
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Cardiovasc. Med. , 10 February 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1021937
 Yezi Chai1†
Yezi Chai1† Meng Jiang1*
Meng Jiang1* Yaohui Wang2†
Yaohui Wang2† Qiming Liu1
Qiming Liu1 Qifan Lu1
Qifan Lu1 Zhengyu Tao1
Zhengyu Tao1 Qizhen Wu1
Qizhen Wu1 Wenjin Yin2*
Wenjin Yin2* Jinsong Lu2*
Jinsong Lu2* Jun Pu1*
Jun Pu1*Background and aim: Cardiotoxicity has become the most common cause of non-cancer death among breast cancer patients. Pyrotinib, a tyrosine kinase inhibitor targeting HER2, has been successfully used to treat breast cancer patients but has also resulted in less well-understood cardiotoxicity. This prospective, controlled, open-label, observational trial was designed to characterize pyrotinib’s cardiac impacts in the neoadjuvant setting for patients with HER2-positive early or locally advanced breast cancer.
Patients and methods: The EARLY-MYO-BC study will prospectively enroll HER2-positive breast cancer patients who are scheduled to receive four cycles of neoadjuvant therapy with pyrotinib or pertuzumab added to trastuzumab before radical breast cancer surgery. Patients will undergo comprehensive cardiac assessment before and after neoadjuvant therapy, including laboratory measures, electrocardiography, transthoracic echocardiography, cardiopulmonary exercise testing (CPET), and cardiac magnetic resonance (CMR). To test the non-inferiority of pyrotinib plus trastuzumab therapy to pertuzumab plus trastuzumab therapy in terms of cardiac safety, the primary endpoint will be assessed by the relative change in global longitudinal strain from baseline to completion of neoadjuvant therapy by echocardiography. The secondary endpoints include myocardial diffuse fibrosis (by T1-derived extracellular volume), myocardial edema (by T2 mapping), cardiac volumetric assessment by CMR, diastolic function (by left ventricular volume, left atrial volume, E/A, and E/E’) by echocardiography, and exercise capacity by CPET.
Discussion: This study will comprehensively assess the impacts of pyrotinib on myocardial structural, function, and tissue characteristics, and, furthermore, will determine whether pyrotinib plus trastuzumab is a reasonable dual HER2 blockade regimen with regard to cardiac safety. Results may provide information in selecting an appropriate anti-HER2 treatment for HER2-positive breast cancer.
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT04510532
Breast cancer is the most commonly diagnosed tumor and is also the most malignant female tumor (1). Advances in pharmacotherapies have resulted in tremendous progress in breast cancer treatment (2). However, survival benefits are increasingly offset by adverse drug reactions, particularly cardiotoxicity. Cardiotoxicity can result in cancer therapy-related cardiac dysfunction (CTRCD), myocarditis, arrythmias, and other adverse outcomes (3, 4). Remarkably, cardiotoxicity may occur during various stages of cancer, at different ages, and even in patients who are considered as theoretically low risk at baseline (5–8). Hence, anticancer drugs with less cardiotoxicity are urgently needed.
HER2-directed drugs have become a cornerstone for the treatment of HER2-positive breast cancer, and they have shown less cardiotoxicity than traditional anthracyclines (2). Currently, dual HER2 blockade with trastuzumab and pertuzumab is recommended by the National Comprehensive Cancer Network (NCCN) guidelines as an important neoadjuvant therapy component for patients with early or locally advanced HER2-positive breast cancer (9). However, studies have recently shown that this regimen has a higher incidence of clinical heart failure than trastuzumab alone (10).
Pyrotinib is a small-molecule tyrosine kinase inhibitor (TKI) that simultaneously inhibits HER2 homodimerization and heterodimerization. After demonstrating significant anticancer activity in the PHOEBE trial and other clinical trials, the National Medical Products Administration of China approved pyrotinib in 2020 for the treatment of HER2-positive advanced or metastatic breast cancer (11). As a promising HER2-targeted agent, adding pyrotinib to trastuzumab and neoadjuvant chemotherapy further improved the pathological complete response (pCR) rate up to 69.81% in patients with early or locally advanced breast cancer in a phase II NeoATP trial (12). To date, the adverse drug reactions of pyrotinib include adverse gastrointestinal reactions and hand-foot syndrome. Unfortunately, no research has yet evaluated its cardiovascular impacts.
Cardiac imaging, including echocardiography and cardiac magnetic resonance (CMR), is critical for cardiac assessment in breast cancer patients. Of the cardiac functional parameters, left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) derived from echocardiography are the most widely used to define CTRCD. Up to 39.1% of breast cancer patients exposed to trastuzumab and anthracycline presented with CTRCD during treatment or follow-up (13). Furthermore, CMR, the non-invasive reference technique for myocardial volumetric and functional assessment, is also capable of offering additional information on cardiac tissue properties. Using CMR, increased intracellular matrix can be detected by T1-derived extracellular volume (ECV) quantification (14), and myocardial edema can be identified on CMR T2-mapping images (15, 16). Hence, multi-modality cardiac imaging can enable a comprehensive picture of myocardial features after anti-cancer therapy.
