- 1Department of Cardiology, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, China
- 2Department of Cardiology, Lanzhou University Second Hospital, The Second Clinical Medical College of Lanzhou University, Lanzhou, China
Background: Antiarrhythmic drugs (AADs) are frequently prescribed following catheter ablation (CA) for atrial fibrillation (AF). However, to date, there is a lack of large-scale, multicenter controlled studies that have confirmed the efficacy of AADs in reducing the incidence of late recurrence of AF after CA. Furthermore, the optimal duration of short-term use of AADs after CA remains a controversial topic.
Methods: PubMed, Embase, Cochrane Library, CNKI, and ClinicalTrials.gov were searched until April 25, 2022. We conducted a meta-analysis of randomized controlled trials (RCTs) to assess the efficacy of blanking period AADs in predicting both early and late recurrence of AF. In addition, Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess the quality of evidence in this meta-analysis.
Results: 12 RCTs with 3,625 patients were included in this meta-analysis. Short-term use of AADs after AF ablation reduced the risk of early recurrence of AF compared with the no-AADs group. In the subgroup analysis of AADs use time, it was found that only using AADs for more than 2 months can reduce the early recurrence of AF after CA. However, when compared with the no-AADs group, short-term use of AADs after CA did not reduce the incidence of late recurrence of AF.
Conclusions: Short-term use of AADs (more than 2 months) can reduce the early recurrence but not the late recurrence of AF after CA.
Introduction
Atrial fibrillation (AF) is a common arrhythmia in clinical practice that increases the risk of stroke and heart failure. As of 2019, there were approximately 59.7 million patients of AF (including atrial flutter) worldwide (1). While the lifetime risk of AF was previously about one in four (2, 3), recent studies have reported that one in three people of European ancestry over 55 has AF (4, 5). The EAST-AFNET4 study showed that in patients with newly diagnosed AF within 1 year, the incidence of major cardiovascular events in early rhythm control was lower than that in the conventional treatment group (mainly ventricular rate control). Additionally, the maintenance of sinus rhythm is higher in the early rhythm control group. Catheter ablation (CA) can be used as the first treatment for AF. However, due to proarrhythmic milieu caused by CA lesions, AF recurrence is common within the first few months of post-ablation. Because of “AF begets AF”, those patients with early recurrence after post-ablation are also more likely to have a late recurrence (6). This notion has also been confirmed that post-ablation blanking period episodes are independent predictors of AF recurrence (7). Therefore, the use of AADs after AF ablation aim not only to reduce early recurrence but also to reduce late recurrence. However, most of the existing studies have shown that the use of AADs cannot reduce the late recurrence of AF after CA. It should be noted that the sample size of previous studies is not enough to clarify this issue, so this conclusion is controversial.
Xu et al. (8) conducted a meta-analysis to evaluate the efficacy of AADs and found that short-term use of AADs could reduce the incidence of early recurrence of AF but could not prevent late recurrence of AF. However, the study had several limitations. First, this meta-analysis included few studies, only six randomized controlled trials (RCTs), which resulted in an insufficient sample size. Second, sources of heterogeneity were not adequately analyzed (Subgroup analysis was not sufficient and meta regressions were not performed). Third, exploration of the stability of the results is not enough (Subgroup analysis was not sufficient and sensitivity analysis was not performed). Finally, an assessment of publication bias was not performed. In addition, they did not evaluate a more meaningful indicator: the use time of AADs in the blanking period after CA of AF, which is crucial for clinical treatment. To shed further light on this issue, we conducted a meta-analysis that, in addition to ameliorating the deficits mentioned above, explored the duration of drug use to reduce AF early recurrence after CA. Simultaneously, we evaluated the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to facilitate its clinical application.
Methods
This meta-analysis was performed according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist guided the protocol reporting (9). There was no registered protocol for this meta-analysis.
Literature search strategy
PubMed, Embase, Cochrane Library, CNKI, and ClinicalTrials.gov were searched until April 25, 2022. The systematic electronic searches were conducted using exploded Medical Subject Headings (MeSH) terms and the corresponding keywords in Title, Abstract, or All Fields. The search terms used in this meta-analysis were (MeSH exp “Atrial Fibrillation”, and keywords “atrial fibrillation OR atrial fibrillat* OR auricular fibrillat* OR atrium fibrillat* OR AF”), (MeSH exp “AntiArrhythmia Agents”, and keywords “Antiarrhythmia agents OR anti-arrhythmia drugs OR antiarrhythmi* OR procainamide OR disopyramide OR mexiletine OR flecainide OR propafenone OR bisoprolol OR esmolol OR amiodarone OR dofetilide OR sotalol OR ibutilide OR azimilide OR moricizine OR cibenzoline”), and (MeSH exp “Catheter Ablation” and keywords “catheter ablation OR radiofrequency OR cryoablation OR PVI OR pulmonary vein isolation”). We applied filters to restrict the type of trials to RCTs involving human subjects only, without any language restrictions. To ensure that no relevant articles were overlooked, we conducted a subsequent search on April 28, 2022. In addition, we manually searched the references in the included literature to identify potential eligible trials.
