- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Endocrinology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 3Functional Examination Section, Gansu Provincial Maternity and Child-Care Hospital (Gansu Provincial Hospital), Lanzhou, China
- 4Clinical Experimental Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
Importance: Cardiac-specific death from radiation caused by radiation therapy (RT) in patients with malignant tumors has received extensive attention, however, little is known regarding the potential cardiotoxic effects of RT in patients with non-malignant tumors.
Objectives and methods: In this study, we used the SEER data to explore the incidence of post-radiation cardiovascular complications in patients with non-malignant tumors of central nervous system (CNS), and identify the influencing factors of cardiac-specific death.
Results: Ultimately 233, 306 patients were included (97.8% of patients had brain tumors and 2.2% had spinal cord tumors). For patients with non-malignant tumors of CNS, RT {yes (odds ratio [OR] 0.851, 95% confidence interval [CI] 0.774–0.936, p = 0.001, before propensity score matching (PSM); OR 0.792, 95% CI 0.702–0.894, p < 0.001, after PSM) vs. no} was associated with lower risk of cardiac-specific death, other clinical features affecting cardiac death similar to those in patients with non-malignant tumors of CNS receiving RT. For patients with non-malignant tumors of CNS receiving RT, female, married status, Hispanic ethnicity, surgery, and tumor site (brain exclude nerve and endocrine, nervous system) were associated with lower risks of cardiac-specific death, while earlier year of diagnosis, older age of diagnosis, Black, larger tumor and bilateral tumor were risk factors for cardiac-specific death.
Conclusions: Our study shows the influencing factors for cardiac-specific death in patients with non-malignant tumors of CNS, and found RT is associated with lower risk of cardiac-specific death. These results can facilitate the identification of patients with non-malignant tumors of CNS who can benefit from RT while avoiding cardiovascular events. In addition, this study helps to enhance the clinical use of RT in these populations, especially in patients who may have impaired cardiac function due to CNS tumors.
Introduction
Radiation therapy (RT) is the first line of treatment for specific cancers, and although effective against tumor cells, is also associated with multiple side effects. For instance, RT significantly increases the risk of cardiovascular complications and death (1), especially in patients with thoracic tumors and Hodgkin's lymphoma (2–4). Therefore, heart disease from radiation is a major concern in cancer therapy (5).
In addition to malignant tumors, non-malignant (benign and borderline malignancy) tumors have also received extensive attention. Non-malignant tumors growths are routinely treated by low-dose or medium-dose radiation (6). However, little is known regarding the potential cardiotoxic effects of RT in patients with non-malignant tumors (7), not to mention in patients with non-malignant tumors of central nervous system (CNS).
The National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database is a population-based coordinated state cancer registry, which collates the demographic and clinical data of cancer patients from multiple regions across the United States (8). Since the SEER database also includes information on patients with non-malignant tumors (9), it is a potentially useful resource for exploring the additional cardiac effects of RT in this population (8, 9). In this study, we used the SEER data to explore the incidence of post-radiation cardiovascular complications in patients with non-malignant tumors of CNS, and identify the influencing factors of cardiac-specific death (10, 11).
Patients and methods
Data retrieval
The “Incidence” package of SEER database was accessed using SEER*Stat version 8.4.0.1, and data representing nearly 30% of the US population from 18 US registries collated between 2000 and 2019 was retrieved. The patients included in the analysis were 18 years of age and older with benign tumors or borderline malignancies (by selecting Behavior code ICD-O-3 = “Benign”, “Borderline malignancy” in the option of Site and Morphology), with or without RT. Cardiac-specific death included all heart diseases that led to death (corresponding variable in the SEER database is COD to site rec KM = “Diseases of Heart”), and patients were divided into non-cardiac and cardiac death groups. Ethical approval and patient consent were not required since the SEER data is publicly available (12).
Statistical analysis
The missing data (Supplementary Table 1) were filled with multiple imputation, and variable (tumor size) not suitable for multiple imputation was imputed using expectation maximization. Categorical variables were expressed as frequencies and proportions. Pearson's chi-square test was used to compare variables with T (all theoretical numbers) ≥ 5 and n (total sample size) ≥ 40, continuity-corrected chi-square test was used if T ranged between ≥ 1 and < 5 and n was ≥ 40, and Fisher's test was used for variables with T < 1 or n < 40. Continuous variables that met normal distribution were expressed as mean ± SD, and compared by t-test if also homoscedastic. Otherwise, these variables were expressed as median (interquartile range [IQR]) and compared by Mann-Whitney U test. The factors significantly influencing cardiac-specific death were screened by univariate logistic regression, and used in multivariate analysis (“enter” method) to identify the independent risk factors. Sensitivity analysis of clinical features affecting cardiac-specific death was stratified by tumor nature and using logistic regression analysis. All statistical tests were two-sided, and p < 0.05 was considered statistically significant. SPSS (version, 27.0; SPSS, Chicago, Illinois, USA) was used for all statistical analyses.
Propensity score matching
PSM is used in clinical research to improve comparability between groups in retrospective observational studies by adjusting for confounding factors (13). A propensity 1:1 matching analysis was performed using in this study by STATA (version, 17.0; STATA, Texas, USA) to minimize bias, followed by the statistical analyses as described above.
