgical procedures predominantly included extended end-to-end anastomosis (n = 20), extended end-to-side anastomosis (n = 62), end-to-end anastomosis (n = 47), end-to-side anastomosis (n = 30), pulmonary autograft patch aortoplasty (n = 20), aortic arch release (n = 10), and others (n = 8). Among them, the surgical procedures with isolated CoA included end-to-end anastomosis (n = 11), end-to-side anastomosis (n = 4), aortic arch release (n = 2), extended end-to-end anastomosis (n = 1), and extended end-to-side anastomosis (n = 1) (Table 5). The surgical management of different types of non-isolated CoA is shown in Table 6. Out of the 197 cases, 191 cases (97.0%) survived after surgical repair, and in-hospital mortality occurred in six patients (3.0%). One patient died from renal failure and had a preductal CoA with severe mitral stenosis, PDA, VSD, and ASD. The other five patients died from heart failure: two patients had DORV, VSD, ASD, and PDA, one of which died during the operation while the other patient died post-surgery in addition to respiratory failure; one other patient had abnormal origin of the right pulmonary artery with postoperative pulmonary hemorrhage; one with VSD and huge ASD died as severe arrhythmia and gastrointestinal bleeding; one with preductal CoA, ASD had severe bradycardia and died with the pacemaker. The postoperative pressure gradient was significantly decreased at post-operation before discharge, 6 months, 1 year, and 3 years (Figure 4). Although the TTE systolic pressure gradient in some patients was higher than 20 mmHg, most had no clinical symptoms, and TTE indicated a normal left heart function without significant recoarctation. Therefore, no patient underwent re-operation diagnosed with recoarctation. When the pressure gradient was higher than 40 mmHg, patients received clinical consultation.
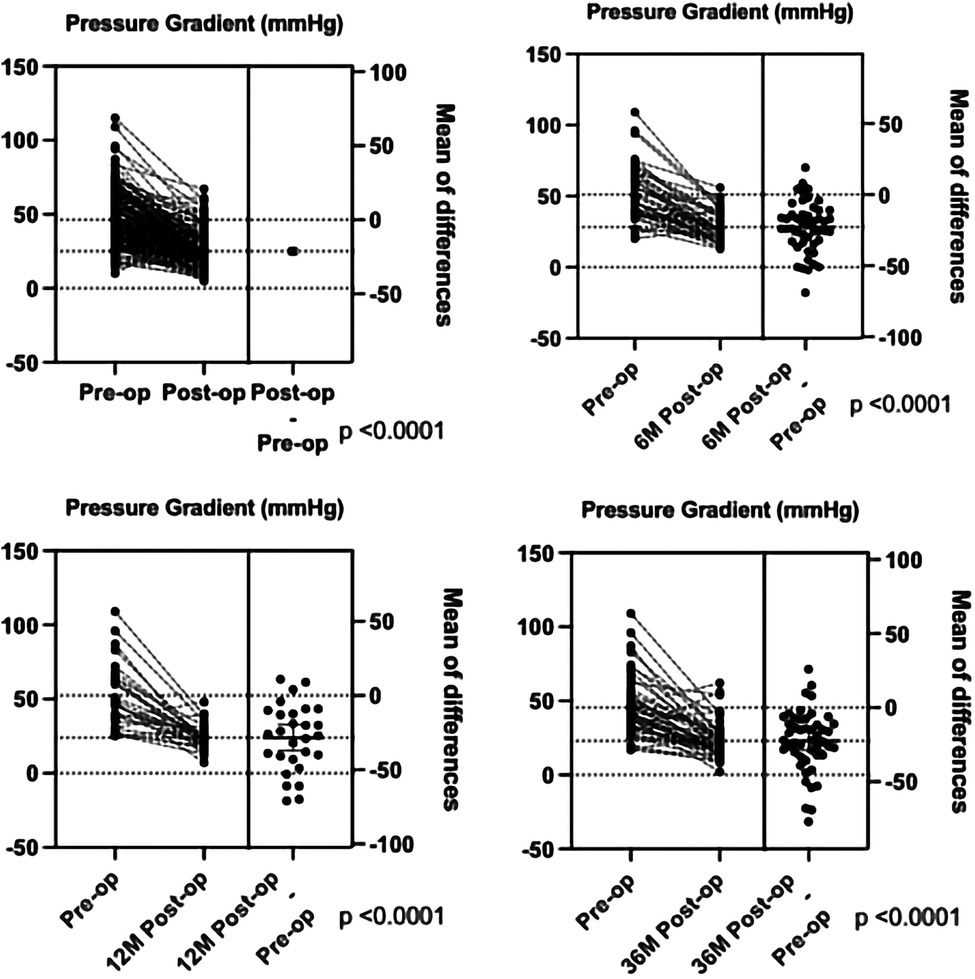
Figure 4. The comparison of the pressure gradient between pre-operation and post-operation before discharge, 6 months, 1 year, and 3 years by TTE.
