- Division of Vascular Surgery, Department of General Surgery, Changhai Hospital, Naval Medical University, Shanghai, China
Background: Thoracic endovascular aortic repair, initially intended for thoracic aortic disease treatment, has extended its application to the proximal zone of the aorta. However, the safety and surgical outcomes of extending the proximal landing zone into the ascending aorta (zone 0) in selected cases remain unknown. Thus, we performed a systematic review and meta-analysis of zone 0 thoracic endovascular aortic repair (TEVAR) to obtain a deeper understanding of its safety, outcomes, and trends over time.
Methods: A literature search was performed using PubMed, EMBASE, and Web of Science databases in accordance with the preferred reporting items for systematic reviews and meta-analyses guidelines, from January, 1997 to January, 2022. Only studies involving zone 0 TEVAR were included. The retrieved data from the eligible studies included basic study characteristics, 30-day/in-hospital mortality rate, indications, comorbidities, stent grafts, techniques, and complications. Summary effect measures of the primary outcomes were obtained by logarithmically pooling the data with an inverse variance-weighted fixed-effects model.
Results: Fifty-three studies with 1,013 patients were eligible for analysis. The pooled 30-day/in-hospital mortality rate of zone 0 TEVAR was 7.49%. The rates of post-operative stroke, type Ia endoleak, retrograde type A aortic dissection, and spinal cord ischemia were 8.95, 9.01, 5.72, and 4.12%, respectively.
Conclusions: Although many novel stent grafts and techniques targeting zone 0 TEVAR are being investigated, a consensus on technique and device selection in zone 0 TEVAR is yet to be established in current practice. Furthermore, the post-operative stroke rate is relatively high, while other complication rates and perioperative death rate are comparable to those of TEVAR for other aortic zones.
1. Introduction
Thoracic endovascular aortic repair (TEVAR) has become a viable treatment option for thoracic aortic pathologies over the past years (1, 2). The proximal landing zone (PLZ) of the stent graft has been extended from the descending to the ascending aorta (zone 0) to ensure a sufficient and healthy PLZ. Following the development of surgical devices and improvement in supra-aortic vessels revascularization techniques, studies investigating the feasibility and safety of TEVAR with zone 0 landing have been conducted.
Unlike TEVAR for other aortic zones, zone 0 TEVAR lacks high-quality evidence to support its use. Studies on zone 0 TEVAR mostly comprise case reports, case series, and retrospective studies. As no standard off-the-shelf stent grafts dedicated to zone 0 TEVAR are available, the safety, feasibility, and efficacy of zone 0 TEVAR with off-label use of thoracic or custom-made stent grafts are yet to be studied (3). Thus, a timely and comprehensive understanding of the safety and outcomes of zone 0 TEVAR is necessary before further promotion of its application. This systematic review and meta-analysis aimed to provide a comprehensive overview of the current application of zone 0 TEVAR, including its indications, stent grafts, procedures, and post-operative complications.
2. Methods
2.1. Search methodology
The systematic review conformed to the preferred reporting items for systematic review and meta-analyses statement standards (4). A search in PubMed, EMBASE, and Web of Science databases from January, 1997 to January, 2022 was made using set algorithms. The search algorithm in PubMed was “((((landing zone) AND (ascending aorta)) OR (‘landing' [All Fields] AND ‘zone' [All Fields] AND (‘zone' [All Fields] AND ‘0' [All Fields]))) OR (‘stent graft' [All Fields] AND (‘zone' [All Fields] AND ‘0' [All Fields]))) OR (‘endovascular' [All Fields] AND (‘zone' [All Fields] AND ‘0' [All Fields])).” The search algorithm used for EMBASE was “1. TEVAR; 2. Zone 0; 3. Ascending aorta; 4. 2 OR 3; 5. 1 AND 4.” The search algorithm in Web of Science was “((TS = (zone 0)) OR TS = (ascending aorta)) AND TS = (TEVAR).”
2.2. Inclusion and exclusion criteria
The studies included were published in English, containing zone 0 TEVAR, and clinical studies or cohort case reports. Studies containing only zone 0 TEVAR with prosthetic ascending aorta as the PLZ and studies, in which the reported number of cases of zone 0 TEVAR were <5, were excluded from the analysis.
2.3. Data extraction
Two researchers independently extracted data. The authors, publication date, study region, research type, number of cases, recruitment time, sex, age, 30-day/in-hospital mortality, 30-day/in-hospital mortality rate, pathology, stent graft, technique, and complications were retrieved from the eligible studies.
2.4. Statistical analysis
Data on stent graft, procedure and complications were summarized. Excel software (Microsoft Corp, Redmond, WA, USA) and Review Management 5.4 (The Cochrane. Collaboration, Oxford, UK) were used to record, analyze, conduct the meta-analysis, and tabulate clinical data. A meta-analysis was performed for perioperative mortality of zone 0 TEVAR and post-operative stroke, type Ia endoleak, spinal cord ischemia (SCI), and retrograde type A dissection (RTAD). Summary effect measures of post-operative complications and perioperative death rates were obtained by logarithmically pooling the data with an inverse variance-weighted fixed-effects model and presented with a 95% confidence interval (CI). Heterogeneity of the summary effects measures was assessed with the I2 test and considered heterogeneous when I2 was >50%. A 2-sided P-value of <0.05 was considered statistically significant.
3. Results
3.1. Study selection
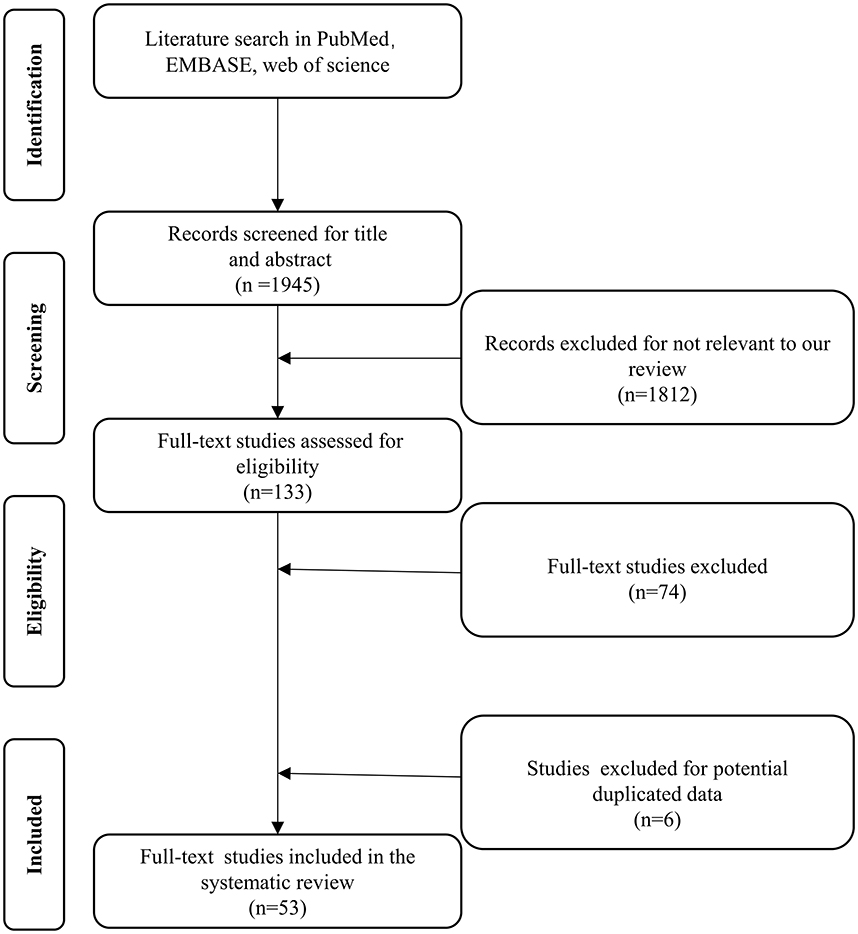
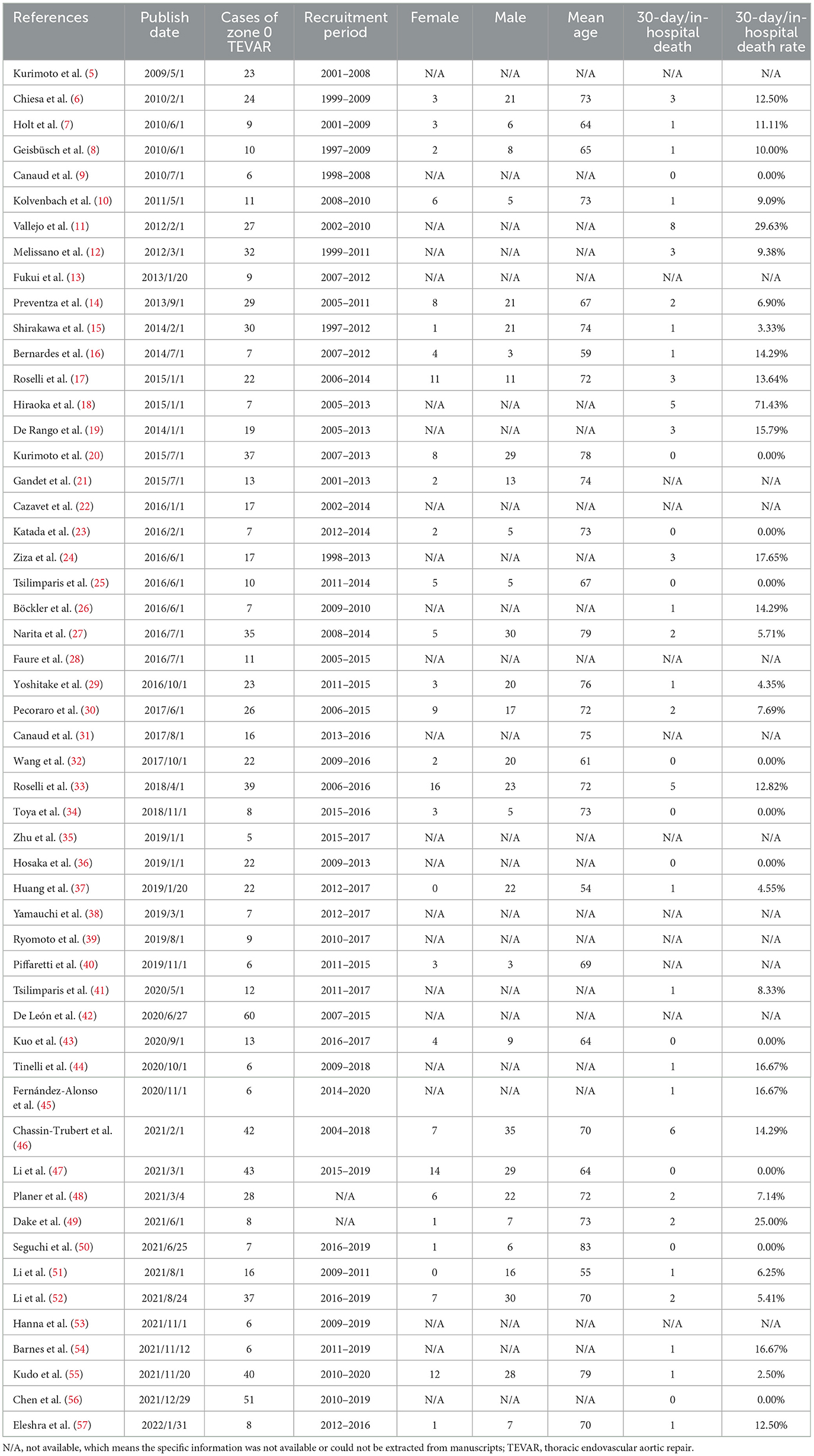
A total of 1,812 studies were retrieved from PubMed, EMBASE, and Web of Science between January, 1997 and January, 2022. A total of 133 studies were considered eligible according to the inclusion criteria, of which 74 were excluded according to the exclusion criteria (Figure 1). Six studies were excluded because they were duplicates. Fifty-three studies, with a total number of 1,013 cases, were included in the final analysis (Table 1). The present study included 48 retrospective and 5 prospective studies.
