
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 September 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.984092
This article is part of the Research Topic Novel and Emerging Therapies in Acute and Chronic Heart Failure View all 12 articles
 Nikolaos Katsiadas1†
Nikolaos Katsiadas1† Andrew Xanthopoulos2*†
Andrew Xanthopoulos2*† Grigorios Giamouzis2
Grigorios Giamouzis2 Spyridon Skoularigkis2
Spyridon Skoularigkis2 Niki Skopeliti2
Niki Skopeliti2 Evgenia Moustaferi3
Evgenia Moustaferi3 Ioannis Ioannidis4
Ioannis Ioannidis4 Sotirios Patsilinakos1
Sotirios Patsilinakos1 Filippos Triposkiadis2
Filippos Triposkiadis2 John Skoularigis2
John Skoularigis2Background: Recent studies suggest that the pivotal mechanism of sodium glucose co-transporter-2 inhibitors (SGLT-2i) favorable action in patients with heart failure (HF) and type 2 diabetes mellitus (DM) is the stimulation of erythropoiesis via an early increase in erythropoietin (EPO) production which leads to hematocrit rise. Red blood cell distribution width (RDW) is a simple hematological parameter which reflects the heterogeneity of the red blood cell size (anisocytosis). Since, EPO has been also implicated in the pathophysiology of RDW increase, the current mechanistic study examined the effect of SGLT-2i administration on red blood cells size (RDW) in patients with HF and DM.
Methods: The present was a prospective single-center study. Patients (N=110) were randomly assigned to dapagliflozin (10 mg a day on top of antidiabetic treatment) or the control group. Inclusion criteria were: (a) age > 18 years, (b) history of type 2 DM and hospitalization for HF exacerbation within 6 months. The evaluation of patients (at baseline, 6 and 12 months) included clinical assessment, laboratory blood tests, and echocardiography. Data were modeled using mixed linear models with dependent variable the RDW index. In order to find factors independently associated with prognosis (1-year death or HF rehospitalization), multiple logistic regression was conducted with death or HF rehospitalization as dependent variable.
Results: An RDW increase both after 6 and after 12 months was observed in the SGLT-2i (dapagliflozin) group (p < 0.001 for all time comparisons), whereas RDW didn't change significantly in the control group. The increase in RDW was positively correlated with EPO, while negatively correlated with ferritin and folic acid (p < 0.005 for all). Baseline RDW was significantly associated with 1-year death or rehospitalization, after adjusting for group (SGLT-2i vs. control), age, gender, smoking and BMI at baseline.
Conclusion: RDW increased with time in patients with HF and DM who received SGLT-2i (dapagliflozin). The increased RDW rates in these patients may stem from the induction of hemopoiesis from dapagliflozin. Baseline RDW was found to be independently associated with outcome in patients with HF and DM.
Red blood cell distribution width is a simple parameter of the complete blood count (CBC) which reflects the heterogeneity of the red blood cell size (anisocytosis) and has been traditionally used for the classification of several types of anemia (1, 2). Over the last decade, high RDW values have been associated with adverse outcomes in patients with cardiovascular disease including stable coronary artery disease, acute coronary syndromes, stroke, diabetes mellitus (DM), and heart failure (HF) (3–7). DM is a major risk factor for new-onset HF and vice versa (8). An increase in Hemoglobin A1C (HbA1C) of 1% correlates to an increment of 8% in HF, whereas diabetic patients have an almost twofold increased risk of HF (9, 10). Interestingly, it has been reported that red blood cell distribution width (RDW) is a good prognostic marker in patients with HF and DM (11). Reduced iron mobilization, oxidative stress, renal failure as well as ineffective erythropoiesis, have been implicated in the pathophysiology of RDW increase (12). However, the exact pathophysiological mechanism of RDW increase in HF, DM, and other pathological states, remain unclear (11).
