
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 09 February 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1038337
This article is part of the Research TopicInsights in Cardiovascular Imaging: 2022View all 29 articles
 Djawid Hashemi1,2,3*
Djawid Hashemi1,2,3* Patrick Doeblin2,3
Patrick Doeblin2,3 Moritz Blum4
Moritz Blum4 Karl Jakob Weiss2,3
Karl Jakob Weiss2,3 Matthias Schneider1,2
Matthias Schneider1,2 Rebecca Beyer2,3
Rebecca Beyer2,3 Burkert Pieske1,2,3
Burkert Pieske1,2,3 Hans-Dirk Duengen1,2
Hans-Dirk Duengen1,2 Frank Edelmann1,2
Frank Edelmann1,2 Sebastian Kelle1,2,3
Sebastian Kelle1,2,3Aims: Heart failure (HF) does not only reduce the life expectancy in patients, but their life is also often limited by HF symptoms leading to a reduced quality of life (QoL) and a diminished exercise capacity. Novel parameters in cardiac imaging, including both global and regional myocardial strain imaging, promise to contribute to better patient characterization and ultimately to better patient management. However, many of these methods are not part of clinical routine yet, their associations with clinical parameters have been poorly studied. An imaging parameters that also indicate the clinical symptom burden of HF patients would make cardiac imaging more robust toward incomplete clinical information and support the clinical decision process.
Methods and results: This prospective study conducted at two centers in Germany between 2017 and 2018 enrolled stable outpatient subjects with HF [n = 56, including HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), and HF with preserved ejection fraction (HFpEF)] and a control cohort (n = 19). Parameters assessed included measures for external myocardial function, for example, cardiac index and myocardial deformation measurements by cardiovascular magnetic resonance imaging, left ventricular global longitudinal strain (GLS), the global circumferential strain (GCS), and the regional distribution of segment deformation within the LV myocardium, as well as basic phenotypical characteristics including the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the 6-minute walk test (6MWT). If less than 80% of the LV segments are preserved in their deformation capacity the functional capacity by 6MWT (6 minutes walking distance: MyoHealth ≥ 80%: 579.8 ± 177.6 m; MyoHealth 60–<80%: 401.3 ± 121.7 m; MyoHealth 40–<60%: 456.4 ± 68.9 m; MyoHealth < 40%: 397.6 ± 125.9 m, overall p-value: 0.03) as well as the symptom burden are significantly impaired (NYHA class: MyoHealth ≥ 80%: 0.6 ± 1.1 m; MyoHealth 60–<80%: 1.7 ± 1.2 m; MyoHealth 40–<60%: 1.8 ± 0.7 m; MyoHealth < 40%: 2.4 ± 0.5 m; overall p-value < 0.01). Differences were also observed in the perceived exertion assessed by on the Borg scale (MyoHealth ≥ 80%: 8.2 ± 2.3 m; MyoHealth 60–<80%: 10.4 ± 3.2 m; MyoHealth 40–<60%: 9.8 ± 2.1 m; MyoHealth < 40%: 11.0 ± 2.9 m; overall p-value: 0.20) as well as quality of life measures (MLHFQ; MyoHealth ≥ 80%: 7.5 ± 12.4 m; MyoHealth 60–<80%: 23.4 ± 23.4 m; MyoHealth 40–<60%: 20.5 ± 21.2 m; MyoHealth < 40%: 27.4 ± 24.4 m; overall p-value: 0.15)–while these differences were not significant.
Conclusion: The share of LV segments with preserved myocardial contraction promises to discriminate between symptomatic and asymptomatic subjects based on the imaging findings, even when the LV ejection fraction is preserved. This finding is promising to make imaging studies more robust toward incomplete clinical information.
Patients with heart failure (HF) are at high risk for mortality and hospitalization and have a high burden of symptoms that alter their function and health-related quality of life (QoL) (1–5). QoL and functional capacities contributing to QoL are major goals in monitoring and treating patients with HF. Although patients with HF and a low QoL may have a higher potential for improvement, they may also be in a stage of the disease that is too advanced to improve (6). Therefore it is important to measure other contributing parameters associated with QoL, disease state and prognosis to better assess the patients (7). QoL measures are often evaluated in clinical trials as patient reported outcome measures become increasingly important, but they are rarely implement in clinical routine. Hence, identifying routine measures reflecting insights into QoL as well as functional capacities will contribute to an improved assessment of HF patients.
Various dimensions including physical capacity influence QoL. Routine measures to assess physical capacities include the semi-objective 6-minute walk tests and the subjective New York Heart Association (NYHA) functional class, often used in clinical trials but rarely in daily routine (8).
Cardiovascular magnetic resonance imaging (CMR) is a comprehensive technique, increasingly accessible and providing not only a high resolution of information on functional cardiac parameters but also information of tissue characteristics. Measurements of cardiac contractility and the assessment of myocardial deformation by strain analyses are an emerging and promising tool to better characterize patients compared to traditional parameters, e.g., left ventricular ejection fraction (LVEF) (9). While LVEF as well as routine strain measurements provide a global impression of cardiac contractility, recent studies showed the relevance of both myocardial deformation per se and the distribution of its impairment assessed by strain measurements characterizes HF patients in more detail (10). The added value of strain compared to LVEF in HF has been highlighted in HFpEF where LVEF is preserved. The MyoHealth score has been introduced as a parameter highlighting the heterogeneity of regions with altered myocardial deformation compared to preserved regional myocardial strain values (10–12). The score is calculated by the ratio of LV segments with preserved myocardial deformation to the total number of LV segments in a 37 segment LV model (10). It has been shown that cardiac remodeling does not present itself simultaneously across all LV segments in HF (10). Various reasons lead to the regional differences of both systolic and diastolic changes including shearing stress induced diffuse fibrosis, altered local gene expression patterns or global metabolic changes of cardiomyocytes (13–16).
We hypothesize that the better characterization of cardiac deformation in HF patients provides information on QoL and functional capacity. Therefore, we aim to evaluate the association of cardiac deformation assessed by the MyoHealth score and parameters for QoL and functional capacity in this analysis.