Here, we registered the EARLY-MYO-BC study in order to describe pyrotinib’s cardiac impacts and to further determine whether novel dual HER2 blockade using pyrotinib plus trastuzumab is non-inferior to the NCCN-recommended regimen (pertuzumab plus trastuzumab) with regard to cardiac safety during the course of neoadjuvant treatment.
The EARLY-MYO-BC (Early Assessment of Myocardial Injury in Patients Receiving Neoadjuvant Pyrotinib Therapy with Early or Locally Advanced HER2-Positive Breast Cancer) is a prospective, open-label, observational study (ClinicalTrials.gov identifier NCT04510532) that will enroll patients with early or locally advanced HER2-positive breast cancer from the Department of Breast Surgery at the Renji Hospital for cardiac assessment from the initiation of neoadjuvant pyrotinib or pertuzumab therapy. The registry has been approved by the local Ethics Committee of Renji Hospital. All patients will provide fully informed written consent. The study protocol conforms with the principles of the 2013 Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guideline.
Patients are eligible for this trial if they meet all of the following criteria: (1) female patients between 18 and 70 years of age; (2) newly diagnosed and histologically confirmed early or locally advanced HER2-positive invasive breast cancer (Stage IIA-IIIC); (3) no signs of cardiac discomfort; (4) normal range of baseline cardiac biomarkers [troponin I (TNI), brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP)], electrocardiography (ECG), and baseline LVEF ≥ 50% from echocardiography; (5) able to complete face-to-face follow-up visits during neoadjuvant therapy; (6) provide signed written informed consent.
The exclusion criteria are as follows: (1) metastatic breast cancer confirmed histologically or by imaging tests; (2) prior history of medical anticancer treatments; (3) documented coronary heart disease or cardiomyopathy; (4) angina pectoris requiring medication; (5) clinically significant valvular disease; (6) unstable arrhythmias; (7) uncontrolled blood pressure; (8) severe chronic or acute renal failure (glomerular filtration rate < 30 ml/min/1.73 m2); (9) severe liver diseases; (10) prior history of immunodeficiency or organ transplantation; (11) pregnant or lactating; (12) unable to take or absorb tablets; (13) contraindications to CMR; (14) any other medical condition assessed by the investigator as inappropriate to participate.
A trained researcher will be responsible for providing all necessary information about this study to the potential participants. It will be made clear to the participants that they are under no obligation to take part in the trial, and their usual care will not be affected by their decision. All participants can withdraw their consent during the trial without providing a reason or explanation. They will be given a sheet with contact details for the research team and instructions on what to do if they wish to withdraw or require further information.
The study flowchart is shown in Figure 1. Cardiac assessments are scheduled to be conducted at baseline and before radical breast cancer surgery. Four cycles of neoadjuvant therapy will be given before radical breast cancer surgery and each cycle will last for 28 days.