Our cohort consisted of 197 patients with a male:female ratio of 1.87:1, slightly lower than the reported literature. The pathophysiological mechanisms that underlie CoA remain poorly understood and have been described to be multifactorial. These hypotheses include the following: (1) abnormal embryogenetic development, (2) reduced vessel blood flow, and (3) aberrant duct-like arterial tissue constriction around the aortic lumen at the isthmus (6, 19). One study suggested that coarctation frequently occurs at the ductal position (20). However, another study showed that infants and young children had more of the preductal CoA type (21), which aligns with our results. Our clinical data were composed of 174 infants less than 3 years old, which suggests that preductal CoA commonly presents in infancy. This is important because if patients, particularly newborns, have severe aortic coarctation, their left ventricle cannot efficiently pump blood against the high pressure in the aorta (1), and thus, it leads to hypertrophy. CoA is often accompanied by other cardiovascular anomalies. In this study, 19 patients had isolated CoA, and 178 children had CoA combined with other congenital cardiovascular malformations, consistent with the previous data (13, 22). In the literature, bicuspid aortic valve is the most associated abnormality that affects ≤75% of all CoA cases (23). However, our results suggested that PDA was the most frequent cardiovascular lesion associated with CoA. The treatment options for CoA include surgery and endovascular intervention, including surgical, balloon angioplasty, and stent treatment (24, 25). Because of our retrospective study design, all our patients had undergone surgery based on preoperative TTE and CTA results, with 41.4% of the patients undergoing extended end-to-end anastomosis and extended end-to-side anastomosis, 39.4% of the patients with end-to-end anastomosis and end-to-side anastomosis, 13.1% of the patients with the patch, including patch aortoplasty, pericardial patch and GORETEX patch. The surgical decision-making depended on the type of CoA and associated cardiac malformations.
In this group, 184 cases were correctly diagnosed by TTE with nine cases undiagnosed and the remaining four cases misdiagnosed as IAA. The limitations of TTE included poor acoustic window, narrow field of view, and operator dependence. Two CoA cases with PDA and VSD were misdiagnosed, which we suggest was because the forward blood flow velocity in the descending aorta did not change significantly and thus no multicolored flow signal was detected by color Doppler. Vergales et al. suggested that a PDA in addition to CoA might affect the physiologic obstruction in neonates (26). Therefore, coarctation in newborns might not be present until the ductal tissues insert completely. In CoA with VSD, the left ventricular end-diastolic pressure is increased by the ventricular preloading, and it is shown as cardiac hypertrophy and left heart dysfunction, which is caused by the progressive of pulmonary venous and arterial hypertension (27). Severe CoA can be distinguished from IAA if there is a long stenotic section. The patients with IAA exhibit blood flow interruption between the ascending aorta and the descending aorta. Thus, the blood that flows into the descending aorta is solely supplied by the PDA. Most diagnoses of IAA are accompanied by ascending aortic arch dystrophy and moderate to severe pulmonary hypertension (28). However, this is not usually associated with pulmonary hypertension unless other cardiovascular abnormalities exist (29). Several imaging methods have been used to detect CoA. Conventional angiography was regarded as the gold standard for clinical assessment of the aorta. However, it is invasive and requires considerable radiation exposure (30). In addition, magnetic resonance imaging is a common tool for vascular diseases, but is limited by its high cost, long examination times, and patients with claustrophobia (31). TTE often serves as the first-line imaging tool for assessing cardiovascular function. It has been widely used for diagnosing CoA and other accompanying cardiovascular lesions due to its low cost and radiation-free properties (32).
