
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 25 August 2022
Sec. Lipids in Cardiovascular Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.988698
This article is part of the Research Topic Implications of Lipids and Modified Lipoproteins in Atherogenesis: From mechanisms towards novel diagnostic and therapeutic targets View all 16 articles
The amount of physical activity (PA) people practice everyday has been reducing in the last decades. Sedentary subjects tend to have an impaired lipid plasma profile with a higher risk of atherosclerosis and related cardio- and cerebrovascular events. Regular PA helps in both primary and secondary cardiovascular prevention because of its beneficial effect on the whole metabolism. Several studies reported lower levels of plasma lipids in trained subjects, but the precise mechanisms by which PA modulates lipoproteins remain only partially described. Thereupon, proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serin protease whose main function is to reduce the amount of low-density lipoprotein cholesterol (LDL-C) receptors, with the direct consequence of reducing LDL-C uptake by the liver and increasing its circulating pool. Accordingly, recently developed PCSK9 inhibitors improved cardiovascular prevention and are increasingly used to reach LDL-C goals in patients at high CV risk. Whether PA can modulate the levels of PCSK9 remains partially explored. Recent studies suggest PA as a negative modulator of such a deleterious CV mediator. Yet the level of evidence is limited. The aim of this review is to summarize the recent reports concerning the regulatory role of PA on PCSK9 plasma levels, highlighting the beneficial role of regular exercise on the prevention of atherosclerosis and overall CV health.
Regular exercise has been recommended to improve both quality and quantity of life in different clinical settings. The importance of fitness for a healthier life is particularly important nowadays, since the modern society tends to underestimate the time spent sitting in front of device screens, with almost two billion of physically inactive subjects worldwide (1). From being nomadic hunter gatherers, we became settled agriculturalists. Nowadays, we tend to spend even less time outdoor with many works are now available online and can be done at home using an internet connection. In this context, the recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) pandemic did not help. Epidemiological data report that the total amount of physical activity (PA) has progressively reduced in the last decades (2), meanwhile the number of obese subjects almost tripled worldwide (3). Obesity is a well-known cardiovascular risk factor and is associated with a high number of comorbidities involving most organs of our bodies.
However, physical activity might be aerobic or anaerobic. The aerobic training consists in exercises involving large group of muscles in a rhythmic and dynamic manner for a long time, while the anaerobic training in a more intense activity, exerted in a shorted time, and involving a restricted group of muscles (4). Even though, the aerobic physical exercise has always been regarded as healthier and therefore preferable, recent evidence shows that both aerobic and anaerobic physical activity may have beneficial effect on the cardiovascular system (4). Regular PA has protective effects against cardiovascular diseases and all-cause mortality (5) and atherosclerosis is negatively modulated by regular exercise (6). Even slight increase of daily amount of PA, in the magnitude of 30 min of light- to vigorous-intensity PA per day can significantly improve cardiovascular health (7). Greater results are obtained with regular high-intensity exercise training (8).
Our body has a greater ability to face PA and starvation periods rather than an excess of caloric intake and sedentarism. We have many hormonal pathways that can mobilize depots and increase circulating glucose and lipid levels, instead the machinery to reduce their levels is rather limited. As a result, the excess of caloric intake associated with low exercise training favor the development of metabolism impairment. The direct consequence is the slowly progression toward the so-called “X syndrome,” better known as the metabolic syndrome. This syndrome is associated with several cardiovascular diseases (9) and sudden cardiac death (10). CV prevention therefore finds a cornerstone in strategies aiming at regulating lipid levels by reducing low-density lipoprotein cholesterol (LDL-C) and increasing high-density lipoprotein cholesterol (HDL-C). PA was shown to have beneficial effects on lipid profile (with sex-related differences), especially when coupled with better dietary habits (11, 12). When this is not enough to reach the target lipoprotein levels suggested by the guidelines, pharmacological approaches including statins and PCSK9 inhibitors are suggested. In consideration of the recent paradigm for cholesterol levels “the lower the better,” the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors drugs such as alirocumab and evolocumab to reduce circulating LDL-C in patient at intermediate to very high risk is getting more and more common in the clinical practice.
PCSK9 is a serine protease whose main role is to favorite the catabolism of LDL-C receptor on the hepatocyte surface thereby reducing LDL-C internalization and increasing its circulating pool (13, 14). Reducing the PCSK9 plasma levels therefore leads to reduced circulating cholesterol levels with direct inhibitory effects on the atherosclerotic process. Yet, PCSK9 recently showed pleiotropic, non-LDL-C-mediated, effects that are nowadays known to mediate some of the anti-atherosclerotic effects of PCSK9 inhibitors (i.e., through inflammation) (15, 16). In fact, low grade inflammation is associated with PCSK9 transcription, especially in metabolic syndrome patients (17). Furthermore, inflammation favorite the expression of PCSK9 in the endothelial cells and vascular smooth muscle cells (18).
