
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 16 February 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1038738
This article is part of the Research Topic Inflammatory Factors in Coronary Heart Disease: Mechanism, diagnosis and therapy View all 12 articles
Background: Coronary heart disease (CHD) is characterized by forming of arterial plaques composed mainly of lipids, calcium, and inflammatory cells. These plaques narrow the lumen of the coronary artery, leading to episodic or persistent angina. Atherosclerosis is not just a lipid deposition disease but an inflammatory process with a high-specificity cellular and molecular response. Anti-inflammatory treatment for CHD is a promising therapy; several recent clinical studies (CANTOS, COCOLT, and LoDoCo2) provide therapeutic directions. However, bibliometric analysis data on anti-inflammatory conditions in CHD are lacking. This study aims to provide a comprehensive visual perspective on the anti-inflammatory research in CHD and will contribute to further research.
Materials and methods: All the data were collected from the Web of Science Core Collection (WoSCC) database. We used the Web of Science’s systematic tool to analyze the year of countries/regions, organizations, publications, authors, and citations. CiteSpace and VOSviewer were used to construct visual bibliometric networks to reveal the current status and emerging hotspot trends for anti-inflammatory intervention in CHD.
Results: 5,818 papers published from 1990 to 2022 were included. The number of publications has been on the rise since 2003. Libby Peter is the most prolific author in the field. “Circulation” was ranked first in the number of journals. The United States has contributed the most to the number of publications. The Harvard University System is the most published organization. The top 5 clusters of keywords co-occurrence are inflammation, C-reactive protein, coronary heart disease, nonsteroidal anti-inflammatory, and myocardial infarction. The top 5 literature citation topics are chronic inflammatory diseases, cardiovascular risk; systematic review, statin therapy; high-density lipoprotein. In the past 2 years, the strongest keyword reference burst is “Nlrp3 inflammasome,” and the strongest citation burst is “Ridker PM, 2017 (95.12).”
Conclusion: This study analyzes the research hotspots, frontiers, and development trends of anti-inflammatory applications in CHD, which is of great significance for future studies.
In recent decades, inflammation has been a growing concern in atherosclerotic coronary artery disease. Meanwhile, the basic and clinical anti-inflammation studies in coronary artery disease have been widely explored (1). In 1986, Professor Russell Ross (2) explicitly stated that atherosclerosis is an inflammatory disease and excessive defensive response to injury. In 1996, Michael A Mendall (3) investigated the relationship between chronic low-grade systemic inflammation (c-reactive protein, CRP) and coronary artery disease through a cross-sectional study. The results suggest that the body’s response to inflammation may affect the development of atherosclerosis in the middle-aged population. In 1999, John Danesh (4) explored the relevance of low-grade inflammatory processes to cardiovascular disease and vascular risk factors. It was concluded that hypersensitive C-reactive protein (hs-CRP) is a strong predictor of future cardiovascular events. CANTOS confirms the relationship between inflammation and coronary heart disease, and reducing inflammation reduces the risks of heart disease (5). This hypothesis has been continuously proven scientifically, from pathological studies of the blood vessel wall to epidemiological studies of circulating inflammatory factors in preliminary intervention studies. More studies are focusing on the mechanisms of anti-inflammatory action in coronary heart disease and exploring new therapeutic approaches for anti-inflammatory drugs (6–9).
Bibliometry is the cross-science of quantitatively analyzing all knowledge carriers using mathematical and statistical methods (10). Bibliometric analysis can capture literature groups’ characteristics and hot trends within a topic domain (11). Therefore, a comprehensive understanding can be gained using bibliometric analysis methods, which greatly help scientific research. In recent years, bibliometric analysis has played a role in medicine with the surge of medical papers (12). However, the bibliometric analysis of inflammation in CHD is still lacking. In this paper, we conducted bibliometric research on anti-inflammation use in CHD to explore its development trends.
The literature data was collected from the Web of Science Core Collection (WoSCC) database through the Science Citation Index Expanded (SCI-E) on August 9, 2022. Our search strategy was: TS = (antiinflammatory or anti-inflammatory or anti-inflammation) AND TS = (coronary heart disease or unstable angina pectoris or angina pectoris or Acute coronary syndrome or heart failure or myocardial infarct) AND PY = (1990–2022) AND LA = (English). Only articles and reviews met the requirement and were included. The literature search was conducted by two authors independently (JL Z and CY J). After data normalization, all documents, including the complete records and cited references, were exported in pure text format. All valid data were imported to VOSviewer and CiteSpace for visual analysis. We analyze the essential characteristics of the literature. Microsoft Excel 2019 was used to predict the growth trend of publications in 2022. Figure 1 shows the literature’s prediction graph and the literature’s screening graph.
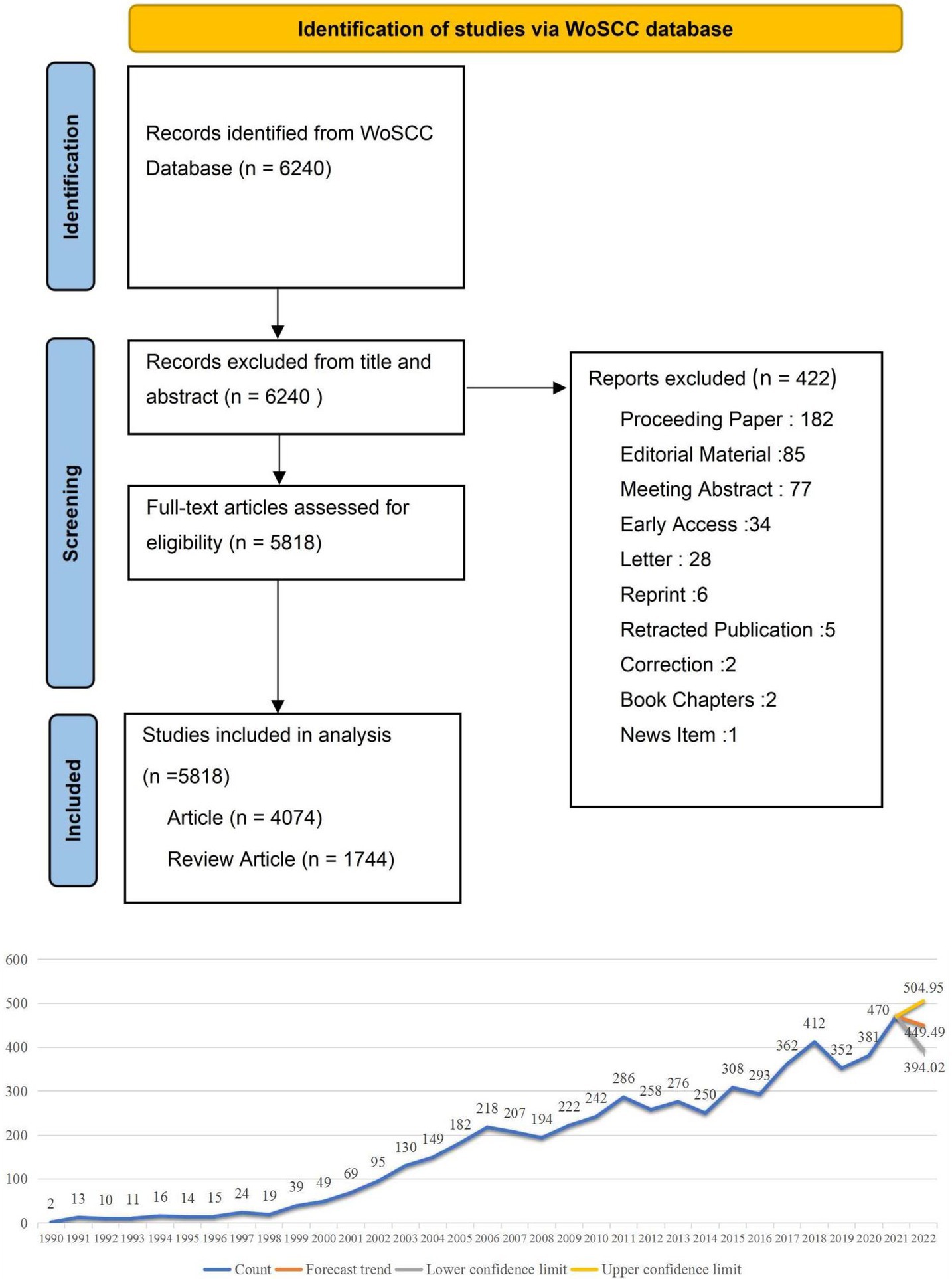
Figure 1. The literature’s prediction graph and the literature’s screening flow chart. This is the trend chart of the distribution and growth of the literature, and we predict that publications in 2022 were between 394 and 504.
VOSviewer is a procedure for building and viewing bibliometric maps (13). It can be used to build author, journal, or keyword maps based on co-occurrence data (14). CiteSpace focuses on analyzing the potential knowledge contained in the scientific literature (15). It can be used to visualize the comprehensive research situation over a certain period and to predict the development trend of the related field (16). CiteSpace has certain advantages in revealing the dynamic development law of the discipline and the research frontier (17). VOSviewer can be selected to draw the knowledge map in presenting the relationship between the subject themes (18).
A total of 5,818 articles focus on anti-inflammatory studies in CHD. Among the most prolific authors, Libby Peter (USA) was ranked first with 28 articles, followed by Aukrust, Pal (Norway), and Ridker, Paul M (USA), with 27 and 26 articles, respectively. Anker, Stefan D (Germany) and Tousoulis, Dimitris (Greece) were ranked fourth and fifth with 22 and 20 articles, respectively. For the details of all literature, see Supplementary material 1.
In terms of publications, they have now been published in over 1,458 journals. Circulation has published 98 articles on the application of anti-inflammatory in CHD. This was followed by PloS One (84 papers), Atherosclerosis (79 papers), International Journal of Cardiology (68 papers), and Current Pharmaceutical Design (65 papers). Impact Factor (IF) is a quantitative index representing a journal’s impact and general evaluation of international journals’ academic level and publication quality (19). According to the latest impact factor published in 2022, Circulation had the highest impact factor of 39.918, followed by Atherosclerosis with an IF of 6.847.
The literature on anti-inflammatory intervention in CHD has been published in 103 countries and regions. The United States leads the way, with 1,708 publications, followed by China (929 publications), Italy (496 publications), Germany (397 publications), And England (394 publications).
Among the research institutions, 4,959 institutions were involved in the research field. According to the statistical analysis, Harvard University ranked first with 213 articles, followed by the University of California System (149 publications), Brigham and Women’s Hospital (144 publications), University of London (124 publications), and Institut National De La Sante Et De La Recherche Medicale Inserm (111 publications). In conclusion, the top three institutions are all research institutions in the US, where Brigham and Women’s Hospital are affiliated hospitals under Harvard Medical School. The US remains a leader in anti-inflammatory intervention for coronary heart disease, and Harvard University’s system ranks first in various organizations’ publications.
The top 20 most cited references besides the clinical guidelines are shown in Table 1. The top 3 papers were all from the relevant Harvard University team. The first paper comes from Peter Libby’s review in Circulation (Inflammation and atherosclerosis) (20), published in 2002. His review of Inflammation in atherosclerosis was published in Nature in the same year (21). The total citation frequency of the two articles reached 12,576 times. It is clearly stated that inflammation is a therapeutic target in atherosclerosis. In 1997, Paul M. Ridker published in The New England Journal of Medicine (NEJM) in “Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men” (22), suggesting that anti-inflammatory drugs may have a clinical benefit in preventing cardiovascular disease. In 2017, Paul M. Ridker published “Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease” (23) in NEJM. This randomized, double-blind, placebo-controlled international multi-center clinical trial created the first new era of anti-inflammatory treatment of atherosclerotic diseases and was cited more than 4,000 times.