Selection criteria
Published studies meeting the following criteria were included: (1) Patients: AF patients underwent CA with pulmonary vein isolation (PVI)-based strategy; (2) Intervention: patients were treated with AADs within the first 3 months after CA (blanking period); (3) Comparison: patients were not on AADs treatment, either placebo or usual care; (4) Outcome: early recurrence of atrial arrhythmia lasted more than 30 s within the first 3 months after CA, and late recurrence of atrial arrhythmia lasted more than 30 s post the 3 months after CA; and (5) Study type: all articles included were RCTs. Exclusion criteria: (1) We excluded duplicate reports; (2) We excluded conference abstracts unless they were accompanied by a full-text publication in a peer-reviewed journal; (3) We excluded animal experiments; (4) We excluded performed additional atrioventricular node ablation, pacing therapy, and surgical ablation patients.
Study inclusion and data extraction
Two reviewers (G.L. and G.C.) conducted initial searches, removed duplicates, and screened titles and abstracts to identify eligible articles. In instances where there were discrepancies in the inclusion of literature, the full-text article was obtained to determine eligibility. Any uncertainties or disagreements were resolved through discussion and consensus. Data collection was performed by G.L. and independently confirmed by other authors (G.C. and D.Z.). Additionally, we also reviewed supplementary appendices of included RCTs and contacted the corresponding authors to verify extracted data and request the unavailable data, if needed. All discrepancies were resolved by discussion and consensus. The scheduled primary outcome was the early recurrence, and the secondary outcome was the late recurrence of AF.
Risk of bias assessment and grading quality of evidence
The risk of bias was assessed in duplicate by independent reviewers (G.L. and G.C.) using the Cochrane risk-of-bias tool (10). A study was considered high risk if only one domain of the trials had rated as high risk; the study was regarded as low risk if all domains had rated as low risk; otherwise, they were considered at unclear risk of bias. The quality of the evidence in this meta-analysis was independently assessed (G.L. and G.C.) according to the GRADE.
Statistical analysis
Statistical analyses were performed using Stata 14.0 and Review Manager 5.4. Relative risk (RR) was used as the effect size indicator for enumeration data. Point estimates and 95% confidence interval (CI) were calculated for dichotomous outcomes. The heterogeneity of the included studies was analyzed using the Q test (test level α = 0.1), and the I2 statistic was used to quantify the heterogeneity between studies. I2 values between 25% and 50% were considered mild heterogeneity, between 50% and 75% were considered moderate heterogeneity, and those above 75% were considered high heterogeneity (11). Regardless of the value of I2, the Mantel-Haenszel method was used to pool the RR and 95% CI with the random-effects model. If there was significant heterogeneity in this meta-analysis, subgroup analyses are applied to identify sources of heterogeneity. Prespecified subgroup analyses included time of drug use, publication time of literature, the sample size of individual studies, and the risk of individual studies. In addition, sensitivity analysis was applied to assess the effect of individual or small sample studies on the overall effect size. If the heterogeneity was significant and could not be resolved by the above methods, the meta-analysis was abandoned, and only qualitative analysis was performed. Since the visual assessment of whether a funnel plot was symmetric could be subjective, we used the Egger test to detect potential publication bias (12). P-value < 0.05 was considered statistically significant unless previously defined, such as p-value < 0.1 indicated statistically significant for heterogeneity test.
Results
Literature search
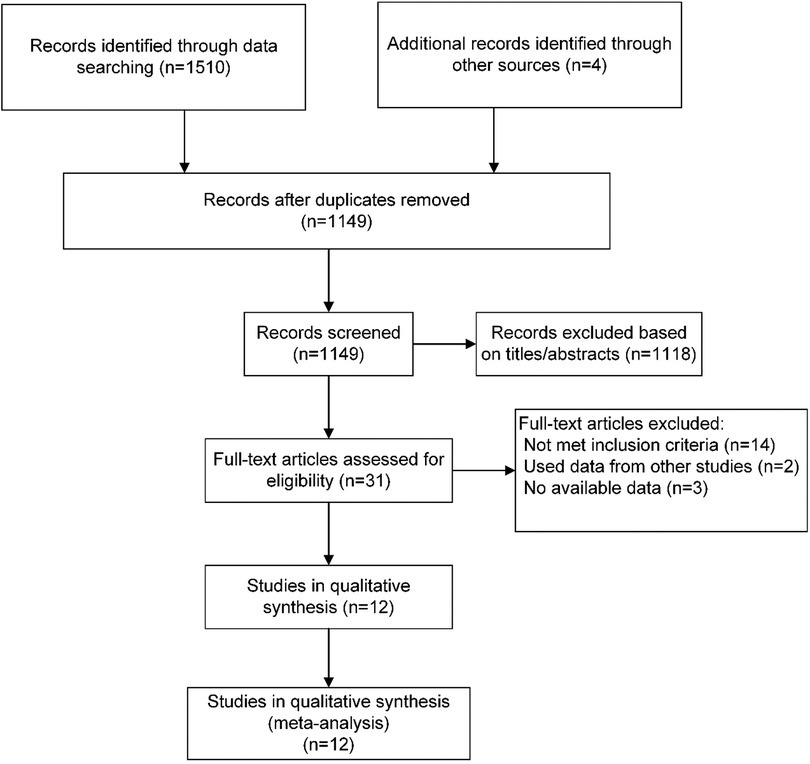
The literature search and selection results are shown in the PRISMA flowchart (Figure 1). Our initial search yielded 1,510 articles. After removing duplicates and screening titles and abstracts, 31 articles were considered likely to meet the inclusion criteria. After a full-text review, 12 published articles from 11 RCTs were finally included in this meta-analysis. Two articles were derived from the same study with different results at 6 weeks and 6 months.
