- 1Department of Ultrasound, Beijing Anzhen Hospital, Beijing Institute of Heart, Lung, and Blood Vessel Diseases, Capital Medical University, Beijing, China
- 2Department of Rheumatology and Immunology, Beijing Anzhen Hospital, Beijing Institute of Heart, Lung, and Blood Vessel Diseases, Capital Medical University, Beijing, China
Objective: The goal of this study is to use superb microvascular imaging (SMI) to observe neovascularization in the carotid vessel wall to identify potential Takayasu’s arteritis (TAK) inflammation markers.
Methods: Bilateral carotid arteries from 96 patients with TAK were imaged by a Doppler ultrasound and SMI. The one-way analysis of variance (ANOVA) was used to document significant differences between the activity and inactivity stages of TAK and the factors closely related to its activity in the binary logistics regression equation. Clinical and laboratory data included age, gender, duration of disease, treatment history, NIH score, erythrocyte sedimentation rate (ESR), and high-sensitivity C-reactive protein. Imaging data included the arterial wall thickness, degree of lesion, SMI grade, and arterial aneurysm formation.
Results: There were 45 patients in the active TAK stage and 51 in the inactive stage. The one-way ANOVA showed significant differences in SMI (p = 0.001) and ESR (p = 0.022) between the active and inactive groups. The binary logistics regression analysis showed that SMI was an independent risk factor for TAK activity (B = −1.505, S.E = 0.340, Wald = 19.528, OR = 0.222 95%, CI = 0.114–0.433, p < 0.01). Using SMI G1 or G2 as the cutoff values for the diagnosis of active TAK, the positive predictive value, sensitivity, and specificity were 60 and 86%, 84% and 56%, and 54% and 92%, respectively.
Conclusion: The SMI grade is a potential marker of disease activity in patients with TAK.
Introduction
Takayasu’s arteritis (TAK) is a chronic, nonspecific, large vessel vasculitis primarily affecting the aorta and its major branches, and it occurs relatively often in younger women (age < 50 years) (1, 2). Contrast-enhanced ultrasound (CEUS) can semi-quantitatively evaluate the formation of carotid wall angiogenesis. Therefore, it has been proposed that ultrasonographic imaging of microbubble development in thickened carotid walls may be useful for the assessment of carotid inflammation in TAK (3, 4). Recently, Toshiba developed an innovative Doppler ultrasound technology called superb microvascular imaging (SMI) using the Aplio™ 500 ultrasound system (Toshiba Medical Systems Corporation, Tochigi, Japan), enabling the visualization of slow-flow vessels without the need for a contrast medium (5, 6). Previous work has shown that SMI is well correlated and consistent with CEUS in its ability to detect plaque neovascularization (7–9). Microvascular hyperplasia is the most characteristic pathological change in the active TAK. Therefore, the goal of this study is to use contrast-free SMI imaging to observe tiny blood vessels that proliferate in the vessel wall to identify potential imaging markers reflecting TAK active inflammation.
Materials and methods
Study participants
We prospectively studied patients with TAK who were in-patients of the rheumatology department of the Beijing Anzhen Hospital from October 2015 to September 2021. The study was approved by the institutional review board and all enrolled patients provided written informed consent. A total of 96 patients with a clinical diagnosis of TAK with carotid artery involvement, based on physical examinations, laboratory studies, and surgical pathologic examinations, were evaluated using vascular sonography. Some patients underwent computed tomographic angiography, magnetic resonance imaging (MRI), and other imaging examinations. The diagnosis of TAK was made using the 1990 standard for TAK from the American College of Rheumatology (10), the Antineutrophil Cytoplasmic Antibody 2012 Workshop on Takayasu Arteritis (1), and the revised nomenclature for vasculitis from the 2012 International Chapel Hill Consensus Conference (11).
Carotid ultrasonography
All patients received standard vascular ultrasound examination of the bilateral subclavian, carotid, and vertebral arteries within 24 h of admission using a 4–9 MHz linear-array transducer with SMI capabilities (Aplio 500, Toshiba Medical, Tokyo, Japan). Measurements were recorded by two well-trained technicians who were blinded to all information about the study participants.
