
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 20 February 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1070450
This article is part of the Research Topic New Insights and Future Prospects of Atrial Cardiomyopathy View all 5 articles
 Anna Brand1,2,3*†
Anna Brand1,2,3*† Elena Romero Dorta1†
Elena Romero Dorta1† Adrian Wolf1
Adrian Wolf1 Daniela Blaschke-Waluga1
Daniela Blaschke-Waluga1 Ute Seeland3,4
Ute Seeland3,4 Claudia Crayen5
Claudia Crayen5 Sven Bischoff1
Sven Bischoff1 Isabel Mattig1
Isabel Mattig1 Henryk Dreger1,3
Henryk Dreger1,3 Karl Stangl1,3
Karl Stangl1,3 Vera Regitz-Zagrosek3,6,7
Vera Regitz-Zagrosek3,6,7 Ulf Landmesser2,3
Ulf Landmesser2,3 Fabian Knebel1,3,8
Fabian Knebel1,3,8 Verena Stangl1,3
Verena Stangl1,3Purpose: The predictive value of maximum left atrial volume index (LAVI), phasic left atrial strain (LAS) and other standard echocardiographic parameters assessing left ventricular (LV) diastolic function to discriminate a future worsening of diastolic function (DD) in patients at risk is unclear. We aimed to prospectively assess and compare the clinical impact of these parameters in a randomly selected study sample of the general urban female population.
Methods and results: A comprehensive clinical and echocardiographic evaluation was performed in 256 participants of the Berlin Female Risk Evaluation (BEFRI) trial after a mean follow up time of 6.8 years. After an assessment of participants’ current DD status, the predictive impact of an impaired LAS on the course of DD was assessed and compared with LAVI and other DD parameters using receiver operating characteristic (ROC) curve and multivariate logistic regression analyses. Subjects with no DD (DD0) who showed a decline of diastolic function by the time of follow-up showed a reduced LA reservoir (LASr) and conduit strain (LAScd) compared to subjects who remained in the healthy range (LASr 28.0% ± 7.0 vs. 41.9% ± 8.5; LAScd −13.2% ± 5.1 vs. −25.4% ± 9.1; p < 0.001). With an area under the curve (AUC) of 0.88 (95%CI 0.82–0.94) and 0.84 (95%CI 0.79–0.89), LASr and LAScd exhibited the highest discriminative value in predicting worsening of diastolic function, whereas LAVI was only of limited prognostic value [AUC 0.63 (95%CI 0.54–0.73)]. In logistic regression analyses, LAS remained a significant predictor for a decline of diastolic function after controlling for clinical and standard echocardiographic DD parameters, indicating its incremental predictive value.
Conclusion: The analysis of phasic LAS may be useful to predict worsening of LV diastolic function in DD0 patients at risk for a future DD development.
The importance of assessing the left atrium (LA) in evaluating left ventricular (LV) diastolic function is well established (1–3). The presence of early staged LV diastolic dysfunction (DD) has been related to worse outcome and higher risk for developing heart failure with preserved ejection fraction (HFpEF) (4, 5). Latest updated recommendations of the 2016 guidelines of the American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) propose a simplified algorithm that predominantly focuses on the detection of increased LV filling pressures (LVFP) including early diastolic transmitral flow velocity in relation to tissue velocity during early diastole (E/e′), e′, maximum LA volume index (LAVI), and estimated pulmonary artery systolic pressure (6). Although this approach provides an adequate diagnostic accuracy when compared to invasively measured LVFP (7, 8), the presence and severity of LV DD remains undetermined in many patients (9–11). In addition, DD parameters that offer predictive information are so far lacking. While the current algorithm proposed in the ASE/EACVI guidelines yields high specificity for the detection of clinically overt DD (6), LA deformation analysis using 2D speckle tracking echocardiography (2D STE) may add important diagnostic value due to its high sensitivity to reveal subtle myocardial alterations (4) and its ability to detect gradual decline in myocardial diastolic performance (3). An increased LAVI has been related to persistent chronic elevation of LV/LA pressures; however, as the LA size may take time to remodel, LAVI has been shown to be an insensitive parameter in the early phases of DD (3, 9–11). In contrast, functional changes appear at prompt stages of DD (4, 9) providing not only a diagnostic advantage but also being helpful for categorizing its severity (10, 11).
The standardization of LA strain (LAS) nomenclature and analysis by 2D STE as well as the definition of normal reference ranges have been recently published (12, 13) making it a tool ready to use for clinical practice. The predictive value of phasic LAS is well established for risk assessment and for guiding therapeutic strategies in the setting of atrial fibrillation (14, 15), cardiac amyloidosis (16), and in acute heart failure (17). There is emerging evidence that LASr could be of important prognostic value in HFpEF, as well (18, 19). However, prospective data are scarce and the predictive impact of LAS alterations on the development of DD is still unclear. In the face of the increasing clinical and economic burden of advanced DD, the implementation of echocardiographic parameters that are not limited to a reliable diagnostic accuracy, but also offer predictive information seems crucial to identify patients at risk for a future development of DD.
