- 1Division of Cardiology, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 2Cardiovascular Research Center, National Yang-Ming Chiao Tung University, Taipei, Taiwan
- 3Institute of Clinical Medicine, National Yang-Ming Chiao Tung University, Taipei, Taiwan
- 4Department of Cardiology, Thoraxcenter, Erasmus Medical Center, Rotterdam, Netherlands
- 5Department of Critical Care Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 6Healthcare and Services Center, Taipei Veterans General Hospital, Taipei, Taiwan
Background: There are few reports published on the comparison of the resting full-cycle ratio (RFR) and fractional flow reserve (FFR) on the assessment of the severity of coronary stenosis. We aimed to investigate the diagnostic accuracy of RFR for detection of functionally significant coronary lesions.
Methods: This was an observational, retrospective, single-center study. We evaluated both RFR and FFR for 277 coronary lesions of 235 patients who underwent coronary angiography. Patients presenting with chronic coronary syndrome, unstable angina, or non-ST-elevation myocardial infarction were included.
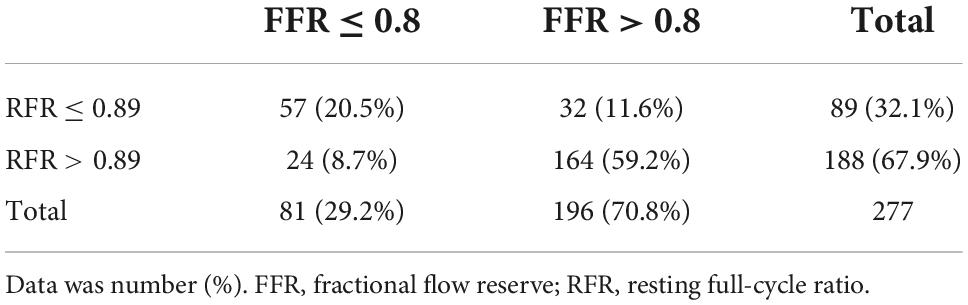
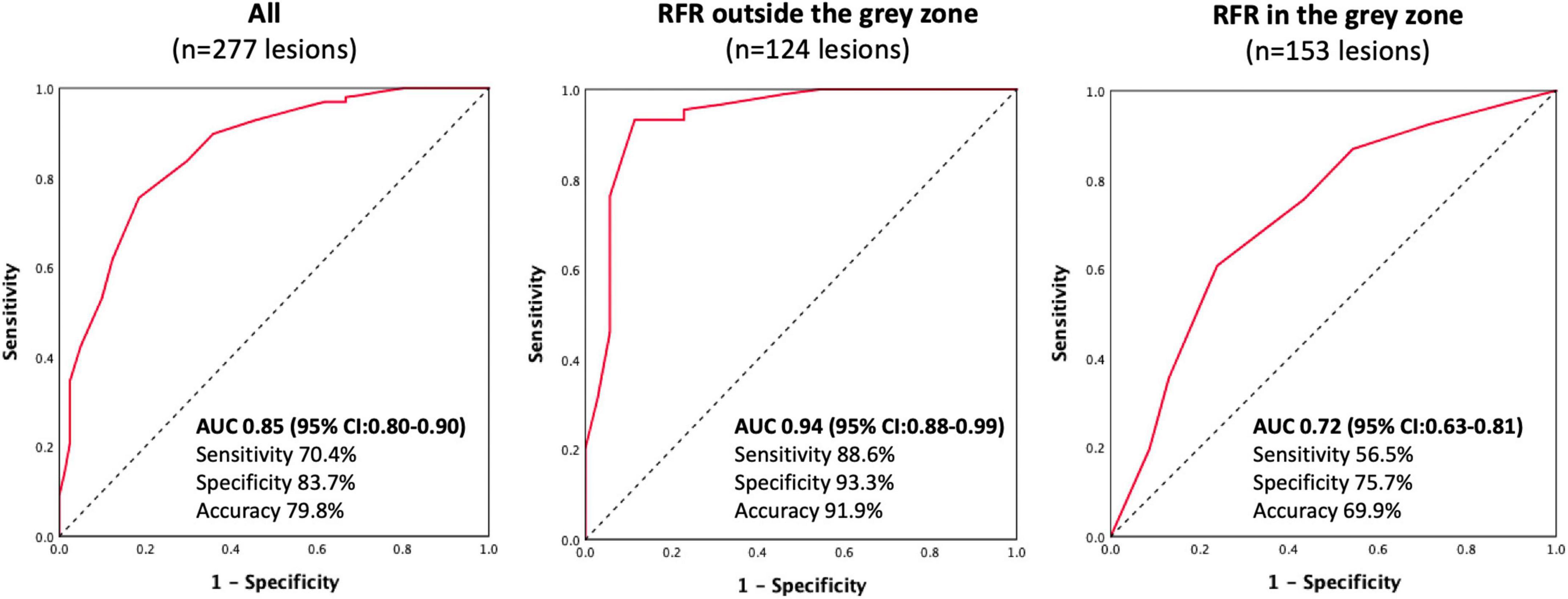
Results: The mean FFR and RFR values were 0.84 ± 0.08 and 0.90 ± 0.08, respectively. RFR significantly correlated with FFR (r = 0.727, P < 0.001). The agreement rate between the FFR and RFR was 79.8% (221/277). The diagnostic performance of RFR vs. FFR was accuracy 79.8%, sensitivity 70.4%, specificity 83.7%, positive predictive value 64.0%, and negative predictive value 87.2%. The discriminative power of RFR to identify lesions with FFR ≤ 0.80 was acceptable when the RFR value was within the gray zone [0.86 ≤ RFR ≤ 0.93; AUC: 0.72 (95% CI:0.63–0.81)], while it was excellent when the RFR value was out of the gray zone [RFR > 0.93 or < 0.86; AUC: 0.94 (95% CI:0.88–0.99)].
Conclusion: RFR was significantly correlated with FFR in the assessment of intermediate coronary stenosis. An RFR-FFR hybrid approach increases the diagnostic accuracy of RFR in the detection of functionally significant lesions.
Introduction
Fractional flow reserve (FFR) is a pressure wire-based physiology index used to assess coronary stenoses under maximal hyperemia. Currently, the use of FFR to guide coronary revascularization has been assigned a class I (level of evidence A) indication in the European guidelines (1). The Fractional Flow Reserve vs. Angiography for Multivessel Evaluation (2) (FAME) and FAME 2 studies (3) have demonstrated that the FFR-guided percutaneous coronary intervention (PCI) strategy is associated with a significantly lower rate of cardiovascular events in patients with stable coronary artery disease. Despite accumulating evidence to support the use of FFR, the adoption rate of FFR remains limited in clinical practice for several reasons, such as adenosine-related side effects, prolonged procedure time, additional equipment needed, and cost-related issues (4).