Big data reveals that cooperation between high-level academics or research institutions can produce more effective results (24). The analysis of literature authors and their cooperative network is conducive to grasping the cooperation between high-yielding authors and academic groups in this research field (25, 26).
In the national cooperation analysis, there are three main trends. First, the United States and China as the central core cluster. Second, it has three obvious geographical advantages, the American continent research cluster, the research cluster of European countries, and the East Asian research cluster, and third, there are spatio-temporal change trends. Before 2014, mainly in Europe and America and other developed countries, after 2014, China and developing countries in Asia began to emerge (Figure 2A).
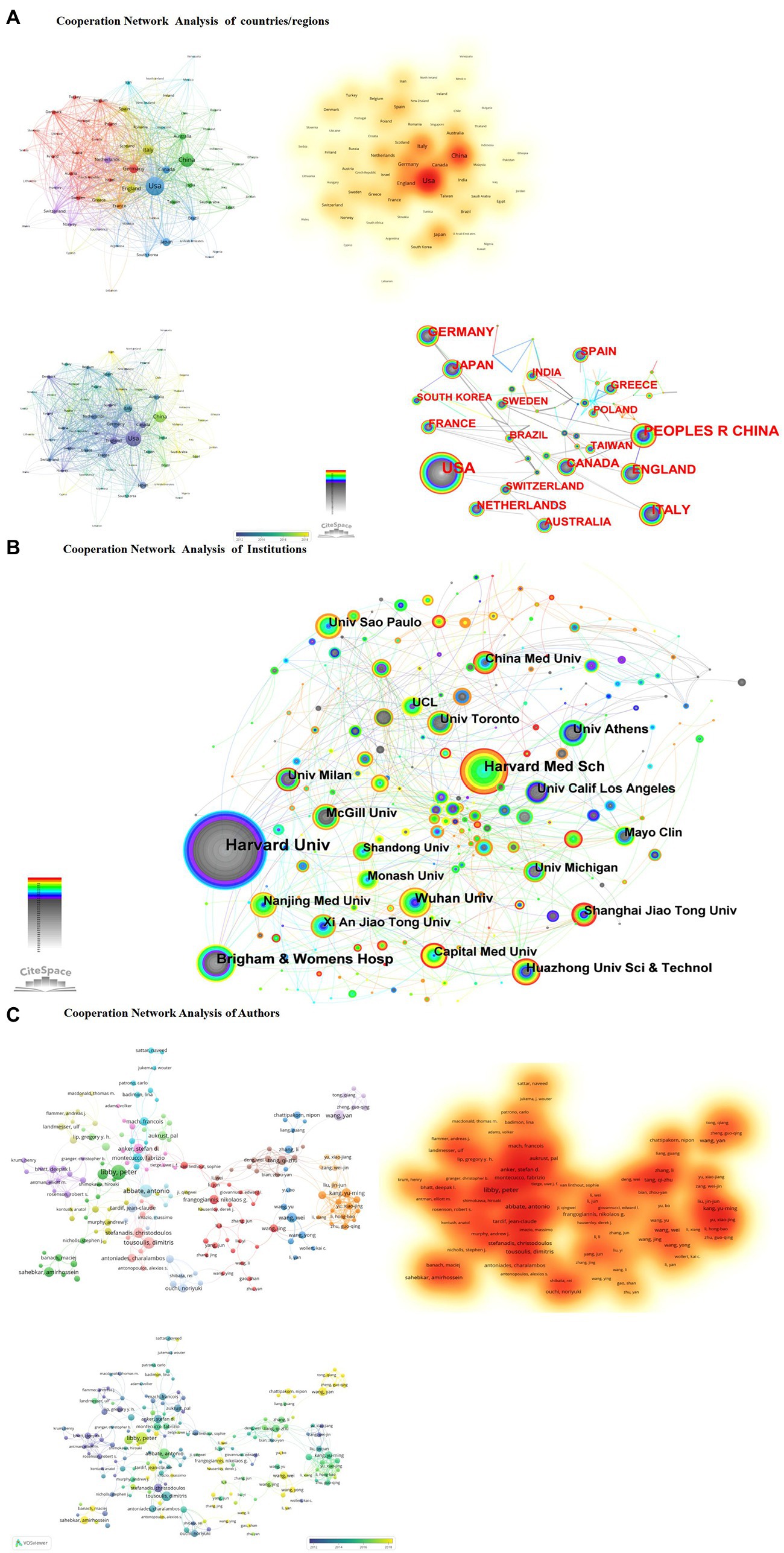
Figure 2. (A) Cooperation network analysis of countries/regions. (B) Cooperation network analysis of institutions. (C) Cooperation network analysis of authors.
In the analysis of institutional cooperation, from the perspective of the frequency of institutional collaboration, the Harvard University system was dominated in the early stage. Harvard Medical School, Brigham & Women’s Hosp participated; after 2010, the University of Amsterdam, Wuhan University, and Nanjing Medical University participated in the research. China followed closely in the study, with a small-scale but systematic cluster of research institutions (Figure 2B).
In the author’s cooperation analysis, there are two apparent characteristics; Developed countries such as Europe and the United States have presented a research pattern of the cooperative cluster formed by a group of Harvard University experts led by Peter Libby and Paul M. Ridker. Developing countries, represented by Chinese experts’ concentrated research, including Qizhu Tang’s (27–33) and Wei Wang’s (34–39) team, have characteristics that emphasize the role of traditional medicine and natural products in the anti-inflammatory treatment of CHD (Figure 2C).
Academic journals serve as a vehicle for disseminating disciplinary knowledge, and the relationship between the journals cited in each discipline can reflect the flow of knowledge between journals. Journal coupling analysis refers to the reference situation where two pieces of literature are cited together (40), i.e., if two articles cite the same literature simultaneously, there is a coupling relationship between the two articles. Coupling analysis connects journals from the perspective of knowledge absorption, exploring the academic classification, determining the core or peripheral status of periodicals, the disciplinary nature of journals, and the degree of correlation among various domains. Journal co-citation refers to the co-citation of two articles that appear in the reference list of a third cited article (41). Journal co-citation analysis examines associations between journals from the perspective of knowledge output, and looking at co-citation relationships between journals provides a glimpse into the scholarly communication patterns of disciplinary research.
All journals had a total of 346,140 citations. From the total citations of the journals, Circulation ranked first with 21,817 citations, followed by NEJM. NEJM published only nine publications, but these nine references’ total citations reached 14,256 times. Lancet published 12 related articles with a total frequency of 6,285 citations. Among the latest journal impact factor, the Circulation score was 39.918, ranking second in Cardiology and Cardiovascular Medicine. The lancet score was 202.731, and the NEJM impact factor was indexed third in the Medicine disciplines, at 176.079. Although the number of publications is low, internationally renowned academic journals greatly influence co-citation (Figure 3A).
The journal coupling method can be used to analyze the relationship between journals, help journal classification and explore the internal knowledge structure of the disciplines. The larger node also shows the more significant influence of the journals. We screened journals with a minimum citation count of five times, yielding 294 publications. In the journal coupling analysis, we can find that; Early years focus on the association of traditional publishing journals. Moving on to open access journals after 2015. With an apparent trend, early years as Circulation, American Journal of Cardiology, Atherosclerosis, International Journal of Cardiology, Current Pharmaceutical Design’s multiple non-open access journals, as the leading core group. Journal coupling density maps formed at later stages, including coupled clusters with open access journals (Figure 3B).
Keywords are the values used to identify specific data items in the literature, mainly to briefly and accurately describe the article’s topic, and are primarily used for indexing or cataloging (42). Therefore, we can understand publications’ characteristics and evolution trends by analyzing the changes in keywords.
A keyword merged coexistence network with 459 nodes and 5,024 links were constructed using CiteSpace. The parameters of the software are set as follows. Time slicing: from 1990 to 2022, 1 year per slice. Node types: reference. Selection criteria: select the top 50 levels of most occurred items from each slice. Cluster analysis was performed based on these keywords
and the results are shown in (Figure 4A). The smaller the cluster tag number
the larger the cluster size. According to the cluster results analysis
the critical clusters are coronary heart disease
c-reactive protein
cardiovascular disease
myocardial infarction
rheumatoid arthritis
and gastrointestinal toxicity. According to the VOSviewer analysis of the keyword cooperative network and keyword density
the top five core keywords are inflammation
C-reactive protein
coronary heart disease
nonsteroidal antiinflammatory
and myocardial infarction (Figure 4B)
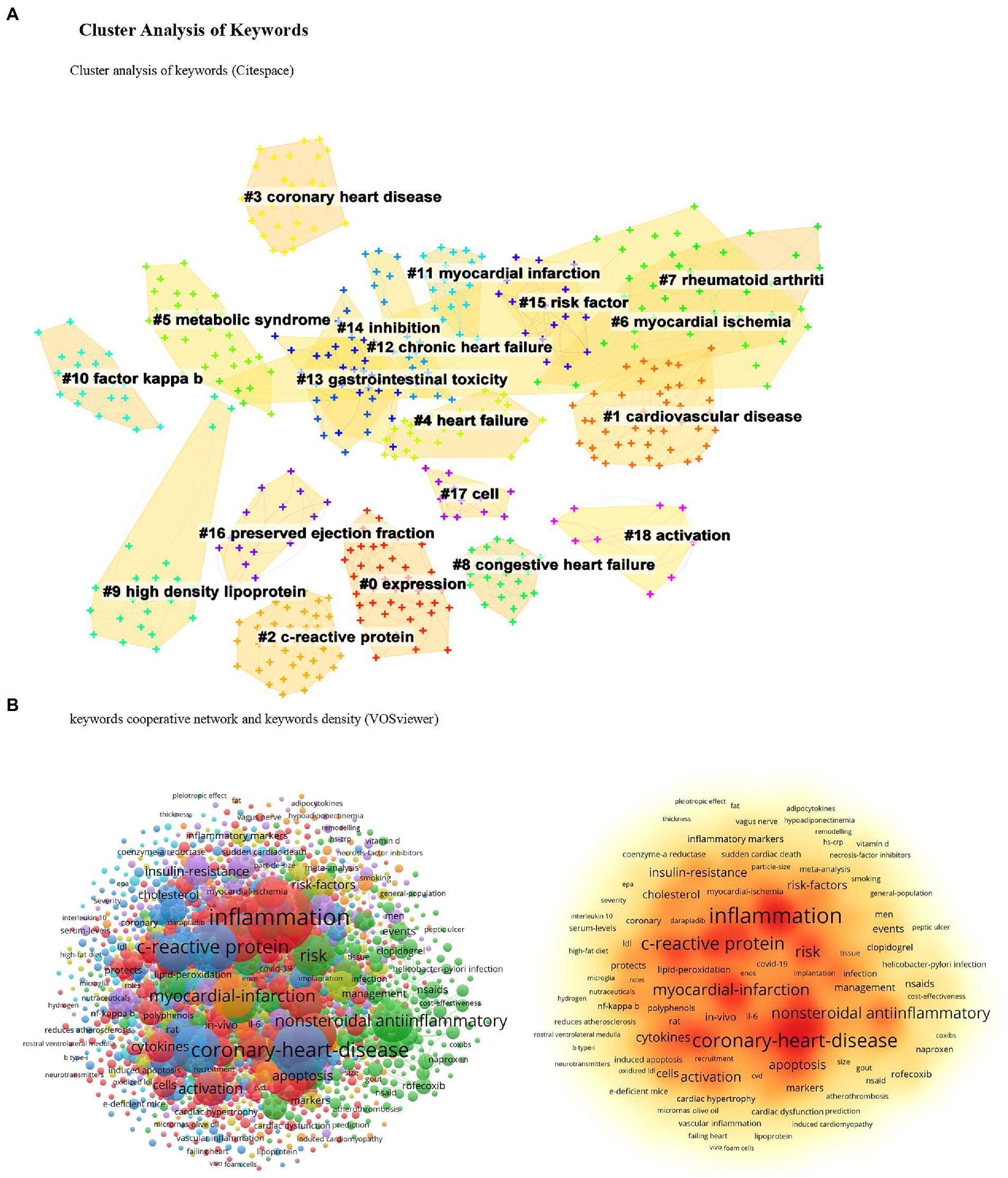
Figure 4. Cluster analysis of keywords. (A) Cluster analysis of keywords (Citespace). (B) Keywords cooperative network and keywords density (VOSviewer).