Results
Patient characteristics
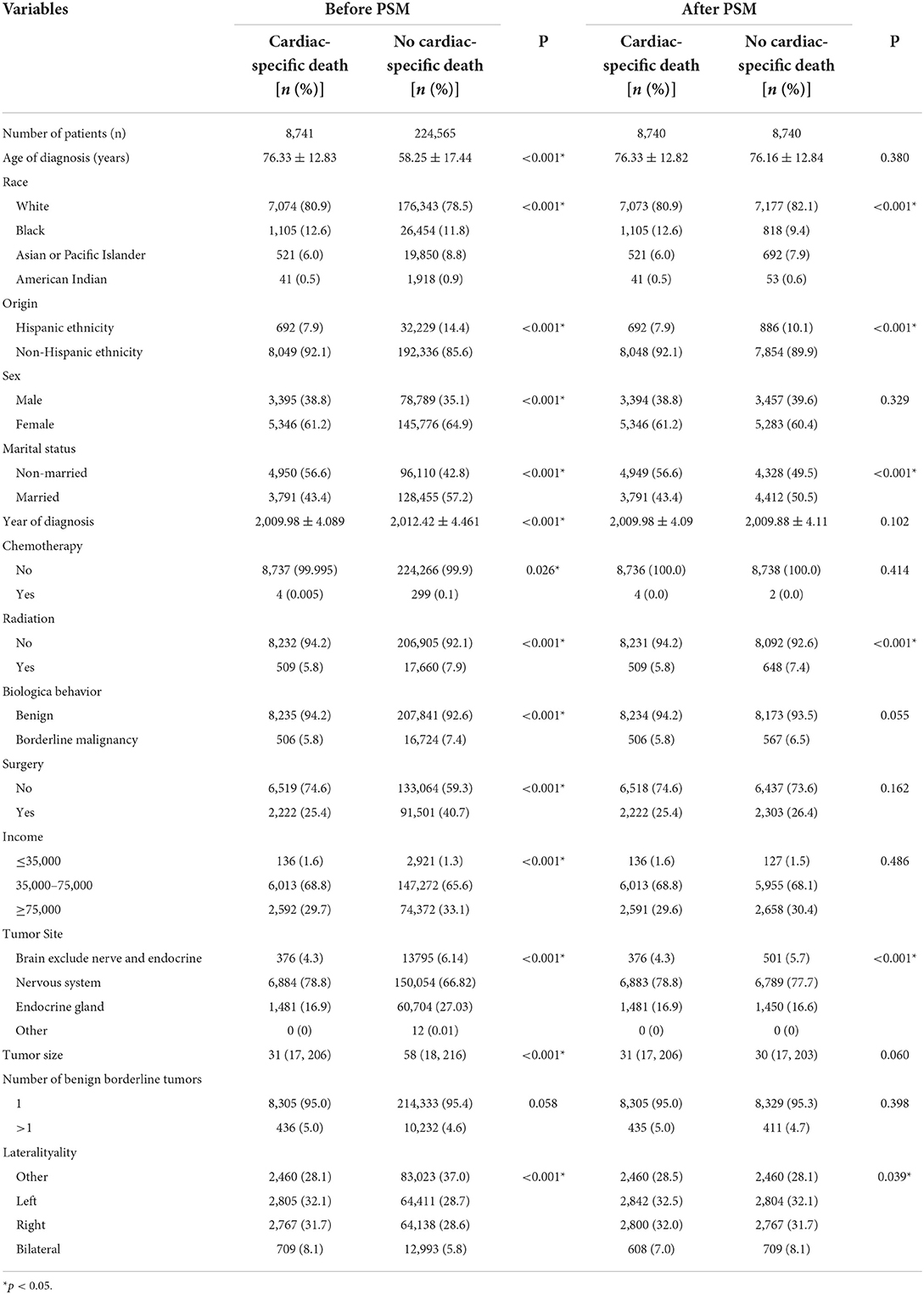
A total of 233, 306 patients with non-malignant tumors of central nervous system (CNS) were included in the study. The average age of the patients at diagnosis was >55 years (cardiac-specific death group [76.33 ± 12.83]/no cardiac-specific death group [58.25 ± 17.44]), and the patients were predominantly White (80.9/78.5%), non-Hispanic ethnicity (92.1/85.6%), female (61.2/64.9%), married (3,791 [43.4%]/128, 455 [57.2%]), and middle-class (68.8/65.6%). The tumors were mainly located in the nervous system (78.8/66.8%). Less than half of patients underwent surgery (25.4/40.7%), a small percentage of patients had received RT (5.8/7.9%), and fewer patients were treated by chemotherapy (0.005/0.13%). There were significant differences between the cardiac and non-cardiac death groups except for the number of borderline tumors (Table 1). Patients with non-malignant tumors of CNS receiving RT had similar baseline characteristics to the patients with non-malignant tumors of CNS. The difference of characteristics between the cardiac and non-cardiac death groups were mainly in age of diagnosis, origin, marital status, year of diagnosis, and surgery (Table 2). In addition, cardiac-specific death was the leading cause of death among patients with non-malignant tumors of CNS receiving RT or not, with statistically different in incidence (RT [15.2%] vs. non-RT [17.5%], p = 0.001).