3.2. Pathology and comorbidities
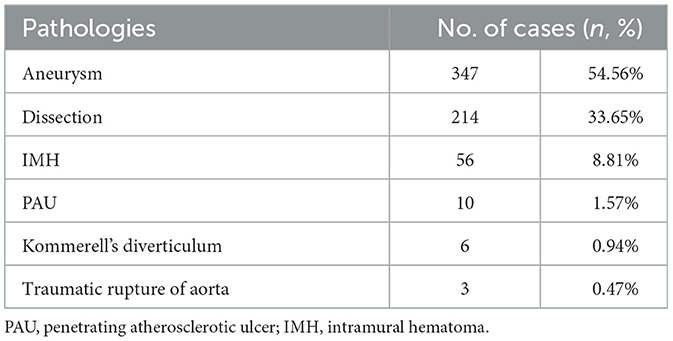
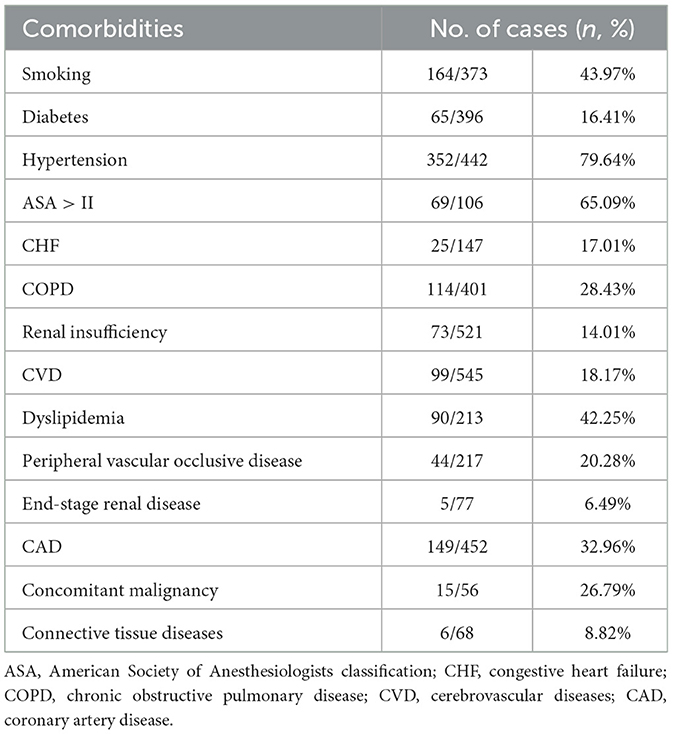
The type of aortic pathologies and comorbidities are summarized in Tables 2, 3, respectively. Indications for zone 0 TEVAR of 636 cases from 32 studies were disclosed, and included aneurysm (n = 347, 54.56%), aortic dissection (n = 214, 33.65%), intramural hematoma (n = 56, 8.81%), penetrating atherosclerotic ulcer (n = 10, 1.57%), Kommerell's diverticulum (n = 6, 0.94%), and traumatic aortic rupture (n = 3, 0.47%).
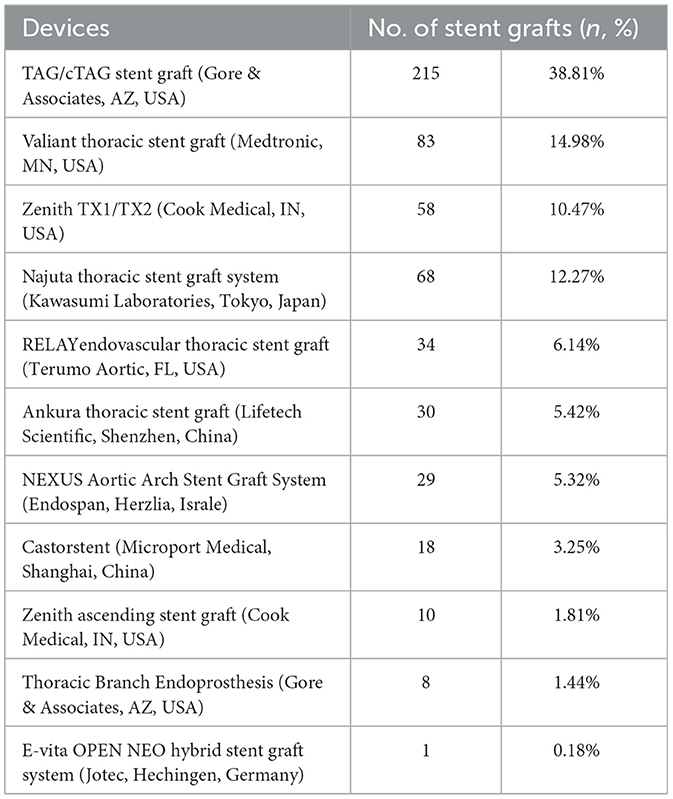
3.3. Stent graft
Stent grafts used in zone 0 TEVAR are summarized in Table 4. A total of 554 stent grafts from 25 studies were analyzed. The most frequently used stent graft in zone 0 TEVAR was TAG/c-TAG stent graft (W.L. Gore and Associates, AZ, USA) (n = 215, 38.81%) followed by Valiant thoracic stent graft (Medtronic, MN, USA) (n = 83, 14.98%), Zenith TX1/TX2 (Cook Medical, IN, USA) (n = 58, 12.27%), Najuta (Kawasumi Laboratories, Tokyo, Japan) (n = 68, 12.27%), RELAY endovascular thoracic stent graft (Terumo Aortic, FL, USA) (n = 34, 6.14%), Ankura thoracic stent graft (Lifetech Scientific, Shenzhen, China) (n = 30, 5.42%), NEXUS Aortic Arch Stent Graft System (Endospan, Herzlia, Israel) (n = 29, 5.32%), Castor (Microport Medical, Shanghai, China) (n = 18, 3.25%), Thoracic Branch Endoprosthesis (Gore & Associates, AZ, USA) (n = 8, 1.44%), Zenith ascending stent graft (Cook Medical, IN, USA) (n = 10, 1.81%), and E-vita (Jotec, Hechingen, Germany) (n = 1, 0.18%). In 337 (60.83%) cases, stent grafts had a proximal bare-metal portion. Three novel stent grafts dedicated to zone 0 TEVAR had been introduced in the reviewed studies (25, 34, 48). Zenith ascending stent graft is aimed at the ascending aorta and has no branches or fenestrations for supra-aortic vessels. Najuta thoracic stent graft system is a fenestrated stent graft with one to three fenestrations, which can preserve all supra-aortic vessels. NEXUS Aortic Arch Stent Graft System is a novel single branch, two stent graft system used for endovascular aortic arch repair.
