- 1Department of Vascular Surgery, Zhongshan Hospital, Fudan University, Xiamen, China
- 2Department of Vascular Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Vascular Surgery Institute of Fudan University, Shanghai, China
- 4National Clinical Research Center for Interventional Medicine, Shanghai, China
- 5Fudan Zhangjiang Institute, Shanghai, China
Background: Currently, the optimal technique to revascularize the left subclavian artery (LSA) during thoracic endovascular aortic repair (TEVAR) remains controversial. Our study seeks to characterize early and late clinical results and to assess the advantages and disadvantages of endovascular vs. surgical strategies for the preservation of LSA.
Methods: PubMed, Embase and Cochrane Library searches were conducted under the PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) standards. Only literature published after January 1994 was included. Studies reporting on endovascular revascularization (ER), surgical revascularization (SR) for LSA preservation were included. 30-day mortality and morbidity rates, restenosis rates, and rates of early and late reintervention are measured as outcomes.
Results: A total of 28 studies involving 2,759 patients were reviewed. All articles were retrospective in design. Single-arm analysis found no significant statistical differences in ER vs. SR in terms of 30-day mortality and perioperative complication rates. The mean follow-up time for the ER cohort was 12.9 months and for the SR cohort was 26.6 months, respectively. Subgroup analysis revealed a higher risk of perioperative stroke (4.2%) and endoleaks (14.2%) with the chimney technique compared to the fenestrated and single-branched stent approaches. Analysis of the double-arm studies did not yield statistically significant results.
Conclusion: Both ER and SR are safe and feasible in the preservation of LSA while achieving an adequate proximal landing zone. Among ER strategies, the chimney technique may presents a greater risk of neurological complications and endoleaks, while the single-branched stent grafts demonstrate the lowest complication rate, and the fenestration method for revascularization lies in an intermediate position. Given that the data quality of the included studies were relatively not satisfactory, more randomized controlled trials (RCTs) are needed to provide convincing evidence for optimal approaches to LSA revascularization in the future.
Introduction
Thoracic endovascular aortic repair (TEVAR) has attracted much attention as a procedure for treating most descending aortic pathologies (1). Standard TEVAR recommends a proximal anchoring zone of no less than 20 mm (2). In many cases, stents will need to be released proximally to cover the left subclavian artery (LSA) in order to expand the landing zone (3). However, direct coverage of LSA during TEVAR may pose a higher incidence of various complications, leading to devastating outcomes including stroke, spinal cord ischemia, or left upper extremity ischemia (4, 5).
In recent years, more and more studies have shown that performing revascularization of the LSA rather than direct coverage may lead to a more favorable overall prognostic outcome (6, 7). Prophylactic LSA revascularization allowing better preservation of normal perfusion through vital branches and thus reducing the risk of potential complications (8). There is a general consensus that revascularization of the LSA should always be performed in most of the case, especially elective surgery (9).
Traditional revascularization usually takes the form of surgical revascularization (SR) such as graft bypass or transposition (10). Endovascular revascularization (ER) techniques such as chimney grafts, fenestrated stent-grafts and single-branched stent-grafts, have developed as an alternative approach over the past two decades (11–13). Open surgery can provide definite results, but may theoretically result in more trauma; whereas, the endovascular technique is currently undergoing different stages of trials and its outcomes are yet to be confirmed.
Controversy still existed regarding the optimal choice of revascularization to minimize the incidence of perioperative complications during TEVAR (12). The objective of the present study was to carry out a meta-analysis of all published studies in an effort to obtain quantitative insight into the impact of various revascularization techniques on the outcome of TEVAR.
Materials and methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement was used to guide the conduct of this study (14). We performed a comprehensive search on the following databases: PubMed, Embase, the Cochrane Library. We have no restrictions on the type of publications. With regard to the year of publication, we restricted the period to papers published after January 1994. The search was updated to June 1, 2021. The primary search terms were (i) “revascularization”; (ii) “TEVAR” OR “TEVAR”; and (iii) MeSH term: “Subclavian Artery” which was found in the MeSH hierarchy. Both the “AND” and “OR” operators combined with distinct search terms were used to ensure the integrity of the search results. Relevant articles were also checked in the reference list.
Inclusion and exclusion criteria
In order to select studies for inclusion in this review, the following criteria were considered: (i) language in English; (ii) more than 2 patients; (iii) publication between January 1994 and August 2021 with adequate data on postprocedural complications and outcomes; (iv) both prospective and retrospective studies. Exclusion criteria were as follows: (i) studies that did not involve humans; (ii) publications categorized as abstracts, letters, editorials, experts’ opinions, reviews, case reports, or technical notes; guidelines; and technical notes; (iii) studies lacking sufficient data for analysis; (iv) duplicate articles or identical data.
Data extraction
Each candidate study was reviewed by the investigators and the data was then extracted. We retrieved full-text versions of articles that were unable to be categorized by title and abstract alone.
The following items were recorded for each study: Early outcomes including 30-day mortality; stroke rates; spinal cord ischemia rates; endoleak rates; left limb ischemia rates and early reintervention rates. Mid-term or long-term outcomes: follow-up length; cumulative patency rates; late reintervention rates; restenosis or re-occlusion rates.
The definitions of measurements and treatments associated with TEVAR are strictly consistent with reporting standards for thoracic endovascular aortic repair (TEVAR) (15). Comprehensive analysis focused primarily on perioperative mortality, stroke, spinal cord ischemia, and endoleak. The three above-mentioned authors completed a quality assessment based on GRADE—Grading of Recommendations, Assessments, Development and Evaluations (16).