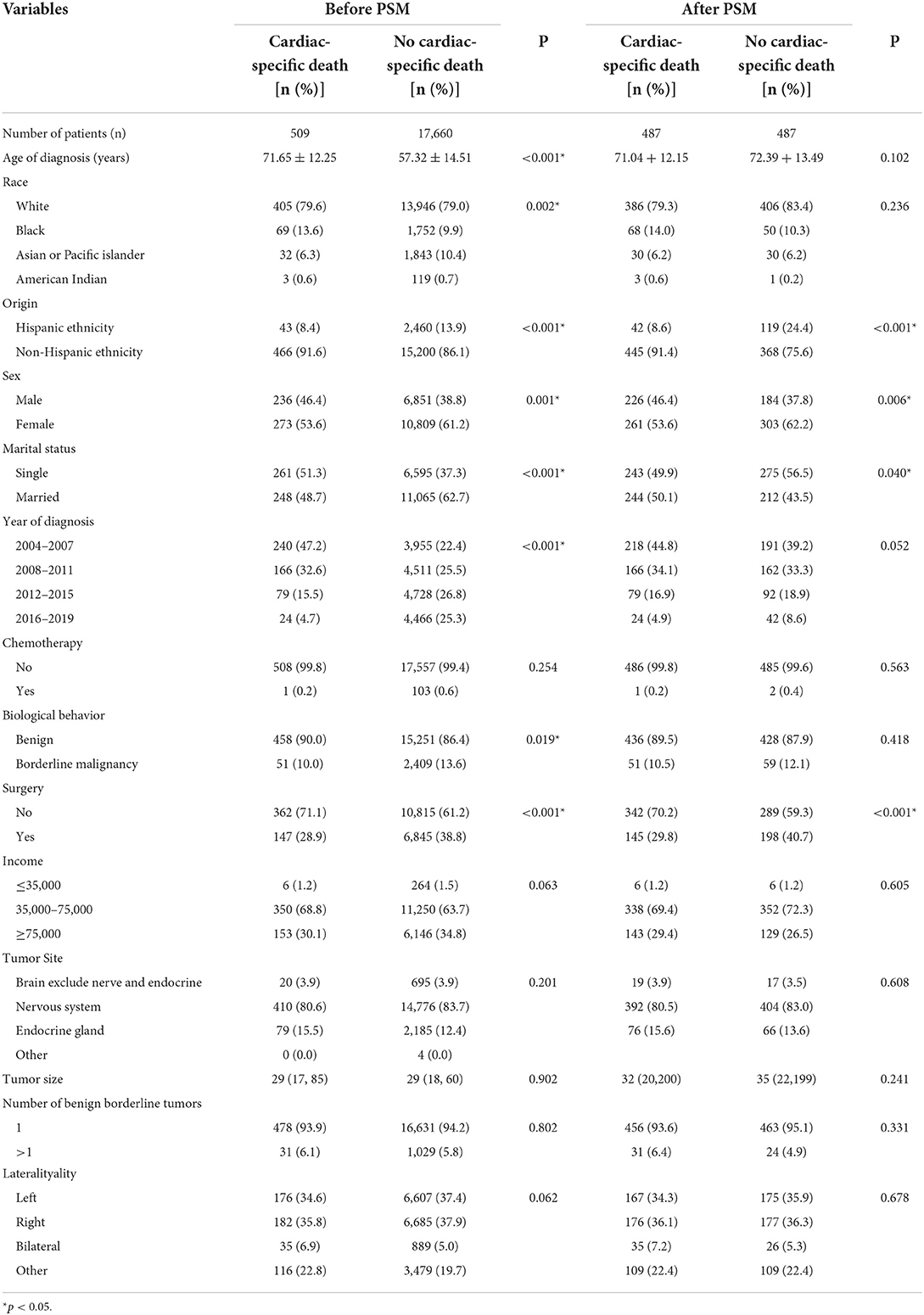
Table 2. Characteristics of patients included in patients with non-malignant tumors of CNS receiving RT.
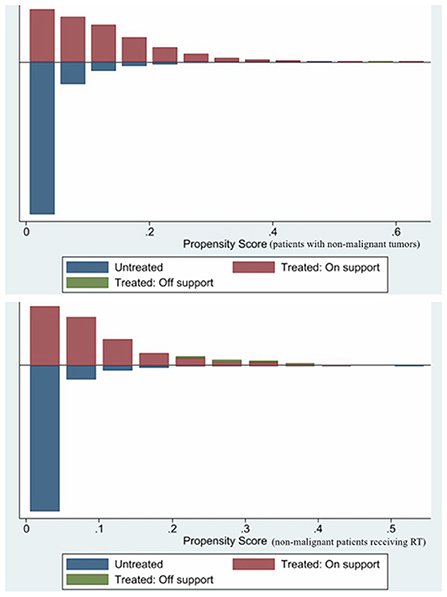
PSM was used to minimize the possible bias between the two groups. After 1:1 matching, 17, 480 patients with non-malignant tumors of CNS and 974 patients with non-malignant tumors of CNS receiving RT still retaining. Comparing the post-PSM with the pre-PSM, the differences in variables (age of diagnosis, sex, year of diagnosis, RT, biological behavior of tumor, surgery, income, tumor site, and tumor size) between the cardiac and non-cardiac death groups were significantly reduced in patients with non-malignant tumors of CNS (Table 1), the same is true for variables (age of diagnosis, race, marital status, year of diagnosis, and biological behavior of tumor) in patients with non-malignant tumors of CNS receiving RT (Table 2). Figure 1 shows distribution of propensity scores.
Risk factors of cardiac-specific death in patients with non-malignant tumors of CNS
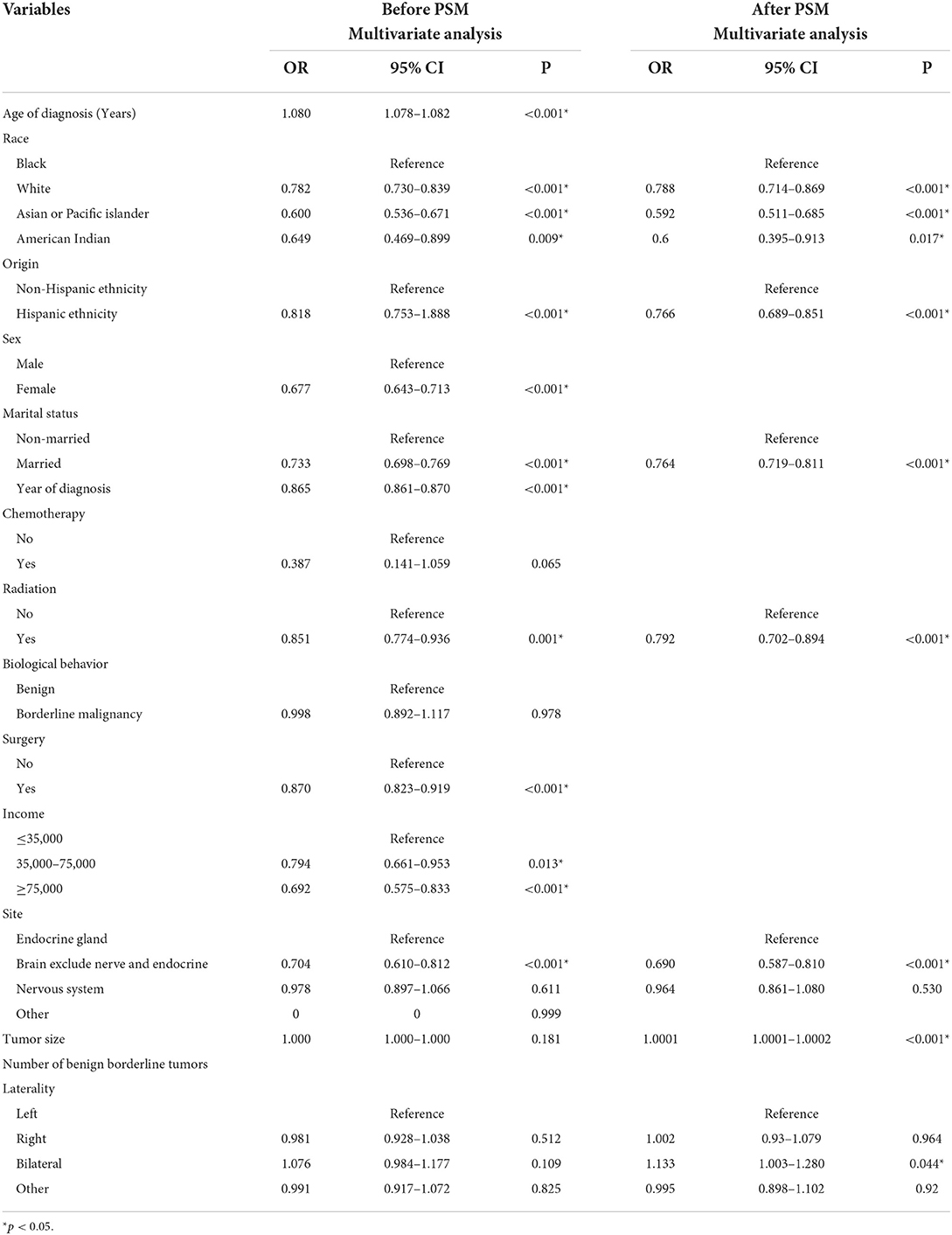
Multivariate logistic regression analysis showed that RT {yes (odds ratio [OR] 0.851, 95% confidence interval [CI] 0.774–0.936, p = 0.001, before PSM; OR 0.792, 95% CI 0.702–0.894, p < 0.001, after PSM) vs. no} was associated with lower risk of cardiac-specific death among patients with non-malignant tumors of CNS.
Besides, before PSM, race (White [OR 0.782, 95% CI: 0.730–0.839, p < 0.001], Asian or Pacific Islander [OR 0.600, 95% CI: 0.536–0.671, p < 0.001], American Indian [OR 0.649, 95% CI: 0.469–0.899, p = 0.009] vs. Black), origin (Hispanic ethnicity [OR 0.818, 95% CI: 0.753–1.888, p < 0.001] vs. non-Hispanic ethnicity), sex (female [OR 0.677, 95% CI: 0.643–0.713, p < 0.001] vs. male), marital status (married [OR 0.733, 95% CI: 0.698–0.769, p < 0.001] vs. non-married), year of diagnosis (OR 0.865, 95% CI: 0.861–0.870, p < 0.001), surgery (OR 0.870, 95% CI: 0.823–0.919, p < 0.001), higher income (≥75,000 [OR 0.692, 95% CI: 0.575–0.833, p < 0.001]; 35,000–75,000 [OR 0.794, 95% CI: 0.661–0.953, p = 