CiteSpace provides burst detection that can perceive significant changes in references and keywords over a certain period (43). We screened the top 20 keywords and the 25 references according to burst intensity (Figure 5). The strongest keywords were “coronary heart disease (20.73),” and most recently “heart failure (18.28),” nonsteroidal anti-inflammatory drug (17.51), oxidative stress (16.62), c reactive protein (12.18), tumor necrosis factor (10.36). In addition, the Nlrp3 inflammasome in the last 2 years strongly references the sudden hot spot, reflecting the current hot spot trend. Moreover, the most cited citation burst is “Ridker PM, 2017 (95.12),” ranked by citation time. The top five are Tardif JC, 2019 (52.59), Ridker PM, 2019 (37.9), Ridker PM, 2018 (22.82), Prabhu SD, 2016 (23.51), and Ridker PM, 2017 (95.12).
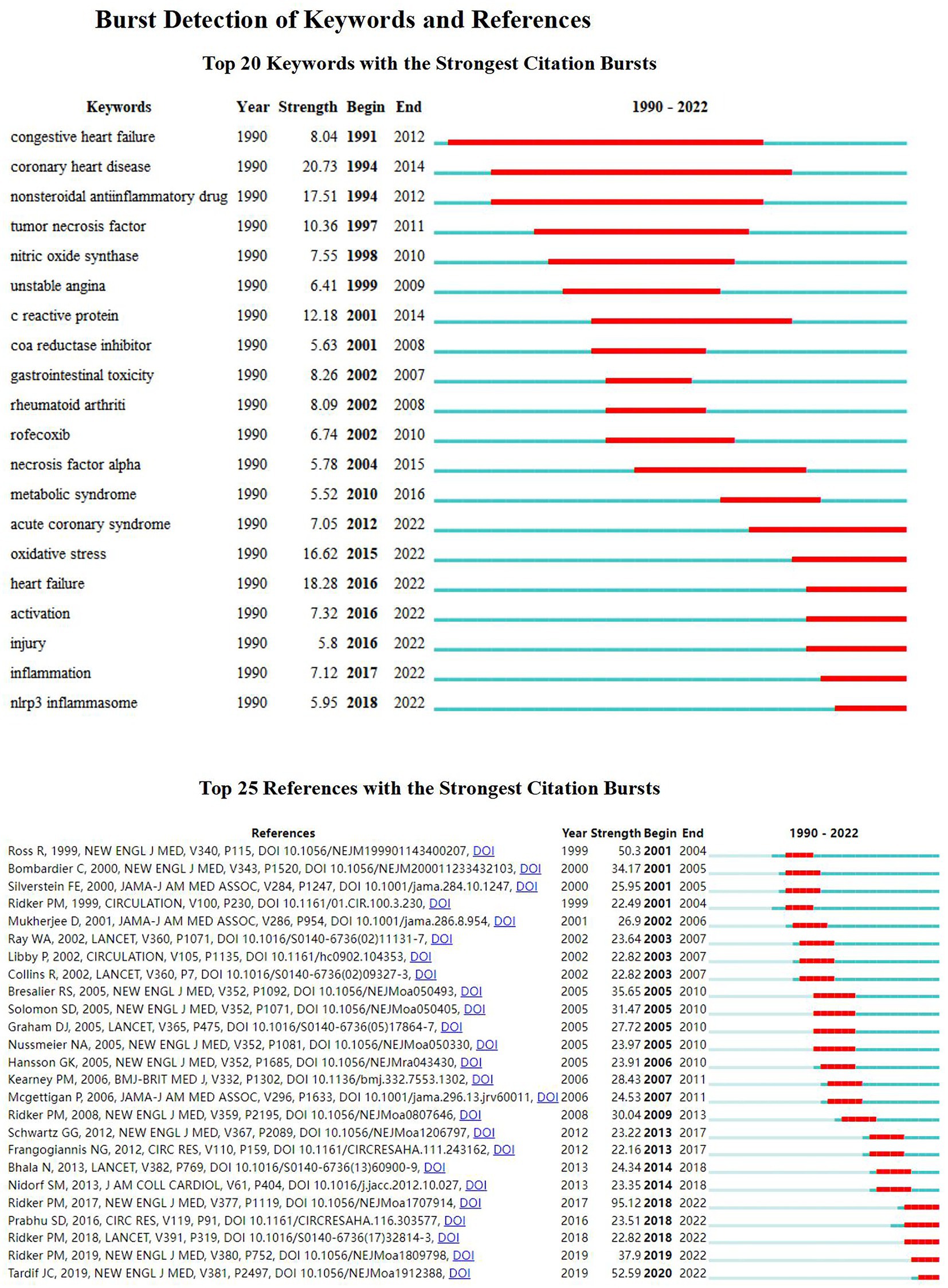
Figure 5. Burst detection of keywords and references. Top 20 keywords with the strongest citation bursts. Top 25 references with the strongest citation bursts.
The strongest keywords were “coronary heart disease (20.73),” and most recently “heart failure (18.28), nonsteroidal anti-inflammatory drug (NSAIDs) (17.51), oxidative stress (16.62), c reactive protein (12.18), and tumor necrosis factor (10.36). In addition, the Nlrp3 inflammasome in the last 2 years is a strong reference to the sudden hot spot, reflecting the current hot spot trend.
Moreover, the most cited citation burst is “Ridker PM, 2017 (95.12),” ranked by citation time; the top five are Tardif JC, 2019 (52.59), Ridker PM, 2019 (37.9), Ridker PM, 2018 (22.82), Prabhu SD, 2016 (23.51) and Ridker PM, 2017 (95.12).
According to the analysis of study topics, reference co-citation clustering is a superior function of CiteSpace, enabling us to study issues and hotspot trends comprehensively. Each cluster is considered to represent a hot frontier of research. Therefore, we used CiteSpace to describe the cluster view (Figure 6A) and the timeline view (Figure 6B) of the reference co-citations to analyze the trend of anti-inflammatory applications in CHD. The parameters of the software are set as follows. The top 50 papers (TOP = 50) references of each time section were also extracted to construct a literature co-citation network. Contains 4,146 lines and 964 nodes. The analysis yielded a cluster modularity value of 0.799 (Q value), reflecting that the clustering is apparent.
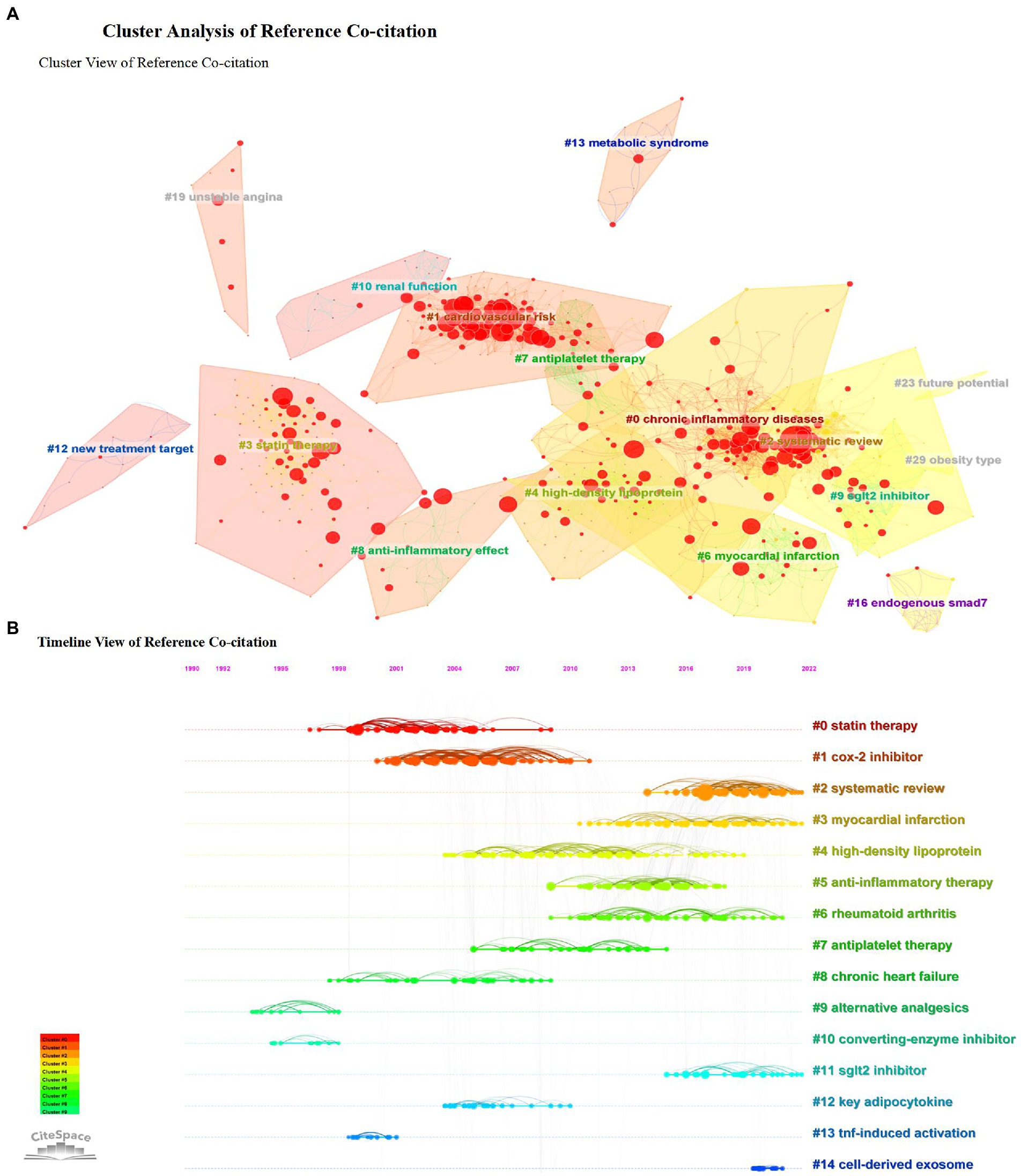
Figure 6. Cluster analysis of reference co-citation. (A) Cluster view of reference co-citation. (B) Timeline view of reference co-citation.
The clustering results jointly cited by references give us some hints: early attention focus on the anti-inflammatory effects of statins (44–46), recent hot topics pay more attention to the study of combined diseases and comorbidities, to explore the common mechanism of anti-inflammation in coronary heart disease and other significant chronic non-communicable complex diseases such as diabetes and obesity (47–49). In addition, the application of new anti-inflammatory drugs is also one of the hot concerns (50–53).