Statistical analysis
Data analysis was conducted following the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions (17). The comparative studies (ER vs. SR) were pooled as risk differences (RD) with 95% confidence intervals (CI) and analyzed in a meta-analysis. Single-arm studies were analyzed in a pooled proportion meta-analysis, these data and single proportions were calculated as overall proportions with 95% CI in order to summarize postoperative data and outcomes in each study. The R package Meta were implemented to conduct all analyses (18). Every single proportions were transformated by Freeman-Tukey Double arcsine transformation and passed the normality test before final calculations. A combination of fixed- and random-effects models were used for sensitivity analyses, as well as to calculate the pooled RD and 95% CIs. A subgroup analysis has been carried out in order to examine and explain the diversity of results (heterogeneity) among the various studies. The I2 statistic was used to assess heterogeneity across the studies. For case of high heterogeneity (I2 > 50%), a random-effects model was applied, while in case of low heterogeneity (I2 < 50%), a fixed-effect model was applied. Sensitivity analyses were performed by comparing the output of both fixed- and random-effects models. Visual analyses of funnel plots and Egger regression tests were performed to evaluate publication bias. Statistical significance was defined as a p-value of < 0.05 with two-sided CI. All statistical analyses were carried out using R software (version 4.1.3).1
Results
Data from overall studies
Search results and study selection
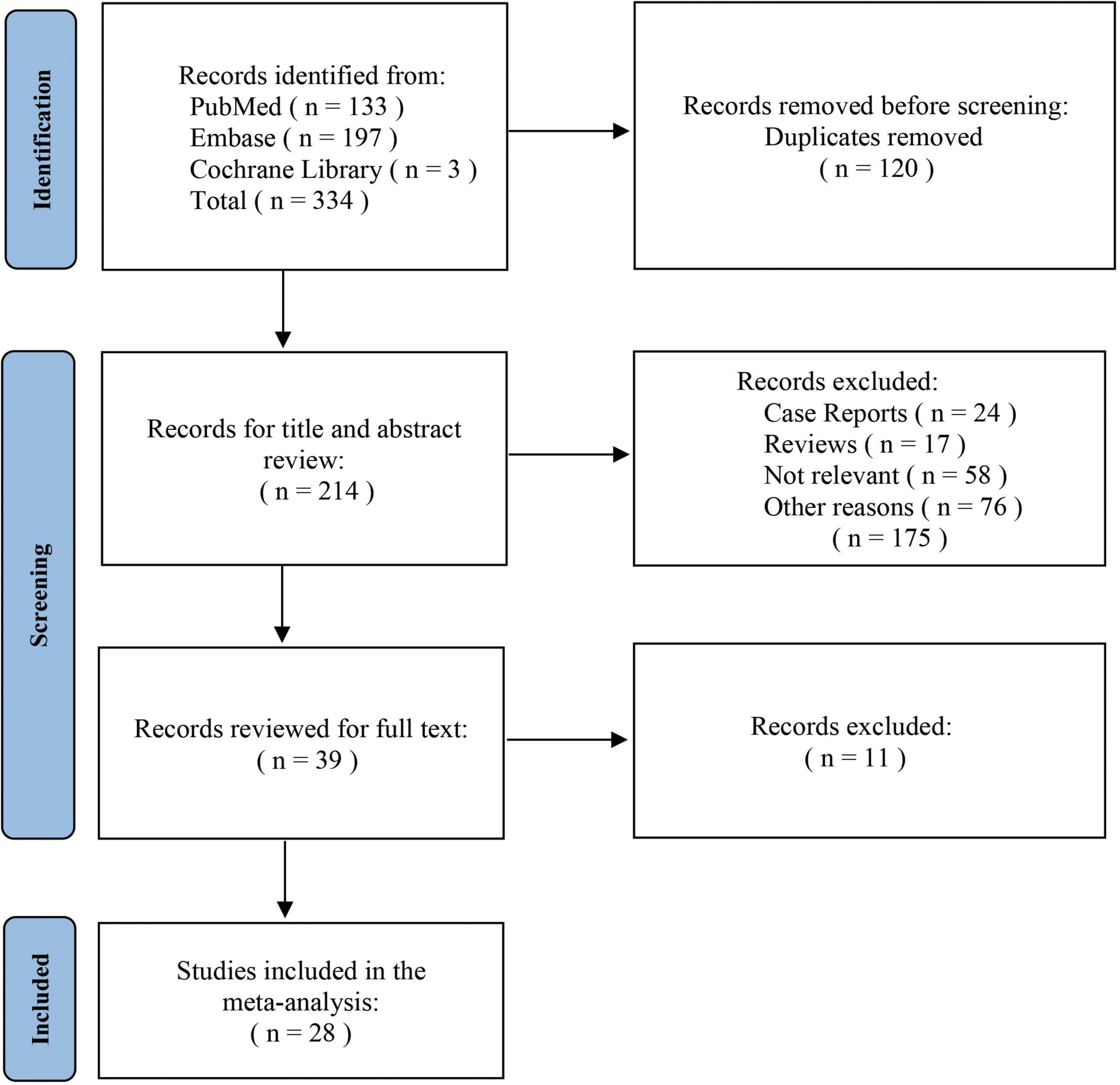
The initial search identified 334 studies associated with the topic. Of these, 214 publications remained after calculating duplications. After screening titles and abstracts, 24 case reports, 17 reviews and 134 publications that did not meet the inclusion criteria were excluded. All remaining 39 studies were retrieved for full text, of which 28 articles were finally included for the analysis (19–46). The screening procedure for this review is depicted in Figure 1. The meta-analysis included a total of 2,759 patients. ER data were derived from 12 studies involving 360 patients to 22 studies involving 2,399 patients for the SR cohort. The number of cases included in cohorts implementing chimney method, fenestration technique and single-branched stent were 72, 194, and 94, respectively.
Early outcomes
Cumulative 30-day mortality was 0.4% (95% CI: 0.0–1.9%) for ER and 2.8% (95% CI: 1.3–4.9%) for SR, respectively. The incidence of early stroke was 0.1% (95% CI: 0.0–1.2%) for ER and 4.1% (95% CI: 2.6–5.8%) for SR. In ER cohort, spinal cord ischemia was observed in 0.0% (95% CI: 0.0–0.8%) of the cases, while in SR, the incidence was 1.7% (95% CI: 1.1–2.4%). Left arm claudication was recorded respectively, in ER and SR with 0.0% (95% CI: 0.0–0.3%) and 1.7% (95% CI: 0.4–3.4%). As for Endoleak, the incidence in ER is 8.3% (95% CI: 2.1-17.1%) and the incidence in SR is 11.2% (95% CI: 4.4–20.5%; Table 1). The overall proportions of early reinterventions were 2.0% (95% CI: 0.0–17.0%) for ER and 9.4% (95% CI: 7.3–11.7%; Table 2) for SR.
