
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 04 October 2022
Sec. Lipids in Cardiovascular Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.993633
This article is part of the Research TopicImplications of Lipids and Modified Lipoproteins in Atherogenesis: From mechanisms towards novel diagnostic and therapeutic targetsView all 16 articles
Very-low-density lipoprotein (VLDL) is the only lipoprotein containing apolipoprotein B that is secreted from the liver, where VLDL is assembled from apolipoproteins, cholesterol, and triglycerides. The primary function of VLDL is to transport cholesterol and other lipids to organs and cells for utilization. Apart from its role in normal biologic processes, VLDL is also known to contribute to the development of atherosclerotic cardiovascular disease. Large VLDL particles, which are subclassified according to their size by nuclear magnetic resonance spectrometry, are significantly correlated not only with atherosclerosis, but also with insulin resistance and diabetes incidence. VLDL can also be subclassified according to surface electrical charge by using anion-exchange chromatography. The most electronegative VLDL subclass is highly cytotoxic to endothelial cells and may contribute to coronary heart disease. In addition, electronegative VLDL contributes to the development of atrial remodeling, especially in patients with metabolic syndrome, which is an established risk factor for atrial fibrillation. In this review, we focus on the VLDL subclasses that are associated with apolipoprotein alterations and are involved in cardiometabolic disease. The postprandial enhancement of VLDL’s pathogenicity is a critical medical issue, especially in patients with metabolic syndrome. Therefore, the significance of the postprandial modification of VLDL’s chemical and functional properties is extensively discussed.
Very-low-density lipoprotein (VLDL) is a precursor to intermediate-density lipoprotein (IDL), which subsequently forms low-density lipoprotein (LDL). Density-gradient ultracentrifugation is the standard method used to isolate VLDL and other major lipoproteins, including chylomicrons, IDL, LDL, and high-density lipoprotein (HDL) from serum or plasma (1, 2). The lipid core of VLDL consists of triglycerides (TGs, 50–70% of particle mass), cholesterol ester (10–25%), and fatty acids (<10%). The major core protein of VLDL is apolipoprotein (apo)B100; other proteins include apoCI, apoCII, apoCIII, and apoE. These surface apolipoproteins also serve as ligands for cell-surface receptors and coordinators for lipolysis (3).
VLDL functions as a cargo carrier, transporting cholesterol, TGs, and proteins to peripheral cells for essential bioactivities. In the liver, TGs and cholesterol are incorporated with apoB100, which affects the lipid abundance and size of secreted VLDL (4). After VLDL is secreted, it is hydrolyzed by lipoprotein lipase (LPL), which is present in the capillary endothelium or associated with VLDL receptors, and transformed into VLDL remnant and IDL. HDL then takes up apoCII from VLDL remnant and IDL, and cholesterol ester transfer protein (CETP) exchanges their TGs and phospholipids with cholesterol. IDL can be taken up by the liver via the LDL receptor or after being transformed into LDL upon losing apoE and TGs (3). VLDL is a TG-rich lipoprotein, and its assembly and metabolism are affected by insulin resistance and long-term nutrient excess (5). VLDL also modulates nitric oxide signaling, which is essential for vascular smooth muscle relaxation and blood pressure control (6). In addition, VLDL enhances phospholipase D activity by increasing cytosolic calcium levels and stimulates aldosterone synthesis in the adrenal gland (7). Therefore, VLDL does not only serve as a lipid cargo carrier, but it also modulates lipid-related blood pressure regulation.
The diameter of VLDL particles can be measured using nuclear magnetic resonance (NMR) spectrometry. To classify VLDL subfractions by particle diameter, most studies have used a simplified classification system with different categories of average diameter. The quantitative analysis of serum or plasma lipoprotein subfractions requires high reproducibility. Such reproducibility has been examined by pooling quality control plasma lipoprotein samples and comparing NMR results among 11 spectrometers and 5 laboratories. In total, 16 subclasses were identified: 6 for VLDL, 6 for LDL, and 4 for HDL (8). However, a consensus has not been reached with respect to standard diameter ranges for classifying VLDL subfractions. For instance, in the study by Garvey et al. (9), three categories were defined as follows: large VLDL (>60nm), intermediate VLDL (35–60 nm), and small VLDL (<35 nm). In the study by Phillips et al. (10), the categories were defined as follows: large VLDL (including chylomicrons, if present, >60 nm), medium VLDL (42–60 nm), and small VLDL (<42 nm). Wang et al. (11) used six categories of VLDL as follows: largest (including chylomicrons, ± 75 nm), very large (average diameter, 64.0 nm), large (53.6 nm), medium (44.5 nm), small (36.8 nm), and very small (31.3 nm) VLDL.
In 1988, Avogaro et al. (12) first characterized LDL on the basis of surface electrical charge rather than particle size by using anion-exchange chromatography to separate LDL into LDL(+) and LDL(–). In addition, Yang et al. (13) and Chen et al. (14) divided LDL into five subfractions according to electrical charge, called L1-L5. Similarly, Chen et al. also used the same method of anion-exchange chromatography to separate VLDL into five subfractions, called V1-V5 (15) (Table 1).
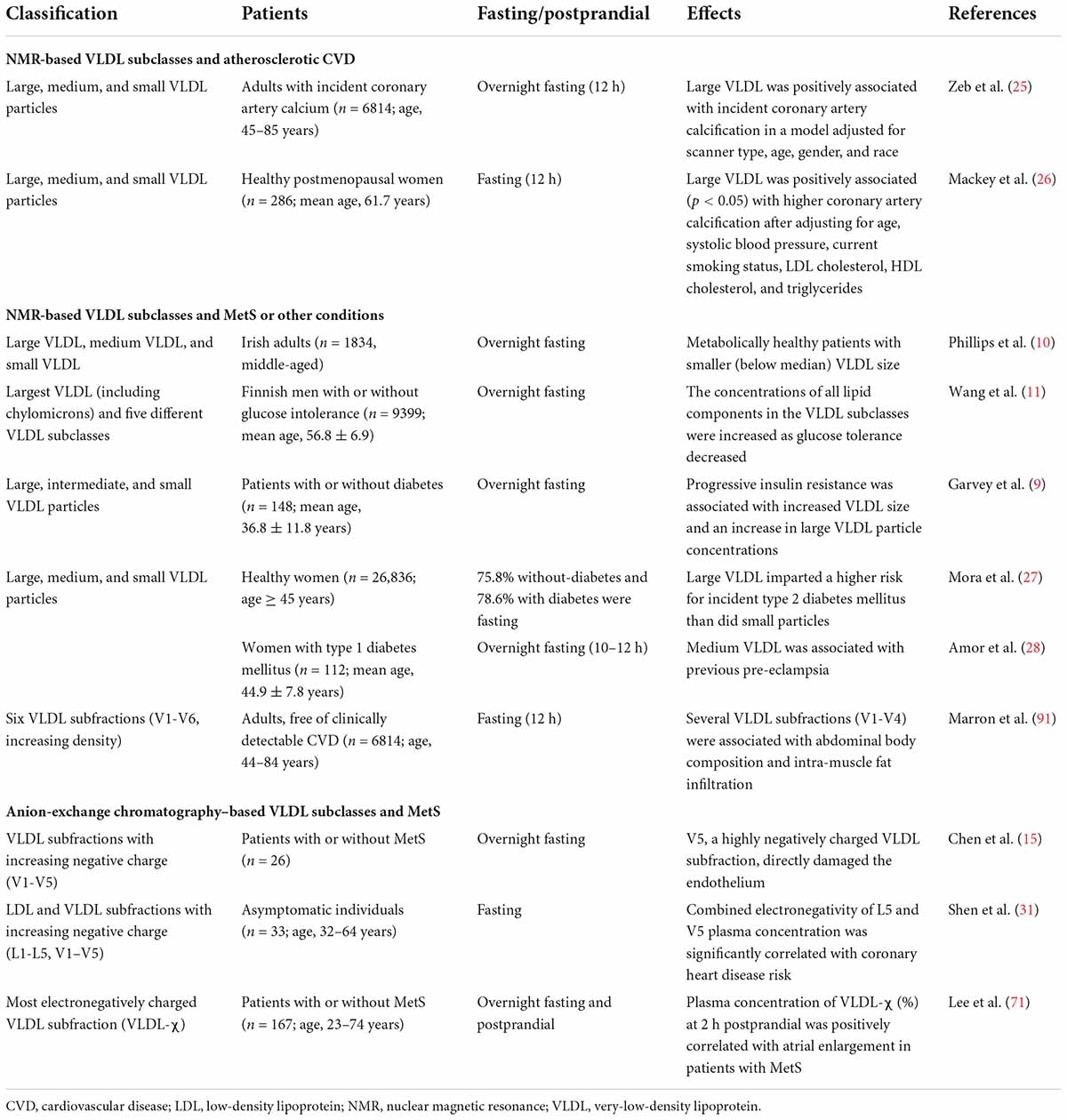
Table 1. VLDL subclassified by size and electrical charge and the effects of VLDL subclasses on atherosclerotic CVD, MetS, and other conditions.
