- 1Peking University Clinical Research Institute, Peking University First Hospital, Beijing, China
- 2Peking University Clinical Research Institute Heart and Vascular Health Research Center at Peking University Shougang Hospital, Beijing, China
- 3Key Laboratory of Molecular Cardiovascular Sciences (Peking University), Ministry of Education, Beijing, China
- 4Peking University Third Hospital, Beijing, China
- 5Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Objective: Several clinical trials have indicated that statins stabilize and reverse atherosclerotic plaque. However, different studies have provided inconsistent findings regarding mechanisms and influencing factors of plaque regression under statin therapy. Apart from lipid-lowering effect, statins have pleiotropic effects including anti inflammation in humans. In this study, meta-analysis and meta-regression were used to determine the effects of statin medications on coronary plaque volume. Meanwhile, to assess whether statins promote plaque regression effect was related to their anti-inflammatory ability, the impact of CRP/hsCRP reduction during statin therapy on plaque regression was investigated.
Methods: Up to June 15, 2022, a systematic PubMed, EMBASE, and Cochrane search was performed for randomized controlled trials that assessed treatment effect using total atheroma volume (TAV), percent atheroma volume (PAV), or plaque volume (PV). Only CRP/hsCRP and LDL-C values reported before and after treatment were considered.
Results: 12 studies (2,812 patients with heart and/or vascular disease) fulfilled the inclusion criteria and were included in the systematic review. A meta-analysis of 15 statin-treated arms reported a significant reduction in change of TAV/PV [standardized mean difference (SMD): –0.27, 95% confidence intervals (–CI): –0.42, –0.12, p < 0.001], compared with the control arms. Another meta-analysis of 7 trials also found that patients in the intervention group had a significant reduction in change of PAV (SMD: -0.16, 95% CI: –0.29, –0.03, p = 0.019), compared with those in the control group. Meta-regressionanalysis revealed that the percent change of CRP/hsCRP was significantly associated with SMD in change of TAV/PV after adjusting for percent change of LDL-C, age, gender and study duration. Meta-regression analysis showed that percent change of CRP/hsCRP statistically influenced SMD in change of PAV, when percent change of CRP/hsCRP was included separately. However, the percent change of CRP/hsCRP was not significantly associated with SMD of PAV change after adjusting for all covariates.
Conclusion: In conclusion, statin therapy is beneficial for plaque regression. Statins promote plaque regression, which might be associated to their anti-inflammatory ability.
Introduction
Cardiovascular diseases are considered the leading causes of death worldwide. Among them, coronary heart disease (CHD) has garnered considerable attention due to its high prevalence and burden. The pathological basis of CHD is atherosclerosis, which is characterized by the accumulation of lipids and cholesterol in the artery’s subintima and progressive chronic inflammation of the fibrotic plaque on the wall of great and medium arteries (1). Assessment of coronary artery plaques provides clinical information regarding the progression of disease and the risk of experiencing future adverse cardiovascular events (2). In recent studies, indicators including total atheroma volume (TAV), percent atheroma volume (PAV), or plaque volume (PV) have been widely used to assess plaque burden (3).
Coronary plaque regression has a significant positive correlation with low density lipoprotein cholesterol (LDL-C). As important lipid-lowering drugs, several studies have demonstrated that statin drugs promote coronary atheroma stabilization and regression in patients with acute coronary events or stable coronary disease (4). Among those studies, recent clinical studies have demonstrated that statins can reduce plaque burden by demonstrating a reduction in TAV, PAV, and PV (5). Currently, statins are widely used to prevent atherosclerotic cardiovascular disease (ASCVD). Numerous studies have shown that statins are effective in reducing LDL-C, and the risk of death and recurrent coronary and cardiovascular events in those with a history of ASCVD (6). Meanwhile, statin therapy is a first-line treatment for the primary prevention of ASCVD in patients with elevated low-density lipoprotein cholesterol levels (≥ 190 mg/dL), those with diabetes mellitus, those who are 40–75 years of age, and those determined to be at sufficient ASCVD risk after a clinician–patient risk discussion (7).
As the mechanism of vascular inflammation is gradually elucidated, numerous evidences have demonstrated that C-reactive protein (CRP) and high-sensitivity C-reactive protein (hsCRP) may play direct pathogenic roles in atherosclerosis (8, 9). Initially, statin drugs were used primarily to reduce blood lipids. With the deepening of research, its non-lipid-lowering effects, such as the anti-inflammatory effect of statins on the coronary plaque volume, have become the focus of recent studies. Ridker et al. discovered that rosuvastatin (20 mg/d) and placebo were administered to randomly selected healthy people with elevated hs-CRP but no evidence of hyperlipidemia. After an average follow-up of 1.9 years, the hs-CRP level in the treatment group decreased by 37% compared with that in the control group, implying that statins may have anti-atherosclerosis functions via anti-inflammatory mechanisms (10). Numerous clinical trials, such as the Air Force/Texas Coronary Atherosclerosis Prevention (AFCAPS/TexCAPS) study, the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial, and the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22 (PROVE IT-TIMI 22) trial, have demonstrated that statins reduce hsCRP levels independently of lowering LDL-C levels. In a trial with canakinumab for atherosclerotic disease, the rate of cardiovascular event recurrence was significantly lower in the treated group than in the placebo group, implying that reducing inflammation without affecting lipid levels can reduce cardiovascular disease risk (11).
Statin therapy was shown to be beneficial in reducing CRP/hsCRP. However, few studies have attempted to investigate the relationship between the degree of CRP/hsCRP reduction associated with changes in coronary plaque burden during statins treatment. To answer the question of whether the CRP/hsCRP lowering effect of statins could delay or reverse the progression of atherosclerosis, we conducted this study. The aim of the present study was to provide a systematic review and meta-regression analysis to examine the impact of statins on CRP/hsCRP reduction on coronary plaque burden assessed with TAV, PAV, and PV. At the same time, we analyzed the joint effects of LDL-C and CRP/hsCRP changes on plaques.
Methods
This work followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and amendments to the Quality of Reporting of Meta-analyses (QUOROM) statement (12, 13).
Search strategy and study selection
For this meta-analysis, we conducted a search in PubMed, EMBASE and the Cochrane Library to identify studies relevant to this topic from their inception to June 15, 2022. The study selection was performed independently by 2-group investigators (CLL, YJM as group 1, and RH, DJ as group 2) using highly sensitive strategy. Disagreements were resolved by consensus with a senior author (WXX). Here we show the search strategy of PubMed: “[(statin) OR (hydroxy-methyl-glutaryl-CoA) OR (HMG-COA) OR (pravastatin) OR (lovastatin) OR (simvastatin) OR (Atorvastatin) OR (fluvastatin) OR (Rosuvastatin) OR (Pitavastatin)] AND [(intravascular ultrasound) OR (IVUS) OR (plaque) OR (atheroma)] AND [(intravascular ultrasound) OR (IVUS) OR (coronary)] AND (Clinical Trial[ptyp]).” Supplementary Table 1 shows details of the search syntax.