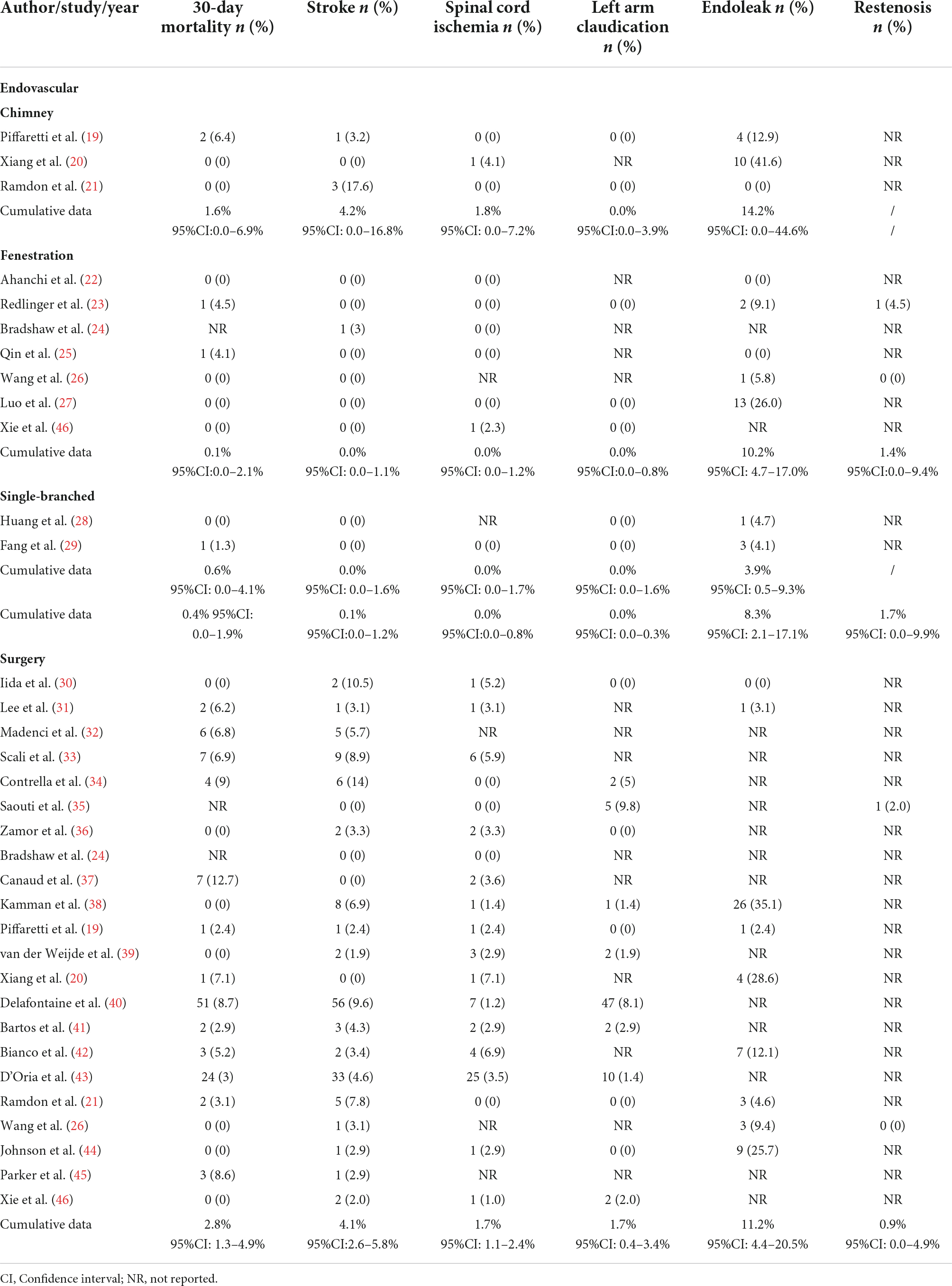
Table 1. Data on early and late outcomes following endovascular revascularization and surgical revascularization.
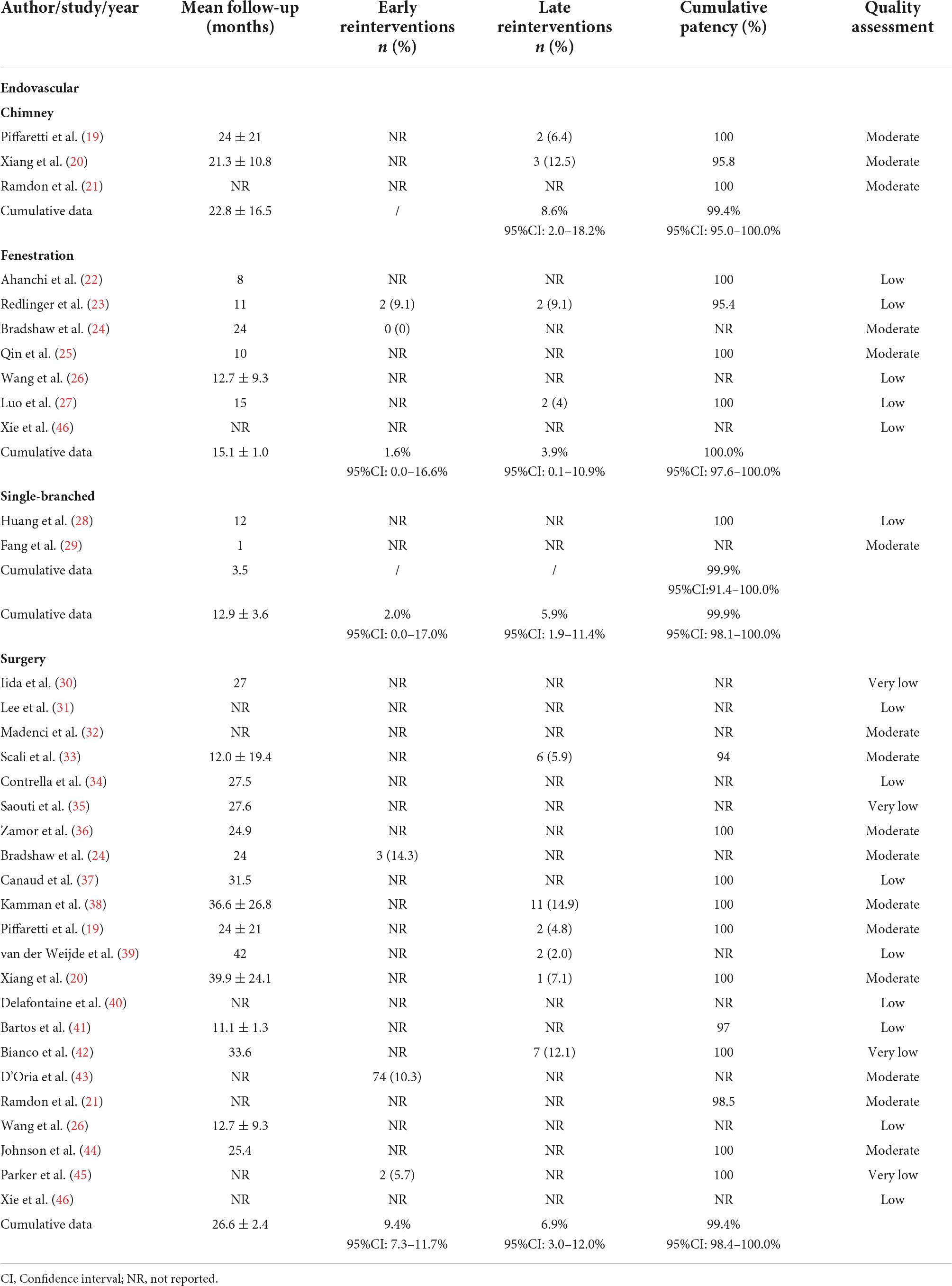
Table 2. Follow-up times, reinterventions and patency rates after endovascular revascularization and surgical revascularization.