0.013] vs. ≤35,000), and tumor site (brain exclude nerve and endocrine [OR 0.704, 95% CI: 0.610–0.812, p < 0.001] vs. endocrine gland) were associated with lower risks of cardiac-specific death among patients with non-malignant tumors of CNS, while age of diagnosis (OR 1.080, 95% CI: 1.078–1.082, p < 0.001) was a risk factor for cardiac-specific death (Table 3).
After PSM, race (White [OR 0.788, 95% CI: 0.714–0.869, p < 0.001], Asian or Pacific Islander [OR 0.592, 95% CI: 0.511–0.685, p < 0.001], American Indian [OR 0.6, 95% CI: 0.395–0.913, p = 0.017] vs. Black), origin (Hispanic ethnicity [OR 0.766, 95% CI: 0.689–0.851, p < 0.001] vs. non-Hispanic ethnicity), marital status (married [OR 0.764, 95% CI: 0.719–0.811, p < 0.001] vs. non-married), and tumor site (brain exclude nerve and endocrine [OR 0.690, 95% CI: 0.587–0.810, p < 0.001] vs. endocrine gland) were associated with lower risks of cardiac-specific death among patients with non-malignant tumors of CNS, while tumor size (OR 1.0001, 95% CI: 1.0001–1.0002, p < 0.001) and laterality (bilateral [OR 1.133, 95% CI: 1.003–1.280, p = 0.044] vs. left) were risk factors for cardiac-specific death (Table 3).
Risk factors of cardiac-specific death in patients with non-malignant tumors receiving RT
Before PSM, race (White [OR 0.699, 95% CI: 0.527–0.927, p = 0.013], Asian or Pacific Islander (OR 0.460, 95% CI: 0.294–0.720, p = 0.001] vs. Black), sex (female [OR 0.627, 95% CI: 0.517–0.761, p < 0.001] vs. male), marital status (married [OR 0.60, 95% CI: 0.495–0.728, p < 0.001] vs. non-married), and tumor site (nervous system [OR 0.650, 95% CI: 0.427–0.989, p = 0.044] vs. endocrine gland) were associated with lower risks of cardiac-specific death among patients with non-malignant tumors receiving RT, while older age of diagnosis (OR 1.085, 95% CI: 1.076–1.094, p < 0.001) and earlier year of diagnosis (2004–2007 [OR 12.409, 95% CI: 8.085–19.048, p < 0.001], 2008–2011 [OR 7.42, 95% CI: 4.8–11.472, p < 0.001], 2012–2015 [OR 3.263, 95% CI: 2.053–5.186, p < 0.001] vs. 2016-2019) were risk factors for cardiac-specific death (Table 4, Figure 2).