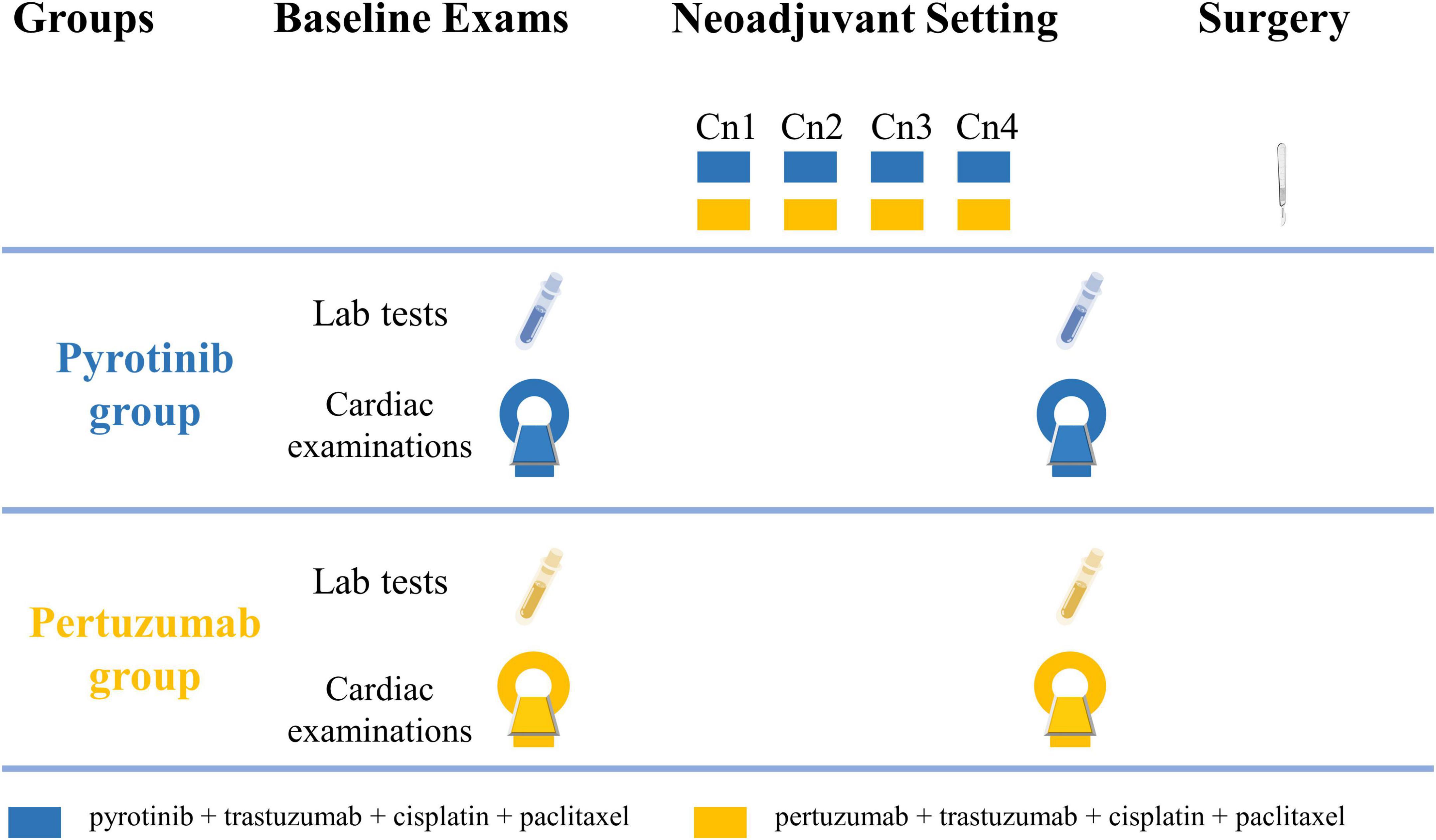
Figure 1. Study flowchart. Cardiac examinations include electrocardiography, echocardiography, cardiac magnetic resonance, and cardiopulmonary exercise testing. Cn, neoadjuvant cycle.
Pyrotinib group treatment regimen: Cisplatin (25 mg/m2 at day 1, 8, 15), paclitaxel (80 mg/m2 at day 1, 8, 15, 22), and trastuzumab (4 mg/kg at loading dose, then 2 mg/kg per week) will be administered intravenously to patients in the ambulatory chemotherapy ward. Patients will take 400 mg pyrotinib orally once per day.
Pertuzumab group treatment regimen: Cisplatin, paclitaxel, and trastuzumab will be administered to patients with the same regimen as the pyrotinib group. Pertuzumab will be intravenously given at an 840 mg loading dose, followed by 420 mg every 3 weeks in the ambulatory chemotherapy ward.
To obtain baseline normal myocardial values for comparison, age- and gender-matched healthy volunteers without cardiovascular diseases (CVDs) will be recruited as negative controls.
Demographic features including age and body mass index (BMI) will be prospectively collected at baseline, as well as blood pressure, heart rate, risk factors for cardiovascular events (e.g., hypertension, hyperlipidemia, diabetes, current smoking, and family history of early onset CVD), tumor location, tumor stage, and history of cancer and other diseases. Baseline cardiac risk assessment will be performed using the statement-recommended algorithm (17).
Laboratory measurements will include: (1) cardiac markers (TNI, BNP, NT-proBNP, and soluble ST2); (2) complete blood counts (white blood cell counts, hemoglobin, hematocrit, and platelet counts); (3) hepatic function markers (alanine aminotransferase, aspartate aminotransferase, and total bilirubin); (4) renal function markers (creatinine and blood urea nitrogen); (5) lipid profile (triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein); (6) fasting glucose; (7) inflammatory markers (C-reactive protein, interleukin-1β, interleukin-6, interleukin-8, and tumor necrosis factor-α).
ECG, echocardiography, cardiopulmonary exercise testing (CPET), and CMR data will be recorded in the study database.
A resting 12-lead ECG will be used following the current guideline-recommended standard protocols to record the cardiac electrophysiology of breast cancer patients (18).
Echocardiography will be performed to obtain left ventricular (LV) morphology and function (Vivid E95 ultrasound system, GE Vingmed Ultrasound, Horten, Norway) following the standard recommendation (19). Two level 3 sonographers (who are blinded to the results of the other examinations) will analyze the images separately. Echocardiographic measurements include left atrial (LA) dimension, LV end-diastolic and end-systolic dimensions, LV septal and posterior wall thicknesses, and LVEF. Myocardial strain [global circumferential strain (GCS), GLS and global radial strain (GRS)] will be used to assess tissue deformation.
Diastolic function will be assessed by the ratio between early (E) and atrial (A) peak diastolic velocities of LV inflow (E/A), the ratio between E and peak early (E’) tissue Doppler of the mitral annulus (E/E’), and the LA dimension. Within 24 h of the ECG and echocardiographic examination, a CMR scan and CPET will be scheduled.