Accordingly, the aim of our longitudinal study was to prospectively assess the clinical value of phasic LAS alterations to predict a worsening of diastolic function over time in a randomly selected sample of study participants without clinically overt DD.
The Berlin Female Risk Evaluation (BEFRI) trial comprised a randomly selected urban female population aged 25–74 years. A detailed description of the BEFRI design has been already published (20). In 2013 and 2014, 473 women participants received a comprehensive transthoracic echocardiography comprising the prospective evaluation of phasic LA function. Data regarding the echocardiographic measurements, focusing on LV DD, have been released in detail previously (4). The study was approved by the institutional ethics committee of Charité-Universitätsmedizin Berlin (EA/2085/19) and all participants gave informed written consent. A subsample from this initial study data is used here as baseline measurements.
We reinvited every woman who participated in the BEFRI echo study feasible for the analysis of LA structure and function for follow-up examinations. These took place between October 2019 and December 2020. Next to the assessment of demographical and clinical data, an extensive transthoracic echocardiography was performed with the focus on DD assessment as well as on LA and LV strain analysis. Sample characteristics at baseline and follow-up are presented in Table 1. Somatometric measurements and clinical data for the larger sample at baseline have been already described in detail (4, 20).
A comprehensive transthoracic echocardiographic examination was performed using the same system applied for baseline examinations (Vivid E9 system, GE Vingmed, Horton, Norway, with an M5S 1.5- to 4.5-MHz transducer). The predefined echocardiographic study protocol can be inspected in the Supplementary material. Routine echocardiographic and Doppler data were obtained in accordance with the current ASE guidelines (6, 21). Standard parameters to assess diastolic function included LAVI; diastolic transmitral inflow velocities derived from pulsed wave-Doppler signal as well as the deceleration time; the septal, lateral, or average early diastolic mitral annular velocity (e′) assessed by pulsed-wave tissue Doppler; and E/e′ ratio. The RV-RA pressure difference was estimated from the maximum transvalvular velocity of the tricuspid regurgitation during systole.
2D STE strain studies were analyzed offline using the EchoPAC v203 software (GE Healthcare). Global peak systolic longitudinal LV strain (LV GLS) was determined from apical 4-chamber, 2-chamber, and long-axis views (17 segment LV model). Phasic LAS was assessed as proposed by the recent EACVI recommendations (12) from an LA focused apical 4-chamber-view, avoiding foreshortening. Three cardiac cycles were recorded for each view and stored for offline analysis. Gain, depth, and frame rate (60–80 frames/s) were optimized for image acquisition. The region of interest was placed on the atrial walls, distributing the interatrial septum and atrial free wall into six segments. LAS was analyzed QRS-triggered. LASr was identified from the plotted average strain curve as the maximum amplitude during ventricular systole. LA conduit strain (during passive LV filling; LAScd) and LA contraction strain (during peak atrial contraction; LASct) were calculated from the generated strain curve as previously described (4, 12, 22) (Figure 1).
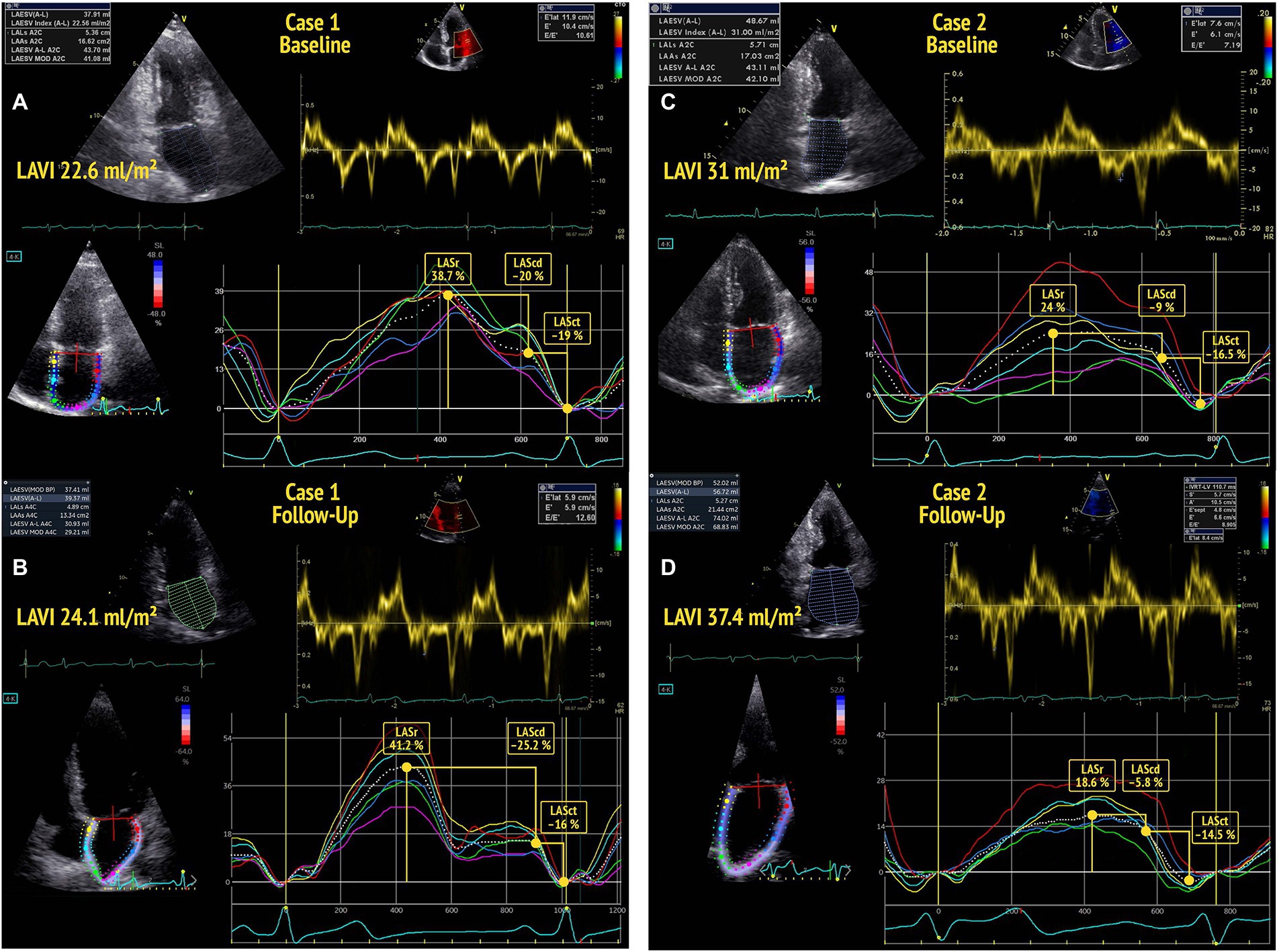