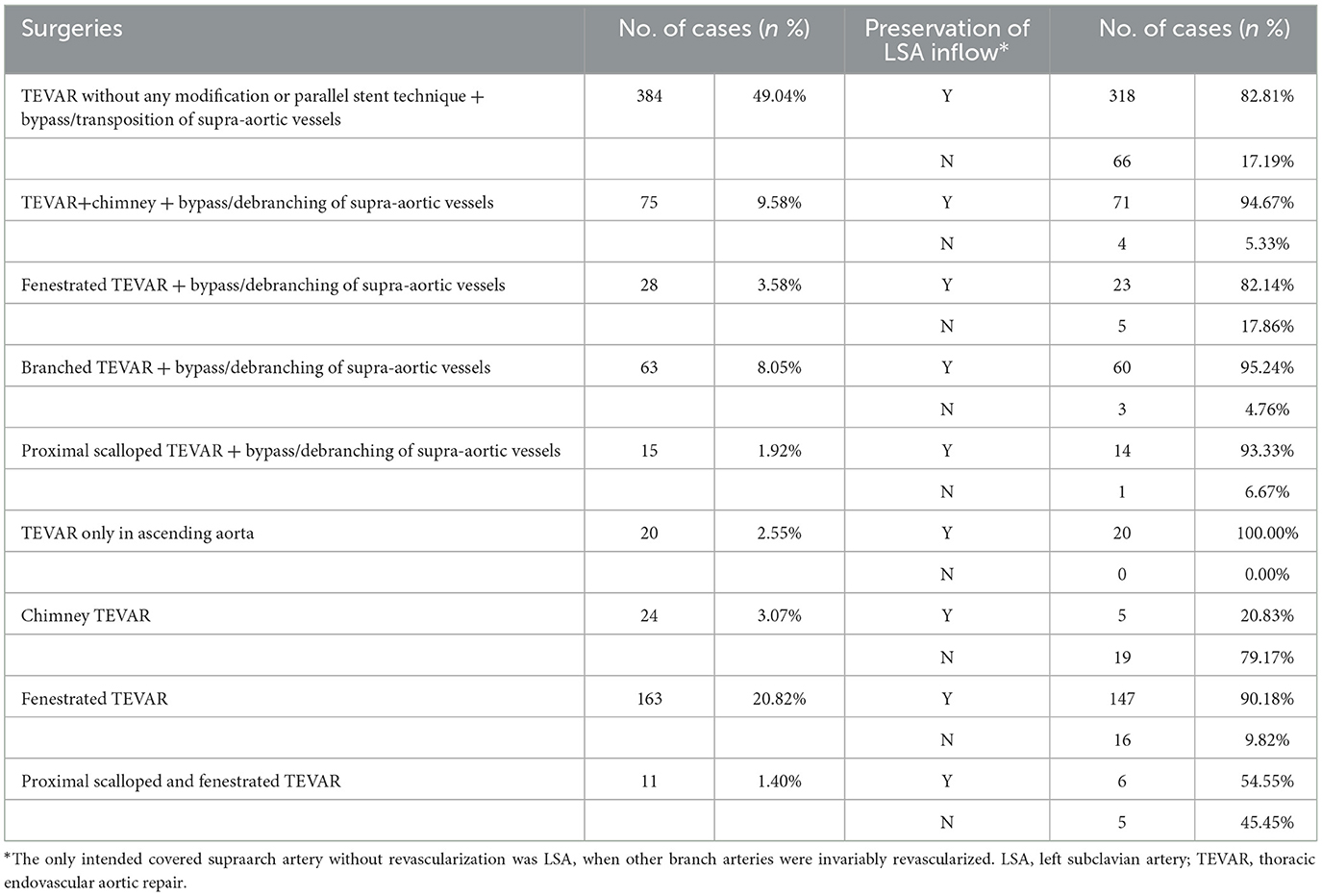
3.4. Procedure
The procedures performed in 783 cases from 46 studies were classified as following: 1. TEVAR without any modification or parallel stent technique + bypass/debranching of supraaortic vessels (n = 384, 49.04%); 2. TEVAR + chimney + bypass/debranching of supraaortic vessels (n = 75, 9.58%); 3. fenestrated TEVAR + bypass/debranching of supraaortic vessels (n = 28, 3.58%); 4. branched TEVAR + bypass/debranching of supraaortic vessels (n = 63, 8.05%); 5. proximal scalloped TEVAR + bypass/debranching of supraaortic vessels (n = 15, 1.92%); 6. TEVAR only in ascending aorta (n = 20, 2.55%); 7. chimney TEVAR (n = 24, 3.07%); 8. fenestrated TEVAR (n = 163, 20.82%); and 9. proximal scalloped and fenestrated TEVAR (n = 11, 1.40%) (Table 5). Figure 2 illustrates the techniques used in zone 0 TEVAR. Out of the 783 cases, the inflow of the left subclavian artery (LSA) was preserved in 642 (81.99%) cases. Among the 191 cases treated with fenestrated TEVAR, pre-operative fenestrations were used in 44 (23.04%), back table fenestrations in 93 (48.69%), laser-in situ fenestrations in 43 (22.51%), and needle-in situ fenestrations in 9 (4.71%) cases, while fenestration techniques were not disclosed in 2 (1.05%) cases.
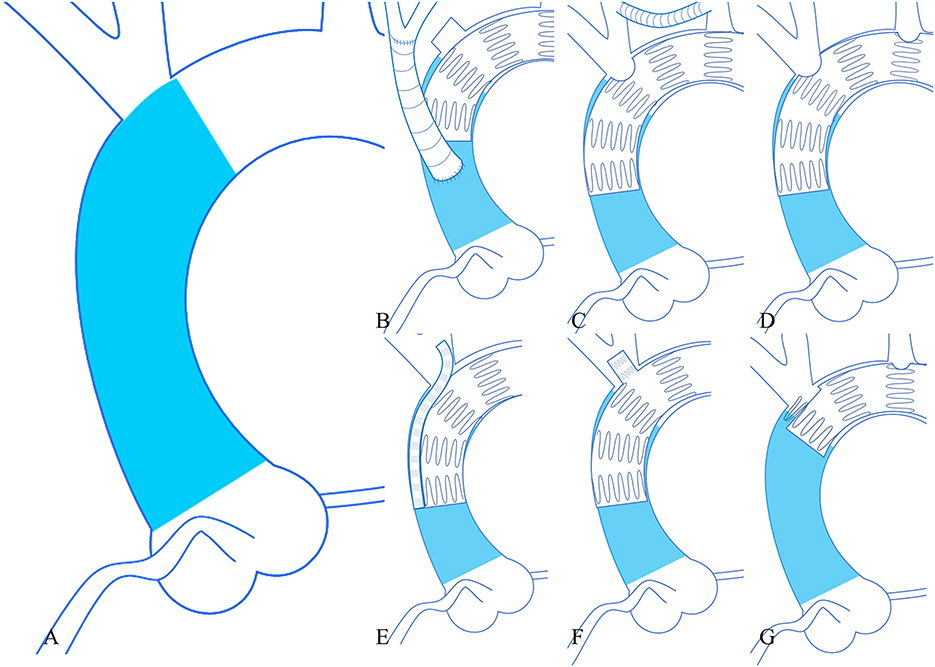
Figure 2. Six techniques used in zone 0 TEVAR. (A) (Left larger image) the area of zone 0 (blue). (B) (Upper left) TEVAR + debranching procedure. (C) (Upper middle) TEVAR + bypass procedure. (D) (Upper right) fenestrated TEVAR. (E) (Lower left) chimney TEVAR. (F) (Lower middle) branched TEVAR. (G) (Lower right) proximal scalloped and fenestrated TEVAR. TEVAR, thoracic endovascular aortic repair.
3.5. Perioperative mortality and post-operative complications
The pooled 30-day/in-hospital death rate was 7.49% (95% CI, 5.45–9.52, P < 0.00001, I2 = 22%, Figure 3). Data on the causes, characteristics, and outcomes of stroke, SCI, type Ia endoleak, and RTAD were collected, and the incidences were obtained by logarithmically pooling the data with an inverse variance-weighted fixed-effects model. The most common post-operative complication was stroke (8.95%, 95% CI, 6.44–11.46, P < 0.00001, I2 = 46%, Figure 4), followed by type Ia endoleak (9.01%, 95% CI, 5.77–12.25, P < 0.00001, I2 = 35%, Figure 5), RTAD (5.72%, 95% CI, 2.67–8.77, P = 0.0002, I2 = 0%, Figure 6), and SCI (4.12%, 95% CI, 1.89–6.35, P = 0.0003, I2 = 0%, Figure 7).
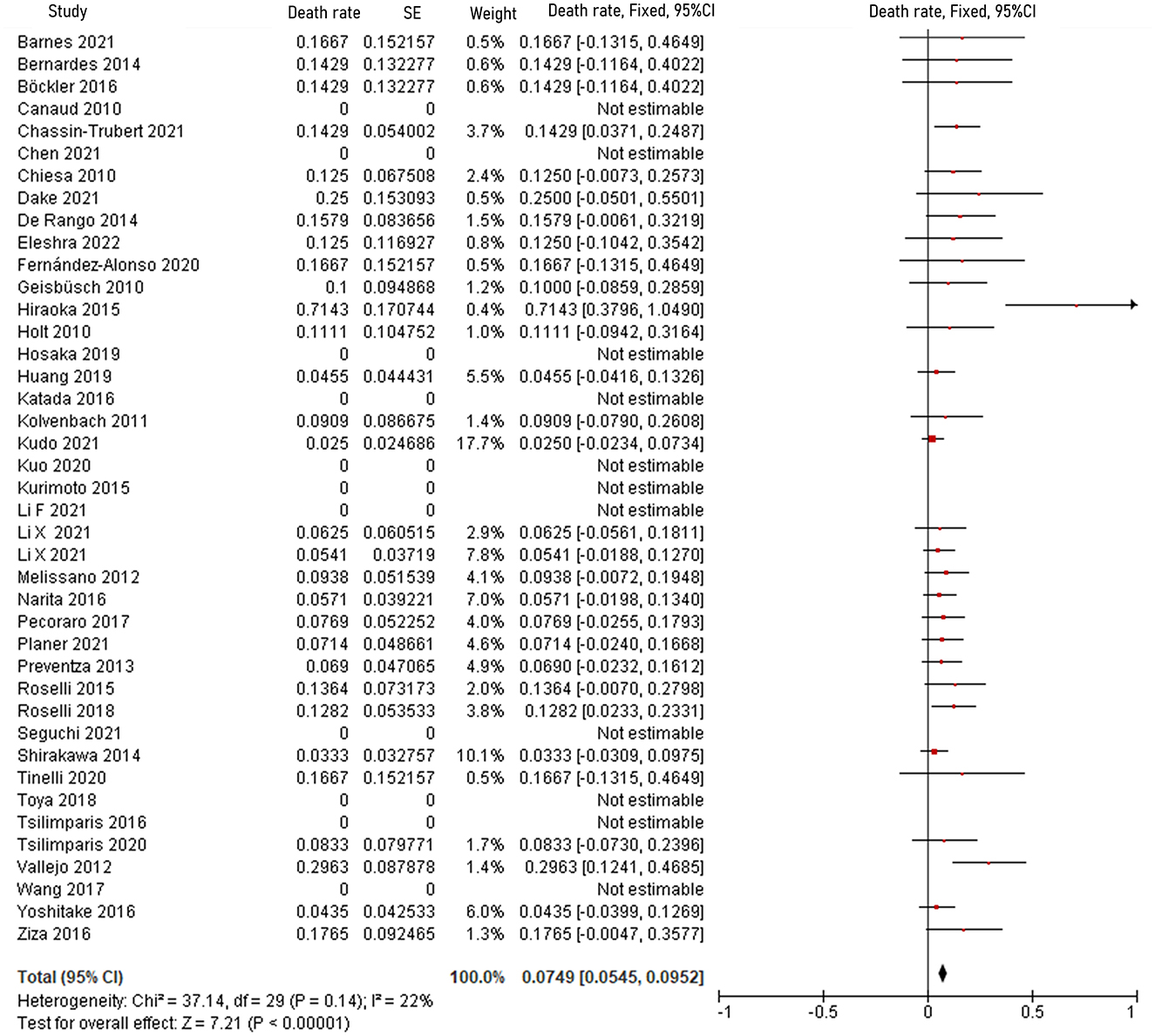
Figure 3. Forest plot shows the fixed-effects proportion meta-analysis for perioperative death rate. CI, confidence interval; SE, standard error.
