
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 September 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.989012
 Kyu-Yong Ko†
Kyu-Yong Ko† Ji-Hun Jang†
Ji-Hun Jang† Seong-Huan Choi
Seong-Huan Choi Yong-Soo Baek
Yong-Soo Baek Sung Woo Kwon
Sung Woo Kwon Sang-Don Park
Sang-Don Park Seong-Ill Woo
Seong-Ill Woo Dae-Hyeok Kim
Dae-Hyeok Kim Sung-Hee Shin*
Sung-Hee Shin*Background: Left atrial (LA) remodeling is associated with adverse cardiovascular events, including heart failure (HF) and stroke in patients with atrial fibrillation (AF). However, there are limited data on the value of right atrial (RA) remodeling in this population. We investigated the prognostic role of RA enlargement in patients with non-valvular AF.
Methods and results: We analyzed 254 consecutive patients (age = 69 ± 12years, male:female = 165:89, mean left ventricular ejection fraction = 58.0 ± 7.2%) with non-valvular AF who underwent two-dimensional echocardiography from a single center. RA and LA volumes were measured from apical views and indexed to the body surface areas (right atrial volume index [RAVI] and left atrial volume index [LAVI]) and RAVI > 30mL/m2 and LAVI > 34mL/m2 were considered as enlarged. The relationship between RA enlargement and composite clinical outcome of hospitalization for HF (HHF), stroke, systemic embolism, or death from any cause was assessed. Right atrial (RA) enlargement was associated with older age and more frequent prevalence of persistent or permanent AF. During a median follow-up of 47.1 months, 77 patients (30%) had experienced primary composite outcome. In a multivariable model, RA enlargement, but not LA enlargement, was independently associated with adverse clinical outcomes even after adjusting for clinical and echocardiographic factors {adjusted hazard ratio [HR], 1.90 [95% confidence interval (CI), 1.14–3.18], p = 0.014 for primary composite outcome; adjusted HR, 2.70 [95% CI, 1.27–5.67], p = 0.001 for HHF or all cause death}.
Conclusion: RA enlargement was independently associated with an increased risk of HF, stroke, systemic embolization or death in patients with non-valvular AF, suggesting that RA volume can be helpful in assessing future cardiovascular risk in this population.
Atrial fibrillation (AF) is one of the most common arrhythmias. It affects approximately 6% of the population older than 65 years, and it is related to increased cardiovascular (CV) mortality and morbidity including heart failure (HF) and stroke (1–3).
It is common to have an enlarged left atrial (LA) size in patients with AF. While AF can increase LA size, LA structural change can also be related to AF development or recurrence after cardioversion or catheter ablation (4–6). Furthermore, impaired LA contraction in AF can result in stasis of blood and increase thromboembolic risk including stroke (7–9). The importance of LA remodeling has been well known in patients with various CV diseases (10–12). Although several studies have previously demonstrated that increased RA volume is a predictor of poor prognosis and low functional status in patients with HF, data are still limited on the value of RA remodeling in patients with AF (13, 14). In this study, we assessed whether RA volume was related to clinical outcomes in patients with non-valvular AF.
We retrospectively enrolled 582 consecutive patients with AF who performed two-dimensional (2D)-echocardiography from May 2016 to February 2018 in our single institution and were followed up regularly. Patients were excluded if they had significant intrinsic valvular heart disease which is more than moderate degree on echocardiography, chronic obstructive pulmonary disease, malignancy, cardiac tumors, pheochromocytoma, severe renal failure requiring dialysis, cardiomyopathies, congenital cardiac abnormalities, rhythm disturbance requiring permanent pacemakers, left ventricular (LV) ejection fraction (EF) less than 45%, regional wall motion abnormalities, a poor acoustic window for adequate RA and LA assessment by echocardiography, or lost to follow-up.
AF was diagnosed by 12-lead electrocardiography (ECG) or 24-hour Holter monitoring. Paroxysmal AF and persistent AF were defined as an episode of self-terminating AF that lasts less than 7 days and longer than 7 days, respectively. Permanent AF is defined as AF that has been accepted by patients and physicians and has no further attempts to restore or maintain sinus rhythm (15–17).
In initially enrolled patients, 135 patients were excluded because of cardiomyopathies, LVEF <45%, intrinsic valvular disease, congenital heart disease, implanted pacemaker, or cardiac tumor. An additional 50 subjects were excluded due to pulmonary disease, severe renal failure, pheochromocytoma or malignancy. Among the remaining 397 patients, there was insufficient image quality for RA volume measurement, including substantial foreshortening in 121 patients and 22 patients had missing clinical data. Finally, 254 patients (mean age 69 ± 12 years, male:female = 165:89, mean LVEF = 58.0 ± 7.2%) were included in our analysis (Figure 1).
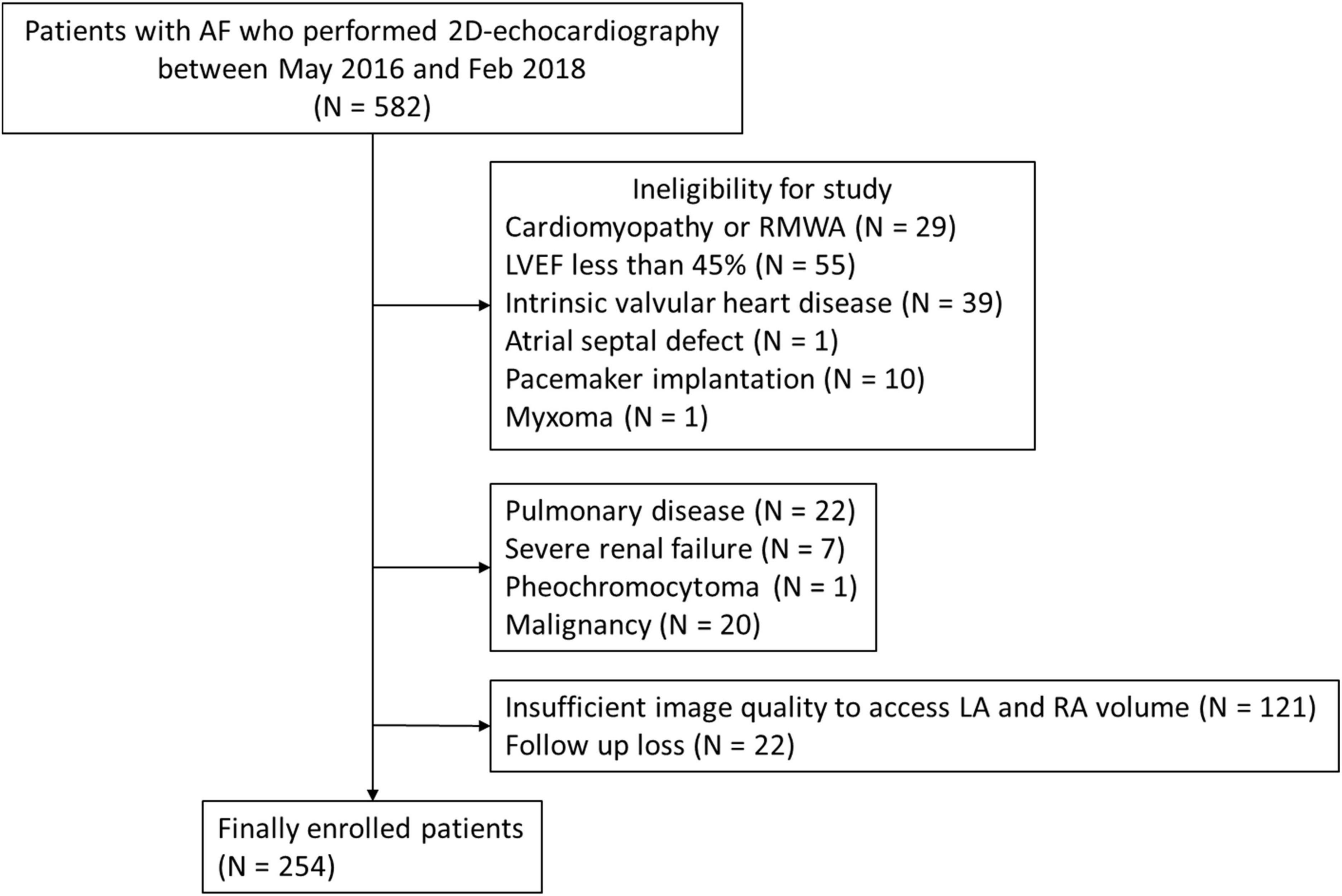
Figure 1. Study flow chart. AF, atrial fibrillation; LA, left atrium; LVEF, left ventricular ejection fraction; RA, right atrium; RWMA, regional wall motion abnormality.