Apolipoproteins are chemically unique, maintaining the structural integrity and functional specificity of different lipoprotein particles in lipid transport processes. Therefore, lipoproteins can be classified immunochemically according to their apolipoprotein composition (16). The two major classes of apolipoprotein-based families are apoA-containing and apoB-containing lipoproteins. VLDL, along with IDL and LDL, is an apoB-containing lipoprotein family. The apoB-containing lipoproteins can be divided into several subfamilies, including cholesterol ester-rich lipoprotein (LP-B) and TG-rich lipoproteins (16).
The physiologic basis for the differences in composition, structure, and function among VLDL particles is important because these differences can strongly influence the atherogenic properties of VLDL. Moreover, abnormal VLDL can adversely affect vascular or cardiac cells (see below), which has important implications. In this review, we present a summary of the emerging evidence for VLDL in promoting cardiometabolic diseases and highlight how the subclassification of VLDL can be used to distinguish VLDL particles that are pathogenic from those that are physiologically necessary.
Plasma LDL-cholesterol (LDL-C) alone is not sufficient to predict all non-atherosclerotic and atherosclerotic cardiovascular disease (ASCVD). Aside from LDL-C, VLDL cholesterol (VLDL-C) is also known to contribute to the development of ASCVD. Plasma VLDL-C is the primary component of non–HDL-cholesterol (HDL-C) (17) and is a predictor of ASCVD independent of LDL cholesterol (LDL-C) (18–20).
Prenner et al. (18) used cardiac electron beam computed tomography scanning to assess coronary artery calcification, which is an independent predictor of CVD risk, in a population of high-risk patients with type 2 diabetes. Their results showed that VLDL-C is an independent risk factor for coronary artery calcification, particularly in women. Furthermore, this association was independent of circulatory TG levels (18). In patients with type 2 diabetes who previously underwent coronary stent implantation, an elevated VLDL-C level >0.52 mmol/L was independently associated with in-stent restenosis (hazard ratio = 3.01) (21). Iannuzzi et al. (22) used ultrasound to measure carotid intima–media thickness in postmenopausal women and showed that VLDL-C was the lipoprotein most strongly associated with subclinical atherosclerosis. In addition, evidence from clinical studies has consistently indicated a causal role for TG-rich lipoproteins such as VLDL in ASCVD. An updated consensus statement regarding the current understanding of the role of TG-rich lipoproteins and their remnants in ASCVD has been published recently (5).
Large VLDL particles have a greater association with the incidence of atherosclerosis than do smaller VLDL particles (Table 1). The size-based subclassification of lipoproteins is performed by the NMR analyzer, which uses characteristic signals of lipoprotein subclasses with different sizes as the basis for quantification. A set of purified standards is required for converting signal amplitudes to specific particle concentrations (23). The standards for VLDL are isolated by using a combination of ultracentrifugation and agarose gel filtration, and the size distribution is determined by using electron microscopy (2).
VLDL circulates in the blood for about 4 h before it is converted to IDL and then LDL (24). Lipolytic remodeling is responsible for the down-sizing of the largest VLDL particles and their conversion to IDL and LDL. Unlike small LDL, large VLDL was associated with an increased risk of incident coronary artery calcification and calcium score progression during follow-up (25). Likewise, in relatively healthy postmenopausal women, large VLDL was positively associated with coronary artery calcification, suggesting that the measurement of lipoprotein subclasses may improve the prediction of coronary artery disease beyond using the conventional lipid panel (26).
In addition, the size of VLDL was shown to be correlated with insulin resistance and diabetes mellitus (11, 27) (Table 1). In a prospective study by Mora et al. (27) of 26,836 initially healthy women followed for 13 years, large VLDL particles were found to predict type 2 diabetes. Likewise, Wang et al. (11) reported in a population study of 9399 Finnish men that abnormal glucose tolerance and new onset type 2 diabetes were associated with an increase in VLDL particles, with the exception of very small VLDL. Conversely, a lower number of large VLDL particles was shown to be the most significant predictor of metabolic health in adults, regardless of body mass index and obesity status (10). Garvey et al. (9) described the effects of insulin resistance and type 2 diabetes on the particle size and concentration of lipoprotein subclasses. Their results showed that progressive insulin resistance was associated with increased VLDL size. Compared with individuals who have normal insulin sensitivity, patients with insulin resistance or diabetes showed increased concentrations of large VLDL particles, but no change in medium VLDL or small VLDL particle concentrations. For patients with type 1 diabetes, medium VLDL particle concentration was independently associated with previous pre-eclampsia during pregnancy after adjusting for age and statin use (28).
Lipoprotein particles can be separated according to charge by using anion-exchange chromatography. L5, which is the most electronegatively charged subfraction of LDL, induces endothelial apoptosis through the lectin-like oxidized LDL receptor-1 (LOX-1) in the absence of the LDL receptor (LDLR) (29). Similarly, the most electronegative subfraction of VLDL, V5, was shown to induce endothelial apoptosis and was the subfraction most rapidly internalized into endothelial cells (15). In addition, patients with metabolic syndrome (MetS) were found to have increased levels of electronegative VLDL. VLDL isolated from patients with MetS induced brain inflammation with glial cell activation in mice, suggesting that electronegative VLDL can promote cognitive dysfunction (30). Furthermore, Shen et al. (31) further confirmed that the most electronegative human plasma LDL (i.e., L5) and VLDL (i.e., V5) are highly atherogenic. In their study, the combined electronegativity of L5 and plasma concentration of V5 was significantly correlated with coronary heart disease risk in an age-adjusted analyses of asymptomatic individuals. Moreover, when human aortic endothelial cells were treated with L5 + V5 and L1 + V1, L5 + V5 induced significantly greater senescence-associated–β-galactosidase activity than did L1 + V1. In ApoE–/– mice, aortic lipid accumulation and cellular senescence were associated with the electronegativity of LDL and VLDL (31).
By 1972, the primary structures, including protein and DNA sequences, had been determined for almost all apolipoproteins (AI, AIV, B, CI, CII, CIII, D, E, I, and J) (16). VLDL particles containing apoE, apoCI, apoCIII, and apoAV have been shown to affect VLDL metabolism, site utilization, and atherogenicity. In the following sections, each lipoprotein is briefly described.
Emerging evidence supports that the compositional change of apolipoproteins in VLDL affects its atherogenicity (Table 2). VLDL is one of several major lipoproteins containing apoE, which is a specific ligand for cysteine-binding repeats of the VLDL receptor (VLDLR). VLDLR is widely expressed throughout the body, including the heart, skeletal muscle, adipose tissue, and brain, and it has an important role in the uptake and metabolism of apoE-containing TG-rich lipoproteins. ApoE is a polymorphic protein arising from three alleles at a single gene locus (32). The enrichment of apoE content in VLDL has been shown to protect against coronary heart disease (33).