This study was a prospective study conducted at two centers in Berlin, Germany, the Charité—University Medicine Berlin and the German Heart Centre Berlin, between 2017 and 2018. Its rationale and design have been previously described (10, 17–20).
Briefly, subjects were screened for diagnosed HF and an age of at least 45 years. The initial diagnosis of HF should have been older than 30 days; the patients were required to be in a stable state with no changes in their HF medication and no HF hospitalization within the previous 7 days. HFrEF was defined as diagnosis of HF, increased N terminal pro brain natriuretic peptide (NT-proBNP) (>220 pg/mL) and LVEF < 40%, HFmrEF as the diagnosis of HF, increased NT-proBNP (>220 pg/mL) and 40% ≥ LVEF < 50% as well as HFpEF as diagnosis of HF, increased NT-proBNP (>220 pg/mL) and LVEF ≥ 50% at the time of study inclusion. We did not distinguish between the causes for HF for recruiting patients (10).
Additionally, we recruited subjects without HF or advanced cardiovascular (CV) diseases as controls.
All studies included complied with the Declaration of Helsinki, the protocols were approved by the responsible ethics committees, and all patients gave written informed consent. It was registered at the German Clinical Trials Register (DRKS, registration number: DRKS00015615). The detailed inclusion and exclusion criteria are listed on the webpage of the DRKS.
As previously described, all CMR images were acquired using a 1.5 T (Achieva, Philips Healthcare, Best, The Netherlands) magnetic resonance imaging (MRI) scanner with a five-channel cardiac surface coil in a supine position. All study participants were scanned using the identical comprehensive imaging protocol. The study protocol included initial scouts to determine cardiac imaging planes. Cine images were acquired using a retrospectively gated cine-CMR in cardiac short-axis, vertical long-axis, and horizontal long-axis orientations using a steady-state free precession sequence for volumetry (10, 20). The calculation of the cardiac indices (CIs) is based on the volumetry of the ventricles. Fast strain-encoded (fast-SENC) MRI was used for strain evaluation, as it has been shown to enable quantification of longitudinal and circumferential strain in free breathing and with high reproducibility (21). Images were blinded to strain analysis, cine, and volumetric measurements, respectively. We waived reproducibility analyses based on an analysis that highlighted the robustness of fast-SENC analyses regarding intraobserver and reproducibility variabilities (22).
All images were analyzed offline using commercially available software in accordance with the recent consensus document for quantification of LV function using CMR (23). In the analysis, we included 2 chamber, 3chamber, and 4chamber cine images, and respectively, three preselected mid-ventricle slices from the LV short-axis stack. Image analysis was performed using the software Medis® Suite MR (Medis medical imaging systems, Leiden, The Netherlands, version 3.1) for voluinterme measurements and the software MyoStrain (Myocardial Solutions, Inc., Morrisville, NC, USA, version 5.0) for fast-SENC strain measurements.
The study population was not only categorized by traditional HF entities, but also by the ratio of myocardial segments with preserved deformation to the total number of myocardial segments (n = 37), described as MyoHealth score (illustrated in S1 of Supplementary material; 10–12). Briefly, the MyoHealth score assess the 37 segments of the LV separately, whether the myocardial deformation is altered, i.e., whether the strain value of that segment is >−17%. The MyoHealth score is the proportion of LV segments with preserved and not altered myocardial deformation from the total 37 segments. The MyoHealth entities introduced include 4 groups: MyoHealth > 80%, MyoHealth 60–<80%, MyoHealth 40–<60%, and MyoHealth < 40%.
Based on the MyoHealth score distribution following parameters were assessed: QoL, 6-minute walk test (6MWT) key parameters as well as the New York Heart Association (NYHA) functional classification.
Patients were instructed to cover the maximum distance in 6 min (6-minute walk distance, 6MWD) at a self-graded walking speed, pausing to rest when needed. The test was supervised by the same study staff to minimize the variability. The 6MWD as well as the level of perceived exertion indicated as specific level on the Borg score were recorded. The functional capacities indicated by the New York Heart Association (NYHA) functional classification were also part of the baseline information collected.
In accordance with the study protocol, study participants completed a QoL questionnaire, the Minnesota Living with Heart Failure Questionnaire (MLHFQ) (24).
Statistical analysis was carried out with R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Normality of variables was assessed by visual assessment of normality curves and the Shapiro–Wilk test. Comparison between groups for continuous variables was performed with a one-way ANOVA for normally distributed data. When a significant P-value was obtained using one-way ANOVA, the group means were examined by the Holm–Bonferroni method. Values of P < 0.05 were considered statistically significant. For the comparison of categorical variables between the groups were used the χ2 test.
The ratio of non-altered myocardial deformation was assessed in 71 patients. The baseline characteristics of these patients have been previously reported, in brief the details are presented in Tables 1, 2 (10, 17–20). The difference in the sex distribution between the groups is not significant (χ2 = 5.21, p = 0.157, n = 71) in Table 1. Due to the non-major numbers in each group, we refrain from further testing. Table 2 shows the increasing global strain values, including both global circumferential (GCS) and longitudinal strain (GLS), with smaller MyoHealth values. Simultaneously, the cardiac index remains on the same level across all groups.
The 6MWD was significantly different across groups separated by the MyoHealth score (overall p-value: 0.03). Figure 1 shows the longest 6MWD in the group with the preserved MyoHealth score (MyoHealth ≥ 80%: 579.8 ± 177.6 m) and shorter distances in the other groups (MyoHealth 60–<80%: 401.3 ± 121.7 m; MyoHealth 40–<60%: 456.4 ± 68.9 m; MyoHealth < 40%: 397.6 ± 125.9 m). S2 of Supplementary material highlights the major role of the MyoHealth score in the prediction of the 6MWD when compared to LVEF and the LV global longitudinal strain.