This study design was approved by the Institutional Review Board (INHAUH2022-01-034-000) and was conducted in compliance with the ethical principles outlined in the Declaration of Helsinki.
Standard echocardiographic parameters were measured in accordance with the American Society of Echocardiography guidelines (18). Standard 2D, M-mode, and Doppler images were acquired from parasternal, apical, and subcostal views. The measurements were averaged over 5 cardiac cycles. LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were measured and LVEF was calculated using the modified Simpson’s method from apical four-chamber and two-chamber views. LV mass was calculated using the linear method from M-mode images. LA volume was measured from apical four and two-chamber views and RA volume was measured in apical four-chamber view at the end of LV systole by using the disks summation technique. LV mass, LA volume, and RA volume were indexed for body size by dividing by the body surface area. RA volume index (RAVI) > 30mL/m2 and LA volume index (LAVI) > 34mL/m2 were considered as enlarged, based on the European Association of Cardiovascular Imaging guideline (19). Early mitral inflow (E) velocity, deceleration time of the E wave, and septal mitral annular velocity (e’) were obtained from tissue Doppler imaging. Systolic pulmonary arterial pressure (SPAP) was calculated from the tricuspid regurgitation (TR) jet velocity and estimated RA pressure.
Clinical factors including age, gender, height, weight, history of HF, hypertension, diabetes mellitus, cerebral vascular events, rhythm control strategy, and type of AF were assessed. The patients were followed up regularly every 1–3 months, sometimes every 6 months, as determined by their physicians with a median follow-up of 47.1 [18.3–57.8] months. We evaluated the relationship of RA enlargement to clinical outcome. Primary clinical outcome included composite of hospitalization for HF (HHF), stroke, systemic embolism, or death from any causes. We additionally assessed the association between RA enlargement and a composite of HHF or all cause death. HHF was defined as the presentation of typical signs and symptoms requiring intravenous diuretics use and overnight hospitalization. Stroke was defined as sudden onset of neurologic deficit with new cerebral infarction on the brain magnetic resonance imaging. Systemic embolism was defined as acute vascular occlusion of an extremity or organ as documented by computed tomography.
Continuous variables are described as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as number and percentages. Student t-test or Mann–Whitney U test was used for continuous variables, and the Chi-squared test or Fisher’s exact test was used for categorical variables to compare the baseline characteristics between the groups. The Kaplan-Meier analysis and log-rank test were used to compare event-free survival rates between the two groups, which were divided based on the RAVI. Cox proportional hazard model was additionally performed to assess the relationship between RA enlargement and clinical outcome. The proportional hazards assumption was tested for all analyses. The multivariable models adjusted for demographic and clinical covariates including age, gender, body mass index (BMI), diabetes, hypertension, type of AF, LA enlargement and LVEF, which were significantly related to clinical outcome or clinically relevant. To avoid overfitting, additional adjustment was made for LV mass index (LVMI), E/e’, SPAP and TR. We used spline regression model to assess the continuous relationship of RAVI with clinical outcomes. To clarify the effect of RA enlargement on clinical events independent of LA enlargement, we additionally performed a sensitivity analysis for the relationship between RA enlargement and clinical outcomes in patients with LA enlargement.
For all analyses, a 2-sided p ≤ 0.05 was considered statistically significant. R statistical software (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria) was used for the analysis.
Table 1 showed the baseline characteristics and echocardiographic parameters of the study population with and without RA enlargement. Patients with enlarged RA were older (72 ± 11 years vs. 67 ± 13 years, p < 0.001), had lower BMI (23.7 ± 3.3kg/m2 vs. 25.1 ± 3.2kg/m2, p < 0.001), and had persistent or permanent AF more frequently (89.5% vs. 57.9%, p < 0.001) than those without RA enlargement. In addition, RA enlargement was associated with greater LV mass and LA volume, higher SPAP, and more significant TR (108.1 ± 21.9 g/m2 vs. 100.6 ± 22.3 g/m2, p = 0.002 for LVMI; 69.1 ± 23.2 mL/m2 vs. 44.7 ± 18.9 mL/m2, p < 0.001 for LAVI; 35.3 ± 11.4 mmHg vs. 29.5 ± 7.2 mmHg, p < 0.001 for SPAP; 17.3% vs. 2.8%, p = 0.006 for moderate or severe TR). Prescription rate of anticoagulants tended to be higher in patients with RA enlargement, but it was not statistically significant. The proportion of patients with a history of HF hospitalization was only 3.5%, which was comparable between the groups.
During a median follow-up of 47.1 months, 77 patients (30%) experienced primary composite endpoint. 37 patients (15%) were hospitalized for HF, 41 patients (16%) were hospitalized for stroke, 6 patients (2%) had systemic embolization, and death occurred in 6 patients (2%).
