
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 02 February 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1054666
Background: Approximately half of patients with heart failure have a preserved ejection fraction (HFpEF). To date, only SGLT-2i, ARNi, and MRAs treatments have been shown to be effective for HFpEF. Exercise intolerance is the primary clinical feature of HFpEF. The aim of this meta-analysis was to explore the effect of inorganic nitrate/nitrite supplementary therapy on the exercise capacity of HFpEF patients.
Methods: We searched PubMed, Embase, Cochrane Library, OVID, and Web of Science for eligible studies for this meta-analysis. The primary outcomes were peak oxygen consumption (peak VO2), exercise time, and respiratory exchange ratio (RER) during exercise. The secondary outcomes were cardiac output, heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure, and systemic vascular resistance during rest and exercise, respectively.
Results: A total of eight randomized-controlled trials were enrolled for this meta-analysis. We found no benefit of inorganic nitrate/nitrite on exercise capacity in patients with HFpEF. Inorganic nitrate/nitrite compared to placebo, did not significantly increased peak VO2 (MD = 0.361, 95% CI = −0.17 to 0.89, p = 0.183), exercise time (MD = 9.74, 95% CI = −46.47 to 65.95, p = 0.734), and respiratory exchange ratio during exercise (MD = −0.003, 95% CI = −0.036 to 0.029, p = 0.834). Among the six diameters reflecting cardiac and artery hemodynamics, inorganic nitrate/nitrite can lower rest SBP, rest/exercise DBP, rest/exercise MAP, and exercise SVR, but has no effect in cardiac output and heart rate for HFpEF patients.
Conclusion: Our meta-analysis suggested that inorganic nitrate/nitrite supplementary therapy has no benefit in improving the exercise capacity of patients with HFpEF, but can yield a blood pressure lowering effect, especially during exercise.
Approximately half of patients with heart failure have a preserved ejection fraction (HFpEF) (1, 2). HFpEF occurs almost exclusively in older population, and many are asymptomatic at rest and show abnormalities only during exercise (3–5). To date, only SGLT-2i, ARNi, and MRAs treatments have been shown to be effective for HFpEF (6–8).
Exercise intolerance is the primary clinical feature of HFpEF and is responsible for the severely reduced quality of life of these patients (9–12). However, the mechanism of this limitation has not been understood completely. Compared with heart failure with reduced ejection fraction, HFpEF has a distinct pathophysiology characterized by ventricular diastolic dysfunction. During exercise, a normally functioning left ventricle can diastole to a larger volume with no increase in filling pressure, but in individuals with HFpEF, left ventricle filling pressure with exercise increases remarkably, producing symptoms of dyspnea (13–16). Not only have diastolic dysfunction been identified, but evidence exists for abnormalities in peripheral arteries and skeletal muscle. The impaired exercise vasodilatory reserve and reduction in skeletal muscle perfusion may contribute significantly to exercise intolerance of HFpEF patients (17–19). Multiple lines of evidence suggest that the impaired perfusion results are due in large part to low availabilities in nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling (18, 20).
Traditionally, endogenous NO was thought to generate exclusively by NO synthases (21). In recent years, however, the nitrate–nitrite–NO pathway has been recognized as an important alternative in vivo source of NO (22, 23). Intriguingly, tissue hypoxia and acidosis can enhance the reduction of nitrite to NO, a condition that exercise develops. This suggests that nitrate/nitrite might better target hemodynamic derangements developed during exercise in people with HFpEF (24–27). Moreover, compared to organic nitrates such as isosorbide mononitrate and dinitrate, inorganic nitrate/nitrite is less likely to cause hypotension/headache and rarely develops tolerance (28, 29), which provides a considerable promise for the use of inorganic nitrate/nitrite in the treatment of HFpEF.
Multiple randomized controlled studies have investigated the effect of inorganic nitrate/nitrite on exercise capacity and cardiac hemodynamics in patients with HFpEF, with the administration duration ranging from acute to short-term. Interventions of these studies were also various, including intravenous sodium nitrite, inhaled nitrite, oral potassium nitrate, and NO3-rich beetroot juice (BRJ). However, the conclusions were inconsistent. Some studies demonstrated a positive effect of inorganic nitrate/nitrite in exercise capacity in patients with HFpEF (21, 25, 26, 30), while others did not (28, 29, 31, 32). To date, no study has summarized the results of relevant trials and thus the conclusion is unclear. Accordingly, we conducted the current meta-analysis of randomized controlled trials to explore the clinical viability of inorganic nitrate/nitrite on exercise performance and cardiac hemodynamics in patients with HFpEF, providing clinicians with new thoughts of HFpEF pharmacological therapeutics.
A systematic search was conducted on PubMed, Embase, Cochrane Library, OVID, and Web of Science for eligible studies published up to December 31, 2020 using the following search terms: (“nitrate” or “azotate” or “nitrite”) and (“heart failure” or “cardiac failure” or “heart decompensation”) and (“preserved” or “normal”). Similar searches were made on clinicaltrials.gov to ensure no bias caused by unpublished trials. We also manually screened the reference lists of key articles to further identify potential eligible studies. There is no restriction in primary outcomes or language.