CPET will be conducted to evaluate the cardiac-pulmonary function of breast cancer patients by continuously measuring breath-by-breath expired gases before, during, and after exercise. Testing will start with 3-min unloaded cycling and gradually achieve peak exercise using the 10 Watt/minute incremental ramp protocol. Patients are encouraged to maintain exercise until exhaustion. The test will be considered valid with a perceived exertion rating >18 on the Borg scale or a respiratory exchange ratio > 1.1 (20–22). A 12-lead ECG and blood pressure measurement will be recorded every 3 min. The typical CPET variables include peak oxygen uptake, peak metabolic equivalent, ventilatory efficiency, oxygen pulse, peak blood pressure, and anaerobic threshold.
CMR will be performed using a Prisma 3.0-Tesla magnetic resonance (MR) scanner (Siemens Healthineers, Germany). The imaging protocol is consistent with the statement from the Society for Cardiovascular Magnetic Resonance (23). The full imaging protocol will include standard cine imaging for cardiac morphological and functional assessment, T1 mapping and late gadolinium enhancement (LGE) for myocardial fibrosis assessment, and T2 mapping for myocardial edema assessment. Two cardiologists (with >8 years of experience in CMR and blinded to the clinical information) are responsible for image post-processing using CVI42 5.13.9 software (Circle Cardiovascular Imaging, Calgary, Canada).
Cine imaging: Standard cine images will be acquired with end-expiratory breath hold using retrospectively gated, balanced, steady-state, free precession sequences in three long-axis planes and sequential short-axis slices from the atrioventricular ring to the LV apex. The typical cine sequence parameters are as follows: field of view (FOV), 360 × 360 mm; repetition time (TR), 3.39 ms; echo time (TE), 1.49 ms; flip angle (FA), 58°; voxel size, 1.4 × 1.4 × 8.0 mm3. The LV endocardial and epicardial borders are end-diastole and end-systole contoured to accomplish cardiac volumetric and strain analysis. All volumetric indices and mass will be indexed to body surface area. Trabeculations and papillary muscles will be included in LV mass calculations and excluded from LV volumes. Myocardial strain will also be assessed. GCS and GRS will be obtained in the short-axis view at the apical, midventricular, and basal levels, and GLS will be obtained in the long-axis view (2-, 3-, and 4-chamber images).
T2 mapping: T2 mapping will be performed with a T2-prepared, single-shot, multi-echo, steady-state free precession (SSFP) technique, and three T2P-SSFP images will be obtained in the short axis at the basal, mid, and apical levels. Typical sequence parameters are as follows: FOV, 360 × 360 mm; TR, 3.15 ms; TE, 1.38 ms; FA, 12°; matrix, 224 × 160; voxel size, 1.6 × 1.6 × 8.0 mm3, bandwidth, 1175 Hz/Px.
Pre- and post-contrast T1 mapping: A SSFP, single breath-hold, modified Look-Locker inversion recovery sequence will be used for T1 mapping before and 15 min after contrast application. Three slices will be obtained for pre- and post-contrast T1 mapping in the short axis at the basal, mid, and apex. Typical parameters are as follows: FOV, 360 × 360 mm; TR, 2.44 ms; TE, 1.12 ms; FA 35°; voxel size, 1.4 × 1.4 × 8.0 mm3; 160 phase-encoding steps; sensitivity encoding factor = 2. Regions of interest will be placed on the entire myocardium to obtain T1 values, but will exclude the blood pool, papillary muscles, chordae, and trabeculations. To detect interstitial matrix changes ECV is calculated based on the combination of pre- and post-contrast T1 values with the formula: ECV = (ΔR1myocardium/ΔR1blood) • (1 - hematocrit) where R1 = 1/T1 time (24).
LGE: A LGE scan will be performed 10 min after intravenous administration of gadolinium contrast and will employ a phase-sensitive reconstruction (PSIR) covering the entire left ventricle from basal to apex in short-axis views. Typical parameters are as follows: TR, 3.87 ms; TE, 1.52 ms; FA, 20°; voxel size, 0.8 × 0.8 × 8.0 mm3. LGE positive is defined as hyperenhancement on the myocardium (>5 × S.D. of reference region).
To determine whether pyrotinib is non-inferior to pertuzumab with regard to cardiac safety when combined with trastuzumab during the course of neoadjuvant therapy, our primary endpoint is designed to compare the relative change in GLS between the two groups by echocardiography. The secondary endpoints are assessed by CMR and CPET including the relative changes in (1) myocardial diffuse fibrosis (by T1-derived ECV), (2) myocardial edema (by T2 mapping), (3) myocardial volumetric and diastolic function (by LV volume, LA volume, E/A, E/E’) and (4) exercise capacity between the two groups.
One-fourth of the patients will be randomly chosen for imaging measurement reproducibility assessment 1 month after the first measurement. The interobserver and intraobserver imaging measurement reproducibility will be assessed by intraclass correlation analyses.