Kaplan-Meier curve showed that the higher estimated event rate in patients with RA enlargement than those without RA enlargement (Figure 2, Log rank p < 0.001). In univariate Cox proportional hazard analysis, age, gender, BMI, hypertension, diabetes, type of AF, and rhythm control strategy were associated with composite outcome (Table 2). Regarding echocardiographic parameters, RA enlargement, LVMI, E/e’, pulmonary hypertension, and moderate or severe TR were related to clinical events {Table 2, Hazard Ratio (HR), 2.34 [95% Confidence interval (CI), 1.48–3.70], p < 0.001 for primary composite outcome of RA enlargement; HR, 2.75 [95% CI, 1.44–5.24], p = 0.002 for HHF or all cause death of RA enlargement}. After adjusting for clinical and echocardiographic parameters, RA enlargement and LVEF were independently associated with adverse clinical events (Figure 3 and Tables 3, 4, and, adjusted HR, 1.90 [95% CI, 1.14–3.18], p = 0.014 for primary composite outcome of RA enlargement; adjusted HR, 2.70 [95% CI, 1.27–5.67], p = 0.001 for HHF or all cause death of RA enlargement). The association of RA enlargement with primary composite outcome remained significant after additional adjusting for LVMI, E/e’, pulmonary hypertension, or TR. In contrast, LA enlargement was not significantly associated with adverse events.
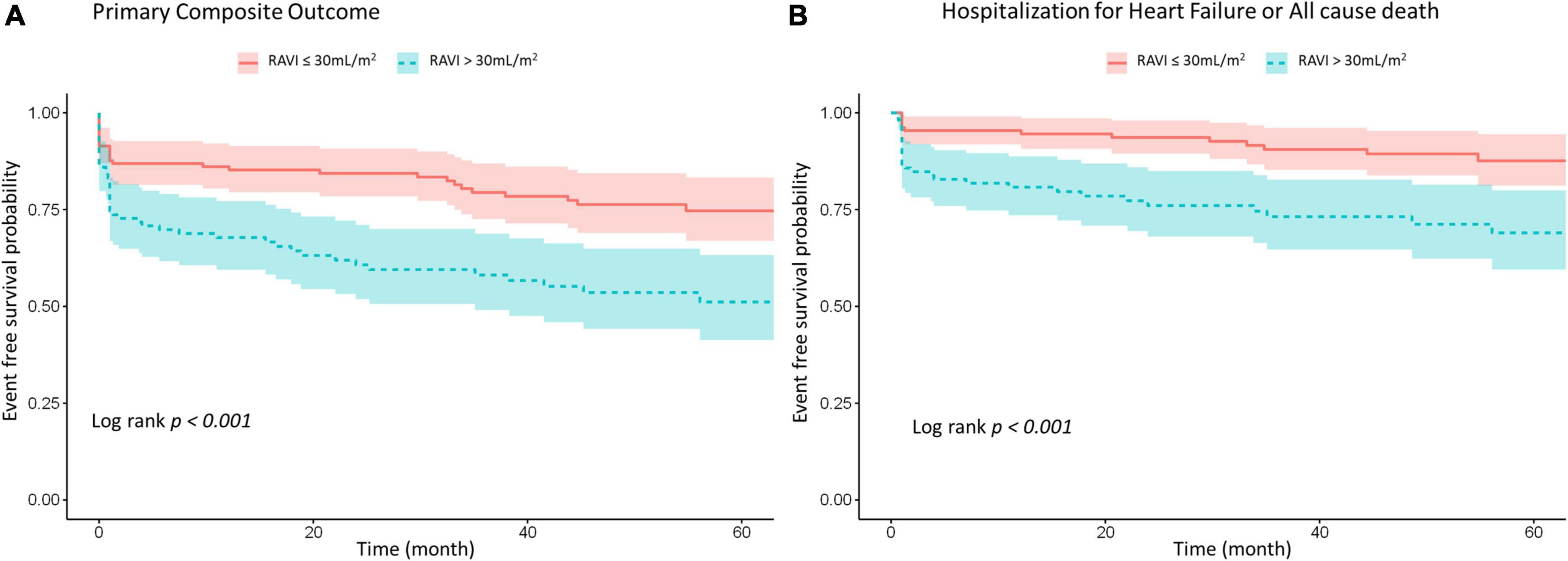
Figure 2. Kaplan–Meier survival analysis for (A) primary composite outcome of HHF, stroke, systemic embolism or all cause death and (B) composite of HHF or all cause death according to RA size. HHF, hospitalization for heart failure; RA, right atrium; RAVI, right atrial volume index.
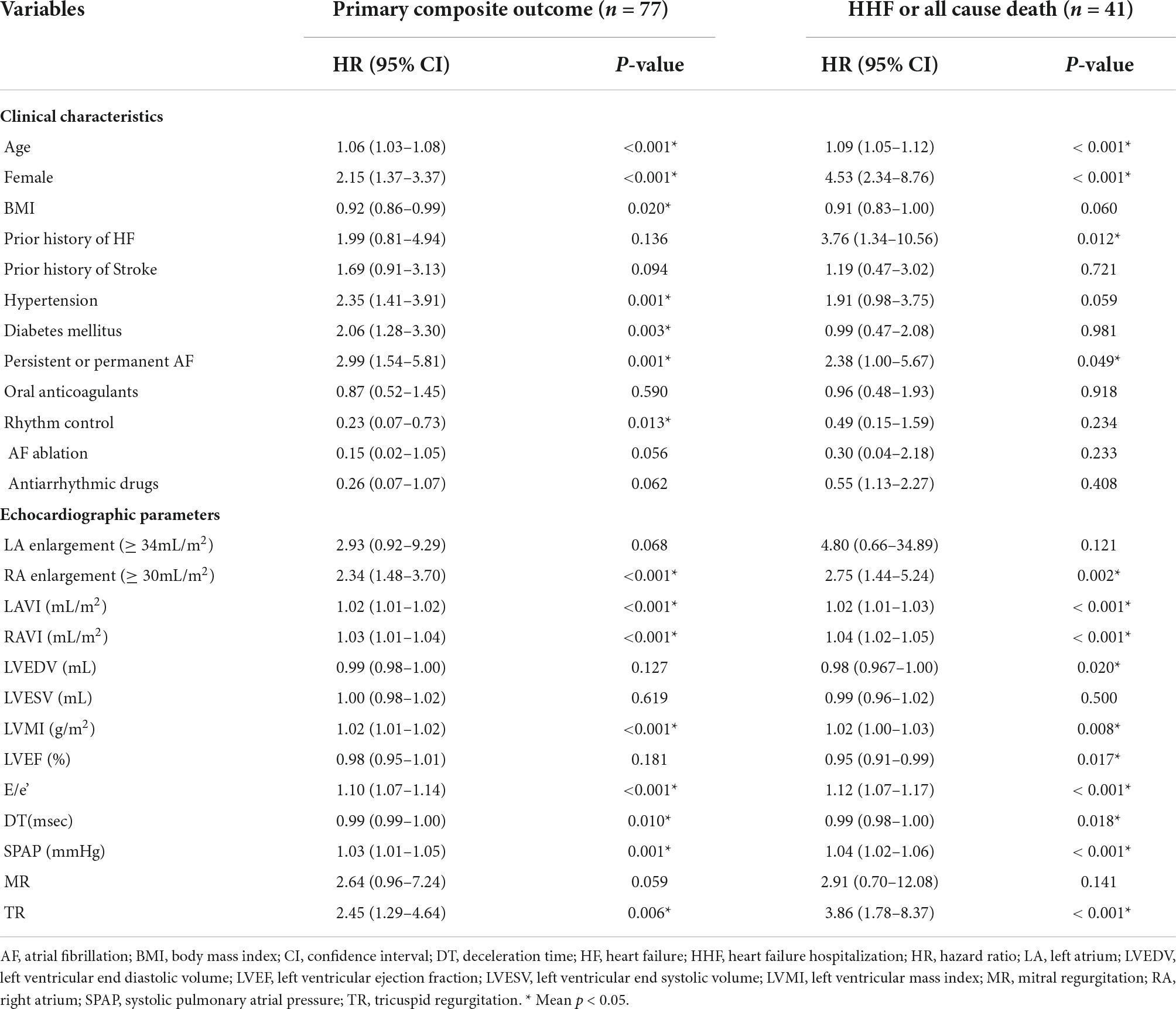
Table 2. Univariate Cox proportional hazard analysis for primary composite outcomes of HHF, stroke, systemic embolism or all cause death, and composite end-point of HHF or all cause death.
