
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 24 August 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.977747
 Amirmohammad Khalaji1,2,3†
Amirmohammad Khalaji1,2,3† Amir Hossein Behnoush1,2,3†
Amir Hossein Behnoush1,2,3† Mana Jameie1,3,4
Mana Jameie1,3,4 Ali Sharifi5
Ali Sharifi5 Ali Sheikhy1,3,4
Ali Sheikhy1,3,4 Aida Fallahzadeh1,3,4
Aida Fallahzadeh1,3,4 Saeed Sadeghian1,2
Saeed Sadeghian1,2 Mina Pashang1,2
Mina Pashang1,2 Jamshid Bagheri1
Jamshid Bagheri1 Seyed Hossein Ahmadi Tafti1
Seyed Hossein Ahmadi Tafti1 Kaveh Hosseini1,3*
Kaveh Hosseini1,3*Background: As the era of big data analytics unfolds, machine learning (ML) might be a promising tool for predicting clinical outcomes. This study aimed to evaluate the predictive ability of ML models for estimating mortality after coronary artery bypass grafting (CABG).
Materials and methods: Various baseline and follow-up features were obtained from the CABG data registry, established in 2005 at Tehran Heart Center. After selecting key variables using the random forest method, prediction models were developed using: Logistic Regression (LR), Support Vector Machine (SVM), Naïve Bayes (NB), K-Nearest Neighbors (KNN), Extreme Gradient Boosting (XGBoost), and Random Forest (RF) algorithms. Area Under the Curve (AUC) and other indices were used to assess the performance.
Results: A total of 16,850 patients with isolated CABG (mean age: 67.34 ± 9.67 years) were included. Among them, 16,620 had one-year follow-up, from which 468 died. Eleven features were chosen to train the models. Total ventilation hours and left ventricular ejection fraction were by far the most predictive factors of mortality. All the models had AUC > 0.7 (acceptable performance) for 1-year mortality. Nonetheless, LR (AUC = 0.811) and XGBoost (AUC = 0.792) outperformed NB (AUC = 0.783), RF (AUC = 0.783), SVM (AUC = 0.738), and KNN (AUC = 0.715). The trend was similar for two-to-five-year mortality, with LR demonstrating the highest predictive ability.
Conclusion: Various ML models showed acceptable performance for estimating CABG mortality, with LR illustrating the highest prediction performance. These models can help clinicians make decisions according to the risk of mortality in patients undergoing CABG.
Cardiovascular diseases -particularly coronary artery disease (CAD)-are the leading worldwide cause of death (1). Coronary artery bypass graft (CABG) surgery and angioplasty are the two primary revascularization methods used to treat CAD patients.
Several scores have been proposed for the assessment of cardiac operative risk, such as the European system for cardiac operative risk calculation (Euro-SCORE I and II) and the Society of Thoracic Surgeons (STS) (2–4). However, these scoring systems mainly evaluate in-hospital, and operative mortality and therefore are not generalizable to longer mortality. Many contributors, including previous medical comorbidities and procedural factors, are associated with higher mortality in patients undergoing CABG (5).
Machine learning (ML) models have been designed to predict outcomes in the cardiovascular medicine (6). These models have shown promising results compared to traditional risk scores with some advantages (7). In these artificial intelligence-based methods, the strongest predictors can be selected to train the system to predict outcomes using supervised learning. Afterward, the learning method is tested on unseen data for evaluation (8).
In light of this information, we aimed to use and compare different ML methods to predict one-to-five-year mortality after CABG.
In this registry-based retrospective cohort study, baseline data from all adult patients (≥18 years old) in the Tehran Heart Center CABG databank enrolled from 2005 through 2015 were reviewed. The study was approved by the ethics committee of Tehran Heart Center (IR.TUMS.VCR.REC.1400.11.23). Due to the retrospective design of the study and data anonymization, the informed consent requirement was waived.
Various predictors were used for this study. Demographic variables included age, gender, and body mass index (BMI). Preoperative variables consisted of serum hemoglobin (Hb), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), total cholesterol, triglycerides (TG), creatinine (Cr), left ventricular ejection fraction (EF), diabetes, hypertension, opium consumption, smoking status, prior myocardial infarction (MI), preoperative heart failure (HF), and chronic obstructive pulmonary disease (COPD). The definitions were consistent with prior studies on this population (9). Perioperative variables, including cardiopulmonary pump utilization and ventilation hours, were assessed.
The primary outcome was mortality in one-year post-CABG. Secondary outcomes were two-, three-, four-, and five-year mortality.
At first, we omitted subjects if they had (1) any missing variable values (1,217 out of total 18,118), and or (2) implausible values such as: Hb < 5 or > 25, LDL-C > 400, TG < 20 or > 1200, HDL-C < 5 or > 100, and Cr < 0.2 or > 15. Excluding missing values was due to a sufficient sample number. For each endpoint during follow-ups, survivors with less follow-up duration than the cut point were excluded.
The study population was randomly assigned to the training cohort (70% of the patients) and the test cohort (30% of the sample) to validate the predictive models.
We ran a feature selection algorithm on the training data to select the most appropriate features. Top features were obtained from random forest model prediction in the train data using k-fold cross-validation (k = 5). In case of a strong correlation between two variables (confirmed by Pearson correlation r) that were also clinically related, the stronger predictor was used to set up the models.