Selection criteria
Studies were included according to the following criteria: (a) randomized controlled trials (RCTs); (b) investigating the impact of statin therapy on plaque volume using IVUS; (c) reporting at least one of the following data: TAV, PV, and PAV; (d) with a follow-up longer than or equal to 6 months; (e) reporting LDL-C at baseline and the end of the study or reporting data of percent change of LDL-C; (d) reporting CRP or hsCRP before and after statin treatment (or percent change of CRP/hs-CRP).
Exclusion criteria included the following: (a) duplicate publication or secondary analyses of the same study population; (b) lack of sufficient information on baseline or follow-up IVUS data, LDL-C data, and CRP/hsCRP data.
Data extraction quality appraisal
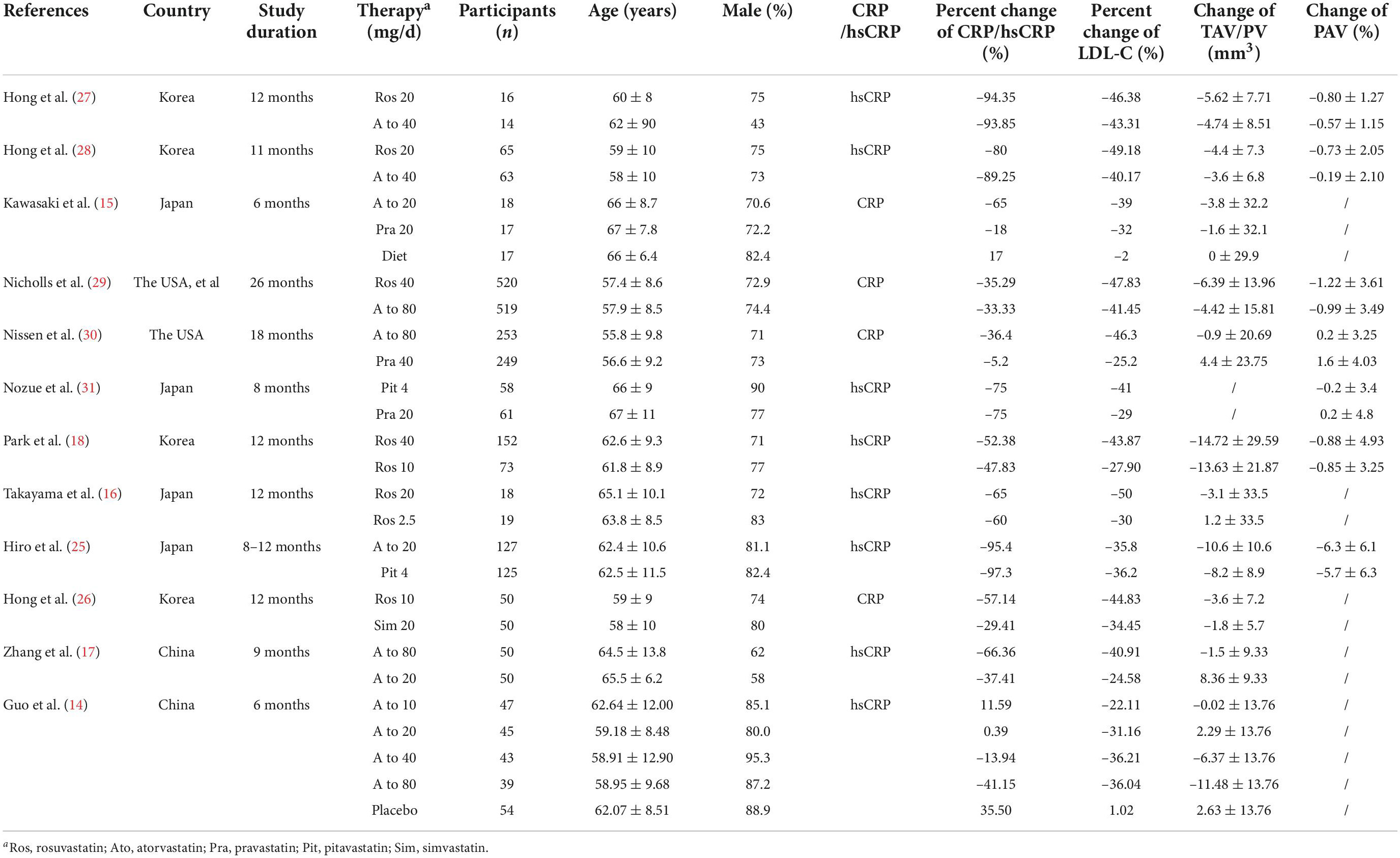
The data were extracted from each study using standard tables. The extracted data included the following: study characteristics (the first author, title, publication time, number of patients, country, and study duration), patient characteristics (age and sex), intervention, control, method characteristics (randomization, blind implementation, and follow-up loss), and patient outcomes. For patient outcomes, we extracted TAV, PAV, or PV data as measured using IVUS technique, LDL-C data, CRP, hsCRP data (including values at baseline and endpoint) and other useful information.
After data extraction, we conducted statistical analysis to calculate change of TAV, change of PV, change of PAV, percent change of LDL-C, percent change of CRP, and percent change of hsCRP. Articles reported mean values and standard deviation (SD) of change of TAV/PV/PAV, the original number was entered. Some studies (14–17) did not report SD values, which were filled by using the SD of the baseline data of the control group. 1 study (18) provided standard error (SE) rather than SD, and then SD value was calculated based on SE value. If the IVUS efficacy endpoints were reported as medians, with distribution-free 95% confidence intervals (CI), the median reported in the original text was extracted, and SD was calculated by formula.
In terms of LDL-C, if the article reported percent change of LDL-C, the original number was entered; otherwise, percent change of LDL-C was calculated using the following formula:
Percent change of CRP and percent change of hsCRP were calculated using the same approach. Supplementary Table 2 shows details of data extraction.
Two independent authors (RH and DRG) assessed the risk of bias in each included study. According to Cochrane’s indications, un-blinded, independent reviewers evaluated the quality of included studies using pre-specified forms (risk of bias table), including seven examined fields: random sequence generation (selection bias); allocation sequence concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and other potential sources of bias.