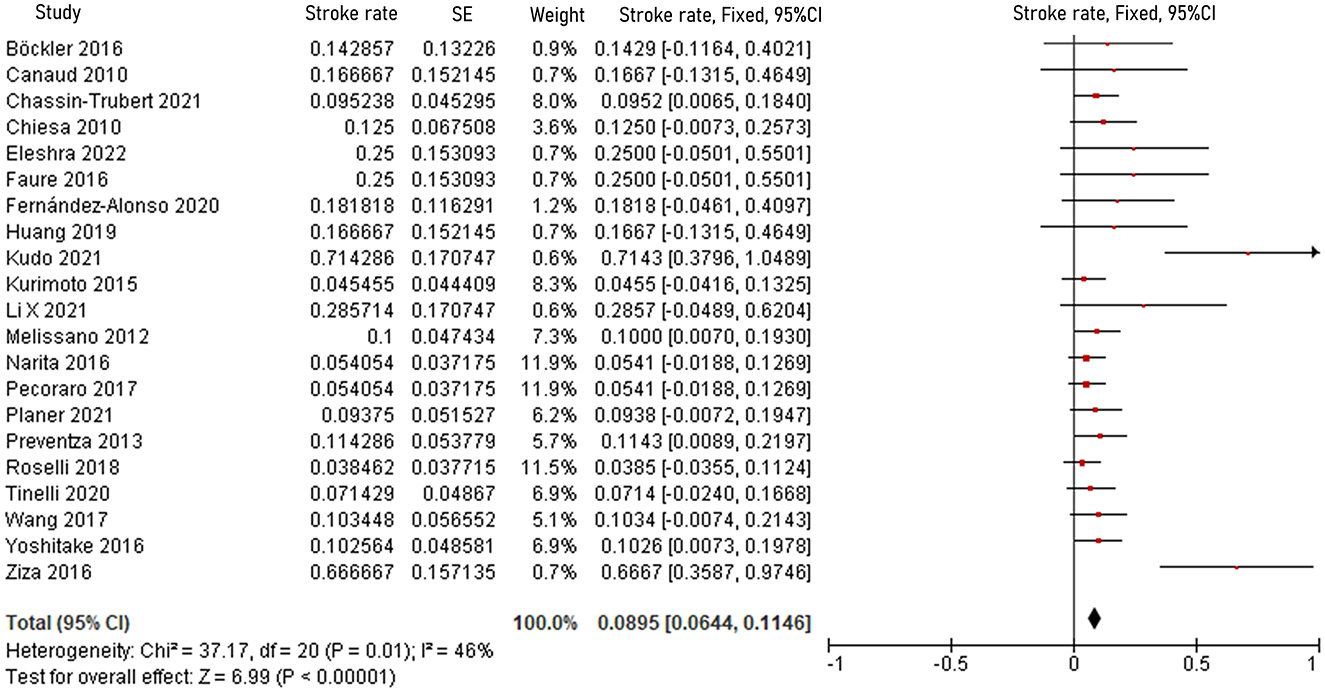
Figure 4. Forest plot showing the fixed-effects proportion meta-analysis for post-operative stroke rate. CI, confidence interval; SE, standard error.
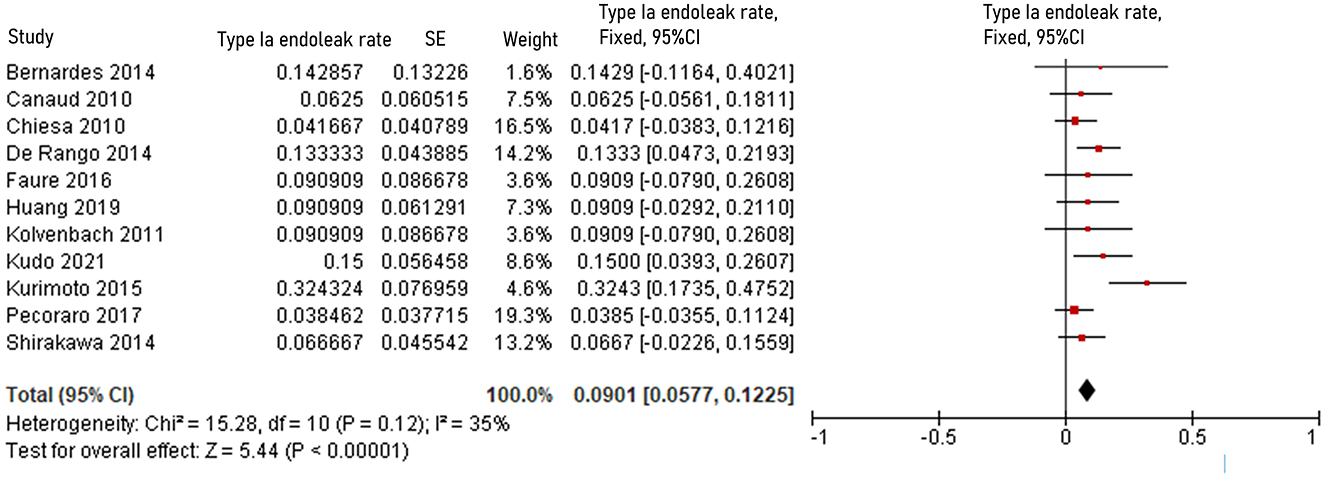
Figure 5. Forest plot showing the fixed-effects proportion meta-analysis for type Ia endoleak rate. CI, confidence interval; SE, standard error.
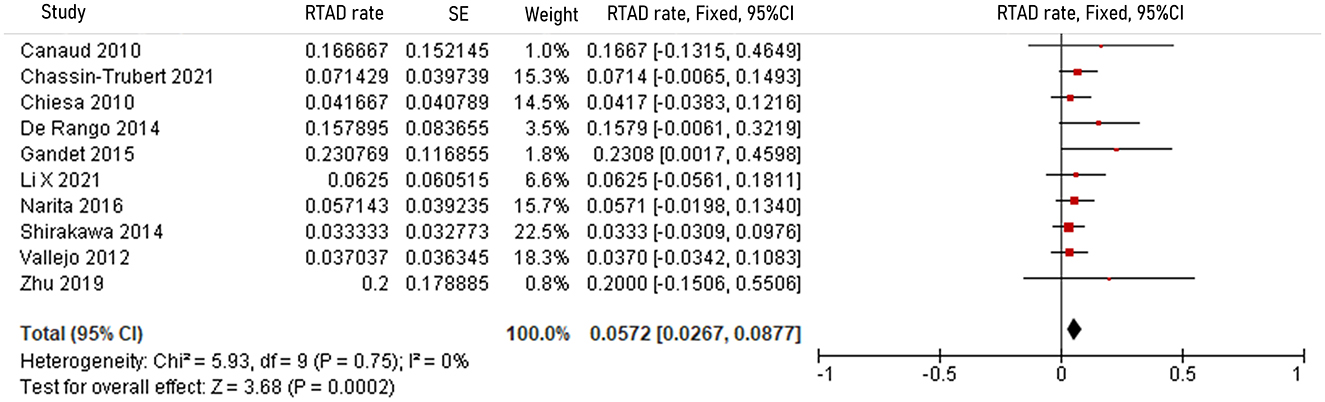
Figure 6. Forest plot showing the fixed-effects proportion meta-analysis for RTAD rate. CI, confidence interval; SE, standard error; RTAD, retrograde type A dissection.
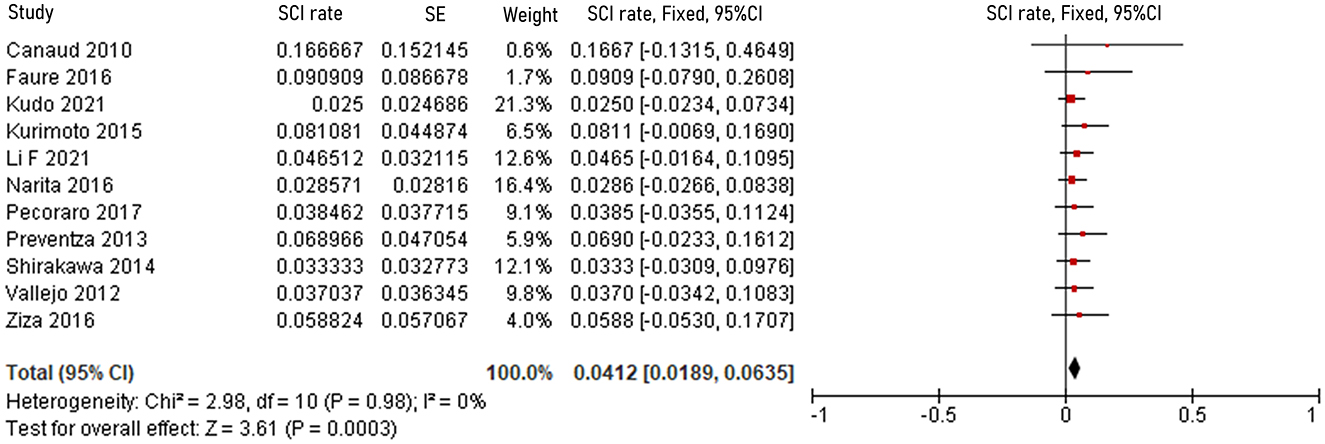
Figure 7. Forest plot showing the fixed-effects proportion meta-analysis for RTAD rate. CI, confidence interval; SE, standard error; RTAD, retrograde type A dissection.
The causes and outcomes of stroke in 26 studies are presented in Table 6. Causes were disclosed in 11 studies, and outcomes were disclosed in 16 studies. Atherosclerotic plaque was considered the principal cause of stroke in six studies (12, 18, 26, 29, 45, 55). Other possible causes suggested by the authors included migration and compression of chimney stents, LSA dissection, debranching of LSA, and lower left ventricular ejection fraction (27, 32, 39, 44). Based on available data, it appears that stroke was the cause of death in 12 of the 65 patients who suffered from this complication (6, 14, 18, 26, 37, 44, 45).