Coronary heart disease has become the highest mortality disease in the world. The number of cardiovascular diseases in China is 290 million, containing 11 million coronary heart diseases, and morbidity trends are increasing yearly (54, 55). With the development of research, it found that inflammation plays an important role in the development of CHD. The inflammatory response is an essential mechanism of CHD and significantly impacts the progression of coronary atherosclerotic plaque and adverse cardiovascular events (56, 57). The inflammatory response accelerates the formation of atherosclerotic plaques. Some inflammatory factors reduce the tensile strength of the plaque fiber cap and increase the necrotic lipid core, leading to damaged endothelium and plaque rupture (58–60). Meanwhile, anti-inflammatory treatment has been proven effective in the secondary prevention of coronary heart disease, reducing acute coronary events and improving the prognosis (61, 62).
The number of anti-inflammatory interventions in CHD-related studies has generally increased over the past 30 years. It shows that inflammation, as a critical pathological change during atherosclerosis progression, is attracting increasing attention from researchers (63). Early studies confirmed the correlation between CRP and inflammatory mechanisms in CHD. Acute phase CRP has been shown to reflect systemic and possible vascular inflammation and to predict future cardiovascular events in asymptomatic individuals. In addition, CRP promotes the release of TNF-α, IL-1β, and IL-6 from macrophages and foam cells in the neointima (64). The release of these pro-inflammatory factors promotes atherosclerosis and recruits early monocytes and lymphocytes in the intima (65). One study (66) showed that serum hs-CRP levels were significantly higher in patients with moderate and severe arterial stenosis than in patients with mild stenosis. A panel of experts from the Centers for Disease Control and Prevention and the American Heart Association (67) has recommended hs-CRP as the clinical test with the highest reliability for assessing and predicting the risk of cardiovascular disease. Hs-CRP has become an independent predictor of atherosclerosis and cardiovascular disease, classifying individuals into three risk categories based on hs-CRP levels. Low <1 mg/l; medium 1–3 mg/l; and high >3 mg/l. Each standard deviation increase in hs-CRP concentration increases the risk by 45%, so the level of hs-CRP can be applied to distinguish low-risk from high-risk for atherosclerosis and cardiovascular disease.
In addition, there is a class of clusters focused on rheumatoid arthritis, which is not exploring the comorbid mechanisms of RA and CHD, but instead uses anti-inflammatory drugs to treat rheumatoid arthritis. Recent studies have focused on mechanistic intervention in cardiovascular disease with the anti-inflammatory drugs allopurinol (68, 69) and colchicine (70–74), promising agents for intervention in cardiovascular disease (75). Moreover, there have been concentrated studies on gastrointestinal toxicity of NSAIDs (76–78), such as targeting gastrointestinal side effects of anti-inflammatory medications COX-2 inhibitors (79, 80), focusing on the drug’s safety, and preventing adverse events (81, 82).
According to the time trend analysis of the keyword strongest burst, from the late 1990s, the anti-inflammatory application of traditional indicators (tumor necrosis factor, CRP) was concerned. Around 2010, research focused on NSAIDs’ use in CHD, including the gastrointestinal toxicity of NSAIDs (77, 83). In the past 5 years, more attention has been paid to the mechanism of the inflammasome in coronary heart disease (84), and the NLRP3 inflammasome-driven IL-1 release has led to atherosclerotic progression and accelerated vascular inflammatory response (85, 86). The research on inflammasome may become one of the research hotspots for future anti-inflammatory interventions in CHD. Although hs-CRP can be used clinically as a biomarker for risk prediction, and high levels of hs-CRP are associated with adverse cardiovascular outcomes after acute coronary syndrome (ACS) (87), most mechanistic studies suggest that CRP itself is unlikely to be an ideal target for specific anti-inflammatory therapies (88). Upstream movement of the inflammatory cascade from CRP to IL-6 to IL-1 offers new therapeutic opportunities for atherosclerosis protection (89). IL-1β, a critical pro-inflammatory cytokine, is involved in various autoimmune inflammatory responses (90). The level of IL-1β is synergistically regulated by Toll-like receptors (TLRs) and Nod-like receptors (NLRs). Activation of TLRs induces the synthesis of precursor IL-1β and precursor interleukin-18, and the activation of NLRs induces assembly in the host cell cytoplasm to form a multimeric protein complex, the inflammasome. The inflammasome is central to the production of IL-1β and IL-18.
The NLRP3 inflammasome/IL-1β signaling pathway plays an important role in the development of AS. Caspase-1, IL-1β, and IL-18, related components of the NLRP3 inflammasome signaling pathway, were highly expressed in atherosclerotic plaques, and the expression levels were higher in vulnerable plaques than in stable plaques (91), indicating that the NLRP3 inflammasome pathway plays an essential role in the development of AS and affects plaque stability. Cytokine release inhibitory drug 3 (MCC 950), a selective inhibitor of the NLRP3 inflammasome (92), was shown to reduce the maximum stenosis significantly, mean plaque size and volume, minimize macrophage aggregation and inflammatory factor levels, and increase plaque stability in the aorta of apoE−/− mice (93). MCC 950 blocks the NLRP3 inflammasome/IL-1β signaling pathway from upstream, reducing the production of not only IL-1β but also inflammatory factors such as IL-1α and IL-18 simultaneously (94). Theoretically, it is a safer and more effective therapeutic measure than the anti-IL-1β monoclonal antibody canakinumab and has good research prospects. Targeted modulation of the NLRP3 inflammasome/IL-1β is expected to be one of the hot studies for future anti-inflammatory interventions in the prevention and treatment of CHD (95, 96).
Similar results were shown according to the timeline of the research topic. The early 90s focused on traditional mechanisms such as tumor necrosis factor (97, 98) and the mechanism of a nuclear transcription factor (99–101) in atherosclerosis. Several cytokines, including TNF-α, TGF-β, and different interleukins, are involved in developing various inflammatory cardiac pathologies (102, 103). It was found that the combined action of the NF-κB signaling pathway and IL-23/IL-17 inflammatory axis allows IL-1β and TNF-α to accumulate in macrophage foam cells and inflammatory responses, both of which are involved in the pathological development of CHD and related diseases (104).
Around 2000, attention was paid to the anti-inflammatory effects of statins in patients by regulating CRP in CHD (105–108). Despite aggressive statin therapy, publications (109, 110) show that inflammation may be an important driver of residual cardiovascular risk in coronary artery disease. Due to the inability of lipid-lowering to slow the progression of atherosclerosis completely, the identification of inflammatory biomarkers as independent risk factors for cardiovascular disease events has facilitated trials using anti-inflammatory strategies to treat atherosclerosis (111, 112). Since 2010, the focus has been on the mechanisms of NSAIDs in CHD, especially pilot studies using anti-rheumatoid arthritis drugs such as colchicine (62, 113) and methotrexate (114) to intervene in CHD and modulate the level of inflammation. Different from the keyword burst, in the last 5 years, on the one hand, attention has been paid to clinical studies, and the integration of evidence has been carried out to evaluate the evidence systematically (115, 116). On the other hand, studies on the potential association between macrophages and anti-inflammatory have been carried out, which will help drive the formation of new therapies (117–119). In addition, the anti-inflammatory efficacy of fish oil in cardiovascular diseases is included as an area of exploration in nutrition (120–122).
The references with the strongest citation bursts revealed that recent attention has focused on the anti-inflammatory clinical studies of Canakinumab and colchicine in cardiovascular diseases (61, 123–126). The CANTOS study (23) enrolled 10,061 patients from 39 countries with myocardial infarction combined with elevated hs-CRP (>2 mg/l). Canakinumab is a selective, high-affinity, fully humanized monoclonal antibody that targets the inhibition of interleukin-1β (IL-1β). The study showed that canakinumab could further reduce adverse cardiovascular events with myocardial infarction on top of lipid-lowering drug therapy (57, 127, 128). The CANTOS confirmed the clinical importance of the pro-atherosclerotic of IL-1β and identified the IL-1 to IL-6 to CRP inflammatory pathway as a central target for atherosclerotic protection. These data support further drug discovery of atherosclerotic thrombosis therapies targeting IL-18 or IL-6. Due to the role of IL-1β in promoting various pro-inflammatory factors previously, the search for signaling pathways upstream of IL-1β (e.g., NLRP3 inflammasome inhibitors) and possible inflammation targets for intervention has become a hot topic of current research (129). In a follow-up study (130) of 4,833 CANTOS participants, inhibition of the IL-6 signaling pathway was associated with reducing cardiovascular events and all-cause mortality. IL-6 is involved in the pathogenesis of multiple inflammatory diseases, and plasma IL-6 levels strongly predict future vascular events independent of traditional risk factors (131). The results of this study also suggest that lower IL-6 ratios may lead to a lower proportion of cardiovascular events.
Allopurinol is commonly used as a first-line agent to lower serum uric acid and prevent acute attacks in patients with gout, and cardiovascular benefits have also been reported (68, 69). A population-based case–control study (132) found that allopurinol was associated with a lower risk of non-fatal acute myocardial infarction and that the longer patients took the drug, the greater the reduction in infarction risk, suggesting additional cardiovascular protection. In a recent ALL-HEART trial conducted in the United Kingdom (133), allopurinol combined with conventional therapy did not improve cardiovascular outcomes (non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death) in patients with ischemic heart disease. Therefore, the trial shows that allopurinol may not be recommended for the secondary prevention of cardiovascular events in patients with ischemic heart disease. New evidence for the cardiovascular benefit of allopurinol remains to be further investigated.
Colchicine is widely used in clinical practice for gout (113, 134, 135). The LoDoCo (136) study suggested that colchicine reduced the relative risk of the primary endpoint event (acute coronary syndrome, out-of-hospital cardiac arrest, or non-cardiogenic embolic ischemic stroke) by 67% in 532 patients with stable coronary artery disease treated with low-dose colchicine (0.5 mg/day) (HR, 0.33; 95% CI, 0.18 to 0.59; p < 0.001). The COLCOT (137) published in 2019 is a large randomized controlled trial (RCT) evaluating the effect of the colchicine group (0.5 mg/day) on recurrent cardiovascular events in patients who had a myocardial infarction within 30 days. The results showed a significant 23% (HR, 0.77; 95% CI, 0.61 to 0.96; p = 0.02) reduction in the risk of the primary endpoint event (including cardiovascular death, cardiac arrest, non-fatal myocardial infarction, non-fatal stroke, and urgent revascularization due to angina). The results of a CT coronary angiography study (138) of colchicine intervention in ACS showed that low-dose colchicine treatment was effective in modifying coronary plaques with ACS and that the anti-inflammatory properties of colchicine may drive the improvement in plaque morphology. Colchicine may be beneficial as an additional secondary prevention drug in patients post-ACS. Recent meta-analyses (139–142) have shown that colchicine positively reduces the incidence of MACE, MI, stroke, and revascularization and decreases cardiovascular events, inflammatory markers, hs-CRP, and IL-6 in patients with coronary artery disease. But with a higher incidence of gastrointestinal distress and no effect on all-cause mortality. In a recent Australian study (143), patients with ACS were treated with colchicine 0.5 mg/d twice daily for the first month, then 0.5 mg daily for 11 months, and after 1 year of follow-up, there was no significant difference in the primary adverse event composite endpoint in patients taking colchicine compared to the placebo. After 2 years follow-up (144), the primary adverse cardiovascular event endpoint incidence was significantly lower. This sustained effect may be attributed to colchicine’s anti-inflammatory and plaque-modulating properties, reducing the potential development of high-risk plaque volume and ischemic complications. Since the drug was only used for 12 months, the results sustained over 2 years suggest that colchicine may have a legacy effect. Several studies with different trial designs, including colchicine dose, the timing of administration, and the different endpoint events, may have influenced the trial results. More comprehensive and in-depth studies are needed to provide definitive evidence for the clinical use of colchicine. In summary, these findings initially suggest an opportunity to reduce the burden of coronary heart disease in patients using either drug targeting IL-1β or other inflammatory inhibitory pathways (145). Future trials of other new anti-inflammatory agents may help to understand the role of anti-inflammation in the prevention of severe cardiovascular disease events in high-risk patients. We list recent ongoing clinical studies of anti-inflammatory interventions for coronary artery disease being recruited by ClinicalTrials.gov in Supplementary material 2.