CTA is now considered a more reliable technique than TTE for the diagnosis of aortic diseases, because it allows better visualization, especially in the detection of extracardiac-vascular abnormalities, with short acquisition time and high spatial resolution (4). With the recent advances in modern scanning techniques, the overall radiation burden required for CTA has significantly reduced, which means that this examination is relatively safe for infants and young children. However, considering that young patients require lifelong follow-up, radiologists should be careful with the use of ionizing radiation in serial examinations. In the current study, the accuracy rate of CTA for the diagnosis of CoA was significantly higher than that of TTE (p = 0.01). The multi-directional and multi-angle reconstructed images from CTA accurately detected the origin and morphology of the great vessels, and their relationship with the heart, which might compensate for the limitations of TTE. Although CTA was superior to TTE when assessing extracardiac-vascular anomalies, the associated cardiovascular lesions remain difficult to detect by CTA alone. TTE failed to diagnose 34 associated cardiovascular malformations, which included 12 HAA, 10 vascular malformations, 3 PDA, 2 VSD, 1 ASD, 1 aortic valve hypoplasia, 2 PFO, and 1 atrial septal aneurysm, 1 collateral and 1 vascular malformation (pulmonary artery origin).
In comparison, CTA misdiagnosed 72 associated cardiovascular malformations, which included 20 PFO, 19 vascular-related malformations, 14 VSD, 13 valvular lesions, 1 EFE, 1 PLSVC, 1 atrial septal aneurysm, 1 DCLV, 1 DCRV, and 1 DORV. These data suggest that TTE was better than CTA in detecting associated cardiovascular lesions, presumably because TTE provides hemodynamic information. In addition, TTE combined with color Doppler can evaluate blood shunting, valvular anomalies, and other intracardiac structures.
Furthermore, TTE has been widely used in postoperative follow-up of patients with repaired CoA for recoarctation. Currently, we use TTE to screen patients with suspected CoA by anatomic structural abnormality, the increased flow velocity of the descending aorta, and assess the intracardiac malformations, using CTA to confirm the diagnosis of CoA, and then managing the surgical plans. The in-hospital mortality rate was 4.9% in this group, which is higher than a large cohort that analyzed 2,424 CoA cases from 43 centers. The main reason for the deaths was attributed to congenital heart disease or other complications. Regarding long-term survival, the outcomes of patients with repaired CoA in infancy were excellent, but they had a small ongoing mortality risk in the early childhood (24). As recoarctation and high blood pressure may occur after repair (22, 33), it is important to continue long-term clinical and cardiovascular imaging follow-up in this population. We showed a significantly decreased pressure gradient after the operation, similar at 6 months, 1 year, and 3 years follow-ups. Although some patients still had systolic pressure gradient higher than 20 mmHg, no patient underwent the second operation with significant recoarctation in our follow-up. We recommend that if patients have no significant clinical symptoms and have normal left heart function, then they can undergo dynamic observation by TTE.
The median age of death is 31 years if 60%–80% of newborns are undiagnosed. Increased left ventricular afterload can cause the potential downstream effects (34, 35). CoA represents a form of pressure afterload that affects the LV. Correction of CoA before irreversible LV dysfunction is vital, which is more crucial if associated with other cardiovascular malformations. LV longitudinal strain is decreased, and LV maximum torsion is elevated in children with moderate LV pressure load secondary to LV obstructive lesions such as mild CoA, which changes rapidly after a successful interventional treatment resulting in LV maximum torsion reduction (36). In another study, the ejection fraction remained normal, but a decrease in strain was measured. Distinct left ventricular outflow tract obstruction (LVOTO) demonstrates different strain values. It is important for an early heart failure treatment to be performed in cases of LV aberrations before the onset of functional abnormalities (37). However, one study showed that non-neonatal CoA repair may be more beneficial than neonatal CoA repair in terms of LV systolic dysfunction recovery, but this may be confounded as neonates intervened during the neonatal period may have more severe CoA, which leads to circulatory problems and early intervention (38). When the PDA closes after birth, severe CoA will lead to aortic obstruction with hypoperfusion of the lower body, renal dysfunction, and metabolic acidosis. Increased afterload of the LV may lead to LV failure. Prostaglandin E is needed to keep the PDA open for lower body perfusion. Milder cases of CoA are important because hypertension and compensatory LV hypertrophy due to increased LV afterload may occur later in life (6). Limited restrictions are indicated for patients with high blood pressure, recoarctation, and aorta dilatation. There is an excellent survival but with a small ongoing mortality risk into early adulthood (39), and lifelong risk of developing aortic aneurysm, hypertension, and cerebral aneurysms. Therefore, patients diagnosed with CoA should seek lifelong follow-up with a cardiologist. Lifelong regular cardiology evaluations are important to monitor for later complications (24). Adequate and timely diagnosis of CoA is crucial for a good prognosis, as early treatment is associated with lower risks of long-term morbidity and mortality (6). Overall, our retrospective study presented the diagnosis and surgical management and outcome follow-up in the large Chinese population and summarized the surgical prognosis of CoA.