Sodium glucose co-transporter-2 inhibitors (SGLT-2i) are a new class of antidiabetic drugs which reduce hyperglycemia through inhibition of glucose reabsorption in the renal proximal tubules (8). SGLT-2i administration has been associated with favorable cardiovascular outcomes (13, 14). Although several hypotheses have been proposed (including diuresis and natriuresis, reduction in blood pressure and afterload, direct effects on myocardial sodium and calcium handling, alterations in myocardial energetics, and improved progenitor cell response), recent studies in patients with type 2 DM suggest that the pivotal mechanism of SGLT-2i favorable action is the stimulation of erythropoiesis via an early increase in erythropoietin (EPO) production which leads to hematocrit (Ht) rise (15, 16). Since erythropoietin has been implicated in the pathophysiology of RDW increase as well, the current mechanistic study examined the effect of SGLT-2i administration on red blood cells size (RDW) in patients with HF and DM.
The present was a prospective single-center study which took place during the period from 4-2020 to 7-2021 on the Cardiology Department of Konstantopoulio General Hospital (Greece). Patients were randomly assigned (Random allocation software) (17) to dapagliflozin (10 mg a day on top of antidiabetic treatment) or the control group (no change in antidiabetic treatment) (1:1) in an open-label fashion. Inclusion criteria were: (a) age > 18 years, (b) history of type 2 DM and hospitalization for HF exacerbation within 6 months. Exclusion criteria were: (a) current or prior treatment with SGLT-2i or gucagon-like peptide 1 (GLP1) agonists, (b) blood transfusions or ferrum or folic acid or vitamin B12 administration the last 6 months, (c) glomerular filtration rate (GFR) < 30 mL/min/1.73 m2, (d) active cancer, or (e) predicted survival <1-year. NK and EM generated the random allocation sequence, enrolled participants, and assigned participants to the study groups.
The evaluation of patients (at baseline, 6 and 12 months) included clinical assessment, laboratory blood tests, and echocardiography. Levels of Ht and RDW were measured with the use of the Unicel DxH 600, Beckman USA analyzer on samples obtained for standard of care evaluation. The normal reference range for age, sex, and ethnicity using NHANES III normal range data for RDW in the hospital laboratory was 11.5–15% with intra-assay variation of 2.6% and inter-assay variation of 1.5% (18). Ferrum, ferritin, vitamin B12, folic acid and EPO measured with the use of Access 2, Beckman USA analyzer, while HbA1C, glucose, urea, creatinine, electrolytes, serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), brain natriuretic peptide (BNP), uric acid, and troponin with Dimension EXL, Siemens analyzer. Echocardiography was performed and reviewed by two independent echocardiographers, with the use of GE, HealthcareVivid e95.
This study conforms to the principles outlined in the Declaration of Helsinki and was approved by the institutional review committee. All patients provided written their informed consent.
Diabetes mellitus was defined as HbA1C ≥ 6.5% and history of antidiabetic treatment (19–22). Heart failure was defined as a clinical syndrome consisting of cardinal symptoms (e.g., breathlessness, ankle swelling, and fatigue) that may be accompanied by signs (e.g., elevated jugular venous pressure, pulmonary crackles, and peripheral oedema). It is due to a structural and/or functional abnormality of the heart that results in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise (23, 24).
The aim of this study was to compare the RDW longitudinal changes between the group of patients who received SGLT-2i (dapagliflozin) and the control group. Furthermore, we examined the association between RDW changes with time and clinical parameters that were known from the literature to be associated with RDW. Lastly, we investigated whether baseline RDW was independently associated with the combined endpoint of death or HF rehospitalization at 12 months. The study follow up was 1-year.