The inclusion criteria are as follows: (1) study design: randomized controlled trial, (2) study population: patients diagnosed with HFpEF, (3) intervention: inorganic nitrate/nitrite, (4) comparator: placebo or control, and (5) reported outcomes of exercise capacity or cardiac hemodynamics. HFpEF was defined as symptoms of chronic heart failure (dyspnea and/or fatigue) and preserved left ventricular ejection fraction (≥50%). Three indicators of exercise capacity were considered as the primary outcomes: peak oxygen consumption (peak VO2), respiratory exchange ratio (VCO2/VO2) during exercise and exercise time. The secondary outcomes were parameters of cardiac and arterial hemodynamics, including cardiac output, heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure, and systemic vascular resistance at rest and exercise, respectively. Studies that did not reported any of the outcomes mentioned above were excluded. Two investigators (Lv-F, Zhang-JY) independently reviewed the titles and abstracts of studies identified by the search strategy, and studies that satisfied the inclusion criteria were entered into the full-text assessment. When articles were only abstracts, efforts were made to contact the authors for full-text, if failed, we would eventually exclude these articles due to the insufficient data and potential significant bias.
All included studies were evaluated for quality by two investigators independently, with disagreements resolved by discussion. The risk of bias was assessed using the criteria proposed by the Cochrane back review group (33). The level of evidence was assessed based on the guidelines of the GRADE working group (34).
The following data were extracted from each selected study: RCT design (parallel or crossover), number of participants per arm, nature of intervention, exercise pattern, age, sex, race, cardiac function, complication, basic medication, and outcomes of interest before and after intervention.
Data were pooled in a meta-analysis in the forms of forest plots. Given that all the outcomes were continuous variables, the combined estimates were presented as mean difference (MD) and 95% CI. If the units are not uniform, a standard mean difference (SMD) will be used. Heterogeneity between studies was assessed using Chi square test and magnitude by calculating I2 statistic, with I2 > 50% regarded as indicating moderate-to-high heterogeneity (35). A random-effect or fixed-effect model was used depending on the heterogeneity calculated. A sensitivity analysis was performed by excluding one study each time, in order to evaluate the effect of single study on the overall estimates. Publication bias was assessed by constructing a funnel plot of each study’s effect size against the standard error. The funnel plot asymmetry was assessed using Begg and Egger’s tests, with a p-value <0.1 considered as significant publication bias. We also used the trim-and-fill computation to estimate the impact of publication bias on the interpretation of results (36). All statistical tests were performed with Stata (version 12.0).
Through a literature searching, we identified 421 studies, of which eight RCTs with 335 patients were eventually included in the current meta-analysis (Figure 1). The eight RCTs were all published after 2014, comparing the effect of inorganic nitrate/nitrite on HFpEF with that of placebo (21, 25, 26, 28–32). Four trials were parallel-group design with baseline characteristics well matched in two arms (25, 26, 30, 32). The other four were cross-over design, meaning that the baseline characteristics in two arms were exactly the same (21, 28, 29, 31). Four trials (25, 26, 28, 30) used sodium nitrite (intravenous or inhaled) as intervention and the other four (21, 29, 31, 32) used nitrate-rich beetroot juice (BRJ) as intervention. Five trials (21, 25, 26, 29, 30) looked at the acute effects of nitrate/nitrite while other threes (28, 31, 32) investigated the short-term effects (≥1 week). One trial (32) compared BRJ with placebo on a background of supervised exercise. The exercise pattern for testing varied among studies, including maximal-effort exercise, submaximal-effort exercise (20/45-W workload) and upright cycle ergometry. The detailed characteristics of the eight studies are shown in Table 1.
All studies included were prospective randomized controlled trials with relatively high quality. Through the evaluation of quality of evidence using the GRADE system (37), only two studies remained high quality. The other six studies all had different degrees of degradation and two were downgraded to a quality of very low. The results of quality assessment of the included studies were shown in Table 2.
We found no benefit of inorganic nitrate/nitrite on exercise capacity in patients with HFpEF (Table 3). Inorganic nitrate/nitrite compared to placebo, did not significantly increased peak VO2 (MD = 0.361, 95% CI = −0.17 to 0.89, p = 0.183; Figure 2A), exercise time (MD = 9.74, 95% CI = −46.47 to 65.95, p = 0.734; Figure 2B), or either respiratory exchange ratio during exercise (MD = −0.003, 95% CI = −0.036 to 0.029, p = 0.834; Figure 2C). For peak VO2 and exercise time, subgroup analyses were conducted according to RCT design (parallel or cross-over), intervention subtype (nitrate or nitrite), and treatment duration (acute or short-term, also interpreted as “single administration” or “repeated administration”). As a result, no significant results were obtained for each subgroup (Figure 2). Neither nitrate (MD = 0.02, 95% CI = −1.03 to 0.99, p = 0.97) nor nitrite (MD = 0.50, 95% CI = −0.27 to 1.02, p = 0.20) was effective in increasing peak VO2 compared to placebo. Similarly, for exercise time, there was no difference either between nitrate and placebo (MD = 30.83, 95% CI = −50.99 to 121.64, p = 0.51) or between nitrite and placebo (MD = −12.0, 95% CI = −59.1 to 35.1, p = 0.62). As can be seen from our subgroup analysis according to treatment duration, the improvement of peak VO2 or exercise time was also absent in the acute effect of inorganic nitrate/nitrite (MD = 25.32, 95% CI = −19.24 to 66.73, p = 0.52; MD = 48, 95% CI = −139.83 to 235.83, p = 0.64). There was no subgroup analysis for respiratory exchange ratio due to its limited number of included studies. No statistically significant between-study heterogeneity was detected for primary outcomes. Further sensitivity analysis of each outcome showed that the exclusion of each study did not alter the significance of the corresponding pooled MD, suggesting that the results were robust. For publication bias, the shape of the funnel plot of each outcome was visually symmetric, then statistical assessment by Egger test suggested no significant publication bias in all pooled studies (p = 0.245; 0.681; 0.825; Figure 3).