The SMI classification
The SMI classification was made based on the CEUS classification standard previously described by Deyama et al. (12). Neovascularization within the carotid wall was identified as continuous or discontinuous short lines, dots, or short rod-shaped, medium to strong, echogenic and was graded as G0 = no visible short lines, dots, or short rod-shaped, medium to strong, echogenic within the wall; G1 = moderate visible short lines, dots, or short rod-shaped, medium to strong, echogenic within the wall; G2 = extensive visible short lines, dots or short rod-shaped, medium to strong, echogenic within the wall. For each patient, the carotid wall with the highest grade of neovascularization was selected for the majority of the analyses.
Clinical data collection
Baseline date
Clinical data included age, gender, duration of disease, pharmacologic treatment history (hormones and/or immunosuppressants), and the NIH score. Ultrasound data included the SMI grade, thickness of the involved arterial wall (The typical “macaroni” sign in transverse section consists of three layers of “strong - medium (low) - strong” echo. The vertical distance between the outer edges of two strong echo lines at the thickest part of the vessel wall was measured), and degree of lesion (1 = concentric thickening of the carotid wall, 2 = stenosis of lumen, 3 = occlusion of the lumen).
Laboratory data
The erythrocyte sedimentation rate (ESR) and high-sensitivity C-reactive protein (hsCRP) levels were measured and recorded during the ultrasound examination. ESR was measured using the modified Westergren method and the hsCRP levels were measured using immunofluorescence turbidimetry (DiaSys Diagnostic Systems GmbH, Alte Strasse 9, 65,558, Holzheim, Germany). The normal range for ESR was defined as 0–15 mm/h for male patients and 0–20 mm/h for female patients. Elevated hsCRP was defined as >5 mg/L.
Criteria of active TAK
The criteria for patients with TAK in the active stage were as follows (13): among patients with TAK, two or more of the following four factors indicated that the disease had aggravated and was active: (1) systemic manifestations, such as fever and joint and muscle involvement (except for other reasons); (2) manifestations of vascular inflammation or ischemia, such as intermittent claudication, diminished or absent pulse, vascular murmur, vascular pain, or bilateral upper or lower limb blood pressure asymmetry; (3) increased ESR and/or hsCRP levels, excluding active infection; (4) typical angiographic abnormality.
NIH score
The NIH scores were based on the National Institutes of Health (NIH) criteria (13). The disease status of patients with TAK was determined to be a score of 1 on the NIH scale if they had one of the features of the criteria, and it was determined to be a score of 2 on the NIH scale if they met two of the above criteria, and so on in the same way.
Statistical methods
The statistical software package SPSS 22.0 (IBM, Armonk, United States) was used for data analysis. The data were summarized as mean ± standard deviation (SD) and median (interquartile range) for continuous variables and the number of subjects (percent) for categorical variables. The one-way analysis of variance (ANOVA) was used to record the significant differences between the two groups and the factors closely related to TAK activity into the logistics regression equation. The stepwise forward method was used to screen the variables, and p < 0.05 was considered statistically significant.
Results
Patient characteristics
There were 45 patients in the active TAK stage and 51 in the inactive stage. Those 96 patients with TAK underwent ultrasonic scanning, and the findings were compared to clinical characteristics and laboratory values (Table 1). Mean of ESR were higher in the active TAK compared to the inactive TAK group [13.11 (SD: 16.72) vs. 6.84 (SD: 4.44), p = 0.022, respectively]. Median SMI grades were higher in the active TAK compared to the inactive TAK group [2 (IQR: 1–2) vs. 0 (IQR: 0–1), p = 0.001, respectively]. The binary logistics regression analysis showed that SMI was an independent risk factor for TAK activity (B = 1.564, S.E = 0.355, Wald = 19.436, OR = 4.778, 95% CI = 2.384–9.576, p < 0.01). The SMI G1 or G2 were used as the cut-off values for the diagnosis of the active TAK stage, and the diagnostic efficacy is shown in Table 2.