Whether PCSK9 mediates some of the beneficial effects of PA on plasma lipoproteins remains to be fully investigated. Recent evidence showed that PCSK9 plasma levels are regulated by regular exercise in both animal models and humans, as reported in Table 1. Although, the strength of this association and the possible pathways are not precisely described. The purpose of this review is to highlight the role of exercise training on PCSK9 plasma levels, as a prevention strategy against atherosclerosis. We searched PubMed and Web of Science for the following keywords: “proprotein convertase/subtilisin kexin type,” “PCSK9” in combination with “exercise” and “physical activity.” Additionally, a list of recent pre-clinical and clinical studies concerning this topic is reported as a table and discussed.
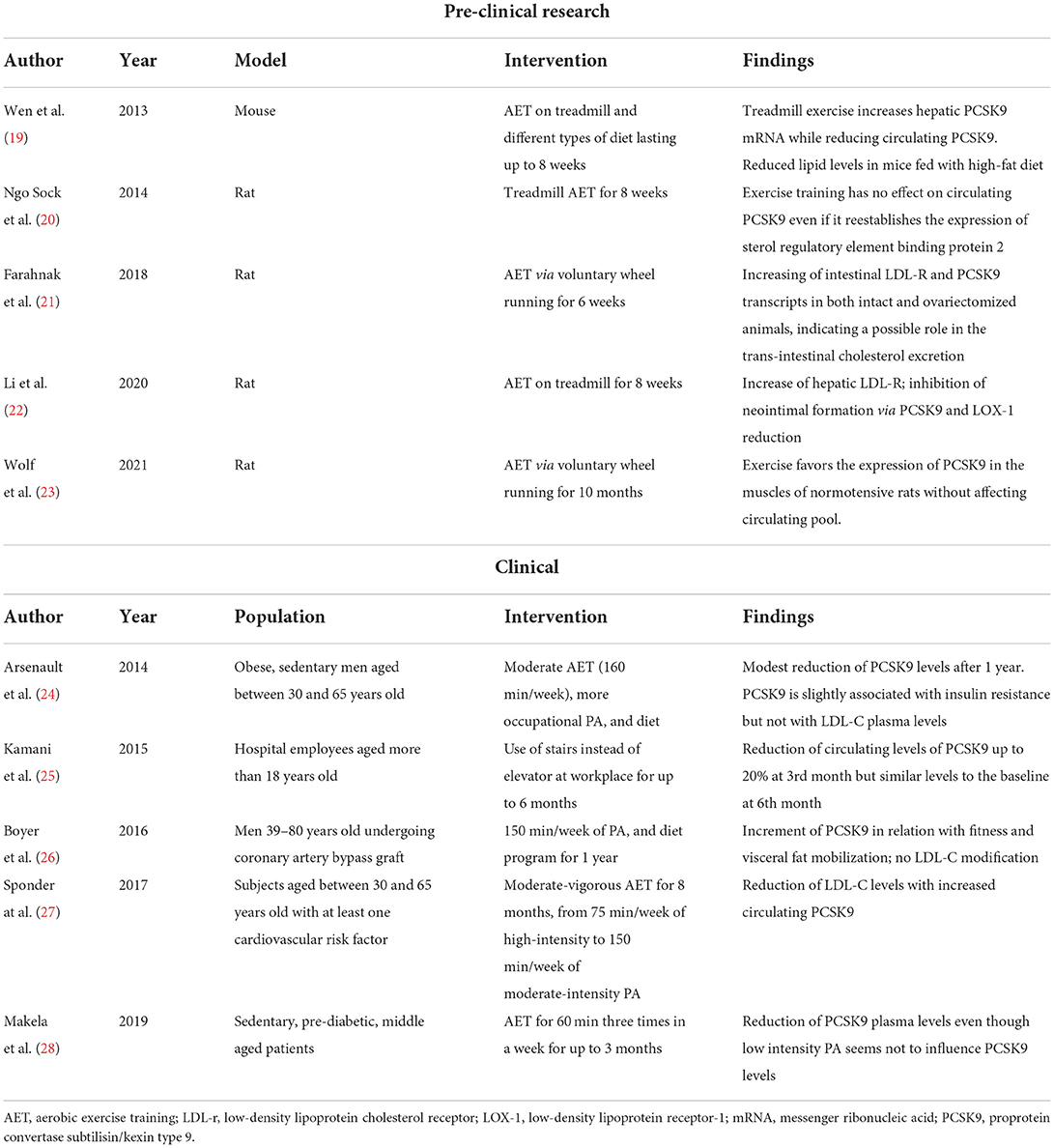
Table 1. Summary of recent pre-clinical and clinical studies evaluating the effect of exercise training on PCSK9.