Figure 1. Baseline echocardiography of two participants (A,C) showing normal left atrial volume index (LAVI) (<34 ml/m2) and normal diastolic function according to the 2016 ASE guidelines. The participant in A shows a left atrial reservoir strain (LASr) >34% at baseline and no deterioration of diastolic function by the time of follow-up (B). The participant in C presents with a low baseline LASr and shows a decline of diastolic function (DD0 to DD1) by the time of follow-up echocardiography (D).
Diastolic function was categorized by an experienced cardiologist blinded for the clinical data in accordance with the recent ASE/EACVI recommendations on LV diastolic function (6). The following parameters were used for classification: (a) septal or lateral e′ velocity of <7 cm/s or <10 cm/s; or average e′ <9 cm/s; (b) E/e′ >14; (c) RV-RA pressure difference >31 mmHg (corresponding to a TR velocity >2.8 m/s); or (d) LAVI >34 ml/m2. Study participants who fulfilled 50% of these diagnostic criteria were graded to have signs of impaired diastolic function (DD1) while subjects meeting >50% of these criteria were assigned to the DD2 group (overt DD) (6). All others fell in the group without DD (DD0).
Applying these criteria to data of both measurement occasions let us define a progression variable that indicates whether the DD status of the woman deteriorated (DD0 to DD1 or DD1 to DD2; labeled “progression”) or remained the same (stable in DD0 or DD1; labeled “no progression”).
Descriptive statistics for baseline and follow-up measures were computed for the full sample and subgroups based on a combination of baseline DD status and DD progression.
To learn more about which baseline measures are associated with a worsening of diastolic function, we performed subsequent backward stepwise logistic regression analyses. In a first step, we build a reference model that included age, BMI, arterial hypertension, diabetes, renal function, average e′, E/e′ and LAVI as predictors and “progression” as the outcome. Starting with this full model, we then deleted terms with a backwards algorithm based on the AIC. The only remaining predictors were age, LASr, and hypertension. The model we report contains in addition e′ and LAVI to demonstrate the relative performance of the competing echo measures. In an extended model, a LAS measure was then added. Because the two LAS indicators – LASr and LAScd – are highly correlated (−0.85), we decided to include only LASr or LAScd, respectively, as predictor. All predictors were centered at their mean value. Models were compared using a likelihood ratio test, information criteria, and Pseudo- R2 (Nagelkerke) measures. A p-value <0.05 was considered statistically significant. In addition, receiver operating characteristic (ROC) curve analyses were performed to assess the diagnostic value of phasic LASr, LAScd, LAVI, and e′ velocity. All analyses were done in R (R Core Team, 2021) with usage of the additional package pROC (23, 24).
Mean follow-up time was 6.8 years (353.1 weeks; interquartile range 343.1–361.1 weeks). Of 449 study subjects reinvited in written form for follow-up examinations, 332 gave written consent (response rate 73.9%). Of these 332 participants, two visits were canceled due to unexpected other medical procedures, nine patients were lost to follow-up, and 63 scheduled follow-up examinations were canceled due to restrictions in relation to the SARS-CoV-2-pandemic situation. Of the 258 participants who were included in the study, two were excluded from analysis due to the development of moderate to severe mitral regurgitation and atrial fibrillation, five because of poor acoustic window, and two due to reduced speckle tracking quality of the LA wall resulting in a total follow-up sample size of 249 women.