Imaging characteristics of carotid ultrasound
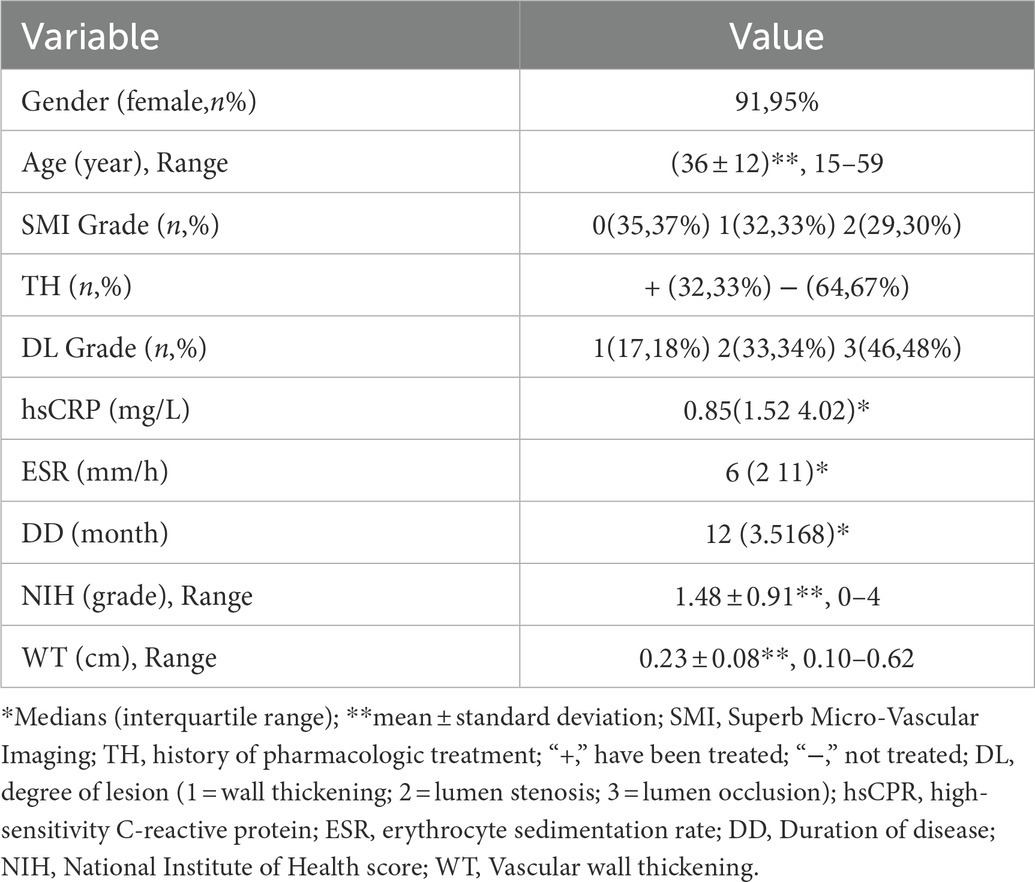
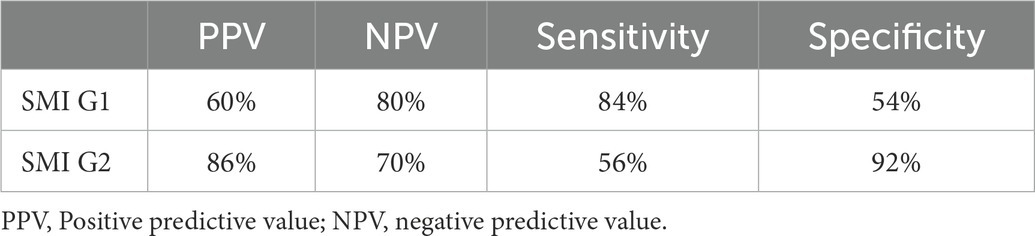
Artery walls with concentric thickening, narrowing of the vascular diameter, and stenosis or occlusion of the lumen could be observed by carotid ultrasound (Figures 1, 2). In addition, carotid arterial aneurysms were readily observed (Figure 1D). SMI was able to display low blood flow signals in the lumen (Figures 1E,F, 2C,D) and concentric thickened walls of affected carotids (Figures 1B–D) that color Doppler could not. SMI can observe the collateral flow (the meandering, twisted, narrow blood flow signals) in the thickened vessel walls (Figures 2E,F). SMI improved image quality, enhanced the vessel wall and lumen, and gave a better definition of the borders of the vascular lesion (Figures 2A,B). Furthermore, we observed moving bright spots and linear flow within the vascular lesions that were principally visualized on the adventitial side of the vessels (Figure 1C), and we believe these signals represent neovessels of the neovascularization that could be visualized by SMI, only 55% could be visualized using color Doppler.