In conclusion, integrating the burst of the keywords and the thematic timeline, the current research is focused on the mechanism of anti-inflammation and anti-inflammatory drugs in CHD, and the association between inflammatory vesicles NLRP3 levels and coronary heart disease risk is one of the hot topics (84). In addition, supplementing dietary nutrients and trace elements (146), including omega-3 fatty acids (147–149), provides a nutritional perspective for anti-inflammatory intervention in cardiovascular disease. A recent study has also found (150) that ferroptosis plays a crucial role in the development of CHD and that antioxidants may be the most promising inhibitors of ferroptosis in widespread use. Ferroptosis inhibition is a good option for treating CHD. Moreover, smartphone-based applications (151) for health management in the anti-inflammatory treatment of coronary heart disease can help bridge the digital divide and may be one of the next hot spots in the post-COVID-19 era.
To the best of our knowledge, this is the first study summarizing the research progress on anti-inflammatory in CHD studies by bibliometric analysis, intuitively presenting contributors, collaboration networks, research hotspots, and development prospects through visualization. This paper analyzes the research trends and hot spots of anti-inflammation in CHD. Researchers can refer to the research trends and grasp the current research hotspots. Meanwhile, researchers can adjust the study design according to the research hotspots to make the study more innovative and feasible. The future can focus on three main points to concentrate on exploration.
1. Inflammatory mechanisms in cardiovascular diseases. Recent studies demonstrating that anti-inflammatory interventions can prevent atherosclerotic complications have only scratched the surface of the potential for developing new therapies. Targeting IL-1β highlights the inflammatory vesicle pathway as a promising avenue for further therapeutic interventions (152). NLRP3 inflammasome and the downstream cytokines IL-1β (153), IL-18 (154, 155), and IL-6 (156) are attractive candidates for intervention.
2. Clinical studies of targeted anti-inflammation. The inflammatory process affects all stages of the atherosclerotic plaque life cycle and is a well-established target for intervention in the disease. CANTOS confirms that IL-1β is a tempting target for anti-inflammatory therapy in CHD and suggests that patients with residual inflammation risk (RIR) are the main population for anti-inflammatory therapy (157). It shows that future anti-inflammatory treatment should move from macro to precision anti-inflammatory therapeutics (158–162). Macrophages are involved in the entire process of atherosclerosis formation, progression, and regression (163). They are the primary inflammatory cells involved in atherosclerosis, and their retention within the arterial tubes is necessary for atherosclerosis (164). The accumulation and functional activation of macrophages in the subintima and the secretion of various pro-inflammatory factors lead to the progression of plaques into chronic complex lesions. Therapeutic strategies that promote the conversion of the macrophage phenotype to an anti-inflammatory phenotype may benefit the prognosis of atherosclerotic cardiovascular disease. Several new anti-inflammatory and anti-cytokine agents, including but not limited to direct upstream inhibitors of the NLRP3 inflammasome, and natural inhibitors of IL-6, can be expected to be used in atherosclerosis by targeting the NLRP3, IL-1, IL-6 to CRP pathway. The way to incorporate these anti-inflammatory agents in practice is long and challenging. Still, discovering potential inflammatory targets demonstrates the importance of addressing this factor for CHD risk prevention.
3. Natural products as anti-inflammatory supplements. Recently, anti-inflammatory nutritional supplements, including fish oil, have attracted widespread attention. Inflammation-induced by dietary components is usually chronic and often caused by alterations in the intestinal flora (165). Therefore, microbial-targeted therapies, such as probiotics, prebiotics, and synbiotics, have great potential in systemic inflammatory diseases. Besides, there is a class of studies (166–168) focusing on the mechanisms of action of natural products in anti-inflammatory intervention for CHD. Evidence suggests that medical plants’ phenolics, and saponins could reduce inflammatory reactions. In addition, various nutritional components within plant flavonoids (169–172), antioxidant vitamins (173–176), and fruit polyphenols (177–179) have the potential to modulate susceptibility to chronic inflammation.
However, it is important to consider that our study still has these limitations. First, this study only contains literature written in English and no literature in other languages, which may bias the study results. Second, we only retrieved data from the WoSCC database, which may lead to incomplete literature collection. Still, it is noteworthy that the academic community recognizes WoSCC as one of the most authoritative literature data platforms covering most studies. Third, the parameter setting and analysis methods of CiteSpace software lack systematic standards, which may lead to discrepancies in the results, so we used CiteSpace combined VOSviewer to achieve a complete visual presentation. Furthermore, articles published in the past 2 years with high impact factor publications were less cited. Therefore, some recently published papers with high quality should have been included in the analysis of highly cited articles.
This study systematically analyzes the global research results of anti-inflammatory intervention for CHD over the past 30 years. It explores the already published papers’ hotspots, frontiers, and trends. Overall, the literature on anti-inflammatory intervention in coronary heart disease has increased yearly. It is expected that nearly 500 articles will be published in the whole year 2022. Further investigation into the interaction between inflammatory mechanisms may be a future research direction, and the treatment’s screening and efficacy evaluation of anti-inflammatory drugs is the focus. Our results summarize the current status of the study and are of great significance for future clinicians and researchers to condense their research directions.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
JH and HT designed this study. JZ and CJ collected all the articles and wrote the manuscript. CJ carried out software operation and figure drawing. XZ contributed to the final version. All authors contributed to the article and approved the submitted version.
The work is supported by the National Key R&D Program of China (2019YFC1708501), the National Natural Science Foundation of China (82074316), and the scientific and technological innovation project of Foshan City in the field of traditional Chinese medicine (2020001005585) and the Fundamental Research Funds for the Central Public Welfare Research Institutes (YZ-202142 and YZ-202218).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1038738/full#supplementary-material
1. Poredos, P, and Jezovnik, MK. Markers of preclinical atherosclerosis and their clinical relevance. Zentralbl Phlebol. (2015) 44:247–56. doi: 10.1024/0301-1526/a000439
2. Ross, R. The pathogenesis of atherosclerosis--an update. N Engl J Med. (1986) 314:488–500. doi: 10.1056/NEJM198602203140806
3. Mendall, MA, Patel, P, Ballam, L, Strachan, D, and Northfield, TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. (1996) 312:1061–5. doi: 10.1136/bmj.312.7038.1061
4. Danesh, J. Smoldering arteries? Low-grade inflammation and coronary heart disease. JAMA. (1999) 282:2169–71. doi: 10.1001/jama.282.22.2169
5. Ali, M, Girgis, S, Hassan, A, Rudick, S, and Becker, RC. Inflammation and coronary artery disease: from pathophysiology to Canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Coron Artery Dis. (2018) 29:429–37. doi: 10.1097/MCA.0000000000000625
6. Hatcher, H, Planalp, R, Cho, J, Tortia, FM, and Torti, SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. (2008) 65:1631–52. doi: 10.1007/s00018-008-7452-4
7. Middleton, E Jr, Kandaswami, C, and Theoharides, TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. (2000) 52:673–751.
8. Mehu, M, Narasimhulu, CA, and Singla, DK. Inflammatory cells in atherosclerosis. Antioxidants (Basel). (2022) 11:2. doi: 10.3390/antiox11020233
9. Gong, HJ, Zhou, L, Ye, DY, Gao, X, Li, YL, Qi, XY, et al. Novel dual inhibitors of secretory phospholipase a(2) and sphingomyelin synthase: design, synthesis and evaluation. Lett Drug Design Discov. (2016) 13:1025–32. doi: 10.2174/1570180813666160805155116
10. Durieux, V, and Gevenois, PA. Bibliometric indicators: quality measurements of scientific publication. Radiology. (2010) 255:342–51. doi: 10.1148/radiol.09090626
11. Bornmann, L, and Mutz, R. Growth rates of modern science: a bibliometric analysis based on the number of publications and cited references. J Assoc Inf Sci Technol. (2015) 66:2215–22. doi: 10.1002/asi.23329
12. Liao, HC, Tang, M, Luo, L, Li, CY, Chiclana, F, and Zeng, XJ. A bibliometric analysis and visualization of medical big data research. Sustainability. (2018) 10:166. doi: 10.3390/su10010166
13. van Eck, NJ, and Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
14. van Eck, NJ, and Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. (2017) 111:1053–70. doi: 10.1007/s11192-017-2300-7
15. Chen, CM. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
16. Synnestvedt, MB, Chen, C, and Holmes, JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Ann Symp Proc. (2005) 2005:724–8.
