
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 20 September 2022
Sec. Cardiovascular Genetics and Systems Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.985121
 Ru Li1†
Ru Li1† Huan Zhang1†
Huan Zhang1† Fan Tang1
Fan Tang1 Chengcheng Duan1
Chengcheng Duan1 Dan Liu2
Dan Liu2 Naqiong Wu3
Naqiong Wu3 Yonghong Zhang1
Yonghong Zhang1 Laiyuan Wang2*
Laiyuan Wang2* Xingbo Mo1,4*
Xingbo Mo1,4*Background: Single nucleotide polymorphisms that affect RNA modification (RNAm-SNPs) may have functional roles in coronary artery disease (CAD). The aim of this study was to identify RNAm-SNPs in CAD susceptibility loci and highlight potential risk factors.
Methods: CAD-associated RNAm-SNPs were identified in the CARDIoGRAMplusC4D and UK Biobank genome-wide association studies. Gene expression and circulating protein levels affected by the RNAm-SNPs were identified by QTL analyses. Cell experiments and Mendelian randomization (MR) methods were applied to test whether the gene expression levels were associated with CAD.
Results: We identified 81 RNAm-SNPs that were associated with CAD or acute myocardial infarction (AMI), including m6A-, m1A-, m5C-, A-to-I- and m7G-related SNPs. The m6A-SNPs rs3739998 in JCAD, rs148172130 in RPL14 and rs12190287 in TCF21 and the m7G-SNP rs186643756 in PVT1 were genome-wide significant. The RNAm-SNPs were associated with gene expression (e.g., MRAS, DHX36, TCF21, JCAD and SH2B3), and the expression levels were associated with CAD. Differential m6A methylation and differential expression in FTO-overexpressing human aorta smooth muscle cells and peripheral blood mononuclear cells of CAD patients and controls were detected. The RNAm-SNPs were associated with circulating levels of proteins with specific biological functions, such as blood coagulation, and the proteins (e.g., cardiotrophin-1) were confirmed to be associated with CAD and AMI in MR analyses.
Conclusion: The present study identified RNAm-SNPs in CAD susceptibility genes, gene expression and circulating proteins as risk factors for CAD and suggested that RNA modification may play a role in the pathogenesis of CAD.
Coronary artery disease (CAD) is the leading global cause of death (1). As an archetypal common complex disease, a person's risk of developing CAD is modulated by a combination of genetic and environmental factors (2). Family-based and twin-pair studies showed that CAD is partly heritable due to genetic and possibly epigenetic factors, and its broad-sense heritability has been estimated to be between 40 and 60% (3). Over the past decade, great progress has been made in understanding the genetics of CAD (4). Genome-wide association studies (GWASs) have provided valuable clues to the understanding of the pathophysiology of this complex disease. More than 100 CAD-associated loci have been identified by previous large-scale GWAS (5–11). Some genetic variants in the identified loci have been shown to affect gene expression. However, most of their functions remain largely unknown.
Since the first discovery of modifiable nucleosides, more than 170 distinct RNA modification types have been detected in all types of RNA molecules (12, 13). Modifiable RNA modifications are involved in the regulation of various biological processes in eukaryotic cells (14). In the study of posttranscriptional gene regulation, RNA modifications are becoming increasingly important. Several RNA modification types, including m6A (N6-adenosine methylation), m6Am (N6,2'-O-dimethyladenosine), m7G (N7-methylguanosine), m1A (N1-adenosine methylation), m5C (5-methylcytidin), m5U (5-methyluridine), Nm (ribose 2'-O-methylation), pseudouridine and A-to-I RNA editing, have been explored since sufficiently sensitive high-resolution transcriptome-wide techniques are available. N6-methyladenosine (m6A) is the most common reversible RNA methylation that plays roles in nearly every aspect of the mRNA lifecycle and in various disease processes (15). m6A methylation has become a research hotspot. Recently, genetic variants, e.g., RNA modification-related SNPs (RNAm-SNPs), that impact several types of RNA modifications by changing the modification target sites or RNA sequences around modifiable nucleotides have been highlighted (16). The RNAm-SNPs in CAD-associated genomic loci may have impacts on RNA modifications and may be putative functional variants for CAD. However, studies on the relationship between RNAm-SNPs and CAD are very scarce.
The identification of RNAm-SNPs as functional variants should help with the translation of GWAS signals into causal mechanisms and clinical applications. The aim of this study was to evaluate the impacts of RNAm-SNPs on CAD using publicly available GWAS summary datasets. Quantitative trait locus (QTL) analysis, cell experiments and Mendelian randomization (MR) analysis were performed to identify the role of the RNAm-SNPs.
The RNAm-SNPs annotation information was obtained in the RMVar database (http://rmvar.renlab.org/download.html), which contains 1,678,126 RNAm-SNPs related to the nine kinds of RNA modifications (m6A, m5C, m5U, m7G, m1A, m6Am, 2′-O-Me, A-to-I and pseudouridine) (17). In the RMVar database, the RNAm-SNPs are classified into three categories according to confidence levels: high, medium and low. RNAm-SNPs determined by single-nucleotide resolution experiments (e.g., miCLIP, m6A-Label-seq, BS-Seq and DART-seq) have high confidence. RNAm-SNPs obtained from MeRIP-Seq experiments were defined as having a medium confidence level. m6A sites with low confidence levels were predicted by a statistical model.
We obtained functional interpretations for CAD GWAS signals by integrating novel RNA modification annotations (i.e., RNAm-SNPs) and summary data from the CARDIoGRAMplusC4D CAD GWAS (7). The GWAS examined the associations between genome-wide SNPs and CAD risk in approximately 185 thousand individuals, mainly (77%) of European descent. The raw data used in our analysis were summary statistics downloaded at http://www.cardiogramplusc4d.org/data-downloads/. The dataset embodied effect size (beta), standard error and P values of associations between approximately 9.5 million SNPs and CAD.
We also annotated RNAm-SNPs in UK Biobank GWAS summary data of acute myocardial infarction (AMI, ICD-10 code: I21). The UK Biobank AMI GWAS included 8,764 AMI patients and 443,500 controls (18). GWAS summary data of AMI (ICD-10 code: I21) were obtained at http://geneatlas.roslin.ed.ac.uk/downloads/. The dataset comprises summary statistics of the genome-wide associations between 9,113,133 imputed variants and AMI analyzed in the linear mixed models. Detailed information on the GWAS can be identified in previous UK Biobank publications (19).
When the GWAS summary data files were downloaded, the CAD-associated SNPs were selected (P < 1.0 × 10−4 were considered). Then, the dataset containing the CAD-associated SNPs was combined with the RNAm-SNP annotation files based on the “rs ID numbers” column using the “merge” function of the R program. By this combination, the SNPs in GWAS summary datasets were annotated according to the RNA modification annotations of the RNAm-SNP sets. Then, RNAm-SNPs associated with CAD were identified.
RNA modification plays a critical role in gene expression regulation. Therefore, CAD-associated RNAm-SNPs may influence gene expression by affecting RNA modification. We performed gene expression QTL (eQTL) analysis to further elaborate the association between the CAD-associated RNAm-SNPs and mRNA expression levels in several types of tissues. We carried out cis-acting eQTL analysis in different cells and tissues to obtain functional evidence for the RNAm-SNPs by searching data from the HaploReg browser (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php) (20). Signals in 10 relevant tissues (coronary artery, aortic artery, tibial artery, atrial appendage, left ventricle, liver, visceral omentum adipose, subcutaneous adipose, adrenal gland and pancreas) and whole blood cells were considered.
We performed a summary data–based Mendelian randomization (SMR) (21) analysis to identify associations between gene expression levels and CAD by integrating eQTL data from the GTEx project (22) with the CARDIoGRAMplusC4D GWAS data. The eQTL datasets that contain summary statistics of gene expression levels in 10 relevant tissues (coronary artery, aorta artery, tibial artery, atrial appendage, left ventricle, liver, visceral omentum adipose, subcutaneous adipose, adrenal gland and pancreas) and whole blood cells were used in the analysis (http://cnsgenomics.com/software/smr/#DataResource). The CAD summary statistics required in the SMR analysis were sorted from the CARDIoGRAMplusC4D GWAS datasets. In SMR analysis, the top-associated eQTL for each gene was used as an instrumental variable to examine association with CAD.