Data analysis and synthesis
Continuous variables were expressed as mean ± SD, whereas categorical variables were expressed as n (%). Heterogeneity among individual studies was assessed with the Q-test and quantified with the I2 statistic (range: 0–100%). I2 represents the proportion of the total variance that can be attributed to heterogeneity of true study effects (19). The heterogeneity was regarded as low if I2 ≤ 25%, as moderate if I2 in the range of 26–74% and as high if I2 ≥ 75% (20). When a study is gathered from the published literature, the random-effects model is generally a more plausible match. For the random-effects model allows the true effect size may vary from study to study. In addition, the standard error of the summary effect and the confidence intervals for the summary effect are wider under the random-effects model than under the fixed-effect model (21). Thus, we performed meta-analysis to pool estimates using random effects model. Meta-analysis with continuous outcome variables was performed, and the effect of statin therapy (vs. control) on change of TAV, PV, and PAV at the end of follow-up was estimated as standardized mean difference (SMD) and 95% CI. If p < 0.05 and the 95% CI did not include zero, the point estimate of SMD was considered statistically significant. To avoid double-counting of subjects and consequent unit-of-analysis error in trials with more than one treatment arm, the control group was evenly divided (where possible) (10). Since the units (mm3) of change of TAV and change of PV were the same, we combined these two indicators for data synthesis.
To explore the link between the dependent variable and the covariate, meta-regression is often used. We hypothesized that the included studies may have shown differences according to the percent change of CRP/hsCRP, percent change of LDL-C, age, gender and study duration of the patients. To evaluate the possible impact of these factors on the results of the meta-analysis, we established model with the change of TAV/PV or change of PAV as the dependent variable. In particular, change in TAV/PV was our primary outcome, and change in PAV was the secondary outcome.
Funnel plot analysis and Begg’s and Egger’s tests were performed to evaluate potential publication bias. Sensitivity analysis was conducted to assess the stability of studies. Sensitivity analysis was conducted using leave-one-out method, i.e., removing one study each time and repeating the analysis. Statistical analyses were carried out using meta packages in R version 4.1.2 (2021-11-01) and risk of bias was evaluated with Review Manager (RevMan 5.3; Cochrane Collaboration).
Result
Flowchart of included studies
The initial literature search retrieved 1,313 articles. After the removal of duplicates, the titles and abstracts of 805 articles were carefully checked, leading to the exclusion of 666 articles for failing to meet the inclusion criteria. Initially, 139 articles were selected, and their full texts were evaluated. Of them, 124 articles were excluded: 22 because CRP/hsCRP levels were not reported, 12 because plaque evaluation (TAV, PAV, or PV) was not performed, 50 because they were not RCTs, 31 because statins were not used, and 9 because of repeated trials. A total of 15 articles entered the third round of evaluation. One was excluded due to a discrepancy between the number of participants receiving statins and the number of people participating in IVUS measurements (22). And two were excluded because of data quality: in one study, CRP was reported, but the indicators of the control group declined significantly (23); in another study, the SD at baseline and follow-up varied greatly and the reported difference value was inconsistent with the calculated difference value (24). Overall, this analysis included 12 trials (14–18, 25–31) Figure 1 summarizes the study selection process.

Figure 1. Flowchart for study. RCTs, randomized controlled trials; IVUS, intravenous ultrasound; CRP, C-reactive protein; hsCRP, high-sensitivity C-reactive protein; TAV, total atheroma volume; PAV, percent atheroma volume; PV, plaque volume.
Characteristics of included studies
The study characteristics are reported in Table 1. A total of 2,812 subjects were included in the 12 eligible studies. Included studies were published between 2004 and 2016 and were reported from China, the USA, Korea and Japan. The largest study had a population size of 1,039 subjects while the smallest study recruited 30 subjects. The mean age of the participants ranged from 55.8 to 67.0 years.
12 trials with 16 treatment arms were included. 8 treatment arms used atorvastatin (dose range: 10–80 mg/day; duration of treatment: 24–72 weeks), 6 treatment arms used rosuvastatin (dose range: 10–40 mg/day; duration of treatment: 44–104 weeks), 1 treatment arm used pravastatin (dose: 20 mg/day; duration of treatment: 24 weeks), and 1 treatment arm used pitavastatin (dose: 4 mg/day; duration of treatment: 32 weeks).
IVUS was used in all studies to evaluate plaque volume. In addition to 1 study (24) 11 studies reported change of TAV/PV, and 7 studies reported change of PAV. As described in the data extraction section, percent change of CRP/hsCRP and percent change of LDL-C were reported in all studies.
Overall, random sequence generation was observed in 6 studies, 4 of them reported allocation concealment. 3 trials were double-blinded, and 8 studies performed blinded assessments of the outcomes. Moreover, 2 studies existed incomplete outcome data because of a high attrition rate. Supplementary Figure 1 shows details of the risk of bias assessment.
Effect of statin therapy on change of TAV/PV
11 trials (n = 2,696) including 15 comparisons reported change of TAV/PV. Compared with control arms, our meta-analysis showed that 15 treatment arms revealed a significant decrease in change of TAV/PV (SMD: –0.27, 95% CI: –0.42, –0.12, p < 0.001), with a moderate heterogeneity (Q = 27.55, df = 17, p = 0.02, I2 = 49.2%). Figure 2 presents the combined results of the 15 head-to-head comparisons in this meta-analysis.