Figure 1. The superb microvascular imaging (SMI) of the carotid artery. (A) SMI G0, no visible short lines, dots, or short sticks of medium-high echogenic within the wall of the carotid artery (Left: Transverse section, Right: Longitudinal section). The asterisk marks the bloodstream in the carotid artery canal. The arrow shows the thickened wall of the carotid artery. (B) SMI G1, moderate visible short lines, dots, or short sticks of medium-high echogenic within the wall. The arrow shows neovessels in the thickened wall of the carotid artery (Left: Transverse section, right: Longitudinal section). (C) SMI G2, extensive visible short lines, dots, or short sticks of medium-high echogenic within the wall of the carotid artery. The arrow shows neovessels in the thickened wall of the carotid artery (Left: Transverse section, right: Longitudinal section). (D) Carotid lumen expansion and aneurysm formation (Left: Transverse section, Right: Longitudinal section). The asterisk marks the bloodstream in the carotid artery canal. The arrow shows neovessels in the thickened wall of the carotid artery aneurysm. (E) Color Doppler flow imaging showing carotid stenosis and segmental occlusion. (F) In contrast with (E), SMI was able to clearly show low blood flow signals in the same carotid stenosis and segmental occlusion cavities.
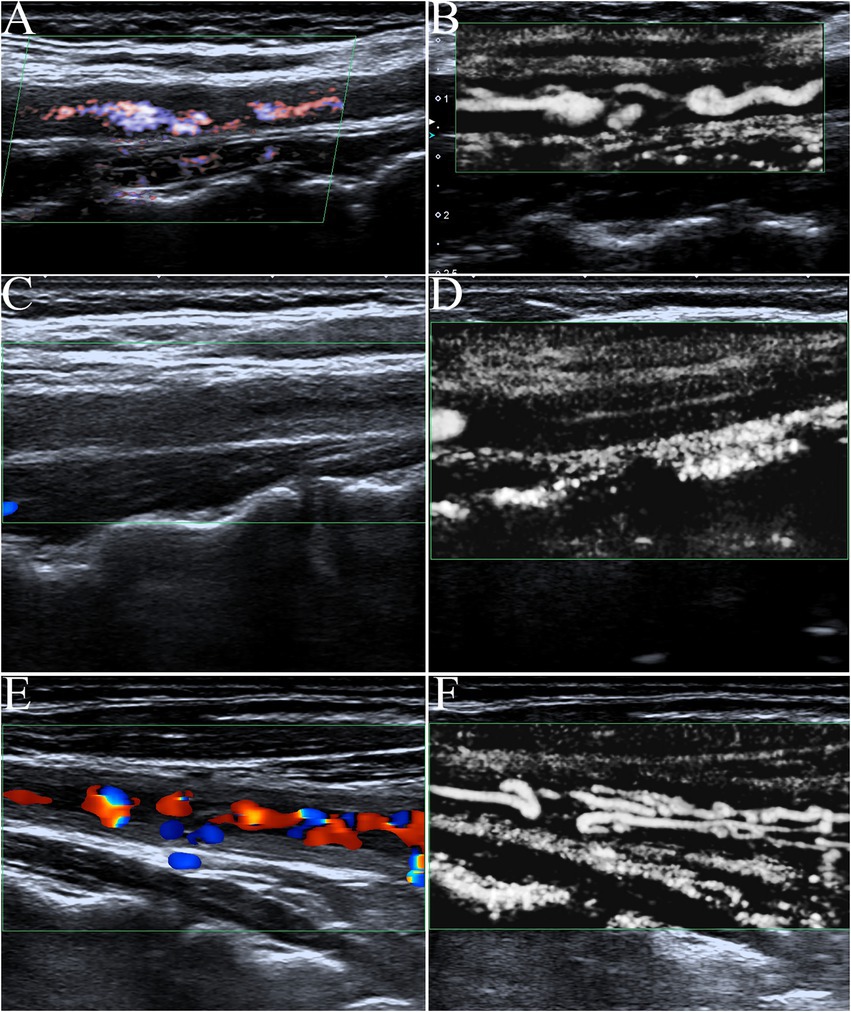
Figure 2. The contrast of color Doppler ultrasonography and SMI in blood flow display. (A) Advanced dramatic flow imaging showing carotid stenosis. (B) In contrast with (A), SMI was able to clearly show low blood flow signals in the same carotid stenosis cavities. SMI improved image quality with a greater enhancement of vessel wall lumen and higher definition of the borders of the vascular lesion. (C) The affected carotid artery wall had varying degrees of diffuse thickening like macaroni signs, and color Doppler flow imaging showed occlusion of the carotid lumen. (D) In contrast with (C), SMI could display very low blood flow signals in the lumen of the occluded carotid (fine lines) that color Doppler could not. (E) Color Doppler flow imaging showed intermittent, meandering, tortuous, and narrow blood flow signals in a fuzzy carotid lumen. (F) In contrast with (C), SMI could clearly show the again unobstructed, meandering, tortuous, and narrow blood flow signals in the thickened vessel walls of the same carotid artery as twining wool.
Discussion