Trials characteristics and risk of bias assessment
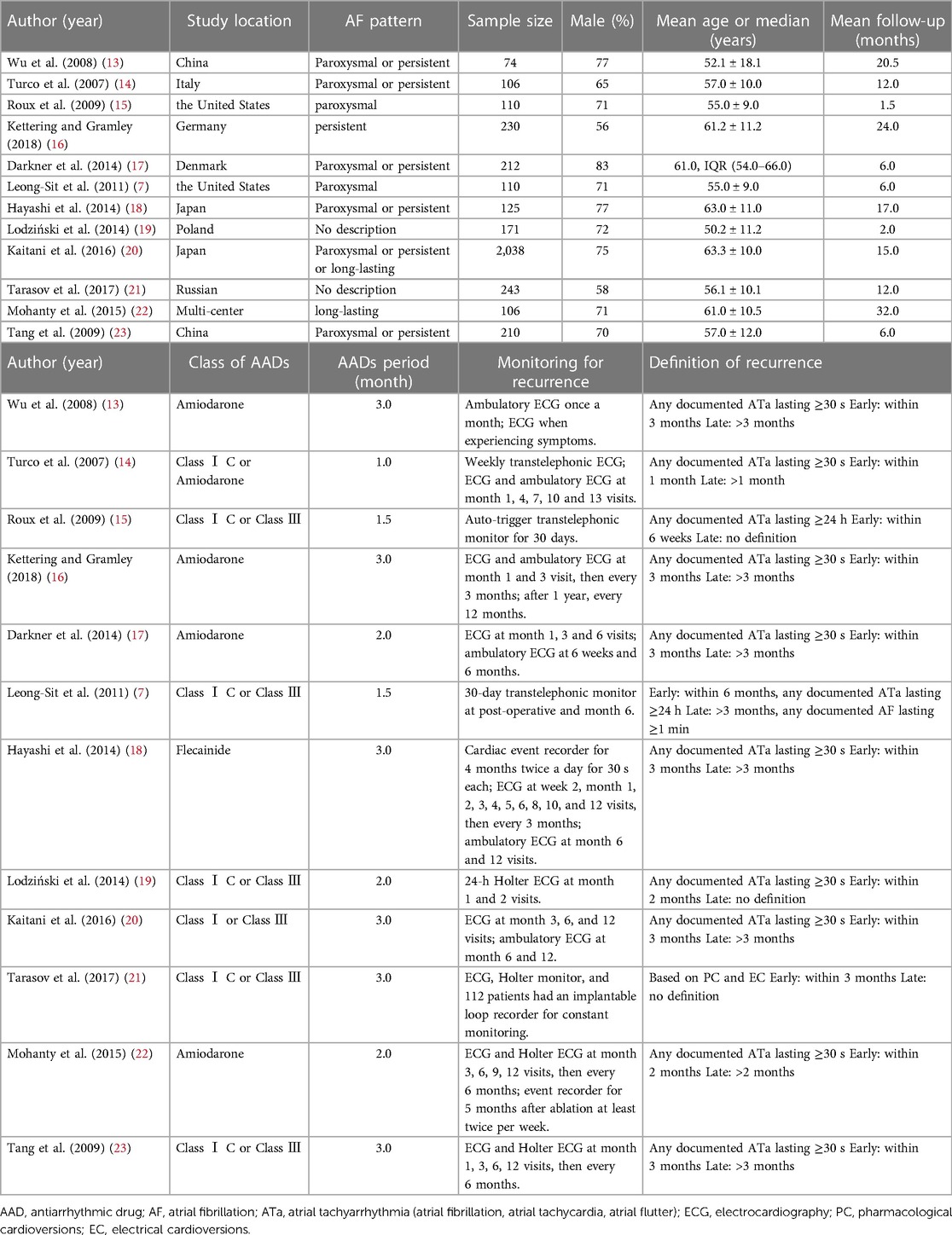
The main characteristics of included 12 RCTs with 3,625 patients are shown in Table 1. The sample size of trials ranged from 74 to 2,038. A total of 1,937 patients were administered AADs, while 1,689 patients received no-AADs treatment. The primary AAD used was amiodarone. All patients underwent CA using a PVI-based strategy. Patients in the AAD group received drug treatment lasting between 6 weeks and 3 months. The RCTs had a mean follow-up duration ranging from 3 to 28 months. Detailed information on the risk of bias is presented in Figures 2, 3.