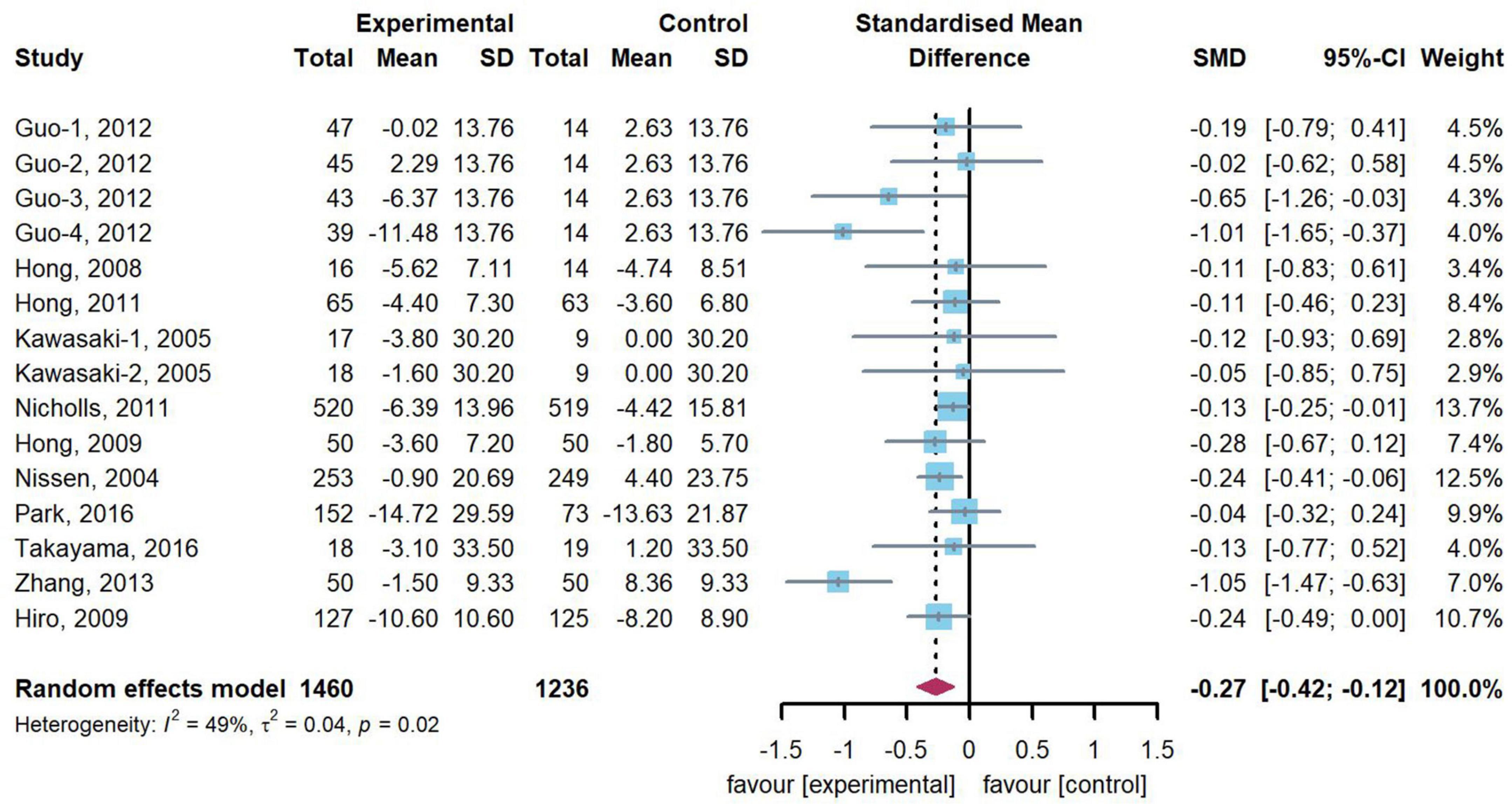
Figure 2. Forest plot of change of TAV/PV. A meta-analysis of 15 statin-treated arms reported a significant reduction in change of TAV/PV [standardized mean difference (SMD): –0.27, 95% confidence intervals (CI): –0.42, –0.12], compared with the control arms.
Effect of statin therapy on change of percent atheroma volume
7 studies (n = 2,295) reported change of PAV. Heterogeneity test of data from 7 studies shown moderate heterogeneity (Q = 10.19, df = 6, p = 0.12, I2 = 41.1%) and random effect model was adopted. Compared with those in the control group, this meta-analysis indicated that patients in the intervention group have a significant reduction in change of PAV (SMD: –0.16, 95% CI: –0.29, –0.03, p = 0.019). Figure 3 presents the combined results of 7 studies in this meta-analysis.

Figure 3. Forest plot of change of PAV. A meta-analysis of 7 studies reported a significant reduction in change of PAV [standardized mean difference (SMD): –0.16, 95% confidence intervals (CI): –0.29, –0.03], compared with the control.
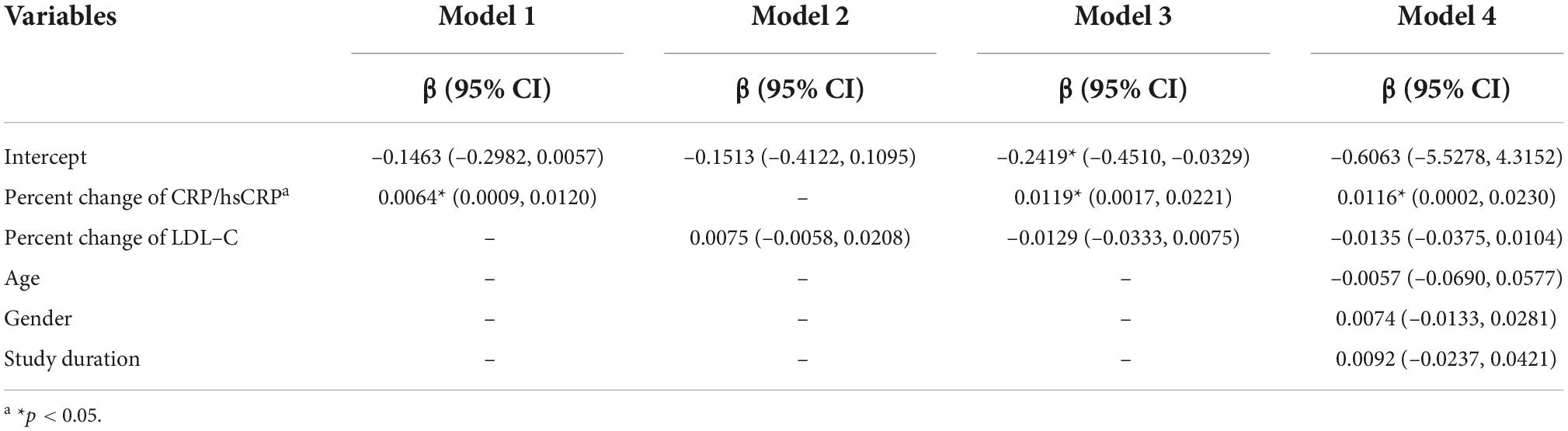
Meta-regression for standardized mean difference in change of TAV/PV
Meta-regression was then employed to test whether the percent change of CRP/hsCRP was associated with the change of TAV/PV. The results of the meta-regression analysis are given in Table 2. Model 1 demonstrates that the impact of percent change of CRP/hsCRP on change of TAV/PV was statistically significant (p = 0.024). The regression coefficient of this independent variable was β = 0.0064 (95% CI: 0.0009–0.0120). Model 2 analyzed the influence of percent change of LDL-C on change of TAV/PV. The results showed that percent change of LDL-C had no significant effect on change of TAV/PV (p = 0.268). Model 3 incorporates percent changes of CRP/hsCRP and LDL-C. Only percent change of CRP/hsCRP was associated with change of TAV/PV (β = 0.0119, 95% CI: 0.0017–0.0221, p = 0.022). In Model 4, we entered percent change of CRP/hsCRP, percent change of LDL-C, age, gender and study duration. Among them, only percent change of CRP/hsCRP statistically influenced the dependent variable (p = 0.046).