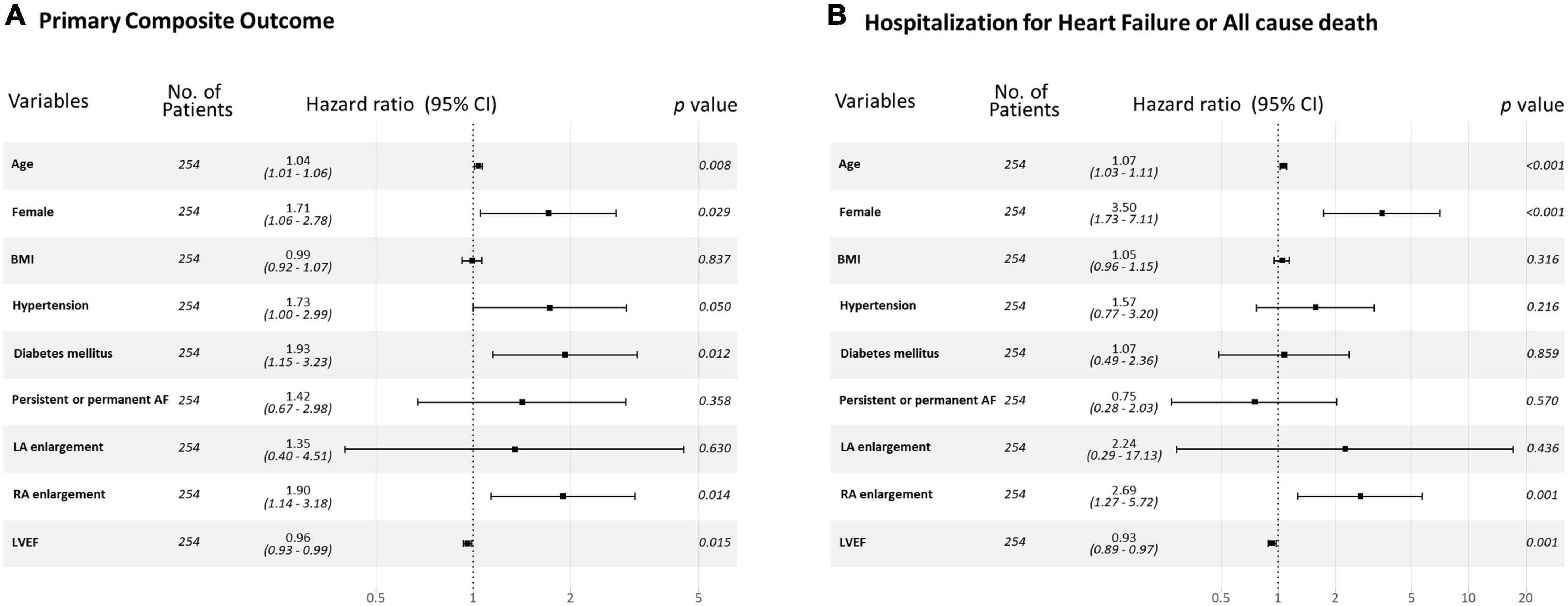
Figure 3. Forest plot for (A) primary composite outcome and (B) a composite of HHF or all cause death for each variable. HHF, hospitalization for heart failure.
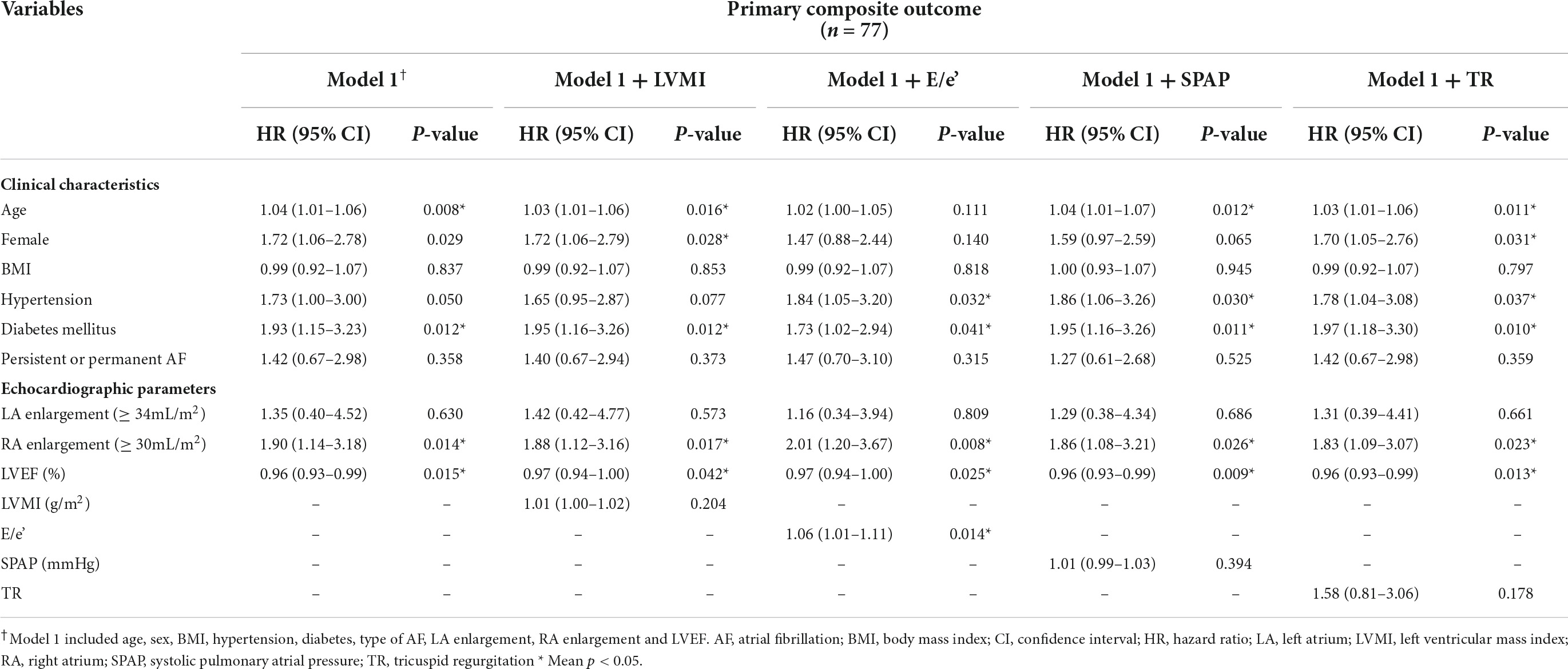
Table 3. Multivariate Cox proportional hazard analysis for primary composite outcomes of HHF, stroke, systemic embolism or all cause death.