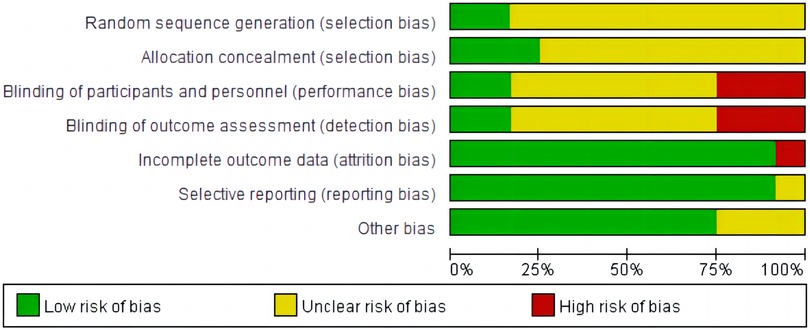
Figure 2. Risk of bias for this meta-analysis: judgments about each risk of bias item presented as percentages across all included randomized controlled trials.
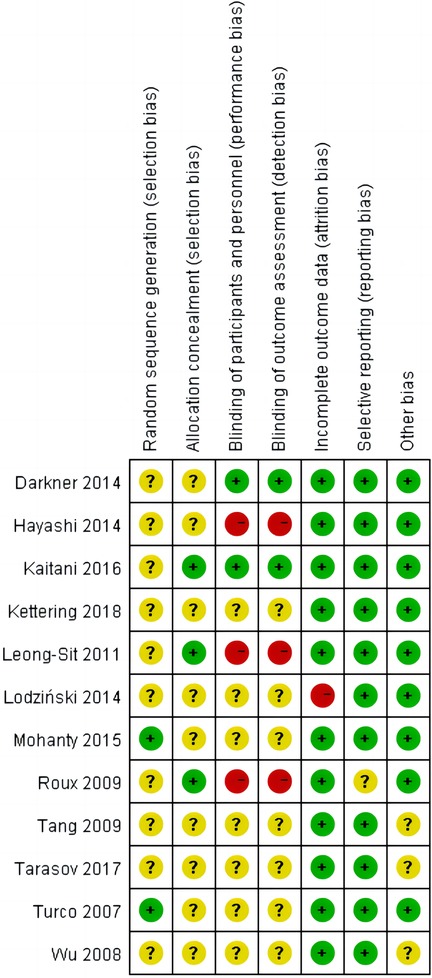
Figure 3. Summary of risk of bias summary of the included randomized controlled trials: details about each risk of bias item for each included trial. Green = low risk of bias, Yellow = unclear risk of bias, Red = high risk of bias.
Primary outcomes
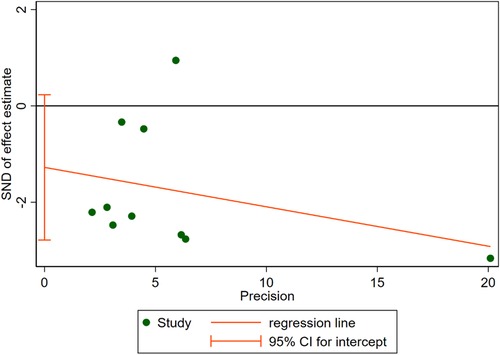
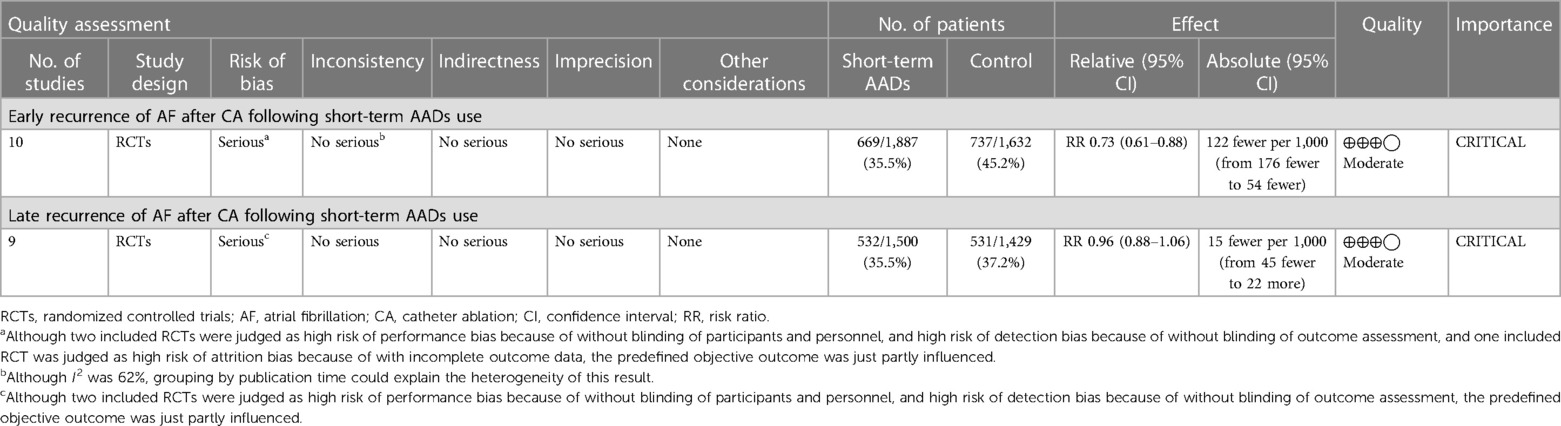
10 RCTs including a total of 3,519 patients were analyzed to provide relevant evidence regarding the primary outcomes of early recurrence of AF. Short-term use of AADs significantly reduced the risk of AF recurrence after CA in the blanking period compared to the no-AADs prescription group (RR, 0.72; 95% CI, 0.59–0.89; p = 0.002; Figure 4), with moderate heterogeneity (I2 = 62%; phet = 0.005). Given the moderate heterogeneity, subgroup analysis was performed to evaluate the influence of different groups on primary outcomes. When grouped by publication time <2014 and ≥2014, it can be seen that the I2 of both groups decreased to less than 50%, and publication time can be considered the main source of heterogeneity in this meta-analysis (Supplementary Figure S1). In addition, when grouped by drug use time, it can be found that drug use time ≤2 months is ineffective (RR, 0.67; 95% CI, 0.43–1.06; p = 0.09) for early recurrence of AF, while drug use time >2 months is effective (RR, 0.75; 95% CI, 0.62–0.91; p = 0.004; Supplementary Figure S2). Further, when grouped by sample size and risk of study, respectively, it can be found that the subgroup with a sample size of less than 200 and the subgroup with a high risk of study both obtained the same conclusion that the use of AADs in the blanking period is ineffective for the early recurrence of AF. The subgroup with a sample size of more than 200 and the subgroup with no-high risk of study obtained the opposite conclusion (Supplementary Figures S3, S4). To assess the robustness of this meta-analysis, a sensitivity analysis consecutively excluding one trial each time was applied to assess the impact of individual studies. As a result, no trials had significant effect on the pooled estimate and 95% CI (Supplementary Figure S5). We conducted an assessment of publication bias using the Egger test (Figure 5) and found no evidence of potential publication bias.