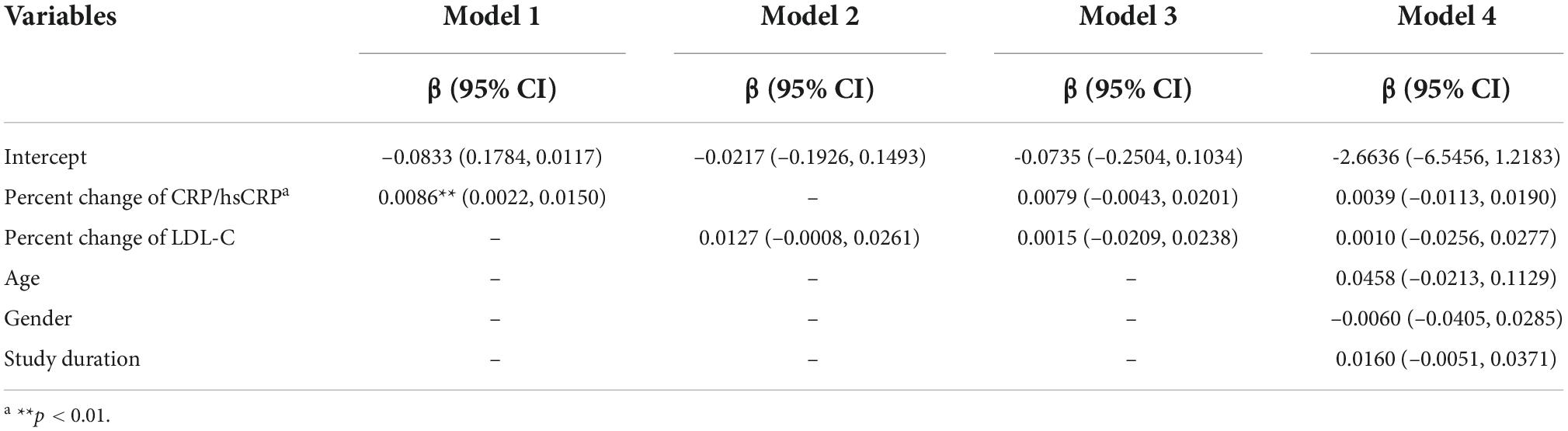
Meta-regression for standardized mean difference in change of percent atheroma volume
Similarly, we performed another meta-regression to explore how the percent change of CRP/hsCRP affects change of PAV. The results of the meta-regression analysis are given in Table 3. Model 1 used the percent change of CRP/hsCRP as an independent variable. The results indicated that the percent change of CRP/hsCRP (β = 0.0086, 95% CI: 0.0022–0.0150) affects PAV change (p = 0.009). When the percent change of CRP/hsCRP was higher, change of PAV was greater. Model 2 shows that the percent change of LDL-C was not significantly associated with PAV change (p = 0.066). In Model 3 (both percent change of CRP/hsCRP and percent change of LDL-C were included as independent variables) and Model 4 (independent variables including percent change of CRP/hsCRP, percent change of LDL-C, age, gender and study duration), multivariable meta-regression analyses did not reveal any significance between independent variables and the change of PAV.
Publication bias and sensitivity analysis
Although Begg’s rank correlation (p = 0.7290) and Egger’s linear regression (p = 0.2323) tests were not significant, the funnel plot was asymmetric, implying potential publication bias in reporting the effect of statin therapy on change of TAV/PV. Regarding the impact of statin therapy on change of PAV, the number of studies was insufficient to conduct Begg’s test and Egger’s tests. However, the funnel plot also indicated potential publication bias. Funnel plots are presented in Supplementary Figures 2, 3.
Sensitivity analysis by excluding one study each time confirmed that the pooled estimate was consistent among studies with balanced weight. Additional sensitivity analyses are presented in Supplementary Figures 4, 5.
Discussion
This meta-analysis comprised RCTs using IVUS to measure coronary plaque burden and reporting results of TAV, PAV, or PV changes. The present meta-analysis demonstrated that (1) quantitative synthesis revealed a decrease in TAV/PV and PAV levels after statin treatment compared with the control. All studies included in the meta-analysis were RCTs, further confirming that statins are effective drugs for reducing the volume of atherosclerotic plaque in coronary arteries; (2) Meta-regressions showed that the percent change of CRP/hsCRP reduction was associated with a significant reduction in change of TAV/PV after statin therapy. After adjusting for percent change of LDL-C, age, gender and study duration, this association still existed. These findings indicate that the reduction in CRP/hsCRP levels might play an important role in the beneficial effects of statins on the progression of the atherosclerotic plaque. To the best of our knowledge, this study firstly investigated the association between CRP/hsCRP change and atherosclerotic plaque reduction using meta-regressions analyses.
Statins are HMG-COA reductase inhibitors. They reduce CHD incidence due to their lipid-regulating and extra-lipid-regulating effects and are important drugs for the primary and secondary prevention of CHD (32, 33). The benefits of statins have been demonstrated to be based on stabilization and/or reversal of atherosclerotic plaque (34–37). Particularly since the introduction of IVUS technology, numerous studies have used it as an important tool for studying coronary plaque. IVUS has recently become the main tool to study the effects of statins on coronary atherosclerotic plaque, and the data obtained by IVUS served as the primary endpoint in several studies (38, 39).
Recent studies suggest that LDL-C accumulates abnormally in the vascular wall due to endothelial cell dysfunction. In addition, LDL-C can be converted into oxidized low-density lipoprotein cholesterol (oxLDL-C), eventually promoting plaque progression (40). This implies that LDL-C change is a potential factor affecting plaque regression. A post hoc analysis found that statin therapy was associated with regression of coronary atherosclerosis when LDL-C was substantially reduced and high density lipoprotein cholesterol was increased by more than 7.5% (41). As a result, we separately included percent change of LDL-C as an independent variable to establish a simple linear regression model, and the results showed that LDL-C change did not influence the result. Moreover, when the percent change of CRP/hsCRP, percent change of LDL-C, age, gender and study duration were simultaneously taken as independent variables to establish the regression model, only the percent change of CRP/hsCRP had a significant impact on TAV/PV. These results indicated that in the included RCTs studies using statins as intervention drugs, the ability of statins to reduce TAV/PV is probably affected by their effect of reducing CRP/hsCRP instead of reducing LDL-C. The greater the reduction in CRP/hsCRP from baseline after statin treatment, the greater the reduction in TAV/PV. After adjusting for covariates (percent change of LDL-C, age, gender, and study duration), this association still existed. A previous study that analyzed the effect of pitavastatin treatment on changes of plaque volume had similar findings to our study. It demonstrated that TAV and PAV decreased more significantly in patients with reduction in hs-CRP ≥ 1 mg/dl than in those with reduction in hs-CRP < 1 mg/dl (42).
Various factors influence the degree of plaque regression under statin therapy. For instance, the statin drug type (43), plaque composition (44), and patient’s age and gender (45). In addition, clinical trials using IVUS demonstrated a linear relationship between LDL-C levels and reductions in atheroma burden under statin treatment (46). Despite the well-established causal role of LDL-C in the pathogenesis of atherosclerosis, our findings do not seem to support a reduction in TAV/PV relying on LDL-C levels. Recent investigations have demonstrated that changes in LDL-C levels are unrelated to plaque progression/regression following ezetimibe treatment (47). This is consistent with our research conclusions. However, the percent change of CRP/hsCRP was not significantly associated with SMD in change of PAV after adjusting for the percent change of LDL-C, age, gender and study duration. This could be because only seven trials were included in the regression analysis. The instability of research outcomes is caused by insufficient research data and an excessive number of independent variables.