Quantitative variables were expressed as mean (Standard Deviation) or as median (interquantile range). Qualitative variables were expressed as absolute and relative frequencies. For the comparison of proportions chi-square tests were used. Independent samples Student's t-tests and Mann-Whitney tests were used for the comparison of quantitative variables between the two groups. Wilcoxon test was used for the time comparisons of RDW, in each group separately. Data were modeled using mixed linear models with dependent variable the RDW index. Primarily, the regression equation included terms for time, group, the interaction term of time and group as well as all characteristics that differed significantly between the two groups at baseline (Model 1). Secondly, another model was constructed with terms for time, group, the interaction term of time and group as well as all characteristics that were known from literature to be associated with RDW (Model 2). Adjusted regression coefficients (β) with standard errors (SE) were computed from the results of the mixed models. Hypothesized interactions of variables in the models were checked. Log transformations were used in RDW due to lack of normal distribution. In order to find factors independently associated with prognosis, multiple logistic regression was conducted with 1-year death or HF rehospitalization as dependent variable. As independent variables RDW (at baseline), group (SGLT-2i vs. control), gender, age, smoking and body mass index (BMI) (at baseline) were used. Adjusted odds ratios (OR) with 95% confidence intervals (95% CI) were computed from the results of the logistic regression analyses. ROC curves (Receiver operating characteristic curves) were used in order to estimate the prognostic value of RDW regarding poor prognosis (1-year death or HF rehospitalization), for each group separately. Sensitivity and specificity were calculated for optimal cut-offs. The area under the curve (AUC) was also calculated. The association of ferritin with C-reactive protein (CRP), white blood cells (WBC), ferrum and soluble transferrin receptor (STFR) was investigated via mixed linear regression models having ferritin as dependent variable. All reported p-values are two-tailed. Statistical significance was set at p < 0.05 and analyses were conducted using SPSS statistical software (version 22.0).
This study randomized 110 patients (124 patients were screened, but 14 patients were excluded, mainly due to inclusion/exclusion criteria) (Figure 1; Supplementary material, CONSORT 2010 Checklist). The overall baseline patient characteristics, as well as baseline characteristics split by the group type (dapagliflozin vs. control group), are presented in Table 1. Patients in the control group were older, had higher heart rate, urea, creatinine and uric acid compared to those on dapagliflozin. On the other hand, patients on dapagliflozin exhibited higher platelet, HbA1C and GFR values than the control group. The other baseline characteristics were not different between the 2 study groups. One patient from the control group was lost to follow up and there were no missing values.
RDW was similar at baseline for both groups (p = 0.974). At 6 and at 12 months the SGLT-2i (dapagliflozin) group had significantly greater values compared to the control group (p = 0.018 and p = 0.001; Figure 2). Also, it was found that in the control group RDW was similar throughout the follow-up period, while in the SGLT-2i group there were significant increases between the consecutive measures as well as between baseline and last follow-up measurement (p < 0.001 for all time comparisons) (Figure 2).
After adjusting for all characteristics that differed significantly between the two groups at baseline (Model 1), RDW still increased significantly only in the SGLT-2i group over the follow-up period (Table 2). Age was positively correlated with RDW index. All other parameters included in the analysis were not significantly associated with RDW.
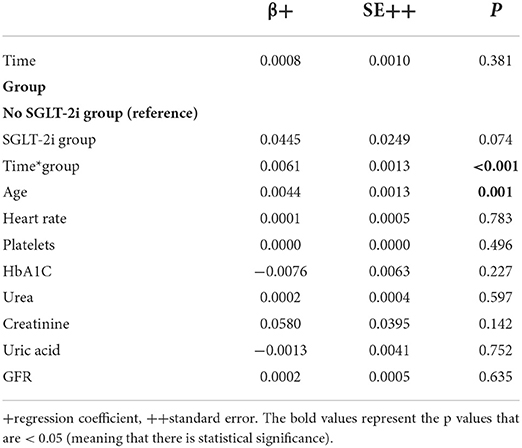
Table 2. Mixed linear regression results with RDW as dependent variable (after logarithmic transformation) and group, time, interaction term of group and time, and all characteristics differed significantly at baseline as independent variables (Model 1).
The analysis was repeated including parameters known from the literature that could be associated with RDW (Model 2, Table 3). It was found that RDW continued to increase significantly only in the SGLT-2i group over the follow-up period. Also, EPO was significantly and positively associated with RDW. On the other hand, ferritin and folic acid were significantly and negatively associated with RDW. Age, gender, hemoglobin, B12, uric acid, BMI, GFR, antiplatelets, and HbA1C were not significantly associated with RDW.