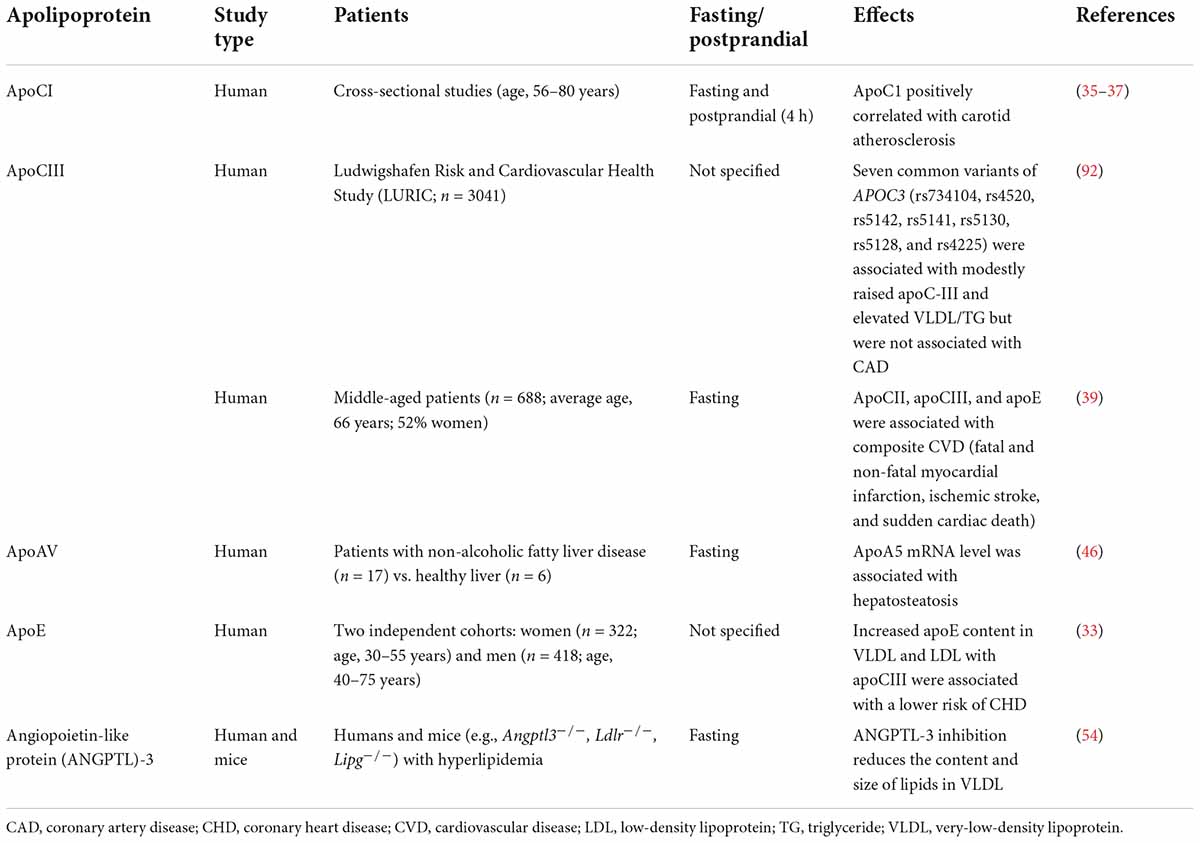
Table 2. Clinical studies showing altered VLDL apolipoproteins in patients with metabolic and atherogenic diseases.
Primarily associated with HDL in the fasting state, apoCI transiently attaches to the surface of TG-rich lipoproteins such as chylomicrons and VLDL postprandially. ApoCI modulates several enzymes involved in lipoprotein metabolism and can reduce the uptake of VLDL by inhibiting its binding to VLDLR (34). The increased intima-media thickness of the common carotid artery indicates early atherosclerosis and was found to be associated with apoCI content in postprandial TG-rich lipoproteins (35, 36). In addition, the number of apoCI molecules per VLDL particle in the fasting state was associated with the plaque size of carotid atherosclerosis (37). ApoCI was also shown to be correlated with cholesterol enrichment in VLDL particles and the delayed clearance of TG-rich lipoproteins (37). In hypercholesterolemic rabbits, the constitutive expression of human apoCI provided protection against serious atherosclerosis (38). This benefit was found to be related to the inhibition of plasma cholesteryl ester transfer protein (CETP) activity (38). These findings support that apoCI enrichment attenuates the atherogenicity of VLDL particles.
ApoCIII has been suggested to be a central regulator of TG-rich lipoprotein metabolism (39). A direct association of apoCIII with atherosclerosis was revealed by clinical genetic studies and studies showing that loss-of-function mutations in APOC3 are associated with low TG levels (40) and a reduced incidence of ischemic CVD (41). Increased plasma levels of apoCIII are associated with increased levels of VLDL, IDL particles, and TGs (42). In human monocytic THP-1 cells, apoCIII activated protein kinase C alpha (PKCα) and transforming protein RhoA, which resulted in β1-integrin activation and promoted endothelial cell adhesion. These results suggested that apoCIII not only modulates lipoprotein metabolism, but may also contribute to atherosclerosis development (43). The antisense apoCIII inhibitor volanesorsen, which reduces apoCIII levels by >75% and plasma TGs levels, inhibits apoCIII synthesis in the liver (44). However, the indication for the clinical use of volanesorsen is limited to patients with familial chylomicronaemia syndrome for preventing pancreatitis; therefore, its effect on reducing CVD remains undetermined (39).
In contrast to APOC3, genotype combinations of common APOA5 variants (c.-1131 T > C, S19 W, and c.*31C > T) are associated with elevated TG levels and increased CHD risk (45). In addition, patients with non-alcoholic fatty liver disease have elevated apoAV expression, which promotes hepatic TG storage in lipid droplets but decreases VLDL secretion by the liver (46). ApoAV also accelerates TG-rich lipoprotein uptake by the liver (47). However, the mechanism by which apoAV regulates circulatory VLDL metabolism remains largely unknown.
A key feature of large VLDL is the overproduction of TGs in the liver, which may occur for several years before the onset of type 2 diabetes (27). In the liver, the biogenesis of VLDLs and the assembly of apolipoproteins are complex and highly regulated processes (4). A major source of TG synthesis is the endoplasmic reticulum (ER) lumen, where TGs are assembled with apoB100 to form lipid-poor primordial VLDL particles. This process is facilitated by microsomal triglyceride transfer protein (MTP) (4), which transfers both neutral and polar lipids to form VLDL particles (Figure 1). Whether and how MTP is modulated in patients with insulin resistance and diabetes remain unclear.
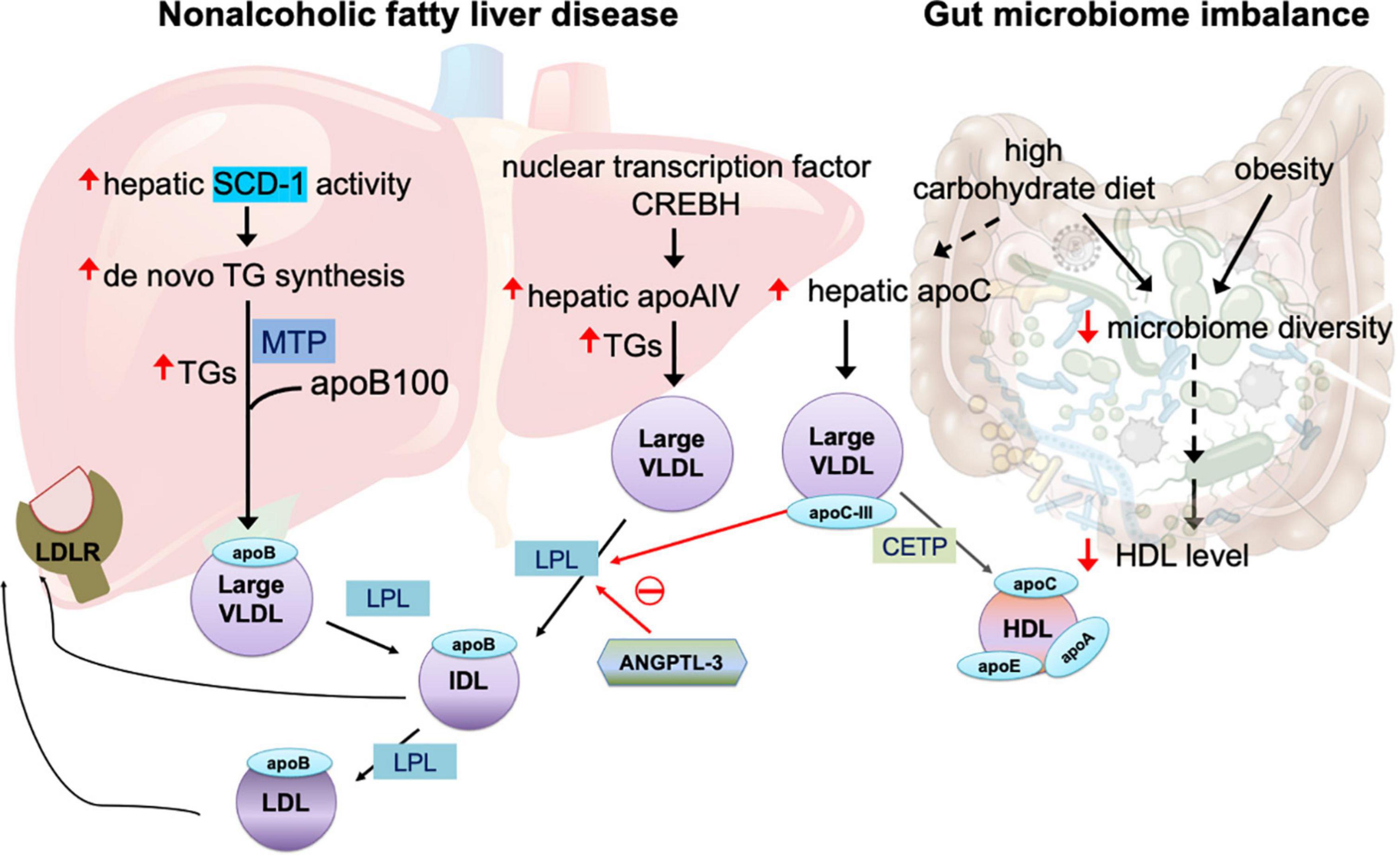
Figure 1. Mechanisms of large very-low-density lipoprotein (VLDL) in non-alcoholic fatty liver disease and gut microbiome imbalance. The overproduction of triglycerides (TGs) is related to increased activity of hepatic stearol-CoA desaturase (SCD)-1, which converts saturated fatty acids to monosaturated fatty acids that serve as the substrate for the synthesis of de novo TGs. The assembly of TGs with apolipoprotein (apo)B100 is facilitated by microsomal triglyceride transfer protein (MTP). In non-alcoholic fatty liver disease, the nuclear transcription factor cAMP-responsive element-binding protein H (CREBH) is upregulated, in turn increasing expression of hepatic apoAIV, which promotes the assembly of TG-rich, large VLDL. Angiopoietin-like protein family 3 (ANGPTL3) inhibits the enzyme activity of lipoprotein lipase (LPL), which is essential for breakdown of TGs in VLDL utilization. Both intermediate-density lipoprotein (IDL) and LDL particles are recognized by LDL receptor (LDLR) expressed in the liver. LPL activity is also inhibited by apoCIII. Large VLDL promotes plasma CETP-induced remodeling of TG-rich HDL. A high-carbohydrate diet and obesity impair microbiome diversity, which is related to reduced plasma HDL levels and increased hepatic apoCIII production that in turn inhibit LPL activity and enhance the abundance of large VLDL in the circulation.