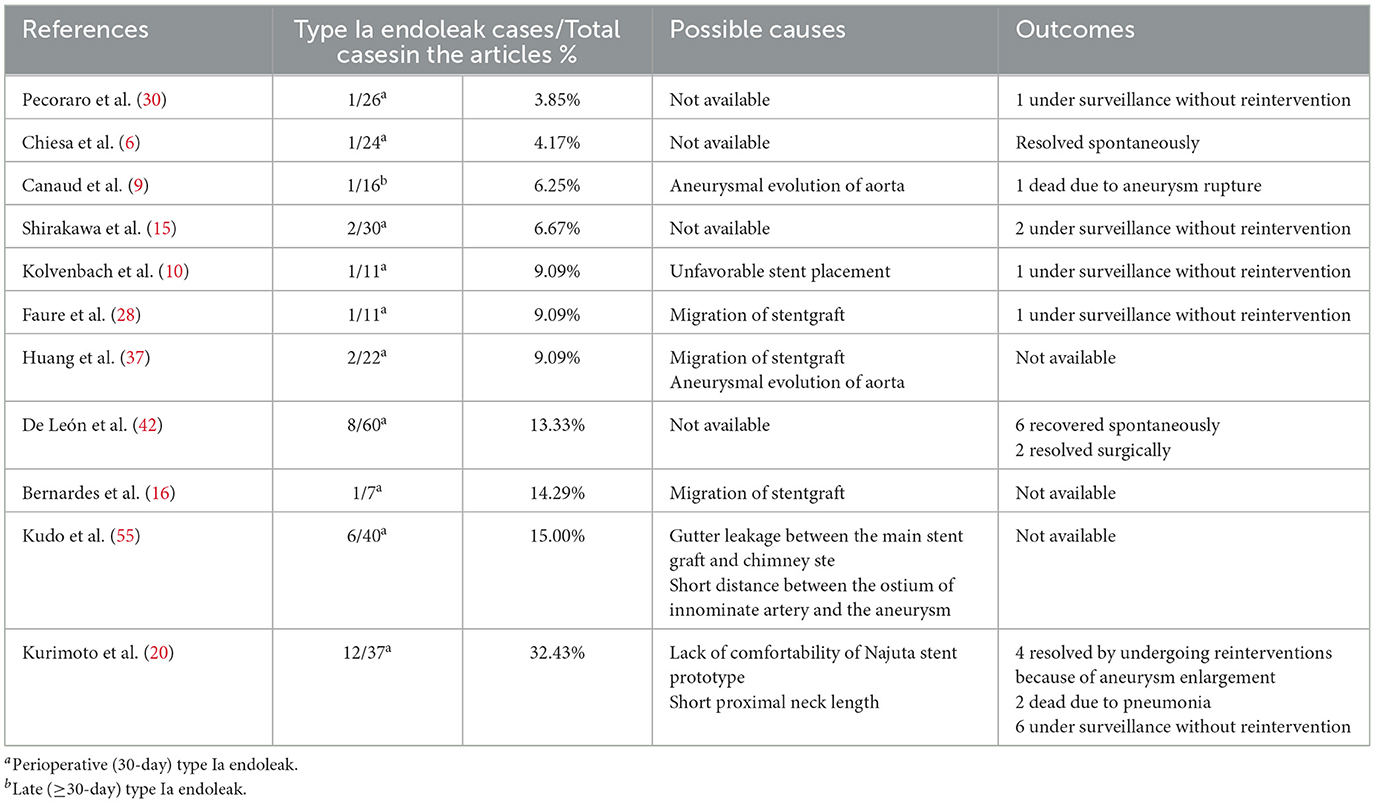
Causes and outcomes of type Ia endoleak in 11 studies are shown in Table 7. Causes were disclosed in seven studies, while outcomes were disclosed in eight studies. Migration of stent graft was considered the principal cause of type Ia endoleak in three studies (16, 28, 37). Other possible causes suggested by the authors included aneurysmal evolution of aorta, unfavorable stent placement, gutter leakage between the main stent graft and chimney stent, short distance between the ostium of innominate artery and the aneurysm, lack of comfortability of Najuta stent prototype, and short proximal neck length (10, 20, 31, 37, 55). Based on available data, 17 of the 36 patients who presented with type Ia endoleak were treated conservatively, while in 5 patients type Ia endoleak lead to aneurysm enlargement, which ruptured and caused death in 1 patient (6, 10, 15, 20, 28, 30, 42).
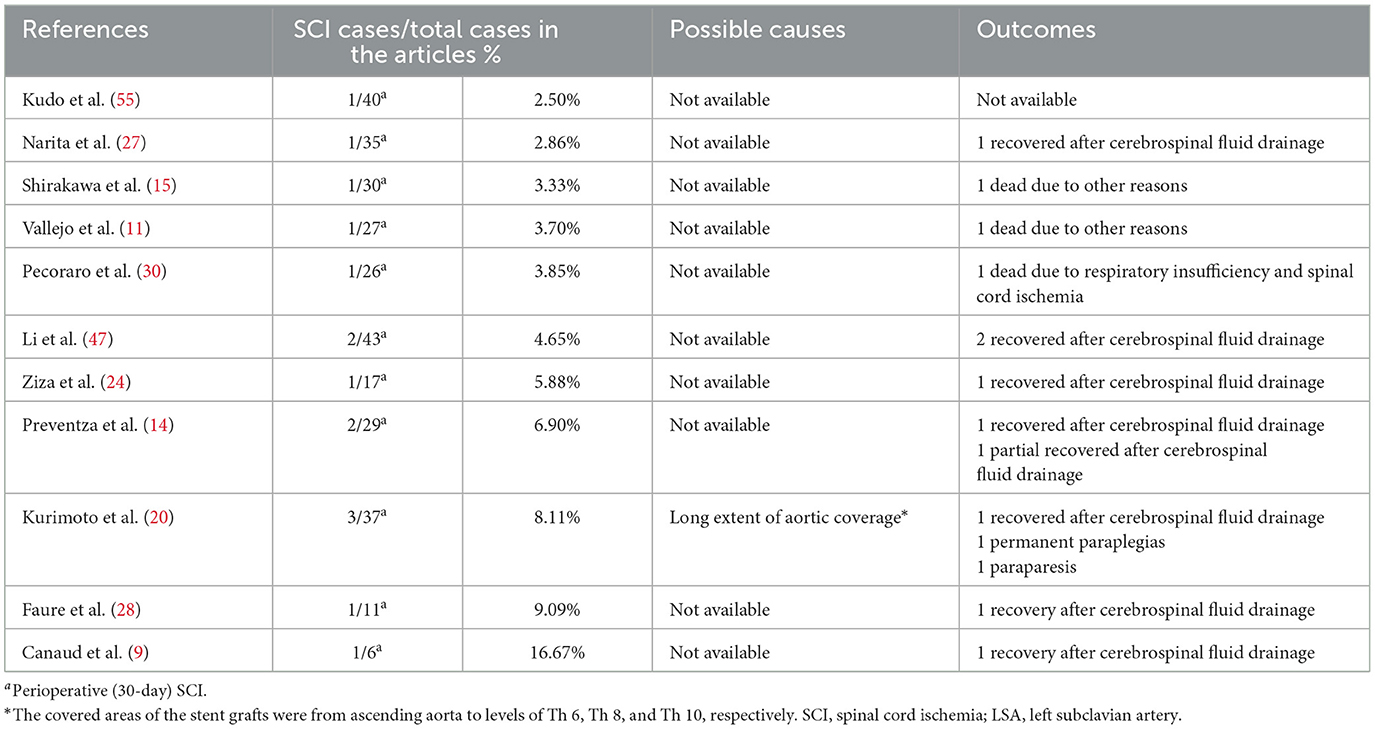
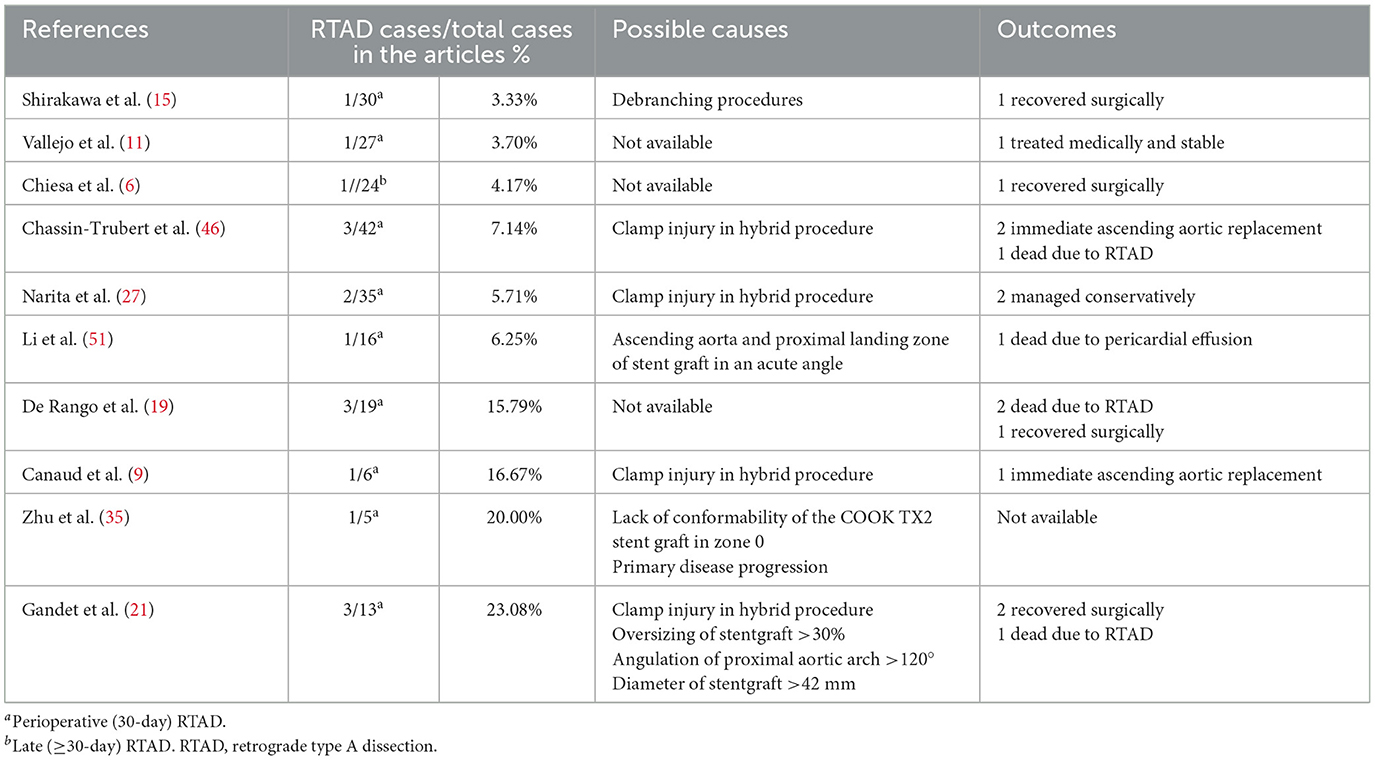
Tables 8, 9 show the causes and outcomes of SCI and RTAD in 11 and 9 studies, respectively. One study suggested that SCI might have been caused by LSA coverage and long extent of aortic coverage (20). Outcomes of SCI were disclosed in 10 studies. Three out of 15 SCI patient were left with long-term sequelae (20, 30). Causes of RTAD were disclosed in seven studies, and outcomes were disclosed in nine studies, with clamp injury in hybrid procedure being considered the principal cause in four manuscripts (9, 21, 27, 46). An acute angle formed by the ascending aorta and PLZ, lack of conformability of the COOK TX2 stent graft in zone 0, primary disease progression, >30% oversizing of the stent graft, angulation of the proximal aortic arch >120° and stent graft diameter >42 mm have also been suggested as possible causes of RTAD (21, 35, 51). Four patients died of complications related to RTAD, and based on the available data eight out of 17 patients received reintervention (6, 9, 15, 19, 21, 46).