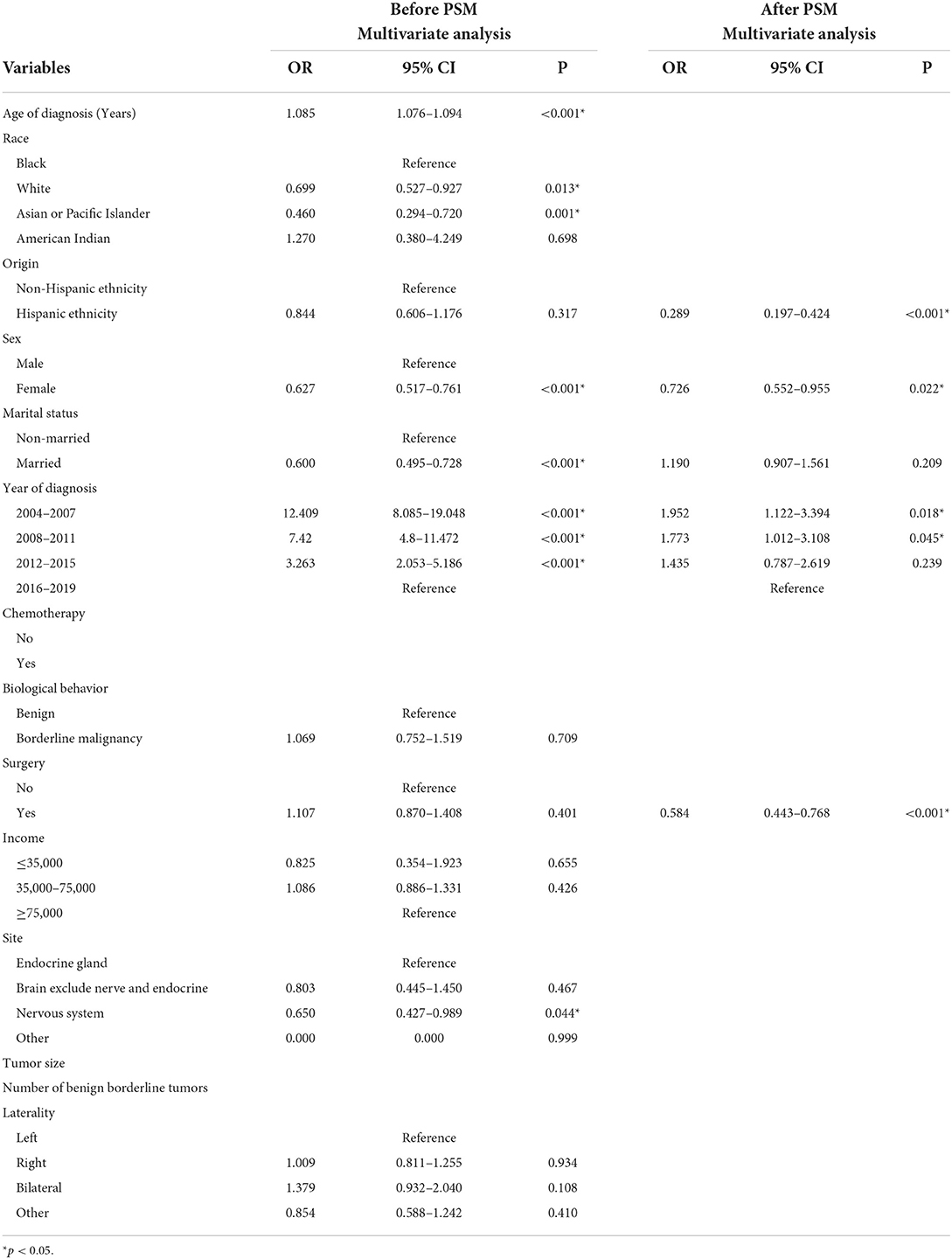
Table 4. Multivariate logistics regression analyses among patients with non-malignant tumors receiving RT.
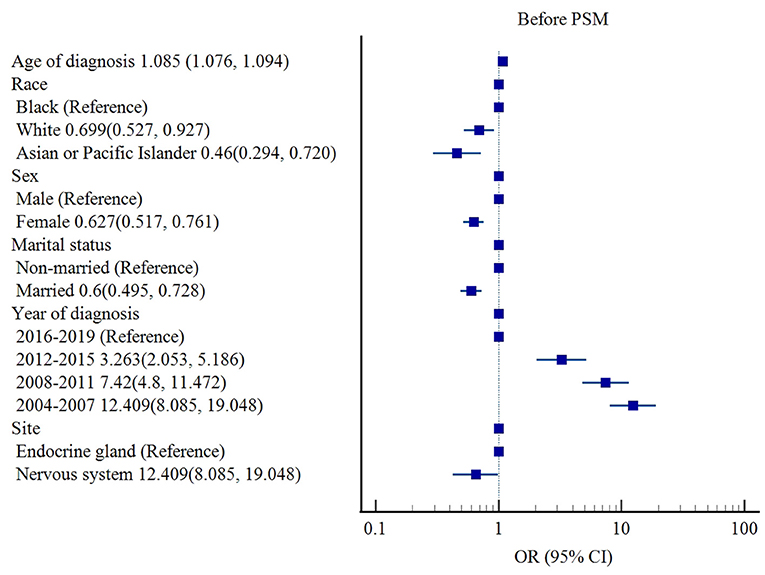
Figure 2. Influencing factors of cardiac-specific death in patients with non-malignant tumors of CNS receiving RT before PSM.
After PSM, origin (Hispanic ethnicity [OR 0.289, 95% CI: 0.197–0.424, p < 0.001] vs. non-Hispanic ethnicity), sex (female [OR 0.726, 95% CI: 0.552–0.955, p = 0.022] vs. male), and surgery (OR 0.584, 95% CI: 0.443–0.768, p < 0.001) were associated with lower risks of cardiac-specific death among patients with non-malignant tumors receiving RT, while earlier year of diagnosis (2004–2007 [OR 1.952, 95% CI: 1.122–3.394, p = 0.018], 2008–2011 [OR 1.773, 95% CI: 1.012–3.108, p = 0.045] vs. 2016–2019) was a risk factor for cardiac-specific death (Table 4, Figure 3).