SMR software (version 0.712) was used with default parameters. Genotype data from HapMap r23 CEU were used as a reference panel to calculate the correlation matrix for SNPs analyzed in SMR. The significance threshold was adjusted with Bonferroni correction. The genome-wide significance threshold for the SMR analysis was 5.0 × 10−5. Meanwhile, the heterogeneity in dependent instruments (HEIDI) test was performed to test the ‘no horizontal pleiotropy' assumption, the basic assumption of MR study. The HEIDI test was conducted to examine whether there was a single causal SNP affecting CAD and gene expression. Multiple testing correction is not required in the HEIDI test, as Zhu et al. did in the original paper of this method (21). Genes without heterogeneity (PHEIDI ≥ 0.05) were considered. The results of the HEIDI tests are presented in the Supplementary Tables S1–S6.
We attempted to determine whether the interference of RNAm-SNPs on m6A methylation and gene expression affects CAD. FTO is an RNA demethylase whose major substrate is m6A methylation and is an important regulator of mRNA expression. Overexpression of FTO decreased m6A levels on RNA (23). We therefore compared the effect of FTO overexpression on m6A modification and the expression of the genes identified to be affected by the RNAm-SNPs in human aorta smooth muscle cells (HASMCs). The HASMCs were purchased from ScienCell (Catalog #6110, San Diego, CA, USA). On receiving them, cells were seeded into each well of 6-well plates at a concentration of 2 × 106 cells per plate and cultured in Smooth Muscle Cell Medium supplemented with 2% fetal bovine serum (Catalog #0010; Thermo Fisher Scientific), 1% smooth muscle cell growth supplement (Catalog #1152) and 1% penicillin/streptomycin solution (Catalog #0503). Cells were grown at 37°C in a humidified 5% CO2 incubator. The medium was replaced every 48 h. When cells were cultured to subconfluence, they were subcultured with 0.25% trypsin-EDTA (Gibco, Life Technology, USA) at a ratio of 1:3. HASMCs from passages 5 to 7 were used in the following experiments. For adenovirus-mediated overexpression, Ad-FTO (Vigenebio, Shandong, China) was added to the culture medium, and HASMCs were transfected with Ad-FTO at a multiplicity of infection of 100 at 37°C. The transfected HASMCs were incubated for an additional 48 h. A recombinant adenovirus encoding enhanced GFP (Ad-GFP) was used as a negative control.
RNA was extracted from peripheral blood mononuclear cells (PBMCs) of five CAD patients and five healthy controls (24) and from FTO-overexpressing HASMCs (n = 3) and control HASMCs (n = 3) for MeRIP-seq and RNA-seq. The MeRIP-seq experiment was conducted by Guangzhou Epibiotek Co., Ltd. (Guangzhou, China) in compliance with our previously published procedure (24) with slight modifications. Briefly, RNAs were fragmented into 100 nt fragments after removing rRNA with a Ribo-Zero rRNA Removal Kit (Illumina, MRZG12324). A strand-specific RNA library was constructed using 10 ng fragmented RNA in accordance with the UTP method. The remaining fragmented RNA was incubated with anti-m6A polyclonal antibody (Synaptic Systems, 202003) in immunoprecipitation (IP) buffer at 4°C for 2 h. The mixture was then immunoprecipitated with protein-A beads (Thermo Fisher Scientific) at 4°C for an additional 2 h. Then, the immunoprecipitated RNA was eluted from the beads with N6-methyladenosine (Berry & Associates, PR3732) in IP buffer and extracted with TRIzol reagent (Thermo Fisher Scientific, 15596026). Purified RNA (the m6A IP and the input samples without IP sample each) was used for RNA-seq library construction with the NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina® (NEB, #E7760). The IP library (MeRIP-seq) and input library (RNA-seq) were each subjected to an Illumina HiSeq 4000 sequencer (Illumina, Inc.) with 150 bp paired-end reads. The harvested paired-end reads were analyzed by Q30. When the Q30 > 80%, the reads were qualified. The clean reads of all libraries were obtained after trimming 3' adaptors, and low-quality reads were removed by Cutadapt software (v1.9.3) (25); then, the reads were aligned by using Hisat2 software (v2.0.4) (26). MACS software (27) was used to identify methylated peaks on RNAs. DiffReps software (28) was used to identify differentially methylated lncRNAs/mRNAs.
We further examined the differential expression of the identified genes in PBMCs. Transcriptome-wide lncRNA and mRNA expression profiles of PBMCs of 93 CAD patients and 48 healthy controls were obtained. Details of our transcriptome-wide lncRNA and mRNA expression profile were described in a previous publication (29). The microarray data were normalized and analyzed using GeneSpring software V12.0 (Agilent Technologies). Differential expression of the identified CAD-related genes between CAD cases and controls was tested by comparing mean gene expression levels between cases and controls using t tests. The P value was two-tailed. Probes with fold change > 2.0 and FDR <0.05 were considered differentially expressed.
To look for proteins affected by the RNAm-SNPs, we carried out pQTL analysis in peripheral blood by using data from a published pQTL study, the INTERVAL study. This pQTL study tested the associations between approximately 10 million genetic variants and circulating levels of 2,994 proteins measured in 3,301 individuals of European descent (30). The association data used in the pQTL analysis were downloaded at http://www.phpc.cam.ac.uk/ceu/proteins/.
To shed light on the pathological significance of the identified proteins in CAD, functional enrichment analyses were performed using the DAVID database (Database for Annotation, Visualization and Integrated Discovery, https://david.ncifcrf.gov/). GO and KEGG pathway analyses were performed to cluster functional genes into different biological processes in which the genes coding the identified proteins were involved. DAVID contains a set of integrated biological knowledge bases and analytic tools for extracting biological significance from large gene/protein lists in a systematic way (31, 32). The results of the functional enrichment analyses are presented in bubble diagrams, which were created by using the “ggplot2” R package.
Finally, we attempted to prove that the proteins identified in pQTL analysis were associated with CAD. Five MR methods, including the weighted median (33), inverse-variance weighted (IVW) (34), MR-Egger (35), MR-PRESSO (MR pleiotropy residual sum and outlier) (36) and CAUSE (Causal Analysis Using Summary Effect estimates) (37), were employed to examine the causal associations between circulating protein levels and CAD. In a fixed-effects meta-analysis model, the IVW method combines the ratios of SNP-exposure to SNP-outcome estimates from each instrument variable (34). If more than 50% of the weights for the SNPs come from valid SNPs, the weighted median estimation can produce a consistent assessment (33). The intercept of the MR-egger further tested horizontal pleiotropy (35). The weighted median, IVW and MR-Egger analyses were performed by applying the “mr_allmethods” function in the “MendelianRandomization” R package (38). MR-PRESSO is a method that systematically detects and corrects horizontal pleiotropic outliers in MR testing through three steps: the MR-PRESSO global test, the MR-PRESSO outlier test and the MR-PRESSO distortion test. The outlying genetic variants were identified by applying this method (36). The source code and documents for MR-PRESSO are available at https://github.com/rondolab/MR-PRESSO. The default parameters were used for the MR-PRESSO analysis. The parameters were left to default in the MR-PRESSO analysis.
The requisite data (i.e., SNP rs number, beta, standard error, and P value) were extracted from each of the CAD GWASs and pQTL studies mentioned above and then merged by SNP to form a plain file with 7 columns (i.e., SNP rs number, beta for protein, standard error for protein, P value for protein, beta for CAD, standard error for CAD and P value for CAD) for the MR analysis using the R language. We sorted out the pQTLs with P < 5.0 × 10−6 as potential instrumental variables. The selection criterion was set to 5.0 × 10−6 because 5.0 × 10−8 would lead to too few instrumental variables. We harmonized the genetic association between the pQTL and CAD GWAS to ensure that they reflected the same effect allele. We then conducted linkage disequilibrium clumping on these SNPs to obtain the independent pQTL (LD r2 <0.001, within 10,000 kb) for each protein. Linkage disequilibrium clumping was realized through the “clump_data” function provided by the “TwoSampleMR” R package with reference to the 1000 Genomes EUR population.