Late outcomes
The mean follow-up time was 12.9 months for a total of 322 patient-years in the ER cohort, while in the SR cohort, it was 26.6 months for a total of 1,717 patient-years of follow-up. The mean follow-up length in the three subgroups of the ER cohort were 22.8 months in chimney, 15.1 months in fenestration and 3.5 months in single-branched stent-grafts group, respectively. Only three of the SR studies reported a follow-up period exceeded 3 years. The cumulative rate of late reintervention after ER was 5.9% (95% CI: 1.9–11.4%) and 6.9% (95% CI: 3.0–12.0%) after SR. The restenosis rate during the follow-up period was 1.7% (95% CI: 0.0–9.9%; Table 1) in ER and 0.9% (95% CI: 0.0–4.9%) in SR. Pooled estimates of overall patency rates were 99.9% (95% CI: 98.1–100.0%) and 99.4% (95% CI: 98.4–100.0%), respectively (Table 2).
Subgroup analysis
The ER group was divided into three cohorts according to different methods of intervention, chimney grafts, fenestration group and single-branched stent-grafts. Subgroup analysis show that 30-day mortality was 1.6% (95% CI: 0.0–6.9%), 0.1% (95% CI: 0.0–2.1%) and 0.6% (95% CI: 0.0–4.1%), respectively. Stroke rate was 4.2% (95% CI: 0.0–16.8%) for chimney, 0.0% (95% CI: 0.0–1.1%) for fenestration and 0.0% (95% CI: 0.0–1.6%) for single-branched stent-grafts. No case of left limb ischemia has been reported in any subgroup. Pooled result indicated significant differences in the incidence of endoleaks among chimney, fenestration, and single-branched groups, with a cumulative data of 14.2% (95% CI: 0.0–44.6%), 10.2% (95% CI: 4.7–17.0%) and 3.9% (95% CI: 0.5–9.3%; Table 1). Notably, the chimney-grafts method showed a relatively high incidence of almost all the complications analyzed in this study, whereas single-branched stent-grafts showed relatively better outcomes. There were no significant subgroup differences for left arm claudication and cumulative patency rates among the ER group. Analysis of early reinterventions and restenosis rates were not feasible due to a lack of data.
Data from comparative studies
Study selection
Six controlled retrospective studies were included, involving 438 patients contributed to comparative meta-analyses. All six included studies used bypass surgery as the method of LSA revascularization, with the chimney-grafts as the control group in three of the studies and the fenestration method as the control group in the remaining three studies.
Outcomes
No statistically significant results were observed among the six studies described above, including 30-day mortality, stroke, endoleaks and spinal cord ischemia, etc. (Supplementary Figures 1–8).
Publication bias
In terms of funnel plot and Egger testing, the results of the visual analysis did not reveal any indications of publication bias within the SR group. Only left arm claudication was identified as a significant risk of bias in the ER studies (Supplementary Figures 9–38).
Discussion
Over the last decade, several studies have suggested the potential benefits of LSA revascularization, a procedure that has been shown to reduce the incidence of postoperative complications compared to direct coverage (47–49). Since there is still no consensus on the advantages and disadvantages of different methods of LSA revascularization, this systematic review was conducted to attempt to answer this question by comparing the complications associated with each technique.
Our study reviewed contemporary methods and clinical outcomes of 2,759 patients from 28 individual studies for LSA revascularization during TEVAR, indicating that performing ER or SR during TEVAR has comparable effects on the outcomes. LSA revascularization with open surgical procedures used to be the most widely applied revascularization approach (50).
Although LSA revascularization through bypass or transposition surgery is technically sophisticated, it entails excessive trauma and compromises the minimally invasive concept of TEVAR. Besides, open surgery can lead to potential local complications, such as irritation of the brachial plexus nerve (41). In comparison, LSA revascularization using endovascular approaches is in line with the minimally invasive concept of the TEVAR procedure and the technology is well established (29). Actually, the indications for complete endovascular aortic reconstruction are also expanding (51). Therefore, we cautiously believe that the ER methods may have some advantages in avoiding unnecessary incisions and trauma to patients.
Another advantage of ER is the shorter surgical time requirement as compared to open surgery (46). When performing ER, the stent-graft is also more tolerant of DSA imaging accuracy and proficiency of manipulation (52).
In terms of ER of LSA, currently there are three main methods: chimney techniques, in situ fenestration procedures, and single-branched stent grafts. The subgroup analysis indicates that all three methods present acceptable clinical results, although there are still some notable differences in some aspects. This subtle difference merits a careful examination in order to identify their strengths and latent weaknesses.