Several non-hyperemic physiological indices have been developed to avoid the side effects of adenosine and reduce the procedural aspects of physiology assessment, including the instantaneous wave-free ratio (iFR) (5–8) and resting full-cycle ratio (RFR)(9). It has been confirmed in randomized control trials that iFR-guided revascularization was non-inferior to FFR-guided revascularization in terms of the risk of major adverse cardiac events (10, 11). RFR was developed to detect the lowest distal coronary pressure (Pd) to aortic pressure (Pa) ratio (Pd/Pa) within the entire cardiac cycle. The VALIDATE RFR study (9) reported that the diagnostic accuracy of RFR was equivalent to that of iFR and the lowest Pd/Pa ratio was outside of diastole in 12% of all cardiac cycles, suggesting that RFR may detect clinically significant lesions that would be missed by other methods dedicated to a specific segment of the cardiac cycle.
Nevertheless, the correlation between RFR and FFR on functional assessment has rarely been reported. Therefore, this study aimed to investigate the correlation between pressure wire-based FFR and RFR in the functional assessment of coronary stenoses in the real-world practice.
Materials and methods
Study design and participants
The present study was an observational, retrospective, single-center study that compared FFR and RFR. Between May 01, 2019, and May 24, 2022, patients presenting with chronic coronary syndrome, unstable angina, or non-ST elevation myocardial infarction who underwent both RFR and FFR assessment at baseline were eligible. The physiological assessment was performed at the operator’s discretion based on the coronary angiograms and clinical information. Coronary physiology was assessed for intermediate-degree of stenosis defined by visual assessment. In patients with acute coronary syndrome, RFR and FFR were performed for the assessment of non-culprit lesions.
Coronary physiology assessment
RFR and FFR were performed according to the standard local clinical practice in the catheterization laboratory. RFR was measured using a 0.014 PressureWire™ X Guidewire (Abbott Vascular Inc., Santa Clara, CA, USA) positioned distal to the target lesion. The QUANTIEN™ or OPTIS™ Integrated System (Abbott Vascular Inc., Santa Clara, CA, USA) was used for measurement. RFR was defined as the lowest Pd/Pa ratio during the entire cardiac cycle (9). After RFR measurement, FFR was measured under hyperemia by