We finally applied the CAUSE (an R package) method to differentiate correlated pleiotropy from causal effects for proteins that passed the four MR tests described above. The CAUSE method accounts for both correlated and uncorrelated horizontal pleiotropic effects using summary statistics of genome-wide SNPs (37). CAUSE included as much information from all of the genome-wide variants as possible, even weakly associated variants. Genome-wide summary statistics from the pQTL study and GWASs described above were used in CAUSE analysis. In the analyses, nuisance parameters were estimated using 1,000,000 SNPs. Other parameters were left as the defaults.
We selected RNAm-SNPs from the CAD GWAS datasets according to the annotation information of the RNAm-SNPs. We found 37 RNAm-SNPs that were associated with CAD at P < 1.0 × 10−4, including 34 m6A-, 1 m1A- and 2 m7G-related SNPs. These 37 RNAm-SNPs mapped to 32 protein coding and noncoding genes.
A total of 923 m6A-SNPs among the 9.5 million SNPs were associated with CAD at P < 0.05 (Figure 1). Among them, 34 m6A-SNPs, including 10 high, 12 medium and 12 low (prediction) confidence levels, were associated with CAD at P < 1.0 × 10−4. Twenty-seven (79.4%) of them were functional loss, while 7 (20.6%) were functional gain m6A-SNPs. One m6A-SNP, rs1811351, mapped to the pseudogene SERBP1P3, and the remaining 33 m6A-SNPs mapped to 29 protein-coding genes. Of these 33 m6A-SNPs mapped to protein coding genes, 10 (30.3%) were exonic, 9 (27.3%) were in the 3'-UTR, 2 (6.1%) were in the 5'-UTR and 12 (36.4%) were intronic. Of note, the m6A-SNP rs3739998 (missense variant) at the sixth exon of JCAD (KIAA1462) was significantly associated with CAD at the genome-wide level (P = 2.44 × 10−9). This SNP is a m6A functional loss and belongs to the medium confidence category. We noticed that rs3739998 is not the top SNP in this locus (Figure 2A). In addition, we also found m6A-SNPs in other well-characterized CAD susceptibility genes, such as MRAS (Figure 3A), LPL, TCF21, MYH11 and SMG6.
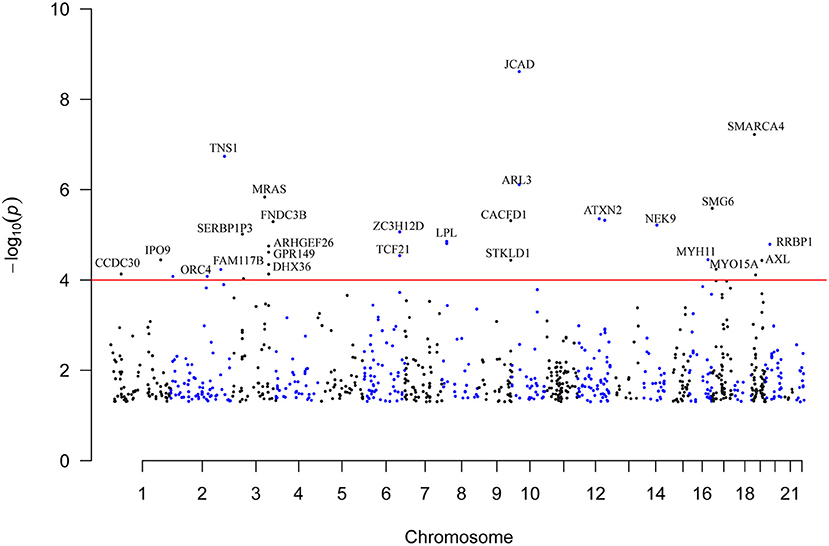
Figure 1. Genome-wide distribution of the identified CAD-associated m6A-SNPs. This is a Manhattan plot that shows the P values of associations between m6A-SNPs and CAD. The x-axis is chromosome positions. The y-axis is the -log10 P values of the associations. The P value information was obtained from the summary dataset of the CAD GWAS. The red line indicates the significance level of 1.0 × 10−4.
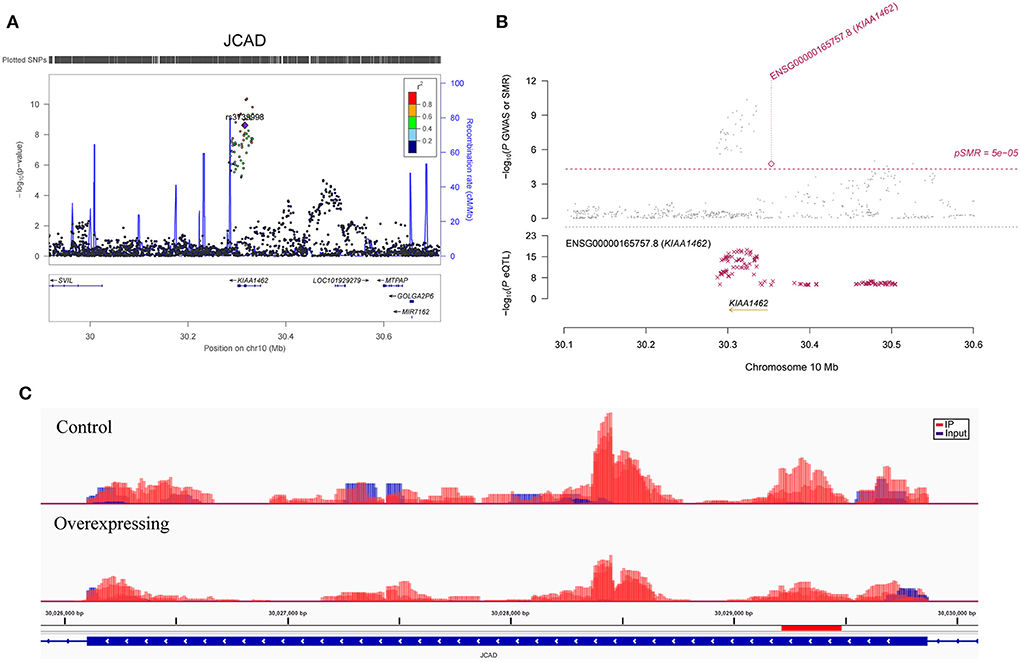
Figure 2. Association between the JCAD gene and CAD. (A) The m6A-SNP rs3739998 in the JCAD (KIAA1462, reference assembly: GRCh37.p13) gene was associated with CAD; (B) SNPs in JCAD were strongly associated with the expression level of JCAD in aortic artery tissue, and the expression level of JCAD in aortic artery tissue was associated with CAD (reference assembly: GRCh37.p13); (C) The m6A methylation peaks in the sixth exon of JCAD in FTO-overexpressing and control HASMCs (reference assembly: GRCh38.p14).