In this study, we investigated 96 patients to study the carotid SMI in evaluating the activity of TAK. The statistical analysis showed that the SMI and ESR had significant difference between the active and inactive groups. The median of SMI and ESR in the active group were significantly higher than those in the inactive group. And more importantly, SMI was an independent risk factor for TAK activity(OR = 4.778), indicating it as a potential marker of disease activity for patients.
TAK is a chronic, nonspecific, large vessel vasculitis with a global distribution but increased incidence in Asian ethnicities and higher morbidity in Asia (2, 14). It has been proposed that cellular immunity directed against vascular endothelial cells drives the inflammatory damage to large vessels, resulting in wall thickening, stenosis, occlusions, dilatations, and aneurysms (15). The newly formed feeding arteries in the wall of the diseased segment are closely related to the inflammatory response in the active stage (3). Whether TAK is in the active stage or not is important for clinical treatment. The active stage means that clinical treatment should be provided to control the inflammatory process. Vessel wall changes in inflammatory diseases could be evaluated using arterial angiography, computerized tomography (CT) scan, MRI, positron emission tomography (PET), and combined PET–CT scan (16). Higher disease activity in TAK does not translate into more severe vascular damage (17). However, previous imaging examinations focused more on evaluating the extent of vascular damage and involvement, and ignored the neovascularization in active inflammatory lesions tube wall. Using Duplex ultrasonography means that the resolution is theoretically superior, at least at high ultrasound frequencies (18–20). Duplex ultrasonography may be used for frequent controls due to ease of use, lack of radiation, and low costs.
SMI is a new ultrasound imaging technology developed based on color Doppler. It uses intelligent filtering technology, only filters out clutter, identifies and retains small blood vessels (diameter ≥ 0.1 mm) at very low velocities (minimum 0.8 cm/s), and is almost unaffected by angle. In the case of low velocity and high gain, it can still maintain the characteristics of less blood overflow and show the microvascular structure more clearly and completely. The existing Doppler modes are unable to distinguish motion artifacts from actual blood flow. With SMI, it is possible to analyze the characteristics of such motion artifacts and extract only the clinically relevant information (13). Compared with conventional Doppler techniques, SMI offers better detail resolution, faster frame rates, less clutter, and fewer flash artifacts (5, 12). In this study, the ability of color Doppler to display microvessels in the lesion was significantly lower than that of SMI.