intravenous (IV) adenosine infusion or intracoronary (IC) bolus injection of adenosine. For IV adenosine infusion, an injection pump was used to achieve a dose of 140 μg/kg/min continuously for 3 min through a large cubital or femoral vein. For IC adenosine injection, the dose of IC adenosine was initiated from 60 to 120 μg for the right coronary artery (RCA) and 120 μg for the left coronary artery. The dose of IC adenosine was escalated subsequently (180, 240, 360, or 480 μg) and at least two increasing doses of IC adenosine were used to achieve hyperemia when FFR > 0.80. The values of FFR/RFR were adjudicated by two interventionalists.
Clinical endpoints
The primary endpoint of this study was the vessel-oriented composite endpoint (VOCE) during clinical follow-up, defined as a composite of all-cause death, vessel related non-fatal myocardial infarction (MI), and target vessel revascularization (TVR). MI was defined according to the Third Universal Myocardial Infarction definition (12). TVR was defined as any repeat percutaneous intervention or surgical bypass of the target vessel. VOCE was analyzed hierarchically.
Statistical methods
Categorical variables are presented as percentages and numbers. Continuous variables are presented as the mean ± standard deviation. Spearman’s correlation coefficient was used to evaluate the relationship between the FFR and RFR. The receiver operating characteristic curve and the area under the curve (AUC) were used to estimate the discriminative value of RFR for functionally significant coronary lesions (FFR ≤ 0.80). Survival curves were constructed using the Kaplan-Meier estimates. A two-sided p-value of less than 0.05 was considered to indicate statistical significance. Data were analyzed using SPSS software (version 25, SPSS, Chicago, IL, USA).
Results
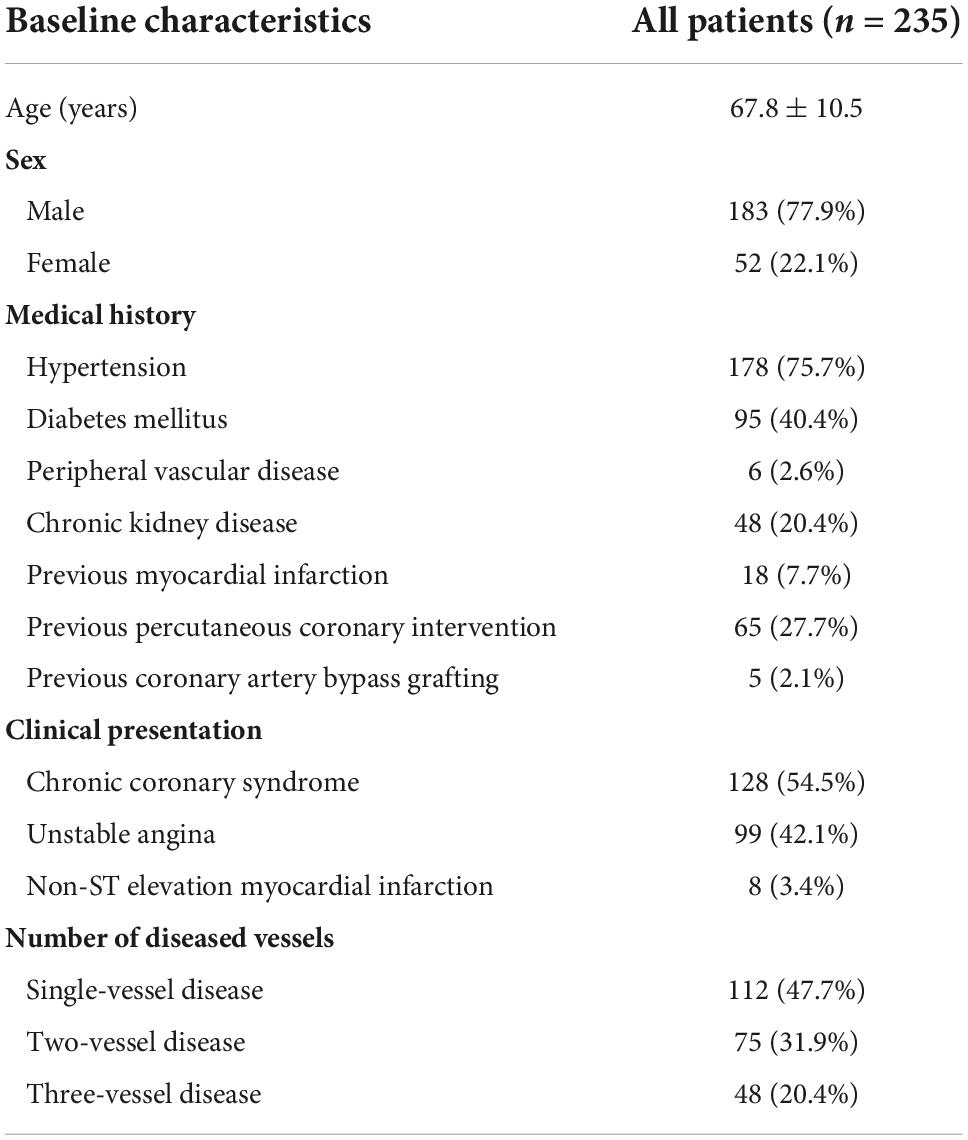
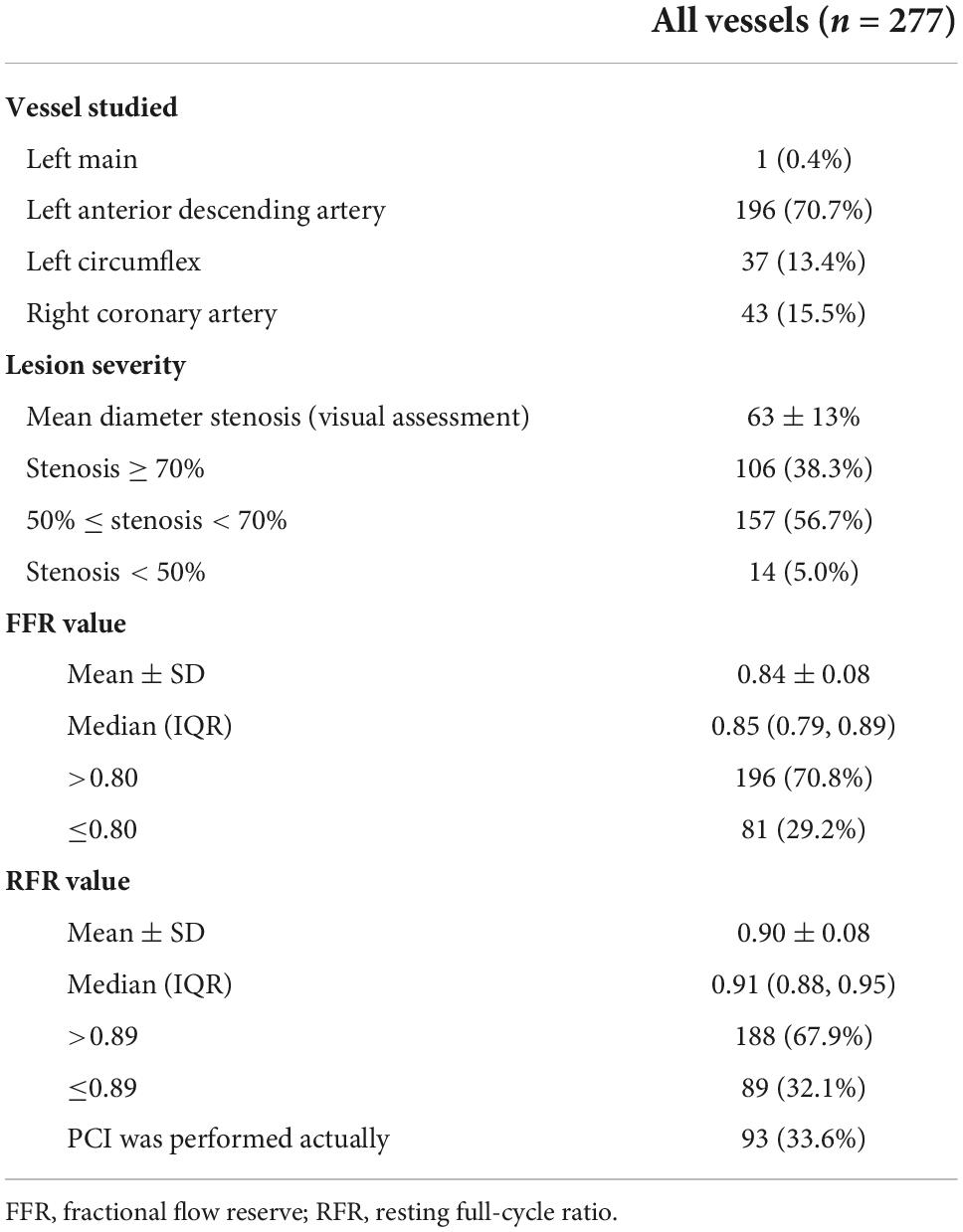
A total of 277 lesions from 235 patients were included in the analysis. The baseline characteristics of eligible patients are shown in Table 1. The mean age was 67.8 ± 10.5 years, 77.9% were male, 40.4% had diabetes, 54.5% presented with chronic coronary syndrome, and 40% had unstable angina. Coronary stenoses were mainly located in the left anterior descending artery (70.7%). The majority (56.6%) of lesions had diameter stenosis between 50 and 70% by visual assessment. The mean FFR and RFR values were 0.84 ± 0.08 and 0.90 ± 0.08, respectively (Table 2).