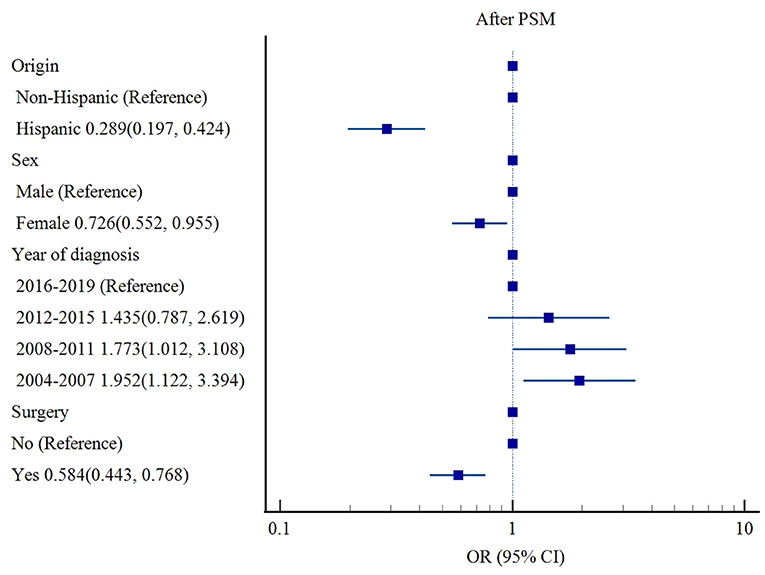
Figure 3. Influencing factors of cardiac-specific death in patients with non-malignant tumors of CNS receiving RT after PSM.
Sensitivity analysis
Clinical features affecting cardiac-specific death in patients with non-malignant tumors of CNS receiving RT were analyzed separately according to the nature of the tumor.
The results showed that among patients with benign tumors, before PSM, race (White [OR 0.715, 95% CI: 0.527–0.97, p = 0.031], Asian or Pacific Islander (OR 0.521, 95% CI: 0.33–0.824, p = 0.005] vs. Black) and sex (female [OR 0.62, 95% CI: 0.506–0.761, p < 0.001] vs. male) were associated with lower risks of cardiac-specific death, while older age of diagnosis (OR 1.089, 95% CI: 1.079–1.098, p < 0.001) and earlier year of diagnosis (2004–2007 [OR 12.695, 95% CI: 7.876–20.464, p < 0.001], 2008–2011 [OR 8.16, 95% CI: 5.03–13.238, p < 0.001], 2012–2015 [OR 3.766, 95% CI: 2.26–6.275, p < 0.001] vs. 2016–2019) were risk factors for cardiac-specific death. After PSM, origin (Hispanic ethnicity [OR 0.285, 95% CI: 0.188–0.43, p < 0.001] vs. non-Hispanic ethnicity), sex (female [OR 0.695, 95% CI: 0.523–0.923, p = 0.012] vs. male), and surgery (OR 0.536, 95% CI: 0.394–0.73, p < 0.001) were associated with lower risks of cardiac-specific death, while earlier year of diagnosis (2004-2007 [OR 2.118, 95% CI: 1.151–3.895, p = 0.016], 2008–2011 [OR 2.125, 95% CI: 1.145–3.943, p = 0.017] vs. 2016–2019) was a risk factor for cardiac-specific death.
Among patients with borderline malignancy tumors, before PSM, older age of diagnosis (OR 1.06, 95% CI: 1.038–1.083, p < 0.001) and earlier year of diagnosis (2004–2007 [OR 13.702, 95% CI: 5.107–36.758, p < 0.001], 2008–2011 [OR 4.419, 95% CI: 1.547–12.622, p = 0.006] vs. 2016–2019) were risk factors for cardiac-specific death. There was no statistically significant influences factor for cardiac-specific death after PSM.
Discussion
Since the clinical benefits of RT for cancer therapy can be outweighed by RT-induced cardiotoxicity, the clinical importance of heart disease from radiation has been recognized by several major radiation oncology societies (14, 15). Meanwhile, it is crucial to explore strategies that can reduce the risk of heart disease from radiation (5). Previous studies have reported greater burden of cardiovascular risk factors and increased incidence of cardiovascular disease in patients with head or neck malignancies after undergoing RT (e.g., heart failure [hazard ratio 1.44, 95% CI: 1.18–1.76, p = 0.0005, coronary artery disease/myocardial infarction [hazard ratio 1.52, 95% CI: 1.03–2.24, p = 0.0357) (16–18). However, above situation may not need to be considered too much in patients receiving RT for non-malignant tumors of CNS, because our findings indicate that RT is associated with lower risk of cardiac-specific death in patients with non-malignant tumors of CNS. Furthermore, the cardiac-specific mortality was lower in patients that receive RT compared to the non-RT patients among patients with non-malignant tumors of CNS (2.8 vs. 3.8%, p < 0.001).
The parameters and outcomes of RT differ considerably between malignant and non-malignant tumors. Firstly, the dose and duration of RT are generally less for non-malignant tumors (19). Secondly, apart from the relatively high incidence of radiation-induced heart failure and a similar number of radiation-induced secondary cancers, primary malignant tumors are prone to post-radiation recurrence that may affect cardiac function through various ways (5). On the other hand, recurrence of non-malignant tumors after radiotherapy is less frequent. Finally, patients with non-malignant tumors rarely undergo chemotherapy, and therefore avoid the potential cardiotoxic effects of drugs. Altogether, the above factors may lead to different additive effects of RT in different nature of tumors. The growth and expansion of benign tumors compress the surrounding organs and constrict blood vessels, which can lead to dysfunction in various organs, including the brain. Natelson proposed the concept of a neuro-cardiological axis in 1985, which is based on the complex interplay between the nervous system of the brain and the cardiovascular system (20–22). For example, the CNS controls cardiovascular function through sympathetic and parasympathetic signals, and lesions in the cranial nervous can impair the structure and function of the cardiovascular system (23), ranging from myocardial damage to even cardiac death (24). Therefore, the cardio protective effect of RT observed in our study can be attributed to the reduction in tumor growth, which in turn minimized the risk of brain dysfunction and the ensuing brain-heart syndrome. Unlike chest irradiation, which elicited more cardiovascular events on the left side than on the right side (25, 26), patients with non-malignant tumors of CNS on the right side of head had no reduction in cardiac-specific death compared to those with tumors on the left side. However, bilateral tumors increased the risk of cardiac deaths. Further study is need to elucidate the mechanisms underlying this phenomenon.