Because of its large size (average diameter >60 nm), VLDL is shifted from the ER membrane to the cis Golgi for cargo selection and vesicle formation. However, the utilization of vesicular carrier proteins for VLDL remains an ongoing subject of investigation (4). It has been suggested that VLDL exits the hepatic ER in a specialized vesicle (i.e., the VLDL transport vesicle), which can accommodate a particle diameter of up to 100–200 nm (48).
Patients with non-alcoholic fatty liver disease have increased hepatic stearol-CoA desaturase (SCD)-1 activity, which converts saturated fatty acids to monosaturated fatty acids that serve as a major substrate for the synthesis of de novo TGs and other lipids (49). How the abundance of TGs and the degree of TG desaturation are controlled or regulated during VLDL synthesis remain undetermined.
Hepatic apoAIV expression, which is regulated by nuclear transcription factor cAMP-responsive element-binding protein H (CREBH), is correlated with hepatic TG content in patients with chronic liver steatosis (50). CREBH activation plays key roles in hepatic steatosis by upregulating apoAIV during VLDL assembly in the ER and promotes the assembly of large and TG-enriched VLDL particles (50) (Figure 1). In addition to its expression in the liver, apoAIV is predominantly expressed in human enterocytes to facilitate intestinal chylomicron assembly and is highly upregulated after a fatty meal (51).
The utilization of VLDL and the breakdown of TGs in organs require the key enzyme lipoprotein lipase (LPL) to generate free fatty acids. The inhibition of lipolysis increases the size of circulating VLDL. Several members of the angiopoietin-like protein (ANGPTL) family regulate the activity of LPL. ANGPTL3, ANGPTL4, and ANGPTL8 are upregulated in patients with type 2 diabetes and obesity (52). In a group of patients who received RNA inhibition therapy with antisense oligonucleotides targeting ANGPTL3, protein levels of ANGPTL3 were reduced by as much as 84.5% from baseline 6 weeks after injection, while levels of TGs were reduced by 63.1%, VLDL cholesterol by 60.0%, and apoCIII by 58.8% (53). In mice, ANGPTL3 inhibition reduced TG content in the liver and retarded atherosclerosis progression (53). Endothelial lipase, which reduces LDL-C via an LDLR-independent mechanism, is essential for phospholipid reduction in VLDL and LDL (54). In LDLR–/– mice, ANGPTL3 inhibition caused a marked reduction in the TG content of VLDL. Furthermore, in ApoE–/– mice, ANGPTL3 inhibition promoted VLDL clearance with the involvement of multiple remnant receptors (54). However, in the liver, ANGPTL3 did not perturbate apoB lipidation and hepatic VLDL assembly (54). These findings suggest that ANGPTL3 governs VLDL catabolism and largely affects VLDL lipid content and size. On the other hand, endothelial lipase exerts anti-atherogenic effects by enhancing the catabolism of β-VLDLs (55), which are cholesterol-rich chylomicron and VLDL remnants that accumulate in the plasma of patients with type III dysbetalipoproteinemia (56). In elderly patients, the removal of TG-rich lipoprotein remnants is delayed, but TG breakdown is unchanged. Whether VLDL receptor function is impaired and whether ANGPTL3 is involved in aging-related, delayed VLDL removal remain unknown.
The reverse-remnant cholesterol transport mechanism, which is the acquisition of VLDL surface components by HDL during LPL-mediated lipolysis, plays an important role in VLDL catabolism (57). HDL affects the lipolysis of VLDL TGs and the release of surface lipids, free cholesterol, phospholipids, and exchangeable apoE, apoCII, and apoCIII from VLDL during lipolysis (58). HDL can also be classified into subpopulations according to size, apolipoprotein content, charge, mass, and density. Although subpopulations of both large and small HDL particles increased VLDL TG lipolysis efficiency and surface material removal from VLDL, the small, protein-enriched HDL particles exhibited a greater effect on this process and promoted a more efficient release of surface components, thereby affecting the properties of the generated remnants. Loss of apoC proteins from VLDL during lipolysis promoted the metabolism of apoB-containing lipoprotein because both apoCII and apoCIII inhibit the binding of apoB lipoproteins to the LDLR (58).
Increased TG content has been suggested to decrease the stability of HDL, VLDL, and LDL via several mechanisms. First, TGs have a direct destabilizing effect on lipoprotein particles from the CETP-induced remodeling of TG-rich HDL. Second, TGs have indirect effects that enhance spontaneous and enzymatic hydrolysis and oxidation. Third, products of the aforementioned processes, particularly free fatty acids, further augment lipoprotein destabilization and fusion. TGs are also involved in the substantial release of proteins from lipoproteins. Finally, the combination of destabilized LDL and VLDL enhances their retention in the arterial wall, triggering atherosclerosis (59).
Genetic variants have been associated with lipoprotein subclasses. Among those, the common variant rs73059724 resulted in small VLDL particles with fewer phospholipids (60). The variant rs73059724 is located on chromosome 19 and is associated with the promoter and intron of HIF3A, which regulates the cellular uptake of cholesterol esters and VLDL by promoting hypoxic conditions. In addition, HIF3A hypermethylation is associated with increased adiposity in Asian infants and children (61, 62). These findings suggest that HIF3A may regulate VLDL particle size. Furthermore, DNA methylation at HIF3A may explain the prenatal influences on adiposity. In another recent genetic study, Li-Gao et al. (63) investigated postprandial metabolomics and found that the ANKRD55 locus led by the rs458741:C variant was strongly associated with extremely large VLDL, body composition, and the incidence of diabetes. This finding illuminates the strong genetic linkage between VLDL modification and insulin resistance.
Vojinovic et al. (64) showed in a prospective population-based cohort of 2309 individuals that 32 microbial families and genera in gut microbiota were associated with size-defined subfractions of VLDL, HDL, serum lipid values, and glycolysis-related metabolites. Among the 32, 18 microbial families and genera were significantly associated with VLDL particles of various sizes (extra small, small, medium, large, very large, and extremely large) (64). Another recent study showed that, in healthy individuals, low microbiota diversity was associated with obesity, abdominal obesity, and low HDL-C level (65). These reports suggest that gut microbiota imbalance may be involved in the alteration of VLDL particle size. Thus, the source of altered VLDL particles is presumably the intestines, although the real origin of altered VLDL particles may be diet. In animals and humans, a high-carbohydrate diet results in the elevation of large TG-enriched VLDL particles, along with the enrichment of apoC proteins. Carbohydrate intake increases hepatic secretory rates of VLDL TGs without changing the secretion of apoB, which together lead to large and dense VLDL particles (66).