Demographic and clinical characteristics of the total sample and subgroups depending on initial status and progression are shown in Table 1. Of the 249 participants, 29 showed DD1 at baseline. Of those 29 women, six (20.7%) had progressed to DD2 by the time of the follow-up examination. Of the 220 women with initially normal diastolic function, 34 (15.5%) had developed DD1 or DD2. The women whose diastolic function had deteriorated were older, had a higher BMI, showed higher levels of natriuretic peptides, and suffered more often from cardiovascular risk factors or diseases (Table 1).
Descriptive statistics for echocardiographic measures and myocardial mechanics at baseline and follow-up can be found in Table 2. Participants who developed DD over time showed a reduced LASr and LAScd value at baseline compared to women whose diastolic function remained in the normal range, while the average LAVI was significantly higher but – according to the currently recommended cut off value of 34 ml/m2 – still in the normal range (Table 2; Figure 2; Supplementary Figure 1). Echocardiographic parameters including myocardial mechanics at baseline and at the time of follow-up are shown in Table 2. With regard to follow-up data, previously healthy women who developed DD over time showed a significantly lower LASr and LAScd and a markedly increased LAVI by the time of follow-up (Table 2). Differences in clinical and echocardiographic characteristics between groups were tested using analysis of variance for continuous variables and the Fisher’s exact test for categorial variables. Intra- and interobserver variability of phasic LAS measurements are published and have been shown to be very low (4).

Figure 2. Left atrial reservoir strain (LASr) is significantly reduced (A), while left atrial volume index (LAVI) is markedly higher, but still below the recommended cut-off value of 34 ml/m2 (B) in study participants with normal diastolic function at baseline who then showed a deterioration of diastolic function (progress) by follow-up. ***p < 0.001; **p = 0.004.
With an AUC of 0.88 (95% CI 0.82–0.94) and an AUC of 0.84 (95% CI 0.79–0.89), LASr and LAScd values showed a high discriminative power in predicting a decline of diastolic function over time. These LA function parameters performed significantly better than the echocardiographic standard parameter LAVI (AUC 0.63; 95% CI 0.54–0.73; see Figure 3). Average e′ held a good discriminative value, as well (AUC of 0.80 with 95% CI 0.74–0.86; not graphically represented).
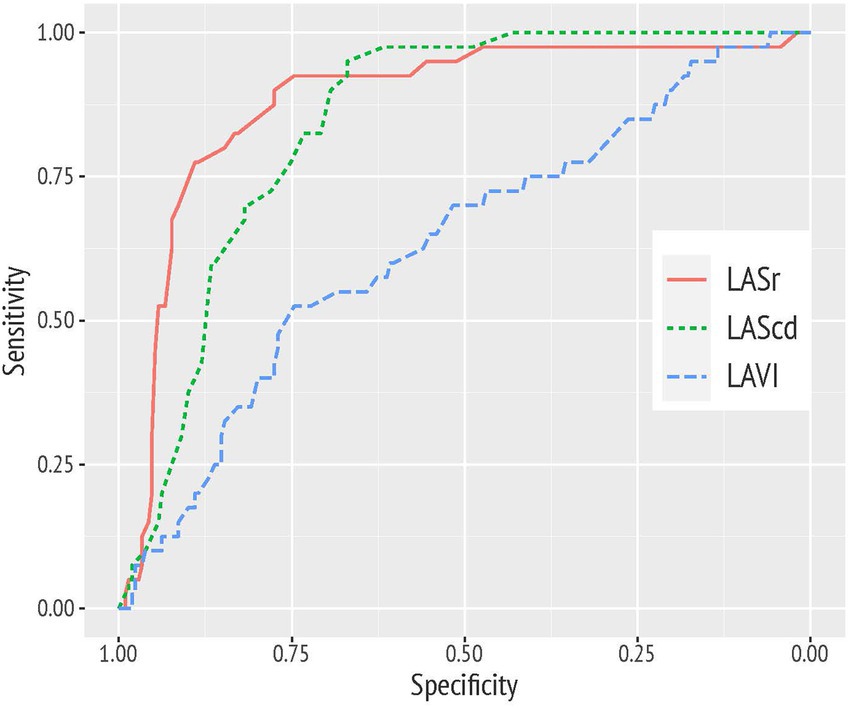
Figure 3. Receiver operating characteristic (ROC) curve analysis demonstrating the superior diagnostic value of left atrial reservoir strain (LASr) and conduit strain (LAScd) compared to left atrial volume index (LAVI) to discriminate study participants with a worsening of diastolic function over time.
For a cut point of <34%, LASr was associated with a sensitivity of 90% and a specificity of 78% in predicting the worsening of diastolic function. For LAScd, respective sensitivity and specificity were 83 and 73% for a cut off value >−17.5%.