Cancer patients have a consistently higher risk of death due to CVD, compared to the general population, and the risk increases with age (27). Furthermore, younger age at treatment, longer follow-up, and cardiac exposure also increase the risk of fatal cardiovascular diseases (5). In our study, older age of diagnosis and earlier year of diagnosis were risk factors for cardiac-specific death in patients with non-malignant tumors of CNS receiving RT. Furthermore, cardiovascular mortality is usually higher among patients with African ancestry, and lower among the Caucasian, Asian and Hispanic populations (28–31). Consistent with this, we found that Caucasian, Asian or Pacific Islander ethnicities were associated with lower risks of post-radiation cardiac-specific death in patients with non-malignant tumors of CNS receiving RT. According to the European Society of Cardiology, lower socioeconomic status may continue to contribute to increased the rate of cancer and the risk of death from cardiovascular diseases in cancer survivors (32). However, we found that the income level had no significant impact on the incidence of cardiac-specific death in patients with non-malignant tumors of CNS receiving RT. Other protective factors of radiation-induced cardiac-specific death in our cohort were the female (33) and married status. Marital stress, or single/divorced status increased the risk of cardiovascular death (34, 35). However, tumor size, number of benign borderline tumors, tumor site, and the biological behavior of tumor did not significantly affect cardiac-specific death in patients with non-malignant tumors of CNS receiving RT.
To further control for confounding factors and improve comparability between groups for more robust results, we matched the patients' baseline data using PSM (1:1) and then reanalyzed the matched data. Hispanic ethnicity and surgery were associated with lower risks of cardiac-specific death in patients with non-malignant tumors of CNS receiving RT. The differences in these results may be attributed to the smaller subset of patients after PSM, although the data was more comparable and the results more credible. Despite its advantages, PSM cannot fully represent the total population, Therefore, it is necessary to evaluate the pre- and post-PSM results for clinical studies. In addition, sensitivity analysis showed that in patients with benign tumors of CNS receiving RT, clinical features affecting cardiac-specific death were consistent with the overall results. However, in patients with borderline malignancy tumors of CNS, only age of diagnosis and year of diagnosis were influential factors for cardiac-specific death. This may be due to the small number (7.3%) of borderline malignancy tumors, which has a smaller contribution on the overall study results. Therefore, our findings are primarily reflective the situation in benign tumors. Overall, in patients with non-malignant tumors of CNS receiving RT, most factors affecting cardiac death were similar to those in patients with malignancies, except for some differences.
Factors affecting cardiac-specific death in patients with non-malignant tumors of CNS were similar to those in patients with non-malignant tumors of CNS receiving RT, except for married, earlier year of diagnosis, and tumor site (brain exclude nerve and endocrine) were consistently associated with lower risks of cardiac-specific death, lower income and larger tumor were risk factors for cardiac-specific death.
Limitation
Although this is the first study analyzing the additional effect of RT on cardiovascular risks in patients with non-malignant tumors of CNS, it has some limitations that ought to be considered. Firstly, since we used SEER data that has been collated from different sources, the information may have been incomplete and some cardiac deaths may have been classified as unexplained deaths because they are not clear. Secondly, cardiac-specific death. Secondly, cardiac-specific deaths may be associated with other confounding factors that were not studied or not included in the SEER database. Thirdly, as a large number of patients did not experience cardiac-specific death, thus preventing the use of COX proportional hazards mode (censored values over 90%). Finally, the retrospective design of the study invariably led to bias.
Conclusion
RT is associated with lower risk of cardiac-specific death in patients with non-malignant tumors of CNS, which indicates that the effect of radiation on the heart depends on the biological behavior of tumor. Hispanic ethnicity, female, married status, surgery and tumor site (nervous system) were the protective factors, whereas Black, older age of diagnosis and earlier year of diagnosis were risk factors for cardiac-specific death. These results can facilitate the identification of patients with non-malignant tumors of CNS who can benefit from RT while avoiding cardiovascular events. In addition, this study helps to enhance the clinical use of RT in these populations, especially in patients who may have impaired cardiac function due to CNS tumors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RW and HY contributed to the conception and design of the study. LW and XZ provided administrative support. RW and YZ contributed to the collection and assembly of data. LM, YW, and JW did the literature search and applied the inclusion and exclusion criteria. RW, HY, and YZ analyzed and interpreted the data. RW, LW, and XZ contributed to the writing of the report. All authors approved the final version of the report.
Funding
This study was supported by the Talent Introduction Funding Project of The First Affiliated Hospital of Jinan University (No. 808026) and Basic Scientific Research Project of Central University of Jinan University (No. 2162301).