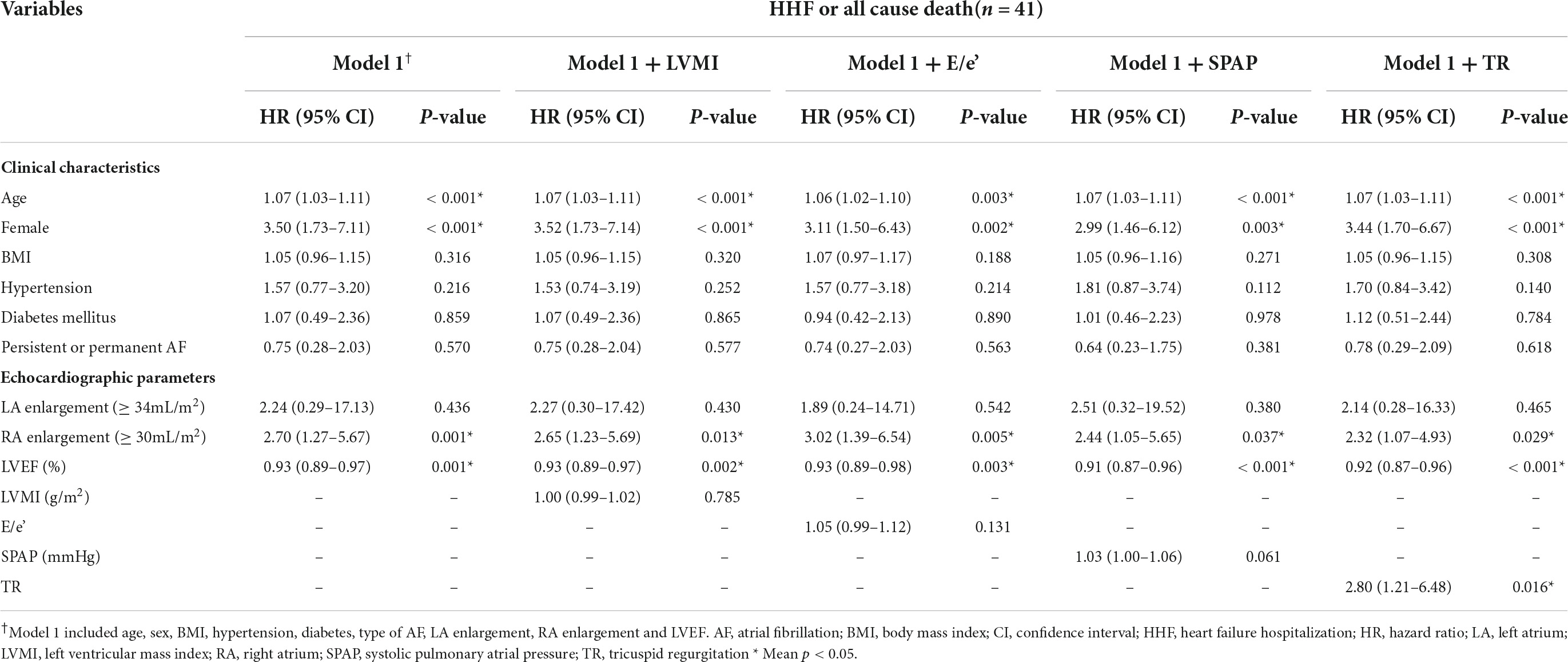
Table 4. Multivariate Cox proportional hazard analysis for composite end–point of HHF or all cause death.
Rhythm control strategy was not significantly associated with adverse events in multivariable analysis (p = 0.12 for primary composite outcome, p = 0.77 for HHF or all cause death). When adding the rhythm control to the standard model in a multivariable model, RA enlargement was consistently associated with adverse clinical outcomes (adjusted HR, 1.95 [95% CI, 1.16–3.25], p = 0.011 for primary composite outcome; adjusted HR, 2.68 [95% CI, 1.27–5.69], p = 0.010 for HHF or all cause death).
When assessing RA volume as continuous variables, RAVI showed a linear association with the clinical outcome (Figure 4). Higher value of RAVI was associated with a higher risk of both primary composite outcome and a composite of HHF or all cause death in multivariable model (adjusted HR, 1.02 [95% CI, 1.00–1.03], p = 0.007 for primary composite outcome per 1mL/m2; adjusted HR, 1.04 [95% CI, 1.02–1.06], p < 0.001 for HHF or all cause death per 1mL/m2) whereas LAVI was not associated clinical outcomes.