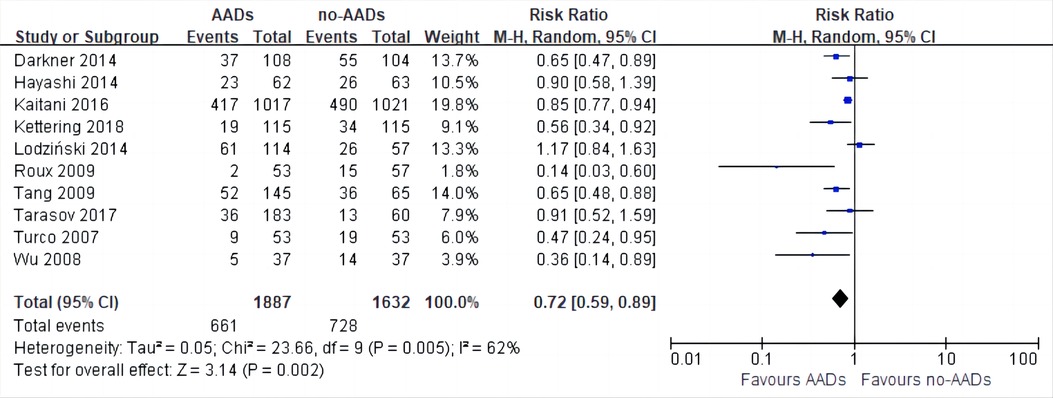
Figure 4. Effect of short-term AADs use versus no-AADs prescription after atrial fibrillation ablation on early recurrence of atrial arrhythmias.
Secondary outcomes
For late recurrence of AF, 2,929 patients were included in nine RCTs. Compared with no-AADs prescription group, short-term use of AADs did not reduce the risk of late recurrence of AF after CA (RR, 0.96; 95% CI, 0.88–1.06; p = 0.45; Supplementary Figure S6), with mild heterogeneity (I2 = 29%; phet = 0.19). Although such heterogeneity is acceptable, we further evaluated the stability of this meta-analysis. Similar methods were used to evaluate the late recurrence of AF. We performed the same subgroup and sensitivity analyses in the late recurrence of AF. All results showed that short-term use of AADs cannot reduce the risk of late recurrence of AF (Supplementary Figures S7–S11).
GRADE profile evidence
The GRADE profile evidence for this meta-analysis in the primary and secondary outcomes is presented in Table 2.
Discussion
The main innovations of this meta-analysis are as follows: (1) the topic of this meta-analysis is an essential issue in clinical practice, but it is controversial whether to use AADs in the blanking period after the CA of AF. At the same time, there is insufficient evidence for guidelines recommending the use of AADs in the blanking period. (2) The meta-analysis on this topic is not updated after 2016. Only six RCTs were included in the studies by Xu et al. (8) and Chen et al. (24), the numbers of studies were too small, and there was no way to evaluate publication bias while we addressed this issue. In addition, we adequately evaluated the stability and heterogeneity in this meta-analysis. (3) The previous guidelines and expert consensus did not recommend the specific time of AADs use during the blanking period. Our study demonstrated that even preventing early recurrence of AF requires at least 2 months of AADs use, which helps guide clinical practice.