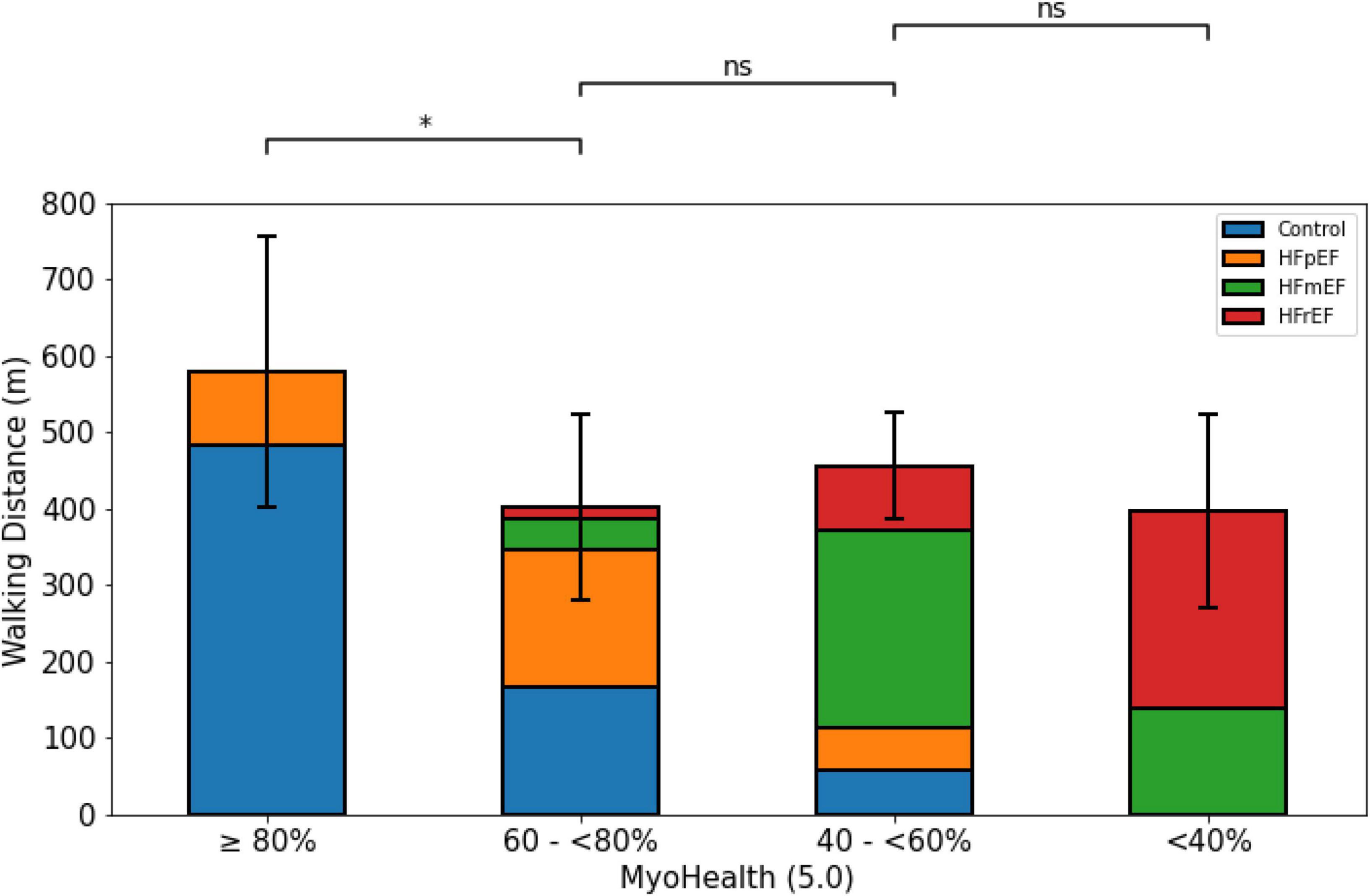
Figure 1. 6-minute walk distance across MyoHealth groups. MyoHealth: ratio of myocardial segments with preserved deformation to the total number of myocardial segments. MyoHealth ≥ 80% vs. MyoHealth 60–<80%: p = 0.03; MyoHealth 60–<80% vs. MyoHealth 40–<60%: p = 0.31; MyoHealth 40–<60% vs. MyoHealth < 40%: p = 0.59. *Significant; ns, not significant.
Figure 2 illustrates the level of perceived exertion at the end of the 6MWT. The overall comparison revealed no difference between the groups (MyoHealth ≥ 80%: 8.2 ± 2.3 m; MyoHealth 60–<80%: 10.4 ± 3.2; MyoHealth 40–<60%: 9.8 ± 2.1; MyoHealth < 40%: 11.0 ± 2.9; overall p-value: 0.20).