The sample size calculation is based on our preliminary research. The mean relative changes in GLS before and after treatment of 10 patients receiving pyrotinib and 10 patients receiving pertuzumab were 6.15 and 5.00 with standard deviations (SDs) of 8.10 and 7.73, respectively. Assuming a non-inferiority margin of 3.8, a sample ratio (n pyrotinib/n pertuzumab) of 1, a type I error (one-sided) of 5%, a power of 80%, and a missing rate of 10%, a final sample size of at least 61 subjects in each group will be required to detect the non-inferiority of pyrotinib to pertuzumab with regard to cardiac safety.
Data will be tested for normal distribution by the Kolmogorov–Smirnov test. Normally distributed data are expressed as mean ± SD. Non-normally distributed data are expressed by median and interquartile range (IQR). Categorical data are expressed as frequencies and percentages. Non-inferiority will be assessed on the primary endpoint to compare the differences between the two groups in terms of relative changes in GLS from baseline to completion of neoadjuvant therapy. Baseline characteristics and secondary endpoints will be analyzed using Student’s t-test or Mann–Whitney U test for continuous variables that are normally or non-normally distributed, respectively, or using chi-square test or Fisher exact test for categorical data. Logistic regression will be employed to identify the predictive value of baseline clinical features (age, BMI, prior history of hypertension and hyperlipidemia, etc.), biomarkers (TNI, BNP, NT-proBNP, ST2, etc.), and imaging indicators in CTRCD development. The interobserver reliability will be evaluated by intraclass correlation coefficient (ICC) for continuous variable and Kappa value for categorical variable. Except for the non-inferiority test of the primary endpoint, all reported P-values are two-sided; those under 0.05 are considered to be statistically significant. Analyses are performed using SPSS 26.0 and R software (version 3.4.1).
To the best of our knowledge, the EARLY-MYO-BC registry is the first to explore pyrotinib’s cardiac impacts in the neoadjuvant setting for patients with early or locally advanced HER2-positive breast cancer.
Over the past decades, tremendous advances have been made in pharmacotherapies for breast cancer, especially in targeted therapy, reducing the 10-year risk of dying from breast cancer by 6.4% in patients diagnosed with early HER2-positive breast cancer (2, 25). These advances in anti-cancer therapies, however, came with an increased frequency of cardiotoxic events that must be addressed. Survivors were revealed to have approximately 2-fold higher risk of CVD-related mortality than the general population 8 years after breast cancer diagnosis (26). Cancer therapy-related cardiotoxicity might exacerbate underlying heart disease or initiate de novo cardiac problems. Furthermore, cardiotoxicity can complicate and even cause interruption or discontinuation of cancer therapy, which significantly reduces breast cancer patients’ survival benefit (27, 28). Indeed, up to 11% of early breast cancer patients exposed to trastuzumab experienced an interruption of therapy due to cardiotoxicity (28). Therefore, early identification of anticancer drug-related cardiotoxicity characteristics and severity is extremely valuable in rationally selecting treatment strategies to avoid severe cardiac injury and cancer treatment discontinuity.
The optimal breast cancer therapy is driven by molecular subtype. For HER2-positive breast cancer, anti-HER2 therapy, especially dual HER2 blockade (trastuzumab and pertuzumab), has been recommended as part of the preferred neoadjuvant therapy by the NCCN (9). Notably, the incidence of cardiotoxicity varies widely among anti-HER2 drugs depending on the nature of the drugs, therapy duration, and underlying patient comorbidities. CTRCD has been described in 3–10% of early breast cancer patients treated with trastuzumab and in 19% treated with trastuzumab plus anthracyclines (2, 29). The mechanism of trastuzumab-induced cardiotoxicity involves inhibition of the neuregulin-1/ErbB signaling pathway, which disequilibrates cardiomyocyte homeostasis and leads to myocardial injury (30, 31). Other anti-HER2 drugs, such as lapatinib, resulted in a lower incidence of CTRCD than trastuzumab (2–9% with lapatinib), probably due to their distinct binding epitopes on the extracellular domain of the HER2 receptor (30, 32–34). Thus, anti-HER2 therapies have different mechanisms and incidences of cardiac injury. For dual HER2 blockade, the cardiotoxicity might be synergistic. Specifically, dual HER2 blockade combining trastuzumab and pertuzumab resulted in a higher incidence of heart failure compared with trastuzumab alone (10, 35). Hence, it is imminent to identify an optimal combination of HER2-directed agents that balance efficacy and cardiotoxicity.
Pyrotinib is an oral small-molecule TKI of multiple HER family members, enabling concomitant blockade of HER2 homodimerization and heterodimerization. In patients with HER2-positive relapsed or metastatic breast cancer, pyrotinib was shown to prolong the median progression-free survival more significantly than lapatinib (12.5 months vs 6.8 months) (11). As a novel dual HER2 blockade regimen in the neoadjuvant setting, pyrotinib combined with trastuzumab demonstrated potent antitumor activity with a pCR rate of 55.1–69.81%, higher than the pCR rate of 54.0% obtained with trastuzumab combined with pertuzumab (12, 36, 37). However, the cardiotoxicity of pyrotinib has rarely been studied in single or dual HER2 blockade regimens. We propose that if the combination of trastuzumab and pyrotinib shows non-inferiority to the combination of trastuzumab and pertuzumab with regard to cardiac safety, the former regimen might serve as an alternative option for dual HER2 blockade in the neoadjuvant setting.