31 of 34 (91.2%) of the previously healthy women who – according to recent ASE/EACVI-recommendations – showed a decrease of diastolic function by follow-up showed a reduced baseline LASr according to our ROC analysis (LASr <34%). In contrast, only 6 of the 34 women (17.6%) featured a LAVI >34 ml/m2, 17 (50.0%) showed an average e′ <9.0 cm/s (1). Vice versa, of the 63 participants who were classified to have DD0 and a LASr <34% at the time of baseline examinations, 31 then developed DD at follow-up (49%) compared to only 2% (3 of 157) participants with a baseline LASr ≥34%. Estimation of PA pressure and assessment of E/e′ was not helpful in discriminating patients with development of DD since PA pressure was not assessable in 157 patients, and baseline E/e′ was <14 in 248 participants.
When comparing the reference model with established measures and the extended model, including LASr improved the model as indicated by a significant Likelihood Ratio Test (χ2(1) = 28.9, p < 0.001), a lower AIC (181.1 vs. 154.2), and a higher Nagelkerke Index (0.35 vs. 0.49).
The results of the extended model are presented in Table 3. In this model, LASr (Table 3) remained a significant predictor of a decline of diastolic function when correcting for age, hypertension, e′ average, and LAVI. LAScd was significantly associated with a worsening of diastolic function, as well (Supplementary Table 1); however, including LAScd in addition to LASr in the analysis did not improve the extended model (data not shown). In addition, the conditional effects of LASr are illustrated in Figure 4.
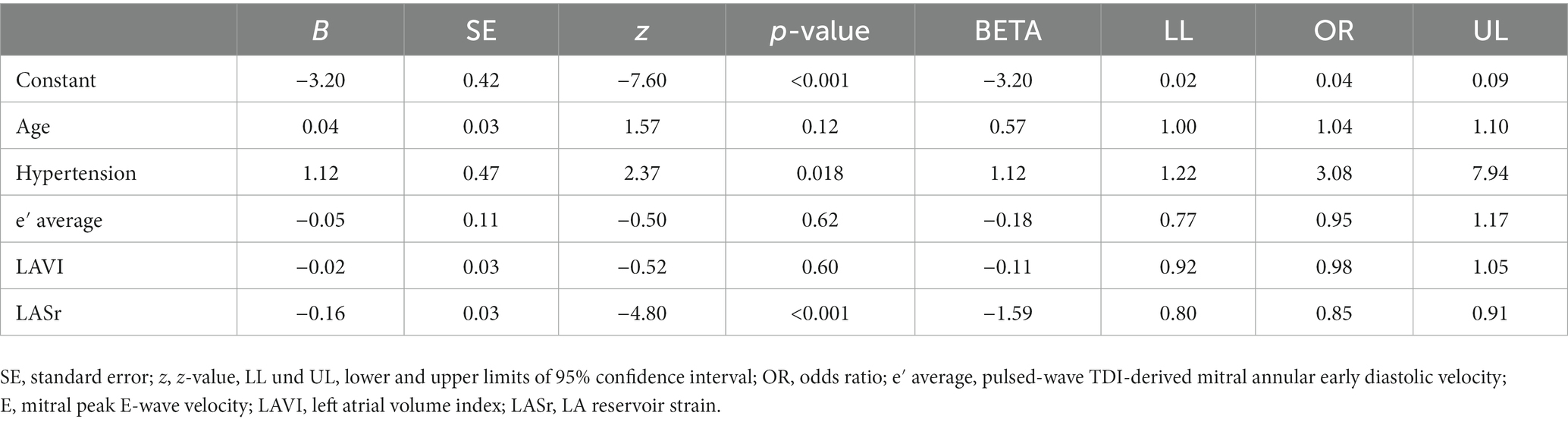
Table 3. Predictors of diastolic function worsening over time; multivariate logistic regression analysis.
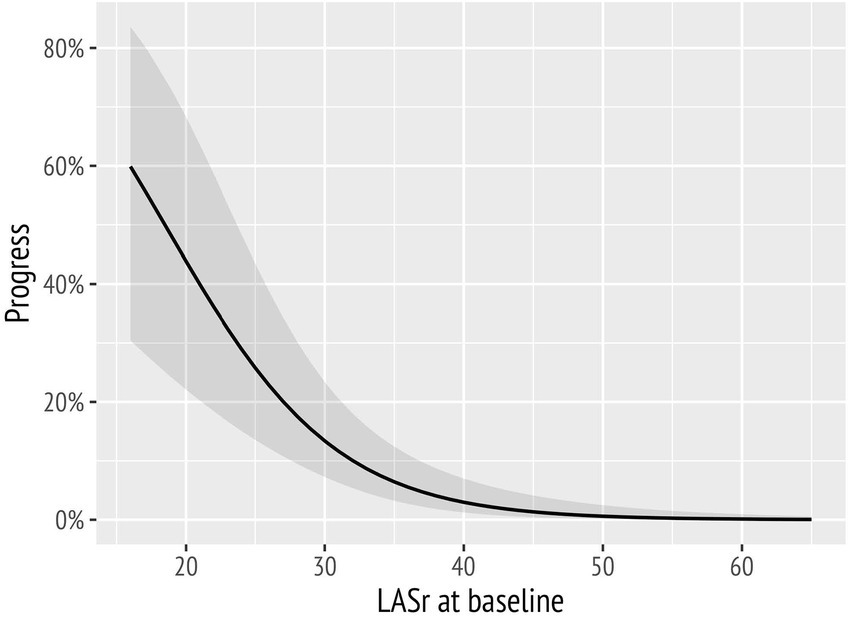
Figure 4. Probability of diastolic function worsening over time markedly increased with decreasing baseline left atrial reservoir strain (LASr) values.