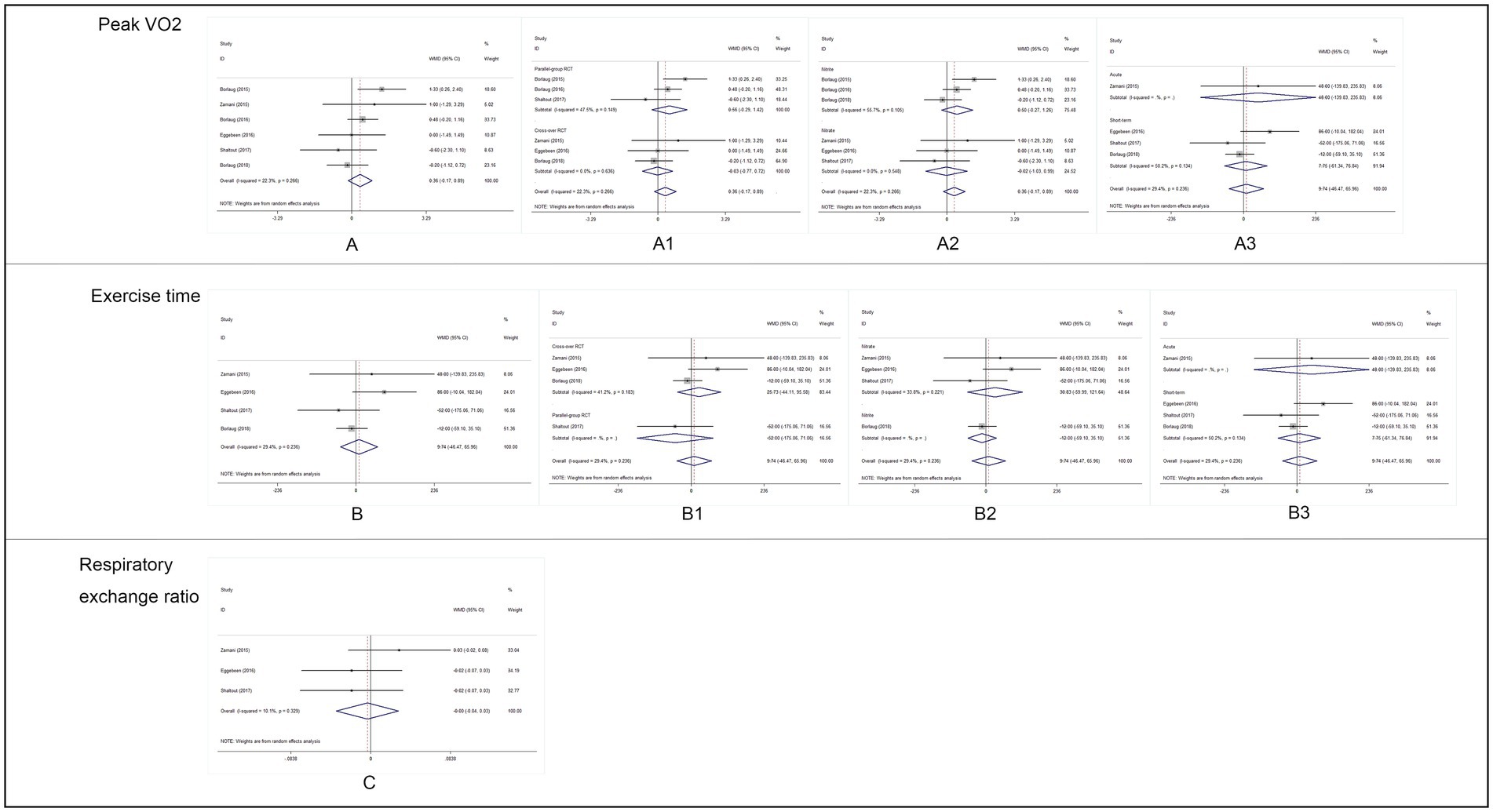
Figure 2. Analysis results of primary outcomes. (A) Forest plot of peak VO2, (A1): subgroup analysis by RCT design, (A2): subgroup analysis by intervention drug, and (A3): subgroup analysis by intervention duration. (B) Forest plot of exercise time, (B1): subgroup analysis by RCT design, (B2): subgroup analysis by intervention drug, (B3): subgroup analysis by intervention duration. (C): Forest plot of respiratory exchange ratio (RER) during exercise.