Postprandial hypertriglyceridemia is a hallmark of dyslipidemia in patients with type 2 diabetes. Recently, it has been suggested that postprandial dyslipidemia is equally as important as the estimation of lipids in the fasting state, particularly for patients with type 2 diabetes (67). Mora et al. (27) characterized lipoprotein particles according to size in fasting and non-fasting states by using NMR, noting similar results between LDL and HDL particles. However, compared with fasting VLDL, non-fasting large VLDL particles carried much higher risk for diabetes. In the Copenhagen General Population Study, in which NMR spectrometry was used to analyze the lipids of 9293 individuals, the results showed that VLDL and IDL particles contained one-third of plasma cholesterol in the non-fasting state (68). Postprandial TGs are carried by primarily chylomicron and VLDL remnants, which are ligands of the VLDL receptor involved in macrophage foam cell formation during the development of atherosclerosis (69).
VLDL utilization serves as the major energy source for the heart. Under physiologic conditions, approximately 70% of the heart’s energy is derived from fatty acid oxidation (70). Lee et al. (71) showed that postprandial VLDL is independently correlated with atrial enlargement, indicating that postprandial VLDL is a risk factor for atrial fibrillation (Table 1). In a prospective study of individuals with MetS (n = 87) and without MetS (n = 80), they found that negatively-charged VLDL (2-h postprandial VLDL-χ, concentration in %), waist and hip circumferences, body mass index, and blood pressure were positively correlated with left atrial diameter. After adjusting for obesity and blood pressure, 2-h postprandial VLDL-χ, but not fasting VLDL, was independently correlated with left atrial diameter. Each 1% increase in VLDL-χ correlated with an incremental left atrial diameter increase of 0.23 cm. Nakajima et al. (72) showed that postprandial VLDL has a higher affinity to the VLDL receptor, with better internalization into cells than non-postprandial VLDL. With these findings in mind, postprandial modified VLDL has been suggested as a therapeutic target for atrial remodeling in patients with MetS (54).
VLDL composition, especially in the postprandial state, is influenced by meals and eating habits. Guerrero et al. (73) described the effects of a sucrose-enriched diet on elevated levels of VLDL-cholesterol and TGs, insulin resistance, and hepatic steatosis in male Wistar rats. In addition, Drorna et al. (74) reviewed the available evidence for the impact of high-fructose intake on health. In healthy individuals, the consumption of up to 1.5 g fructose/kg body weight per day for 4 weeks resulted in increased plasma TG concentrations (74). In addition to elevating TG levels, high fructose intake can induce hepatic steatosis, insulin resistance, and hyperuricemia (74). It is very likely that high fructose intake can alter VLDL particles with respect to size and TG richness. After a single high-fat meal, postprandial changes in TGs and VLDL can be significant in men with abdominal obesity compared with non-obese men (75). However, no such difference was observed between obese and non-obese women (75), suggesting sex-based differences in postprandial VLDL secretion during the reproductive stage.
In patients with existing cardiometabolic risks, 8-week nutritional intervention with a high polyphenol diet can significantly reduce the postprandial lipid content of large VLDL after a high-fat test meal (76). Another study showed that the consumption of a diet composed of fruit, avocado, whole grains, and trout for 8 weeks can reduce fasting insulin and VLDL and lower the postprandial increase in TGs and VLDL (77). With respect to the intake of fish, notable differences were seen in the NMR lipoprotein profile of the three main n-3 fatty acid subtypes: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and a-linolenic acid (ALA). Only a high intake of EPA significantly reduced VLDL particles and VLDL TGs (78). In addition, the reduction of apoCIII expression is believed to be the mechanism underlying the TG-lowering effects of omega-3 carboxylic acids, which contain 50–60% EPA and 15–25% DHA, as well as other active omega-3 free fatty acids (79). Fasting per se is beneficial for VLDL modification. In a study of 40 relatively healthy, middle-aged individuals, long-term fasting improved the postprandial lipid profile, especially with respect to the concentrations of large VLDL particles, which are significantly decreased after 7 and 14 days of fasting (80). Nevertheless, the impact of nutritional intervention on clinical cardiovascular outcomes warrants long-term observation and follow-up.
In addition to nutritional intervention, synbiotic and probiotic supplements that improve gut microbiome imbalance have shown potential for decreasing serum VLDL-C levels (81). In addition, several oral anti-diabetic drugs have been identified that promote beneficial effects on VLDL metabolism. Pioglitazone, a PPAR-γ activator, was shown to facilitate LPL activity and promote the clearance of VLDL (82). Furthermore, glucagon-like peptide 1 (GLP-1) agonist reduced TG levels in the liver and the VLDL secretion rate (83).
Commonly used lipid-lowering drugs, although not specifically VLDL-targeted, have also been shown to help reduce VLDL. HMG-CoA reductase inhibitors (i.e., statins) reduce one-third of VLDL-TGs and more than 40% of apoCIII levels (84). In addition, peroxisome proliferator-activated receptor-α (PPAR-α) agonists (i.e., fibrates), which are prescribed primarily for managing hypertriglyceridemia, reduce VLDL-apoCIII levels, as well (84). Similar to selective estrogen receptor modulators, the first selective PPAR-α modulator (SPPARMα) LY-518674, which targets the receptor–cofactor binding profile of the PPARα ligand, modulates tissue- and gene-selective responses. In clinical phase II/III trials, this SPPARMα agonist reduced TG and apoCIII levels by about 50% (85). Proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors, which reduce the degradation of LDL receptors and promote LDL uptake in the liver, also upregulate VLDL receptors and reduce VLDL levels. PCSK9 inhibitors have also been shown to preferentially modify the size and apolipoprotein composition of VLDL particles (86).
Several lipid-lowering agents are under development, including CETP inhibitor (87), microsomal triglyceride transfer protein (MTTP) inhibitor (88), and antisense oligonucleotides targeting the genes encoding apoB100 (88) and apoCIII (89). These therapeutics are currently being tested in clinical trials. Monoclonal antibody targeting ANGPTL3 has been shown to robustly reduce VLDL levels but at the expense of elevating LDL levels (90). In addition, ARO-ANG3 is an siRNA-based medication that inhibits the hepatic translation of ANGPTL3 mRNA [102]. These new medications have the potential to produce favorable effects on VLDL structure and metabolism.
Independent of LDL-C, VLDL’s atherogenic properties are associated with TG abundance, which largely affects particle size, apolipoprotein content alteration, electrical charge, and lipid composition, especially in the postprandial state (Figure 2). With adverse modification, VLDL facilitates ectopic lipid accumulation, which has been observed in the liver, heart, and skeletal muscles. To elucidate the pathogenic roles of VLDL in cardiovascular diseases, the issues of modification, in both fasting and postprandial states, should be taken into consideration. To improve adversely modified VLDL, nutritional intervention, especially through the reduction of fructose content in food, should be widely recommended, especially for patients with insulin resistance and cardiometabolic risks. However, interpreting data from only the size-based, charge-based, or apolipoprotein-based classified VLDL does not provide complete knowledge or information about lipids in health and diseases. To obtain a more comprehensive understanding of the lipid transport and metabolism process, methodologies are needed that can reflect the complex immunochemical and functional properties of all apolipoprotein-containing lipoproteins in the blood.