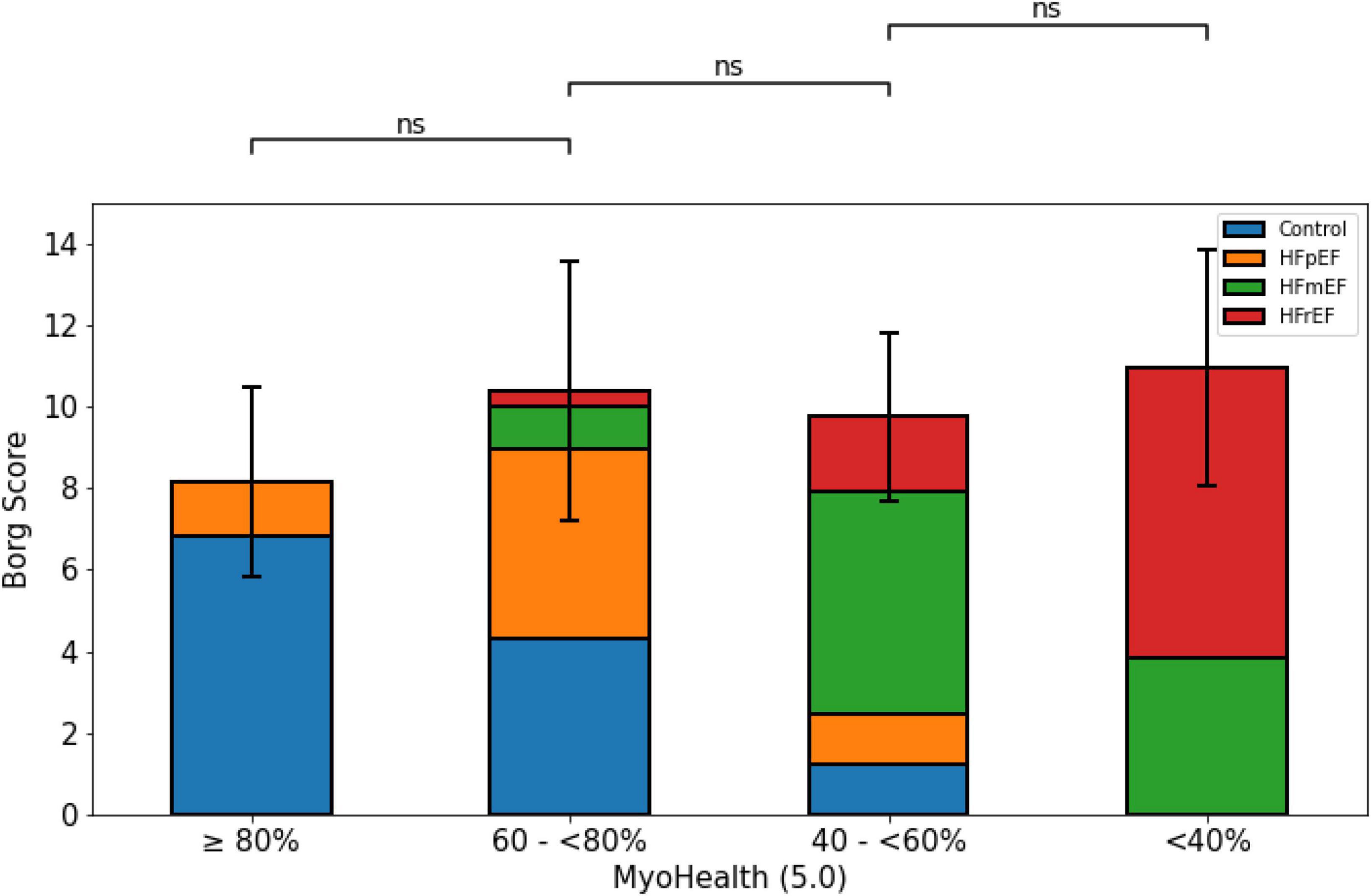
Figure 2. Perceived exertion at the end of the 6-minute walk test across MyoHealth groups. MyoHealth ≥ 80% vs. MyoHealth 60–<80%: p = 0.45; MyoHealth 60–<80% vs. MyoHealth 40–<60%: p = 1; MyoHealth 40–<60% vs. MyoHealth < 40%: p = 0.43. ns, not significant.
Figure 3 demonstrates the QoL measure assessed by the MLHFQ with lower numbers indicating a higher QoL. It shows the lowest MLHFQ score values in the group with preserved myocardial deformation. However, the comparison did not reveal a reliable difference across the study population (MyoHealth ≥ 80%: 7.5 ± 12.4; MyoHealth 60–<80%: 23.4 ± 23.4; MyoHealth 40–<60%: 20.5 ± 21.2; MyoHealth < 40%: 27.4 ± 24.4; overall p-value: 0.15).
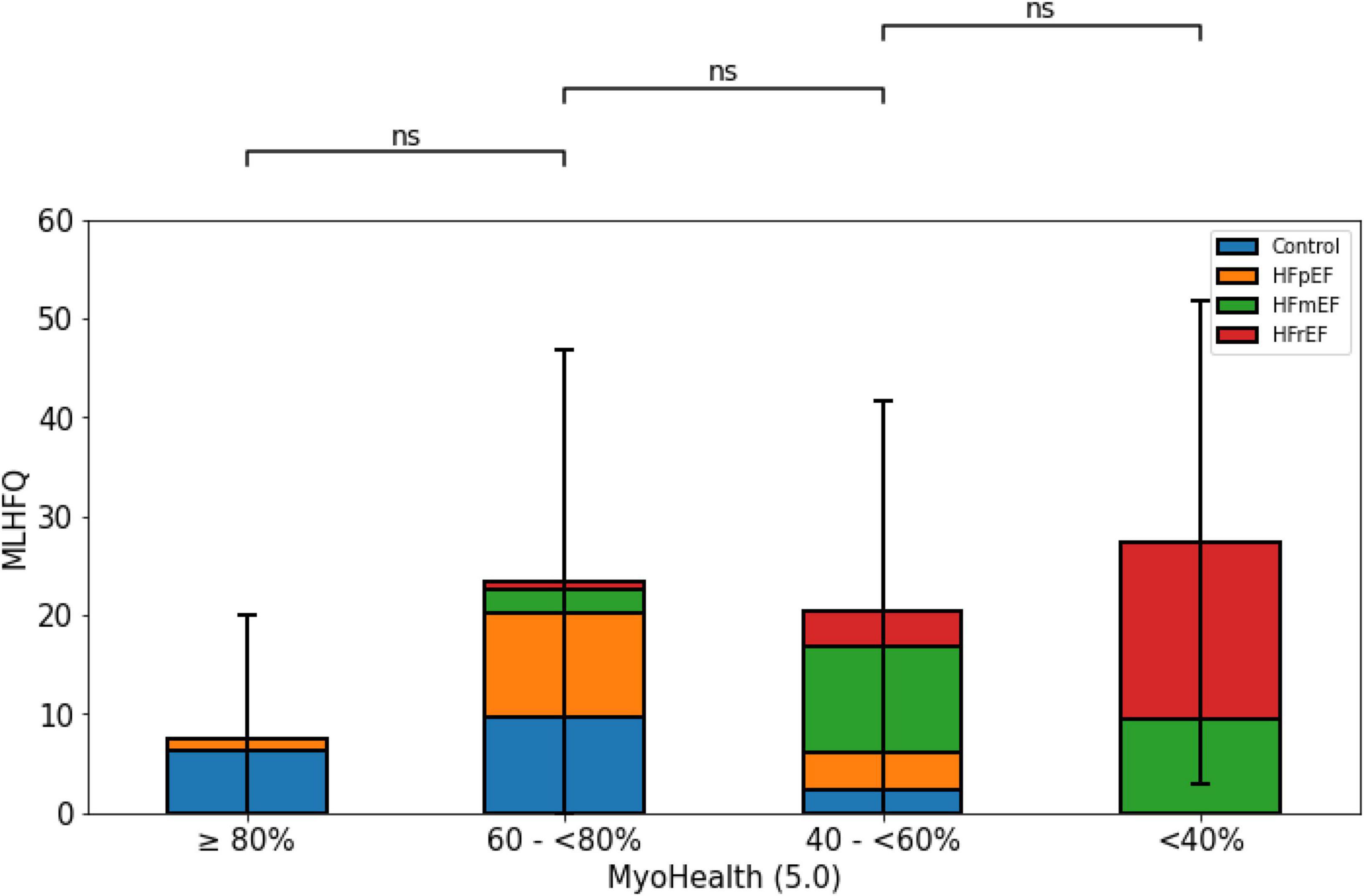
Figure 3. Quality of life by MLHFQ across MyoHealth groups. MyoHealth ≥ 80% vs. MyoHealth 60–<80%: p = 0.15; MyoHealth 60–<80% vs. MyoHealth 40–<60%: p = 1; MyoHealth 40–<60% vs. MyoHealth < 40%: p = 1. ns, not significant.