Outcomes of cardiac and artery hemodynamics diameters were pooled for analysis (Table 3). In general, we yield an equivocal result. Our analysis showed that compared to placebo, inorganic nitrate/nitrite can lower rest SBP (MD = −7.91, 95% CI = −11.25 to −4.56, p < 0.001), rest/exercise DBP (MD = −2.96, 95% CI = −3.73 to −2.20, p < 0.001; MD = −3.93, 95% CI = −4.99 to −2.82, p < 0.001), rest/exercise MAP (MD = −2.91, 95% CI = −3.65 to −2.17, p < 0.001; MD = −4.11, 95% CI = −7.11 to −1.11, p = 0.007), and exercise SVR (MD = −74.43, 95% CI = −129.85 to −19.01, p = 0.008) in patients with HFpEF. However, no significant difference was found for cardiac output and HR, either in rest or during exercise. Subgroup analyses by study design, intervention drug, and treatment duration were performed for outcomes of HR, SBP, and DBP during exercise. Consequently, there was still no significant difference in exercise HR in acute treatment (MD = −0.02, 95% CI = −0.761 to 0.723, p = 0.96) or short-term treatment (MD = 1.68, 95% CI = −6.06 to 9.42, p = 0.67), or in nitrate (MD = 1.92, 95% CI = −3.15 to 6.92, p = 0.46), or nitrite (MD = −0.045, 95% CI = −0.79 to 0.70, p = 0.91). All the general statistical syntheses of secondary outcomes were shown in Figure 4. However, the subgroup analyses for exercise SBP and DBP showed that it was nitrite (MD = −6.17, 95% CI = −11.32 to −1.01, p = 0.019; MD = −4.07, 95% CI = −5.18 to −2.95, p < 0.001) rather than nitrate (MD = −1.40, 95% CI = −11.13 to −8.33, p = 0.78; MD = −2.40, 95% CI = −6.06 to 1.25, p = 0.198) that was able to lower exercise blood pressure. Moreover, the antihypertensive effect induced by inorganic nitrate/nitrite appeared to be acute (or transient) rather than persistent, because the exercise SBP and DBP was significantly lowered only in the acute treatment (MD = −6.17, 95% CI = −11.32 to −1.01, p = 0.019; MD = −4.07, 95% CI = −5.18 to −2.95, p < 0.001) but no in the short-term treatment (MD = −1.40, 95% CI = −11.13 to −8.33, p = 0.78; MD = −2.40, 95% CI = −6.06 to 1.25, p = 0.198). All the general statistical syntheses of secondary outcomes were shown in Figure 5. Still, sensitivity analyses were conducted for each outcome, and no results were changed after any extraction of studies. In analyses for exercise/rest CO, exercise/rest SBP, and rest HR, publication bias on Egger test were found (p = 0.017, 0.044, 0.015, 0.054, and 0.017, respectively). However, further trim-and-fill test indicated that the estimates were not impacted by these publication bias (i.e., no trimming done because data unchanged).
The results of this meta-analysis show that compared with placebo, inorganic nitrate/nitrite therapy cannot improve peak VO2, respiratory exchange ratio, or exercise time, which means it has no benefits in improving the exercise capacity of HFpEF patients. However, analyses of hemodynamics indexes show that inorganic nitrate/nitrite can temporarily lower exercise blood in HFpEF.
When the mechanism for a disease is understood, the corresponding treatment comes into being. Although the mechanism of HFpEF has not yet been fully clarified, several major theories have been formed. Multiple lines of indirect trial evidence suggest that systematic microvascular inflammation plays an important role in the development of HFpEF (38–42). In HFpEF, microvascular inflammation caused by comorbidities reduces the availability of cyclic guanosine monophosphate (cGMP), thereby decreases nitric oxide (NO) activity and blocks actin phosphorylation, which ultimately damages adjacent cardiomyocytes (1). Accordingly, therapeutic trials aimed at the NO-cGMP pathway have been conducted as an investigation for treatment of HFpEF. Organic nitrates such as isosorbide mononitrate have been proved unable to lead a better quality of life or submaximal exercise capacity for patients with HFpEF (43). Furthermore, it may lead to hypotension due to excessive preload reduction and paradoxically cause endothelial dysfunction. In contrast to the organic nitrates that require aldehyde dehydrogenase and other enzymes for activation (44), there is no tolerance with nitrate–nitrite (23). Due to fewer side effects and enhanced pathway in the presence of hypoxia and acidosis, an increasing number of researchers are turning their attention to the field of inorganic nitrate/nitrite treatment for HFpEF. Although numerous trials have been conducted, the results have not been conclusive. This is why we conduct the current meta-analysis, and what we found may provide an answer for the current puzzle.
Exercise testing with ventilatory expired gas analysis has been acting as a valuable tool for assessing patients with heart failure (HF) (43–45). Peak exercise oxygen uptake (peak VO2), the standard for assessing cardiovascular fitness, plays an important role in prognosis and risk stratification among patients with chronic heart failure (CHF) (46–49). It is the gold-standard indicator of functional capacity and is depressed in patients with HFpEF (50–53). The elevated cardiac filling pressure in HFpEF result in reduction in peak VO2, promoting symptoms of dyspnea and limiting oxygen delivery, which ultimately impact exercise capacity. We therefore use peak VO2 as the primary indicator of exercise tolerance for patients in our study. Respiratory exchange ratio (RER), defined as the ratio of VCO2 to VO2, depends mostly on the skeletal muscle energy metabolism (54). In HF patients, overactivation of intramuscular ergoreceptors can induce excessive ventilatory response (i.e., hyperventilation), thereby yielding a reduced ventilatory efficiency with a higher RER even during submaximal exercise (55, 56). Reliable evidence suggested that high RER during exercise, particularly at anaerobic (AT) threshold workload, is associated with poor clinical outcome in HF patients (57). Consequently, the value of RER was used in our study as another important indicator to assess the exercise capacity. Finally, exercise time is the most intuitive indicator to evaluate patients’ exercise ability and the most important endpoint that intervention drugs need to target.