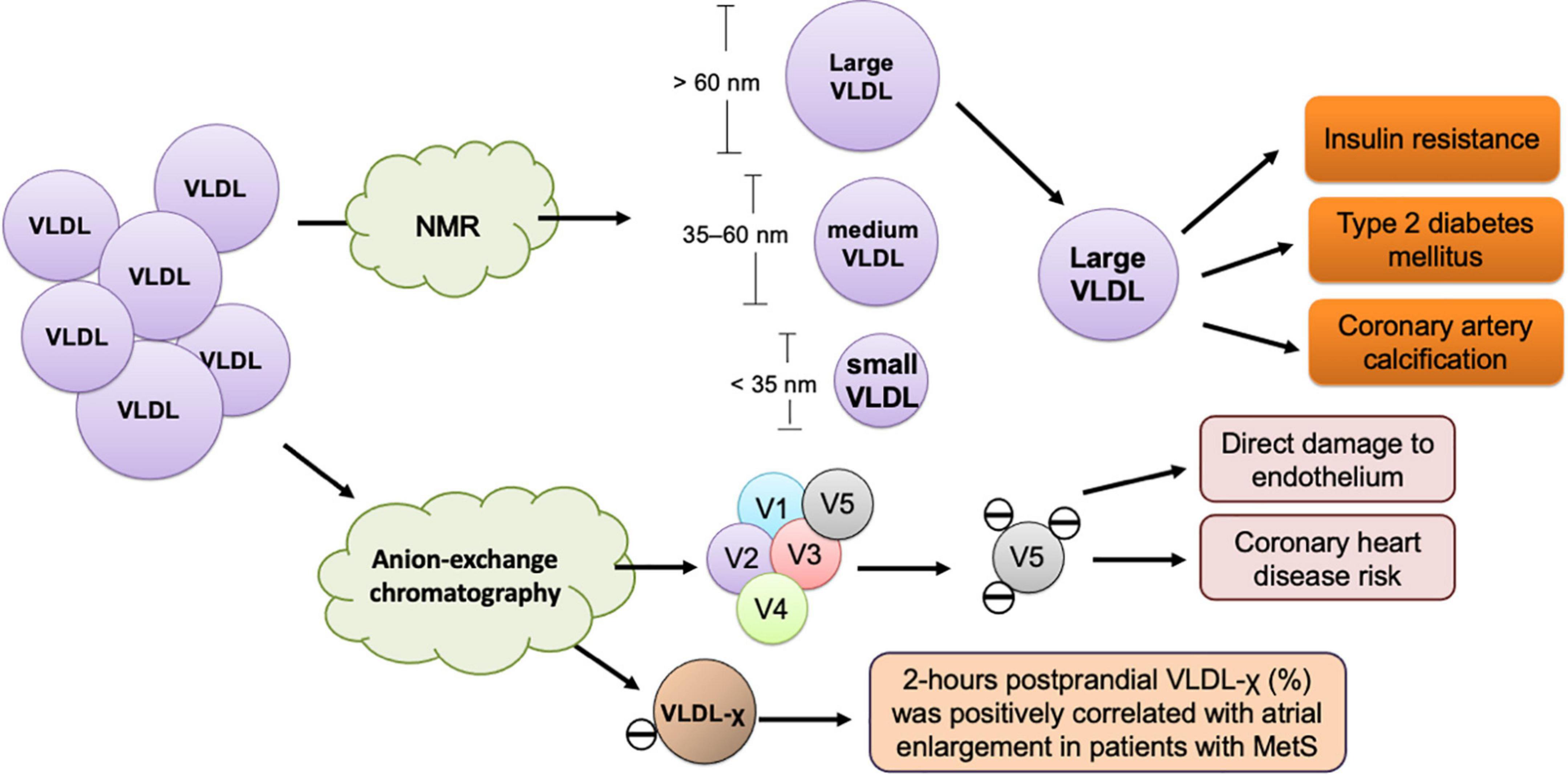
Figure 2. Size- and charge-defined subfractions of VLDL and their association with cardiometabolic diseases. The size-defined classification of VLDL according to particle diameter is performed using nuclear magnetic resonance (NMR) spectrometry. Large VLDL, which has a diameter larger than 60 nm, is associated with insulin resistance, type 2 diabetes mellitus, and coronary artery calcification. VLDL-χ or V5, the most negatively-charged subfraction of VLDL, is isolated and measured using anion-exchange chromatography. VLDL-χ or V5 causes direct damage to the endothelium and associated with coronary heart disease risk and atrial myopathy in metabolic syndrome (MetS).
H-CL contributed to the conceptualization of the study, participated in funding acquisition, and wrote the manuscript. AA and C-HC reviewed and edited the manuscript. All authors have approved the submitted version and agreed to be personally accountable for their own contributions.
This study was supported by Kaohsiung Medical University Hospital (KMUH109-9R11 and KMUH110-0R11), Taiwan Ministry of Science and Technology Grants (MOST 109-2314-B-037-111-MY3), KMU Global Networking Talent Plan (110KMUOR01), and National Health Research Institutes NHRI (NHRI-EX107-10724SC, NHRI-EX108-10724SC, NHRI-EX109-10724SC, and NHRI-EX110-10724SC).
We thank Ms. Yi-Lin Shiao for her artwork shown in the schematic figure, and Nicole Stancel, Ph.D., ELS(D), of Scientific Publications at the Texas Heart Institute, for her contributions to the editing of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Niimi M, Yan H, Chen Y, Wang Y, Fan J. Isolation and analysis of plasma lipoproteins by ultracentrifugation. J Vis Exp. (2021). doi: 10.3791/61790
2. Chapman MJ, Goldstein S, Lagrange D, Laplaud PM. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res. (1981) 22:339–58.
3. Huang JK, Lee HC. Emerging evidence of pathological roles of very-low-density lipoprotein (VLDL). Int J Mol Sci. (2022) 23:4300.
4. Tiwari S, Siddiqi SA. Intracellular trafficking and secretion of VLDL. Arterioscl Thromb Vasc Biol. (2012) 32:1079–86.
5. Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. (2021) 42:4791–806. doi: 10.1093/eurheartj/ehab551
6. Magnifico MC, Oberkersch RE, Mollo A, Giambelli L, Grooten Y, Sarti P, et al. VLDL induced modulation of nitric oxide signalling and cell redox homeostasis in HUVEC. Oxid Med Cell Longev. (2017) 2017:2697364. doi: 10.1155/2017/2697364
7. Tsai YY, Rainey WE, Bollag WB. Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production. J Endocrinol. (2017) 232:R115–29.
8. Jimenez B, Holmes E, Heude C, Tolson RF, Harvey N, Lodge SL, et al. Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by (1)H NMR spectroscopy in a multilaboratory trial. Anal Chem. (2018) 90:11962–71. doi: 10.1021/acs.analchem.8b02412
9. Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. (2003) 52:453–62.
10. Phillips CM, Perry IJ. Lipoprotein particle subclass profiles among metabolically healthy and unhealthy obese and non-obese adults: does size matter? Atherosclerosis. (2015) 242:399–406. doi: 10.1016/j.atherosclerosis.2015.07.040
11. Wang J, Stancakova A, Soininen P, Kangas AJ, Paananen J, Kuusisto J, et al. Lipoprotein subclass profiles in individuals with varying degrees of glucose tolerance: a population-based study of 9399 Finnish men. J Intern Med. (2012) 272:562–72. doi: 10.1111/j.1365-2796.2012.02562.x
12. Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. (1988) 8:79–87.
13. Yang CY, Raya JL, Chen HH, Chen CH, Abe Y, Pownall HJ, et al. Isolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteins. Arterioscler Thromb Vasc Biol. (2003) 23:1083–90. doi: 10.1161/01.ATV.0000071350.78872.C4
14. Chen CH, Jiang T, Yang JH, Jiang W, Lu J, Marathe GK, et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation. (2003) 107:2102–8. doi: 10.1161/01.CIR.0000065220.70220.F7
15. Chen CH, Lu J, Chen SH, Huang RY, Yilmaz HR, Dong J, et al. Effects of electronegative VLDL on endothelium damage in metabolic syndrome. Diabetes Care. (2012) 35:648–53. doi: 10.2337/dc11-1623
16. Alaupovic P. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol. (1996) 263:32–60.
17. Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. Apolipoprotein B and Non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol. (2021) 77:1439–50.
18. Prenner SB, Mulvey CK, Ferguson JF, Rickels MR, Bhatt AB, Reilly MP. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis. (2014) 236:244–50. doi: 10.1016/j.atherosclerosis.2014.07.008
19. Ren J, Grundy SM, Liu J, Wang W, Wang M, Sun J, et al. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: the results of a 15-Year Chinese Multi-Provincial Cohort Study (CMCS). Atherosclerosis. (2010) 211:327–32. doi: 10.1016/j.atherosclerosis.2010.02.020
20. Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. (2006) 98:1363–8.
21. Qin Z, Zheng FW, Zeng C, Zhou K, Geng Y, Wang JL, et al. Elevated levels of very low-density lipoprotein cholesterol independently associated with in-stent restenosis in diabetic patients after drug-eluting stent implantation. Chin Med J. (2017) 130:2326–32. doi: 10.4103/0366-6999.213575
22. Iannuzzi A, Giallauria F, Gentile M, Rubba P, Covetti G, Bresciani A, et al. Association between Non-HDL-C/HDL-C ratio and carotid intima-media thickness in post-menopausal women. J Clin Med. (2021) 11:78. doi: 10.3390/jcm11010078
23. Petersen M, Dyrby M, Toubro S, Engelsen SB, Norgaard L, Pedersen HT, et al. Quantification of lipoprotein subclasses by proton nuclear magnetic resonance-based partial least-squares regression models. Clin Chem. (2005) 51:1457–61. doi: 10.1373/clinchem.2004.046748
24. Nakajima K, Nakano T, Tokita Y, Nagamine T, Inazu A, Kobayashi J, et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin Chim Acta. (2011) 412:1306–18.