This study was limited by its retrospective design where some patients had incomplete clinical data, and not all patients had genetic testing performed. Furthermore, only the raw diameter of the CoA was recorded as there is no adequate and available Z-score COA calculator for the Chinese population when quantifying the TTE results. We only assessed patients who underwent surgical intervention and not balloon or stent catheterization. Since this was surgery, these cases were probably more extreme compared with those who ended with a catheterization treatment. For the pressure gradient follow-ups by echocardiography, as this was a retrospective study, the follow-up rate of the surgical outcome was lower than expected at 6 months, 1 year, and 3 years.
This study demonstrates that the diagnostic accuracy of CTA for CoA was higher than that of TTE, and TTE was superior to CTA when detecting other cardiovascular lesions. The combined use of CTA and TTE may improve the preoperative diagnostic accuracy of CoA and associated cardiovascular malformations, respectively, providing a more comprehensive assessment for the clinical management and surgical decision-making. TTE was beneficial for post-operation follow-up and clinical management.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University (protocol code 2015.91 and approved 5 August 2015). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
XJ and LS designed this project. XZ, DD, TR, LL, and XJ participated in diagnosing the clinical cases. TG and FZ collected the clinic data. TG, FZ, and LS prepared the manuscript. LS and XJ supervised the study and worked on editing the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by the National Natural Science Foundation of China (Grant No. 81301300), Project of Chongqing Municipal Human Resources and Social Security Bureau (cx2019065), and Chongqing Health Commission Appropriate Technology Extension Project (2021jstg007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gach P, Dabadie A, Sorensen C, Quarello E, Bonello B, Pico H, et al. Multimodality imaging of aortic coarctation: from the fetus to the adolescent. Diagn Interv Imaging. (2016) 97(5):581–90. doi: 10.1016/j.diii.2016.03.006
2. Pádua LMS, Garcia LC, Rubira CJ, de Oliveira Carvalho PE. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. (2012) 16(5):CD008204. doi: 10.1002/14651858.cd008204.pub2
3. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39(12):1890–900. doi: 10.1016/S0735-1097(02)01886-7
4. Budoff MJ, Shittu A, Roy S. Use of cardiovascular computed tomography in the diagnosis and management of coarctation of the aorta. J Thorac Cardiovasc Surg. (2013) 146(1):229–32. doi: 10.1016/j.jtcvs.2013.01.024
5. Singh S, Hakim FA, Sharma A, Roy RR, Panse PM, Chandrasekaran K, et al. Hypoplasia, pseudocoarctation and coarctation of the aorta—a systematic review. Heart Lung Circ. (2015) 24(2):110–8. doi: 10.1016/j.hlc.2014.08.006
6. Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart. (2017) 103:1148–55. doi: 10.1136/heartjnl-2017-311173
7. Hoffman JIE. The challenge in diagnosing coarctation of the aorta. Cardiovasc J Afr. (2018) 29(4):252–5. doi: 10.5830/CVJA-2017-053
8. Ganigara M, Doshi A, Naimi I, Mahadevaiah GP, Buddhe S, Chikkabyrappa SM. Preoperative physiology, imaging, and management of coarctation of aorta in children. Semin Cardiothorac Vasc Anesth. (2019) 23(4):379–86. doi: 10.1177/1089253219873004