This longitudinal study demonstrates that phasic LAS entails predictive value in evolving DD. Phasic LAS analysis was associated with a higher diagnostic accuracy in predicting diastolic function worsening than LAVI and other echocardiographic parameters in study participants with a normal diastolic function at baseline. Furthermore, subjects with baseline LASr <34% demonstrated a significantly increased risk of developing impaired diastolic function over time. The analysis of LA mechanics may accordingly be not only useful as a diagnostic but also as a predictive tool in the echocardiographic assessment of diastolic function.
Current guidelines (6) still recommend to assess LAVI due to its large body of evidence in cardiovascular risk stratification (25). Although not yet included in the ASE/EACVI recommendations, the analysis of LA function might significantly improve the predictive impact of the echocardiographic assessment in diverse clinical conditions. Our data additionally suggest that alterations of phasic LAS, and not of LAVI, may be useful to identify “healthy” patients at risk for future DD development.
Strain alterations of all three phases of the LA cycle have been widely described in DD and there is reliable evidence that LAS may worsen even in absence of LA volume enlargement (1, 3, 9). LASr hence constitutes an accurate, feasible and robust parameter in the evaluation of DD and has recently been proposed as a sensitive and specific parameter to be included in the diagnostic algorithm of DD (26). This may lead to an improvement in terms of detection and classification of the severity of DD, especially in situations where classification cannot be unequivocally achieved by current guideline algorithms (“gray zone” or indeterminate range) (27–29). As previously shown in the BEFRI trial (4), all three LAS components exhibit specific alterations in different DD stages. We were able to confirm these findings in the present follow-up study. In line with these findings, Morris et al. (9) demonstrated higher sensitivity of LAS analyses compared with LAVI in detecting DD, as well. In their study, 62% of patients with DD presented a LASr <23%, while only 34% showed a LAVI >34 ml/m2. Furthermore, the addition of LASr to the standard algorithms improved the detection of DD by 10% (9). The fact that LA mechanics are essential to preserve LV function (30) and the close relationship to symptom development (9, 31, 32) brings LAS assessment in the focus of early detection methods of DD (26). Our study further demonstrates that decreased phasic LAS may be of predictive value with respect to the worsening of diastolic function.
There is increasing evidence that analysis of LA function may play a key role as a prognostic marker in patients with advanced DD. For example, the TOPCAT trial demonstrated that a reduction of LASr was associated with a higher risk of HF hospitalization and cardiovascular death (18). In the previously mentioned study by Morris et al. (9), a LASr <23% was linked to worse NYHA class and higher risk of hospitalization for HF. Similarly, LASr was found to be an independent predictor of death and cardiovascular hospitalization in 308 HFpEF patients (32).
To our knowledge, the present study is the first to prospectively investigate the predictive value of phasic LAS alterations with respect to a decline of diastolic function. LASr and LAScd appeared superior to predict worsening of diastolic function over time than LAVI and other traditional DD parameters. Consequently, phasic LAS analysis may contribute to an improved diagnostic work up and risk stratification in patients with normal diastolic function but at risk for future diastolic function worsening.
Diastolic function was strictly graded according to the current ASE recommendations on diastolic function with all its inherent limitations, i.e., providing a good specificity with regard to overt clinical DD while potentially missing a gradual decrease of diastolic function. Following rigorously the criteria of the current DD grading scheme, phasic LAS analysis proved superior to LAVI in predicting a decline of diastolic function in patients without DD; this diagnostic strength suggests a potential role in the management of patients at risk in future. Age-related cut offs in DD have recently been defined and found to be of prognostic significance in patients with mild DD (33). As another limitation, our analyses were not stratified by age. However, in multivariate analyses controlling for age among other factors, LASr and LAScd remained significantly associated with a worsening of diastolic function over time. Due to the study design of the BEFRI study, only women were included in the analyses. Although previous reports have found no differences between LAS in men and women (13, 34) the predictive value has yet to be demonstrated in studies of men. Due to restrictions of the pandemic SARS-CoV-2 situation, more than 60 patients were unpredictably lost to follow up. However, the results of the ROC and logistic regression analyses showed a strong additive predictive utility of phasic LAS analysis on the course of DD that warrant further studies, preferably including both, men and women. In addition, the clinical impact of our data needs to be investigated by larger future trials which yield sufficient power to demonstrate associations between LAS alterations and the development of HF signs and symptoms, preferably using a LA-dedicated strain tracking software that was not yet available for clinical use at the time of baseline investigations.
Our data demonstrated a high discriminative value of phasic LAS analysis to predict a decline of diastolic function in study participants with normal baseline diastolic function in a longitudinal study design. The integration of phasic LAS analysis into the current echocardiographic algorithm to assess diastolic function may be useful for predicting the deterioration of LV diastolic function and therefore for identifying patients at risk for a future development of DD.