4. Discussion
To meet the challenges posed by the lack of dedicated stent grafts for zone 0 TEVAR, numerous procedures and novel devices have been developed. Techniques range from hybrid surgery to fenestrated, chimney, and branched TEVAR, while newer devices try to provide simpler and safer procedures. According to the reviewed studies, no clear protocol was shown regarding the selection technique or device for zone 0 TEVAR.
Stroke is the most common post-operative complication after zone 0 TEVAR (8.95%). Two meta-analyses of TEVAR for descending thoracic aortic diseases performed by Karaolanis et al. and Allmen et al. suggested that stroke rates in TEVAR for descending aortic aneurysm and type B dissection were 4.1 and 4.4%, respectively, lower than those in zone 0 TEVAR (58, 59). A meta-analysis from 2019 involving 989 patients undergoing total arch replacement with frozen elephant trunk also showed a lower stroke rate (8.95 vs. 2.38%) (60). Six authors suggested detachment of atherosclerotic plaque debris in the aortic arch, induced by manipulation of the guide-wire or stent graft delivery system was the cause of stroke (12, 18, 26, 29, 45, 55). Three authors suggested that the occlusion of supra-aortic vessels owing to migration or compression of stents, and the dissection of supra-aortic vessels could be a further cause (24, 32, 44). Other possible causes included prolonged procedural time, lower left ventricular ejection fraction, and increased blood loss (27, 39). While no author in reviewed studies attributed stroke to planned LSA coverage without revascularization, the meta-analysis made by Chen et al. and Karaolanis et al. reported a significant reduction in stroke rate when the covered LSA had been revascularized (59, 61). However, the stroke rate in the planned LSA coverage without revascularization remains unknown in reviewed studies. To prevent stroke, some authors introduced procedures such as mini-cardiopulmonary bypass support and temporary inflow blockage of branch vessels (39, 50). The principal goal of these interventions is to prevent atheromatous debris from accessing the cerebral blood supply. Given 8.95% stroke rate and 18.46% (12/65) stroke-related death in the review, post-operative stroke prevention must be considered more when planning zone 0 TEVAR (6, 14, 18, 26, 37, 44, 45).
The incidence of type Ia endoleak in zone 0 TEVAR is 9.01% in the review. That is slightly lower than that (10.07%) reported in the multicenter study, involving 1, 18, 43, 55, and 22 cases of zone 0, 1, 2, 3, 4 TEVAR, conducted by Hammo et al. (62) in 2019. In the aortic arch, the varied lengths of the outer and inner curves pose a barrier to the stent graft's stability. This leads a bird-beak configuration after TEVAR increasing the risk of type Ia endoleak. However, Kudo et al. (63) proposed that bird-beak configuration did not occur during the early or late periods after zone 0 TEVAR. Causes suggested by the authors include migration, unfavorable deployment, and lack of flexibility of the stent graft (10, 16, 20, 28, 37). Reinterventions, including open or endovascular surgery, are appropriate treatments for endoleak (20, 42). De León et al. (42) classified type Ia endoleak as “fast” and “slow” based on the time needed to visualize the aneurysmal sac during arteriogram. Based on their observation, they postulated that slow endoleak tends to resolve naturally within 1 year after TEVAR. Of 36 patients with type Ia endoleak, one died of aneurysm rupture and 7 resolved spontaneously indicating that active surveillance and timely treatment can lead to favorable results in selected cases (6, 9, 42).
The incidence of SCI is 4.12% in zone 0 TEVAR, near to that (4.5%) reported by Uchida, which included 7,309 patients treated by TEVAR in 2014 (64). There were no concrete explanations for SCI or risk factors in the reviewed studies. In theory, sacrifice of LSA inflow is a risk factor for SCI; however, absence of detailed information impeded analysis in this review. In this review, only one SCI patient had their LSA covered without revascularization (20). Nonetheless, previous studies have suggested that LSA revascularization in selected patients might prevent SCI (34). Authors also provided other preventive procedures including prophylactic cerebrospinal fluid drainage and maintenance of higher mean arterial pressure (18, 34). In this review, 8 cases of SCI were successfully treated with cerebrospinal fluid drainage. Only one case of permanent paraplegia was recorded, in a patient implanted with a long aortic stent, with LSA coverage (20).
The rate of post-operative RTAD in the present review is 5.72%, lower than that reported by Chen et al. in their meta-analysis. Their study, conducted in 2018, highlighted the higher risk of RTAD in zone 0 TEVAR compared to zones 1, 2, 3, and 4 (8.12 vs. 2.57, 2.66, 0.67,% and 0.67%, respectively) (65). The anatomy of the arch and lack of comfort with newer stent grafts might contribute to the high rate of RTAD in zone 0 TEVAR. In four reviewed studies, RTAD patients had undergone hybrid procedures, and the leading cause suggested by the authors was clamp injury while debranching or bypassing branch vessels (9, 21, 27, 46). Oversizing of the stent graft, a large diameter of the stent graft, and an acute angle formed by the ascending aorta and PLZ contribute to RTAD according to some authors (21, 51). Although previous studies suggested proximal bare-metal configuration as a risk factor for RTAD, this configuration was present in 60.56% of cases in the reviewed studies and did not correlate with the rate of RTAD (65, 66). Prevention of RTAD mainly focuses on pre-operative planning and stent graft design. Chassin-Trubert et al. (46) suggested that, in selected hybrid procedures, rapid right ventricular pacing might decrease the risk of RTAD following zone 0 TEVAR. Given the 4/17 (23.5%) related-death rate and 8/17 (47.0%) reintervention rate, surgeons should consider reintervention as soon as RTAD is discovered (6, 9, 15, 19, 21, 46).
In the present review, the overall 30-day/in-hospital death rate of zone 0 TEVAR is 7.49%, close to that reported for all zone TEVAR (8.07%) in a systematic review by Ramdass in 2015 (67). But it exceeds the rates after TEVAR for descending thoracic aortic disease, as shown by Naazie in 2022 and Harris in 2020 (4.2% in 2,141 patients and 4.0% in 1,784 patients, respectively) (68, 69). If arch disease is taken into account, the 30-day rate of death in this review is lower than that reported for frozen elephant trunk in a multicenter study by Leone (437 patients, 14.9%), and higher than that reported for open total arch replacement in a meta-analysis performed in 2016 (2,880 patients, 5.3%) (70, 71).
The present meta-analysis and systemic review shows some limitations. First, most studies were conducted in a single center and lacked specific clinical data on individual patients. Second, most of the reviewed studies focused on one or two techniques, leading significant reporting biases. Finally, the recruitment time in reviewed studies with high heterogeneity inhibited further analysis of yearly trends. Consequently, to obtain a complete picture of zone 0 TEVAR, more exhaustive investigations on zone 0 TEVAR are required in the future.
5. Conclusion
Despite the absence of stent grafts dedicated for zone 0 TEVAR, novel stent grafts and various techniques targeting zone 0 TEVAR are currently being investigated and developed. However, there is still no consensus on technique and device selection for zone 0 TEVAR in current practice. Furthermore, the post-operative stroke rate is relatively high, while other complications and perioperative death rate are comparable to those of TEVAR for other aortic zones. The small number of studies aimed at zone 0 TEVAR calls for more comprehensive and detailed clinical studies to improve the informed decision-making in patients who may benefit from zone 0 TEVAR.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL and LZ contributed to conception and design of the study, organized the data extraction and statistical analysis, and wrote sections of the manuscript. LZ wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TEVAR, Thoracic endovascular aortic repair; PLZ, Proximal landing zone; SCI, Spinal cord ischemia; RTAD, Retrograde type A aortic dissection; PAU, Penetrating atherosclerotic ulcer; IMH, Intramural hematoma; LSA, Left subclavian artery.