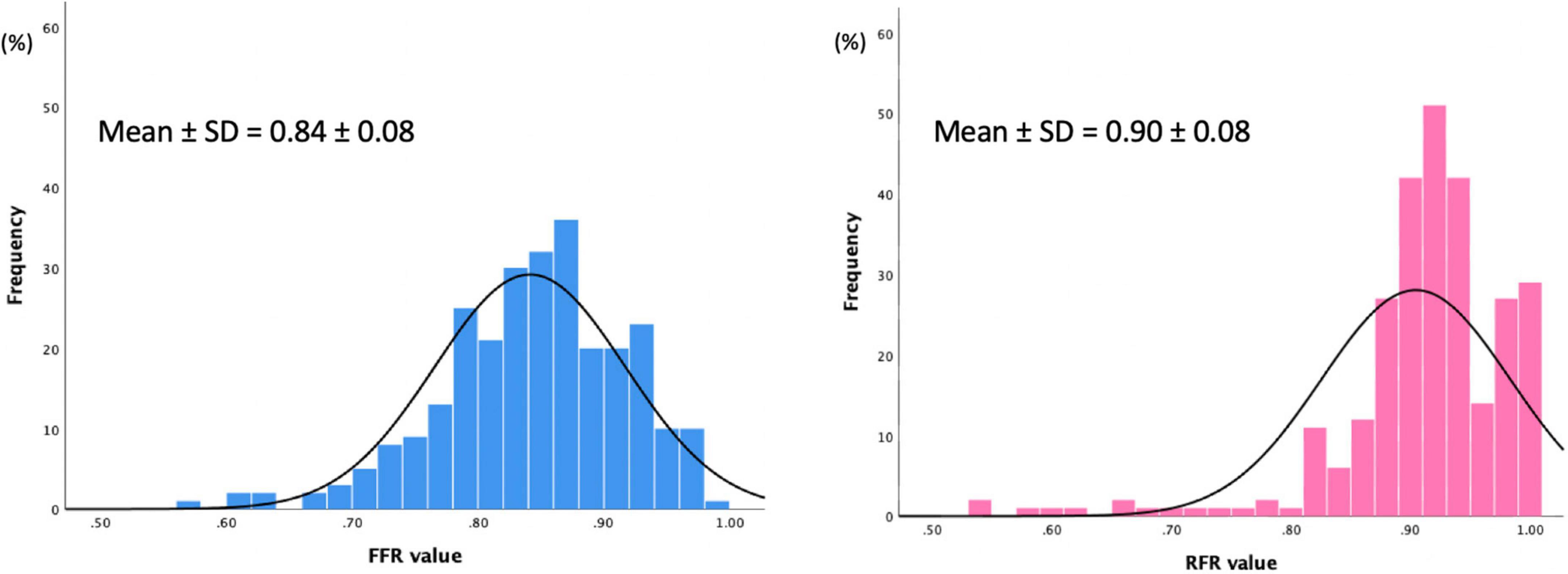
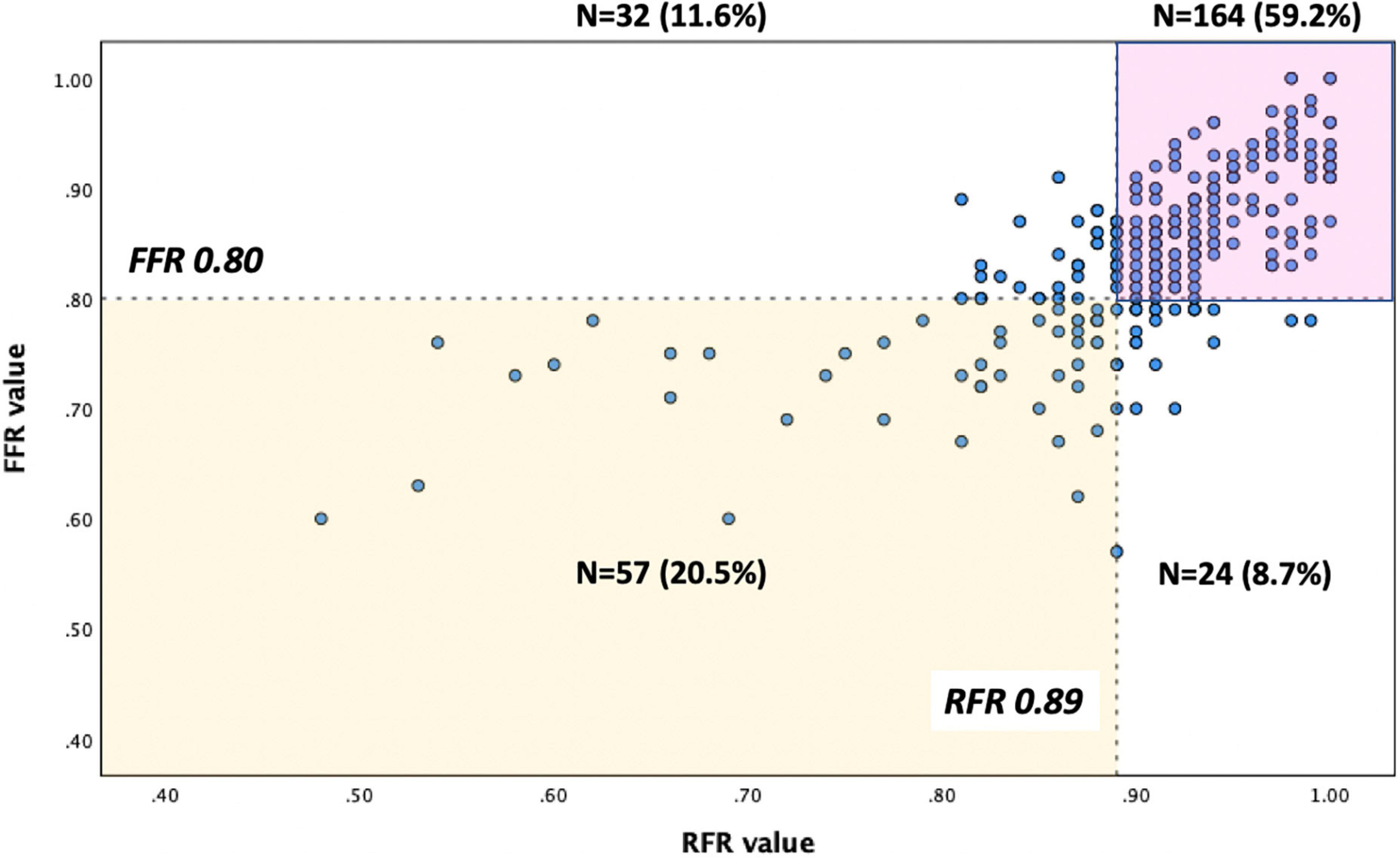
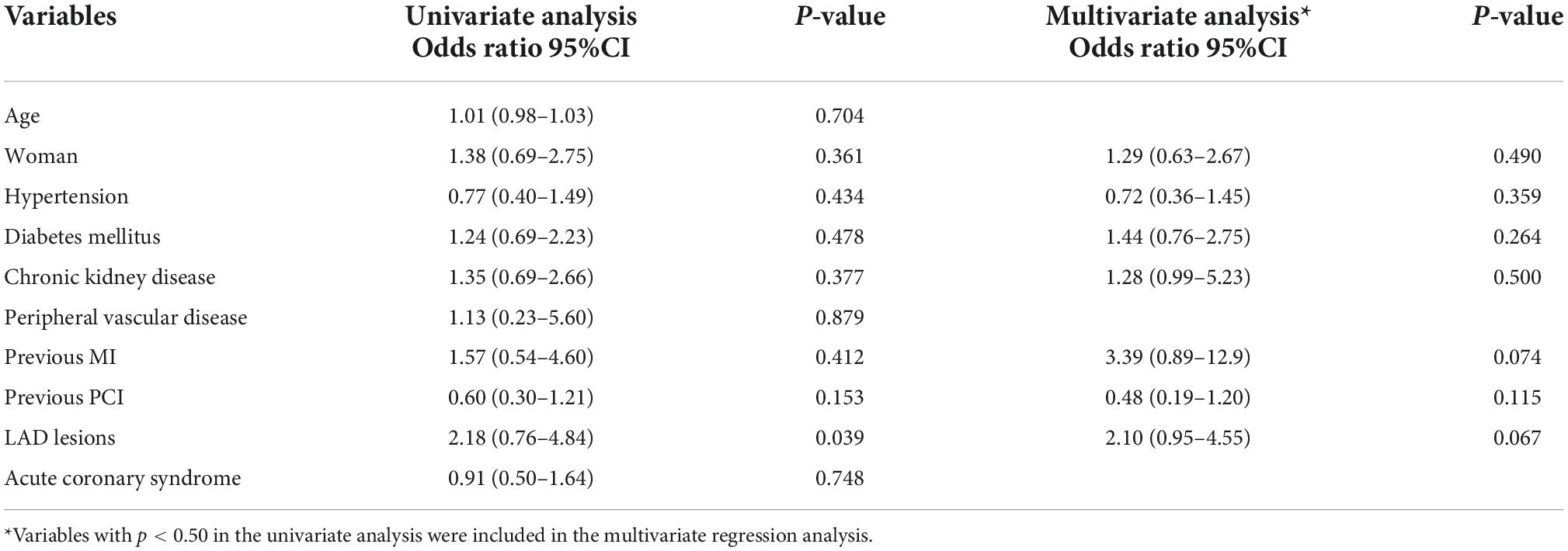
The distributions of FFR and RFR values are shown in Figure 1. RFR was highly positively correlated with FFR (r = 0.727, P < 0.001) (Figure 2). Using the cut-off value of RFR ≤ 0.89 to define a functionally significant lesion, the diagnostic concordance between RFR and FFR was 79.8% (Table 3). The univariate and multivariate regression analysis were performed to identify predictors of discordance between RFR and FFR. Prior MI [Odds Ratio (OR), 3.39; 95% confidence interval (CI), 