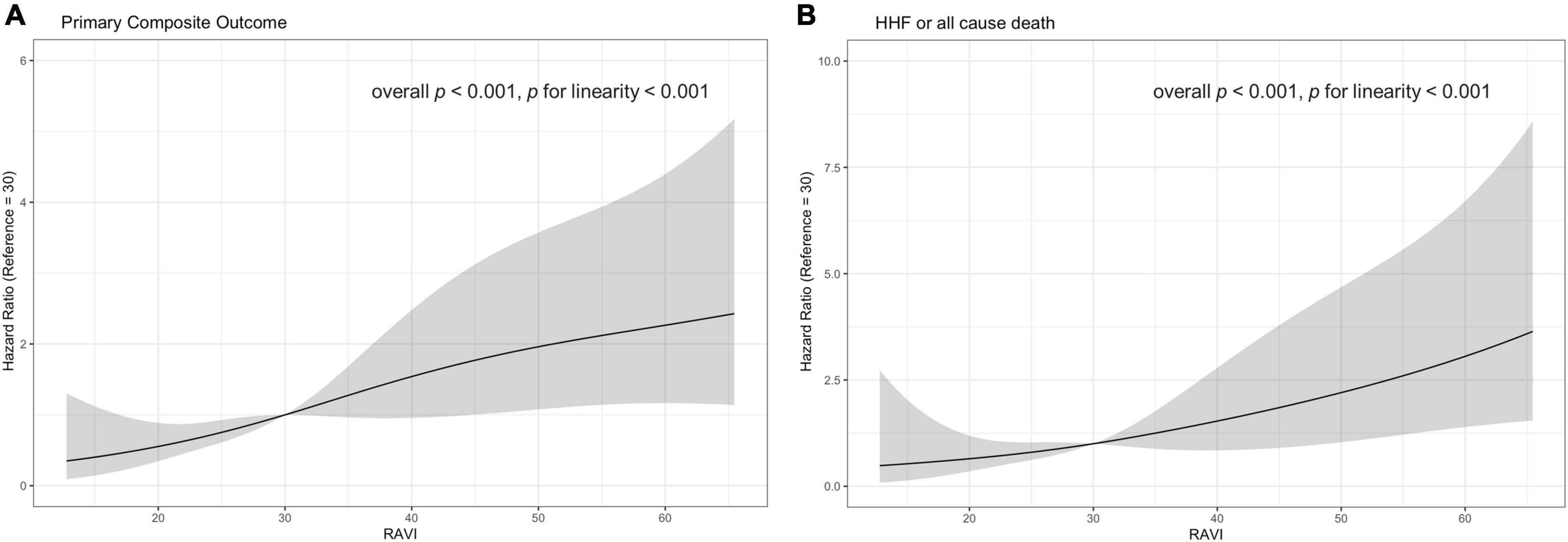
Figure 4. Estimated hazard ratio with RAVI and (A) primary composite outcome (B) a composite outcome of HHF or all cause death. The solid curves indicating unadjusted estimates and colored curves indicating 95% confidence limits. HHF, hospitalization for heart failure; RAVI, right atrial volume index.
When the patients were divided by RA and LA enlargement, patients with RA enlargement had adverse events more frequently than in those without RA enlargement among patients with LA enlargement (Figure 5). The event rates were approximately 1.7-fold higher for primary composite outcome and 1.9-fold higher for HFF or all cause death in the group with RA enlargement when compared with those without RA enlargement among the patients with LA enlargement.
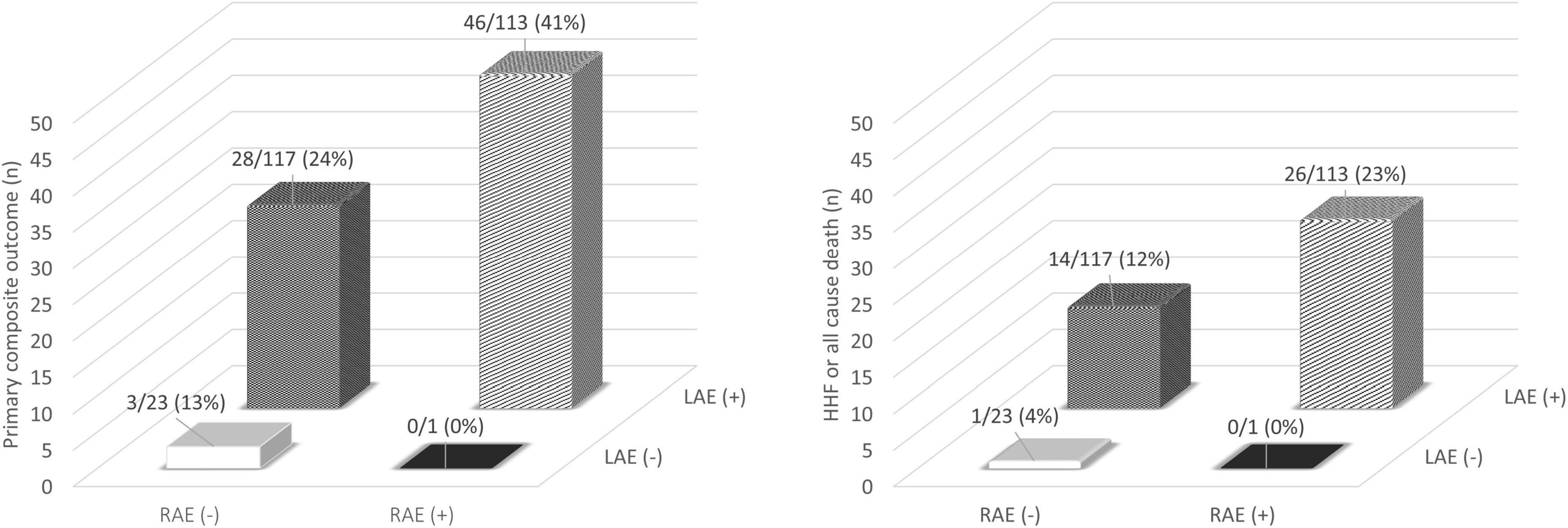
Figure 5. Incidence of adverse clinical outcome classified by atrial enlargement. HHF, hospitalization for heart failure; LAE, left atrial enlargement; RAE, right atrial enlargement.
In our study, RA enlargement was related to older age, female gender, lower body weight, and longer duration of AF in patients with non-valvular AF. In addition, RA size was associated with LVMI, elevated E/e’, SPAP and tricuspid regurgitation. After adjusting for clinical and echocardiographic factors, RA enlargement, but not LA enlargement, was related to higher rates of clinical events. Among patients with LA enlargement, patients with RA enlargement had more clinical events than those without RA enlargement.
Atrial remodeling including atrial structural, architectural, contractile, or electrophysiological changes is crucial pathophysiologic findings in AF mechanisms (17, 19). While there are numerous studies regarding importance of LA structural and functional changes in AF, little is known about the factors which can attribute to RA enlargement and the prognostic value of RA remodeling in patients with AF. RA acts as a reservoir for systemic venous return when the tricuspid valve is closed. RA operates as a passive conduit in early diastole when the tricuspid valve is open and as an active conduit in late diastole during atrial contraction (19). In AF, decreased atrial contractile function and increased atrial compliance result in subsequent atrial remodeling and these changes will affect both LA and RA (20–22). RA size and its pressure can increase progressively in patients with AF, which can lead to an enlargement of the tricuspid annulus. At the same time, LV diastolic dysfunction and elevated LA pressure may increase the afterload of the right ventricle. These changes also may lead to a functional TR by participating in this vicious circle (23). Indeed, we observed that RA enlargement was related to LAVI, E/e’, and SPAP which can reflect LV diastolic dysfunction and elevated LA pressure, as well as TR grade in our data. We also showed RA enlargement was more significantly related to clinical events including HHF, stroke, systemic embolization or mortality than LA enlargement in patients with non-valvular AF. Although data about clinical utility of RA remodeling in patients with AF is limited, several literatures have reported the role of RA in patients with AF. Prior data have shown that the RA volume is superior to the LA volume in predicting AF recurrences at 6 months after direct current cardioversion (24). RA size is a significant predictor of arrhythmia recurrence after pulmonary vein isolation when compared with LA size (25, 26). Additionally, RA size is associated with stroke occurrence in patients with AF (27).