The datasets presented in this article are not readily available because of continued data analyses. Requests to access the datasets should be directed to YW5uYS5icmFuZEBjaGFyaXRlLmRl.
The studies involving human participants were reviewed and approved by Charité-Universitätsmedizin Berlin (EA/2085/19). The patients/participants provided their written informed consent to participate in this study.
AB: funding of study, conception and design, data collection, supervision of study, analysis and interpretation, writing the manuscript, critical revision of manuscript, and funding. ER: data collection, analysis and interpretation, writing the manuscript. AW and DB-W: data collection, analysis and interpretation, critical revision of manuscript. US: funding of study, conception and design, critical revision of manuscript. CC: statistical analysis, analysis and interpretation, critical revision of manuscript. SB: analysis and interpretation, critical revision of manuscript. IM: data collection, analysis and interpretation, critical revision of manuscript. HD, KS, VR-Z, UL, and FK: conception and design, analysis and interpretation, critical revision of manuscript. VS: conception and design, analysis and interpretation, writing the manuscript, critical revision of manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the DZHK (German Center for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research). Grant number 81Z0100211.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1070450/full#supplementary-material
1. Thomas, L, Muraru, D, Popescu, BA, Sitges, M, Rosca, M, Pedrizzetti, G, et al. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. (2020) 33:934–52. doi: 10.1016/j.echo.2020.03.021
2. Gentile, F, Ghionzoli, N, Borrelli, C, Vergaro, G, Pastore, MC, Cameli, M, et al. Epidemiological and clinical boundaries of heart failure with preserved ejection fraction. Eur J Prev Cardiol. (2021) 29:1233–43. doi: 10.1093/eurjpc/zwab077
3. Thomas, L, Marwick, TH, Popescu, BA, Donal, E, and Badano, LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1961–77. doi: 10.1016/j.jacc.2019.01.059
4. Brecht, A, Oertelt-Prigione, S, Seeland, U, Rücke, M, Hättasch, R, Wagelöhner, T, et al. Left atrial function in preclinical diastolic dysfunction: two-dimensional speckle-tracking echocardiography-derived results from the BEFRI trial. J Am Soc Echocardiogr. (2016) 29:750–8. doi: 10.1016/j.echo.2016.03.013
5. Pieske, B, Tschöpe, C, de Boer, RA, Fraser, AG, Anker, SD, Donal, E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40:3297–317. doi: 10.1093/eurheartj/ehz641
6. Nagueh, SF, Smiseth, OA, Appleton, CP, Byrd, BF 3rd, Dokainish, H, Edvardsen, T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
7. Lancellotti, P, Galderisi, M, Edvardsen, T, Donal, E, Goliasch, G, Cardim, N, et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI euro-filling study. Eur Heart J Cardiovasc Imaging. (2017) 18:961–8. doi: 10.1093/ehjci/jex067
8. Andersen, OS, Smiseth, OA, Dokainish, H, Abudiab, MM, Schutt, RC, Kumar, A, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. (2017) 69:1937–48. doi: 10.1016/j.jacc.2017.01.058
9. Morris, DA, Belyavskiy, E, Aravind-Kumar, R, Kropf, M, Frydas, A, Braunauer, K, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. J Am Coll Cardiol Img. (2018) 11:1405–15. doi: 10.1016/j.jcmg.2017.07.029
10. Singh, A, Addetia, K, Maffessanti, F, Mor-Avi, V, and Lang, RM. LA strain for categorization of LV diastolic dysfunction. J Am Coll Cardiol Img. (2017) 10:735–43. doi: 10.1016/j.jcmg.2016.08.014
11. Mandoli, GE, Sisti, N, Mondillo, S, and Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: have we finally found the missing piece of the puzzle? Heart Fail Rev. (2020) 25:409–17. doi: 10.1007/s10741-019-09889-9
12. Badano, LP, Kolias, TJ, Muraru, D, Abraham, TP, Aurigemma, G, Edvardsen, T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19:591–600. doi: 10.1093/ehjci/jey042
13. Sugimoto, T, Robinet, S, Dulgheru, R, Bernard, A, Ilardi, F, Contu, L, et al. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. (2018) 19:630–8. doi: 10.1093/ehjci/jey018
14. Jasic-Szpak, E, Marwick, TH, Donal, E, Przewlocka-Kosmala, M, Huynh, Q, Gozdzik, A, et al. Prediction of AF in heart failure with preserved ejection fraction: incremental value of left atrial strain. J Am Coll Cardiol Img. (2021) 14:131–44. doi: 10.1016/j.jcmg.2020.07.040
15. Park, JJ, Park, JH, Hwang, IC, Park, JB, Cho, GY, and Marwick, TH. Left atrial strain as a predictor of new-onset atrial fibrillation in patients with heart failure. J Am Coll Cardiol Img. (2020) 13:2071–81. doi: 10.1016/j.jcmg.2020.04.031