0.89–12.9; p = 0.074] and LAD lesion (OR 2.10; 95% CI, 0.95–4.55, p = 0.067) tended to be predictors of RFR/FFR discordance (Table 4).
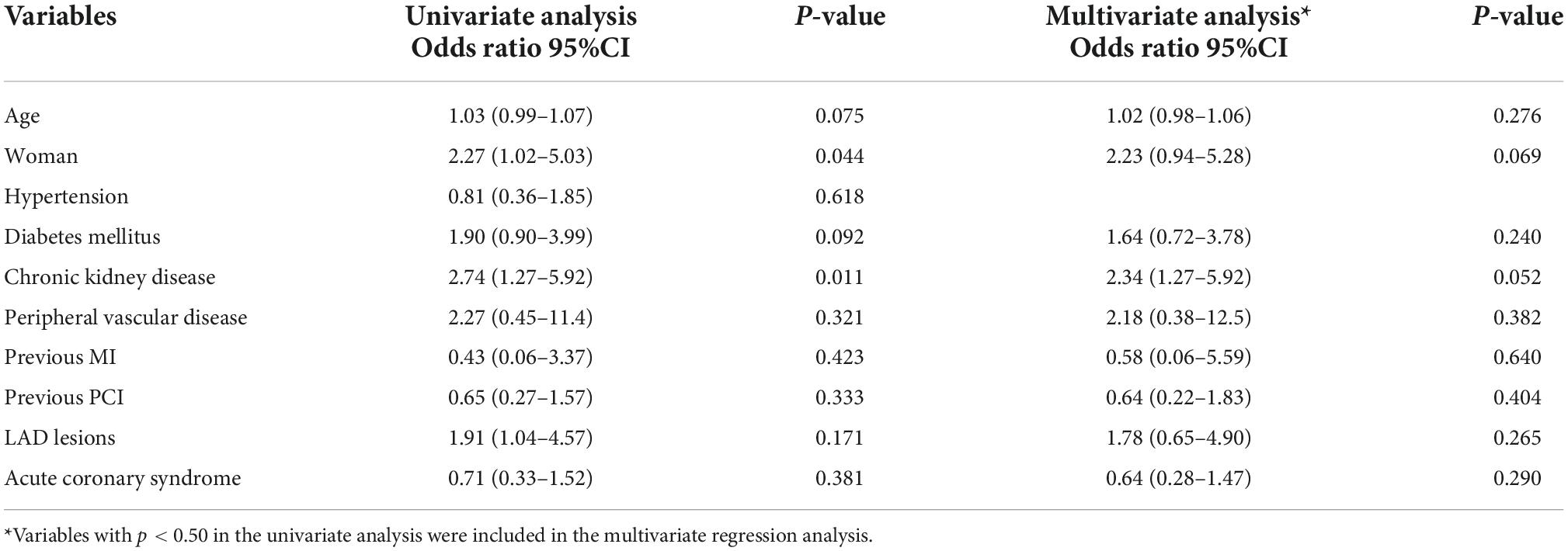
The diagnostic performance of RFR vs. FFR was reported to have a diagnostic accuracy of 79.8%, sensitivity 70.4%, specificity 83.7%, positive predictive value 64.0%, and negative predictive value 87.2%. A sub-analysis was performed to identify predictors of false positive RFR results (RFR ≤ 0.89/FFR > 0.80). Chronic kidney disease tended to be a predictor of low RFR/high FFR (OR 2.34; 95% CI, 1.27–5.92, p = 0.052) (Table 5).