Contrast-enhanced ultrasound can dynamically display the microvascular perfusion information of tissues in real-time, which is the “gold standard” for displaying the microvessels flow of organs. However, CEUS can only show the blood flow of the lesion during the filling time of the contrast agent (about 5–8 min), and there is a very short time limit for the filling and regression of the contrast agent. Therefore, CEUS cannot comprehensively display the microflow of extensive diffuse lesions in a sufficient time. SMI examination without the need for a contrast medium is not limited by filling time and phase, which ensures a detailed and comprehensive observation of the microflow of all diseased vessel walls. Moreover, SMI is significantly more economical and feasible than CEUS.
SMI is increasingly being used for vascular imaging indications (21, 22). In this study, our ability to detect neovascularization in the thickened vessel wall of patients with TAK using SMI technology was similar to previous reports (Magnoni M, Schinkel AF) that used contrast-enhanced ultrasound (3, 4). Currently, ESR and hsCPR are often used to assess aortic disease activity, but studies have shown that ESR and hsCPR can be normal in patients in the active stage. Therefore, a normal ESR does not represent the extinction of vascular wall inflammation and the cessation of lesions, and both values are non-specific markers of inflammation, lacking specificity for the evaluation of vascular inflammation (23–26). Studies have shown that the evaluation of vascular inflammation using CEUS is more sensitive than acute phase reactants (27). The results of our study showed that feeding vessels in the wall of the involved carotid artery detected using SMI were an independent risk factor for predicting the active stage of TAK. This is consistent with the pathophysiological changes of proliferation and expansion of nutrient vessels in active TAK and the results of previous studies (28–30).
In Sato W’s study, arterial wall vascularization detected using SMI predicted the fluorodeoxyglucose (FDG) uptake with 100% sensitivity, 97% specificity, 83% positive predictive value, and 100% negative predictive value (n = 9,54 sites). Therefore, it was concluded that carotid artery hypervascularity detected using SMI is a potential marker of disease activity in patients with TAK (31). In our study, the SMI G1 and G2 were used as cut-off values to diagnose active TAK. The diagnostic efficacy was lower than that of the above studies because the control criterion was clinical NIH ≥ 2 scores, rather than the FDG uptake. In theory, SMI is consistent with the FDG uptake in detecting vessel wall vascularization. SMI allows the dynamic assessment of carotid wall vascularization, which is a potential marker of disease activity in patients with TAK.
This study has several limitations. First,the SMI technique used to evaluate neovascularization in the vessel walls of patients with TAK was not compared to blank control and other gold standard methods. MRI or contrast-enhanced ultrasound should be used for direct comparison with SMI in future studies. In addition, SMI-based diagnosis of vessel wall neovascularization was semi-quantitative instead of quantitative, which introduced subjectivity to the data analysis pipeline. The sensitivity and specificity concluded from this small sample-size study remained further testify in clinic. Finally, the only SMI technology utilized was the Toshiba ultrasound system. However, SMI technology can indeed detect the proliferation of tiny vessels with smaller inner diameter and slower flow that color Doppler cannot detect.
Conclusion
In summary, SMI was an independent risk factor for TAK activity, indicating it as a potential marker of disease activity for patients with TAK.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Anzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
F-JL and W-PC: conception and design of the research, analysis and interpretation of the data, and critical revision of the manuscript for intellectual content. F-JL and YC: acquisition of data and statistical analysis. F-JL: writing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SMI, Superb microvascular imaging; TAK, Takayasu’s arteritis; ANOVA, One-way analysis of variance; ESR, Erythrocyte sedimentation rate; CEUS, Contrast-enhanced ultrasound; MRI, Magnetic resonance imaging; hsCRP, High-sensitivity C-reactive protein; NIH, National Institutes of Health; SD, Standard deviation.