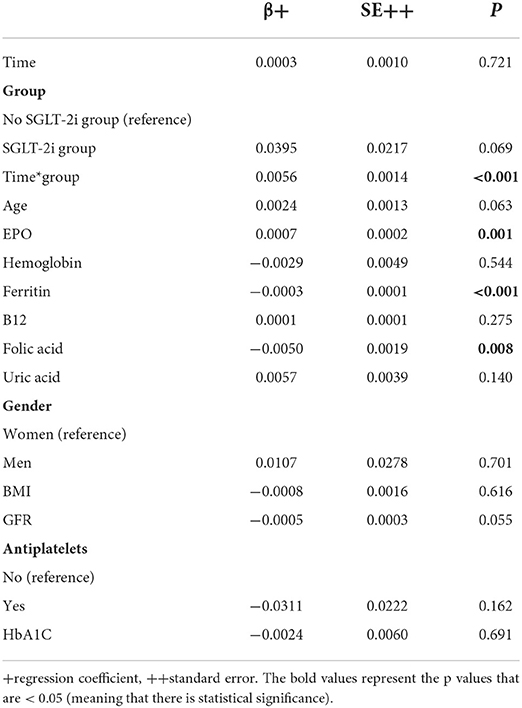
Table 3. Mixed linear regression results with RDW as dependent variable (after logarithmic transformation) and group, time, interaction term of group and time, age, EPO, Hb, Ferritin, B12, folic acid, uric acid, gender, BMI, GFR, antiplatelets, and HbA1C as independent variables (Model 2).
Altough, the percentage of 1-year death or HF rehospitalization was numerically lower in the SGLT-2i group (8.9% for the dapagliflozin group vs. 16.7% for the control group), there was no significant difference between the two groups (p = 0.223; Supplementary Table 1).
RDW was significantly and independently associated with 1-year death or HF rehospitalization, after adjusting for age, group, gender, smoking and BMI at baseline. More specifically, greater RDW values at baseline were significantly associated with greater probability of death or rehospitalization, i.e., worse prognosis. All other independent factors were not found to be significantly associated with prognosis (Table 4).
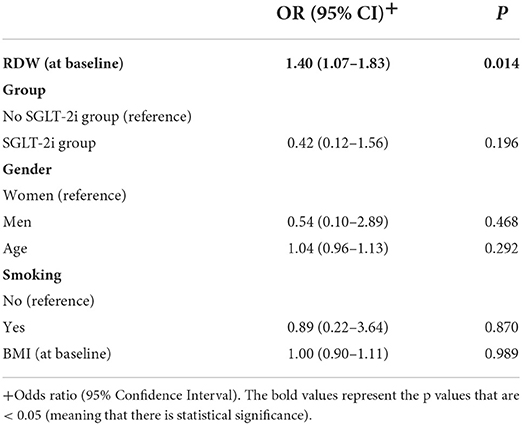
Table 4. Multiple logistic regression results with 1-year death or HF rehospitalization as dependent variable.
The prognostic value of RDW is listed in the Supplementary Figure 1. Baseline RDW was of prognostic significance regarding the combined endpoint of 1-year death/rehospitalization in both groups [AUC 0.82, 95% CI (0.68–0.96), p = 0.019 in the dapagliflozin group and AUC 0.72, 95% CI (0.56–0.87), p = 0.042 in the control group]. No significant difference was observed between the aforementioned AUCs (p = 0.349). Optimal RDW cut-off for the dapagliflozin group was 15.7%, with 80% sensitivity and 70.6% specificity. For the control group, optimal RDW cut-off value was 14.8%, with 77.8% sensitivity and 57.8% specificity.
Greater WBC and ferrum values were significantly associated with greater ferritin values. On the contrary, soluble transferrin receptor (STFR) was significantly negatively associated with ferritin. CRP was not significantly associated with ferritin (Supplementary Table 2).
No serious adverse events (diabetic ketoacidosis, symptoms of volume depletion, renal events, major hypoglycemia, fracture, lower limb amputations, Fournier's gangrene), and adverse events leading to discontinuation of the study drug were reported. Urinary tract infection was reported in two patients (one in the dapagliflozin group and 1 in the control group).
The present study demonstrated a correlation between the SGLT-2i (dapagliflozin) use, and an increase in the rates of RDW in patients with HF and DM both after 6 and after 12 months against those not on SGLT-2i (control group). It was equally noted that the increase in RDW was positively correlated with factors such as EPO, while negatively correlated with ferritin and folic acid. Lastly, baseline RDW proved to be independently associated with outcomes (death or HF rehospitalization) at 1-year, in both groups.