The discriminative power of RFR for functionally significant coronary lesions (FFR ≤ 0.80) was excellent. AUC was 0.85 (95% CI:0.80–0.90) (Figure 3). A total of 153 lesions had an RFR value in the gray zone (0.86 ≤ RFR ≤ 0.93), while 124 lesions had an RFR value outside the gray zone (RFR < 0.86 or RFR > 0.93). The diagnostic performance of RFR was excellent for FFR ≤ 0.80, when RFR was outside the gray zone [AUC: 0.94 (95% CI: 0.88–0.99), P < 0.0001]. However, the diagnostic performance of RFR was only acceptable when RFR was in the gray zone [AUC: 0.72 (95% CI: 0.63–0.81), P < 0.0001], assuming that FFR is always accurate.
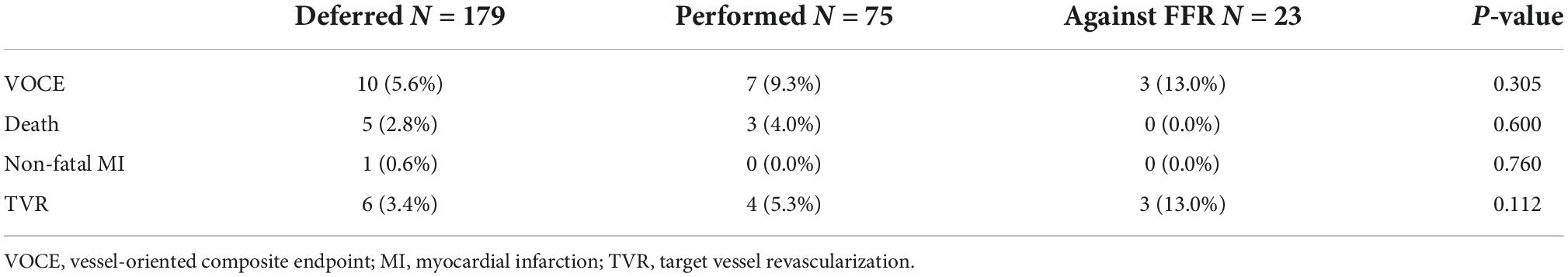
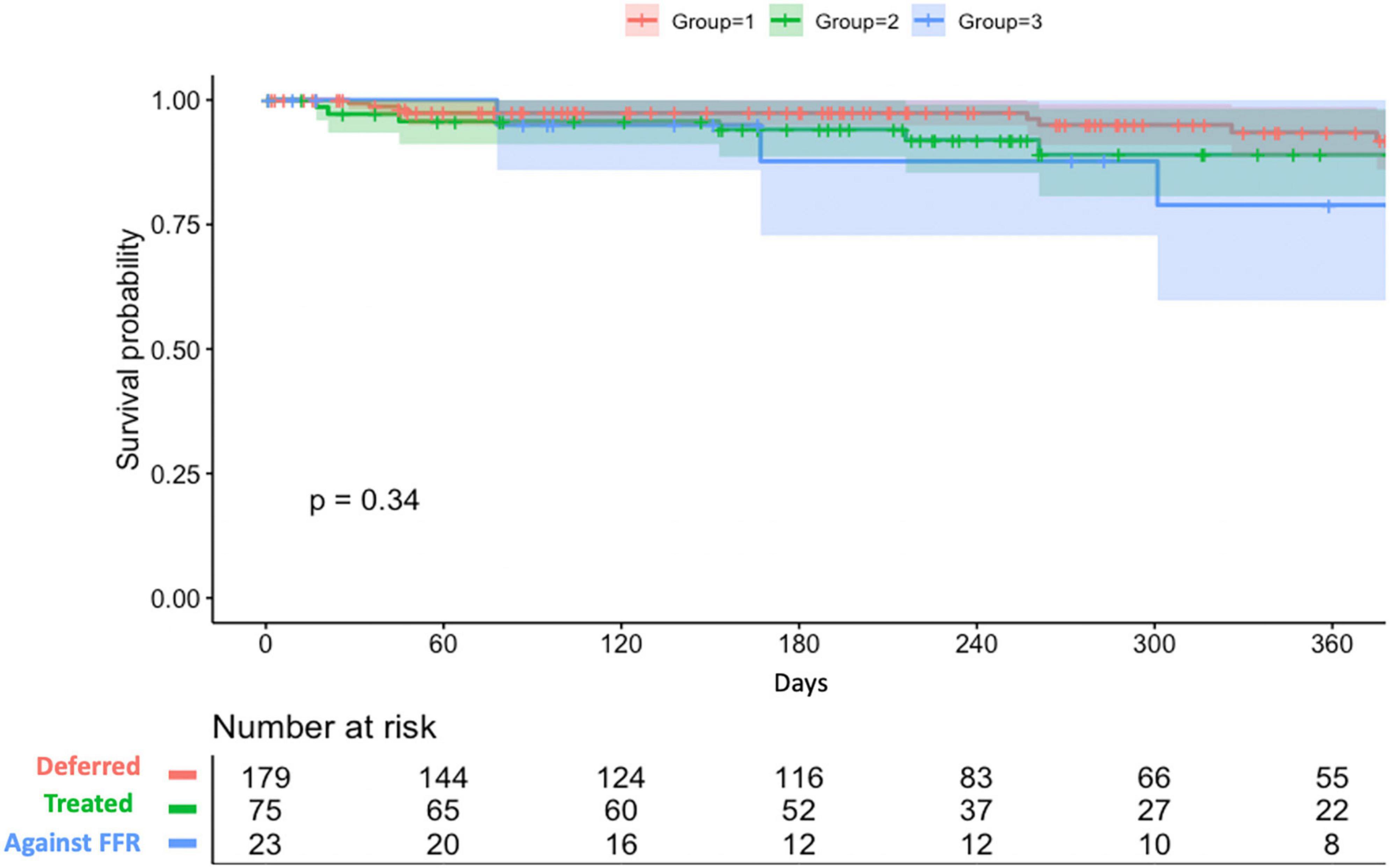
Overall, 91.7% (254/277) of lesions were treated according to their RFR and FFR result (179 lesions were deferred and 75 lesions were treated by PCI). On the other hand, 23 lesions were treated against to FFR result (PCI was done for 19 lesions with FFR > 0.80 and 4 lesions with FFR ≤ 0.80 were only under medical therapy). Among 94 lesions treated by PCI, post-PCI functional assessment was performed for 25 lesions (26.6%). The mean post-PCI FFR and RFR values were 0.87 and 0.91, respectively. Clinical outcomes stratified by actual treatments were shown in Table 6. During a mean follow-up of 258 ± 182 days, the VOCE-free survival rate was numerically higher in the deferred group than the treated group and against FFR group (93.5% vs. 89% vs. 78.9%, P = 0.34) (Figure 4).