Figure 4 displays the significant association of the NYHA functional class and the proportion of preserved myocardial segments. While the NYHA class was lowest in the group with a preserved MyoHealth score, it was similarly higher in the groups with decreased score values (MyoHealth ≥ 80%: 0.6 ± 1.1; MyoHealth 60–<80%: 1.7 ± 1.2; MyoHealth 40–<60%: 1.8 ± 0.7; MyoHealth < 40%: 2.4 ± 0.5; overall p-value < 0.01).
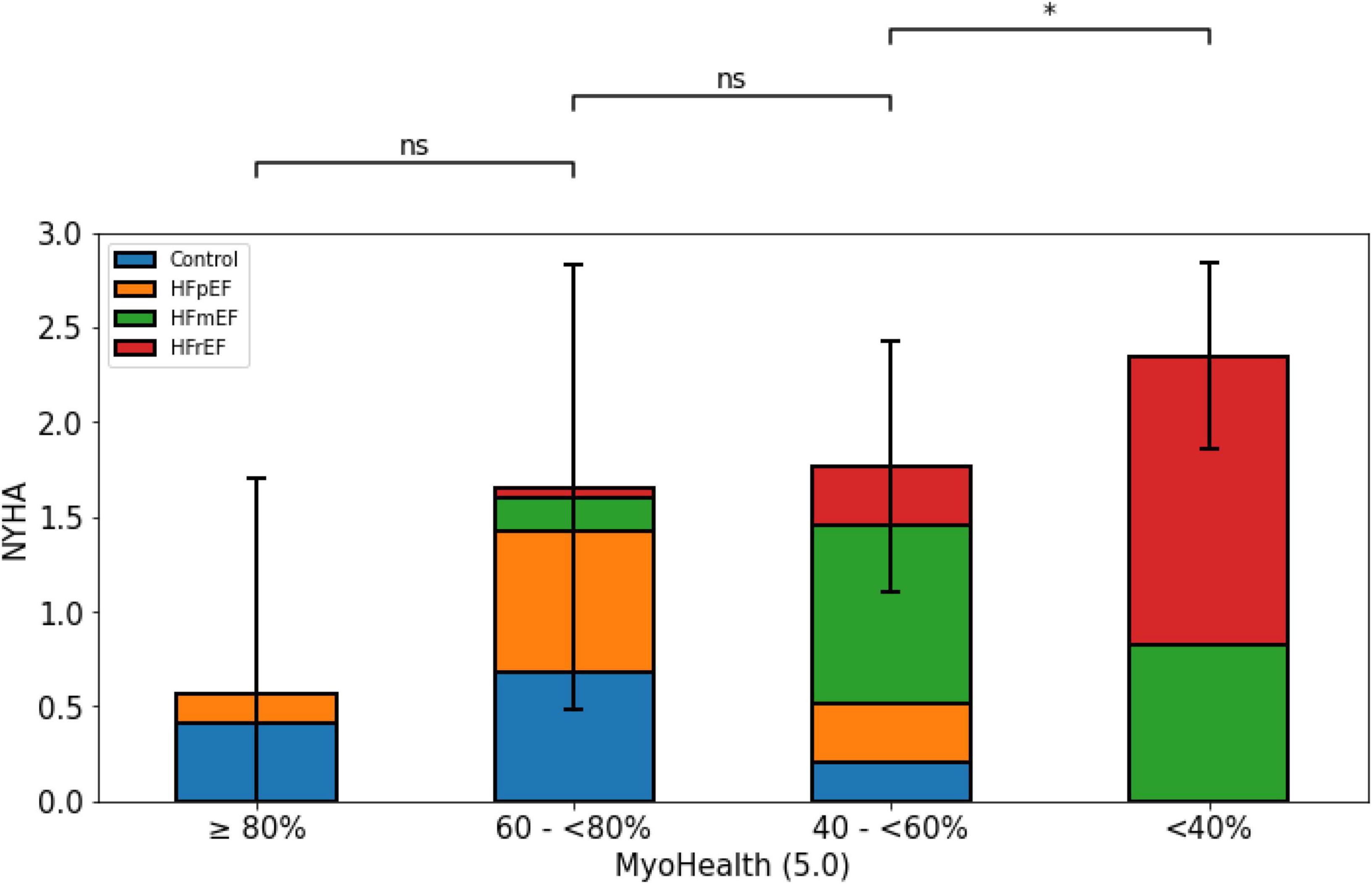
Figure 4. Limitations and symptom burden by NYHA functional class across MyoHealth groups. MyoHealth ≥ 80% vs. MyoHealth 60–<80%: p = 0.15; MyoHealth 60–<80% vs. MyoHealth 40–<60%: p = 1; MyoHealth 40–<60% vs. MyoHealth < 40%: p < 0.01. *Significant; ns, not significant.