References
1. Manetta F, Newman J, Mattia A. Indications for thoracic endovascular aortic repair (TEVAR): a brief review. Int J Angiol. (2018) 27:177–84. doi: 10.1055/s-0038-1666972
2. Baikoussis NG, Antonopoulos CN, Papakonstantinou NA, Argiriou M, Geroulakos G. Endovascular stent grafting for ascending aorta diseases. J Vasc Surg. (2017) 66:1587–601. doi: 10.1016/j.jvs.2017.07.064
3. Muetterties CE, Menon R, Wheatley GR. A systematic review of primary endovascular repair of the ascending aorta. J Vasc Surg. (2018) 67:332–42. doi: 10.1016/j.jvs.2017.06.099
4. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
5. Kurimoto Y, Kawaharada N, Ito T, Baba T, Ohori S, Watanabe A, et al. Less-invasive management of left subclavian artery in stent-grafting for distal aortic arch disease. Interact Cardiovasc Thorac Surg. (2009) 8:548–52. doi: 10.1510/icvts.2008.192484
6. Chiesa R, Melissano G, Tshomba Y, Civilini E, Marone EM, Bertoglio L, et al. Ten years of endovascular aortic arch repair. J Endovasc Ther. (2010) 17:1–11. doi: 10.1583/09-2884.1
7. Holt PJ, Johnson C, Hinchliffe RJ, Morgan R, Jahingiri M, Loftus IM, et al. Outcomes of the endovascular management of aortic arch aneurysm: implications for management of the left subclavian artery. J Vasc Surg. (2010) 51:1329–38. doi: 10.1016/j.jvs.2009.10.131
8. Geisbüsch P, Kotelis D, Hyhlik-Dürr A, Hakimi M, Attigah N, Böckler D. Endografting in the aortic arch - does the proximal landing zone influence outcome? Eur J Vasc Endovasc Surg. (2010) 39:693–9. doi: 10.1016/j.ejvs.2010.03.018
9. Canaud L, Hireche K, Berthet JP, Branchereau P, Marty-Ané C, Alric P. Endovascular repair of aortic arch lesions in high-risk patients or after previous aortic surgery: midterm results. J Thorac Cardiovasc Surg. (2010) 140:52–8. doi: 10.1016/j.jtcvs.2009.09.022
10. Kolvenbach RR, Karmeli R, Pinter LS, Zhu Y, Lin F, Wassiljew S, et al. Endovascular management of ascending aortic pathology. J Vasc Surg. (2011) 53:1431–7. doi: 10.1016/j.jvs.2010.10.133
11. Vallejo N, Rodriguez-Lopez JA, Heidari P, Wheatley G, Caparrelli D, Ramaiah V, et al. Hybrid repair of thoracic aortic lesions for zone 0 and 1 in high-risk patients. J Vasc Surg. (2012) 55:318–25. doi: 10.1016/j.jvs.2011.08.042
12. Melissano G, Tshomba Y, Bertoglio L, Rinaldi E, Chiesa R. Analysis of stroke after TEVAR involving the aortic arch. Eur J Vasc Endovasc Surg. (2012) 43:269–75. doi: 10.1016/j.ejvs.2011.12.009
13. Fukui D, Wada Y, Komatsu K, Fujii T, Ohashi N, Terasaki T, et al. Innovative application of available stent grafts in Japan in aortic aneurysm treatment-significance of innovative debranching and chimney method and coil embolization procedure. Ann Vasc Dis. (2013) 6:601–11. doi: 10.3400/avd.oa.13-00070
14. Preventza O, Bakaeen FG, Cervera RD, Coselli JS. Deployment of proximal thoracic endograft in zone 0 of the ascending aorta: treatment options and early outcomes for aortic arch aneurysms in a high-risk population. Eur J Cardiothorac Surg. (2013) 44:446–52. Discussion 452–3. doi: 10.1093/ejcts/ezt068
15. Shirakawa Y, Kuratani T, Shimamura K, Torikai K, Sakamoto T, Shijo T, et al. The efficacy and short-term results of hybrid thoracic endovascular repair into the ascending aorta for aortic arch pathologies. Eur J Cardiothorac Surg. (2014) 45:298–304. Discussion 304. doi: 10.1093/ejcts/ezt391
16. Bernardes RC, Navarro TP, Reis FR, Lima LC, Monteiro EL, Procopio RJ, et al. Early experience with off-the-shelf endografts using a zone 0 proximal landing site to treat the ascending aorta and arch. J Thorac Cardiovasc Surg. (2014) 148:105–12. doi: 10.1016/j.jtcvs.2013.07.049
17. Roselli EE, Idrees J, Greenberg RK, Johnston DR, Lytle BW. Endovascular stent grafting for ascending aorta repair in high-risk patients. J Thorac Cardiovasc Surg. (2015) 149:144–51. doi: 10.1016/j.jtcvs.2014.07.109
18. Hiraoka A, Chikazawa G, Tamura K, Totsugawa T, Sakaguchi T, Yoshitaka H. Clinical outcomes of different approaches to aortic arch disease. J Vasc Surg. (2015) 61:88–95. doi: 10.1016/j.jvs.2014.06.121
19. De Rango P, Cao P, Ferrer C, Simonte G, Coscarella C, Cieri E, et al. Aortic arch debranching and thoracic endovascular repair. J Vasc Surg. (2014) 59:107–14. doi: 10.1016/j.jvs.2013.07.010
20. Kurimoto Y, Maruyama R, Ujihira K, Nishioka N, Hasegawa K, Iba Y, et al. Thoracic endovascular aortic repair for challenging aortic arch diseases using fenestrated stent grafts from zone 0. Ann Thorac Surg. (2015) 100:24–32. Discussion 32–3. doi: 10.1016/j.athoracsur.2015.01.071
21. Gandet T, Canaud L, Ozdemir BA, Ziza V, Demaria R, Albat B, et al. Factors favoring retrograde aortic dissection after endovascular aortic arch repair. J Thorac Cardiovasc Surg. (2015) 150:136–42. doi: 10.1016/j.jtcvs.2015.03.042
22. Cazavet A, Alacoque X, Marcheix B, Chaufour X, Rousseau H, Glock Y, et al. Aortic arch aneurysm: short- and mid-term results comparing open arch surgery and the hybrid procedure†. Eur J Cardiothorac Surg. (2016) 49:134–40. doi: 10.1093/ejcts/ezv024
23. Katada Y, Kondo S, Tsuboi E, Rokkaku K, Irie Y, Yokoyama H. Endovascular total arch repair using in situ fenestration for arch aneurysm and chronic type A dissection. Ann Thorac Surg. (2016) 101:625–30. doi: 10.1016/j.athoracsur.2015.07.032
24. Ziza V, Canaud L, Molinari N, Branchereau P, Marty-Ané C, Alric P. Thoracic endovascular aortic repair: a single center's 15-year experience. J Thorac Cardiovasc Surg. (2016) 151:1595–603.e7. doi: 10.1016/j.jtcvs.2015.12.030
25. Tsilimparis N, Debus ES, Oderich GS, Haulon S, Terp KA, Roeder B, et al. International experience with endovascular therapy of the ascending aorta with a dedicated endograft. J Vasc Surg. (2016) 63:1476–82. doi: 10.1016/j.jvs.2015.12.027
26. Böckler D, Brunkwall DJ, Taylor PR, Mangialardi N, Hüsing J, Larzon T. Thoracic endovascular aortic repair of aortic arch pathologies with the conformable thoracic aortic graft: early and 2 year results from a European Multicentre Registry. Eur J Vasc Endovasc Surg. (2016) 51:791–800. doi: 10.1016/j.ejvs.2016.02.006
27. Narita H, Komori K, Usui A, Yamamoto K, Banno H, Kodama A, et al. Postoperative outcomes of hybrid repair in the treatment of aortic arch aneurysms. Ann Vasc Surg. (2016) 34:55–61. doi: 10.1016/j.avsg.2015.11.041
28. Faure EM, Canaud L, Marty-Ané C, Alric P. Hybrid aortic arch repair for dissecting aneurysm. J Thorac Cardiovasc Surg. (2016) 152:162–8. doi: 10.1016/j.jtcvs.2016.03.020
29. Yoshitake A, Hachiya T, Okamoto K, Kitahara H, Kawaguchi S, Nakatsuka S, et al. Postoperative stroke after debranching with thoracic endovascular aortic repair. Ann Vasc Surg. (2016) 36:132–8. doi: 10.1016/j.avsg.2016.02.039
30. Pecoraro F, Lachat M, Hofmann M, Cayne NS, Chaykovska L, Rancic Z, et al. Mid-term results of zone 0 thoracic endovascular aneurysm repair after ascending aorta wrapping and supra-aortic debranching in high-risk patients. Interact Cardiovasc Thorac Surg. (2017) 24:882–9. doi: 10.1093/icvts/ivx016
31. Canaud L, Baba T, Gandet T, Narayama K, Ozdemir BA, Shibata T, et al. Physician-modified thoracic stent-grafts for the treatment of aortic arch lesions. J Endovasc Ther. (2017) 24:542–548. doi: 10.1177/1526602817714206
32. Wang T, Shu C, Li QM, Li M, Li X, He H, et al. First experience with the double chimney technique in the treatment of aortic arch diseases. J Vasc Surg. (2017) 66:1018–27. doi: 10.1016/j.jvs.2017.02.035
33. Roselli EE, Idrees JJ, Johnston DR, Eagleton MJ, Desai MY, Svensson LG. Zone zero thoracic endovascular aortic repair: a proposed modification to the classification of landing zones. J Thorac Cardiovasc Surg. (2018) 155:1381–9. doi: 10.1016/j.jtcvs.2017.11.054
34. Toya N, Ohki T, Fukushima S, Shukuzawa K, Ito E, Murakami Y, et al. Case series of aortic arch aneurysm in patients with bovine arch treated with proximal scalloped and fenestrated stent graft. Cardiovasc Intervent Radiol. (2018) 41:1648–53. doi: 10.1007/s00270-018-2058-1
35. Zhu J, Dai X, Noiniyom P, Luo Y, Fan H, Feng Z, et al. Fenestrated thoracic endovascular aortic repair using physician-modified stent grafts (PMSGs) in zone 0 and zone 1 for aortic arch diseases. Cardiovasc Intervent Radiol. (2019) 42:19–27. doi: 10.1007/s00270-018-2079-9
36. Hosaka A, Motoki M, Kato M, Sugai H, Okubo N. Quantification of aortic shagginess as a predictive factor of perioperative stroke and long-term prognosis after endovascular treatment of aortic arch disease. J Vasc Surg. (2019) 69:15–23. doi: 10.1016/j.jvs.2018.03.425
37. Huang W, Ding H, Jiang M, Liu Y, Huang C, Yang X, et al. Outcomes of chimney technique for aortic arch diseases: a single-center experience with 226 cases. Clin Interv Aging. (2019) 14:1829–40. doi: 10.2147/CIA.S222948
38. Yamauchi T, Kubota S, Hasegawa K, Ueda H. Clinical results and medical costs of thoracic endovascular aortic repair in patients over 80 years of age. J Artif Organs. (2019) 22:61–7. doi: 10.1007/s10047-018-1073-y