To date, assessment of cancer therapy-related cardiotoxicity has relied largely on serum biomarker measures (TNI, BNP, NT-proBNP, etc.) and cardiac imaging tests (echocardiography, CMR, etc.) (17, 38, 39). As the most commonly used cardiac biomarkers, TNI and BNP play a limited role in detecting early subclinical cardiac impairment since biomarker abnormality usually suggests a present or irreversible myocardial injury (40, 41). Although NT-proBNP has been shown to predict future heart failure in the general population, evidence-based data for NT-proBNP regarding the detection of cancer therapy-related cardiotoxicity is still lacking (42–47). Clinically, LVEF is the mainstay of imaging metrics for cardiac function evaluation. However, relying solely on LVEF assessment to identify cardiotoxicity, especially in those presenting with early subclinical cardiac dysfunction, is far from adequate. GLS, an emerging imaging metric, provides quantitative assessment for the early detection of pre-clinical heart failure in cancer patients and has been recommended by the Cardio-Oncology Council of the European Society of Cardiology (17). GLS reduction helps to predict subsequent cardiotoxicity in cancer patients before the decrease of LVEF (48–50). We therefore selected GLS change during neoadjuvant therapy as our primary endpoint. Furthermore, previous literature revealed that the increase in chemotherapy-induced myocardial fibrosis (indicated by T1-derived ECV) and edema (indicated by T2 mapping) on CMR images implied cardiotoxicity when LVEF was still within normal range (14–16). With this in mind, the secondary endpoints in our study further explore the myocardial tissue alterations in patients treated with pyrotinib or pertuzumab.
In conclusion, this study aims to comprehensively assess pyrotinib’s cardiac impacts and, further, to determine whether pyrotinib plus trastuzumab is non-inferior to pertuzumab plus trastuzumab with regard to cardiac safety. The myocardial structure, function, and tissue characteristics will be explored, and the findings will provide valuable information for oncologists with respect to selecting appropriate treatment options for patients with breast cancer.
The studies involving human participants were reviewed and approved by the local Ethics Committee of Renji Hospital. The patients/participants provided their written informed consent to participate in this study.
YC and YW: design, patient recruitment, data acquisition and analysis, manuscript drafting and revision, and approval of the final version of the manuscript. QML, QFL, ZT, and QW: data acquisition. MJ, WY, JL, and JP: study concept and design, study supervision, drafting, revision, and approval of the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This study received funding support from the National Natural Science Foundation of China (U21A20341, 81971570, 81930007, 82103695, and 82173115), Shanghai Outstanding Academic Leaders Program (18XD1402400), National Science Fund for Distinguished Young Scholars (81625002), Shanghai Academic/Technology Leader Program (21XD1432100) and Shanghai Science and Technology Commission Program (20Y11910500), Clinical Research Plan of SHDC (SHDC2020CR2025B and SHDC2020CR3003A), Shanghai Rising-Star Program (No. 22QC1400200), Shanghai Jiao Tong University (YG2019ZDA12, YG2019QNA28, PYIII20-09, and RJPY-LX-002), and University of Shanghai for Science and Technology (10-20-302-425).
The authors are indebted to the support from the Innovative Research Team of High-level Local Universities in Shanghai.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. (2011) 365:1273–83. doi: 10.1056/NEJMoa0910383
3. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. (2021) 43:280–99. doi: 10.1093/eurheartj/ehab674
4. Lyon A, Lopez-Fernandez T, Couch L, Asteggiano R, Aznar M, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. (2022) 43:4229–361.
5. Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. Death causes in breast cancer patients. Ann Oncol. (2012) 23:604–10. doi: 10.1093/annonc/mdr160
6. Patnaik J, Byers T, DiGuiseppi C, Dabelea D, Denberg T. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. (2011) 13:R64. doi: 10.1186/bcr2901
7. Zhang X, Pawlikowski M, Olivo-Marston S, Williams K, Bower J, Felix A. Ten-year cardiovascular risk among cancer survivors: the National Health and Nutrition Examination Survey. PLoS One. (2021) 16:e0247919. doi: 10.1371/journal.pone.0247919
8. Thavendiranathan P, Abdel-Qadir H, Fischer H, Camacho X, Amir E, Austin P, et al. Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. (2016) 34:2239–46. doi: 10.1200/JCO.2015.65.1505
9. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer v7. (2021). Available online at: www.nccn.org (accessed February 3, 2023).