Acknowledgments
Thanks to Prof. Lyu Jun from the Clinical Research Department of The First Affiliated Hospital, Jinan University, Guangzhou, China and thanks to the Guangzhou Key Laboratory of Basic and Translational Research on Chronic Diseases, The First Affiliated Hospital, Jinan University, Guangzhou, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.991621/full#supplementary-material
References
1. Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. (2003) 13:346–56. doi: 10.1016/S1053-4296(03)00026-2
2. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van't Veer MB, Baaijens MHA, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. (2007) 109:1878–86. doi: 10.1182/blood-2006-07-034405
3. de Vries S, Schaapveld M, Janus CPM, Daniëls LA, Petersen EJ, van der Maazen RWM, et al. Long-Term Cause-Specific Mortality in Hodgkin lymphoma patients. J Natl Cancer Inst. (2021) 113:760–9. doi: 10.1093/jnci/djaa194
4. Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. (2007) 33:578–593. doi: 10.1016/j.ctrv.2007.07.011
5. Andratschke N, Maurer J, Molls M, Trott K-R. Late radiation-induced heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol. (2011) 100:160–166. doi: 10.1016/j.radonc.2010.08.010
6. Torres Royo L, Antelo Redondo G, Árquez Pianetta M, Arenas Prat M. Low-Dose radiation therapy for benign pathologies. Rep Pract Oncol Radiother. (2020) 25:250–4. doi: 10.1016/j.rpor.2020.02.004
7. Little MP, Kleinerman RA, Stovall M, Smith SA, Mabuchi K. Analysis of dose response for circulatory disease after radiotherapy for benign disease. Int J Radiat Oncol Biol Phys. (2012) 84:1101–9. doi: 10.1016/j.ijrobp.2012.01.053
8. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: toward strengthening the critical relationship. Am J Surg Pathol. (2016) 40:e94–e102. doi: 10.1097/PAS.0000000000000749
9. Healy MA, Morris AM, Abrahamse P, Ward KC, Kato I, Veenstra CM. The accuracy of chemotherapy ascertainment among colorectal cancer patients in the surveillance, epidemiology, and end results registry program. BMC Cancer. (2018) 18:481. doi: 10.1186/s12885-018-4405-7
10. Wu WT Li YJ, Feng AZ Li L, Huang T, Xu A-D, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. (2021) 8:44. doi: 10.1186/s40779-021-00338-z
11. Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. (2020) 13:57–69. doi: 10.1111/jebm.12373
12. Guan X, Hu H, Chen W, Jiang Z, Liu Z, Zhao Z, et al. Comparison of long-term outcome between hemicolectomy and partial colectomy in the elderly: a large population-based study. Oncotarget. (2017) 8:51076–85. doi: 10.18632/oncotarget.16993
13. Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. (2015) 16:286–96. doi: 10.3348/kjr.2015.16.2.286
14. Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. (2009) 73:980–7. doi: 10.1016/j.ijrobp.2008.11.016
15. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
16. Boulet J, Peña J, Hulten EA, Neilan TG, Dragomir A, Freeman C, et al. Statin Use and risk of vascular events among cancer patients after radiotherapy to the thorax, head, and neck. J Am Heart Assoc. (2019) 8:e005996. doi: 10.1161/JAHA.117.005996
17. Mahmood SS, Nohria A. Cardiovascular complications of cranial and neck radiation. Curr Treat Options Cardiovasc Med. (2016) 18:45. doi: 10.1007/s11936-016-0468-4
18. O'Connor MM, Mayberg MR. Effects of radiation on cerebral vasculature: a review. Neurosurgery. (2000) 46:138–149. doi: 10.1093/neurosurgery/46.1.138
19. McKeown SR, Hatfield P, Prestwich RJ, Shaffer RE, Taylor RE. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol. (2015) 88:20150405. doi: 10.1259/bjr.20150405
20. Natelson BH. Neurocardiology. An interdisciplinary area for the 80s. Arch Neurol. (1985) 42:178–84. doi: 10.1001/archneur.1985.04060020096022
21. Chatterjee S, ECG. changes in subarachnoid haemorrhage: a synopsis. Neth Heart J. (2011) 19:31–4. doi: 10.1007/s12471-010-0049-1
22. Kukla P, Jastrzebski M, Praefort W. J-wave-associated ventricular fibrillation in a patient with a subarachnoid haemorrhage. Europace. (2012) 14:1063–4. doi: 10.1093/europace/eur410
23. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res. (2017) 121:451–68. doi: 10.1161/CIRCRESAHA.117.311170
24. Tahsili-Fahadan P, Geocadin RG. Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res. (2017) 120:559–72. doi: 10.1161/CIRCRESAHA.116.308446
25. Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. (2007) 25:3031–7. doi: 10.1200/JCO.2006.08.6595
26. Boero IJ, Paravati AJ, Triplett DP, Hwang L, Matsuno RK, Gillespie EF, et al. Modern radiation therapy and cardiac outcomes in breast cancer. Int J Radiat Oncol Biol Phys. (2016) 94:700–8. doi: 10.1016/j.ijrobp.2015.12.018
27. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. doi: 10.1093/eurheartj/ehz766
28. Ohman RE, Yang EH, Abel ML. Inequity in Cardio-Oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. J Am Heart Assoc. (2021) 10:e023852. doi: 10.1161/JAHA.121.023852
29. Mo X, Zhou M, Yan H, Chen X, Wang Y. Competing risk analysis of cardiovascular/cerebrovascular death in T1/2 kidney cancer: a SEER database analysis. BMC Cancer. (2021) 21:13. doi: 10.1186/s12885-020-07718-z
30. Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and ethnic disparities in early-stage lung cancer survival. Chest. (2017) 152:587–97. doi: 10.1016/j.chest.2017.03.059
31. Connor AE, Kaur M, Sheng JY, Hayes JH. Racial disparities in mortality outcomes among women diagnosed with breast cancer in Maryland: Impact of cardiovascular disease and clinical characteristics. Cancer. (2022) 128:727–36. doi: 10.1002/cncr.33889
32. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, et al. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. (2020) 41:12–85. doi: 10.1093/eurheartj/ehz859
33. Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. (1984) 5:433–58. doi: 10.1146/annurev.pu.05.050184.002245
34. Orth-Gomér K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. Jama. (2000) 284:3008–14. doi: 10.1001/jama.284.23.3008
Keywords: radiation therapy, non-malignant tumors, benign tumors, borderline tumors, central nervous system, cardiac-specific death
Citation: Wang R, Ye H, Zhao Y, Ma L, Wei J, Wang Y, Zhang X and Wang L (2022) Effect of radiotherapy on cardiac-specific death in patients with non-malignant tumors of central nervous system and related clinical features. Front. Cardiovasc. Med. 9:991621. doi: 10.3389/fcvm.2022.991621
Received: 11 July 2022; Accepted: 22 September 2022;
Published: 06 October 2022.
Edited by:
Rod Skinner, Newcastle University, United KingdomReviewed by:
Nickolas Stabellini, Case Western Reserve University, United StatesPanshak Dakup, Pacific Northwest National Laboratory (DOE), United States
Copyright © 2022 Wang, Ye, Zhao, Ma, Wei, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Wang, bmQ2Njg4NjY4OEBnbWFpbC5jb20=; Xiaofang Zhang, ODU3MTU0NTg2QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ruxin Wang
Ruxin Wang Haowen Ye
Haowen Ye Yongting Zhao
Yongting Zhao Li Ma
Li Ma Jinjing Wei
Jinjing Wei Ying Wang1
Ying Wang1