Our main focus was on stroke and endoleak. Specifically, when the chimney method is applied to LSA revascularization, it results in an increased rate of postoperative complications. We identified that the incidence of stroke (4.2%; 95% CI: 0.0–16.8%) and endoleak (14.2%; 95% CI: 0.0–44.6%) was markedly higher in the chimney group. In comparison, LSA revascularization with single-branched stent-grafts demonstrated a considerably lower perioperative complication rates than expected, with no perioperative strokes recorded and the incidence of endoleak was only 3.9% (95% CI: 0.5–9.3%). In particular, it should be noted that despite this result appearing to be extremely favorable, the findings obtained by this study have a great deal of bias, as the number of single-branch stents reported is the lowest of all techniques.
Fenestrated technique has been associated with modest outcomes, with the incidence of stroke was 0.0% (95% CI: 0.0–1.5%) and the incidence of endoleak was 10.2% (95% CI: 4.7–17.0%), which was relatively high compared to the result of the single-branched stent-graft group.
The chimney technique is a widely accepted and utilized method for LSA revascularization in TEVAR. It is a much more technically accessible approach to implement than fenestration technique and using single-branched stent-graft, yet a mounting number of studies suggest that it may increase the risk of endoleaks and reintervention rates (53, 54). According to one relevant publication, endoleaks are responsible for the majority of secondary interventions after TEVAR (55). In the current review, the perioperative endoleak rate of the chimney technique is 14.2% (95% CI: 0.0–44.6%), significantly higher than the average incidence in the ER (8.3%; 95% CI: 2.1–17.1%) and SR (11.2%; 95% CI: 4.4–20.5%) groups. It is also the highest of the three ER methods. Even though early reintervention rates were not recorded in the included studies, one previous study reported a rate of 11% of type I endoleaks associated with the chimney technique, and 42% of these patients required early reintervention (56). Moreover, we also found a late reintervention rate of 8.6% (95% CI: 2.0–18.2%) among the chimney group, which is higher than the cumulative rate of ER (5.9%; 95% CI: 1.9–11.4%) and SR (6.9%; 95% CI: 3.0–12.0%) group. Based on the findings of this analysis, it has been speculated that the high incidence of endoleaks may be one of the main factors leading to reintervention. Considering that reintervention rates were also higher in groups with a higher incidence of endoleak, we felt that it was essential to consider the potential risk associated with the chimney method.
Fenestration is another technique commonly used for LSA revascularization. There are two types of fenestration techniques, pre-fenestration and in situ fenestration, each of which may be appropriate in certain clinical situations. In the current meta-analysis, all the cases in other studies except two were totally complemented in situ fenestration technique (26, 46). An increasing number of studies have described the high repeatability and low perioperative mortality and morbidity of this procedure (25, 57, 58). Current evidence obtained from this analysis suggests that the incidence of 30-day mortality and early outcomes in the fenestrated cohort is close to the overall results of ER group. It is worth mentioning that the incidence of endoleak of the fenestration method is 10.2% (95% CI: 4.7–17.0%), which is relatively close to the average incidence of endoleak in the ER group derived in this analysis, while the incidence level is lower compared to the chimney group. This variation is understandable because the fenestration technique does not cause gutter endoleaks and has a relatively sufficient landing zone, which explains the significantly lower incidence of endoleaks reported in previous studies (59, 60). In comparison, it should be noted that the fenestration technique may result in an increased incidence of type III endoleak, which may be explained by the fact that the sealing zone between the connecting stent-graft and the LSA is relatively short and requires strong adhesion to the aortic wall (59). In general, considering the relatively low fenestration-related morbidity demonstrated shown in this analysis, the fenestration technique remains a safe, reproducible and durable procedure for LSA revascularization.
The single-branched stent-graft includes a main body and a branch graft that by design reduces the risk of endoleaks (28). It has been hypothesized that due to the superior stability of the single-branch stent and its ability to maintain structural integrity, the incidence of endoleaks should be reduced. This is in accordance with the results obtained in this analysis. According to results from a previous multicenter study, single-branched stent-grafts were found to be an effective technique for reconstructing the distal aortic arch (61). Additionally, we also noticed that almost all perioperative complications showed promising results with single-branched stent during TEVAR, including no occurrence of stroke, spinal cord ischemia, or left arm claudication during the in-hospital period or 30 days after the procedure. Moreover, Fang et al. noted that single-branched stent-grafts are simpler to implement than fenestration, suggesting that the method has good feasibility (29). Limited evidence so far suggests that the method has very promising potential for broader application. Nevertheless, this does not necessarily mean that the single-branch stent method is the most effective option. From our current review, the postoperative follow-up time of the cohort with branched stent-grafts was considerably shorter than the other groups undergoing LSA revascularization. As a result, the lack of long-term data is likely to have contributed to the underlying bias. Additionally, custom stent grafts are often required to compensate for the heterogeneity of the aortic arch anatomy, complicating the establishment of large-scale clinical studies.
Due to the distinct and independent characteristics of each method, we cannot extrapolate which is the more reliable solution. Their full potential has yet to be demonstrated, and it is reasonable to expect more details to be documented and worked out in clinical practice. Taking all these factors into account, a feasible option for the method of LSA revascularization during TEVAR should be carefully chosen based on the patients’ indications, while considering efficacy, practicality, and the practitioners’ expertise. As a result, this is probably the best way to ensure a viable landing zone while maintaining the blood supply to the LSA, allowing patients to benefit from TEVAR as fully as possible.
Limitations
Several limitations were identified in the current analysis that need to be carefully considered. Firstly, since no relevant randomized controlled trials (RCTs) were retrieved, all of the included studies were retrospective in design. Secondly, the data collected are primarily based on English-language studies, and therefore information from published research papers in other languages is not included. Thirdly, the heterogeneity between groups and subgroups is relatively significant, and the amount of literature used to conduct subgroup analyses varies considerably. Finally, the baseline characteristics of the patients that included in the analysis were not fully taken into account, furthermore, the data used in the analysis are partially missing and incomplete, contributing to bias in this study. In light of the above limitations, additional research is needed in the future to derive more accurate and reliable results.