In this study, aiming to better characterize HF patients and imaging parameters indicating their symptom burden, we found that the proportion of myocardial segments with preserved myocardial deformation indicates better functional capacity. Furthermore, this exploratory analysis suggests an association of the MyoHealth score with quality of life surrogate parameters in larger study population.
Table 2 shows the consistency of our data. The cardiac index remains on a similar level across all groups who were either healthy subjects or HF patients in a stable outpatient condition. The global strain values (GCS and GLS) were increasing with smaller MyoHealth score values, as the MyoHealth score per se represents the proportion of segments with preserved strain values.
Our observational study of consecutive patients with HF as well as control subjects was carefully designed to better characterize myocardial contraction. After focusing on the contraction pattern and describing the onset of changes in HF in interventricular septal segments, we sought to highlight that CMR scans in HF are not only relevant to characterize HF but to better phenotype patients with regards to parameters limiting their lives on a daily basis (10).
Given the high prevalence of HF and a nearly half of these patients showing nearly normal values with regards to traditional HF parameters, e.g., LVEF, there is an unmet need for innovative tools to diagnose patients and identify those at risk (8). In this analysis we sought to identify a parameter that reflects both an insight to the cardiac contraction with its regional differences and key parameters limiting patients’ everyday experience, functional capacity, and quality of life (8, 25).
The MyoHealth score allows for the estimation of the clinical condition when functional capacity data is not available. If the signal with regards to the QoL assessed by the MLHFQ prove feasible and the differences are significantly different in a larger study population, this might lead to fewer resources than assessing the symptom burden systematically aside from the image acquisition, e.g., by conducting a 6MWT or questionnaires for QoL.
Impaired functional capacity, often assessed by 6MWT, is the main symptom in patients with HF, regardless of LVEF (26–28). With the 6MWD being relevantly reduced in both patients and subjects with a reduced ratio of segments with preserved myocardial deformation, as seen in Figure 1, we could show the association of an innovative parameter reflecting the myocardial contraction with its regional disparities. The validity of this finding is shown in Figure 2, as patients in all groups reached a comparable level of exhaustion indicated by values on the Borg scale. The comparable values on the Borg scale indicate that patients were tested with the same rigor to maintain a comparable, adjusted intensity to test their capacities.
While there is some evidence indicating a worse prognosis based on CMR characteristics in HFpEF, the association with the symptom burden remains to be further explored (29–31).
The HFA-PEFF algorithm introduced imaging parameters to diagnose HFpEF, e.g., E/e’, tricuspid regurgitation velocity, left atrial volume index, and LV wall thickness (32). In brief, the HFA-PEFF algorithm leads to a score value for an individual patient based on functional, morphological and biomarker parameters, that may diagnose HFpEF, exclude HFpEF or indicate further investigation for a diagnosis by stress testing. Many of the highlighted functional and morphological parameters are derived from echocardiography and cannot be acquired reliably by routine CMR techniques. Nonetheless, it has been shown that the application of a CMR stress testing technique is a feasible strategy for further investigations in suspected HFpEF patients (33). All these efforts aim to better understand patients with a symptom burden leading to a suspected diagnosis of HF and a preserved traditional parameter, LVEF. Identifying those with altered cardiac function, like our analysis of regional differences of myocardial deformation, is at the core of recent findings, which aspire to lead to an earlier diagnosis as well as a better differentiation of the broad spectrum of patients diagnosed with HFpEF.
HFA-PEFF parameters as well as other HFpEF criteria have been often analyzed for a prognostic value regarding survival, but only recently a few studies focused on functional capacity (26, 34, 35). These analyses focused on clinical features and their impact on the 6MWT (26). Clinical information is often neither at the disposal of the physician reporting on cardiac imaging nor of the team managing the patient, which stresses the relevance of this analysis highlighting a method to increase the robustness of images to interpret.
With regards to the QoL the greatest difference could be observed between the group with preserved MyoHealth score and the impaired groups, as illustrated in S1, indicating that this parameter indicates QoL. While many patients with HF suffer from depression, QoL was only recently used as a clinical trial outcome parameter in HF—very rarely in cardiac imaging and CMR studies (36–40).
Figure 4 reflects the harmony of the presented data, as the subjects with a preserved ratio of altered myocardial deformation are those with the lowest symptom burden with regards to the subjective NYHA classification.
The main limitation of our study is that we cannot provide prognostic information of the subjects and patients examined. Nonetheless, the main objective of this analysis was to evaluate the predictive value of regional myocardial deformation on functional capacity as well as the quality of life in HF patients, especially HFpEF.
However, our explorative analyses to better understand the differences between the study subgroups are limited due to the smaller subgroup sizes the more fragmented they become. Comparing HF entities from different subgroups against each other within our analyses (Figures 1–4) will include subgroups with very few subjects, which restricts massively the claim of transferability. Further analyses of our exploratory indications require replication in larger cohorts. Due to this restriction, we also did not perform a post hoc subject sex or age matched analysis, as it would lead to even smaller numbers of subgroup subjects.
The small numbers also limit some of our analyses where we see quantitative difference, which do not reach the level of statistical significance, e.g., the assessment of QoL by the MLHFQ. Revisiting this analysis in a larger study population would lead to results that are more reliable. However, we believe that these not significant results are a signal to further analyses.
The results of this study promise an association between regional LV strain impairment and the symptom burden of HF patients with regards to functional capacity and quality of life, especially relevant in patients with a preserved LVEF that requires future prospective validation.