Our study set up three subgroup analyses based on RCT design, intervention drug, and intervention duration, respectively. We believe that such grouping can maximize the source of heterogeneity and minimize the inter-group heterogeneity, thus making the results more authentic. Considering that HFpEF is a chronic disease and has no self-healing tendency, many trials have applied cross-over design to better rule out the effects of confounding factors. Because the cross-over RCT possesses a higher level of evidence than the parallel-group RCT, it is necessary to separate the results from the two types of RCT. Then, although nitrate and nitrite both ultimately convert to NO through nitrate–nitrite–NO pathway (58–60), they are ingested in different ways. It is noteworthy that the ingested dietary NO3− needs to be reduced to bioactive NO2− by bacteria in the oral cavity before the NO2− is taken up by the plasma from the digestive system and eventually converted to NO. In previous studies, inorganic nitrate was conformably ingested orally in the form of concentrated nitrate-rich beetroot juice (BRJ) (21, 29, 31, 32), whereas inorganic nitrite was supplemented using direct nitrite infusion (25) or inhalation (28). Indeed, previous studies focusing on inorganic nitrite (25, 26, 30) trended to drawn more significant results than that of nitrate (29, 31, 32), probably due to the more direct ingesting way of NO2− than NO3− and the first pass metabolism during the conversion from oral NO3− to plasma NO2− (31). Therefore, nitrate and nitrite studies were analyzed separately to investigate whether there is difference in therapeutic effect between the two subtypes. Another factor that induced huge difference between studies was the duration of administration, generally divided into single dose (to observe the instantaneous effect) and repeated doses (to observe the continuous effect) in previous studies. In the study by Eggebeen et al. (31), 1 week of daily dose with BRJ improved submaximal aerobic endurance, whereas no significant effect was found for this outcome with a single, acute BRJ dose. It could be explained by that the acute effects of inorganic nitrate/nitrite may be due to its instantaneous impact on cardiac hemodynamics, yet the long-term effects should depend on its chronic improvement on microvascular function. This is why a subgroup analysis was performed according to whether the administration was single, acute dose or repeated, chronic dose.
To our knowledge, this is the first meta-analysis assessing the inorganic nitrate/nitrite supplementary therapy for HFpEF. Despite the wealth of data showing favorable effects of therapies targeting the inorganic nitrate/nitrite pathway in HFpEF, our study did not find a positive effect of this treatment in exercise capacity. What we found is consistent with a recently published multicenter trial by Borlaug et al. (28), which demonstrated that inhaled sodium nitrite did not improved the clinical status of patients with chronic HFpEF. Our results are also in agreement with several previous studies showing that a dietary nitrate intake in the form of BRJ did not improve exercise intolerance or hemodynamics indicators such as mean arterial pressure, heart rate, or cardiac output in HFpEF patients, although the concentration and duration of BRJ intervention in these studies varied (29, 31, 32). The reasons for the discrepancies between the rationale that nitric oxide possesses the ability of improving microcirculation and cardiac function in patients with HFpEF and the absence of clinical benefit are not clear. Reasonable explanations might be that the half-life of plasma nitrite is too short to maintain a sustained high level of plasma cGMP, whose deficiency has been repeatedly shown to play a key role in the pathogenesis of HFpEF (1, 41). In addition, as each intervention ultimately works through the conversion of the NO3−–NO2−–NO pathway to the final effector—nitric oxide, whether the interventions in the included studies have caused a sufficient increase in circulating nitric oxide is unknown. Furthermore, the duration of the trials studied were relatively brief (up to 4 weeks), which might not have allowed adequate exposure to observe a favorable effect on cardiovascular structure and function. It is worth mentioning that multiple trials with longer duration of administration targeting the inorganic nitrate/nitrite pathway in HFpEF are currently underway (NCT03015402, NCT02980068, NCT02918552, NCT02840799, NCT03289481, and NCT02713126). Results of these trials are expected to provide stronger evidence, which may alter the current finding.
Multiple earlier studies have suggested that inorganic nitrate or nitrite has the trend of lowering arterial blood pressure, especially during exercise (25, 26, 30–32). Our findings confirmed this notion and further demonstrated that the reduction in blood pressure induced by inorganic nitrate/nitrite in HFpEF was instantaneous rather than continuous. It is widely noted that systemic hypertension is highly prevalent in HFpEF. However, whether arterial stiffening is more specific to HFpEF or just common to all hypertensive patients is unknown. For this reason, two earlier studies compared HFpEF subjects with carefully matched hypertensive control subjects in the measurement of arterial stiffness (61, 62). As a result, they both demonstrated that the elevations of arterial pressure and blood flow were more apparent during exercise when HFpEF subjects were compared to hypertensive control subjects, despite lack of discernable difference in resting arterial afterload, revealing that arterial stiffening plays an important role in the pathophysiology of HFpEF, especially during exercise. However, a prior study exploring the blood-pressure-lowering effect of dietary nitrate showed that a 4-week dietary nitrate could provide a sustained BP lowering in patients with hypertension (63), which contradicts with our finding that a long-term BP lowering effect is absent with inorganic nitrate/nitrite supplementation. It must be pointed out that the BRJ the patients received in that study was equal to 6.4 mmol nitrate, a daily dose higher than that of any our included studies. In addition, the arterial hemodynamics characters of HFpEF are more complicated than that of hypertension alone. More long-term outcome studies are needed to be carried out to explore the effect of inorganic nitrate/nitrite on BP lowering in HFpEF patients.