Exercise training consists in a structured and repetitive PA that is practiced regularly. As longtime known, the amount and the quality of exercises and trainings can determine different results ranging from beneficial to even negative results in different settings (29). As a result, one of the major pitfalls in comparing different trials is due to the different type, intensity, and duration of the exercise protocol. Concerning the effect of exercise on cholesterol, several preclinical (30–32) and clinical studies (33–35) found an amelioration of the lipid plasma profile via regular exercise, especially aerobic one.
The modulation of circulating LDL-C in trained animals and humans relates to the adaptation of the body toward a higher metabolic state. Accordingly, the effects of exercise can be seen even acutely. Immediately after PA, LDL-C plasma levels decreases (36, 37). After termination, LDL-C levels tend to return to their basal levels, yet with regular exercise they remain lower on the long-term (38). Concerning the abovementioned adaptations, a higher metabolic state associates with a reduction of PCSK9. Consequently, the reduced catabolism of LDL receptor, increases the uptake of circulating LDL thereby reducing circulating cholesterol levels. Accordingly, recent evidence showed that PA increases the amount of hepatic LDL-C receptor (19, 39). Table 1 summarizes the main evidence proving an effect of PA on PCSK9 levels. As most studies agree on a beneficial lowering effect, an augmentation of PCSK9 levels may coexist together with LDL-C reduction in well-trained people (27). Such mixed effect might be due to many limitations in the analysis and interpretation of the studies. Furthermore, the amount of evidence concerning this topic remains quite limited.
Trained people tend to show higher amount of lean mass and lower levels of visceral fat (40–42). As known today, visceral fat is not only a storage tissue, but it plays a crucial role in the pathophysiology of different diseases (e.g., atherosclerosis) by releasing several mediators with local and systemic effects (i.e., adipocytokines) (Figure 1).
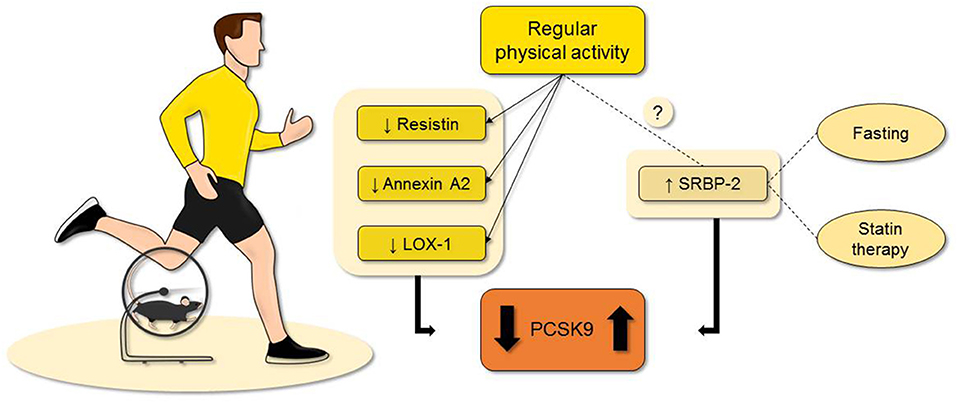
Figure 1. Evidence suggest that regular physical activity can regulate the PCSK9 plasma levels even though few evidence is available at the moment. As possible explanations, the reduction of resistin, annexin A2, and LOX-1 are reported with the most concrete results. Although, PCSK9 can be also augmented in trained subjects. This is probably related to a higher metabolism, a possible undiscovered mechanism, or confounding factors. While it is less known if exercise training affects the plasma levels of SREBP2, both fasting and statin treatment represent two confounding factors when evaluating the possible role of physical activity on PCSK9 levels. LOX-1, lectin-type oxidized low-density lipoprotein receptor-1; Proprotein convertase subtilisin/kexin type 9; SREBP2, sterol regulatory element-binding protein 2.
The hypertrophic visceral adipose tissue of obese patients undergoes degenerative remodeling including hypoxia and macrophage infiltration, favoring the development of inflammation with direct effects on the quality and quantity of released adipocytokines. The reduction of atheroprotective adiponectin (43) and the increased levels of resistin facilitate lipolysis with direct effects on vessel health (44). As exercise was shown to reduce resistin levels (45, 46), such adipokine can be a further link between exercise and PCSK9 since resistin was reported to increase the expression of PCSK9 in hepatocyte thereby modulating LDLR levels and indirectly atherogenesis (47). Furthermore, activation of adiponectin receptors was shown to regulate PCSK9 expression in experimental model of atherosclerosis with direct impact on the disease burden (48). Also, leptin interferes with PCSK9 expression via the activation of STAT3 and p38MAPK pathways (49, 50). Among, adipocytokines, fibroblast growth factor 21 is a peptide implied in fatty acids and glucose metabolism (51) under both physiological and pathological conditions including obesity and metabolic syndrome (52). Acute exercise was shown to increase the circulating levels of fibroblast growth factor 21 (53), which in turn impairs the expression of PCSK9 via the suppression of the hepatic sterol regulatory element binding protein 2 (54). Expressed by a variety of cell types including adipocytes, annexin A2 is an anionic phospholipid-binding proteins of the Ca2+ dependent family that was reported to reduce PCSK9 levels by favoring the modification of its catalytic subunit (55–57). Of interest, annexin A2 levels are elevated in people who regularly practice exercise (25), thereby indicating another possible molecular link.