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethikkommission der Charité – Universitätsmedizin Berlin. The patients/participants provided their written informed consent to participate in this study.
DH, HDD, FE, and SK: conception and design of the study and literature review. DH, MB, and SK: analysis and interpretation of the data. DH: drafting of the manuscript. DH, PD, and MB: data collection. All authors revising and editing the manuscript and approved the submitted version.
This work was supported by the German Centre for Cardiovascular Research (DZHK), funded by the German Federal Ministry of Education and Research, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—CRC-1470—B06, and the Charité – Universitätsmedizin Berlin, Germany, as well as the German Heart Institute Berlin, Germany, and Myocardial Solutions. This research was also supported by an unrestricted research grant from Philips Healthcare. DH received a CMR specific research grant from the DZHK (grant number: 81X3100214).
We wish to express our gratitude toward all participating patients and subjects. We would especially like to acknowledge the contribution of James G. Whayne and Hayden Whayne, who supported this analysis.
SK reported grants and other support by the Philips Healthcare, BioVentrix, Berlin-Chemie, Merck/Bayer, Novartis, Astra Zeneca, Siemens, and Myocardial Solutions outside of the submitted work. SK was also on the advisory board for Merck/Bayer, BioVentrix, and Myocardial Solutions.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1038337/full#supplementary-material
1. Lewis E. Assessing the impact of heart failure therapeutics on quality of life and functional capacity. Curr Treat Options Cardiovasc Med. (2013) 15:425–36. doi: 10.1007/s11936-013-0249-2
2. Butler J, Stebbins A, Melenovsky V, Sweitzer N, Cowie M, Stehlik J, et al. Vericiguat and health-related quality of life in patients with heart failure with reduced ejection fraction: insights from the VICTORIA trial. Circ Heart Fail. (2022) 15:e009337. doi: 10.1161/CIRCHEARTFAILURE.121.009337
3. Vaishnava P, Lewis E. Assessment of quality of life in severe heart failure. Curr Heart Fail Rep. (2007) 4:170–7. doi: 10.1007/s11897-007-0037-y
4. Bui A, Horwich T, Fonarow G. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. (2011) 8:30–41. doi: 10.1038/nrcardio.2010.165
5. Johansson I, Joseph P, Balasubramanian K, McMurray J, Lund L, Ezekowitz J, et al. Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation. (2021) 143:2129–42. doi: 10.1161/CIRCULATIONAHA.120.050850
6. Butler J, Khan M, Mori C, Filippatos G, Ponikowski P, Comin-Colet J, et al. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. (2020) 22:999–1005. doi: 10.1002/ejhf.1810
7. Fitz J, Edelmann F, Hasenfuss G, Sandek A, Nolte K, Hashemi D, et al. Influence of baseline parameters on one-year physical, mental, and health-related quality of life in patients with heart failure and preserved ejection fraction. ESC Heart Fail. (2021) 8:4635–43. doi: 10.1002/ehf2.13593
8. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eu Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
9. Marwick T, Shah S, Thomas J. Myocardial strain in the assessment of patients with heart failure: a review. JAMA Cardiol. (2019) 4:287–94. doi: 10.1001/jamacardio.2019.0052
10. Hashemi D, Motzkus L, Blum M, Kraft R, Tanacli R, Tahirovic E, et al. Myocardial deformation assessed among heart failure entities by cardiovascular magnetic resonance imaging. ESC Heart Fail. (2021) 8:890–7. doi: 10.1002/ehf2.13193
11. Gersak B, Kukec A, Steen H, Montenbruck M, Sostaric M, Schwarz A, et al. Relationship between quality of life indicators and cardiac status indicators in chemotherapy patients. Zdr Varst. (2021) 60:199–209. doi: 10.2478/sjph-2021-0028
12. Steen H, Montenbruck M, Gerzak B, Schwarz A, Kelle S, Giusca S, et al. Cardiotoxicity during cancer treatment causes more regional than global dysfunction: the prefect study. J Am Coll Cardiol. (2020) 75:1824. doi: 10.1016/s0735-1097(20)32451-7
13. Parikh V, Caleshu C, Reuter C, Lazzeroni L, Ingles J, Garcia J, et al. Regional variation in RBM20 causes a highly penetrant arrhythmogenic cardiomyopathy. Circ Heart Fail. (2019) 12:e005371. doi: 10.1161/CIRCHEARTFAILURE.118.005371
14. Achenbach S, Fuchs F, Goncalves A, Kaiser-Albers C, Ali Z, Bengel F, et al. Non-invasive imaging as the cornerstone of cardiovascular precision medicine. Eur Heart J Cardiovasc Imaging. (2022) 23:465–75. doi: 10.1093/ehjci/jeab287
15. Bertero E, Sequeira V, Maack C. Hungry hearts. Circ Heart Fail. (2018) 11:e005642. doi: 10.1161/CIRCHEARTFAILURE.118.005642
16. Voros G, Ector J, Garweg C, Droogne W, Van Cleemput J, Peersman N, et al. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail. (2018) 11:e004953. doi: 10.1161/CIRCHEARTFAILURE.118.004953
17. Tanacli R, Hashemi D, Neye M, Motzkus L, Blum M, Tahirovic E, et al. Multilayer myocardial strain improves the diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail. (2020) 7:3240–5. doi: 10.1002/ehf2.12826
18. Blum M, Hashemi D, Motzkus L, Neye M, Dordevic A, Zieschang V, et al. Variability of myocardial strain during isometric exercise in subjects with and without heart failure. Front Cardiovasc Med. (2020) 7:111. doi: 10.3389/fcvm.2020.00111
19. Tanacli R, Hashemi D, Lapinskas T, Edelmann F, Gebker R, Pedrizzetti G, et al. Range variability in CMR feature tracking multilayer strain across different stages of heart failure. Sci Rep. (2019) 9:16478. doi: 10.1038/s41598-019-52683-8