25. Zeb I, Jorgensen NW, Blumenthal RS, Burke GL, Lloyd-Jones D, Blaha MJ, et al. Association of inflammatory markers and lipoprotein particle subclasses with progression of coronary artery calcium: the multi-ethnic study of atherosclerosis. Atherosclerosis. (2021) 339:27–34. doi: 10.1016/j.atherosclerosis.2021.11.003
26. Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. (2002) 90:71i–6i. doi: 10.1016/s0002-9149(02)02636-x
27. Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. (2010) 59:1153–60.
28. Amor AJ, Vinagre I, Valverde M, Urquizu X, Meler E, Lopez E, et al. Nuclear magnetic resonance lipoproteins are associated with carotid atherosclerosis in type 1 diabetes and pre-eclampsia. Diabetes Metab Res Rev. (2021) 37:e3362. doi: 10.1002/dmrr.3362
29. Lu J, Yang JH, Burns AR, Chen HH, Tang D, Walterscheid JP, et al. Mediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosis. Circ Res. (2009) 104:619–27.
30. Lin YS, Liu CK, Lee HC, Chou MC, Ke LY, Chen CH, et al. Electronegative very-low-density lipoprotein induces brain inflammation and cognitive dysfunction in mice. Sci Rep. (2021) 11:6013. doi: 10.1038/s41598-021-85502-0
31. Shen MY, Hsu JF, Chen FY, Lu J, Chang CM, Madjid M, et al. Combined LDL and VLDL electronegativity correlates with coronary heart disease risk in asymptomatic individuals. J Clin Med. (2019) 8:1193. doi: 10.3390/jcm8081193
32. Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. (2000) 1:507–37.
33. Mendivil CO, Rimm EB, Furtado J, Sacks FM. Apolipoprotein E in VLDL and LDL with apolipoprotein C-III is associated with a lower risk of coronary heart disease. J Am Heart Assoc. (2013) 2:e000130. doi: 10.1161/JAHA.113.000130
34. Fuior EV, Gafencu AV. Apolipoprotein C1: its pleiotropic effects in lipid metabolism and beyond. Int J Mol Sci. (2019) 20:53. doi: 10.3390/ijms20235939
35. Hamsten A, Silveira A, Boquist S, Tang R, Bond MG, de Faire U, et al. The apolipoprotein CI content of triglyceride-rich lipoproteins independently predicts early atherosclerosis in healthy middle-aged men. J Am Coll Cardiol. (2005) 45:1013–7. doi: 10.1016/j.jacc.2004.12.049
36. Hansen JB, Fernandez JA, Noto AT, Deguchi H, Bjorkegren J, Mathiesen EB. The apolipoprotein C-I content of very-low-density lipoproteins is associated with fasting triglycerides, postprandial lipemia, and carotid atherosclerosis. J Lipids. (2011) 2011:271062. doi: 10.1155/2011/271062
37. Noto AT, Mathiesen EB, Brox J, Bjorkegren J, Hansen JB. The ApoC-I content of VLDL particles is associated with plaque size in persons with carotid atherosclerosis. Lipids. (2008) 43:673–9. doi: 10.1007/s11745-008-3193-2
38. Gautier T, Deckert V, Aires V, Le Guern N, Proukhnitzky L, Patoli D, et al. Human apolipoprotein C1 transgenesis reduces atherogenesis in hypercholesterolemic rabbits. Atherosclerosis. (2021) 320:10–8. doi: 10.1016/j.atherosclerosis.2021.01.011
39. Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, et al. Very-low-density lipoprotein–associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J. Am. Coll. Cardiol. (2017) 69:789–800. doi: 10.1016/j.jacc.2016.11.065
40. Wulff AB, Nordestgaard BG, Tybjaerg-Hansen A. APOC3 loss-of-function mutations, remnant cholesterol, low-density lipoprotein cholesterol, and cardiovascular risk: mediation- and meta-analyses of 137 895 individuals. Arterioscl Thromb Vasc Biol. (2018) 38:660–8. doi: 10.1161/ATVBAHA.117.310473
41. Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. (2014) 371:32–41.
42. van Capelleveen JC, Bernelot Moens SJ, Yang X, Kastelein JJP, Wareham NJ, Zwinderman AH, et al. Apolipoprotein CIII levels and incident coronary artery disease risk: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. (2017): doi: 10.1161/ATVBAHA.117.309007
43. Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. (2006) 113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743
44. Graham MJ, Lee RG, Bell TA III, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. (2013) 112:1479–90. doi: 10.1161/CIRCRESAHA.111.300367
45. Dai W, Zhang Z, Yao C, Zhao S. Emerging evidences for the opposite role of apolipoprotein C3 and apolipoprotein A5 in lipid metabolism and coronary artery disease. Lipids Health Dis. (2019) 18:220. doi: 10.1186/s12944-019-1166-5
46. Feng Q, Baker SS, Liu W, Arbizu RA, Aljomah G, Khatib M, et al. Increased apolipoprotein A5 expression in human and rat non-alcoholic fatty livers. Pathology. (2015) 47:341–8. doi: 10.1097/PAT.0000000000000251
47. Zheng XY, Zhao SP, Yan H. The role of apolipoprotein A5 in obesity and the metabolic syndrome. Biol Rev Camb Philos Soc. (2013) 88:490–8.
48. Gusarova V, Brodsky JL, Fisher EA. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J Biol Chem. (2003) 278:48051–8. doi: 10.1074/jbc.M306898200
49. Kotronen A, Seppanen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepaa AL, et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. (2009) 58: 203–8.
50. Cheng D, Xu X, Simon T, Boudyguina E, Deng Z, VerHague M, et al. Very low density lipoprotein assembly is required for cAMP-responsive element-binding protein H processing and hepatic apolipoprotein A-IV expression. J Biol Chem. (2016) 291:23793–803. doi: 10.1074/jbc.M116.749283
51. Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. (2007) 293:G519–24. doi: 10.1152/ajpgi.00189.2007
52. Abu-Farha M, Al-Khairi I, Cherian P, Chandy B, Sriraman D, Alhubail A, et al. Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. Lipids Health Dis. (2016) 15:181. doi: 10.1186/s12944-016-0337-x
53. Graham MJ, Lee RG, Brandt TA, Tai L-J, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. (2017) 377:222-32.
54. Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res. (2020) 61:1271–86. doi: 10.1194/jlr.RA120000888
55. Yan H, Niimi M, Wang C, Chen Y, Zhou H, Matsuhisa F, et al. Endothelial lipase exerts its anti-atherogenic effect through increased catabolism of beta-VLDLs. J Atheroscler Thromb. (2021) 28:157–68. doi: 10.5551/jat.55244
56. Hopkins PN, Wu LL, Schumacher MC, Emi M, Hegele RM, Hunt SC, et al. Type III dyslipoproteinemia in patients heterozygous for familial hypercholesterolemia and apolipoprotein E2. Evidence for a gene-gene interaction. Arterioscler Thromb. (1991) 11:1137–46. doi: 10.1161/01.atv.11.5.1137
57. Kontush A. HDL and reverse remnant-cholesterol transport (RRT): relevance to cardiovascular disease. Trends Mol Med. (2020) 26:1086–100.
58. Wieczorek E, Cwiklinska A, Kuchta A, Kortas-Stempak B, Gliwinska A, Jankowski M. The differential effects of HDL subpopulations on lipoprotein lipase (LPL)-mediated VLDL catabolism. Biomedicines. (2021) 9:1839. doi: 10.3390/biomedicines9121839
59. Jayaraman S, Baveghems C, Chavez OR, Rivas-Urbina A, Sanchez-Quesada JL, Gursky O. Effects of triacylglycerol on the structural remodeling of human plasma very low- and low-density lipoproteins. Biochim Biophys Acta Mol Cell Biol Lipids. (2019) 1864:1061–71.
60. Davis JP, Huyghe JR, Locke AE, Jackson AU, Sim X, Stringham HM, et al. Common, low-frequency, and rare genetic variants associated with lipoprotein subclasses and triglyceride measures in Finnish men from the METSIM study. PLoS Genet. (2017) 13:e1007079. doi: 10.1371/journal.pgen.1007079
61. Wang S, Song J, Yang Y, Zhang Y, Wang H, Ma J. HIF3A DNA methylation is associated with childhood obesity and ALT. PLoS One. (2015) 10:e0145944. doi: 10.1371/journal.pone.0145944
62. Pan H, Lin X, Wu Y, Chen L, Teh AL, Soh SE, et al. HIF3A association with adiposity: the story begins before birth. Epigenomics. (2015) 7:937–50. doi: 10.2217/epi.15.45
63. Li-Gao R, Hughes DA, van Klinken JB, de Mutsert R, Rosendaal FR, Mook-Kanamori DO, et al. Genetic studies of metabolomics change after a liquid meal illuminate novel pathways for glucose and lipid metabolism. Diabetes. (2021) 70:2932–46. doi: 10.2337/db21-0397
64. Vojinovic D, Radjabzadeh D, Kurilshikov A, Amin N, Wijmenga C, Franke L, et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat Commun. (2019) 10:5813. doi: 10.1038/s41467-019-13721-1
65. Kashtanova DA, Klimenko NS, Tkacheva ON, Strazhesko ID, Metelskaya VA, Gomyranova NV, et al. Subfractional spectrum of serum lipoproteins and gut microbiota composition in healthy individuals. Microorganisms. (2021) 9:1461. doi: 10.3390/microorganisms9071461
66. Witztum JL, Schonfeld G. Carbohydrate diet-induced changes in very low density lipoprotein composition and structure. Diabetes. (1978) 27: 1215–29.