Recently described as an important mediator of atherogenesis, lectin-type oxidized low-density lipoprotein receptor-1(LOX-1) may be another mediator of the effect of PA on PCSK9 levels. LOX-1 is a scavenger receptor with important function in oxidized LDL-C uptake by endothelial cells (58). PCSK9 and LOX-1 are both involved in the atherosclerotic process (59) and recent evidence showed that PCSK9 and LOX-1 influence each other in the vascular tissue (18, 60) and are co-related to the atheroma formation. Pre-clinical evidence showed that PA can reduce cholesterol accumulation in atherosclerotic plaques via the reduction of LOX-1 gene expression (61). Both PCSK9 and LOX-1 are reduced upon exercise (22).
Sterol regulatory element binding protein 2 (SREBP2) is a molecule implicated in the synthesis of cholesterol (62) as well as a transcriptional activator of PCSK9 (63, 64). For such reason, SREBP2 is often used to explain the paradoxical association of elevated PCSK9 levels and reduced LDL-C that is seen during fasting periods.
Even though it is not clear whether PA can modulate SREBP2 levels, regular exercise reduces the expression of several enzymes that regulates lipid metabolism in animal models (65, 66). The reduction of SREBP2 levels would end in lower PCSK9 plasma levels, as it happens during fasting (67, 68). Indeed, few days of fasting reduce the amount of circulating PCSK9 but with the unexpected results of increasing the quantity of circulating LDL-C (69).
Furthermore, regular PA can augment the circulating levels of interferon gamma, a cytokine with critical role in regulation of immune system, as showed in both pre-clinical (70) and clinical (71, 72) settings. Recent studies suggested a role for interferon gamma in PCSK9 regulation (73).
An excess of body fat is related to several detrimental is associated with higher risk of insulin resistance (74), and insulin resistance is one of the key element of the metabolic syndrome. Also, higher levels of insulin are associated with a higher expression of PCSK9 via sterol regulatory element binding protein 1c (SREBP-1c) (17). On the other hand, regular physical activity can improve insulin sensitivity (75–77).
First of all, the variations of LDL-C circulating levels can either be a consequence or a cause of PCSK9 variations. In fact, when PCSK9 modulate the amount of LDL-C also LDL-C can directly bind the PCSK9 molecule, causing a direct impairment of its functionality (78). Furthermore, many limitations reside in the evaluation of circulating levels of this mediator as it is not clear whether this is a fair counterpart of its hepatic levels. Also, as most studies investigate the levels of this molecule, they might not directly reflect its activity. Under this point of view, the presence of studies showing augmentation of PCSK9 along with reduction of LDL-C circulating levels might be explained by different PCSK9 functionality. Furthermore, most of the analytic method for detecting PCSK9 use antibodies binding to its mature form. However, the activity of PCSK9 resides in its catalytic processes (79).
As previously mentioned, another great limitation resides in the difficult standardization of exercise protocols. Also, with regard to clinical research, sample sizes are generally small, and some studies has non-negligible unbalanced gender and/or comorbidities differences. The interpretation of the relatively small number of recent clinical trials is also hampered by the fact that often they use holistic interventional program with prescription of both PA and diet. Lastly, the concomitant use of statin therapy can interfere with the interpretation of the results. In fact, statin treatment augments the amount of circulating PCSK9 (80–82), probably because they increase the levels of SREBP2 (83).
Regular exercise is known to ameliorate lipid profile and represent the cornerstone of cardiovascular prevention. Patient that practice regular exercise show a non-negligible reduced risk of developing atherosclerosis and possible cardiovascular events. The relationship between PCSK9 and PA can be a possible explanation with exercise training envisaging a non-pharmacological PCSK9 inhibitor. Indeed, the reduction of adiposity and molecules like LOX-1, annexin A2, fibroblast growth factor 21, and resistin secondary to exercise favor the reduction of PCSK9 in the bloodstream. Yet, at present, several limitations impact on the interpretation of the results including the difficult standardization of exercise protocols.
AT conceived and drafted the manuscript. FM and LL revised it for important intellectual content. All authors read and approved its final version.
LL received a research grant from the Swiss Heart Foundation.