17. Chen, C, and Chen, Y. Searching for clinical evidence in CiteSpace. AMIA Annu Symp Proc. (2005) 2005:121–5.
18. Orduna-Malea, E, and Costas, R. Link-based approach to study scientific software usage: the case of VOSviewer. Scientometrics. (2021) 126:8153–86. doi: 10.1007/s11192-021-04082-y
19. Yu, G, Yang, D-H, and Liang, W. Reliability-based citation impact factor and the manipulation of impact factor. Scientometrics. (2010) 83:259–70. doi: 10.1007/s11192-009-0083-1
20. Libby, P, Ridker, PM, and Maseri, A. Inflammation and atherosclerosis. Circulation. (2002) 105:1135–43. doi: 10.1161/hc0902.104353
21. Libby, P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) 32:2045–51. doi: 10.1161/ATVBAHA.108.179705
22. Ridker, PM, Cushman, M, Stampfer, MJ, Tracy, RP, and Hennekens, CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. (1997) 336:973–9. doi: 10.1056/NEJM199704033361401
23. Ridker, PM, Everett, BM, Thuren, T, MacFadyen, JG, Chang, WH, Ballantyne, C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
24. Stokols, D, Misra, S, Moser, RP, Hall, KL, and Taylor, BK. The ecology of team science - understanding contextual influences on transdisciplinary collaboration. Am J Prev Med. (2008) 35:S96–S115. doi: 10.1016/j.amepre.2008.05.003
25. Landry, R, Traore, N, and Godin, B. An econometric analysis of the effect of collaboration on academic research productivity. High Educ. (1996) 32:283–301. doi: 10.1007/BF00138868
26. Lee, S, and Bozeman, B. The impact of research collaboration on scientific productivity. Soc Stud Sci. (2005) 35:673–702. doi: 10.1177/0306312705052359
27. Duan, MX, Zhou, H, Wu, QQ, Liu, C, Xiao, Y, Deng, W, et al. Andrographolide protects against HG-induced inflammation, apoptosis, migration, and impairment of angiogenesis via PI3K/AKT-eNOS Signalling in HUVECs. Mediat Inflamm. (2019) 2019:1–15. doi: 10.1155/2019/6168340
28. Mou, SQ, Zhou, ZY, Feng, H, Zhang, N, Lin, Z, Aiyasiding, X, et al. Liquiritin attenuates lipopolysaccharides-induced cardiomyocyte injury via an AMP-activated protein kinase-dependent signaling pathway. Front Pharmacol. (2021) 12:648688. doi: 10.3389/fphar.2021.648688
29. Song, P, Shen, DF, Meng, YY, Kong, CY, Zhang, X, Yuan, YP, et al. Geniposide protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway. Free Radic Biol Med. (2020) 152:186–96. doi: 10.1016/j.freeradbiomed.2020.02.011
30. Wan, CX, Xu, M, Huang, SH, Wu, QQ, Yuan, Y, Deng, W, et al. Baicalein protects against endothelial cell injury by inhibiting the TLR4/NF-κB signaling pathway. Mol Med Rep. (2018) 17:3085–91. doi: 10.3892/mmr.2017.8266
31. Wang, HB, Huang, SH, Xu, M, Yang, J, Yang, J, Liu, MX, et al. Galangin ameliorates cardiac remodeling via the MEK1/2-ERK1/2 and PI3K-AKT pathways. J. Cell Physiol. (2019) 234:15654–67. doi: 10.1002/jcp.28216
32. Xie, S, Deng, W, Chen, J, Wu, QQ, Li, H, Wang, J, et al. Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. (2020) 16:12–26. doi: 10.7150/ijbs.37269
33. Zhang, N, Wei, WY, Li, LL, Hu, C, and Tang, QZ. Therapeutic potential of polyphenols in cardiac fibrosis. Front Pharmacol. (2018) 9:122. doi: 10.3389/fphar.2018.00122
34. Li, C, Sui, C, Wang, W, Yan, J, Deng, N, Du, X, et al. Baicalin attenuates oxygen-glucose deprivation/Reoxygenation-induced injury by modulating the BDNF-TrkB/PI3K/Akt and MAPK/Erk1/2 signaling axes in neuron-astrocyte Cocultures. Front Pharmacol. (2021) 12:599543. doi: 10.3389/fphar.2021.599543
35. Li, C, Wang, J, Wang, Q, Zhang, Y, Zhang, N, Lu, L, et al. Qishen granules inhibit myocardial inflammation injury through regulating arachidonic acid metabolism. Sci Rep. (2016) 6:36949. doi: 10.1038/srep36949
36. Li, Y, Li, X, Chen, X, Sun, X, Liu, X, Wang, G, et al. Qishen granule (QSG) inhibits monocytes released from the spleen and protect myocardial function via the TLR4-MyD88-NF-κB p65 pathway in heart failure mice. Front Pharmacol. (2022) 13:850187. doi: 10.3389/fphar.2022.850187
37. Ren, W, Gao, S, Zhang, H, Ren, Y, Yu, X, Lin, W, et al. Decomposing the mechanism of Qishen granules in the treatment of heart failure by a quantitative pathway analysis method. Molecules. (2018) 23:1829. doi: 10.3390/molecules23071829
38. Shao, M, Wang, M, Ma, L, Wang, Q, Gao, P, Tian, X, et al. β-Elemene blocks lipid-induced inflammatory pathways via PPARβ activation in heart failure. Eur J Pharmacol. (2021) 910:174450. doi: 10.1016/j.ejphar.2021.174450
39. Wang, X, Li, W, Zhang, Y, Sun, Q, Cao, J, Tan, N, et al. Calycosin as a novel PI3K activator reduces inflammation and fibrosis in heart failure through AKT-IKK/STAT3 Axis. Front Pharmacol. (2022) 13:828061. doi: 10.3389/fphar.2022.828061
40. Zhang, J, Liu, X, and Wu, L. The study of subject-classification based on journal coupling and expert subject-classification system. Scientometrics. (2016) 107:1149–70. doi: 10.1007/s11192-016-1890-9
41. Henry, S. Co-citation in the scientific literature: a new measure of the relationship between two documents. J Am Soc Inf Sci. (1973) 24:265–9. doi: 10.1002/asi.4630240406
42. Radhakrishnan, S, Erbis, S, Isaacs, JA, and Kamarthi, S. Novel keyword co-occurrence network-based methods to foster systematic reviews of scientific literature. PLoS One. (2017) 12:e0172778. doi: 10.1371/journal.pone.0172778
43. Chen, C. Science mapping: a systematic review of the literature. J Data Inf Sci. (2017) 2:1–40. doi: 10.1515/jdis-2017-0006
44. Scalia, R, and Stalker, TJ. Microcirculation as a target for the anti-inflammatory properties of statins. Microcirculation. (2002) 9:431–42.
45. Shovman, O, Levy, Y, Gilburd, B, and Shoenfeld, Y. Antiinflammatory and immunomodulatory properties of statins. Immunol Res. (2002) 25:271–86. doi: 10.1385/IR:25:3:271
46. Weitz-Schmidt, G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. (2002) 23:482–7. doi: 10.1016/S0165-6147(02)02077-1
47. Neergaard-Petersen, S, Hvas, AM, Kristensen, SD, and Grove, EL. Platelets and antiplatelet therapy in patients with coronary artery disease and diabetes. Semin Thromb Hemost. (2016) 42:234–41. doi: 10.1055/s-0036-1571308
48. Wang, D, Zhang, Y, and Shen, C. Research update on the association between SFRP5, an anti-inflammatory adipokine, with obesity, type 2 diabetes mellitus and coronary heart disease. J Cell Mol Med. (2020) 24:2730–5. doi: 10.1111/jcmm.15023
49. Wolkowicz, P, White, CR, and Anantharamaiah, GM. Apolipoprotein mimetic peptides: an emerging therapy against diabetic inflammation and dyslipidemia. Biomol Ther. (2021) 11:627. doi: 10.3390/biom11050627
50. Kocyigit, D, Gurses, KM, and Tokgozoglu, L. Anti-inflammatory therapy in atherosclerosis. Front Biosci. (2020) 25:242–69. doi: 10.2741/4805
51. Nunes, CDR, Barreto Arantes, M, de Faria, M, Pereira, S, Leandro da Cruz, L, de Souza, PM, et al. Plants as sources of anti-inflammatory agents. Molecules. (2020) 25. doi: 10.3390/molecules25163726
52. Pedro-Botet, J, Climent, E, and Benaiges, D. Atherosclerosis and inflammation. New therapeutic approaches. Med Clin. (2020) 155:256–62. doi: 10.1016/j.medcli.2020.04.024
53. Tasneem, S, Liu, B, Li, B, Choudhary, MI, and Wang, W. Molecular pharmacology of inflammation: medicinal plants as anti-inflammatory agents. Pharmacol Res. (2019) 139:126–40. doi: 10.1016/j.phrs.2018.11.001
54. Bei, Y, Shi, C, Zhang, Z, and Xiao, J. Advance for cardiovascular health in China. J Cardiovasc Transl Res. (2019) 12:165–70. doi: 10.1007/s12265-018-9852-7
55. Zhao, D, Liu, J, Wang, M, Zhang, X, and Zhou, M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16:203–12. doi: 10.1038/s41569-018-0119-4
56. Kaptoge, S, Seshasai, SR, Gao, P, Freitag, DF, Butterworth, AS, Borglykke, A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. (2014) 35:578–89. doi: 10.1093/eurheartj/eht367
57. Golia, E, Limongelli, G, Natale, F, Fimiani, F, Maddaloni, V, Pariggiano, I, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. (2014) 16:435. doi: 10.1007/s11883-014-0435-z
58. Badimon, L, and Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. (2014) 276:618–32. doi: 10.1111/joim.12296
59. Hansson, GK, Robertson, AK, and Söderberg-Nauclér, C. Inflammation and atherosclerosis. Annu Rev Pathol. (2006) 1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100
60. Jebari-Benslaiman, S, Galicia-García, U, Larrea-Sebal, A, Olaetxea, JR, Alloza, I, Vandenbroeck, K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. (2022) 23:3346. doi: 10.3390/ijms23063346
61. Ortega-Paz, L, Capodanno, D, and Angiolillo, DJ. Canakinumab for secondary prevention of coronary artery disease. Futur Cardiol. (2021) 17:427–42. doi: 10.2217/fca-2020-0211
62. Xia, M, Yang, X, and Qian, C. Meta-analysis evaluating the utility of colchicine in secondary prevention of coronary artery disease. Am J Cardiol. (2021) 140:33–8. doi: 10.1016/j.amjcard.2020.10.043
63. Núñez, E, Fuster, V, Gómez-Serrano, M, Valdivielso, JM, Fernández-Alvira, JM, Martínez-López, D, et al. Unbiased plasma proteomics discovery of biomarkers for improved detection of subclinical atherosclerosis. EBioMedicine. (2022) 76:103874. doi: 10.1016/j.ebiom.2022.103874
64. Kosmas, CE, Silverio, D, Sourlas, A, Montan, PD, Guzman, E, and Garcia, MJ. Anti-inflammatory therapy for cardiovascular disease. Ann Transl Med. (2019) 7:147. doi: 10.21037/atm.2019.02.34
65. Ridker, PM. Establishing a clinical basis for hsCRP in the prevention and treatment of cardiovascular disease. Clin Chem. (2010) 56:1186–7. doi: 10.1373/clinchem.2009.142240
66. Assadpour, PM. The correlation between high-sensitivity C-reactive protein (hsCRP) serum levels and severity of coronary atherosclerosis. Int Cardiovasc Res J. (2014) 8:6–8.
67. Pearson, TA, Mensah, GA, Alexander, RW, Anderson, JL, Cannon, RO 3rd, Criqui, M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
68. Kelkar, A, Kuo, A, and Frishman, WH. Allopurinol as a cardiovascular drug. Cardiol Rev. (2011) 19:265–71. doi: 10.1097/CRD.0b013e318229a908
69. Okafor, ON, Farrington, K, and Gorog, DA. Allopurinol as a therapeutic option in cardiovascular disease. Pharmacol Ther. (2017) 172:139–50. doi: 10.1016/j.pharmthera.2016.12.004
70. Akodad, M, Sicard, P, Fauconnier, J, and Roubille, F. Colchicine and myocardial infarction: a review. Arch Cardiovasc Dis. (2020) 113:652–9. doi: 10.1016/j.acvd.2020.04.007
71. Bouabdallaoui, N, and Tardif, JC. Colchicine in the management of acute and chronic coronary artery disease. Curr Cardiol Rep. (2021) 23:120. doi: 10.1007/s11886-021-01560-w
72. Deftereos, SG, Beerkens, FJ, Shah, B, Giannopoulos, G, Vrachatis, DA, Giotaki, SG, et al. Colchicine in cardiovascular disease: in-depth review. Circulation. (2022) 145:61–78. doi: 10.1161/CIRCULATIONAHA.121.056171
73. Gasparyan, AY, Ayvazyan, L, Yessirkepov, M, and Kitas, GD. Colchicine as an anti-inflammatory and cardioprotective agent. Expert Opin Drug Metab Toxicol. (2015) 11:1781–94. doi: 10.1517/17425255.2015.1076391
74. Martínez, GJ, Celermajer, DS, and Patel, S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. (2018) 269:262–71. doi: 10.1016/j.atherosclerosis.2017.12.027
75. Kaul, S, Gupta, M, Bandyopadhyay, D, Hajra, A, Deedwania, P, Roddy, E, et al. Gout pharmacotherapy in cardiovascular diseases: a review of utility and outcomes. Am J Cardiovasc Drugs. (2021) 21:499–512. doi: 10.1007/s40256-020-00459-1
76. Bindu, S, Mazumder, S, and Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. (2020) 180:114147. doi: 10.1016/j.bcp.2020.114147