The main conclusions of this meta-analysis are as follows: (1) The use of AADs in the blanking period can reduce the early recurrence of AF after CA. (2) The use of AADs in the blanking period cannot reduce the late recurrence of AF after CA. Existing studies have shown that 60% of patients with early recurrence automatically return to sinus rhythm, but early recurrence is an independent predictor of AF late recurrence after CA (7, 20, 25). There are two issues that need to be addressed. First, why do patients with early recurrence automatically return to sinus rhythm? Second, AADs can reduce the early recurrence, so they can theoretically reduce the AF late recurrence. However, our study is consistent with previous studies showing that using AADs in the blanking period does not reduce the risk of late recurrence of AF. So how do we interpret this result? Regarding the first issue, there may be the following reasons: (1) Inflammatory response is one of the reasons for the recurrence of AF in the blanking period post-ablation, which is also confirmed by using anti-inflammatory drugs to reduce the early recurrence of AF. In addition, elevated inflammatory cytokines return to baseline within 30 days after CA (26–30). (2) The potential side effect of AF ablation is the imbalance of cardiac autonomic nerve regulation. Studies have found that circumferential pulmonary vein ablation can change the autonomic nerve activity dominating the sinoatrial node, leading to the recurrence of AF, which often disappears within 1 month (31). (3) The electrical conduction of the left atrium and pulmonary veins recover early after CA, which is caused by incomplete ablation lesions. (4) Development of a permanent atrial lesions by scar rigidification may cause delayed cure. In this process, due to the postoperative inflammatory response and autonomic imbalance disappear within 1 month, patients with AF recurrence in the second and third months after CA may be stronger predictors of AF late recurrence than those in the first month. This inference has also been confirmed in some studies (32–34).
As far as the second issue is concerned, the following reasons can partially explain this phenomenon: (1) The most important reason is incomplete PVI, which leads to the restoration of conduction between the left atrium and the pulmonary veins (35, 36); (2) Another common reason is the trigger foci outside the pulmonary veins, which can be mapped and identified after intravenous infusion of high-dose isoproterenol; (3) The experience of CA for AF patients also affects the late recurrence rate of AF. The above problems cannot be effectively solved by the use of AADs.
In addition, we also need to pay attention to the fact that the type of AF and the means of recurrence screening also have an important impact on the late recurrence rate of AF (37, 38). To further exclude the above factors affecting our results, the type of AF and the means of recurrence screening detail are described in our baseline data.
In this meta-analysis, there was moderate heterogeneity (I2 = 62%) for the pooled primary outcome, and further subgroup analysis revealed that the main source of heterogeneity was publication time. After grouping by 2014 as the cutoff value, the heterogeneity of the pooled results was significantly reduced. This result can be explained because there has been some progress in CA of AF during this period. Cardiologists came to recognize that the extent of CA lesions depends on the stability of the catheter, contact pressure, energy output, temperature, and ablation time (39). In addition, it is more important to recognize that the initial pulmonary veins isolation is observed for 20–30 min and to reevaluate that a bidirectional block can improve the permanent isolation rate. Some special patients can be monitored for 60–90 min to perform a reevaluation (40).
When we grouped studies by sample size and risk of bias, respectively, we found that studies with a sample size of less than 200 and studies classified as high risk all concluded that AADs were ineffective in preventing early AF relapse. Meanwhile, studies with a sample size greater than 200 and those classified as non-high risk reached opposite conclusion. This suggests that the relatively small sample size and poor quality of the studies may lead to erroneous conclusions. In fact, the result should be more robust. Furthermore, we found that pooled results heterogeneity was low for secondary outcomes, and the results were robust under all grouping factors.
Another notable finding is that previous studies have shown that continuous AADs use for less than 2 months after CA can reduce incidence of atrial arrhythmias during the blanking period. However, our study confirmed that only continuous application of AADs for more than 2 months could reduce the episodes of the blanking period with a larger sample size. This finding provides valuable insights for clinicians regarding the optimal duration of medication during the blanking period.
Limitations
(1) This meta-analysis does not utilize individual patient data, which limits our ability to identify the presence or absence of effect modification. (2) Many studies included in our meta-analysis were open-label and required additional ECG and/or Holter monitoring if patients developed arrhythmia-related symptoms. Patients in the control group with more ECG and/or Holter monitoring (more likely to experience arrhythmia-related symptoms) had more opportunities to document AF recurrence. (3) The RCTs included in this meta-analysis exhibited differences in terms of the ablation procedures applied, follow-up periods used, physician experience, and type of AADs administered.
Conclusions
Short-term use of AADs post-AF ablation can lower the risk of early recurrence of AF. However, it does not result in a corresponding reduction in the risk of late recurrence of AF. More importantly, the risk of early relapse can be reduced only when AADs are used continuously for 2 months or more in the blanking period.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
GL completed the data analysis and wrote the paper. XG and GC guided this meta-analysis. All authors contributed to the article and approved the submitted version.
Funding
The open access publication fees funded by Lanzhou University. The study was not funded by any other institutions or individuals.