10. Alhussein M, Mokbel A, Cosman T, Aghel N, Yang E, Mukherjee S, et al. Pertuzumab cardiotoxicity in patients with HER2-positive cancer: a systematic review and meta-analysis. CJC Open. (2021) 3:1372–82. doi: 10.1016/j.cjco.2021.06.019
11. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. (2021) 22:351–60. doi: 10.1016/S1470-2045(20)30702-6
12. Yin W, Wang Y, Wu Z, Ye Y, Zhou L, Xu S, et al. Neoadjuvant trastuzumab and pyrotinib for locally advanced HER2-Positive Breast Cancer (NeoATP): primary analysis of a phase II study. Clin Cancer Res. (2022) 28:3677–85. doi: 10.1158/1078-0432.CCR-22-0446
13. Demissei B, Hubbard R, Zhang L, Smith A, Sheline K, McDonald C, et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc. (2020) 9:e014708. doi: 10.1161/JAHA.119.014708
14. Jordan J, Vasu S, Morgan T, D’Agostino R Jr., Melendez G, Hamilton C, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. (2016) 9:e004325. doi: 10.1161/CIRCIMAGING.115.004325
15. Plana J, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging. (2018) 11:1173–86. doi: 10.1016/j.jcmg.2018.06.003
16. Grover S, Leong D, Chakrabarty A, Joerg L, Kotasek D, Cheong K, et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol. (2013) 168:5465–7. doi: 10.1016/j.ijcard.2013.07.246
17. Celutkiene J, Pudil R, Lopez-Fernandez T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. (2020) 22(9):1504–24.
18. Kligfield P, Gettes L, Bailey J, Childers R, Deal B, Hancock E, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. (2007) 115:1306–24. doi: 10.1161/CIRCULATIONAHA.106.180200
19. Nagueh S, Smiseth O, Appleton C, Byrd B III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
20. Jones L, Eves N, Haykowsky M, Freedland S, Mackey J. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. (2009) 10:598–605. doi: 10.1016/S1470-2045(09)70031-2
21. American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. (2003) 167:211–77. doi: 10.1164/rccm.167.2.211
22. Khouri M, Hornsby W, Risum N, Velazquez E, Thomas S, Lane A, et al. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Res Treat. (2014) 143:531–9. doi: 10.1007/s10549-013-2818-1
23. Ordovas K, Baldassarre L, Bucciarelli-Ducci C, Carr J, Fernandes J, Ferreira V, et al. Cardiovascular magnetic resonance in women with cardiovascular disease: position statement from the Society for Cardiovascular Magnetic Resonance (SCMR). J Cardiovasc Magn Reson. (2021) 23:52. doi: 10.1186/s12968-021-00746-z
24. Flett A, Hayward M, Ashworth M, Hansen M, Taylor A, Elliott P, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. (2010) 122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636
25. Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. (2021) 22:1139–50.
26. Ramin C, Schaeffer M, Zheng Z, Connor A, Hoffman-Bolton J, Lau B, et al. All-cause and cardiovascular disease mortality among breast cancer survivors in CLUE II, a long-standing community-based cohort. J Natl Cancer Inst. (2021) 113:137–45. doi: 10.1093/jnci/djaa096
27. D’Hondt V, Canon J, Roca L, Levy C, Pierga J, Le Du F, et al. UCBG 2-04: long-term results of the PACS 04 trial evaluating adjuvant epirubicin plus docetaxel in node-positive breast cancer and trastuzumab in the human epidermal growth factor receptor 2-positive subgroup. Eur J Cancer. (2019) 122:91–100. doi: 10.1016/j.ejca.2019.09.014
28. Yu A, Yadav N, Lung B, Eaton A, Thaler H, Hudis C, et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat. (2015) 149:489–95. doi: 10.1007/s10549-014-3253-7
29. Suter T, Procter M, van Veldhuisen D, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. (2007) 25:3859–65. doi: 10.1200/JCO.2006.09.1611
30. Fedele C, Riccio G, Malara A, D’Alessio G, De Lorenzo C. Mechanisms of cardiotoxicity associated with ErbB2 inhibitors. Breast Cancer Res Treat. (2012) 134:595–602. doi: 10.1007/s10549-012-2103-8
31. De Keulenaer G, Doggen K, Lemmens K. The vulnerability of the heart as apluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. (2010) 106:35–46. doi: 10.1161/CIRCRESAHA.109.205906
32. Leemasawat K, Phrommintikul A, Chattipakorn S, Chattipakorn N. Mechanisms and potential interventions associated with the cardiotoxicity of ErbB2-targeted drugs: insights from in vitro, in vivo, and clinical studies in breast cancer patients. Cell Mol Life Sci. (2020) 77:1571–89. doi: 10.1007/s00018-019-03340-w
33. Guan Z, Xu B, DeSilvio M, Shen Z, Arpornwirat W, Tong Z, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J Clin Oncol. (2013) 31:1947–53. doi: 10.1200/JCO.2011.40.5241
34. Di Leo A, Gomez H, Aziz Z, Zvirbule Z, Bines J, Arbushites M, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. (2008) 26:5544–52. doi: 10.1200/JCO.2008.16.2578
35. von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. (2017) 377:122–31. doi: 10.1056/NEJMoa1703643
36. Hurvitz S, Martin M, Symmans W, Jung K, Huang C, Thompson A, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. (2018) 19:115–26. doi: 10.1016/S1470-2045(17)30716-7
37. Liu Z, Wang C, Chen X, Zhu J, Sun X, Xia Q, et al. Pathological response and predictive role of tumour-infiltrating lymphocytes in HER2-positive early breast cancer treated with neoadjuvant pyrotinib plus trastuzumab and chemotherapy (Panphila): a multicentre phase 2 trial. Eur J Cancer. (2022) 165:157–68. doi: 10.1016/j.ejca.2022.01.022
38. Zamorano J, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
39. Pudil R, Mueller C, Celutkiene J, Henriksen P, Lenihan D, Dent S, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the cardio-oncology study group of the heart failure association and the cardio-oncology council of the European society of cardiology. Eur J Heart Fail. (2020) 22:1966–83.