77. Dajani, EZ, and Islam, K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. (2008) 59:117–33.
78. Marsico, F, Paolillo, S, and Filardi, PP. NSAIDs and cardiovascular risk. J Cardiovasc Med. (2017) 18:e40–e43. doi: 10.2459/JCM.0000000000000443
79. Borer, JS, and Simon, LS. Cardiovascular and gastrointestinal effects of COX-2 inhibitors and NSAIDs: achieving a balance. Arthritis Res Ther. (2005) 7:S14–22. doi: 10.1186/ar1794
80. Funk, CD, and FitzGerald, GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. (2007) 50:470–9. doi: 10.1097/FJC.0b013e318157f72d
81. Harirforoosh, S, Asghar, W, and Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. (2013) 16:821–47. doi: 10.18433/J3VW2F
82. Davies, NM, Reynolds, JK, Undeberg, MR, Gates, BJ, Ohgami, Y, and Vega-Villa, KR. Minimizing risks of NSAIDs: cardiovascular, gastrointestinal and renal. Expert Rev Neurother. (2006) 6:1643–55. doi: 10.1586/14737175.6.11.1643
83. Scheiman, JM, and Hindley, CE. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther. (2010) 32:667–77. doi: 10.1016/j.clinthera.2010.04.009
84. Tong, Y, Wang, Z, Cai, L, Lin, L, Liu, J, and Cheng, J. NLRP3 Inflammasome and its central role in the cardiovascular diseases. Oxidative Med Cell Longev. (2020) 2020:1–8. doi: 10.1155/2020/4293206
85. Abbate, A, Toldo, S, Marchetti, C, Kron, J, Van Tassell, BW, and Dinarello, CA. Interleukin-1 and the Inflammasome as therapeutic targets in cardiovascular disease. Circ Res. (2020) 126:1260–80. doi: 10.1161/CIRCRESAHA.120.315937
86. Grebe, A, Hoss, F, and Latz, E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. (2018) 122:1722–40. doi: 10.1161/CIRCRESAHA.118.311362
87. Denegri, A, and Boriani, G. High sensitivity C-reactive protein (hsCRP) and its implications in cardiovascular outcomes. Curr Pharm Des. (2021) 27:263–75. doi: 10.2174/1381612826666200717090334
88. Ridker, PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. (2016) 67:712–23. doi: 10.1016/j.jacc.2015.11.037
89. Ridker, PM. From C-reactive protein to Interleukin-6 to Interleukin-1: moving upstream to identify novel targets for Atheroprotection. Circ Res. (2016) 118:145–56. doi: 10.1161/CIRCRESAHA.115.306656
90. Zhen, Y, and Zhang, H. NLRP3 Inflammasome and inflammatory bowel disease. Front Immunol. (2019) 10:276. doi: 10.3389/fimmu.2019.00276
91. Shi, X, Xie, WL, Kong, WW, Chen, D, and Qu, P. Expression of the NLRP3 Inflammasome in carotid atherosclerosis. J Stroke Cerebrovasc Dis. (2015) 24:2455–66. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.024
92. Bakhshi, S, and Shamsi, S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: latest evidence and therapeutic outcomes. Int Immunopharmacol. (2022) 106:108595. doi: 10.1016/j.intimp.2022.108595
93. van der Heijden, T, Kritikou, E, Venema, W, van Duijn, J, van Santbrink, PJ, Slütter, B, et al. NLRP3 Inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler Thromb Vasc Biol. (2017) 37:1457–61. doi: 10.1161/ATVBAHA.117.309575
94. Hettwer, J, Hinterdobler, J, Miritsch, B, Deutsch, MA, Li, X, Mauersberger, C, et al. Interleukin-1β suppression dampens inflammatory leucocyte production and uptake in atherosclerosis. Cardiovasc Res. (2022) 118:2778–91. doi: 10.1093/cvr/cvab337
95. Toldo, S, Mezzaroma, E, Buckley, LF, Potere, N, Di Nisio, M, Biondi-Zoccai, G, et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol Ther. (2022) 236:108053. doi: 10.1016/j.pharmthera.2021.108053
96. Zhang, T, Hou, D, He, J, Zeng, X, Liu, R, Liu, L, et al. Oxidative-damaged mitochondria activate GABARAPL1-induced NLRP3 Inflammasomes in an Autophagic-exosome manner after acute myocardial ischemia. Oxidative Med Cell Longev. (2022) 2022:1–19. doi: 10.1155/2022/7958542
97. Chatterjee, S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler Thromb Vasc Biol. (1998) 18:1523–33. doi: 10.1161/01.ATV.18.10.1523
98. Lopes-Virella, MF, and Virella, G. Immune mechanisms of atherosclerosis in diabetes mellitus. Diabetes. (1992) 41:86–91. doi: 10.2337/diab.41.2.S86
99. Brand, K, Page, S, Walli, AK, Neumeier, D, and Baeuerle, PA. Role of nuclear factor-kappa B in atherogenesis. Exp Physiol. (1997) 82:297–304. doi: 10.1113/expphysiol.1997.sp004025
100. Thurberg, BL, and Collins, T. The nuclear factor-kappa B/inhibitor of kappa B autoregulatory system and atherosclerosis. Curr Opin Lipidol. (1998) 9:387–96. doi: 10.1097/00041433-199810000-00002
101. Luo, JY, Liu, F, Fang, BB, Tian, T, Li, YH, Zhang, T, et al. NFKB1 gene mutant was associated with prognosis of coronary artery disease and exacerbated endothelial mitochondrial fission and dysfunction. Oxidative Med Cell Longev. (2022) 2022:1–13. doi: 10.1155/2022/9494926
102. Yang, S, Yuan, HQ, Hao, YM, Ren, Z, Qu, SL, Liu, LS, et al. Macrophage polarization in atherosclerosis. Clin Chim Acta. (2020) 501:142–6. doi: 10.1016/j.cca.2019.10.034
103. Kang, X, Jiao, T, Wang, H, Pernow, J, and Wirdefeldt, K. Mendelian randomization study on the causal effects of tumor necrosis factor inhibition on coronary artery disease and ischemic stroke among the general population. EBioMedicine. (2022) 76:103824. doi: 10.1016/j.ebiom.2022.103824
104. Liu, YC, Zou, XB, Chai, YF, and Yao, YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. (2014) 10:520–9. doi: 10.7150/ijbs.8879
105. Albert, MA, Danielson, E, Rifai, N, Ridker, PM, and Investigators, P. Effect of statin therapy on C-reactive protein levels - the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. (2001) 286:64–70. doi: 10.1001/jama.286.1.64
106. Bickel, C, Rupprecht, HJ, Blankenberg, S, Espiniola-Klein, C, Schlitt, A, Rippin, G, et al. Relation of markers of inflammation (C-reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long-term mortality in patients with angiographically proven coronary artery disease. Am J Cardiol. (2002) 89:901–8. doi: 10.1016/S0002-9149(02)02236-1
107. Chan, DC, Watts, GF, Barrett, PHR, Beilin, LJ, and Mori, TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. Clin Chem. (2002) 48:877–83. doi: 10.1093/clinchem/48.6.877
108. Leiherer, A, Ulmer, H, Muendlein, A, Saely, CH, Vonbank, A, Fraunberger, P, et al. Value of total cholesterol readings earlier versus later in life to predict cardiovascular risk. EBioMedicine. (2021) 67:103371. doi: 10.1016/j.ebiom.2021.103371
109. Puri, R, Nissen, SE, Libby, P, Shao, M, Ballantyne, CM, Barter, PJ, et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. (2013) 128:2395–403. doi: 10.1161/CIRCULATIONAHA.113.004243
110. Dykun, I, Clark, D 3rd, Carlo, J, Lincoff, AM, Menon, V, Nissen, SE, et al. Longitudinal high-sensitivity C-reactive protein and longer-term cardiovascular outcomes in optimally-treated patients with high-risk vascular disease. Am J Cardiol. (2022) 181:1–8. doi: 10.1016/j.amjcard.2022.06.061
111. Bahrami, A, Liberale, L, Reiner, Ž, Carbone, F, Montecucco, F, and Sahebkar, A. Inflammatory biomarkers for cardiovascular risk stratification in familial hypercholesterolemia. Rev Physiol Biochem Pharmacol. (2020) 177:25–52. doi: 10.1007/112_2020_26
112. Luo, Y, Guo, Y, Wang, H, Yu, M, Hong, K, Li, D, et al. Phospholipid nanoparticles: therapeutic potentials against atherosclerosis via reducing cholesterol crystals and inhibiting inflammation. EBioMedicine. (2021) 74:103725. doi: 10.1016/j.ebiom.2021.103725
113. Zhang, FS, He, QZ, Qin, CH, Little, PJ, Weng, JP, and Xu, SW. Therapeutic potential of colchicine in cardiovascular medicine: a pharmacological review. Acta Pharmacol Sin. (2022) 43:2173–90. doi: 10.1038/s41401-021-00835-w
114. Sun, KJ, Liu, LL, Hu, JH, Chen, YY, and Xu, DY. Methotrexate can prevent cardiovascular events in patients with rheumatoid arthritis an updated meta-analysis. Medicine. (2021) 100:e24579. doi: 10.1097/MD.0000000000024579
115. Wang, HM, Jiang, M, Li, X, Zhao, YZ, Shao, JJ, Liu, ZF, et al. Anti-inflammatory therapies for coronary heart disease: a systematic review and meta-analysis. Front Cardiovasc Med. (2021) 8:8. doi: 10.3389/fcvm.2021.726341
116. Wudexi, I, Shokri, E, Abo-Aly, M, Shindo, K, and Abdel-Latif, A. Comparative effectiveness of anti-inflammatory drug treatments in coronary heart disease patients: a systematic review and network meta-analysis. Mediat Inflamm. (2021) 2021:1–17. doi: 10.1155/2021/5160728
117. Essandoh, K, Li, Y, Huo, J, and Fan, GC. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. (2016) 46:122–31. doi: 10.1097/SHK.0000000000000604
118. Hamidzadeh, K, Christensen, SM, Dalby, E, Chandrasekaran, P, and Mosser, DM. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol. (2017) 79:567–92. doi: 10.1146/annurev-physiol-022516-034348
119. Kuznetsova, T, Prange, KHM, Glass, CK, and de Winther, MPJ. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. (2020) 17:216–28. doi: 10.1038/s41569-019-0265-3
120. Giugliano, D, Ceriello, A, and Esposito, K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. (2006) 48:677–85. doi: 10.1016/j.jacc.2006.03.052
121. Simopoulos, AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. (2002) 21:495–505. doi: 10.1080/07315724.2002.10719248
122. Weitz, D, Weintraub, H, Fisher, E, and Schwartzbard, AZ. Fish oil for the treatment of cardiovascular disease. Cardiol Rev. (2010) 18:258–63. doi: 10.1097/CRD.0b013e3181ea0de0
123. Capodanno, D, and Angiolillo, DJ. Canakinumab for secondary prevention of atherosclerotic disease. Expert Opin Biol Ther. (2018) 18:215–20. doi: 10.1080/14712598.2018.1420776
124. Libby, P, and Everett, BM. Novel Antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol. (2019) 39:538–45. doi: 10.1161/ATVBAHA.118.310958
125. Lorenzatti, A, and Servato, ML. Role of anti-inflammatory interventions in coronary artery disease: understanding the Canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Eur Cardiol. (2018) 13:38–41. doi: 10.15420/ecr.2018.11.1
126. Lutgens, E, Atzler, D, Döring, Y, Duchene, J, Steffens, S, and Weber, C. Immunotherapy for cardiovascular disease. Eur Heart J. (2019) 40:3937–46. doi: 10.1093/eurheartj/ehz283
127. Dinarello, CA, Simon, A, and van der Meer, JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. (2012) 11:633–52. doi: 10.1038/nrd3800
128. Iwata, Y, Ito, S, Wakabayashi, S, and Kitakaze, M. TRPV2 channel as a possible drug target for the treatment of heart failure. Lab Invest. (2020) 100:207–17. doi: 10.1038/s41374-019-0349-z