39. Ryomoto M, Tanaka H, Mitsuno M, Yamamura M, Sekiya N, Uemura H, et al. A novel approach to prevent perioperative stroke in patients undergoing debranching thoracic endovascular aortic repair with a mini-cardiopulmonary bypass support. Ann Vasc Surg. (2019) 59:143–9. doi: 10.1016/j.avsg.2018.09.036
40. Piffaretti G, Grassi V, Lomazzi C, Brinkman WT, Navarro TP, Jenkins MP, et al. Thoracic endovascular stent graft repair for ascending aortic diseases. J Vasc Surg. (2019) 70:1384–9.e1. doi: 10.1016/j.jvs.2019.01.075
41. Tsilimparis N, Law Y, Rohlffs F, Spanos K, Debus ES, Kölbel T. Fenestrated endovascular repair for diseases involving the aortic arch. J Vasc Surg. (2020) 71:1464–71. doi: 10.1016/j.jvs.2019.06.205
42. De León AI, Cheng YT, Chen SW, Chu SY, Nan YY, Liu KS. Outcomes of type Ia endoleaks after endovascular repair of the proximal aorta. J Thorac Cardiovasc Surg. (2020) 163:2012.e6. doi: 10.1016/j.jtcvs.2020.06.026
43. Kuo HS, Huang JH, Chen JS. Handmade fenestrated stent grafts to preserve all supra-aortic branches in thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. (2020) 160:629–39.e1. doi: 10.1016/j.jtcvs.2019.07.096
44. Tinelli G, Ferrer C, Giudice R, Ferraresi M, Pogany G, Cao P, et al. Long-term results of hybrid repair techniques for Kommerell's diverticulum. J Vasc Surg. (2020) 72:1213–21. doi: 10.1016/j.jvs.2019.11.052
45. Fernández-Alonso L, Fernández Alonso S, Martínez Aguilar E, Santamarta Fariña E, Alegret Solé J, Atienza Pascual M, et al. Fenestrated and scalloped endovascular grafts in zone 0 and zone 1 for aortic arch disease. Ann Vasc Surg. (2020) 69:360–5. doi: 10.1016/j.avsg.2020.06.009
46. Chassin-Trubert L, Ozdemir BA, Roussel A, Dessertenne G, Castier Y, Ludovic C, et al. Prevention of retrograde ascending aortic dissection by cardiac pacing during hybrid surgery for zone 0 aortic arch repair. Ann Vasc Surg. (2021) 71:48–55. doi: 10.1016/j.avsg.2020.08.136
47. Li F, Wu X, Zhang X, Qin J, Zhao Z, Ye K, et al. Clinical outcomes of distal tapered restrictive covered stent applied in endovascular treatment of aortic dissection involving zone 0. Eur J Vasc Endovasc Surg. (2021) 61:413–21. doi: 10.1016/j.ejvs.2020.11.037
48. Planer D, Elbaz-Greener G, Mangialardi N, Lindsay T, D'Onofrio A, Schelzig H, et al. NEXUS arch: a multicenter study evaluating the initial experience with a novel aortic arch stent graft system. Ann Surg. (2021) 277:e460–6. doi: 10.1097/SLA.0000000000004843
49. Dake MD, Bavaria JE, Singh MJ, Oderich G, Filinger M, Fischbein MP, et al. Management of arch aneurysms with a single-branch thoracic endograft in zone 0. JTCVS Tech. (2021) 7:1–6. doi: 10.1016/j.xjtc.2021.01.011
50. Seguchi R, Kiuchi R, Horikawa T, Tarui T, Sanada J, Ohtake H, et al. Novel brain protection method for zone 0 endovascular aortic repair with selective cerebral perfusion. Ann Vasc Dis. (2021) 14:153–8. doi: 10.3400/avd.oa.21-00025
51. Li X, Zhang L, Song C, Zhang H, Xia S, Li H, et al. Long-term outcomes of thoracic endovascular repair for aortic arch dissection using customized single-branched fenestrated stent-graft. Vasc Endovascular Surg. (2021) 55:577–585. doi: 10.1177/15385744211010446
52. Li X, Zhang L, Song C, Zhang H, Xia S, Li H, et al. Outcomes of total endovascular aortic arch repair with surgeon-modified fenestrated stent-grafts on zone 0 landing for aortic arch pathologies. J Endovasc Ther. (2021) 2021:15266028211036478. doi: 10.1177/15266028211036478
53. Hanna L, Abdullah A, Kashef E, Riga C, Jenkins M, Bicknell C, et al. Four-year results of the Bolton relay proximal scallop endograft in the management of thoracic and thoracoabdominal aortic pathology with unfavorable proximal landing zone. J Vasc Surg. (2021) 74:1447–55. doi: 10.1016/j.jvs.2021.04.027
54. Barnes JA, Wanken ZJ, Columbo JA, Kuwayama DP, Fillinger MF, Suckow BD. Procedure-associated costs and mid-term outcomes of endovascular zone 0 and zone 1 aortic arch repair. Ann Vasc Surg. (2021) 81:98–104. doi: 10.1016/j.avsg.2021.10.026
55. Kudo T, Kuratani T, Shirakawa Y, Shimamura K, Kin K, Sakamoto T, et al. Comparison of the outcomes of total endovascular aortic arch repair between branched endograft and chimney endograft technique in zone 0 landing. J Endovasc Ther. (2021) 2021:15266028211059912. doi: 10.1177/15266028211059912
56. Chen D, Luo M, Fang K, Shu C. Endovascular repair for acute zone 0 intramural hematoma with most proximal tear or ulcer-like projection in the descending aorta. J Vasc Surg. (2021) 75:1561–9. doi: 10.1016/j.jvs.2021.12.055
57. Eleshra A, Heo W, Lee KH, Kim TH, Sim HS, Sharafeldin H, et al. Mid-term outcomes of hybrid debranching endovascular aortic arch repair in landing zones 0–2. Vascular. (2022) 2022:17085381211068230. doi: 10.1177/17085381211068230
58. von Allmen RS, Gahl B, Powell JT. Editor's choice - incidence of stroke following thoracic endovascular aortic repair for descending aortic aneurysm: a systematic review of the literature with meta-analysis. Eur J Vasc Endovasc Surg. (2017) 53:176–84. doi: 10.1016/j.ejvs.2016.10.025
59. Karaolanis GI, Antonopoulos CN, Charbonneau P, Georgakarakos E, Moris D, Scali S, et al. A systematic review and meta-analysis of stroke rates in patients undergoing Thoracic Endovascular Aortic Repair for descending thoracic aortic aneurysm and type B dissection. J Vasc Surg. (2022) 76:292–301.e3. doi: 10.1016/j.jvs.2022.02.031
60. Papakonstantinou NA, Antonopoulos CN, Baikoussis NG, Kakisis I, Geroulakos G. Frozen elephant trunk: an alternative surgical weapon against extensive thoracic aorta disease. A three-year meta-analysis. Heart Lung Circ. (2019) 28:213–22. doi: 10.1016/j.hlc.2018.04.306
61. Chen X, Wang J, Premaratne S, Zhao J, Zhang WW. Meta-analysis of the outcomes of revascularization after intentional coverage of the left subclavian artery for thoracic endovascular aortic repair. J Vasc Surg. (2019) 70:1330–40. doi: 10.1016/j.jvs.2019.03.022
62. Hammo S, Larzon T, Hultgren R, Wanhainen A, Mani K, Resch T, et al. Outcome after endovascular repair of ruptured descending thoracic aortic aneurysm: a national multicentre study. Eur J Vasc Endovasc Surg. (2019) 57:788–94. doi: 10.1016/j.ejvs.2018.10.029
63. Kudo T, Kuratani T, Shimamura K, Sawa Y. Determining the optimal proximal landing zone for TEVAR in the aortic arch: comparing the occurrence of the bird-beak phenomenon in zone 0 vs zones 1 and 2. J Endovasc Ther. (2020) 27:368–76. doi: 10.1177/1526602820914269
64. Uchida N. How to prevent spinal cord injury during endovascular repair of thoracic aortic disease. Gen Thorac Cardiovasc Surg. (2014) 62:391–7. doi: 10.1007/s11748-014-0395-9
65. Chen Y, Zhang S, Liu L, Lu Q, Zhang T, Jing Z. Retrograde type A aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. (2017) 6:e004649. doi: 10.1161/JAHA.116.004649
66. Neuhauser B, Greiner A, Jaschke W, Chemelli A, Fraedrich G. Serious complications following endovascular thoracic aortic stent-graft repair for type B dissection. Eur J Cardiothorac Surg. (2008) 33:58–63. doi: 10.1016/j.ejcts.2007.10.010
67. Ramdass M. TEVAR for symptomatic Stanford B dissection: a systematic review of 30-day mortality and morbidity. Thorac Cardiovasc Surg. (2015) 63:97–112. doi: 10.1055/s-0034-1370760
68. Naazie IN, Gupta JD, Azizzadeh A, Arbabi C, Zarkowsky D, Malas MB. Risk calculator predicts 30-day mortality after thoracic endovascular aortic repair for intact descending thoracic aortic aneurysms in the Vascular Quality Initiative. J Vasc Surg. (2022) 75:833–41.e1. doi: 10.1016/j.jvs.2021.08.056
69. Harris DG, Olson SL, Panthofer AM, Matsumura JS, DiMusto PD. A Frailty-based risk score predicts morbidity and mortality after elective endovascular repair of descending thoracic aortic aneurysms. Ann Vasc Surg. (2020) 67:90–9. doi: 10.1016/j.avsg.2019.10.090
70. Settepani F, Cappai A, Basciu A, Barbone A, Tarelli G. Outcome of open total arch replacement in the modern era. J Vasc Surg. (2016) 63:537–45. doi: 10.1016/j.jvs.2015.10.061
Keywords: zone 0 TEVAR, fenestrated TEVAR, chimney TEVAR, hybrid endovascular aortic repair, endograft, complications after TEVAR
Citation: Zhu L, Li X and Lu Q (2023) A systematic review and meta-analysis of thoracic endovascular aortic repair with the proximal landing zone 0. Front. Cardiovasc. Med. 10:1034354. doi: 10.3389/fcvm.2023.1034354
Received: 01 September 2022; Accepted: 06 February 2023;
Published: 24 February 2023.
Edited by:
Emiliano Chisci, Nuovo Ospedale San Giovanni di Dio, ItalyReviewed by:
Xinsheng Xie, Fudan University, ChinaPiero Farina, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2023 Zhu, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingsheng Lu,  bHVxc0BuZXd2YXNjdWxhci5jbg==
bHVxc0BuZXd2YXNjdWxhci5jbg==
†These authors have contributed equally to this work
 Longtu Zhu†
Longtu Zhu† Xiaoye Li
Xiaoye Li Qingsheng Lu
Qingsheng Lu