Acknowledgments
We would like to acknowledge the members of the Department of Cardiology, Lanzhou University, for interpreting the significance of the results. We also want to thank the corresponding author for giving an idea for this meta-analysis and guiding this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1071950/full#supplementary-material
Supplementary Figure S1
Subgroup analysis of early recurrence of atrial fibrillation after catheter ablation by publication time.
Supplementary Figure S2
Subgroup analysis of early recurrence of atrial fibrillation after catheter ablation by antiarrhythmic drugs (AADs) use time.
Supplementary Figure S3
Subgroup analysis of early recurrence of atrial fibrillation after catheter ablation by sample size.
Supplementary Figure S4
Subgroup analysis of early recurrence of atrial fibrillation after catheter ablation by risk of study.
Supplementary Figure S5
Sensitivity analysis of early recurrence of atrial fibrillation.
Supplementary Figure S6
Effect of short-term antiarrhythmic drugs (AADs) use versus no-AADs prescription after atrial fibrillation ablation on late recurrence of atrial arrhythmias.
Supplementary Figure S7
Subgroup analysis of late recurrence of atrial fibrillation after catheter ablation by publication time.
Supplementary Figure S8
Subgroup analysis of late recurrence of atrial fibrillation after catheter ablation by antiarrhythmic drugs (AADs).
Supplementary Figure S9
Subgroup analysis of late recurrence of atrial fibrillation after catheter ablation by sample size.
Supplementary Figure S10
Subgroup analysis of late recurrence of atrial fibrillation after catheter ablation by risk of study.
Supplementary Figure S11
Sensitivity analysis of late recurrence of atrial fibrillation.
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the rotterdam study. Eur Heart J. (2006) 27(8):949–53. doi: 10.1093/eurheartj/ehi825
3. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the framingham heart study. Circulation. (2004) 110(9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42
4. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njølstad I, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (biomarker for cardiovascular risk assessment in Europe). Circulation. (2017) 136(17):1588–97. doi: 10.1161/CIRCULATIONAHA.117.028981
5. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the framingham heart study. Br Med J. (2018) 361:k1453. doi: 10.1136/bmj.k1453
6. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. (1995) 92(7):1954–68. doi: 10.1161/01.CIR.92.7.1954
7. Leong-Sit P, Roux JF, Zado E, Callans DJ, Garcia F, Lin D, et al. Antiarrhythmics after ablation of atrial fibrillation (5A study): six-month follow-up study. Circ Arrhythm Electrophysiol. (2011) 4(1):11–4. doi: 10.1161/CIRCEP.110.955393
8. Xu B, Peng F, Tang W, Du Y, Guo H. Short-term antiarrhythmic drugs after catheter ablation for atrial fibrillation: a meta-analysis of randomized controlled trials. Ann Pharmacother. (2016) 50(9):697–705. doi: 10.1177/1060028016653140
9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
10. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
12. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
13. Wu G, Jiang H, Huang CX, Yang B, Huang H, Wang YL, et al. Effects of antiarrhythmic drug use on atrial fibrillation recurrence in atrial fibrillation patients post circumferential pulmonary vein ablation. Zhonghua Xin Xue Guan Bing Za Zhi. (2008) 36(7):623–6. doi: 10.3321/j.issn:0253-3758.2008.07.011
14. Turco P, De Simone A, La Rocca V, Iuliano A, Capuano V, Astarita C, et al. Antiarrhythmic drug therapy after radiofrequency catheter ablation in patients with atrial fibrillation. Pacing Clin Electrophysiol. (2007) 30(Suppl 1):S112–115. doi: 10.1111/j.1540-8159.2007.00618.x
15. Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, et al. Antiarrhythmics after ablation of atrial fibrillation (5A study). Circulation. (2009) 120(12):1036–40. doi: 10.1161/CIRCULATIONAHA.108.839639
16. Kettering K, Gramley F. Catheter ablation of persistent atrial fibrillation: beneficial effect of a short-term adjunctive amiodarone therapy on the long-term outcome. Herzschrittmacherther Elektrophysiol. (2018) 29(1):133–40. doi: 10.1007/s00399-017-0498-y
17. Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. (2014) 35(47):3356–64. doi: 10.1093/eurheartj/ehu354
18. Hayashi M, Miyauchi Y, Iwasaki YK, Yodogawa K, Tsuboi I, et al. Three-month lower-dose flecainide after catheter ablation of atrial fibrillation. Europace. (2014) 16(8):1160–7. doi: 10.1093/europace/euu041
19. Lodziński P, Kiliszek M, Ko?luk E, Pi?tkowska A, Balsam P, Kochanowski J, et al. Does a blanking period after pulmonary vein isolation impact long-term results? Results after 55 months of follow-up. Cardiol J. (2014) 21(4):384–91. doi: 10.5603/CJ.a2013.0144
20. Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi T, et al. Efficacy of antiarrhythmic drugs short-term use after catheter ablation for atrial fibrillation (EAST-AF) trial. Eur Heart J. (2016) 37(7):610–8. doi: 10.1093/eurheartj/ehv501
21. Tarasov AV, Reynbakh O, Davtyan KV, Martsevich SY. Comparison of antiarrhythmic drugs clinical effect during early post procedural period after atrial fibrillation ablation. Eur Heart J. (2017) 38:581. doi: 10.1093/eurheartj/ehx502.P2705
22. Mohanty S, Di Biase L, Mohanty P, Trivedi C, Santangeli P, Bai R, et al. Effect of periprocedural amiodarone on procedure outcome in patients with longstanding persistent atrial fibrillation undergoing extended pulmonary vein antrum isolation: results from a randomized study (SPECULATE). Heart Rhythm. (2015) 12(3):477–83. doi: 10.1016/j.hrthm.2014.11.016
23. Tang R, Ma C, Dong J, Liu X, Long D, Yu R, et al. The relationship between antiarrhythmic drugs and recurrence after catheter ablation of atrial fibrillation. Chin J Cardiol Pacing Electrophysiol. (2009) 23:222–5. CNKI:SUN:ZGXZ.0.2009-03-014
24. Chen W, Liu H, Ling Z, Xu Y, Fan J, Du H, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation-A systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One. (2016) 11(5):e0156121. doi: 10.1371/journal.pone.0156121
25. Oral H, Knight BP, Ozaydin M, Tada H, Chugh A, Hassan S, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. (2002) 40(1):100–4. doi: 10.1016/S0735-1097(02)01939-3
26. Kim YR, Nam GB, Han S, Kim SH, Kim KH, Lee S, et al. Effect of short-term steroid therapy on early recurrence during the blanking period after catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. (2015) 8(6):1366–72. doi: 10.1161/CIRCEP.115.002957
27. Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. (2012) 60(18):1790–6. doi: 10.1016/j.jacc.2012.07.031
28. Deftereos S, Giannopoulos G, Efremidis M, Kossyvakis C, Katsivas A, Panagopoulou V, et al. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm. (2014) 11(4):620–8. doi: 10.1016/j.hrthm.2014.02.002
29. Koyama T, Tada H, Sekiguchi Y, Arimoto T, Yamasaki H, Kuroki K, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol. (2010) 56(18):1463–72. doi: 10.1016/j.jacc.2010.04.057
30. Lim HS, Schultz C, Dang J, Alasady M, Lau DH, Brooks AG, et al. Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2014) 7(1):83–9. doi: 10.1161/CIRCEP.113.000876
31. Hsieh MH, Chiou CW, Wen ZC, Wu CH, Tai CT, Tsai CF, et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation. (1999) 100(22):2237–43. doi: 10.1161/01.CIR.100.22.2237
32. Li Z, Wang S, Hidru TH, Sun Y, Gao L, Yang X, et al. Long atrial fibrillation duration and early recurrence are reliable predictors of late recurrence after radiofrequency catheter ablation. Front Cardiovasc Med. (2022) 9:864417. doi: 10.3389/fcvm.2022.864417
33. Bertaglia E, Stabile G, Senatore G, Zoppo F, Turco P, Amellone C, et al. Predictive value of early atrial tachyarrhythmias recurrence after circumferential anatomical pulmonary vein ablation. Pacing Clin Electrophysiol. (2005) 28(5):366–71. doi: 10.1111/j.1540-8159.2005.09516.x
34. Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, Bader G, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. (2008) 5(5):679–85. doi: 10.1016/j.hrthm.2008.01.031
35. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022
36. Sotomi Y, Inoue K, Ito N, Kimura R, Toyoshima Y, Masuda M, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol. (2013) 111(4):552–6. doi: 10.1016/j.amjcard.2012.10.040
37. Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. (2008) 118(24):2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582
38. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. (2009) 2(4):349–61. doi: 10.1161/CIRCEP.108.824789
39. Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. (2013) 6(2):327–33. doi: 10.1161/CIRCEP.113.000374
40. Nakamura K, Naito S, Kaseno K, Tsukada N, Sasaki T, Hayano M, et al. Optimal observation time after completion of circumferential pulmonary vein isolation for atrial fibrillation to prevent chronic pulmonary vein reconnections. Int J Cardiol. (2013) 168(6):5300–10. doi: 10.1016/j.ijcard.2013.08.011
Keywords: atrial fibrillation, antiarrhythmics, clinical trials, catheter ablation, meta-analysis
Citation: Chen G, Li G, Zhang D, Wang X and Guo X (2023) Blanking period antiarrhythmic drugs after catheter ablation for atrial fibrillation: a meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 10:1071950. doi: 10.3389/fcvm.2023.1071950
Received: 17 October 2022; Accepted: 4 July 2023;
Published: 20 July 2023.
Edited by:
Enrico Baldi, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Lisa A. Gottlieb, University of Copenhagen, DenmarkLana Boodhoo, The University of the West Indies St. Augustine, Trinidad and Tobago
© 2023 Chen, Li, Zhang, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueya Guo Z3VveHVleWEyMDA2QDEyNi5jb20=
†These authors share first authorship
 Gang Chen
Gang Chen Guangling Li
Guangling Li Demei Zhang
Demei Zhang Xiaomei Wang
Xiaomei Wang Xueya Guo
Xueya Guo