References
1. Isobe, M. The Asia Pacific meeting on vasculitis and ANCA 2012 workshop on Takayasu arteritis: advances in diagnosis and medical treatment. Clin Exp Nephrol. (2013) 17:686–9. doi: 10.1007/s10157-012-0697-0
2. de Souza, AW, and de Carvalho, JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun. (2014) 48-49:79–83. doi: 10.1016/j.jaut.2014.01.012
3. Magnoni, M, Dagna, L, Coli, S, Cianflone, D, Sabbbadini, MG, and Maseri, A. Assessment of Takayasu arteritis activity by carotid contrast-enhanced ultrasound. Circ Cardiovasc Imaging. (2011) 4:e1–2. doi: 10.1161/CIRCIMAGING.110.960906
4. Schinkel, AF, van den Oord, SC, van der Steen, AF, van Laar, JA, and Sijbrands, EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. (2014) 15:541–6. doi: 10.1093/ehjci/jet243
5. O’Hara, S. Toshiba Medical System. Superb Micro-vascular Imaging (SMI). (2015). Available at: http://medical.toshiba.com/products/ul/general/aplio-500/clinical-applications (Accessed 23 March 2015).
6. Machado, P, and Forsberg, F. Toshiba Medical System. Medical Review Initial experience with a novel microvascular flow imaging technique. (2015). Available at: http://www.toshibamedicalsystems.com (Accessed 23 March 2015)
7. Xie, X, Bai, ZY, Liu, Y, and Zhang, HB. Value of superb micro-vascular imaging in diagnosing carotid artery vulnerable plaque. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (2018) 40:444–9. doi: 10.3881/j.issn.1000-503X.10424
8. Oura, K, Kato, T, Ohba, H, and Terayama, Y. Evaluation of Intraplaque neovascularization using superb microvascular imaging and contrast-enhanced ultrasonography. J Stroke Cerebrovasc Dis. (2018) 27:2348–53. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.023
9. Zhang, HX, Du, JW, Wang, H, Wang, HL, Jiang, JH, Zhao, JJ, et al. Comparison of diagnostic values of ultrasound micro-flow imaging and contrast-enhanced ultrasound for neovascularization in carotid plaques. Exp Ther Med. (2017) 14:680–8. doi: 10.3892/etm.2017.4525
10. Arend, WP, Michel, BA, Bloch, DA, Hunder, GG, Calabrese, LH, Edworthy, SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. (1990) 33:1129–34.
11. Jennette, JC, Falk, RJ, and Bacon, PA. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. (2013) 65:1–11. doi: 10.1002/art.37715
12. Deyama, J, Nakamura, T, Takishima, I, Fujioka, D, Kawabata, KI, Obata, JE, et al. Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J. (2013) 77:1499–507. doi: 10.1253/circj.CJ-12-1529
13. Kerr, GS, Hallahan, CW, Giordano, J, Leavitt, RY, Fauci, AS, Rottem, M, et al. Takayasu arteritis. Ann Intern Med. (1994) 120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004
14. Aydin, SZ, Merkel, PA, and Direskeneli, H. Outcome measures for Takayasu's arteritis. Curr Opin Rheumatol. (2015) 27:32–7. doi: 10.1097/BOR.0000000000000129
15. Seko, Y, Minota, S, Kawasaki, A, Shinkai, Y, Maeda, K, Yagita, H, et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu's arteritis. J Clin Invest. (1994) 93:750–8. doi: 10.1172/JCI117029
16. Ammirati, E, Moroni, F, Pedrotti, P, Scotti, I, Magnoni, M, Bozzolo, EP, et al. Non-invasive imaging of vascular inflammation. Front Immunol. (2014) 5:399. doi: 10.3389/fimmu.2014.00399
17. Karabacak, M, Kaymaz-Tahra, S, Şahin, S, Yıldız, M, Adroviç, A, Barut, K, et al. Childhood-onset versus adult-onset Takayasu arteritis: a study of 141 patients from Turkey. Semin Arthritis Rheum. (2021) 51:192–7. doi: 10.1016/j.semarthrit.2020.10.013
18. Czihal, M, Zanker, S, Rademacher, A, Tatò, F, Kuhlencordt, PJ, Schulze-Koops, H, et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol. (2012) 41:231–6. doi: 10.3109/03009742.(2011).641581
19. Schmidt, WA, and Gromnica-Ihle, E. What is the best approach to diagnosing large-vessel vasculitis? Best Pract Res Clin Rheumatol. (2005) 19:223–42. doi: 10.1016/j.berh.2005.01.006
20. Schmidt, WA. Role of ultrasound in the understanding and management of vasculitis. Ther Adv Musculoskelet Dis. (2014) 6:39–47. doi: 10.1177/1759720X13512256
21. Durmaz, MS, and Sivri, M. Comparison of superb micro-vascular imaging (SMI) and conventional Doppler imaging techniques for evaluating testicular blood flow. J Med Ultrasound. (2018) 45:443–52. doi: 10.1007/s10396-017-0847-9
22. Park, AY, Seo, BK, Cha, SH, Yeom, SK, Lee, SW, and Chung, HH. An innovative ultrasound technique for evaluation of tumor vascularity in breast cancers: superb micro-vascular imaging. J Breast Cancer. (2016) 19:210–3. doi: 10.4048/jbc.2016.19.2.210
23. Holly, L, Woodland, N, Hung, WT, Cordatos, K, and Reuben, A. Influence of fibrinogen and haematocrit on erythrocyte sedimentation kinetics. Biorheology. (1999) 36:287–97.