HF and DM often coexist and share common pathophysiological routes such as oxidative stress, inflammation and the disruption in normal hematopoiesis, conditions which can lead patients in developing anemia of chronic disease (8). EPO is a hormone produced by the kidneys and secreted in situations of hypoxia; its principal function is to induce hematopoiesis (25, 26). The increase in hematopoiesis which happens through erythropoietin results in an increase of the size of the circulating RBCs and consequently in an increase of the rate of RDW (anisocytosis) (27).
SGLT-2i are antidiabetic medications with pleiotropic effects in multiple organs and proven cardiovascular benefits, especially in patients with HF and DM (28). One of their favorable actions is the increase in the levels of Ht although the exact mechanism through which this happens is unknown (29). A sub-analysis of EMPA-HEART CardioLink-6 (Effects of Empagliflozin on Cardiac Structure in Patients With Type 2 Diabetes) randomized clinical trial, including 82 patients with DM and coronary artery disease showed that the administration of empagliflozin was associated with an increase in the EPO levels, a change in the morphology of RBCs (increase in RDW), a decrease in ferritin reserves and an increase in Ht concluding that SGLT-2i induce hematopoiesis through the increase in the secretion of EPO (15). Another randomized study investigated the effect of dapagliflozin on the levels of Ht and hepcidin (a suppressive hormone for hemopoiesis which increases in pre-inflammatory conditions such as DM or HF) in 52 patients with type 2 DM (30). Patients who were on dapagliflozin presented an increase in Ht and EPO, but a decrease in the rate of hepcidin and ferritin. The investigators concluded that dapagliflozin induces hematopoiesis through the mobilization of iron reserves, increase in EPO and suppression of hepcidin (30). The present study demonstrated that dapagliflozin administration increases RDW values in patients with HF and DM and that this increase is associated with the EPO rates. Taking into consideration that the induction of hemopoiesis through the increase of endogenous EPO leads to anisocytosis (and by extension to an increase in RDW), it is possible that these observations suggest the stimulation of hemopoiesis from dapagliflozin in patients with HF and DM. This supposition is reinforced by the fact that a negative correlation between RDW and ferritin as well as folic acid was noted, which suggests a potential mobilization of the reserves of endogenous iron and folic acid for the induction of hemopoiesis as necessary ingredients in the composition of RBCs. Furthermore, the present study revealed a positive association between ferritin values and ferrum as well as WBC (markers of iron and inflammation, respectively), and a negative association between ferritin and STFR (marker of erythropoiesis). Potential mechanisms through which a stimulation of hemopoiesis through SGLT-2i can occur have not yet been clarified (30–32).
Another parameter which has been correlated with higher RDW rates is increased age. In a retrospective study of 1907 healthy subjects, RDW rates increased according to the age group (p < 0.001) while the average rate of RDW was 11% higher in persons aged over 60 years compared to persons aged under 60 years (14.6 vs. 13.2%; p < 0.001) (33). Similar findings were reported in a study utilizing data from 8,089 unique individuals, reporting an RDW increase of 6% from the youngest to oldest age class (34). In the present study, RDW rates were positively correlated with aging in Model 1. Taking into consideration that increase in age parallels an increase in inflammation, oxidative stress as well as deficiencies in basic ingredients for the maturing RBCs such as folic acid, vitamin B12 and iron, it can be suggested that all of the above pathologies lead to disorders in normal hemopoiesis and by extension to presenting anisocytosis (35, 36).
Based on all the above, it could be proposed that the administration of SGLT-2i appears to result in the induction of hemopoiesis in patients with HF and DM (Supplementary Figures 2, 3) resulting in RDW increase. Notably in the present study, the abovementioned cardioprotective mechanism of SGLT-2i didn't result in better outcome in the dapagliflozin group vs. the control group. However, this may be due to the small number of adverse events observed in both groups.