129. Ridker, PM, MacFadyen, JG, Thuren, T, and Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J. (2020) 41:2153–63. doi: 10.1093/eurheartj/ehz542
130. Ridker, PM, Libby, P, MacFadyen, JG, Thuren, T, Ballantyne, C, Fonseca, F, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Eur Heart J. (2018) 39:3499–507. doi: 10.1093/eurheartj/ehy310
131. Huse, C, Anstensrud, AK, Michelsen, AE, Ueland, T, Broch, K, Woxholt, S, et al. Interleukin-6 inhibition in ST-elevation myocardial infarction: immune cell profile in the randomised ASSAIL-MI trial. EBioMedicine. (2022) 80:104013. doi: 10.1016/j.ebiom.2022.104013
132. de Abajo, FJ, Gil, MJ, Rodríguez, A, García-Poza, P, Álvarez, A, Bryant, V, et al. Allopurinol use and risk of non-fatal acute myocardial infarction. Heart. (2015) 101:679–85. doi: 10.1136/heartjnl-2014-306670
133. Mackenzie, IS, Hawkey, CJ, Ford, I, Greenlaw, N, Pigazzani, F, Rogers, A, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet. (2022) 400:1195–205. doi: 10.1016/S0140-6736(22)01657-9
134. Chen, T, Liu, G, and Yu, B. Colchicine for coronary artery disease: a review. Front Cardiovasc Med. (2022) 9:892588. doi: 10.3389/fcvm.2022.892588
135. González, L, Bulnes, JF, Orellana, MP, Muñoz Venturelli, P, and Martínez, RG. The role of colchicine in atherosclerosis: from bench to bedside. Pharmaceutics. (2022) 14:1395. doi: 10.3390/pharmaceutics14071395
136. Nidorf, SM, Eikelboom, JW, Budgeon, CA, and Thompson, PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. (2013) 61:404–10. doi: 10.1016/j.jacc.2012.10.027
137. Tardif, JC, Kouz, S, Waters, DD, Bertrand OF, Diaz, R, Maggioni, AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
138. Vaidya, K, Arnott, C, Martínez, GJ, Ng, B, McCormack, S, Sullivan, DR, et al. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. J Am Coll Cardiol Img. (2018) 11:305–16. doi: 10.1016/j.jcmg.2017.08.013
139. Bytyçi, I, Bajraktari, G, Penson, PE, Henein, MY, and Banach, M. Efficacy and safety of colchicine in patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Br J Clin Pharmacol. (2022) 88:1520–8. doi: 10.1111/bcp.15041
140. Fiolet, ATL, Opstal, TSJ, Mosterd, A, Eikelboom, JW, Jolly, SS, Keech, AC, et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. (2021) 42:2765–75. doi: 10.1093/eurheartj/ehab115
141. Kofler, T, Kurmann, R, Lehnick, D, Cioffi, GM, Chandran, S, Attinger-Toller, A, et al. Colchicine in patients with coronary artery disease: a systematic review and meta-analysis of randomized trials. J Am Heart Assoc. (2021) 10:e021198. doi: 10.1161/JAHA.121.021198
142. Ma, Z, Chen, J, Jin, K, and Chen, X. Colchicine and coronary heart disease risks: a meta-analysis of randomized controlled clinical trials. Front Cardiovasc Med. (2022) 9:947959. doi: 10.3389/fcvm.2022.947959
143. Tong, DC, Quinn, S, Nasis, A, Hiew, C, Roberts-Thomson, P, Adams, H, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation. (2020) 142:1890–900. doi: 10.1161/CIRCULATIONAHA.120.050771
144. Tong, DC, Bloom, JE, Quinn, S, Nasis, A, Hiew, C, Roberts-Thomson, P, et al. Colchicine in patients with acute coronary syndrome: two-year follow-up of the Australian COPS randomized clinical trial. Circulation. (2021) 144:1584–6. doi: 10.1161/CIRCULATIONAHA.121.054610
145. Everett, BM, MacFadyen, JG, Thuren, T, Libby, P, Glynn, RJ, and Ridker, PM. Inhibition of interleukin-1β and reduction in Atherothrombotic cardiovascular events in the CANTOS trial. J Am Coll Cardiol. (2020) 76:1660–70. doi: 10.1016/j.jacc.2020.08.011
146. Yuan, S, Yu, L, Gou, W, Wang, L, Sun, J, Li, D, et al. Health effects of high serum calcium levels: updated phenome-wide Mendelian randomisation investigation and review of Mendelian randomisation studies. EBioMedicine. (2022) 76:103865. doi: 10.1016/j.ebiom.2022.103865
147. Abdelhamid, AS, Brown, TJ, Brainard, JS, Biswas, P, Thorpe, GC, Moore, HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2018) 11:CD003177. doi: 10.1002/14651858.CD003177.pub4
148. Ajith, TA, and Jayakumar, TG. Omega-3 fatty acids in coronary heart disease: recent updates and future perspectives. Clin Exp Pharmacol Physiol. (2019) 46:11–8. doi: 10.1111/1440-1681.13034
149. Jain, AP, Aggarwal, KK, and Zhang, PY. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. (2015) 19:441–5.
150. Fan, X, Li, A, Yan, Z, Geng, X, Lian, L, Lv, H, et al. From iron metabolism to Ferroptosis: pathologic changes in coronary heart disease. Oxidative Med Cell Longev. (2022) 2022:1–14. doi: 10.1155/2022/6291889
151. Paruchuri, K, Finneran, P, Marston, NA, Healy, EW, Andreo, J Jr, Lynch, R, et al. Outcomes of a smartphone-based application with live health-coaching post-percutaneous coronary intervention. EBioMedicine. (2021) 72:103593. doi: 10.1016/j.ebiom.2021.103593
152. Mitroulis, I, Skendros, P, and Ritis, K. Targeting IL-1β in disease; the expanding role of NLRP3 inflammasome. Eur J Intern Med. (2010) 21:157–63. doi: 10.1016/j.ejim.2010.03.005
153. Ridker, PM, MacFadyen, JG, Thuren, T, Libby, P, and Grp, CT. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1 beta inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J. (2020) 41:2153–63. doi: 10.1093/eurheartj/ehz542
154. Robertson, S, Martinez, GJ, Payet, CA, Barraclough, JY, Celermajer, DS, Bursill, C, et al. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci. (2016) 130:1237–46. doi: 10.1042/CS20160090
155. Westerterp, M, Fotakis, P, Ouimet, M, Bochem, AE, Zhang, H, Molusky, MM, et al. Cholesterol efflux pathways suppress Inflammasome activation, NETosis, and Atherogenesis. Circulation. (2018) 138:898–912. doi: 10.1161/CIRCULATIONAHA.117.032636
156. Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. (2012) 8:1237–47. doi: 10.7150/ijbs.4989
157. Ridker, PM. From CANTOS to CIRT to COLCOT to clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation. (2020) 141:787–9. doi: 10.1161/CIRCULATIONAHA.119.045256
158. Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. (2021) 117:2525–36. doi: 10.1093/cvr/cvab303
159. Libby, P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
160. Libby, P. Inflammation in atherosclerosis-no longer a theory. Clin Chem. (2021) 67:131–42. doi: 10.1093/clinchem/hvaa275
161. Sherratt, SCR, Libby, P, Bhatt, DL, and Mason, RP. A biological rationale for the disparate effects of omega-3 fatty acids on cardiovascular disease outcomes. Prostaglandins Leukot Essent Fatty Acids. (2022) 182:102450. doi: 10.1016/j.plefa.2022.102450
162. Soehnlein, O, and Libby, P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. (2021) 20:589–610. doi: 10.1038/s41573-021-00198-1
163. Colin, S, Chinetti-Gbaguidi, G, and Staels, B. Macrophage phenotypes in atherosclerosis. Immunol Rev. (2014) 262:153–66. doi: 10.1111/imr.12218
164. Moore, KJ, Koplev, S, Fisher, EA, Tabas, I, Björkegren, JLM, Doran, AC, et al. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (part 2). J Am Coll Cardiol. (2018) 72:2181–97. doi: 10.1016/j.jacc.2018.08.2147
165. Bilal, M, Ashraf, S, and Zhao, X. Dietary component-induced inflammation and its amelioration by prebiotics, probiotics, and Synbiotics. Front Nutr. (2022) 9:931458. doi: 10.3389/fnut.2022.931458
166. Liang, B, Cai, XY, and Gu, N. Marine natural products and coronary artery disease. Front Cardiovasc Med. (2021) 8:739932. doi: 10.3389/fcvm.2021.739932
167. Hatware, KV, Sharma, S, Patil, K, Rajput, H, and Gupta, G. Therapeutic role of natural agents in the Management of Coronary Artery Disease: a review. J Environ Pathol Toxicol Oncol. (2020) 39:159–77. doi: 10.1615/JEnvironPatholToxicolOncol.2020033025
168. Wu, GS, Li, HK, and Zhang, WD. Metabolomics and its application in the treatment of coronary heart disease with traditional Chinese medicine. Chin J Nat Med. (2019) 17:321–30. doi: 10.1016/S1875-5364(19)30037-8
169. Calderon-Montano, JM, Burgos-Moron, E, Perez-Guerrero, C, and Lopez-Lazaro, M. A review on the dietary flavonoid Kaempferol. Mini-Rev Med Chem. (2011) 11:298–344. doi: 10.2174/138955711795305335
170. Garcia-Lafuente, A, Guillamon, E, Villares, A, Rostagno, MA, and Martinez, JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. (2009) 58:537–52. doi: 10.1007/s00011-009-0037-3
171. McKay, TB, and Karamichos, D. Quercetin and the ocular surface: what we know and where we are going. Exp Biol Med. (2017) 242:565–72. doi: 10.1177/1535370216685187
172. Orhan, IE. Phytochemical and pharmacological activity profile of Crataegus oxyacantha L. (hawthorn) - a Cardiotonic herb. Curr Med Chem. (2018) 25:4854–65. doi: 10.2174/0929867323666160919095519
173. Bolling, BW, McKay, DL, and Blumberg, JB. The phytochemical composition and antioxidant actions of tree nuts. Asia Pac J Clin Nutr. (2010) 19:117–23.
174. Mozos, I, Stoian, D, and Luca, CT. Crosstalk between vitamins a, B12, D, K, C, and E status and arterial stiffness. Dis Markers. (2017) 2017:1–14. doi: 10.1155/2017/8784971
175. Raposo, MFD, and de Morais, A. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. (2015) 125:32–41. doi: 10.1016/j.lfs.2014.09.018
176. Wallert, M, Schmolz, L, Galli, F, Birringer, M, and Lorkowski, S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. (2014) 2:495–503. doi: 10.1016/j.redox.2014.02.002
177. Rasines-Perea, Z, and Teissedre, PL. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules. (2017) 22:68. doi: 10.3390/molecules22010068
178. Singh, B, Singh, JP, Kaur, A, and Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res Int. (2020) 132:109114. doi: 10.1016/j.foodres.2020.109114
Keywords: anti-inflammatory, coronary heart disease, atherosclerosis, C-reactive protein, bibliometric analysis, inflammation
Citation: Zhang J, Ji C, Zhai X, Tong H and Hu J (2023) Frontiers and hotspots evolution in anti-inflammatory studies for coronary heart disease: A bibliometric analysis of 1990–2022. Front. Cardiovasc. Med. 10:1038738. doi: 10.3389/fcvm.2023.1038738
Received: 07 September 2022; Accepted: 17 January 2023;
Published: 16 February 2023.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Yang Ye, Peking University Third Hospital, ChinaCopyright © 2023 Zhang, Ji, Zhai, Tong and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Zhai, ✉ amFtZXN6aGFpMzRAMTYzLmNvbQ==; Hongxuan Tong, ✉ dGhvbmd4dWFuMUAxNjMuY29t; Jingqing Hu, ✉ Z2NwMzA2QDEyNi5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.