Author LL is co-inventor on the International Patent WO/2020/226993 filed in April 2020; the patent relates to the use of antibodies which specifically bind interleukin-1α to reduce various sequelae of ischemia-reperfusion injury to the central nervous system.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. (2012) 380:247–57. doi: 10.1016/S0140-6736(12)60646-1
2. Archer E, Lavie CJ, McDonald SM, Thomas DM, Hebert JR, Taverno Ross SE, et al. Maternal inactivity: 45-year trends in mothers' use of time. Mayo Clin Proc. (2013) 88:1368–77. doi: 10.1016/j.mayocp.2013.09.009
3. NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. (2017) 390:2627–42. doi: 10.1016/S0140-6736(17)32129-3
4. Patel H, Alkhawam H, Madanieh R, Shah N, Kosmas CE, Vittorio TJ. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J Cardiol. (2017) 9:134–8. doi: 10.4330/wjc.v9.i2.134
5. Ekblom-Bak E, Halldin M, Vikstrom M, Stenling A, Gigante B, de Faire U, et al. Physical activity attenuates cardiovascular risk and mortality in men and women with and without the metabolic syndrome - a 20-year follow-up of a population-based cohort of 60-year-olds. Eur J Prev Cardiol. (2021) 28:1376–85. doi: 10.1177/2047487320916596
6. Lazaros G, Oikonomou E, Vogiatzi G, Christoforatou E, Tsalamandris S, Goliopoulou A, et al. The impact of sedentary behavior patterns on carotid atherosclerotic burden: implications from the corinthia epidemiological study. Atherosclerosis. (2019) 282:154–61. doi: 10.1016/j.atherosclerosis.2019.01.026
7. German C, Makarem N, Fanning J, Redline S, Elfassy T, McClain A, et al. Sleep, sedentary behavior, physical activity, and cardiovascular health: MESA. Med Sci Sports Exerc. (2021) 53:724–31. doi: 10.1249/MSS.0000000000002534
8. Karlsen T, Aamot IL, Haykowsky M, Rognmo O. High intensity interval training for maximizing health outcomes. Prog Cardiovasc Dis. (2017) 60:67–77. doi: 10.1016/j.pcad.2017.03.006
9. Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. (2017) 183:57–70. doi: 10.1016/j.trsl.2017.01.001
10. Tirandi A, Carbone F, Montecucco F, Liberale L. The role of metabolic syndrome in sudden cardiac death risk: recent evidence and future directions. Eur J Clin Invest. (2022) 52:e13693. doi: 10.1111/eci.13693
11. Skoumas J, Pitsavos C, Panagiotakos DB, Chrysohoou C, Zeimbekis A, Papaioannou I, et al. Physical activity, high density lipoprotein cholesterol and other lipids levels, in men and women from the ATTICA study. Lipids Health Dis. (2003) 2:3. doi: 10.1186/1476-511X-2-3
12. Pitanga FJG, Griep RH, Almeida MDC, Fonseca M, Souza AR, Silva RC, et al. Association between leisure time physical activity and HDL-C in the elsa-brasil study participants: are there any gender differences in the dose-response effect? Arq Bras Cardiol. (2021) 117:494–500. doi: 10.36660/abc.20200438
13. Glerup S, Schulz R, Laufs U, Schluter KD. Physiological and therapeutic regulation of PCSK9 activity in cardiovascular disease. Basic Res Cardiol. (2017) 112:32. doi: 10.1007/s00395-017-0619-0
14. Luquero A, Badimon L, Borrell-Pages M. PCSK9 functions in atherosclerosis are not limited to plasmatic LDL-cholesterol regulation. Front Cardiovasc Med. (2021) 8:639727. doi: 10.3389/fcvm.2021.639727
15. Giunzioni I, Tavori H, Covarrubias R, Major AS, Ding L, Zhang Y, et al. Local effects of human PCSK9 on the atherosclerotic lesion. J Pathol. (2016) 238:52–62. doi: 10.1002/path.4630
16. Macchi C, Ferri N, Sirtori CR, Corsini A, Banach M, Ruscica M. Proprotein convertase subtilisin/kexin type 9: a view beyond the canonical cholesterol-lowering impact. Am J Pathol. (2021) 191:1385–97. doi: 10.1016/j.ajpath.2021.04.016
17. Ferri N, Ruscica M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: insights on insulin resistance, inflammation, and atherogenic dyslipidemia. Endocrine. (2016) 54:588–601. doi: 10.1007/s12020-016-0939-0
18. Ding Z, Pothineni NVK, Goel A, Luscher TF, Mehta JL. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc Res. (2020) 116:908–15. doi: 10.1093/cvr/cvz313
19. Wen S, Jadhav KS, Williamson DL, Rideout TC. Treadmill exercise training modulates hepatic cholesterol metabolism and circulating PCSK9 concentration in high-fat-fed mice. J Lipids. (2013) 2013:908048. doi: 10.1155/2013/908048