Rhythm control strategy including antiarrhythmic drugs or catheter ablation may effectively reduce the atrial remodeling by minimizing the burden of AF and it has shown to improve cardiovascular outcomes over usual care (28, 29). Rhythm control strategies may affect on the progression of atrial remodeling. In our study, the rhythm control strategy tended to yield better outcomes, however, it did not reach statistical significance probably due to the small number of patients with rhythm control. We additionally confirmed that RA size was consistently associated with clinical outcomes even after adjusting rhythm control strategy as an additional variable in the multivariate model.
The precise mechanisms of association of adverse clinical events in RAVI compared with LAVI are not clear. While RAVI was related to age, LV diastolic dysfunction, greater LV mass index, increased atrioventricular valvular regurgitation, and pulmonary hypertension, it was independently associated with adverse clinical events even after adjusting for clinical and echocardiographic parameters in our study. However, LA enlargement was not significantly related to clinical outcome in our data. Whereas LA enlargement is commonly used to assess LV diastolic dysfunction in patients with a variety of CV diseases, the LA can be enlarged regardless of filling pressure in cases of AF (18). On the other hand, there are some controversies with regards to LA enlargement as a risk factor of thromboembolic complications or the development of HF in patients with AF (6, 30, 31). To avoid the potential effect of factors other than AF on RA remodeling, we excluded the patients with chronic lung disease, intrinsic valvular disease, or other cardiomyopathies. Atrial enlargement is a part of atrial structural remodeling. Decreased atrial contractile function and increased atrial compliance in AF results in subsequent atrial remodeling and these changes will affect both LA and RA. Given the LA and RA volumes were more enlarged in patients with persistent or permanent AF than those with paroxysmal AF, both atrial anatomical remodeling might reflect the burden and the chronicity of AF (32). In addition, RA enlargement might reflect more advanced atrial remodeling rather than being a sensitive marker to hemodynamic loading compared with LA enlargement alone when considering that most patients with enlarged RAVI had an enlarged LAVI in our data. Therefore, RA remodeling which may reflect more chronicity of AF may contribute to worse clinical outcome through vicious pathophysiological cycle for HF and exposure to the persistent thromboembolic risk (33). Electrophysiologic remodeling followed by anatomical remodeling also may play a role in clinical outcome. Progression of atrial enlargement can increase the likelihood of ectopic or reentrant activity and it may produce a substrate for AF maintenance due to RA re-entrant activity, with an underlying substrate prominently involving RA fibrosis and conduction abnormalities (34).
In our study, only 3.5% of the patients had a history of a prior hospitalization for HF, indicating most of our population was in stage A or B according to 2022 ACC/AHA stages of HF (35). While we usually focus on LA enlargement or LV hypertrophy in structural changes in the pre-HF stage, we may need to pay more attention when RA enlargement develops. Further research would be necessary if earlier and more aggressive therapeutic intervention including catheter ablation or specific drugs can modify the relationship between RA remodeling and worse outcome in this population.
Several limitations of our study should be noted. First, our study was a retrospective cohort study and selective bias may occur. Second, we used 2D echocardiography to assess the LA and RA volumes. While 2D echocardiography is most commonly used to assess atrial size, it has inherent limitations because of the use of foreshortened views and asymmetric shapes of the atrium. In our study, we sought to avoid the use of foreshortened images to minimize measurement errors and excluded the patients if the images were not good enough to assess RA volume. Another study has shown that RA volume using single-plane Simpson’s method of disks from 2D echocardiography is comparable to those from MRI, whereas atrial volumes by 2D echocardiography are smaller than those by cardiac magnetic resonance (36, 37). However, good images would be essential in assessing RA volume accurately since it is common not to focus on the RA in clinical echocardiography. Indeed, we excluded 121 patients because of insufficient image quality including foreshortening or endocardial dropout to measure RA volume in our analysis. Third, the study population was relatively small and from a single referral tertiary center. Thus, our study population might be a skewed selected population, rather than representing the general population with AF. Indeed, our population showed more frequent incidence of adverse events than patients with non-valvular AF in the community.
In patients with non-valvular AF, RA enlargement was more significantly related to adverse clinical events than LA enlargement, suggesting that RA size can be helpful in stratifying the risk in this population. Further studies are necessary to confirm the utility of RA size for AF in a larger population and whether specific therapies that reverse RA remodeling would improve clinical outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board (INHAUH2022-01-034-000). The patients/participants provided their written informed consent to participate in this study.
K-YK, J-HJ, S-HC, Y-SB, SWK, S-DP, S-IW, D-HK, and S-HS: conception and design of the work, analysis and interpretation of data, writing of the original draft, and substantial revision of the manuscript. K-YK and J-HJ: data acquisition, analysis and interpretation of data, writing of the original draft, and substantial revision of the manuscript. S-HC, Y-SB, and SWK: data analysis and substantial revision of the manuscript. S-DP, S-IW, and D-HK: cross-checking of the analysis and substantial revision of the manuscript. All authors have contributed to critical revision of the manuscript and approved the submitted version.
This work was supported by the Inha University Research Grant (INHA 67910).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: The rotterdam study. Eur Heart J. (2006). 27:949–53. doi: 10.1093/eurheartj/ehi825
2. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The framingham heart study. Circulation. (1998) 98:946–52. doi: 10.1161/01.cir.98.10.946
3. Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. Risk factors. Stroke. (1997) 28:1507–17.
4. Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. (1997) 96:3157–63. doi: 10.1161/01.Cir.96.9.3157
5. Bollmann A, Husser D, Steinert R, Stridh M, Soernmo L, Olsson SB, et al. Echocardiographic and electrocardiographic predictors for atrial fibrillation recurrence following cardioversion. J Cardiovasc Electrophysiol. (2003) 14:S162–5. doi: 10.1046/j.1540.8167.90306.x
6. Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow-up investigation of rhythm management (Affirm) study. J Am Coll Cardiol (2005) 45:2026–33. doi: 10.1016/j.jacc.2005.03.020
7. Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The fiamingham study. Neurology. (1978) 28:973.
8. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: Time for a new model. Stroke. (2016) 47:895–900. doi: 10.1161/STROKEAHA.115.012004
9. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ Res. (2014) 114:1500–15. doi: 10.1161/CIRCRESAHA.114.303772
10. Tsutsui JM, Dourado PM, Elhendy A, Falcao SN, Goes RM, Chagas AC, et al. Prognostic value of left atrial volume in patients who underwent dobutamine stress echocardiography for known or suspected coronary artery disease. Am Heart J. (2008) 156:1110–6. doi: 10.1016/j.ahj.2008.07.015
11. Tani T, Yagi T, Kitai T, Kim K, Nakamura H, Konda T, et al. Left atrial volume predicts adverse cardiac and cerebrovascular events in patients with hypertrophic cardiomyopathy. Cardiovasc Ultrasound. (2011) 9:34. doi: 10.1186/1476-7120-9-34
12. Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The strong heart study (Shs). Am Heart J. (2006) 151:412–8. doi: 10.1016/j.ahj.2005.04.031
13. Mantziari L, Kamperidis V, Ventoulis I, Damvopoulou E, Giannakoulas G, Efthimiadis G, et al. Increased right atrial volume index predicts low duke activity status index in patients with chronic heart failure. Hellenic J Cardiol. (2013) 54:32–8.
14. Sallach JA, Tang WH, Borowski AG, Tong W, Porter T, Martin MG, et al. Right Atrial Volume Index in Chronic Systolic Heart Failure and Prognosis. JACC Cardiovasc Imaging. (2009) 2:527–34. doi: 10.1016/j.jcmg.2009.01.012
15. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 Aha/Acc/Hrs guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64:e1–76.
16. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 Aha/Acc/Hrs focused update of the 2014 Aha/Acc/Hrs guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2019) 74:104–32.
17. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. Corrigendum To: 2020 Esc guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (Eacts): The task force for the diagnosis and management of atrial fibrillation of the european society of cardiology (Esc) developed with the special contribution of the european heart rhythm association (Ehra) of the Esc. Eur Heart J. (2021) 42:4194. doi: 10.1093/eurheartj/ehab648
18. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur J Echocardiogr. (2016) 17:1321–60.
19. Donal E, Lip GY, Galderisi M, Goette A, Shah D, Marwan M, et al. Eacvi/Ehra expert consensus document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. (2016) 17:355–83. doi: 10.1093/ehjci/jev354
20. Pathak R, Lau DH, Mahajan R, Sanders P. Structural and functional remodeling of the left atrium: Clinical and therapeutic implications for atrial fibrillation. J Atr Fibrillation. (2013) 6:986. doi: 10.4022/jafib.986
21. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. (2002) 54:230–46. doi: 10.1016/s0008-6363(02)00258-4
22. Houltz B, Johansson B, Berglin E, Karlsson T, Edvardsson N, Wandt B. Left ventricular diastolic function and right atrial size are important rhythm outcome predictors after intraoperative ablation for atrial fibrillation. Echocardiography. (2010) 27:961–8. doi: 10.1111/j.1540-8175.2010.01167.x
23. Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography. (2012) 29:140–6. doi: 10.1111/j.1540-8175.2011.01565.x
24. Luong C, Thompson DJS, Bennett M, Gin K, Jue J, Barnes ME, et al. Right atrial volume is superior to left atrial volume for prediction of atrial fibrillation recurrence after direct current cardioversion. Can J Cardiol. (2015) 31:29–35. doi: 10.1016/j.cjca.2014.10.009
25. Sasaki T, Nakamura K, Naito S, Minami K, Koyama K, Yamashita E, et al. The right to left atrial volume ratio predicts outcomes after circumferential pulmonary vein isolation of longstanding persistent atrial fibrillation. Pacing Clin Electrophysiol. (2016) 39:1181–90. doi: 10.1111/pace.12953
26. Akutsu Y, Kaneko K, Kodama Y, Suyama J, Li HL, Hamazaki Y, et al. Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation a pilot study. Circ-Cardiovasc Imag. (2011) 4:524–31. doi: 10.1161/Circimaging.110.962761
27. Tsao HM, Hu WC, Tsai PH, Lee CL, Wang HH, Chang SL, et al. functional remodeling of both atria is associated with occurrence of stroke in patients with paroxysmal and persistent atrial fibrillation. Acta Cardiol Sin. (2017) 33:50–7. doi: 10.6515/Acs20160411a
28. Camm AJ, Naccarelli GV, Mittal S, Crijns HJ, Hohnloser SH, Ma C-S, et al. The increasing role of rhythm control in patients with atrial fibrillation: Jacc state-of-the-art review. J Am Coll Cardiol. (2022) 79:1932–48.
29. Kim D, Yang PS, You SC, Jang E, Yu HT, Kim TH, et al. Comparative effectiveness of early rhythm control versus rate control for cardiovascular outcomes in patients with atrial fibrillation. J Am Heart Assoc. (2021) 10:e023055.
30. Investigators AF. Echocardiographic predictors of stroke in patients with atrial fibrillation: A prospective study of 1,066 patients from 3 clinical trials. Arch Intern Med. (1998) 158:1316–20. doi: 10.1001/archinte.158.12.1316
31. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J Am Coll Cardiol. (2006) 47:1018–23. doi: 10.1016/j.jacc.2005.08.077
32. Moon J, Hong YJ, Shim J, Hwang HJ, Kim JY, Pak HN, et al. Right atrial anatomical remodeling affects early outcomes of nonvalvular atrial fibrillation after radiofrequency ablation. Circ J. (2012) 76:860–7. doi: 10.1253/circj.cj-11-1232
33. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation. (2009) 119:2516–25.
34. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circulation. (2008) 1:62–73.
35. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 Aha/Acc/Hfsa guideline for the management of heart failure: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e263–421.
36. Whitlock M, Garg A, Gelow J, Jacobson T, Broberg C. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol. (2010) 106:1345–50. doi: 10.1016/j.amjcard.2010.06.065
Keywords: atrial fibrillation, left atrium, right atrium, heart failure, stroke
Citation: Ko K-Y, Jang J-H, Choi S-H, Baek Y-S, Kwon SW, Park S-D, Woo S-I, Kim D-H and Shin S-H (2022) Impact of right atrial enlargement on clinical outcome in patients with atrial fibrillation. Front. Cardiovasc. Med. 9:989012. doi: 10.3389/fcvm.2022.989012
Received: 07 July 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Shinichi Okuda, Yamaguchi Prefectural Grand Medical Center, JapanReviewed by:
Fang Wang, Peking University, ChinaCopyright © 2022 Ko, Jang, Choi, Baek, Kwon, Park, Woo, Kim and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung-Hee Shin, c3NoaW5AaW5oYS5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.