9. Lannering K, Bartos M, Mellander M. Late diagnosis of coarctation despite prenatal ultrasound and postnatal pulse oximetry. Pediatrics. (2015) 136(2):e406–12. doi: 10.1542/PEDS.2015-1155
10. Evers PD, Ranade D, Lewin M, Arya B. Diagnostic approach in fetal coarctation of the aorta: a cost-utility analysis. J Am Soc Echocardiogr. (2017) 30(6):589–94. doi: 10.1016/j.echo.2017.01.019
11. Arya B, Bhat A, Vernon M, Conwell J, Lewin M. Utility of novel fetal echocardiographic morphometric measures of the aortic arch in the diagnosis of neonatal coarctation of the aorta. Prenat Diagn. (2016) 36(2):127–34. doi: 10.1002/pd.4753
12. Chang RKR, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. (2008) 162(10):969–74. doi: 10.1001/archpedi.162.10.969
13. Campbell M. Natural history of coarctation of the aorta. Br Heart J. (1970) 32(5):633–40. doi: 10.1136/hrt.32.5.633
14. Doshi AR, Chikkabyrappa S. Coarctation of aorta in children. Cureus. (2018b) 10(12):1–10. doi: 10.7759/cureus.3690
15. Boris JR. Primary-care management of patients with coarctation of the aorta. Cardiol Young. (2016) 26(8):1537–42. doi: 10.1017/S1047951116001748
16. Beckmann E, Jassar AS. Coarctation repair—redo challenges in the adults: what to do? J Vis Surg. (2018) 4:76–76. doi: 10.21037/jovs.2018.04.07
17. Lee MGY, Brizard CP, Galati JC, Iyengar AJ, Rakhra SS, Konstantinov IE, et al. Outcomes of patients born with single-ventricle physiology and aortic arch obstruction: the 26-year Melbourne experience. J Thorac Cardiovasc Surg. (2014) 148(1):194–201. doi: 10.1016/j.jtcvs.2013.07.076
18. Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric council of the American Society of Echocardiography. J Am Soc Echocardiogr. (2006) 19(12):1413–30. doi: 10.1016/j.echo.2006.09.001
19. Karaosmanoglu AD, Khawaja RDA, Onur MR, Kalra MK. CT and MRI of aortic coarctation: pre-and postsurgical findings. Am J Roentgenol. (2015) 204(3):W224–33. doi: 10.2214/AJR.14.12529
20. Vergales J, Gangemi J, Rhueban K, Lim D. Coarctation of the aorta—the current state of surgical and transcatheter therapies. Curr Cardiol Rev. (2013) 9(3):211–9. doi: 10.2174/1573403(113099990032
21. Chang JH, Burrington JD. Coarctation of the aorta in infants and children. J Pediatr Surg. (1972) 7(2):127–35. doi: 10.1016/0022-3468(72)90486-1
22. O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 h blood pressure measurement. Heart. (2002) 88(2):163–6. doi: 10.1136/heart.88.2.163
23. Ghorbani N, Muthurangu V, Khushnood A, Goubergrits L, Nordmeyer S, Fernandes JF, et al. Impact of valve morphology, hypertension and age on aortic wall properties in patients with coarctation: a two-centre cross-sectional study. BMJ Open. (2020) 10(3):1–9. doi: 10.1136/bmjopen-2019-034853
24. O’Brien P, Marshall AC. Coarctation of the aorta. Circulation. (2015) 131(9):e363–5. doi: 10.1161/CIRCULATIONAHA.114.008821
25. Forbes TJ, Kim DW, Du W, Turner DR, Holzer R, Amin Z, et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (congenital cardiovascular interventional study consortium). J Am Coll Cardiol. (2011) 58(25):2664–74. doi: 10.1016/j.jacc.2011.08.053
26. Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation long-term assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. (2007) 134:3. doi: 10.1016/j.jtcvs.2007.04.027
27. Yokoyama U, Ichikawa Y, Minamisawa S, Ishikawa Y. Pathology and molecular mechanisms of coarctation of the aorta and its association with the ductus arteriosus. J Physiol Sci. (2017) 67(2):259–70. doi: 10.1007/s12576-016-0512-x