Conclusion
As new endovascular devices and techniques are developed, complete ER is expected to become the standard of care for the treatment of LSA in the near future. It will, however, take a considerable amount of time for these alternatives to be verified. We cannot yet be certain whether ER will be able to completely replace SR, as there are still a number of uncertainties to be resolved. This review emphasizes the critical role of RCT research in providing insight into the manner in which LSA revascularization is performed during TEVAR. Further well-designed and large-scale studies are required to give more persuasive evidence regarding the optimal technique to LSA revascularization and to serve as the foundation for new treatment guidelines.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YucZ designed the study, collected, analyzed, and wrote the first draft of the manuscript. XX and YY collected data from the included studies and evaluated them according to the aforementioned criteria. CH, EW, and YufZ evaluated and proofread the data obtained from the studies included in this review. PL, ZL, and FM reviewed the statistical analysis methods and results that were conducted. WF and LW conceived and designed the study, coordinated and monitored its conduct, and carefully revised the final manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Science Foundation of China (Grant no. 81970412), the Shanghai Municipal Science and Technology Commission Innovation Fund (Grant no. 22S31904800), the Fujian Province Health Science and Technology Fund (Grant no. 2021GGB030), the Shanghai Municipal Science and Technology Commission Innovation Fund (Grant no. 18441902400), the National Clinical Research Center for Interventional Medicine Fund (Grant no. 2021-004), the Shanghai Municipal Health Commission (Grant no. 20214Y0474), the Xiamen Municipal Health Science and Technology Program Fund (Grant no. 3502Z20194034), the Fudan Zhangjiang Clinical Medicine Innovation Fund (Grant no. KP7202115), and the Zhongshan Hospital’s Talents Supporting Plan (Grant no. 2019ZSGG11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.991937/full#supplementary-material
Footnotes
References
1. Alfson DB, Ham SW. Type B aortic dissections. Cardiol Clin. (2017). 35:387–410. doi: 10.1016/j.ccl.2017.03.007
2. Yoon WJ, Mell MW. Outcome comparison of thoracic endovascular aortic repair performed outside versus inside proximal landing zone length recommendation. J Vasc Surg. (2020) 72:1883–90. doi: 10.1016/j.jvs.2020.03.033
3. Mesar T, Alie-Cusson FS, Rathore A, Dexter DJ, Stokes GK, Panneton JM. A more proximal landing zone is preferred for thoracic endovascular repair of acute type B aortic dissections. J Vasc Surg. (2022) 75:38–46. doi: 10.1016/j.jvs.2021.06.036
4. Conway AM, Qato K, Nhan N, Tran N, Giangola G, Carroccio A. Management of the left subclavian artery in TEVAR for chronic type B aortic dissection. Vasc Endovasc Surg. (2020) 54:586–91. doi: 10.1177/1538574420942353
5. Youssef A, Ghazy T, Kersting S, Leip JL, Hoffmann RT, Kappert U, et al. Management of the left subclavian artery during TEVAR–complications and mid-term follow-up. Vasa. (2018) 47:387–92. doi: 10.1024/0301-1526/a000713
6. Weigang E, Parker JATC, Czerny M, Lonn L, Bonser RS, Carrel TP, et al. Should intentional endovascular stent-graft coverage of the left subclavian artery be preceded by prophylactic revascularisation? Eur J Cardio Thor Surg. (2011) 40:858–68. doi: 10.1016/j.ejcts.2011.01.046
7. Waterford SD, Chou D, Bombien R, Uzun I, Shah A, Khoynezhad A. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thor Surg. (2016) 101:381–9. doi: 10.1016/j.athoracsur.2015.05.138
8. Noor N, Sadat U, Hayes PD, Thompson MM, Boyle JR. Management of the left subclavian artery during endovascular repair of the thoracic aorta. J Endovas Ther. (2008) 15:168–76. doi: 10.1583/08-2406.1
9. D’Oria M, Mani K, DeMartino R, Czerny M, Donas KP, Wanhainen A, et al. Narrative review on endovascular techniques for left subclavian artery revascularization during thoracic endovascular aortic repair and risk factors for postoperative stroke. Int Cardiovasc Thor Surg. (2021) 32:764–72. doi: 10.1093/icvts/ivaa342
10. Hughes GC, Vekstein A. Current state of hybrid solutions for aortic arch aneurysms. Ann Cardiothorac Surg. (2021) 10:731–43. doi: 10.21037/acs-2021-taes-168
11. Spanos K, Tsilimparis N, Rohlffs F, Wipper S, Detter C, Behrendt CA, et al. Total endovascular arch repair is the procedure of the future. J Cardiovasc Surg. (2018) 59:559–71. doi: 10.23736/S0021-9509.18.10412-5
12. Shutze WP, Baxter R, Gable C, Harrington K, Schaffer J, Moore D, et al. Comparison of surgical debranching versus branched endografts in zone 2 thoracic endovascular aortic repair. J Vasc Surg. (2021) 73:50–2. doi: 10.1016/j.jvs.2020.12.047
13. Cheng SWK. Novel endovascular procedures and new developments in aortic surgery. Br J Anaesth. (2016) 117:ii3–12. doi: 10.1093/bja/aew222
14. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 21:339. doi: 10.1371/journal.pmed.1000097
15. Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. (2010) 52:1022–1033e5. doi: 10.1016/j.jvs.2010.07.008
16. Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. (2004) 4:38. doi: 10.1186/1472-6963-4-38
17. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. (2022). Available online at: https://training.cochrane.org/handbook/current (accessed April 15, 2022).
18. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Mental Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
19. Piffaretti G, Pratesi G, Gelpi G, Galli M, Criado FJ, Antonello M, et al. Comparison of two different techniques for isolated left subclavian artery revascularization during thoracic endovascular aortic repair in zone 2. J Endovasc Ther. (2018) 25:740–9. doi: 10.1177/1526602818802581
20. Xiang Y, Huang B, Zhao J, Hu H, Yuan D, Yang Y. The strategies and outcomes of left subclavian artery revascularization during thoracic endovascular repair for type B aortic dissection. Sci Rep. (2018) 8:9289. doi: 10.1038/s41598-018-27588-7
21. Ramdon A, Patel R, Hnath J, Yeh CC, Darling RC. Chimney stent graft for left subclavian artery preservation during thoracic endograft placement. J Vasc Surg. (2020) 71:758–66. doi: 10.1016/j.jvs.2019.05.049
22. Ahanchi SS, Almaroof B, Stout CL, Panneton JM. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J Endovasc Ther. (2012) 19:226–30. doi: 10.1583/11-3770MR.1
23. Redlinger RE, Ahanchi SS, Panneton JM. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J Vasc Surg. (2013) 58:1171–7. doi: 10.1016/j.jvs.2013.04.045
24. Bradshaw RJ, Ahanchi SS, Powell O, Larion S, Brandt C, Soult MC, et al. Left subclavian artery revascularization in zone 2 thoracic endovascular aortic repair is associated with lower stroke risk across all aortic diseases. J Vasc Surg. (2017) 65:1270–9. doi: 10.1016/j.jvs.2016.10.111
25. Qin J, Zhao Z, Wang R, Ye K, Li W, Liu X, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. JAHA. (2017) 6:e004542. doi: 10.1161/JAHA.116.004542
26. Wang M, Dong D, Yuan H, Wang M, Wu X, Zhang S, et al. Hybrid versus in vitro fenestration for preserving the left subclavian artery in patients undergoing thoracic endovascular aortic repair with unfavorable proximal landing zone. Vascular. (2020) 28:42–7. doi: 10.1177/1708538119862952
27. Luo M, Fang K, Fan B, Li Q, Li M, He H, et al. Midterm results of retrograde in situ needle fenestration during thoracic endovascular aortic repair of aortic arch pathologies. J Endovasc Ther. (2021) 28:36–43. doi: 10.1177/1526602820953406
28. Huang H, Jiao Y, Zhang Y, Zhu Y, Liu Z, Qiao T, et al. Implantation of unibody single-branched stent graft for patients with type B aortic dissections involving the left subclavian artery: 1-year follow-up outcomes. Cardiovasc Int Radiol. (2017) 40:1678–86. doi: 10.1007/s00270-017-1748-4
29. Fang C, Wang C, Liu K, Pang X. Early outcomes of left subclavian artery revascularization using castor single-branched stent-graft in the treatment of type B aortic dissection or intramural hematoma. ATCS. (2021) 27:251–9. doi: 10.5761/atcs.oa.20-00166
30. Iida Y, Kawaguchi S, Koizumi N, Komai H, Obitsu Y, Shigematsu H. Thoracic endovascular aortic repair with aortic arch vessel revascularization. Ann Vasc Surg. (2011) 25:748–51. doi: 10.1016/j.avsg.2010.12.018
31. Lee TC, Andersen ND, Williams JB, Bhattacharya SD, McCann RL, Hughes GC. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thor Surg. (2011) 92:97–103. doi: 10.1016/j.athoracsur.2011.03.089
32. Madenci AL, Ozaki CK, Belkin M, McPhee JT. Carotid-subclavian bypass and subclavian-carotid transposition in the thoracic endovascular aortic repair era. J Vasc Surg. (2013) 57:1275–1282.e2. doi: 10.1016/j.jvs.2012.11.044
33. Scali ST, Chang CK, Pape SG, Feezor RJ, Berceli SA, Huber TS, et al. Subclavian revascularization in the age of thoracic endovascular aortic repair and comparison of outcomes in patients with occlusive disease. J Vasc Surg. (2013) 58:901–9. doi: 10.1016/j.jvs.2013.04.005
34. Contrella BN, Sabri SS, Tracci MC, Stone JR, Kern JA, Upchurch GR, et al. Outcomes of coverage of the left subclavian artery during endovascular repair of the thoracic aorta. J Vasc Int Radiol. (2015) 26:1609–14. doi: 10.1016/j.jvir.2015.07.022
35. Saouti N, Hindori V, Morshuis WJ, Heijmen RH. Left subclavian artery revascularization as part of thoracic stent grafting†. Eur J Cardio Thor Surg. (2015) 47:120–5. doi: 10.1093/ejcts/ezu130
36. Zamor KC, Eskandari MK, Rodriguez HE, Ho KJ, Morasch MD, Hoel AW. Outcomes of thoracic endovascular aortic repair and subclavian revascularization techniques. J Am College Surg. (2015) 221:93–100. doi: 10.1016/j.jamcollsurg.2015.02.028
37. Canaud L, Ziza V, Ozdemir BA, Berthet JP, Marty-Ané CH, Alric P. Outcomes of left subclavian artery transposition for hybrid aortic arch debranching. Ann Vasc Surg. (2017) 40:94–7. doi: 10.1016/j.avsg.2016.06.037
38. Kamman AV, Eliason JL, Williams DM, Yang B, Moll FL, Trimarchi S, et al. Impact of left subclavian artery revascularization before thoracic endovascular aortic repair on postoperative cerebrovascular hemodynamics. Ann Vasc Surg. (2018) 46:307–13. doi: 10.1016/j.avsg.2017.06.046
39. van der Weijde E, Saouti N, Vos JA, Tromp SC, Heijmen RH. Surgical left subclavian artery revascularization for thoracic aortic stent grafting: a single-centre experience in 101 patients†. Int CardioVasc Thor Surg. (2018) 27:284–9. doi: 10.1093/icvts/ivy059
40. Delafontaine JL, Hu B, Tan TW, Tang GL, Starnes BW, Virk C, et al. Outcome comparison of TEVAR with and without left subclavian artery revascularization from analysis of nationwide inpatient sample database. Ann Vasc Surg. (2019) 58:174–9. doi: 10.1016/j.avsg.2019.01.005
41. Bartos O, Mustafi M, Andic M, Grözinger G, Artzner C, Schlensak C, et al. Carotid-axillary bypass as an alternative revascularization method for zone II thoracic endovascular aortic repair. J Vasc Surg. (2020) 72:1229–36. doi: 10.1016/j.jvs.2019.11.053
42. Bianco V, Sultan I, Kilic A, Aranda-Michel E, Cuddy RJ, Srivastava A, et al. Concomitant left subclavian artery revascularization with carotid-subclavian transposition during zone 2 thoracic endovascular aortic repair. J. Thor Cardiovasc Surg. (2020) 159:1222–7. doi: 10.1016/j.jtcvs.2019.03.060