Discussion
The present study investigated the diagnostic accuracy of RFR in detecting functionally significant lesions (FFR ≤ 0.80) in patients with chronic coronary syndrome, unstable angina, or non-ST elevation myocardial infarction. The main findings were as follows: (1) RFR had a positive correlation with the FFR. (2) The concordance rate between RFR and FFR was high (79.8%) in our study. (3) The discriminative power of RFR to identify lesions with FFR ≤ 0.80, was acceptable when the RFR value was within the gray zone (0.86 ≤ RFR ≤ 0.93), while it was excellent when the RFR value was outside the gray zone (RFR > 0.93, or < 0.86).
Accumulating data supports the use of FFR to guide revascularization of chronic or acute coronary syndrome (13, 14). To date, non-hyperemic physiological indices, such as iFR and RFR, have been invented to avoid the side effects of adenosine and reduce the procedural time of physiological assessment (9, 10). Previous studies showed that a hybrid iFR-FFR approach for revascularization could increase the adoption of physiology-guided PCI and still have a high agreement with the FFR-only strategy (15, 16). Similarly, our study demonstrated that when the RFR value was in the gray zone, evaluation of FFR was suggested to confirm the result to guide decision-making. When the RFR value was outside the gray zone, the RFR-only strategy could be used for deferring or performing PCI. An RFR-FFR hybrid approach can increase the diagnostic accuracy of RFR in detecting functionally significant lesions and may facilitate the physiological assessment of coronary lesions by simplifying the procedures. Otherwise, a RFR-only strategy with a treatment cut-off of ≤ 0.89 would have resulted in 11.6% of unnecessary PCI and 8.7% of lesions inappropriately deferred in our study cohort. In our study, FFR was almost mandatory following the initial RFR assessment, irrespective of the result. This early experience of our single-center may reflect the fact that RFR is a newly developed physiological index with relatively limited evidence. In fact, adenosine injection can be waived for 44.7% of lesions in our cohort, which can be simply deferred or treated by PCI based on the RFR results only.
Recently, the Resting-Full-Cycle Ratio Comparison vs. Fractional Flow Reserve study provided prospective validation data (17). In the abovementioned study, the diagnostic performance of RFR was diagnostic accuracy 79.0%, sensitivity 76.0%, specificity 80.0%, positive predictive value 68.0%, and negative predictive value 86.0%. These numbers are consistent with our findings and another report (18). It is noteworthy that the positive predictive value of RFR was slightly lower than expected and resulted in false positive result (RFR ≤ 0.89/FFR > 0.80). Wienemann et al. reported that LAD lesions, peripheral artery disease, age, woman and non-focal stenoses were predictors of false positive result of RFR (18). It has been known that several characteristics, such as chronic kidney disease or hemodialysis, affecting coronary flow reserve through microvascular function can influence resting and hyperremic coronary flow velocity (19, 20).
Compared to iFR and FFR, the concordance between RFR and FFR has rarely been reported in the literature. Lee et al. reported that the discrepancy rate between iFR and FFR was approximately 12% of the vessel analyzed, and it was mostly observed in the left anterior descending artery (LAD) (21). Likewise, Goto et al. showed that LM and LAD location, hemodialysis, and peripheral artery disease were associated with a low RFR among patients with a high FFR value (22). Similar findings were observed in our study. The discrepancies between RFR and FFR were 23.4, 8.1, and 13.9% for the LAD, left circumflex artery, and RCA, respectively. The potential explanation is that the large myocardial territory supplied by LM/LAD vessel compared to non-LAD vessels may cause higher coronary flow variation between resting and hyperemic status, which could be responsible for the higher discrepancy rate between resting and hyperemic indices in LM/LAD lesions (23). In our study, the presence of prior MI tended to be a predictor of RFR and FFR discordance. It has been shown that FFR value depends on the mass of viable myocardium not only the degree of coronary stenosis (24). Whether myocardial scarring after infarction may result in discrepancy between resting and hyperemic physiology indices need to be further explored.
Nevertheless, it has been reported that the discrepancy between resting physiological indices and FFR was not associated with the risk of vessel-oriented composite outcomes in deferred lesions. Only when lesions with concordant abnormal results in both resting physiological indices and FFR showed an increased risk of vessel-oriented composite outcomes (25).
Limitations
The present study has some important limitations. First, this was a retrospective observational study, and data were obtained in a real-world practice without an independent core lab for pressure waveform interrogation. Second, the use of FFR or RFR was at the discretion of the treating physicians, and potential selection bias could not be avoided in this setting. Third, the reproducibility of RFR measurement has not yet been reported and warrants further investigation. Lastly, the results from a single-center experience with a limited sample size need to be interpreted cautiously. The adverse event rate was too low to detect any substantial differences.
Conclusion
RFR is significantly correlated with FFR in the assessment of intermediate coronary stenoses. A hybrid approach increases the diagnostic accuracy of RFR for detecting functionally significant lesions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to corresponding author, C-CC.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the Taipei Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
C-CC and P-HH contributed to the conception and design of the study. C-CC, Y-HL, M-JC, Y-WL, Y-LT, R-HC, C-HW, and T-ML contributed to data collection. C-CC and M-JC analyzed and interpreted the data and drafted the report, which was critically revised for important intellectual content by T-ML and P-HH. All authors participated in the work and reviewed and agreed with the content of the article, and approved the final version of the report.
Funding
This study was supported in part by research grants from the Novel Bioengineering and Technological Approaches to Solve Two Major Health Problems in Taiwan Program, sponsored by the Taiwan Ministry of Science and Technology Academic Excellence Program (108-2633-B-009-001) and Taipei Veterans General Hospital (VGH-V100E2-002 and VGHUST103-G7-2-1). The funding institutions took no part in the study design, data collection or analysis, publication intent, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. (2019) 14:1435–534.
2. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve vs. angiography for guiding percutaneous coronary intervention. N Engl J Med. (2009) 360:213–24.
3. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. (2014) 371:1208–17.
4. Gotberg M, Cook CM, Sen S, Nijjer S, Escaned J, Davies JE. The evolving future of instantaneous wave-free ratio and fractional flow reserve. J Am Coll Cardiol. (2017) 70:1379–402.
5. Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine vasodilator independent stenosis evaluation) study. J Am Coll Cardiol. (2012) 59:1392–402. doi: 10.1016/j.jacc.2011.11.003
6. Berry C, van ‘t Veer M, Witt N, Kala P, Bocek O, Pyxaras SA, et al. VERIFY (VERification of instantaneous wave-free ratio and fractional flow reserve for the assessment of coronary artery stenosis severity in everyday practice): a multicenter study in consecutive patients. J Am Coll Cardiol. (2013) 61:1421–7. doi: 10.1016/j.jacc.2012.09.065
7. Jeremias A, Maehara A, Genereux P, Asrress KN, Berry C, De Bruyne B, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. (2014) 63:1253–61. doi: 10.1016/j.jacc.2013.09.060
8. Escaned J, Echavarria-Pinto M, Garcia-Garcia HM, van de Hoef TP, de Vries T, Kaul P, et al. Prospective assessment of the diagnostic accuracy of instantaneous wave-free ratio to assess coronary stenosis relevance: results of ADVISE II international, multicenter study (ADenosine vasodilator independent stenosis evaluation II). JACC Cardiovasc Interv. (2015) 8:824–33. doi: 10.1016/j.jcin.2015.01.029
9. Svanerud J, Ahn JM, Jeremias A, van ‘t Veer M, Gore A, Maehara A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the resting full-cycle ratio (VALIDATE RFR) study. EuroIntervention. (2018) 14:806–14. doi: 10.4244/EIJ-D-18-00342
10. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the Instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. (2017) 376:1824–34.
11. Gotberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous wave-free ratio vs. fractional flow reserve to guide PCI. N Engl J Med. (2017) 376:1813–23.
12. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. (2012) 33:2551–67.
13. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI vs. medical therapy in stable coronary disease. N Engl J Med. (2012) 367:991–1001.
14. Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. (2017) 376:1234–44.
15. Petraco R, Park JJ, Sen S, Nijjer SS, Malik IS, Echavarria-Pinto M, et al. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adoption of physiology-guided coronary revascularisation. EuroIntervention. (2013) 8:1157–65. doi: 10.4244/EIJV8I10A179
16. Shuttleworth K, Smith K, Watt J, Smith JAL, Leslie SJ. Hybrid instantaneous wave-free ratio-fractional flow reserve vs. fractional flow reserve in the real world. Front Cardiovasc Med. (2017) 4:35. doi: 10.3389/fcvm.2017.00035
17. Casanova-Sandoval J, Fernandez-Rodriguez D, Otaegui I, Gil Jimenez T, Rodriguez-Esteban M, Rivera K, et al. Usefulness of the hybrid RFR-FFR approach: results of a prospective and multicenter analysis of diagnostic agreement between RFR and FFR-the RECOPA (REsting full-cycle ratio comparation vs. fractional flow reserve (a prospective validation)) study. J Interv Cardiol. (2021) 2021:5522707. doi: 10.1155/2021/5522707
18. Wienemann H, Meyer A, Mauri V, Baar T, Adam M, Baldus S, et al. Comparison of resting full-cycle ratio and fractional flow reserve in a german real-world cohort. Front Cardiovasc Med. (2021) 8:744181. doi: 10.3389/fcvm.2021.744181
19. Niizuma S, Takiuchi S, Okada S, Horio T, Kamide K, Nakata H, et al. Decreased coronary flow reserve in haemodialysis patients. Nephrol Dial Transplant. (2008) 23:2324–8.
20. Tebaldi M, Biscaglia S, Fineschi M, Manari A, menozzi m, secco gg, et al. fractional flow reserve evaluation and chronic kidney disease: analysis from a multicenter italian registry (the FREAK study). Catheter Cardiovasc Interv. (2016) 88:555–62. doi: 10.1002/ccd.26364
21. Lee JM, Shin ES, Nam CW, Doh JH, Hwang D, Park J, et al. Discrepancy between fractional flow reserve and instantaneous wave-free ratio: clinical and angiographic characteristics. Int J Cardiol. (2017) 245:63–8.
22. Goto R, Takashima H, Ohashi H, Ando H, Suzuki A, Sakurai S, et al. Independent predictors of discordance between the resting full-cycle ratio and fractional flow reserve. Heart Vessels. (2021) 36:790–8.
23. Kobayashi Y, Johnson NP, Berry C, De Bruyne B, Gould KL, Jeremias A, et al. The influence of lesion location on the diagnostic accuracy of adenosine-free coronary pressure wire measurements. JACC Cardiovasc Interv. (2016) 9:2390–9. doi: 10.1016/j.jcin.2016.08.041
24. De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation. (2001) 104:157–62.
25. Lee JM, Rhee TM, Choi KH, Park J, Hwang D, Kim J, et al. Clinical outcome of lesions with discordant results among different invasive physiologic indices- resting distal coronary to aortic pressure ratio, resting full-cycle ratio, diastolic pressure ratio, instantaneous wave-free ratio, and fractional flow reserve. Circ J. (2019) 83:2210–21. doi: 10.1253/circj.CJ-19-0230
Keywords: fractional flow reserve, resting full-cycle ratio, coronary artery disease, percutaneous coronary intervention, coronary physiology
Citation: Chuang M-J, Chang C-C, Lee Y-H, Lu Y-W, Tsai Y-L, Chou R-H, Wu C-H, Lu T-M and Huang P-H (2022) Clinical assessment of resting full-cycle ratio and fractional flow reserve for coronary artery disease in a real-world cohort. Front. Cardiovasc. Med. 9:988820. doi: 10.3389/fcvm.2022.988820
Received: 07 July 2022; Accepted: 10 October 2022;
Published: 25 October 2022.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Łukasz Niewiara, Jagiellonian University Medical College, PolandEric Poon, St Vincent’s Hospital (Melbourne), Australia
Copyright © 2022 Chuang, Chang, Lee, Lu, Tsai, Chou, Wu, Lu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Chin Chang, aG9yYXdhbjI4QGdtYWlsLmNvbQ==; Po-Hsun Huang, aHVuYWdic3ZnaEBnbWFpbC5jb20=
 Ming-Ju Chuang
Ming-Ju Chuang Chun-Chin Chang
Chun-Chin Chang Yin-Hao Lee
Yin-Hao Lee Ya-Wen Lu1,2
Ya-Wen Lu1,2 Cheng-Hsueh Wu
Cheng-Hsueh Wu Po-Hsun Huang
Po-Hsun Huang