Taken together, our results can only support the role of inorganic nitrate/nitrite as an adjunct in the treatment of HFpEF, which seems to declare another drug to be ineffective for HFpEF, again. However, this does not mean our study is meaningless. On the contrary, our research is of great value for it is the first meta-analysis to fully summarize the clinical effects of inorganic nitrate/nitrite on HFpEF. Before this, inorganic nitrate/nitrite administration has long been considered as a promising new therapy being tested in HFpEF. As noted above, although a considerable number of studies have tested inorganic nitrate/nitrite in HFpEF, the question of whether it improves exercise capacity of HFpEF patients remains unanswered. In addition, our study provides a new direction for inorganic nitrate/nitrite treatment on HFpEF, in which thinking about how to administer the drug may be more useful than thinking about whether the drug is effective. Because from our results effects of different administrations of inorganic nitrate/nitrite may be quite different. For example, studies demonstrating positive results all used intravenous or inhaled nitrite, yet those demonstrating negative results almost used oral nitrate (BRJ), which suggests that the rate of administration and the efficiency of absorption greatly influence the therapeutic effect of inorganic nitrate/nitrite on HFpEF. Therefore, although our results generally showed ineffectiveness of inorganic nitrate/nitrite on HFpEF, attempts of different administrations are encouraged, and the question of the usefulness of inorganic nitrate/nitrite treatment can only be answered after enough evidence-based trials with various administrations are conducted.
Our study has several limitations. First, since we only enrolled a total of eight studies, the number of studies for the combination of a single outcome was relatively limited, which limited the persuasiveness of the results to some extent. Second, as we tried to include all the studies assessing therapies targeting inorganic nitrate/nitrite pathway, there was no restriction on the type and duration of intervention, which increased heterogeneity to some extent. To minimize the heterogeneity, we correspondingly conduct subgroup analyses. Third, with only three trials (26, 31, 32) measuring plasma nitrate/nitrite concentrations before and after administration, our study failed to analyze the relationship between elevated plasma nitrate/nitrite levels and intervention outcomes. In other words, whether the negative results we obtained was caused by the fact that the intervention did not cause sufficient elevation of plasma nitrate/nitrite or that elevated plasma nitrate/nitrite did not improve the exercise capacity of patients with HFpEF is unknown from our study.
There is insufficient evidence to support the use of inorganic nitrate/nitrite for improving the exercise performance of patients with HFpEF at this time. But inorganic nitrite may yield a transient blood pressure lowering effect, especially during exercise. More prospective trials testing long-term effect of inorganic nitrate/nitrite therapy are warranted.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
FL and JZ: conceived the idea, data curation, and writing-original draft preparation. FL, JZ and YT: writing-review and editing. YT: supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1.Paulus, WJ, and Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. (2013) 62:263–71. doi: 10.1016/j.jacc.2013.02.092
2.Redfield, MM. Heart failure with preserved ejection fraction. N Engl J Med. (2017) 376:897. doi: 10.1056/NEJMc1615918
3.Kitzman, DW, Gardin, JM, Gottdiener, JS, Arnold, A, Boineau, R, Aurigemma, G, et al. Importance of heart failure with preserved systolic function in patients ≥65 years of age. Am J Cardiol. (2001) 87:413–9. doi: 10.1016/S0002-9149(00)01393-X
4.Owan, TE, Hodge, DO, Herges, RM, Jacobsen, SJ, Roger, VL, and Redfield, MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9. doi: 10.1056/NEJMoa052256
5.Kitzman, DW, Little, WC, Brubaker, PH, Anderson, RT, Hundley, WG, Marburger, CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. (2002) 288:2144–50. doi: 10.1001/jama.288.17.2144
6.Anker, SD, Butler, J, Filippatos, G, Ferreira, JP, Bocchi, E, Böhm, M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
7.Solomon, SD, McMurray, JJV, Anand, IS, Ge, J, Lam, CSP, Maggioni, AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381:1609–20. doi: 10.1056/NEJMoa1908655
8.Pfeffer, MA, Claggett, B, Assmann, SF, Boineau, R, Anand, IS, Clausell, N, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. (2015) 131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255
9.Borlaug, BA, Jaber, WA, Ommen, SR, Lam, CSP, Redfield, MM, and Nishimura, RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. (2011) 97:964–9. doi: 10.1136/hrt.2010.212787
10.Santos, M, Opotowsky Alexander, R, Shah Amil, M, Tracy, J, Waxman Aaron, B, and Systrom, DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension. Circ Heart Fail. (2015) 8:278–85. doi: 10.1161/CIRCHEARTFAILURE.114.001551
11.Andersen Mads, J, Olson Thomas, P, Melenovsky, V, Kane Garvan, C, and Borlaug, BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. (2015) 8:41–8. doi: 10.1161/CIRCHEARTFAILURE.114.001731