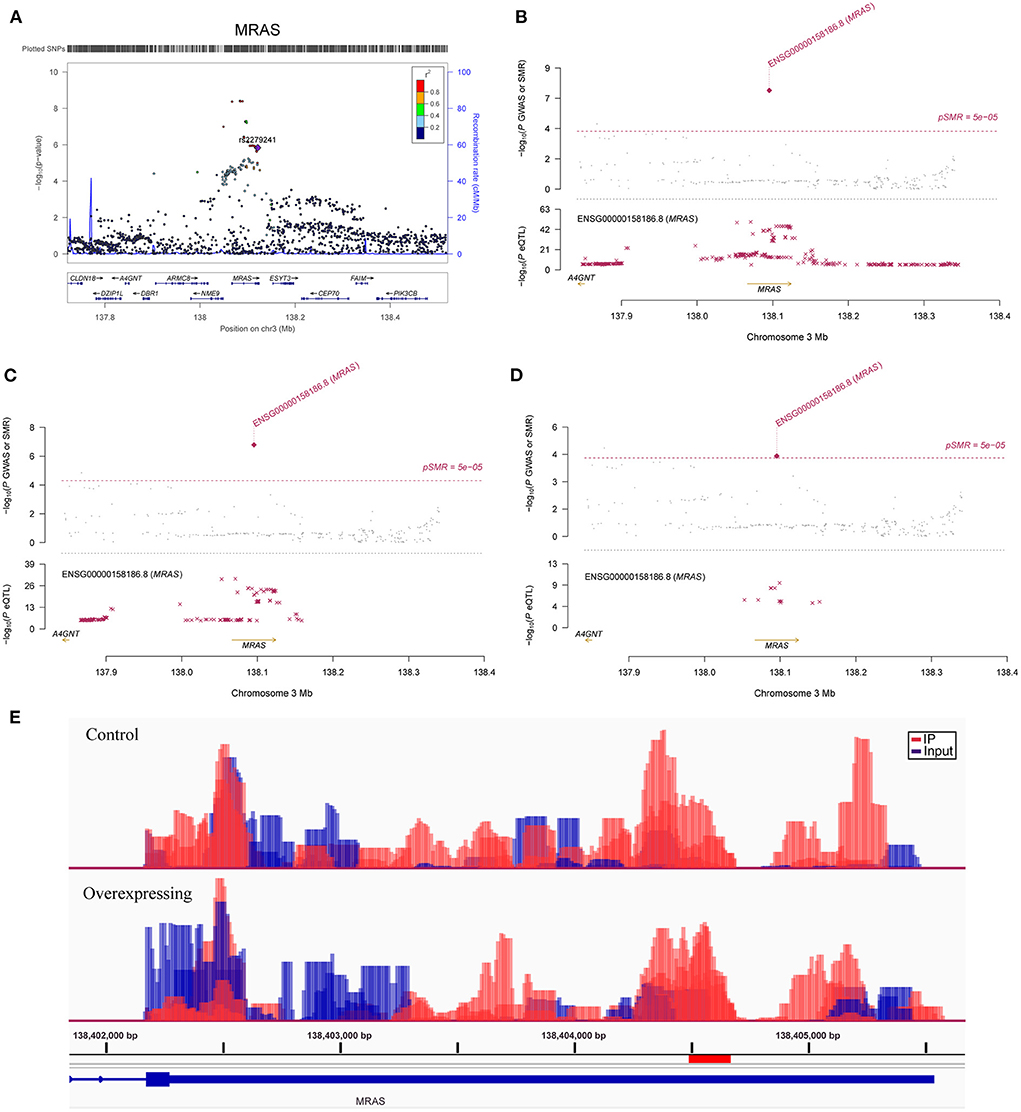
Figure 3. Association between the MRAS gene and CAD. (A) The m6A-SNP rs2279241 (reference assembly: GRCh37.p13) in the MRAS gene was associated with CAD; SNPs in MRAS were strongly associated with the expression level of MRAS, and the expression levels of the MRAS gene in aortic artery (B), tibial artery (C) and coronary artery tissues (D) were associated with CAD (reference assembly: GRCh37.p13); (E) The m6A methylation peaks in the 3'-UTR of MRAS in FTO-overexpressing and control HASMCs (reference assembly: GRCh38.p14).
For m7G modification, we identified nine m7G-SNPs to be nominally associated with CAD. The functional loss m7G-associated SNPs rs2270576 (P = 9.74 × 10−6) in SNF8 and rs8354 (P = 3.01 × 10−5) in the 3'-UTR of ARL3 were associated with CAD. In addition, we found one functional loss m1A-SNP rs897172 at the 5'-UTR of ORC4 that was significantly associated with CAD (P = 8.33 × 10−5).
Furthermore, we tested for an association between the RNAm-SNPs and AMI in the UK Biobank GWAS. We found 49 RNAm-SNPs that were associated with AMI at P < 1.0 × 10−4, including 45 m6A-, 1 m5C-, 1 A-to-I- and 2 m7G-related SNPs. The 45 m6A-SNPs, including 10 high, 15 medium and 20 low (prediction) confidence levels, mapped to 6 noncoding genes and 38 protein coding genes. Among the 39 m6A-SNPs mapped to protein coding genes, 8 (20.5%) were exonic, 13 (33.3%) were in the 3'-UTR, 2 (5.1%) were in the 5'-UTR and 16 (41.0%) were intronic. Three m6A-SNPs, rs764957 in LOC100506178, rs148172130 in RPL14 and rs12190287 in TCF21, were significantly associated with AMI at the genome-wide level (P = 3.95 × 10−10, 2.46 × 10−8 and 3.95 × 10−8, respectively). We found that a missense variant rs72654423 (m6A-SNP) in APOB was nominally associated with AMI (P = 7.27 × 10−5). The m7G-SNP rs186643756 in the lncRNA PVT1 was significantly associated with CAD at the genome-wide level (P = 3.20 × 10−8). For the 37 CAD-associated RNAm-SNPs described above, we found four m6A-SNPs (rs13702 and rs3208305 in the 3'-UTR of LPL, rs12190287 in the 3'-UTR of TCF21 and rs6490162 in the intron of ATXN2) that were associated with AMI (P < 1.0 × 10−4). Taken together, we identified 81 RNAm-SNPs (three types of modification) associated with CAD or AMI at P < 1.0 × 10−4 (Supplementary Table S1).
We further investigated whether the 81 identified RNAm-SNPs were associated with the expression levels of their host genes. Many RNAm-SNPs displayed eQTL effects with the corresponding genes in various types of tissues according to the HaploReg database. Thirty-six eQTLs were found, and 28 of them were significantly associated with the expression of their host genes (cis-eQTLs), including LPL, TCF21, MRAS, JCAD, ATXN2, MAPKAPK5, MYH11, SNF8, MYO15A, IPO9, ORC4, TNS1, IP6K2, SERBP1P3, ARHGEF26, DHX36, PARP12, CACFD1, ARL3, POC1B, NEK9, CFDP1, ZNF652, SMARCA4, CYP4F2 and RRBP1. Specifically, rs2279241 in MRAS was associated with the expression levels of MRAS in subcutaneous adipose, aortic artery and tibial artery tissues; rs12190287 in TCF21 was associated with the expression levels of TCF21 in subcutaneous adipose tissue; rs13702 in LPL was associated with the expression levels of LPL in whole blood cells; rs6490162 in ATXN2 was associated with the expression levels of ATXN2 in whole blood cells and was also associated with the expression levels of SH2B3 in lymphoblastoid and whole blood cells; rs2075511 in MYH11 was associated with the expression levels of MYH11 in whole blood cells; rs2270576 in SNF8 was associated with the expression levels of SNF8 in aortic artery and adrenal gland and was associated with the expression levels of UBE2Z and ATP5G1 in whole blood cells; and two missense variants, rs3739998 and rs2185724, in JCAD were associated with the expression levels of JCAD in the aortic artery. In addition, in skeletal muscle and the tibial artery, rs854766 in the intron of MYO15A was strongly associated with the expression of ALKBH5, which is a m6A demethylase.
In SMR analysis, we found significant associations between gene expression levels in the 11 relevant tissue types and CAD. We set the significance threshold to 5.0 × 10−5 and detected significant associations for FAM117B, MRAS, DHX36, TCF21, JCAD, ARL3, SH2B3, MAPKAPK5, CFDP1, ATP5G1, SMARCA4 and RRBP1 (Supplementary Table S2). We found significant associations between the expression levels of JCAD in the aortic artery (P = 1.73 × 10−5) (Figure 2B), MRAS in the aortic artery (P =4.59 × 10−8) (Figure 3B), tibial artery (P =1.64 × 10−7) (Figure 3C), coronary artery (P = 3.89 × 10−5) (Figure 3D) and atrial appendage (P = 1.43 × 10−5), FAM117B in the aortic artery (P =4.78 × 10−6), DHX36 in whole blood cells (P = 2.02 × 10−5), SH2B3 in whole blood cells (P = 2.04 × 10−5), ATP5G1 in whole blood cells (P =3.03 × 10−6), left ventricle (P =7.32 × 10−6) and atrial appendage (P = 1.66 × 10−5), SMARCA4 in whole blood cells (P = 4.82 × 10−5), RRBP1 in whole blood cells (P = 2.05 × 10−5) and CAD. In addition, suggestive associations were found between the expression levels of DHX36 in whole blood cells (P = 5.96 × 10−5), TCF21 in the tibial artery (P = 8.85 × 10−5) and subcutaneous adipose tissue (P = 7.76 × 10−5), ARL3 in whole blood cells (P = 8.64 × 10−5), MAPKAPK5 in whole blood cells (P = 7.44 × 10−5), and CFDP1 in whole blood cells (P = 8.71 × 10−5) and CAD.