20. Doeblin P, Hashemi D, Tanacli R, Lapinskas T, Gebker R, Stehning C, et al. CMR tissue characterization in patients with HFmrEF. J Clin Med. (2019) 8:1877. doi: 10.3390/jcm8111877
21. Lapinskas T, Zieschang V, Erley J, Stoiber L, Schnackenburg B, Stehning C, et al. Strain-encoded cardiac magnetic resonance imaging: a new approach for fast estimation of left ventricular function. BMC Cardiovasc Disord. (2019) 19:52. doi: 10.1186/s12872-019-1031-5
22. Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou G, et al. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail. (2020) 7:523–32. doi: 10.1002/ehf2.12576
23. Suinesiaputra A, Bluemke D, Cowan B, Friedrich M, Kramer C, Kwong R, et al. Quantification of LV function and mass by cardiovascular magnetic resonance: multi-center variability and consensus contours. J Cardiovasc Magn Reson. (2015) 17:63. doi: 10.1186/s12968-015-0170-9
24. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, et al. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. (2014) 19:359–67. doi: 10.1007/s10741-013-9394-7
25. Mant J, Doust J, Roalfe A, Barton P, Cowie M, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. (2009) 13:1–207. doi: 10.3310/hta13320
26. Berisha-Muharremi V, Henein M, Dini F, Haliti E, Bytyci I, Ibrahimi P, et al. Diabetes is the strongest predictor of limited exercise capacity in chronic heart failure and preserved ejection fraction (HFpEF). Front Cardiovasc Med. (2022) 9:883615. doi: 10.3389/fcvm.2022.883615
27. Nadruz W Jr., West E, Sengelov M, Santos M, Groarke J, Forman D, et al. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc. (2017) 6:e006000. doi: 10.1161/JAHA.117.006000
28. Batalli A, Ibrahimi P, Bytyci I, Ahmeti A, Haliti E, Elezi S, et al. Different determinants of exercise capacity in HFpEF compared to HFrEF. Cardiovasc Ultrasound. (2017) 15:12. doi: 10.1186/s12947-017-0103-x
29. Golukhova E, Bulaeva N, Alexandrova S, Gromova O, Berdibekov B. Prognostic value of characterizing myocardial tissue by cardiac MRI with T1 mapping in HFpEF patients: a systematic review and meta-analysis. J Clin Med. (2022) 11:2531. doi: 10.3390/jcm11092531
30. Schonbauer R, Kammerlander A, Duca F, Aschauer S, Koschutnik M, Dona C, et al. Prognostic impact of left atrial function in heart failure with preserved ejection fraction in sinus rhythm vs. persistent atrial fibrillation. ESC Heart Fail. (2022) 9:465–75. doi: 10.1002/ehf2.13723
31. Pezel T, Hovasse T, Sanguineti F, Kinnel M, Garot P, Champagne S, et al. Long-term prognostic value of stress CMR in patients with heart failure and preserved ejection fraction. JACC Cardiovasc Imaging. (2021) 14:2319–33. doi: 10.1016/j.jcmg.2021.03.010
32. Pieske B, Tschope C, de Boer R, Fraser A, Anker S, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European society of cardiology (ESC). Eur Heart J. (2019) 40:3297–317. doi: 10.1093/eurheartj/ehz641
33. Backhaus S, Lange T, George E, Hellenkamp K, Gertz R, Billing M, et al. Exercise stress real-time cardiac magnetic resonance imaging for noninvasive characterization of heart failure with preserved ejection fraction: the HFpEF-stress trial. Circulation. (2021) 143:1484–98. doi: 10.1161/CIRCULATIONAHA.120.051542
34. Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca H, Henkens M, Heymans S, Beussink-Nelson L, et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. (2020) 22:413–21. doi: 10.1002/ejhf.1614
35. Sotomi Y, Iwakura K, Hikoso S, Inoue K, Onishi T, Okada M, et al. Prognostic significance of the HFA-PEFF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail. (2021) 8:2154–64. doi: 10.1002/ehf2.13302
36. Angermann C, Ertl G. Depression, anxiety, and cognitive impairment: comorbid mental health disorders in heart failure. Curr Heart Fail Rep. (2018) 15:398–410. doi: 10.1007/s11897-018-0414-8
37. Angermann C, Gelbrich G, Stork S, Gunold H, Edelmann F, Wachter R, et al. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. (2016) 315:2683–93. doi: 10.1001/jama.2016.7635
38. Piepoli M, Hussain R, Comin-Colet J, Dosantos R, Ferber P, Jaarsma T, et al. OUTSTEP-HF: randomised controlled trial comparing short-term effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. (2021) 23:127–35. doi: 10.1002/ejhf.2076
39. Armstrong P, Lam C, Anstrom K, Ezekowitz J, Hernandez A, O’Connor C, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. (2020) 324:1512–21. doi: 10.1001/jama.2020.15922
Keywords: heart failure, cardiovascular magnetic resonance imaging, myocardial deformation, quality of life, CMR, score, strain, quantitative
Citation: Hashemi D, Doeblin P, Blum M, Weiss KJ, Schneider M, Beyer R, Pieske B, Duengen H-D, Edelmann F and Kelle S (2023) Reduced functional capacity is associated with the proportion of impaired myocardial deformation assessed in heart failure patients by CMR. Front. Cardiovasc. Med. 10:1038337. doi: 10.3389/fcvm.2023.1038337
Received: 06 September 2022; Accepted: 23 January 2023;
Published: 09 February 2023.
Edited by:
Frédéric Schnell, Centre Hospitalier Universitaire (CHU) de Rennes, FranceReviewed by:
Oliver Bruder, Elisabeth-Krankenhaus Essen, GermanyCopyright © 2023 Hashemi, Doeblin, Blum, Weiss, Schneider, Beyer, Pieske, Duengen, Edelmann and Kelle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Djawid Hashemi,  ZGphd2lkLmhhc2hlbWlAY2hhcml0ZS5kZQ==
ZGphd2lkLmhhc2hlbWlAY2hhcml0ZS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.