20. Ngo Sock ET, Chapados NA, Lavoie JM. LDL receptor and Pcsk9 transcripts are decreased in liver of ovariectomized rats: effects of exercise training. Horm Metab Res. (2014) 46:550–5. doi: 10.1055/s-0034-1370910
21. Farahnak Z, Chapados N, Lavoie JM. Exercise training increased gene expression of LDL-R and PCSK9 in intestine: link to transintestinal cholesterol excretion. Gen Physiol Biophys. (2018) 37:309–17. doi: 10.4149/gpb_2017047
22. Li W, Park H, Guo E, Jo W, Sim KM, Lee SK. Aerobic exercise training inhibits neointimal formation via reduction of PCSK9 and LOX-1 in atherosclerosis. Biomedicines. (2020) 8:92. doi: 10.3390/biomedicines8040092
23. Wolf A, Kutsche HS, Atmanspacher F, Karadedeli MS, Schreckenberg R, Schluter KD. Untypical metabolic adaptations in spontaneously hypertensive rats to free running wheel activity includes uncoupling protein-3 (UCP-3) and proprotein convertase subtilisin/kexin type 9 (PCSK9) expression. Front Physiol. (2021) 12:598723. doi: 10.3389/fphys.2021.598723
24. Arsenault BJ, Pelletier-Beaumont E, Almeras N, Tremblay A, Poirier P, Bergeron J, et al. PCSK9 levels in abdominally obese men: association with cardiometabolic risk profile and effects of a one-year lifestyle modification program. Atherosclerosis. (2014) 236:321–6. doi: 10.1016/j.atherosclerosis.2014.07.010
25. Kamani CH, Gencer B, Montecucco F, Courvoisier D, Vuilleumier N, Meyer P, et al. Stairs instead of elevators at the workplace decreases PCSK9 levels in a healthy population. Eur J Clin Invest. (2015) 45:1017–24. doi: 10.1111/eci.12480
26. Boyer M, Levesque V, Poirier P, Marette A, Mathieu P, Despres JP, et al. Impact of a 1-year lifestyle modification program on plasma lipoprotein and PCSK9 concentrations in patients with coronary artery disease. J Clin Lipidol. (2016) 10:1353–61. doi: 10.1016/j.jacl.2016.08.014
27. Sponder M, Campean IA, Dalos D, Emich M, Fritzer-Szekeres M, Litschauer B, et al. Effect of long-term physical activity on PCSK9, high- and low-density lipoprotein cholesterol, and lipoprotein(a) levels: a prospective observational trial. Pol Arch Intern Med. (2017) 127:506–11. doi: 10.1093/eurheartj/ehx502.P1516
28. Makela KA, Leppaluoto J, Jokelainen J, Jamsa T, Keinanen-Kiukaanniemi S, Herzig KH. Effect of physical activity on plasma PCSK9 in subjects with high risk for type 2 diabetes. Front Physiol. (2019) 10:456. doi: 10.3389/fphys.2019.00456
29. Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr. (1999) 53:514–22. doi: 10.1038/sj.ejcn.1600784
30. Heeren MV, De Sousa LE, Mostarda C, Moreira E, Machert H, Rigatto KV, et al. Exercise improves cardiovascular control in a model of dislipidemia and menopause. Maturitas. (2009) 62:200–4. doi: 10.1016/j.maturitas.2008.12.011
31. Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. (2011) 218:323–9. doi: 10.1016/j.atherosclerosis.2011.06.040
32. Donatto FF, Neves RX, Rosa FO, Camargo RG, Ribeiro H, Matos-Neto EM, et al. Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour-bearing rats. Cytokine. (2013) 61:426–32. doi: 10.1016/j.cyto.2012.10.021
33. Knight S, Bermingham MA, Mahajan D. Regular non-vigorous physical activity and cholesterol levels in the elderly. Gerontology. (1999) 45:213–9. doi: 10.1159/000022090
34. Rigla M, Sanchez-Quesada JL, Ordonez-Llanos J, Prat T, Caixas A, Jorba O, et al. Effect of physical exercise on lipoprotein(a) and low-density lipoprotein modifications in type 1 and type 2 diabetic patients. Metabolism. (2000) 49:640–7. doi: 10.1016/S0026-0495(00)80041-4
35. Ruiz-Ramie JJ, Barber JL, Sarzynski MA. Effects of exercise on HDL functionality. Curr Opin Lipidol. (2019) 30:16–23. doi: 10.1097/MOL.0000000000000568
36. Ferguson MA, Alderson NL, Trost SG, Davis PG, Mosher PE, Durstine JL. Plasma lipid and lipoprotein responses during exercise. Scand J Clin Lab Invest. (2003) 63:73–9. doi: 10.1080/00365510310000510
37. Rahnama N, Younesian A, Mohammadion M, Bambaeichi E. A 90 minute soccer match decreases triglyceride and low density lipoprotein but not high-density lipoprotein and cholesterol levels. J Res Med Sci. (2009) 14:335–41.