24. Dhingra, R, Gona, P, Nam, BH, D’Agostino, RB Sr, Wilson, PWF, Benjamin, EJ, et al. C-reactive protein, inflammatory condition, and cardiovascular disease risk. Am J Med. (2007) 120:1054–62. doi: 10.1016/j.amjmed.2007.08.037
25. Sato, EI, Lima, DN, Santo, BE, and Hata, F. Takayasu’s arteritis treatment and prognosis in a university center in Brazil. Int J Cardiol. (2000) 75:S163–6. doi: 10.1016/S0167-5273(00)00197-2
26. Hoffman, GS, and Ahmed, AE. Surrogate markers of disease activity in patients with Takayasu arteritis: a preliminary report from the international network for the study of the systemic Vasculitides (INSSYS). Int J Cardiol. (1998) 66:S191–4. doi: 10.1016/S0167-5273(98)00181-8
27. Ma, LY, Li, CL, Ma, LL, Cui, XM, Dai, XM, Sun, Y, et al. Value of contrast-enhanced ultrasonography of the carotid artery for evaluating disease activity in Takayasu arteritis. Arthritis Res Ther. (2019) 21:24. doi: 10.1186/s13075-019-1813-2
28. Ito, S, Tahara, N, Hirakata, S, Kaieda, S, Tahara, A, Maeda-Ogata, S, et al. Signal intensity of superb micro-vascular imaging associates with the activity of vascular inflammation in Takayasu arteritis. J Nucl Cardiol. (2020) 27:1063–5. doi: 10.1007/s12350-019-01665-4
29. Svensson, C, Eriksson, P, and Zachrisson, H. Vascular ultrasound for monitoring of inflammatory activity in Takayasu arteritis. Clin Physiol Funct Imaging. (2020) 40:37–45. doi: 10.1111/cpf.12601
30. Sato, W, Sato, T, Iino, T, Seki, K, and Watanabe, H. Visualization of arterial wall vascularization using superb microvascular imaging in active-stage Takayasu arteritis. Eur Heart J Cardiovasc Imaging. (2019) 20:719. doi: 10.1093/ehjci/jey285
Keywords: Takayasu's arteritis, superb microvascular imaging, Doppler ultrasound, clinical study, carotid superb microvascular imaging
Citation: Liu F-J, Ci W-P and Cheng Y (2023) Clinical study of carotid superb microvascular imaging in evaluating the activity of Takayasu’s arteritis. Front. Cardiovasc. Med. 10:1051862. doi: 10.3389/fcvm.2023.1051862
Edited by:
M. Eline Kooi, Maastricht University Medical Centre, NetherlandsReviewed by:
Werner Mess, Maastricht University Medical Centre, NetherlandsOzgur Kasapcopur, Istanbul University-Cerrahpasa, Türkiye
Copyright © 2023 Liu, Ci and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Ju Liu, bGl1ZmVuZ2p1bGZqQDIxY24uY29t
†These authors have contributed equally to this work
 Feng-Ju Liu
Feng-Ju Liu Wei-Ping Ci2†
Wei-Ping Ci2†