67. Chahal J, Gupta S, Chawla SPS, Grewal H. Comparative study on fasting and postprandial lipid profile in type 2 diabetes mellitus. J Family Med Prim Care. (2021) 10:1288–93.
68. Balling M, Langsted A, Afzal S, Varbo A, Davey Smith G, Nordestgaard BG. A third of nonfasting plasma cholesterol is in remnant lipoproteins: lipoprotein subclass profiling in 9293 individuals. Atherosclerosis. (2019) 286:97–104.
69. Takahashi S. Triglyceride rich lipoprotein -LPL-VLDL receptor and Lp(a)-VLDL receptor pathways for macrophage foam cell formation. J Atheroscler Thromb. (2017) 24:552–9. doi: 10.5551/jat.RV17004
70. Niu YG, Evans RD. Very-low-density lipoprotein: complex particles in cardiac energy metabolism. J Lipids. (2011) 2011:189876.
71. Lee HC, Shin SJ, Huang JK, Lin MY, Lin YH, Ke LY, et al. The role of postprandial very-low-density lipoprotein in the development of atrial remodeling in metabolic syndrome. Lipids Health Dis. (2020) 19:210. doi: 10.1186/s12944-020-01386-5
72. Nakajima K, Tokita Y, Tanaka A, Takahashi S. The VLDL receptor plays a key role in the metabolism of postprandial remnant lipoproteins. Clin Chim Acta. (2019) 495:382–93. doi: 10.1016/j.cca.2019.05.004
73. Plazas Guerrero CG, Acosta Cota SJ, Castro Sanchez FH, Vergara Jimenez MJ, Rios Burgueno ER, Sarmiento Sanchez JI, et al. Evaluation of sucrose-enriched diet consumption in the development of risk factors associated to type 2 diabetes, atherosclerosis and non-alcoholic fatty liver disease in a murine model. Int J Environ Health Res. (2021) 31:651–69. doi: 10.1080/09603123.2019.1680817
74. Dornas WC, de Lima WG, Pedrosa ML, Silva ME. Health implications of high-fructose intake and current research. Adv Nutr. (2015) 6:729–37.
75. Sabaka P, Kruzliak P, Gaspar L, Caprnda M, Bendzala M, Balaz D, et al. Postprandial changes of lipoprotein profile: effect of abdominal obesity. Lipids Health Dis. (2013) 12:179.
76. Della Pepa G, Vetrani C, Vitale M, Bozzetto L, Costabile G, Cipriano P, et al. Effects of a diet naturally rich in polyphenols on lipid composition of postprandial lipoproteins in high cardiometabolic risk individuals: an ancillary analysis of a randomized controlled trial. Eur J Clin Nutr. (2020) 74:183–92. doi: 10.1038/s41430-019-0459-0
77. Munoz-Perez DM, Gonzalez-Correa CH, Astudillo-Munoz EY, Porras-Hurtado GL, Sanchez-Giraldo M, Lopez-Miranda J, et al. Alternative foods in cardio-healthy dietary models that improve postprandial lipemia and insulinemia in obese people. Nutrients. (2021) 13:2225. doi: 10.3390/nu13072225
78. Amigo N, Akinkuolie AO, Chiuve SE, Correig X, Cook NR, Mora S. Habitual fish consumption, n-3 fatty acids, and nuclear magnetic resonance lipoprotein subfractions in women. J Am Heart Assoc. (2020) 9:e014963. doi: 10.1161/JAHA.119.014963
79. Morton AM, Furtado JD, Lee J, Amerine W, Davidson MH, Sacks FM. The effect of omega-3 carboxylic acids on apolipoprotein CIII-containing lipoproteins in severe hypertriglyceridemia. J Clin Lipidol. (2016) 10:1442–51.e4. doi: 10.1016/j.jacl.2016.09.005
80. Grundler F, Plonne D, Mesnage R, Muller D, Sirtori CR, Ruscica M, et al. Long-term fasting improves lipoprotein-associated atherogenic risk in humans. Eur J Nutr. (2021) 60:4031–44. doi: 10.1007/s00394-021-02578-0
81. Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. (2014) 49:695–701. doi: 10.1007/s11745-014-3901-z
82. Rodriguez V, Newman JD, Schwartzbard AZ. Towards more specific treatment for diabetic dyslipidemia. Curr Opin Lipidol. (2018) 29:307.
83. Patel VJ, Joharapurkar AA, Shah GB, Jain MR. Effect of GLP-1 based therapies on diabetic dyslipidemia. Curr Diabetes Rev. (2014) 10:238–50.
84. Ooi EM, Ng TW, Watts GF, Chan DC, Barrett PHR. Effect of fenofibrate and atorvastatin on VLDL apoE metabolism in men with the metabolic syndrome. J Lipid Res. (2012) 53:2443–9. doi: 10.1194/jlr.P029223
85. Fruchart JC, Santos RD, Aguilar-Salinas C, Aikawa M, Al Rasadi K, Amarenco P, et al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha) paradigm: conceptual framework and therapeutic potential : a consensus statement from the International Atherosclerosis Society (IAS) and the Residual Risk Reduction Initiative (R3i) Foundation. Cardiovasc Diabetol. (2019) 18:71. doi: 10.1186/s12933-019-0864-7
86. Hollstein T, Vogt A, Grenkowitz T, Stojakovic T, März W, Laufs U, et al. Treatment with PCSK9 inhibitors reduces atherogenic VLDL remnants in a real-world study. Vasc Pharmacol. (2019) 116:8–15. doi: 10.1016/j.vph.2019.03.002
87. Group HTRC. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. (2017) 377:1217–27.
88. Butt WZ, Yee JK. The role of non-statin lipid-lowering medications in youth with hypercholesterolemia. Curr Atheroscler Rep. (2022) 24:379–89. doi: 10.1007/s11883-022-01013-x
89. Zhang J, de Albuquerque Rocha N, McCullough PA. Contribution of ApoCIII to diabetic dyslipidemia and treatment with volanesorsen. Rev Cardiovasc Med. (2018) 19:13–9.
90. Ahmad Z, Pordy R, Rader DJ, Gaudet D, Ali S, Gonzaga-Jauregui C, et al. Inhibition of angiopoietin-like protein 3 with evinacumab in subjects with high and severe hypertriglyceridemia. J Am Coll Cardiol. (2021) 78:193–5. doi: 10.1016/j.jacc.2021.04.091
91. Marron MM, Allison M, Kanaya AM, Larsen B, Wood AC, Herrington D, et al. Associations between lipoprotein subfractions and area and density of abdominal muscle and intermuscular adipose tissue: the multi-ethnic study of atherosclerosis. Front Physiol. (2021) 12:713048. doi: 10.3389/fphys.2021.713048
92. Silbernagel G, Scharnagl H, Kleber ME, Hoffmann MM, Delgado G, Stojakovic T, et al. Common APOC3 variants are associated with circulating ApoC-III and VLDL cholesterol but not with total apolipoprotein B and coronary artery disease. Atherosclerosis. (2020) 311:84–90. doi: 10.1016/j.atherosclerosis.2020.08.017
Keywords: very-low-density lipoprotein, cardiovascular disease, triglycerides, metabolic syndrome, apolipoproteins, cardiometabolic disorders
Citation: Lee H-C, Akhmedov A and Chen C-H (2022) Spotlight on very-low-density lipoprotein as a driver of cardiometabolic disorders: Implications for disease progression and mechanistic insights. Front. Cardiovasc. Med. 9:993633. doi: 10.3389/fcvm.2022.993633
Received: 13 July 2022; Accepted: 12 September 2022;
Published: 04 October 2022.
Edited by:
Kailash Gulshan, Cleveland State University, United StatesReviewed by:
Chia-Feng Liu, Cleveland Clinic, United StatesCopyright © 2022 Lee, Akhmedov and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chu-Huang Chen, Y2NoZW5AdGV4YXNoZWFydC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.