RDW is an important marker of prognosis in HF patients (37, 38). In the present study, the administration of SGLT-2i (dapagliflozin) was associated with the increase of RDW over time. Having in mind, that the cardioprotective actions of SGLT-2i in HF patients are established (39–42), this beneficial effect on prognosis might be mediated by mechanistic actions not comprehensively investigated in the present study, such as the reduction in oxidative stress involving the xanthine oxidase pathway (43, 44). Oxidative stress has also been suggested to increase RDW rates (45, 46). One of the endogenous sources of reactive oxygen species (ROS) is the enzyme xanthine oxidase which catalyzes hypoxanthine to xanthine and xanthine to uric acid. Oxidative stress has an important role in the onset and development of HF and DM (47, 48) while studies have highlighted the prognostic value of uric acid as a potential indicator of increased oxidative stress in both conditions (49–52). However, uric acid may be caused not only by oxidative stress but also by decreased uric acid excretion from the kidneys and high purine diets (53). The present study was not able to demonstrate any association between uric acid and RDW.
The value of RDW as a prognostic indicator in patients with HF and DM has been previously reported (11, 54). The present study adds to the current knowledge by showing that the prognostic value of baseline RDW in patients with HF and Type 2 DM was significant both in patients who started receiving SGLT-2i (dapagliflozin) or not. Therefore, in the era of novel life saving therapies (SGLT-2i) in HF, baseline RDW remains a simple, inexpensive and reliable prognostic marker.
It is well-known from the literature, that a high RDW value in patients with DM and HF is likely a consequence of a chronic inflammatory state involving a reduced use of iron reserves and reduced production of erythropoietin and correlates negatively with prognosis (11, 54). However, the pathophysiological significance of an increased RDW at follow-up in patients treated with SGLT-2i (dapagliflozin) could be different, and likely linked to a greater use of iron reserves and increase in erythropoiesis as demonstrated in the present work (Supplementary Table 3). Large, prospective clinical studies are urgently needed.
The sample size was not large (110 patients), however each patient completing the follow-up had 3 measurements (baseline, 6 and 12 months). Another limitation was the lack of double blind and placebo group design, although patients were treated on top of optimized medical therapy. Consequently, the results of this study should be evaluated with caution and used as a basis for larger studies. Furthermore, the SGLT-2i used in the current study was dapagliflozin and therefore the current observations may not apply to other SGLT-2i. However, the possibility that those mechanisms reflect a “class effect” can't be excluded.
RDW, a simple parameter of a blood count which indicates anisocytosis, has been found in this study to increase in patients with HF and DM who received SGLT-2i (dapagliflozin). The increased RDW rates in these patients may be due to the induction of hemopoiesis from dapagliflozin. RDW was independently associated with 1-year death or HF rehospitalization, in patients with DM and HF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Committee of the Konstantopoulio General Hospital (email: Z3JhbW1hdGVpYS5lcC5zeW12b3VsaW91QGtvbnN0YW50b3BvdWxlaW8uZ3I=). The patients/participants provided their written informed consent to participate in this study.
NK and EM collected the data. AX, GG, II, SP, FT, and JS conceived and designed the study. SP, II, and JS were responsible for the project administration. II, FT, and JS were responsible for the supervision of the study. NK, AX, and JS wrote the main manuscript text. GG, SS, and EM prepared figures. NK (biostatistician) performed the statistical analysis. II, SP, and FT revised the manuscript critically for important intellectual content. All authors reviewed the manuscript, discussed the results, and commented on the manuscript.