16. Huntjens, PR, Zhang, KW, Soyama, Y, Karmpalioti, M, Lenihan, DJ, and Gorcsan, J 3rd. Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. J Am Coll Cardiol Img. (2021) 14:1508–19. doi: 10.1016/j.jcmg.2021.01.016
17. Park, JH, Hwang, IC, Park, JJ, Park, JB, and Cho, GY. Prognostic power of left atrial strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging. (2021) 22:210–9. doi: 10.1093/ehjci/jeaa013
18. Santos, AB, Roca, GQ, Claggett, B, Sweitzer, NK, Shah, SJ, Anand, IS, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. (2016) 9:e002763. doi: 10.1161/CIRCHEARTFAILURE.115.002763
19. Freed, BH, Daruwalla, V, Cheng, JY, Aguilar, FG, Beussink, L, Choi, A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. (2016) 9:e003754. doi: 10.1161/CIRCIMAGING.115.003754
20. Oertelt-Prigione, S, Seeland, U, Kendel, F, Rücke, M, Flöel, A, Gaissmaier, W, et al. Cardiovascular risk factor distribution and subjective risk estimation in urban women—the BEFRI study: a randomized cross-sectional study. BMC Med. (2015) 13:52. doi: 10.1186/s12916-015-0304-9
21. Lang, RM, Badano, LP, Mor-Avi, V, Afilalo, J, Armstrong, A, Ernande, L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
22. Brand, A, Frumkin, D, Hübscher, A, Dreger, H, Stangl, K, Baldenhofer, G, et al. Phasic left atrial strain analysis to discriminate cardiac amyloidosis in patients with unclear thick heart pathology. Eur Heart J Cardiovasc Imaging. (2021) 22:680–7. doi: 10.1093/ehjci/jeaa043
23. Robin, X, Turck, N, Hainad, A, Tiberti, N, Lisacek, F, Sanchez, JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. (2011) 12:77. doi: 10.1186/1471-2105-12-77
24. R Core Team,. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna (2021).
25. Hoit, BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. (2014) 63:493–505. doi: 10.1016/j.jacc.2013.10.055
26. Smiseth, OA, Morris, DA, Cardim, N, Cikes, M, Delgado, V, Donal, E, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2022) 23:e34–61. doi: 10.1093/ehjci/jeab154
27. Flachskampf, FA, and Baron, T. Echocardiographic algorithms for detecting elevated diastolic pressures: reasonable, not perfect. J Am Coll Cardiol. (2017) 69:1949–51. doi: 10.1016/j.jacc.2017.02.022
28. Pearson, AC. Issues with estimating "diastolic function" and left ventricular filling pressure using the new guidelines. J Am Coll Cardiol. (2017) 70:1197–8. doi: 10.1016/j.jacc.2017.05.072
29. Sengupta, PP, and Marwick, TH. The many dimensions of diastolic function: a curse or a blessing? J Am Coll Cardiol Img. (2018) 11:409–10. doi: 10.1016/j.jcmg.2017.05.015
30. Miglioranza, MH, Badano, LP, Mihăilă, S, Peluso, D, Cucchini, U, Soriani, N, et al. Physiologic determinants of left atrial longitudinal strain: a two-dimensional speckle-tracking and three-dimensional echocardiographic study in healthy volunteers. J Am Soc Echocardiogr. (2016) 29:1023–1034.e3. doi: 10.1016/j.echo.2016.07.011
31. Sanchis, L, Gabrielli, L, Andrea, R, Falces, C, Duchateau, N, Perez-Villa, F, et al. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. (2015) 16:62–7. doi: 10.1093/ehjci/jeu165
32. Reddy, YNV, Obokata, M, Egbe, A, Yang, JH, Pislaru, S, Lin, G, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. (2019) 21:891–900. doi: 10.1002/ejhf.1464
33. Granot, Y, Ben-Assa, E, Sapir, O, Laufer-Perl, M, Topilsky, Y, and Rozenbaum, Z. Age-specific mortality risk of mild diastolic dysfunction among hospitalized patients with preserved ejection fraction. Int J Cardiol. (2021) 332:216–22. doi: 10.1016/j.ijcard.2021.03.054
Keywords: left atrial strain, diastolic dysfunction, left atrium, LAVI, BEFRI
Citation: Brand A, Romero Dorta E, Wolf A, Blaschke-Waluga D, Seeland U, Crayen C, Bischoff S, Mattig I, Dreger H, Stangl K, Regitz-Zagrosek V, Landmesser U, Knebel F and Stangl V (2023) Phasic left atrial strain to predict worsening of diastolic function: Results from the prospective Berlin Female Risk Evaluation follow-up trial. Front. Cardiovasc. Med. 10:1070450. doi: 10.3389/fcvm.2023.1070450
Received: 14 October 2022; Accepted: 02 February 2023;
Published: 20 February 2023.
Edited by:
Michal Schäfer, University of Colorado Denver, United StatesReviewed by:
Beata Uziębło-Życzkowska, Military Institute of Medicine, PolandCopyright © 2023 Brand, Romero Dorta, Wolf, Blaschke-Waluga, Seeland, Crayen, Bischoff, Mattig, Dreger, Stangl, Regitz-Zagrosek, Landmesser, Knebel and Stangl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Brand, ✉ YW5uYS5icmFuZEBjaGFyaXRlLmRl
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.