28. Goudar SP, Shah SS, Shirali GS. Echocardiography of coarctation of the aorta, aortic arch hypoplasia, and arch interruption: strategies for evaluation of the aortic arch. Cardiol Young. (2016) 26(8):1553–62. doi: 10.1017/S1047951116001670
29. Hanneman K, Newman B, Chan F. Congenital variants and anomalies of the aortic arch. Radiographics. (2017) 37(1):32–51. doi: 10.1148/rg.2017160033
30. Richards CE, Obaid DR. Low-dose radiation advances in coronary computed tomography angiography in the diagnosis of coronary artery disease. Curr Cardiol Rev. (2019) 15(4):304–15. doi: 10.2174/1573403(15666190222163737
31. Zhao Q, Wang J, Yang ZG, Shi K, Diao KY, Huang S, et al. Assessment of intracardiac and extracardiac anomalies associated with coarctation of aorta and interrupted aortic arch using dual-source computed tomography. Sci Rep. (2019) 9(1):1–7. doi: 10.1038/s41598-019-47136-1
32. Malik SB, Chen N, Parker RA, Hsu JY. Transthoracic echocardiography: pitfalls and limitations as delineated at cardiac CT and MR imaging. Radiographics. (2017) 37(2):383–406. doi: 10.1148/rg.2017160105
33. Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 h blood pressure measurement. (2002) 88(2):163–66. doi: 10.1136/heart.88.2.163
34. Jashari H, Rydberg A, Ibrahimi P, Bajraktari G, Henein MY. Left ventricular response to pressure afterload in children: aortic stenosis and coarctation: a systematic review of the current evidence. Int J Cardiol. (2015) 178:203–9. doi: 10.1016/j.ijcard.2014.10.089
35. Jashari H, Rydberg A, Ibrahimi P, Bajraktari G, Kryeziu L, Jashari F, et al. Normal ranges of left ventricular strain in children: a meta-analysis. Cardiovasc Ultrasound. (2015) 13(1):1–16. doi: 10.1186/s12947-015-0029-0
36. Laser KT, Haas NA, Jansen N, Schäffler R, Palacios Argueta JR, Zittermann A, et al. Is torsion a suitable echocardiographic parameter to detect acute changes in left ventricular afterload in children? J Am Soc Echocardiogr. (2009) 22(10):1121–8. doi: 10.1016/j.echo.2009.06.014
37. Van der Ende J, Vázquez Antona CA, Erdmenger Orellana J, Romero Cárdenas Á, Roldan FJ, Vargas Barrón J. Left ventricular longitudinal strain measured by speckle tracking as a predictor of the decrease in left ventricular deformation in children with congenital stenosis of the aorta or coarctation of the aorta. Ultrasound Med Biol. (2013) 39(7):1207–14. doi: 10.1016/j.ultrasmedbio.2013.02.015
38. Klitsie LM, Roest AAW, Kuipers IM, Van Der Hulst AE, Hazekamp MG, Blom NA, et al. Enhanced characterization of ventricular performance after coarctation repair in neonates and young children. Ann Thorac Surg. (2013) 96(2):629–36. doi: 10.1016/j.athoracsur.2013.04.058
Keywords: coarctation of the aorta, congenital heart disease, transthoracic echocardiography, computed tomographic angiography, surgical outcome
Citation: Gong T, Zhang F, Feng L, Zhu X, Deng D, Ran T, Li L, Kong L, Sun L and Ji X (2023) Diagnosis and surgical outcomes of coarctation of the aorta in pediatric patients: a retrospective study. Front. Cardiovasc. Med. 10:1078038. doi: 10.3389/fcvm.2023.1078038
Received: 23 October 2022; Accepted: 10 July 2023;
Published: 24 July 2023.
Edited by:
John Frank LaDisa, Medical College of Wisconsin, United StatesReviewed by:
Nazmi Narin, Izmir Katip Celebi University, Türkiye© 2023 Gong, Zhang, Feng, Zhu, Deng, Ran, Li, Kong, Sun and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Sun liqun.sun@sickkids.ca Xiaojuan Ji jixiaojuan2003@163.com
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.