The authors thank the participants of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.984092/full#supplementary-material
1. Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. (1991) 9 (Suppl. 1):71–4. doi: 10.1016/0736-4679(91)90592-4
2. Bessman JD, Gilmer PR Jr, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol. (1983) 80:322–6. doi: 10.1093/ajcp/80.3.322
3. Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. (2008) 117:163–8. doi: 10.1161/CIRCULATIONAHA.107.727545
4. Abrahan LLt, Ramos JDA, Cunanan EL, Tiongson MDA, Punzalan FER. Red cell distribution width and mortality in patients with acute coronary syndrome: a meta-analysis on prognosis. Cardiol Res. (2018) 9:144–52. doi: 10.14740/cr732w
5. Feng GH, Li HP, Li QL, Fu Y, Huang RB. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. (2017) 2:172–5. doi: 10.1136/svn-2017-000071
6. Xiong XF, Yang Y, Chen X, Zhu X, Hu C, Han Y, et al. Red cell distribution width as a significant indicator of medication and prognosis in type 2 diabetic patients. Sci Rep. (2017) 7:2709. doi: 10.1038/s41598-017-02904-9
7. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure. Data from the CHARM program and the duke databank. J Am Coll Cardiol. (2007) 50:40–7. doi: 10.1016/j.jacc.2007.02.067
8. Triposkiadis F, Xanthopoulos A, Bargiota A, Kitai T, Katsiki N, Farmakis D, et al. Diabetes mellitus and heart failure. J Clin Med. (2021) 10:3682. doi: 10.3390/jcm10163682
9. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
10. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. (2001) 161:996–1002. doi: 10.1001/archinte.161.7.996
11. Xanthopoulos A, Giamouzis G, Melidonis A, Kitai T, Paraskevopoulou E, Paraskevopoulou P, et al. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc Diabetol. (2017) 16:81. doi: 10.1186/s12933-017-0563-1
12. Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. (2009) 158:659–66. doi: 10.1016/j.ahj.2009.07.024
13. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:422–34. doi: 10.1016/j.jacc.2019.11.031
14. Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. (2020) 19:98. doi: 10.1186/s12933-020-01071-y
15. Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. (2020) 141:704–707. doi: 10.1161/CIRCULATIONAHA.119.044235
16. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
17. Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. (2004) 4:26. doi: 10.1186/1471-2288-4-26
18. Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C, et al. Hematological and iron-related analytes–reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat. (2005) 11:1–156.
19. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. (2003) 26:3160–7. doi: 10.2337/diacare.26.11.3160
20. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2012) 35 (Suppl. 1):S64–71. doi: 10.2337/dc12-s064
21. Authors/Task Force M, Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD). Eur Heart J. (2013) 34:3035–87. doi: 10.1093/eurheartj/eht108
22. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486
23. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
24. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis treatment of acute chronic heart failure: the task force for the diagnosis treatment of acute chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
25. Bhoopalan SV, Huang LJ, Weiss MJ. Erythropoietin regulation of red blood cell production: from bench to bedside and back. F1000Res. (2020) 9:F1000. doi: 10.12688/f1000research.26648.1
26. Suresh S, Rajvanshi PK, Noguchi CT. The many facets of erythropoietin physiologic and metabolic response. Front Physiol. (2019) 10:1534. doi: 10.3389/fphys.2019.01534
27. Hidalgo D, Bejder J, Pop R, Gellatly K, Hwang Y, Maxwell Scalf S, et al. EpoR stimulates rapid cycling and larger red cells during mouse and human erythropoiesis. Nat Commun. (2021) 12:7334. doi: 10.1038/s41467-021-27562-4
28. Takata T, Isomoto H. Pleiotropic effects of sodium-glucose cotransporter-2 inhibitors: renoprotective mechanisms beyond glycemic control. Int J Mol Sci. (2021) 22:4374. doi: 10.3390/ijms22094374
29. Wang X, Fu R, Liu H, Ma Y, Qiu X, Dong Z. The effects of sodium glucose co-transporter (SGLT) 2 inhibitors on hematocrit levels: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med. (2021) 10:6467–81. doi: 10.21037/apm-21-1022
30. Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, et al. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. (2020) 105:dgaa057. doi: 10.1210/clinem/dgaa057