12.Michalska-Kasiczak, M, Bielecka-Dabrowa, A, von Haehling, S, Anker, SD, Rysz, J, and Banach, M. Biomarkers, myocardial fibrosis and co-morbidities in heart failure with preserved ejection fraction: an overview. Arch Med Sci. (2018) 14:890–909. doi: 10.5114/aoms.2018.76279
13.Maeder, MT, Thompson, BR, Brunner-La Rocca, H-P, and Kaye, DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. (2010) 56:855–63. doi: 10.1016/j.jacc.2010.04.040
14.Dhakal Bishnu, P, Malhotra, R, Murphy Ryan, M, Pappagianopoulos Paul, P, Baggish Aaron, L, Weiner Rory, B, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ Heart Fail. (2015) 8:286–94. doi: 10.1161/CIRCHEARTFAILURE.114.001825
15.van Empel, VP, Mariani, J, Borlaug, BA, and Kaye, DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. (2014) 3:e001293. doi: 10.1161/JAHA.114.001293
16.Neumann, FJ, Jander, N, Kienzle, R-P, Hochholzer, W, Zeh, W, Dorfs, S, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. (2014) 35:3103–12. doi: 10.1093/eurheartj/ehu315
17.Haykowsky, MJ, Kouba, EJ, Brubaker, PH, Nicklas, BJ, Eggebeen, J, and Kitzman, DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. (2014) 113:1211–6. doi: 10.1016/j.amjcard.2013.12.031
18.Kream, RM, and Stefano, GB. Interactive effects of endogenous morphine, nitric oxide, and ethanol on mitochondrial processes. Arch Med Sci. (2010) 6:658–62. doi: 10.5114/aoms.2010.17077
19.Haykowsky, MJ, Brubaker, PH, Morgan, TM, Kritchevsky, S, Eggebeen, J, and Kitzman, DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol Ser A. (2013) 68:968–75. doi: 10.1093/gerona/glt011
20.van Heerebeek, L, Hamdani, N, Falcão-Pires, I, Leite-Moreira Adelino, F, Begieneman Mark, PV, Bronzwaer Jean, GF, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. (2012) 126:830–9. doi: 10.1161/CIRCULATIONAHA.111.076075
21.Zamani, P, Rawat, D, Shiva-Kumar, P, Geraci, S, Bhuva, R, Konda, P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. (2015) 131:371–80. doi: 10.1161/CIRCULATIONAHA.114.012957
22.Cosby, K, Partovi, KS, Crawford, JH, Patel, RP, Reiter, CD, Martyr, S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. (2003) 9:1498–505. doi: 10.1038/nm954
23.Dejam, A, Hunter, CJ, Tremonti, C, Pluta, RM, Hon, YY, Grimes, G, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. (2007) 116:1821–31. doi: 10.1161/CIRCULATIONAHA.107.712133
24.Abudiab, MM, Redfield, MM, Melenovsky, V, Olson, TP, Kass, DA, Johnson, BD, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. (2013) 15:776–85. doi: 10.1093/eurjhf/hft026
25.Borlaug, BA, Koepp, KE, and Melenovsky, V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. (2015) 66:1672–82. doi: 10.1016/j.jacc.2015.07.067
26.Borlaug Barry, A, Melenovsky, V, and Koepp, KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. (2016) 119:880–6. doi: 10.1161/CIRCRESAHA.116.309184
27.Simon, MA, Vanderpool, RR, Nouraie, M, Bachman, TN, White, PM, Sugahara, M, et al. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight. (2016) 1:e89620. doi: 10.1172/jci.insight.89620
28.Borlaug, BA, Anstrom, KJ, Lewis, GD, Shah, SJ, Levine, JA, Koepp, GA, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical TrialEffect of inorganic nitrite on exercise capacity in HFpEFEffect of inorganic nitrite on exercise capacity in HFpEF. JAMA. (2018) 320:1764–73. doi: 10.1001/jama.2018.14852
29.Londono-Hoyos, F, Zamani, P, Beraun, M, Vasim, I, Segers, P, and Chirinos, JA. Effect of organic and inorganic nitrates on cerebrovascular pulsatile power transmission in patients with heart failure and preserved ejection fraction. Physiol Meas. (2018) 39:044001. doi: 10.1088/1361-6579/aab2ef
30.Reddy, YNV, Andersen, MJ, Obokata, M, Koepp, KE, Kane, GC, Melenovsky, V, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. (2017) 70:136–48. doi: 10.1016/j.jacc.2017.05.029
31.Eggebeen, J, Kim-Shapiro, DB, Haykowsky, M, Morgan, TM, Basu, S, Brubaker, P, et al. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. (2016) 4:428–37. doi: 10.1016/j.jchf.2015.12.013
32.Shaltout, HA, Eggebeen, J, Marsh, AP, Brubaker, PH, Laurienti, PJ, Burdette, JH, et al. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide. (2017) 69:78–90. doi: 10.1016/j.niox.2017.05.005
33.Furlan, AD, Pennick, V, Bombardier, C, and van Tulder, M, from the Editorial Board of the Cochrane Back Review G. 2009 updated method guidelines for systematic reviews in the Cochrane Back review group. Spine. (2009) 34:1929–41. doi: 10.1097/BRS.0b013e3181b1c99f
34.Balshem, H, Helfand, M, Schünemann, HJ, Oxman, AD, Kunz, R, Brozek, J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
35.Higgins, JPT, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
36.Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
37.Guyatt, GH, Oxman, AD, Sultan, S, Glasziou, P, Akl, EA, Alonso-Coello, P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. (2011) 64:1311–6. doi: 10.1016/j.jclinepi.2011.06.004
38.Lam, CSP, Roger, VL, Rodeheffer, RJ, Borlaug, BA, Enders, FT, and Redfield, MM. Pulmonary hypertension in heart failure with preserved ejection fraction. J Am Coll Cardiol. (2009) 53:1119–26. doi: 10.1016/j.jacc.2008.11.051
39.Lam, CSP, and Brutsaert, DL. Endothelial dysfunction. J Am Coll Cardiol. (2012) 60:1787–9. doi: 10.1016/j.jacc.2012.08.004