38. Kujala UM, Ahotupa M, Vasankari T, Kaprio J, Tikkanen MJ. Low LDL oxidation in veteran endurance athletes. Scand J Med Sci Sports. (1996) 6:303–8. doi: 10.1111/j.1600-0838.1996.tb00475.x
39. Pinto PR, Rocco DD, Okuda LS, Machado-Lima A, Castilho G, da Silva KS, et al. Aerobic exercise training enhances the in vivo cholesterol trafficking from macrophages to the liver independently of changes in the expression of genes involved in lipid flux in macrophages and aorta. Lipids Health Dis. (2015) 14:109. doi: 10.1186/s12944-015-0093-3
40. Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev. (2006) 7:183–200. doi: 10.1111/j.1467-789X.2006.00250.x
41. Dahl-Petersen IK, Brage S, Bjerregaard P, Tolstrup JS, Jorgensen ME. Physical activity and abdominal fat distribution in greenland. Med Sci Sports Exerc. (2017) 49:2064–70. doi: 10.1249/MSS.0000000000001337
42. Jung HC, Jeon S, Lee NH, Kim K, Kang M, Lee S. Effects of exercise intervention on visceral fat in obese children and adolescents. J Sports Med Phys Fitness. (2019) 59:1045–57. doi: 10.23736/S0022-4707.18.08935-1
43. Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. (2019) 7:715–25. doi: 10.1016/S2213-8587(19)30084-1
44. Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. (2018) 137:1391–406. doi: 10.1161/CIRCULATIONAHA.117.029617
45. Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care. (2007) 30:719–21. doi: 10.2337/dc06-1149
46. Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, et al. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. (2008) 19:371–5. doi: 10.1016/j.jnutbio.2007.05.007
47. Melone M, Wilsie L, Palyha O, Strack A, Rashid S. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J Am Coll Cardiol. (2012) 59:1697–705. doi: 10.1016/j.jacc.2011.11.064
48. Sun L, Yang X, Li Q, Zeng P, Liu Y, Liu L, et al. Activation of adiponectin receptor regulates proprotein convertase subtilisin/kexin type 9 expression and inhibits lesions in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. (2017) 37:1290–300. doi: 10.1161/ATVBAHA.117.309630
49. Du Y, Li S, Cui CJ, Zhang Y, Yang SH, Li JJ. Leptin decreases the expression of low-density lipoprotein receptor via PCSK9 pathway: linking dyslipidemia with obesity. J Transl Med. (2016) 14:276. doi: 10.1186/s12967-016-1032-4
50. Macchi C, Greco MF, Botta M, Sperandeo P, Dongiovanni P, Valenti L, et al. Leptin, resistin, and proprotein convertase subtilisin/kexin type 9: the role of STAT3. Am J Pathol. (2020) 190:2226–36. doi: 10.1016/j.ajpath.2020.07.016
51. Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. (2016) 78:223–41. doi: 10.1146/annurev-physiol-021115-105339
52. Xie T, Leung PS. Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am J Physiol Endocrinol Metab. (2017) 313:E292–302. doi: 10.1152/ajpendo.00101.2017
53. Khalafi M, Alamdari KA, Symonds ME, Nobari H, Carlos-Vivas J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: systematic review and meta-analysis. Hormones. (2021) 20:23–33. doi: 10.1007/s42000-020-00245-3
54. Huang Z, Xu A, Cheung BMY. The potential role of fibroblast growth factor 21 in lipid metabolism and hypertension. Curr Hypertens Rep. (2017) 19:28. doi: 10.1007/s11906-017-0730-5
55. Ly K, Saavedra YG, Canuel M, Routhier S, Desjardins R, Hamelin J, et al. Annexin A2 reduces PCSK9 protein levels via a translational mechanism and interacts with the M1 and M2 domains of PCSK9. J Biol Chem. (2014) 289:17732–46. doi: 10.1074/jbc.M113.541094
56. Mayer G, Poirier S, Seidah NG. Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels. J Biol Chem. (2008) 283:31791–801. doi: 10.1074/jbc.M805971200
57. Seidah NG, Poirier S, Denis M, Parker R, Miao B, Mapelli C, et al. Annexin A2 is a natural extrahepatic inhibitor of the PCSK9-induced LDL receptor degradation. PLoS ONE. (2012) 7:e41865. doi: 10.1371/journal.pone.0041865
58. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. (2013) 2013:152786. doi: 10.1155/2013/152786
59. Tam J, Thankam F, Agrawal DK, Radwan MM. Critical role of LOX-1-PCSK9 axis in the pathogenesis of atheroma formation and its instability. Heart Lung Circ. (2021) 30:1456–66. doi: 10.1016/j.hlc.2021.05.085
60. Ding Z, Liu S, Wang X, Deng X, Fan Y, Shahanawaz J, et al. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc Res. (2015) 107:556–67. doi: 10.1093/cvr/cvv178
61. Pinto PR, da Silva KS, Iborra RT, Okuda LS, Gomes-Kjerulf D, Ferreira GS, et al. Exercise training favorably modulates gene and protein expression that regulate arterial cholesterol content in CETP transgenic mice. Front Physiol. (2018) 9:502. doi: 10.3389/fphys.2018.00502
62. Weber LW, Boll M, Stampfl A. Maintaining cholesterol homeostasis: sterol regulatory element-binding proteins. World J Gastroenterol. (2004) 10:3081–7. doi: 10.3748/wjg.v10.i21.3081
63. Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. (2003) 100:12027–32. doi: 10.1073/pnas.1534923100
64. Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res. (2008) 49:399–409. doi: 10.1194/jlr.M700443-JLR200
65. Griffiths MA, Fiebig R, Gore MT, Baker DH, Esser K, Oscai L, et al. Exercise down-regulates hepatic lipogenic enzymes in food-deprived and refed rats. J Nutr. (1996) 126:1959–71.