40. Fallah-Rad N, Walker J, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. (2011) 57:2263–70. doi: 10.1016/j.jacc.2010.11.063
41. Giusca S, Korosoglou G, Montenbruck M, Gersak B, Schwarz A, Esch S, et al. Multiparametric early detection and prediction of cardiotoxicity using myocardial strain, T1 and T2 mapping, and biochemical markers: a longitudinal cardiac resonance imaging study during 2 years of follow-up. Circ Cardiovasc Imaging. (2021) 14:e012459. doi: 10.1161/CIRCIMAGING.121.012459
42. Zardavas D, Suter T, Van Veldhuisen D, Steinseifer J, Noe J, Lauer S, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. (2017) 35:878–84. doi: 10.1200/JCO.2015.65.7916
43. Gardner R, Ozalp F, Murday A, Robb S, McDonagh T. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. (2003) 24:1735–43. doi: 10.1016/j.ehj.2003.07.005
44. Zile M, Claggett B, Prescott M, McMurray J, Packer M, Rouleau J, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. (2016) 68:2425–36. doi: 10.1016/j.jacc.2016.09.931
45. Pfister R, Diedrichs H, Schiedermair A, Rosenkranz S, Hellmich M, Erdmann E, et al. Prognostic impact of NT-proBNP and renal function in comparison to contemporary multi-marker risk scores in heart failure patients. Eur J Heart Fail. (2008) 10:315–20. doi: 10.1016/j.ejheart.2008.01.009
46. Putt M, Hahn V, Januzzi J, Sawaya H, Sebag I, Plana J, et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. (2015) 61:1164–72. doi: 10.1373/clinchem.2015.241232
47. Goldberg L, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. (2006) 113:2851–60. doi: 10.1161/CIRCULATIONAHA.105.600437
48. Thavendiranathan P, Poulin F, Lim K, Plana J, Woo A, Marwick T. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. (2014) 63(25 Pt A):2751–68. doi: 10.1016/j.jacc.2014.01.073
49. Houbois C, Nolan M, Somerset E, Shalmon T, Esmaeilzadeh M, Lamacie M, et al. Serial cardiovascular magnetic resonance strain measurements to identify cardiotoxicity in breast cancer: comparison with echocardiography. JACC Cardiovasc Imaging. (2021) 14:962–74. doi: 10.1016/j.jcmg.2020.09.039
50. Oikonomou E, Kokkinidis D, Kampaktsis P, Amir E, Marwick T, Gupta D, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. (2019) 4:1007–18. doi: 10.1001/jamacardio.2019.2952
Keywords: pyrotinib, cardiotoxicity, HER2-positive breast cancer, therapy-related cardiac dysfunction, echocardiography, magnetic resonance
Citation: Chai Y, Jiang M, Wang Y, Liu Q, Lu Q, Tao Z, Wu Q, Yin W, Lu J and Pu J (2023) Protocol for pyrotinib cardiac safety in patients with HER2-positive early or locally advanced breast cancer–The EARLY-MYO-BC study. Front. Cardiovasc. Med. 10:1021937. doi: 10.3389/fcvm.2023.1021937
Received: 12 September 2022; Accepted: 23 January 2023;
Published: 10 February 2023.
Edited by:
John David Horowitz, The University of Adelaide, AustraliaReviewed by:
Roberta Manganaro, University Hospital of Policlinico G. Martino, ItalyCopyright © 2023 Chai, Jiang, Wang, Liu, Lu, Tao, Wu, Yin, Lu and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Jiang,  amlhbmdtZW5nMDkxOUAxNjMuY29t; Jun Pu,
amlhbmdtZW5nMDkxOUAxNjMuY29t; Jun Pu,  cHVqdW4zMTBAaG90bWFpbC5jb20=; Wenjin Yin,
cHVqdW4zMTBAaG90bWFpbC5jb20=; Wenjin Yin,  Zm9sbG93cm9hZEAxNjMuY29t; Jinsong Lu,
Zm9sbG93cm9hZEAxNjMuY29t; Jinsong Lu,  bHVqanNzQDE2My5jb20=
bHVqanNzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.