40.Lam, CSP, Lim, SL, Brutsaert, DL, De Keulenaer, GW, and Segers, VFM. Cardiac endothelium–myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur Heart J. (2015) 36:2050–60. doi: 10.1093/eurheartj/ehv132
41.Shah Sanjiv, J, Kitzman Dalane, W, Borlaug Barry, A, van Heerebeek, L, Zile Michael, R, Kass David, A, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation. (2016) 134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884
42.Kitzman, DW, Brubaker, P, Morgan, T, Haykowsky, M, Hundley, G, Kraus, WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical TrialDiet vs exercise in obese older patients with HFPEFDiet vs exercise in obese older patients with HFPEF. JAMA. (2016) 315:36–46. doi: 10.1001/jama.2015.17346
43.Redfield, MM, Anstrom, KJ, Levine, JA, Koepp, GA, Borlaug, BA, Chen, HH, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. (2015) 373:2314–24. doi: 10.1056/NEJMoa1510774
44.Münzel, T, Daiber, A, and Gori, T. Nitrate therapy. Circulation. (2011) 123:2132–44. doi: 10.1161/CIRCULATIONAHA.110.981407
45.Ramos, RP, Alencar, MC, Treptow, E, Arbex, F, Ferreira, EM, and Neder, JA. Clinical usefulness of response profiles to rapidly incremental cardiopulmonary exercise testing. Pulm Med. (2013) 2013:359021. doi: 10.1155/2013/359021
46.Jessup, M, Abraham, WT, Casey, DE, Feldman, AM, Francis, GS, Ganiats, TG, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. J Am Coll Cardiol. (2009) 53:1343–82. doi: 10.1016/j.jacc.2008.11.009
47.Szlachcic, J, Masse, BM, Kramer, BL, Topic, N, and Tubau, J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. (1985) 55:1037–42. doi: 10.1016/0002-9149(85)90742-8
48.Stevenson Lynne, W, Couper, G, Natterson, B, Fonarow, G, Hamilton Michele, A, Woo, M, et al. Target heart failure populations for newer therapies. Circulation. (1995) 92:174–81. doi: 10.1161/01.CIR.92.9.174
49.Opasich, C, Pinna, GD, Bobbio, M, Sisti, M, Demichelis, B, Febo, O, et al. Peak exercise oxygen consumption in chronic heart failure: toward efficient use in the individual patient. J Am Coll Cardiol. (1998) 31:766–75. doi: 10.1016/S0735-1097(98)00002-3
50.Swank Ann, M, Horton, J, Fleg Jerome, L, Fonarow Gregg, C, Keteyian, S, Goldberg, L, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients. Circ Heart Fail. (2012) 5:579–85. doi: 10.1161/CIRCHEARTFAILURE.111.965186
51.Mudge, GH, Goldstein, S, Addonizio, LJ, Caplan, A, Mancini, D, Levine, TB, et al. Task force 3: recipient guidelines/prioritization. J Am Coll Cardiol. (1993) 22:21–31. doi: 10.1016/0735-1097(93)90812-F
52.Guazzi, M, Myers, J, and Arena, R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. (2005) 46:1883–90. doi: 10.1016/j.jacc.2005.07.051
53.Reddy, YNV, Olson, TP, Obokata, M, Melenovsky, V, and Borlaug, BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. (2018) 6:665–75. doi: 10.1016/j.jchf.2018.03.003
54.Clark, AL, Poole-Wilson, PA, and Coats, AJS. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. (1996) 28:1092–102. doi: 10.1016/S0735-1097(96)00323-3
55.Okita, K, Yonezawa, K, Nishijima, H, Hanada, A, Ohtsubo, M, Kohya, T, et al. Skeletal muscle metabolism limits exercise capacity in patients with chronic heart failure. Circulation. (1998) 98:1886–91. doi: 10.1161/01.CIR.98.18.1886
56.Balady Gary, J, Arena, R, Sietsema, K, Myers, J, Coke, L, Fletcher Gerald, F, et al. Clinician’s guide to cardiopulmonary exercise testing in adults. Circulation. (2010) 122:191–225. doi: 10.1161/CIR.0b013e3181e52e69
57.Sacrez, A, Roul, G, Sacrez, J, Mossard, JM, Moulichon, ME, Bareiss, P, et al. Exercise peak VO2 determination in chronic heart failure: is it still of value? Eur Heart J. (1994) 15:495–502. doi: 10.1093/oxfordjournals.eurheartj.a060533
58.Lundberg, JO, Weitzberg, E, and Gladwin, MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. (2008) 7:156–67. doi: 10.1038/nrd2466
59.Shiva, S, Huang, Z, Grubina, R, Sun, J, Ringwood Lorna, A, MacArthur Peter, H, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. (2007) 100:654–61. doi: 10.1161/01.RES.0000260171.52224.6b
60.Kim-Shapiro, DB, and Gladwin, MT. Mechanisms of nitrite bioactivation. Nitric Oxide. (2014) 38:58–68. doi: 10.1016/j.niox.2013.11.002
61.Melenovsky, V, Borlaug, BA, Rosen, B, Hay, I, Ferruci, L, Morell, CH, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community. J Am Coll Cardiol. (2007) 49:198–207. doi: 10.1016/j.jacc.2006.08.050
62.Desai, AS, Mitchell, GF, Fang, JC, and Creager, MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. (2009) 15:658–64. doi: 10.1016/j.cardfail.2009.03.006
Keywords: heart failure with preserved ejection fraction (HFpEF), inorganic nitrate, inorganic nitrite, meta-analysis,
Citation: Lv F, Zhang J and Tao Y (2023) Efficacy and safety of inorganic nitrate/nitrite supplementary therapy in heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 10:1054666. doi: 10.3389/fcvm.2023.1054666
Received: 25 October 2022; Accepted: 12 January 2023;
Published: 02 February 2023.
Edited by:
Alexander E. Berezin, Zaporizhia State Medical University, UkraineReviewed by:
Filippos Triposkiadis, University of Thessaly, GreeceCopyright © 2023 Lv, Zhang and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Tao, ✉ dGFveXVhbnl1YW4wNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.