31. Marathias KP, Lambadiari VA, Markakis KP, Vlahakos VD, Bacharaki D, Raptis AE, et al. Competing effects of renin angiotensin system blockade and sodium-glucose cotransporter-2 inhibitors on erythropoietin secretion in diabetes. Am J Nephrol. (2020) 51:349–56. doi: 10.1159/000507272
32. Verma S. Potential mechanisms of sodium-glucose co-transporter 2 inhibitor-related cardiovascular benefits. Am J Cardiol. (2019) 124 (Suppl. 1):S36–44. doi: 10.1016/j.amjcard.2019.10.028
33. Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med. (2014) 52:e197–9. doi: 10.1515/cclm-2014-0353
34. Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med. (2015) 53:2015–9. doi: 10.1515/cclm-2015-0155
35. Xanthopoulos A, Tryposkiadis K, Dimos A, Bourazana A, Zagouras A, Iakovis N, et al. Red blood cell distribution width in elderly hospitalized patients with cardiovascular disease. World J Cardiol. (2021) 13:503–13. doi: 10.4330/wjc.v13.i9.503
36. Nishizaki Y, Yamagami S, Suzuki H, Joki Y, Takahashi S, Sesoko M, et al. Red blood cell distribution width as an effective tool for detecting fatal heart failure in super-elderly patients. Intern Med. (2012) 51:2271–6. doi: 10.2169/internalmedicine.51.7938
37. Carluccio E, Biagioli P, Alunni G, Murrone A, Zingarini G, Coiro S, et al. Non-cardiac factors for prediction of response to cardiac resynchronization therapy: the value of baseline, and of serial changes, in red cell distribution width. Int J Cardiol. (2017) 243:347–53. doi: 10.1016/j.ijcard.2017.05.123
38. Huang YL, Hu ZD, Liu SJ, Sun Y, Qin Q, Qin BD, et al. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS ONE. (2014) 9:e104861. doi: 10.1371/journal.pone.0104861
39. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
40. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022). doi: 10.1056/NEJMoa2206286
41. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. doi: 10.1056/NEJMoa2022190
42. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
43. Novo G, Guarino T, Di Lisi D, Biagioli P, Carluccio E. Effects of SGLT2 inhibitors on cardiac structure and function. Heart Fail Rev. (2022). doi: 10.1007/s10741-022-10256-4
44. Seferovic PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the heart failure association of the European society of cardiology. Eur J Heart Fail. (2020) 22:1495–503. doi: 10.1002/ejhf.1954
45. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. (2017) 2017:8416763. doi: 10.1155/2017/8416763
46. Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. (2022) 11:552. doi: 10.3390/cells11030552
47. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. (2011) 301:H2181–90. doi: 10.1152/ajpheart.00554.2011
48. Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. (2011) 7:313–24. doi: 10.2174/157339911797415585
49. Huang G, Qin J, Deng X, Luo G, Yu D, Zhang M, et al. Prognostic value of serum uric acid in patients with acute heart failure: a meta-analysis. Medicine. (2019) 98:e14525. doi: 10.1097/MD.0000000000014525
50. Ndrepepa G, Braun S, King L, Cassese S, Tada T, Fusaro M, et al. Prognostic value of uric acid in patients with type 2 diabetes mellitus and coronary artery disease. Clin Sci. (2013) 124:259–68. doi: 10.1042/CS20120336
51. Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J Card Fail. (2020) 26:977–84. doi: 10.1016/j.cardfail.2020.08.015
52. Carluccio E, Coiro S, Ambrosio G. Unraveling the relationship between serum uric acid levels and cardiovascular risk. Int J Cardiol. (2018) 253:174–5. doi: 10.1016/j.ijcard.2017.11.035
53. El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: a review. J Adv Res. (2017) 8:487–93. doi: 10.1016/j.jare.2017.03.003
Keywords: sodium glucose co-transporter-2 inhibitors, red blood cell distribution width, mechanisms, aging, oxidative stress, erythropoietin
Citation: Katsiadas N, Xanthopoulos A, Giamouzis G, Skoularigkis S, Skopeliti N, Moustaferi E, Ioannidis I, Patsilinakos S, Triposkiadis F and Skoularigis J (2022) The effect of SGLT-2i administration on red blood cell distribution width in patients with heart failure and type 2 diabetes mellitus: A randomized study. Front. Cardiovasc. Med. 9:984092. doi: 10.3389/fcvm.2022.984092
Received: 01 July 2022; Accepted: 07 September 2022;
Published: 29 September 2022.
Edited by:
Erberto Carluccio, Division of Cardiology and Cardiovascular Pathophysiology, ItalyReviewed by:
Alina Yu Babenko, Almazov National Medical Research Centre, RussiaCopyright © 2022 Katsiadas, Xanthopoulos, Giamouzis, Skoularigkis, Skopeliti, Moustaferi, Ioannidis, Patsilinakos, Triposkiadis and Skoularigis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Xanthopoulos, YW5kcmV3dnhhbnRoQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.