In FTO-overexpressing HASMCs, we aimed to determine which of the CAD loci contain methylation sites, whether the expression of the methylated genes is affected, and which of the CAD-associated m6A-SNPs fall into the differentially methylated peaks. For the genes containing m6A-SNPs, we found that 19 were differentially methylated in FTO-overexpressing HASMCs (fold change > 2.0, FDR <0.05) (Supplementary Table S3). We noticed that key CAD genes, such as JCAD (Figure 2C), MRAS (Figure 3E), MYH11 and SMG6, were differentially methylated, and SMG6 was differentially expressed in FTO-overexpressing HASMCs. We found that the synonymous m6A-SNP rs216196 (chr17: 2299651) was located in differentially methylated peaks (chr17: 2299633 - 2299784) in the SMG6 genes. In addition, we found that UBE2Z and SH2B3 were differentially methylated and differentially expressed in FTO-overexpressing HASMCs. For the differentially methylated genes, the expression levels of MAPKAPK5, MRAS and SH2B3 in the aorta, coronary artery, tibial artery or whole blood cells were associated with CAD in the SMR analysis described above (Supplementary Table S2).
Among the genes that contain the RNAm-SNPs identified above, we further found that ASAP2, MRAS, ARHGEF26, MDN1, MTHFSD, SMG6, MYO15A, OSBPL7, AIRE, FTCD and MAPK11 were differentially methylated (seven upregulated and four downregulated; fold change > 2.0, FDR <0.05) in human PBMCs of five CAD cases and five controls (Supplementary Table S4). In human PBMCs of 93 CAD cases and 48 controls, 42 genes seemed to be differentially expressed (FDR <0.05), including APOB, ATXN2, MYH11, UBE2Z, ATP5G1 and SH2B3 (Supplementary Table S5). However, only APOB passed the significance threshold of fold change > 2.0, FDR <0.05 (fold change = 2.0, FDR = 3.73 × 10−5).
We identified 65 pQTL signals (P < 5.0 × 10 Supplementary Table S6) for six of the identified RNAm-SNPs (rs739468, rs41302673, rs6859, rs3172494, rs11105310 and rs8354). A total of 44 proteins were identified. rs739468 in CACFD1 was associated with circulating levels of 26 proteins; rs41302673 in STKLD1 was associated with circulating levels of 20 proteins. The top signals were the significant associations between rs739468 in CACFD1 and circulating levels of SELE (P = 2.04 × 10−134) and ABO (P = 3.80 × 10−126). rs41302673 in STKLD1 was also associated with circulating levels of SELE (P = 1.0 × 10−89) and ABO (P = 3.16 × 10−84). The 3'-UTR SNP rs8354 in ARL3 was significantly associated with ARL3 levels (P = 2.57 × 10−8).
We performed GO and KEGG pathway analyses in the DAVID database for proteins that were affected by the CAD-associated RNAm-SNPs. We mainly analyzed the 44 proteins related to the RNAm-SNPs. The identified proteins (FLT4, INSR, IL3RA, KDR, MET and TLR4) were enriched in the PI3K-Akt signaling pathway (P = 1.80 × 10−3), Rap1 signaling pathway (P = 1.60 × 10−2), Ras signaling pathway (P = 2.10 × 10−2) and MAPK signaling pathway (P = 3.90 × 10−2) (Figure 4A). The 44 associated proteins were also enriched in 58 biological process GO terms, including peptidyl-tyrosine modification (TPST2, CTF1, CD300A, FLT4, INSR, KDR and MET, P = 1.40 × 10−4), innate immune response-activating signal transduction (CD300A, CD209, PSME1, ICAM2, TLR4 and MBL2, P = 6.0 × 10−4), positive regulation of cell migration (SELP, FLT4, INSR, KDR, SELE, MET and TLR4, P = 1.40 × 10−3), MAPK cascade (CD300A, FLT4, INSR, IL3RA, PSME1, KDR, MET and TLR4, P = 4.50 × 10−3), blood coagulation (SELP, F8, C1GALT1C1, DOCK9 and TLR4, P = 6.70 × 10−3) and so on (Figure 4B).
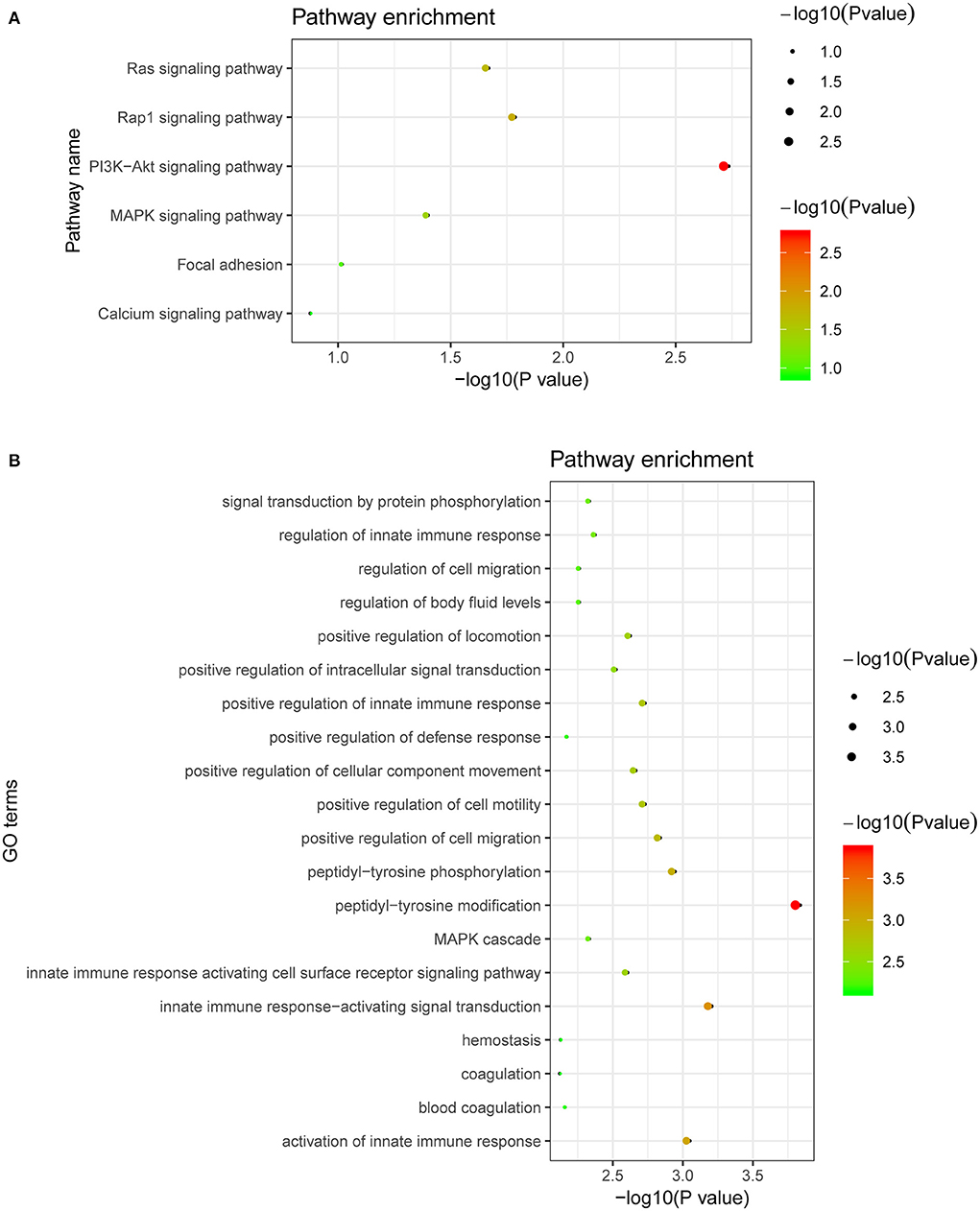
Figure 4. Biological pathways related to the proteins affected by the CAD-associated RNAm-SNPs. (A) KEGG pathway enrichment of the proteins affected by the CAD-associated RNAm-SNPs; (B) The top 20 significant biological process GO terms for the proteins affected by the CAD-associated RNAm-SNPs.