43. D’Oria M, Kärkkäinen JM, Tenorio ER, Oderich GS, Mendes BC, Shuja F, et al. Perioperative outcomes of carotid–subclavian bypass or transposition versus endovascular techniques for left subclavian artery revascularization during nontraumatic zone 2 thoracic endovascular aortic repair in the vascular quality initiative. Ann Vasc Surg. (2020) 69:17–26. doi: 10.1016/j.avsg.2020.05.062
44. Johnson CE, Zhang L, Magee GA, Ham SW, Ziegler KR, Weaver FA, et al. Periscope sandwich stenting as an alternative to open cervical revascularization of left subclavian artery during zone 2 thoracic endovascular aortic repair. J Vasc Surg. (2021) 73:466–475.e3. doi: 10.1016/j.jvs.2020.05.063
45. Parker MH, Colpitts DK, Gilson GF, Ryan L, Mukherjee D. Carotid-axillary bypass as an alternative to carotid-subclavian bypass following coverage of left subclavian artery during TEVAR. Vasc Endovasc Surg. (2021) 55:265–8. doi: 10.1177/1538574420983655
46. Xie W, Xue Y, Li S, Jin M, Zhou Q, Wang D. Left subclavian artery revascularization in thoracic endovascular aortic repair: single center’s clinical experiences from 171 patients. J Cardiothorac Surg. (2021) 16:1593. doi: 10.1186/s13019-021-01593-w
47. Chung J, Kasirajan K, Veeraswamy RK, Dodson TF, Salam AA, Chaikof EL, et al. Left subclavian artery coverage during thoracic endovascular aortic repair and risk of perioperative stroke or death. J Vasc Surg. (2011) 54:979–84. doi: 10.1016/j.jvs.2011.03.270
48. Rehman SM, Vecht JA, Perera R, Jalil R, Saso S, Kidher E, et al. How to manage the left subclavian artery during endovascular stenting for thoracic aortic dissection? An assessment of the evidence. Ann Vascu Surg. (2010) 24:956–65. doi: 10.1016/j.avsg.2010.05.005
49. Karaolanis GI, Antonopoulos CN, Charbonneau P, Georgakarakos E, Moris D, Scali S, et al. A systematic review and meta-analysis of stroke rates in patients undergoing thoracic endovascular aortic repair for descending thoracic aortic aneurysm and type B dissection. J Vasc Surg. (2022) 76:292–301.e3. doi: 10.1016/j.jvs.2022.02.031
50. Tolenaar JL, van Bogerijen GHW, Eagle KA, Trimarchi S. Update in the management of aortic dissection. Curr Treat Options Cardio Med. (2013) 15:200–13. doi: 10.1007/s11936-012-0226-1
51. Tadros RO, Tang GHL, Barnes HJ, Mousavi I, Kovacic JC, Faries P, et al. Optimal treatment of uncomplicated type B aortic dissection: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:1494–504. doi: 10.1016/j.jacc.2019.07.063
52. de Souza LR, Oderich GS, Banga PV, Hofer JM, Wigham JR, Cha S, et al. Outcomes of total percutaneous endovascular aortic repair for thoracic, fenestrated, and branched endografts. J Vasc Surg. (2015) 62:1442–1449.e3. doi: 10.1016/j.jvs.2015.07.072
53. Mangialardi N, Ronchey S, Malaj A, Fazzini S, Alberti V, Ardita V, et al. Value and limitations of chimney grafts to treat arch lesions. J Cardiovasc Surg (Torino). (2015) 56:503–11.
54. Moulakakis KG, Mylonas SN, Avgerinos E, Papapetrou A, Kakisis JD, Brountzos EN, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. (2012) 55:1497–503. doi: 10.1016/j.jvs.2011.10.009
55. Lee CJ, Rodriguez HE, Kibbe MR, Malaisrie SC, Eskandari MK. Secondary interventions after elective thoracic endovascular aortic repair for degenerative aneurysms. J Vasc Surg. (2013) 57:1269–74. doi: 10.1016/j.jvs.2012.10.124
56. Lindblad B, Bin Jabr A, Holst J, Malina M. Chimney grafts in aortic stent grafting: hazardous or useful technique? systematic review of current data. Eur J Vasc Endovasc Surg. (2015) 50:722–31. doi: 10.1016/j.ejvs.2015.07.038
57. Crawford SA, Sanford RM, Forbes TL, Amon CH, Doyle MG. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J Vasc Surg. (2016) 64:244–50. doi: 10.1016/j.jvs.2016.03.445
58. Glorion M, Coscas R, McWilliams RG, Javerliat I, Goëau-Brissonniere O, Coggia MA. Comprehensive review of in situ fenestration of aortic endografts. Eur J Vasc Endovasc Surg. (2016) 52:787–800. doi: 10.1016/j.ejvs.2016.10.001
59. Kasprzak PM, Kobuch R, Schmid C, Kopp R. Long-term durability of aortic arch in situ stent graft fenestration requiring lifelong surveillance. J Vasc Surg. (2017) 65:538–41. doi: 10.1016/j.jvs.2016.05.072
60. Katada Y, Kondo S, Tsuboi E, Rokkaku K, Irie Y, Yokoyama H. Endovascular total arch repair using in situ fenestration for arch aneurysm and chronic type A dissection. Ann Thor Surg. (2016) 101:625–30. doi: 10.1016/j.athoracsur.2015.07.032
61. Patel HJ, Dake MD, Bavaria JE, Singh MJ, Filinger M, Fischbein MP, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the gore thoracic branch endoprosthesis. Ann Thor Surg. (2016) 102:1190–8. doi: 10.1016/j.athoracsur.2016.03.091
Keywords: meta-analysis, left subclavian artery, thoracic endovascular aortic repair, revascularization, endoleak
Citation: Zhang Y, Xie X, Yuan Y, Hu C, Wang E, Zhao Y, Lin P, Li Z, Mo F, Fu W and Wang L (2022) Comparison of techniques for left subclavian artery preservation during thoracic endovascular aortic repair: A systematic review and single-arm meta-analysis of both endovascular and surgical revascularization. Front. Cardiovasc. Med. 9:991937. doi: 10.3389/fcvm.2022.991937
Received: 12 July 2022; Accepted: 29 August 2022;
Published: 15 September 2022.
Edited by:
Qingsheng Lu, Second Military Medical University, ChinaReviewed by:
Ziheng Wu, The First Affiliated Hospital of Zhejiang University, ChinaJian Zhou, Second Military Medical University, China
Copyright © 2022 Zhang, Xie, Yuan, Hu, Wang, Zhao, Lin, Li, Mo, Fu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiguo Fu, ZnUud2VpZ3VvQHpzLWhvc3BpdGFsLnNoLmNu; Lixin Wang, d2FuZy5saXhpbkB6cy1ob3NwaXRhbC5zaC5jbg==
 Yuchong Zhang
Yuchong Zhang Xinsheng Xie1
Xinsheng Xie1 Enci Wang
Enci Wang Yufei Zhao
Yufei Zhao Lixin Wang
Lixin Wang