It has previously been shown that anti-inflammatory therapy alone is beneficial for plaque regression (48). Considering the pleiotropic nature of statins, CRP/hsCRP is an important indicator of the anti-inflammatory effect of statins. Our findings imply that statins promote plaque regression, which is associated with their anti-inflammatory ability. And the effect of plaque regression may not be affected by their ability to regulate LDL-C.
At present, the main mechanisms of plaque formation include vascular endothelial dysfunction, intimal hyperplasia, lipid accumulation, and inflammatory response. Arterial inflammation plays an important role in the initiation and progression of atherosclerosis. Consistent with growing evidence that atherosclerosis is an inflammatory condition and many inflammatory cells, especially macrophages and foam cells can produce a variety of cytokines that may stimulate the hepatic expression of the CRP gene and up-regulate CRP production in the liver (49, 50). Therefore, elevated CRP, elevated hsCRP and changes of some other inflammatory markers may be potentially related to the risk of atherosclerosis development (50, 51). It is thought that the roles of CRP in the development of atherosclerotic plaque are complicated (52). Recent evidence propose that CRP and type oxidized LDL-C after being converted into foam cells stimulate tissue factor before thrombus formation, endothelial cell expression of adhesion molecules, and vascular endothelial dysfunction, all of which contribute to unstable atherosclerotic plaque (53, 54). In addition, several studies have suggested that atherosclerotic plaques also express CRP, and induce macrophage activation (55). Simultaneously, the expression and release of inflammatory factors are regulated to accelerate atherosclerotic plaque formation (56). Other studies also found that smooth muscle cells of atherosclerotic lesions could produce CRP and the locally produced CRP could participate in atherogenesis and the development of cardiovascular complications directly (50, 57). These associations between CRP and atherosclerosis suggest that inhibition of CRP may represent a therapeutic modality for the treatment of cardiovascular disease (49).
In addition to their cholesterol-lowering effects, recent clinical trials have established that the advantages of statins are based on their pleiotropic properties, such as reducing inflammation, stabilizing plaque, improving vascular endothelial function, suppressing vascular smooth muscle proliferation, and so on (58). And the ability to reduce inflammatory markers such as CRP and hsCRP is also included (59). Statins block CRP production by a variety of mechanisms (60). On the one hand, statins suppress CRP production by reducing IL-6, which is involved in stimulating CRP production by liver cells. On the other hand, statins reduce the production of inflammatory mediators from atherosclerotic plaques due to the decrease in LDL-C and consequently oxLDL-C (59, 61). Moreover, a direct interaction between statin molecules and CRP was found in silico evidence (62). Clinical trials also tried to confirm that the effects of statins on lowering CRP/hsCRP levels were beneficial to the prognosis of coronary plaque volume. For instance, an intervention trial evaluating rosuvastatin revealed that rosuvastatin reduced hs-CRP levels by 37% and hs-CRP are indicators of successful treatment with statins (63).
Despite the large body of evidence associating CRP with atherosclerotic lesions in previous studies, there is a lack of a direct correlation between its concentration and the extension of atherosclerosis as determined by imaging techniques (8). Our study indicates that the anti-inflammatory effects of statins may have a positive effect on atherosclerotic plaque regression as measured by the IVUS technique. This result suggests that CRP/hsCRP may be a potential therapeutic target in the process of atherosclerosis during statin therapy. Therefore, future research should continue to further study the effect of statin therapy on anti-inflammatory, including reducing serum CRP/hsCRP levels directly.
This study also has some limitations. First of all, we only searched 3 commonly used databases. It is possible that some studies in other databases and gray literature are overlooked. However, given that PubMed, EMBASE, and the Cochrane library are three most common databases used for meta-analysis and systematic review, our results should be a representative sample (64–66). Second, although the studies included in this meta-analysis were all RCTs and the quality of evidence was relatively higher, not all studies were double-blind trials. It is possible that performance bias is introduced. The meta-regression analysis (SMD in change of PAV as the dependent variable) was performed with 7 trials, which might lead to insufficient statistical power. In addition, this research adopted aggregate study-level data rather than individual-patient-level data. Individual-patient-level data may reflect the actual allocation plan of the subjects and improve the accuracy and integrity of the data. If future research could establish regression model based on individual-patient-level data to analyze the relationship between CRP/hsCRP levels and plaque regression, our research results could be further verified.
Conclusion
In conclusion, our mete-analysis indicated that statins could significantly reduce plaque load measured by TAV/PV and PAV. Further meta-regression revealed that the percent change of CRP/hsCRP was significantly associated with the reduction in plaque volume. However, the percent change of LDL-C was not significantly associated with TAV/PV change or PAV change. Our results support that CRP/hsCRP decrease is crucial in the reduction of TAV/PV during statin treatment. Statins could promote plaque regression through their anti-inflammatory ability and that their ability to reduce plaque volume might be unaffected by their ability to reduce LDL-C. This finding will provide new avenues for future research on plaque regression.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
RH, YM, and WX conceived and designed the study. RH, DJ, YM, CL, and SW performed the statistical analysis. DG, RH, and WX drafted and revised the manuscript. WX and QM were responsible for the integrity of the work as a whole. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81974490) and the 2019 Irma and Paul Milstein Program for Senior Health Research Project Award.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.989527/full#supplementary-material
References
1. McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Progress Lipid Res. (2011) 50:331–47. doi: 10.1016/j.plipres.2011.04.002
2. Han D, Berman DS, Miller RJH, Andreini D, Budoff MJ, Cademartiri F, et al. Association of cardiovascular disease risk factor burden with progression of coronary atherosclerosis assessed by serial coronary computed tomographic angiography. JAMA Netw Open. (2020) 3:e2011444. doi: 10.1001/jamanetworkopen.2020.11444
3. Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, et al. Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis. (2020) 306:85–95. doi: 10.1016/j.atherosclerosis.2020.05.017
4. Masson W, Lobo M, Siniawski D, Molinero G, Masson G, Huerin M, et al. Role of non-statin lipid-lowering therapy in coronary atherosclerosis regression: a meta-analysis and meta-regression. Lipids Health Dis. (2020) 19:111. doi: 10.1186/s12944-020-01297-5
5. van Rosendael AR, van den Hoogen IJ, Gianni U, Ma X, Tantawy SW, Bax AM, et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol. (2021) 6:1257–66.