We tested whether circulating levels of these 44 proteins were causally associated with CAD using five MR methods to support the role of the RNAm-SNPs in CAD. Associations with P < 1.14 × 10−3 were considered significant in this analysis. We found that the associations between circulating levels of ABO, C1GALT1C1, C5orf38, CD209, CEP57, CTF1, F8, GNAI3, GOLM1, IL3RA, LRRN1, PSME1, QSOX2, SELE, VIMP and VPS29 and CAD were significant in weighted median, IVW, MR-Egger and MR-PRESSO analyses (Table 1). Proteins F8, C1GALT1C1 and GNAI3 were enriched in the blood coagulation biological process (P = 2.30 × 10−2). We further examined the potential causal associations between these 16 proteins and AMI. The associations between circulating levels of 12 proteins, including C1GALT1C1, C5orf38, CD209, CEP57, CTF1, F8, GNAI3, LRRN1, PSME1, QSOX2, VIMP and VPS29, and AMI were significant in weighted median, IVW, MR-Egger or MR-PRESSO analyses (Table 1).Therefore, the associations between circulating levels of these 12 proteins (including the three proteins enriched in blood coagulation biological process) and CAD were strengthened.
In the CAUSE analysis, which accounted for correlated and uncorrelated horizontal pleiotropic effects, circulating levels of ABO, C5orf38, CD209, CEP57, CTF1, IL3RA, LRRN1, QSOX2, SELE and VIMP were associated with CAD; C5orf38, CD209, CEP57, CTF1, F8, LRRN1 and VIMP levels were found to be associated with AMI (Table 1). The most significant protein was CTF1 (cardiotrophin-1), which was associated with both CAD (P = 1.10 × 10−4) and AMI (P = 8.09 × 10−3) in the CAUSE analysis (Figure 5).
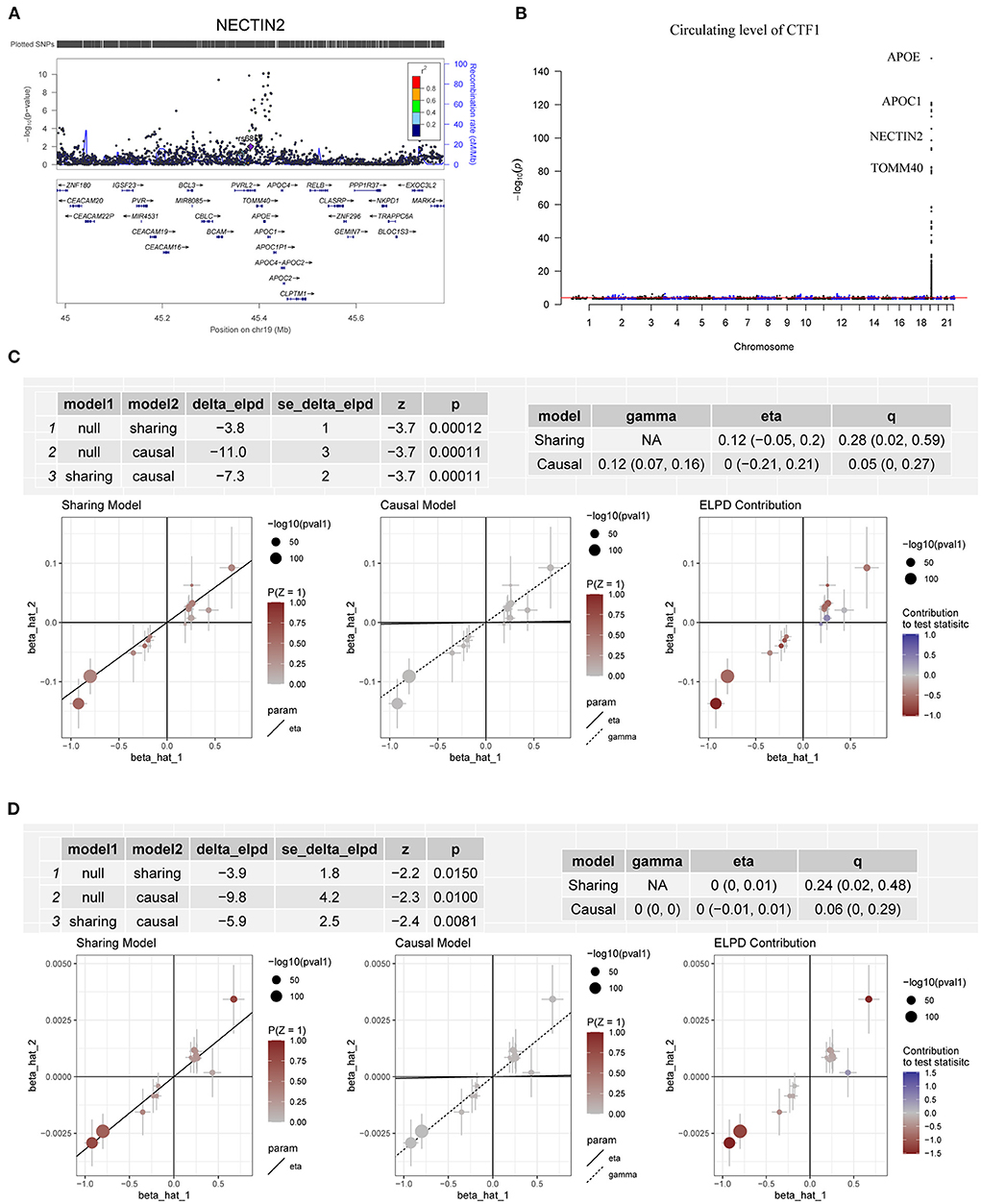
Figure 5. Association between circulating levels of CTF1 and CAD. (A) The m6A-SNP rs6859 in the NECTIN2 gene (PVRL2, in 19q13.32, reference assembly: GRCh37.p13) was associated with CAD; (B) A total of 263 SNPs in 19q13.32 were significantly (P < 5.0 × 10−8) associated with the circulating level of CTF1; (C) The association between CTF1 level and CAD in the CAUSE analysis. The causal model was significantly better than both the null and the sharing models; (D) The association between CTF1 level and AMI in the CAUSE analysis. The causal model was significantly better than both the null and the sharing models.
In the present study, we integrated information from the RMVar database, CAD GWAS and QTL studies to identify CAD-associated RNAm-SNPs related to m6A, m1A, m5C, m7G and A-to-I modification types. Some of the RNAm-SNPs were associated with the expression of the local genes in CAD-relevant tissues, and the genes were differentially expressed in CAD. We also found some of the RNAm-SNPs to be associated with circulating protein levels, and the related proteins were associated with CAD and AMI. This study highlighted the importance of detecting RNAm-SNPs in CAD susceptibility genes by excavating the conveniently available SNP association data of GWAS, and the findings indicated that RNAm-SNPs might be potential functional variants for CAD.
Hundreds of genomic loci associated with CAD have been identified in GWASs. However, the identification of functional variants, causal gene(s) or protein(s) remains a major challenge. The role of m6A methylation in CAD has been shown (24). Some genetic variants in CAD loci may alter m6A methylation and then disturb gene expression regulation (39). Recently, a study integrating m6A QTLs with disease genetics identified 184 GWAS-colocalized m6A QTLs, including 50 muscle/heart m6A QTLs underlying CAD (40). Until now, the functional roles and underlying molecular mechanisms of RNA modification in CAD remained largely unclear. In the present study, we identified many RNAm-SNPs in CAD susceptibility genes and showed that the RNAm-SNPs may have functional roles in gene expression at the mRNA and protein levels. In FTO-overexpressing HASMCs, we showed that CAD susceptibility genes were differentially methylated and that some of the m6A-SNPs were located in a differentially methylated peak (e.g., rs216196 in SMG6). Differential methylation and expression of CAD susceptibility genes between CAD cases and controls were also found in PBMCs. These findings suggest that RNA modification may play critical roles in the pathogenesis of CAD.