66. Fiebig RG, Hollander JM, Ji LL. Exercise down-regulates hepatic fatty acid synthase in streptozotocin-treated rats. J Nutr. (2001) 131:2252–9. doi: 10.1093/jn/131.9.2252
67. Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA. (1998) 95:5987–92. doi: 10.1073/pnas.95.11.5987
68. Persson L, Cao G, Stahle L, Sjoberg BG, Troutt JS, Konrad RJ, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. (2010) 30:2666–72. doi: 10.1161/ATVBAHA.110.214130
69. Browning JD, Horton JD. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J Lipid Res. (2010) 51:3359–63. doi: 10.1194/jlr.P009860
70. Heidarianpour A, Vahidian Rezazadeh M, Zamani A. Effect of moderate exercise on serum interferon-gamma and interleukin-17 levels in the morphine withdrawal period. Int J High Risk Behav Addict. (2016) 5:e26907. doi: 10.5812/ijhrba.26907
71. Vijayaraghava A, K R. Alteration of interferon gamma (IFN-gamma) in human plasma with graded physical activity. J Clin Diagn Res. (2014) 8:BC05–7. doi: 10.7860/JCDR/2014/9502.4440
72. Zamani A, Salehi I, Alahgholi-Hajibehzad M. Moderate exercise enhances the production of interferon-gamma and interleukin-12 in peripheral blood mononuclear cells. Immune Netw. (2017) 17:186–91. doi: 10.4110/in.2017.17.3.186
73. Pirault J, Polyzos KA, Petri MH, Ketelhuth DFJ, Back M, Hansson GK. The inflammatory cytokine interferon-gamma inhibits sortilin-1 expression in hepatocytes via the JAK/STAT pathway. Eur J Immunol. (2017) 47:1918–24. doi: 10.1002/eji.201646768
74. Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. (2013) 5:2019–27. doi: 10.3390/nu5062019
75. Yamanouchi K, Nakajima H, Shinozaki T, Chikada K, Kato K, Oshida Y, et al. Effects of daily physical activity on insulin action in the elderly. J Appl Physiol. (1992) 73:2241–5. doi: 10.1152/jappl.1992.73.6.2241
76. Macias-Cervantes MH, Malacara JM, Garay-Sevilla ME, Diaz-Cisneros FJ. Effect of recreational physical activity on insulin levels in Mexican/Hispanic children. Eur J Pediatr. (2009) 168:1195–202. doi: 10.1007/s00431-008-0907-7
77. Cai C, Zhang Z, McDonald S, Strom C, Skow RJ, May LE, et al. Leisure-Time physical activity before and during pregnancy is associated with improved insulin resistance in late pregnancy. Int J Environ Res Public Health. (2021) 18:4413. doi: 10.3390/ijerph18094413
78. Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem. (2013) 288:8279–88. doi: 10.1074/jbc.M112.421370
79. Farnier M. The role of proprotein convertase subtilisin/kexin type 9 in hyperlipidemia: focus on therapeutic implications. Am J Cardiovasc Drugs. (2011) 11:145–52. doi: 10.2165/11590330-000000000-00000
80. Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. (2008) 49:394–8. doi: 10.1194/jlr.M700437-JLR200
81. Mayne J, Dewpura T, Raymond A, Cousins M, Chaplin A, Lahey KA, et al. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. (2008) 7:22. doi: 10.1186/1476-511X-7-22
82. Sahebkar A, Simental-Mendia LE, Guerrero-Romero F, Golledge J, Watts GF. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: a systematic review and meta-analysis of clinical trials. Diabetes Obes Metab. (2015) 17:1042–55. doi: 10.1111/dom.12536
Keywords: proprotein convertase subtilisin/kexin type 9, physical activity, exercise, inflammation, cardiovascular
Citation: Tirandi A, Montecucco F and Liberale L (2022) Physical activity to reduce PCSK9 levels. Front. Cardiovasc. Med. 9:988698. doi: 10.3389/fcvm.2022.988698
Received: 07 July 2022; Accepted: 08 August 2022;
Published: 25 August 2022.
Edited by:
Tatsuya Sawamura, Shinshu University, JapanReviewed by:
Chiara Macchi, University of Milan, ItalyCopyright © 2022 Tirandi, Montecucco and Liberale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Liberale, bHVjYS5saWJlcmFsZUB1bmlnZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.