6. Ngo-Metzger Q, Zuvekas S, Shafer P, Tracer H, Borsky AE, Bierman AS. Statin use in the U.S. for secondary prevention of cardiovascular disease remains suboptimal. J Am Board Fam Med. (2019) 32:807–17. doi: 10.3122/jabfm.2019.06.180313
7. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2019) 140:e563–95. doi: 10.1161/CIR.0000000000000724
8. Salazar J, Martínez MS, Chávez M, Toledo A, Añez R, Torres Y, et al. C-reactive protein: clinical and epidemiological perspectives. Cardiol Res Pract. (2014) 2014:605810.
9. Sukegawa H, Maekawa Y, Yuasa S, Anzai A, Kodaira M, Takei M, et al. Intensive statin therapy stabilizes C-reactive protein, but not chemokine in stable coronary artery disease treated with an everolimus-eluting stent. Coron Artery Dis. (2016) 27:405–11. doi: 10.1097/MCA.0000000000000375
10. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. (2008) 359:2195–207. doi: 10.1056/NEJMoa0807646
11. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31.
12. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. quality of reporting of meta-analyses. Lancet. (1999) 354:1896–900. doi: 10.1016/S0140-6736(99)04149-5
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
14. Guo S, Wang R, Yang Z, Li K, Wang Q. Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques. Exp Ther Med. (2012) 4:1069–74. doi: 10.3892/etm.2012.722
15. Kawasaki M, Sano K, Okubo M, Yokoyama H, Ito Y, Murata I, et al. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol. (2005) 45:1946–53. doi: 10.1016/j.jacc.2004.09.081
16. Takayama T, Komatsu S, Ueda Y, Fukushima S, Hiro T, Hirayama A, et al. Comparison of the Effect of Rosuvastatin 2.5 mg vs 20 mg on Coronary Plaque Determined by Angioscopy and Intravascular Ultrasound in Japanese With Stable Angina Pectoris (from the Aggressive Lipid-Lowering Treatment Approach Using Intensive Rosuvastatin for Vulnerable Coronary Artery Plaque [ALTAIR] Randomized Trial). Am J Cardiol. (2016) 117:1206–12. doi: 10.1016/j.amjcard.2016.01.013
17. Zhang X, Wang H, Liu S, Gong P, Lin J, Lu J, et al. Intensive-dose atorvastatin regimen halts progression of atherosclerotic plaques in new-onset unstable angina with borderline vulnerable plaque lesions. J Cardiovasc Pharmacol Ther. (2013) 18:119–25. doi: 10.1177/1074248412465792
18. Park SJ, Kang SJ, Ahn JM, Chang M, Yun SC, Roh JH, et al. Effect of statin treatment on modifying plaque composition: a double-blind, randomized study. J Am Coll Cardiol. (2016) 67:1772–83. doi: 10.1016/j.jacc.2016.02.014br
19. Langan D. Assessing heterogeneity in random-effects meta-analysis. Methods Mol Biol. (2022) 2345:67–89. doi: 10.1007/978-1-0716-1566-9_4
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60.
21. Borenstein M, Hedges LV, Higgins JPT, Rothstein HRA. basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
22. Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. (2004) 110:1061–8. doi: 10.1161/01.CIR.0000140261.58966.A4
23. Lee SW, Hau WK, Kong SL, Chan KK, Chan PH, Lam SC, et al. Virtual histology findings and effects of varying doses of atorvastatin on coronary plaque volume and composition in statin-naive patients: the VENUS study. Circ J. (2012) 76:2662–72. doi: 10.1253/circj.cj-12-0325
24. Matsushita K, Hibi K, Komura N, Akiyama E, Maejima N, Iwahashi N, et al. Effects of 4 statins on regression of coronary plaque in acute coronary syndrome. Circ J. (2016) 80:1634–43. doi: 10.1253/circj.CJ-15-1379
25. Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. (2009) 54:293–302.
26. Hong MK, Park DW, Lee CW, Lee SW, Kim YH, Kang DH, et al. Effects of statin treatments on coronary plaques assessed by volumetric virtual histology intravascular ultrasound analysis. JACC Cardiovasc Interv. (2009) 2:679–88. doi: 10.1016/j.jcin.2009.03.015
27. Hong YJ, Jeong MH, Chung JW, Sim DS, Cho JS, Yoon NS, et al. The effects of rosuvastatin on plaque regression in patients who have a mild to moderate degree of coronary stenosis with vulnerable plaque. Korean Circ J. (2008) 38:366-73. doi: 10.4070/kcj.2008.38.7.366
28. Hong YJ, Jeong MH, Hachinohe D, Ahmed K, Choi YH, Cho SH, et al. Comparison of effects of rosuvastatin and atorvastatin on plaque regression in Korean patients with untreated intermediate coronary stenosis. Circ J. (2011) 75:398–406. doi: 10.1253/circj.cj-10-0658
29. Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. (2011) 365:2078–87. doi: 10.14341/2071-8713-5304
30. Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. (2004) 291:1071–80. doi: 10.1001/jama.291.9.1071
31. Nozue T, Yamamoto S, Tohyama S, Umezawa S, Kunishima T, Sato A, et al. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. (2012) 163:191–9.e1. doi: 10.1016/j.ahj.2011.11.004
32. LIPID Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. (2002) 359:1379–87. doi: 10.1016/S0140-6736(02)08351-4
33. Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. (2004) 5:81–7. doi: 10.1016/j.atherosclerosissup.2004.08.027
34. Böse D, von Birgelen C, Erbel R. Intravascular ultrasound for the evaluation of therapies targeting coronary atherosclerosis. J Am Coll Cardiol. (2007) 49:925–32. doi: 10.1016/j.jacc.2006.08.067
35. Endo H, Dohi T, Miyauchi K, Kuramitsu S, Kato Y, Okai I, et al. Clinical significance of non-culprit plaque regression following acute coronary syndrome: a serial intravascular ultrasound study. J Cardiol. (2019) 74:102–8. doi: 10.1016/j.jjcc.2018.12.023
36. Jensen LO, Thayssen P, Pedersen KE, Stender S, Haghfelt T. Regression of coronary atherosclerosis by simvastatin: a serial intravascular ultrasound study. Circulation. (2004) 110:265–70. doi: 10.1161/01.CIR.0000135215.75876.41
37. Yamada T, Azuma A, Sasaki S, Sawada T, Matsubara H. Randomized evaluation of atorvastatin in patients with coronary heart disease: a serial intravascular ultrasound study. Circ J. (2007) 71:1845–50. doi: 10.1253/circj.71.1845
38. Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK, et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med. (2015) 13:229. doi: 10.1186/s12916-015-0459-4
39. D’Ascenzo F, Agostoni P, Abbate A, Castagno D, Lipinski MJ, Vetrovec GW, et al. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: a meta-regression of randomized clinical trials. Atherosclerosis. (2013) 226:178–85. doi: 10.1016/j.atherosclerosis.2012.10.065
40. Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. (2003) 107:2342–7. doi: 10.1161/01.CIR.0000066691.52789.BE
41. Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA-J Am Med Assoc. (2007) 297:499–508. doi: 10.1001/jama.297.5.499
42. Hong YJ, Jeong MH, Ahn Y, Kim SW, Bae JH, Hur SH, et al. Effect of pitavastatin treatment on changes of plaque volume and composition according to the reduction of high-sensitivity C-reactive protein levels. J Cardiol. (2012) 60:277–82. doi: 10.1016/j.jjcc.2012.04.003
43. Qian C, Wei B, Ding J, Wu H, Cai X, Li B, et al. Meta-analysis comparing the effects of rosuvastatin versus atorvastatin on regression of coronary atherosclerotic plaques. Am J Cardiol. (2015) 116:1521–6. doi: 10.1016/j.amjcard.2015.08.010
44. Kwon O, Kang SJ, Kang SH, Lee PH, Yun SC, Ahn JM, et al. Relationship between serum inflammatory marker levels and the dynamic changes in coronary plaque characteristics after statin therapy. Circ Cardiovasc Imaging. (2017) 10:e005934. doi: 10.1161/CIRCIMAGING.116.005934
45. Dai J, Hou J, Xing L, Jia H, Hu S, Soeda T, et al. Is age an important factor for vascular response to statin therapy? A serial optical coherence tomography and intravascular ultrasound study. Coron Artery Dis. (2017) 28:209–17.
46. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA. (2016) 316:2373–84. doi: 10.1001/jama.2016.16951
47. Spence JD, Solo K. Resistant atherosclerosis: the need for monitoring of plaque burden. Stroke. (2017) 48:1624–9. doi: 10.1161/STROKEAHA.117.017392
48. Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. (2012) 126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556
49. Kolke T, Sun HJ, Ichikawa T, Kitajima S, Hatakeyama K, Asada Y, et al. C-reactive protein in atherosclerotic lesions: Its origin and pathophysiological significance. Arterioscl Thromb Vasc Biol. (2005) 25:E63–E. doi: 10.1016/S0002-9440(10)61202-3
50. Zhuang Q, Shen C, Chen Y, Zhao X, Wei P, Sun J, et al. Association of high sensitive C-reactive protein with coronary heart disease: a Mendelian randomization study. BMC Med Genetics. (2019) 20:170. doi: 10.1186/s12881-019-0910-z
51. Badimon L, Pena E, Arderiu G, Padro T, Slevin M, Vilahur G, et al. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front Immunol. (2018) 9:430. doi: 10.3389/fimmu.2018.00430
52. Kandelouei T, Abbasifard M, Imani D, Aslani S, Razi B, Fasihi M, et al. Effect of statins on serum level of hs-CRP and CRP in Patients with Cardiovascular Diseases: a systematic review and meta-analysis of randomized controlled trials. Med Inflamm. (2022) 2022:8732360. doi: 10.1155/2022/8732360
53. Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, Rahimi HR, Tajfard M, Ferns G. Serum C-reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol. (2018) 233:8508–25. doi: 10.1002/jcp.26791
54. Cicci JD, Iyer P, Clarke MM, Mazzella AJ. Aspirin for the primary prevention of cardiovascular disease: a review of the literature and considerations for clinical practice. Cardiol Rev. (2020) 28:98–106.
55. Paffen E, deMaat MPM. C-reactive protein in atherosclerosis: a causal factor? Cardiovasc Res. (2006) 71:30–9.
56. Kones R. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. a comprehensive survey. Drug Design Dev Therapy. (2011) 5:325–80. doi: 10.2147/DDDT.S14934
57. Jabs WJ, Theissing E, Nitschke M, Bechtel JFM, Duchrow M, Mohamed S, et al. Local generation of C-reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation. (2003) 108:1428–31. doi: 10.1161/01.CIR.0000092184.43176.91
58. Dupuis J, Tardif JC, Rouleau JL, Ricci J, Arnold M, Lonn E, et al. Intensity of lipid lowering with statins and brachial artery vascular endothelium reactivity after acute coronary syndromes (from the BRAVER trial). Am J Cardiol. (2005) 96:1207–13. doi: 10.1016/j.amjcard.2005.06.057
59. Arabi SM, Chambari M, Malek-Ahmadi M, Bahrami LS, Hadi V, Rizzo M, et al. The effect of statin therapy in combination with ezetimibe on circulating C-reactive protein levels: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. (2022) 30:1597–615. doi: 10.1007/s10787-022-01053-4
60. Endres M. Statins: potential new indications in inflammatory conditions. Atheroscler Suppl. (2006) 7:31–5.
61. Carlos Arevalo-Lorido J. Clinical relevance for lowering C-reactive protein with statins. Ann Med. (2016) 48:516–24. doi: 10.1080/07853890.2016.1197413
62. Shakour N, Ruscica M, Hadizadeh F, Cirtori C, Banach M, Jamialahmadi T, et al. Statins and C-reactive protein: in silico evidence on direct interaction. Arch Med Sci. (2020) 16:1432–9.
63. Kozlowski B, Narkiewicz K. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. Kardiol Polska. (2009) 67:344–7.
64. Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimers Dement. (2018) 14:1450–9.
65. Wen X, Luo J, Mai Y, Li Y, Cao Y, Li Z, et al. Placebo response to oral administration in osteoarthritis clinical trials and its associated factors: a model-based meta-analysis. JAMA Netw Open. (2022) 5:e2235060. doi: 10.1001/jamanetworkopen.2022.35060
Keywords: statins, regression of atherosclerosis, C-reactive protein, randomized controlled trial, meta-analysis
Citation: Gao D, Hua R, Jiesisibieke D, Ma Y, Li C, Wu S, Ma Q and Xie W (2022) C-reactive protein and coronary atheroma regression following statin therapy: A meta-regression of randomized controlled trials. Front. Cardiovasc. Med. 9:989527. doi: 10.3389/fcvm.2022.989527
Received: 08 July 2022; Accepted: 24 October 2022;
Published: 11 November 2022.
Edited by:
Pietro Scicchitano, ASLBari—Azienda Sanitaria Localedella provincia di Bari (ASL BA), ItalyReviewed by:
Peter Penson, Liverpool John Moores University, United KingdomMallikarjuna Korivi, Zhejiang Normal University, China
Copyright © 2022 Gao, Hua, Jiesisibieke, Ma, Li, Wu, Ma and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Ma, ZnNtYXFpYW5AMTYzLmNvbQ==; Wuxiang Xie, eGlld3V4aWFuZ0Boc2MucGt1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Darui Gao
Darui Gao Rong Hua
Rong Hua Dina Jiesisibieke
Dina Jiesisibieke Yanjun Ma
Yanjun Ma Chenglong Li
Chenglong Li Sijing Wu5
Sijing Wu5 Qian Ma
Qian Ma Wuxiang Xie
Wuxiang Xie