We found several RNAm-SNPs that were associated with gene expression, and the gene expression levels were associated with CAD. The m6A-SNP rs3739998 was associated with JCAD expression in aortic artery tissue, and the expression levels of JCAD in the aortic artery were associated with CAD. JCAD (Junctional cadherin 5 associated, also known as KIAA1462), which encodes the junctional protein associated with CAD, is one of most important GWAS-identified genes for CAD. Genetic variants in the JCAD gene region were significantly associated with CAD and AM (7, 8, 41). A study (42) showed an association of this nonsynonymous SNP with CAD in the German MI Family Study with a combined P = 1.27 × 10−11. Furthermore, Xu et al. (43) demonstrated that JCAD depletion in endothelial cells inhibited the activation of the YAP/TAZ pathway and the expression of downstream proatherogenic genes. They also showed that JCAD regulates YAP/TAZ protein activation by interacting with TRIOBP, an action-binding protein. Therefore, JCAD stabilizes stress fiber formation and thereby plays a functional role in the pathogenesis of CAD. Our study highlighted rs3739998, which may affect JCAD expression, as a potential functional variant for CAD and suggested the possibility of attractive new therapeutic strategies for CAD by targeting JCAD.
To avoid a large number of false positive results, previous GWASs used strict significance thresholds. However, due to the use of strict significance levels, many moderate association signals in the GWAS dataset were ignored. In addition to the genome-wide significant SNP in JCAD, RNAm-SNPs in other important CAD susceptibility genes were also identified, including MRAS and TCF21. These RNAm-SNPs are not genome-wide significant, but they significantly affected the expression levels of MRAS and TCF21, and the expression levels of MRAS and TCF21 were genetically associated with CAD. DHX36, which is involved in the regulation of cardioblast differentiation and proliferation during heart development and plays a role in many biological processes, such as genomic integrity (44), transcriptional regulation (45, 46) and posttranscriptional regulation (47), is another example. We found that four m6A-SNPs in 3q25.2, rs12493885 and rs357504 in ARHGEF26, rs403132 in DHX36 and rs701133 in GPR149, were associated with the expression levels of DHX36. The associations between these RNAm-SNPs and CAD are not genome-wide significant. These variants are proximal to previously described loci associated with CAD, specifically the 3'-UTR variant rs701145 (10) and the intron variants rs1727949 (48) and rs789294 (10). DHX36 expression levels in blood cells were found to be genetically associated with CAD in our SMR analysis. Hence, it is possible that RNA methylation may affect DHX36 gene expression and then have a pathogenic effect on CAD. Overall, these findings suggested that the RNAm-SNPs may affect CAD risk by affecting gene expression and therefore may be functional variants in the CAD loci.
Circulating proteins are druggable targets (49, 50). A great number of studies have been conducted to identify circulation proteins contributing to CAD. However, traditional observational studies have limitations in disentangling which factors causally affect CAD. MR-based studies can address these limitations by use of genetic proxies of putative risk factors when evaluating their associations with disease risk, as they are not subject to reverse causation (51). More importantly, the MR method allows the evaluation of the association between gene expression levels and CAD risk in very large samples by using data from large-scale GWAS. Some of the new MR methods, such as CAUSE, avoid more false positives induced by correlated and uncorrelated horizontal pleiotropic effects (37). As we showed in this study, MR analysis provided robust evidence of the vital roles of several proteins in CAD.
According to the pQTL analysis, we found that the RNAm-SNPs were associated with circulating levels of many proteins, including proteins involved in the Rap1 signaling pathway, Ras signaling pathway, PI3K-Akt signaling pathway and MAPK signaling pathway. Activation of the PI3K/Akt signaling pathway is well known to accelerate the development of atherosclerosis (52). As suggested by a recently published study (53), this pathway plays an important role in the pathology of CAD. The affected proteins play functional roles in biological processes of peptidyl-tyrosine modification, innate immune response-activating signal transduction, positive regulation of cell migration, MAPK cascade, blood coagulation and so on. Afterward, by applying several MR methods, we showed that the proteins affected by the RNAm-SNPs were causally associated with CAD, including proteins function in the blood coagulation process (F8, C1GALT1C1 and GNAI3). The strongest evidence was found for cardiotrophin-1, which was significantly associated with both CAD and AMI in all MR analyses using five methods. The associations between SNPs in 19q13.32 (APOE, APOC1, NECTIN2, TOMM40, BCAM, CEACAM16, CBLC, APOC4, BCL3, RELB, CLPTM1, NKPD1, CEACAM19, PVR, IGSF23, CLASRP, TRAPPC6A and PPP1R37) and CAD and circulating levels of CTF1 contributed to the identification of the causal association between circulating levels of CTF1 and CAD. CTF1 is a procardiogenic factor that has been shown to play important roles in cardiovascular disease (54–56). Identification of this key protein provided a better entry point for studies on the pathological mechanism of CAD. Although the m6A-SNP rs6859 was found in NECTIN2, which encodes nectin cell adhesion molecule 2, functional genetic variants in this locus need to be confirmed in future studies.
Some limitations of the current work need to be acknowledged. First, the data used were mainly from European populations; therefore, the results may need to be extrapolated carefully. Second, although the m6A-SNP dataset was extensive, information on other types of RNA modification was still scarce. Third, the sample size of the pQTL study was relatively small, and therefore, the estimations of the effects of proteins on CAD risk may not be so accurate. Finally, this work is based on statistical evidence, and the functions of the identified RNAm-SNPs and genes have not been validated experimentally. Further experimental studies are required to determine their functions.
In conclusion, the present study identified CAD-associated RNAm-SNPs and suggested that RNA modification may play a role in the pathogenesis of CAD. RNAm-SNPs in CAD susceptibility genes may regulate gene expression at the mRNA (e.g., JCAD, MRAS, TCF21, and DHX36) or protein (e.g., ABO, C5orf38, CD209, CEP57, CTF1, IL3RA, LRRN1, QSOX2, SELE and VIMP) levels. The relationships between RNAm-SNPs, RNA modification, gene expression and CAD have not been clarified in previous studies. Therefore, this study increased our understanding of the genetic association signals identified in the CAD GWAS and identified additional risk factors for CAD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Soochow University. The patients/participants provided their written informed consent to participate in this study.
HZ and XM obtained funding, performed the analyses, and rafted the initial manuscript. XM and LW designed the study. CD, RL, FT, DL, and NW were involved in the study design and/or data collection. XM, YZ, and LW have primary responsibility for the final content. XM is the guarantor of this study. All authors read and approved the final manuscript.
This study was supported by the Natural Science Foundation of China (82173597, 82170480, 82073636, and 81773508), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-008), the Startup Fund from Soochow University (Q413900313, Q413900412), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.985121/full#supplementary-material
1. Musunuru K, Kathiresan S. Genetics of Common, Complex Coronary Artery Disease. Cell. (2019) 177:132–45. doi: 10.1016/j.cell.2019.02.015
2. O'Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med. (2011) 365:2098–109. doi: 10.1056/NEJMra1105239
3. Mayhew AJ, Meyre D. Assessing the heritability of complex traits in humans: methodological challenges and opportunities. Curr Genomics. (2017) 18:332–40. doi: 10.2174/1389202918666170307161450
4. McPherson R. 2018 George Lyman Duff memorial lecture: genetics and genomics of coronary artery disease: a decade of progress. Arterioscler Thromb Vasc Biol. (2019) 39:1925–37. doi: 10.1161/ATVBAHA.119.311392
5. Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. (2011) 43:333–8. doi: 10.1038/ng.784
6. Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. (2013) 45:25–33. doi: 10.1038/ng.2480
7. Nikpay M, Goel A, Won H-H, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47:1121–30. doi: 10.1038/ng.3396
8. Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet. (2017) 49:1385–91. doi: 10.1038/ng.3913
9. Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NGD, Jansen H, et al. Systematic evaluation of pleiotropy identifies 6 further loci associated with coronary artery disease. J Am Coll Cardiol. (2017) 69:823–36. doi: 10.1016/j.jacc.2016.11.056
10. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. (2018) 122:433–43. doi: 10.1161/CIRCRESAHA.117.312086
11. Hartiala JA, Han Y, Jia Q, Hilser JR, Huang P, Gukasyan J, et al. Genome-wide analysis identifies novel susceptibility loci for myocardial infarction. Eur Heart J. (2021) 42:919–33. doi: 10.1093/eurheartj/ehaa1040
12. Yi C, Pan T. Cellular dynamics of RNA modification. Acc Chem Res. (2011) 44:1380–8. doi: 10.1021/ar200057m
13. Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. (2018) 46:D303–7. doi: 10.1093/nar/gkx1030
14. Malbec L, Zhang T, Chen Y-S, Zhang Y, Sun B-F, Shi B-Y, et al. Dynamic methylome of internal mRNA N-methylguanosine and its regulatory role in translation. Cell Res. (2019) 29:927–41. doi: 10.1038/s41422-019-0230-z
15. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. (2017) 169:1187–200. doi: 10.1016/j.cell.2017.05.045
16. Zheng Y, Nie P, Peng D, He Z, Liu M, Xie Y, et al. m6AVar: a database of functional variants involved in m6A modification. Nucleic Acids Res. (2018) 46:D139–45. doi: 10.1093/nar/gkx895
17. Luo X, Li H, Liang J, Zhao Q, Xie Y, Ren J, et al. RMVar: an updated database of functional variants involved in RNA modifications. Nucleic Acids Res. (2021) 49:D1405–12. doi: 10.1093/nar/gkaa811
18. Canela-Xandri O, Rawlik K, Tenesa A. An atlas of genetic associations in UK Biobank. Nat Genet. (2018) 50:1593–9. doi: 10.1038/s41588-018-0248-z
19. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
20. Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. (2012) 40:D930–934. doi: 10.1093/nar/gkr917
21. Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. (2016) 48:481–7. doi: 10.1038/ng.3538
22. Battle A, Brown CD, Engelhardt BE, Montgomery SB. Genetic effects on gene expression across human tissues. Nature. (2017) 550:204–13. doi: 10.1038/nature24277
23. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. (2011) 7:885–7. doi: 10.1038/nchembio.687
24. Deng K, Ning X, Ren X, Yang B, Li J, Cao J, et al. Transcriptome-wide N6-methyladenosine methylation landscape of coronary artery disease. Epigenomics. (2021) 13:793–808. doi: 10.2217/epi-2020-0372
25. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. (2011) 17:10. doi: 10.14806/ej.17.1.200
26. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. (2015) 12:357–60. doi: 10.1038/nmeth.3317
27. Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. (2008) 9:R137. doi: 10.1186/gb-2008-9-9-r137
28. Shen L, Shao NY, Liu X, Maze I, Feng J, Nestler EJ. diffReps: detecting differential chromatin modification sites from ChIP-seq data with biological replicates. PLoS ONE. (2013) 8:e65598. doi: 10.1371/journal.pone.0065598
29. Li L, Wang L, Li H, Han X, Chen S, Yang B, et al. Characterization of LncRNA expression profile and identification of novel LncRNA biomarkers to diagnose coronary artery disease. Atherosclerosis. (2018) 275:359–67. doi: 10.1016/j.atherosclerosis.2018.06.866
30. Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature. (2018) 558:73–9. doi: 10.1038/s41586-018-0175-2
31. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. (2009) 4:44–57. doi: 10.1038/nprot.2008.211
32. Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. (2009) 37. doi: 10.1093/nar/gkn923
33. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
34. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
35. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
36. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
37. Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nature Genet. (2020) 52:740–7. doi: 10.1038/s41588-020-0631-4
38. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–9. doi: 10.1093/ije/dyx034
39. Mo XB, Lei SF, Zhang YH, Zhang H. Detection of m(6)A-associated SNPs as potential functional variants for coronary artery disease. Epigenomics. (2018) 10:1279–87. doi: 10.2217/epi-2018-0007
40. Xiong X, Hou L, Park YP, Molinie B, Gregory RI, Kellis M. Genetic drivers of mA methylation in human brain, lung, heart and muscle. Nat Genet. (2021) 53:1156–65. doi: 10.1038/s41588-021-00890-3
41. Consortium CADG. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. (2011) 43:339–44. doi: 10.1038/ng.782
42. Erdmann J, Willenborg C, Nahrstaedt J, Preuss M, König IR, Baumert J, et al. Genome-wide association study identifies a new locus for coronary artery disease on chromosome 10p11.23. Eur Heart J. (2011) 32:158–68. doi: 10.1016/S1567-5688(10)71034-5
43. Xu S, Xu Y, Liu P, Zhang S, Liu H, Slavin S, et al. The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis. Eur Heart J. (2019) 40:2398–408. doi: 10.1093/eurheartj/ehz303
44. Booy EP, Meier M, Okun N, Novakowski SK, Xiong S, Stetefeld J, et al. The RNA helicase RHAU (DHX36) unwinds a G4-quadruplex in human telomerase RNA and promotes the formation of the P1 helix template boundary. Nucleic Acids Res. (2012) 40:4110–24. doi: 10.1093/nar/gkr1306
45. Giri B, Smaldino PJ, Thys RG, Creacy SD, Routh ED, Hantgan RR, et al. G4 resolvase 1 tightly binds and unwinds unimolecular G4-DNA. Nucleic Acids Res. (2011) 39:7161–78. doi: 10.1093/nar/gkr234
46. Huang W, Smaldino PJ, Zhang Q, Miller LD, Cao P, Stadelman K, et al. Yin Yang 1 contains G-quadruplex structures in its promoter and 5'-UTR and its expression is modulated by G4 resolvase 1. Nucleic Acids Res. (2012) 40:1033–49. doi: 10.1093/nar/gkr849
47. Newman M, Sfaxi R, Saha A, Monchaud D, Teulade-Fichou M-P, Vagner S. The G-quadruplex-specific RNA helicase DHX36 regulates p53 Pre-mRNA 3'-end processing following UV-induced DNA damage. J Mol Biol. (2017) 429:3121–31. doi: 10.1016/j.jmb.2016.11.033
48. Koyama S, Ito K, Terao C, Akiyama M, Horikoshi M, Momozawa Y, et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat Genet. (2020) 1169–77. doi: 10.1038/s41588-020-0705-3
49. Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. (2006) 5:821–34. doi: 10.1038/nrd2132
50. Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. (2017) 16:19–34. doi: 10.1038/nrd.2016.230
51. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
52. Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res. (2009) 82261–71. doi: 10.1093/cvr/cvn325
53. Zuo J, Xu M, Wang D, Bai W, Li G. Role of competitive endogenous RNA networks in the pathogenesis of coronary artery disease. Ann Transl Med. (2021) 9:1234. doi: 10.21037/atm-21-2737
54. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Relationship between plasma level of cardiotrophin-1 and left ventricular mass index in patients with dilated cardiomyopathy. J Am Coll Cardiol. (2001) 38:1485–90. doi: 10.1016/S0735-1097(01)01576-5
55. Zolk O, Ng LL, O'Brien RJ, Weyand M, Eschenhagen T. Augmented expression of cardiotrophin-1 in failing human hearts is accompanied by diminished glycoprotein 130 receptor protein abundance. Circulation. (2002) 106:1442–6. doi: 10.1161/01.CIR.0000033117.39335.DF
Keywords: RNA modification, genome-wide association study, gene expression, cardiotrophin-1, Mendelian randomization
Citation: Li R, Zhang H, Tang F, Duan C, Liu D, Wu N, Zhang Y, Wang L and Mo X (2022) Coronary artery disease risk factors affected by RNA modification-related genetic variants. Front. Cardiovasc. Med. 9:985121. doi: 10.3389/fcvm.2022.985121
Received: 03 July 2022; Accepted: 02 September 2022;
Published: 20 September 2022.
Edited by:
Shizuka Uchida, Aalborg University Copenhagen, DenmarkReviewed by:
Olli-Pekka Smolander, Tallinn University of Technology, EstoniaCopyright © 2022 Li, Zhang, Tang, Duan, Liu, Wu, Zhang, Wang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingbo Mo, eGJtb0BzdWRhLmVkdS5jbg==; Laiyuan Wang, d2FuZ2xhaXl1YW5kd0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.