As one of the challenges in ML is imbalanced data, we used the synthetic minority oversampling technique (SMOTE) to balance our data in the training sample, which was performed after the test/train split as the data in the test sample should be unseen and remain unchanged. SMOTE technique creates synthetic data for the minority group, which was non-survivors in our study, to have an equal number of outcomes. The oversampling strategy, demonstrating the rate of the minority group to the majority, was tuned manually to have the best prediction model. Finally, a standard scaler was used to scale the data of each feature for developing prediction models. This standardizes features by removing the mean and scaling to unit variance. Oversampling and scaling were conducted for each follow-up data separately.
To develop predictive models, six ML methods were utilized: (1) Logistic Regression (LR); (2) K-Nearest Neighbors (KNN); (3) Random Forest (RF); (4) Extreme Gradient Boosting (XGBoost); (5) Naïve Bias (NB) and (6) Support Vector Machine (SVM). All the models were designed using k-fold (k = 5) cross-validation. The parameters used for each model were tuned using the grid search method to increase the accuracy of the prediction. Each model was trained and tested for one-to-five-year mortality.
We evaluated the performance of ML methods using following indices. (1) Sensitivity; (2) specificity; (3) accuracy of prediction; and (4) area under the receiver operating characteristics curve score (ROC-AUC) by plotting true positive against false positive rate. As AUC is a measure of discrimination independent of threshold, we chose it as the major index to compare the performance of models. AUC was interpreted as follows. AUC ≥ 0.9, outstanding discrimination; 0.8 ≤ AUC < 0.9, excellent discrimination; 0.7 ≤ AUC < 0.8, acceptable/fair discrimination; 0.6 ≤ AUC < 0.7, poor discrimination; and AUC < 0.6, no discrimination (10). The threshold determines the cut-off to turn a projected probability into a class label which is normally set at 0.5 (50%). Finally, due to the highly imbalanced outcome and low mortality rate after CABG, the prediction threshold, which is usually set as 0.5, was tuned using k-fold (k = 5) cross-validation to adjust sensitivity and specificity.
Baseline characteristics are represented as mean and standard deviation (SD) or percentage. Data were compared using the Pearson chi-squared test and Fisher’s exact test for categorical variables, and the independent sample t-test for continuous variables. Two-sided p-value < 0.05 was considered statistically significant. All statistical analyses and model development were performed using Python (3.10). LR, NB, RF, SVM, and KNN models were implemented using the scikit-learn (1.0.2) library (11), and XGBoost was developed using the XGBoost (1.6.0) Python library.
The total cohort included 16,850 patients with isolated CABG (age: 67.34 ± 9.67, 73.51% male). Table 1 illustrates the baseline characteristics of the whole cohort. Among 16,620 patients with complete one-year follow-up, 2.81% (n = 468) died, followed by 4.06%, 6.01%, 8.56%, and 12.77% respective cumulative mortality rates at two, three, four, and five years of follow-up. Figure 1 indicates the number of survivors and non-survivors for each follow-up duration. Figure 2 compares the baseline characteristics between survivors and the deceased patients with a one-year follow-up. Non-survivors were more likely to be older and be afflicted with conventional CAD risk factors. Moreover, EF was significantly lower (40.67 ± 10.64 vs. 46.27 ± 9.01, p-value < 0.001), and ventilation hours higher (77.49 ± 152.58 vs. 11.53 ± 14.40) in non-survivors compared to the survivors.

Figure 2. Comparison of baseline and hospitalization characteristics of survivors and non-survivors with one-year follow-up. MI, myocardial infarction; HF, heart failure; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass grafting; PCI, primary cutaneous intervention; PVD, peripheral vascular disease; CVA, cerebrovascular accident; FBS, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; BMI, body mass index; Hb, hemoglobin; EF, ejection fraction.
We used the RF prediction model in the test data using k-fold cross-validation (k = 5) to rank all the features based on their importance. All correlations between the features were assessed by Pearson correlation r. Figure 3 demonstrates the features and their order for developing the models. Eleven features were chosen for predicting one-year mortality based on the RF model (Figure 3). Total ventilation hours and EF were the most predictive variables. Feature selection for secondary endpoints (longer follow-up mortalities) revealed the same predictor features with minor changes in their order.

Figure 3. Feature importance based on the Random Forest model. MI, myocardial infarction; HF, heart failure; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass grafting; PCI, primary cutaneous intervention; PVD, peripheral vascular disease; CVA, cerebrovascular accident; FBS, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; BMI, body mass index; Hb, hemoglobin; EF, ejection fraction.
Models ran for one-to-five-year mortality based on the top features selected and were evaluated on the test dataset. Table 2 compares the predictive values of different models concerning their AUC, accuracy, sensitivity, and specificity. All the models had at least acceptable performance for one-year mortality (10), with LR presenting the highest [AUC (95% CI) = 0.81 (0.77–0.85)] and KNN the lowest discriminatory ability [AUC (95% CI) = 0.72 (0.67–0.76)]. Moreover, all techniques illustrated acceptable performance (AUC > 0.7) for predicting two-to-five-year mortality (10), excluding the SVM model. After tuning for the threshold, the highest sensitivity was obtained in the LR model (72.99%), while the highest specificity and accuracy were calculated as 85.67% and 84.96% for the NB model, respectively.
By the same token, the LR model surpassed other models for predicting two-to-five-year mortality. Figure 4 demonstrates the ROC-AUC for all six models.
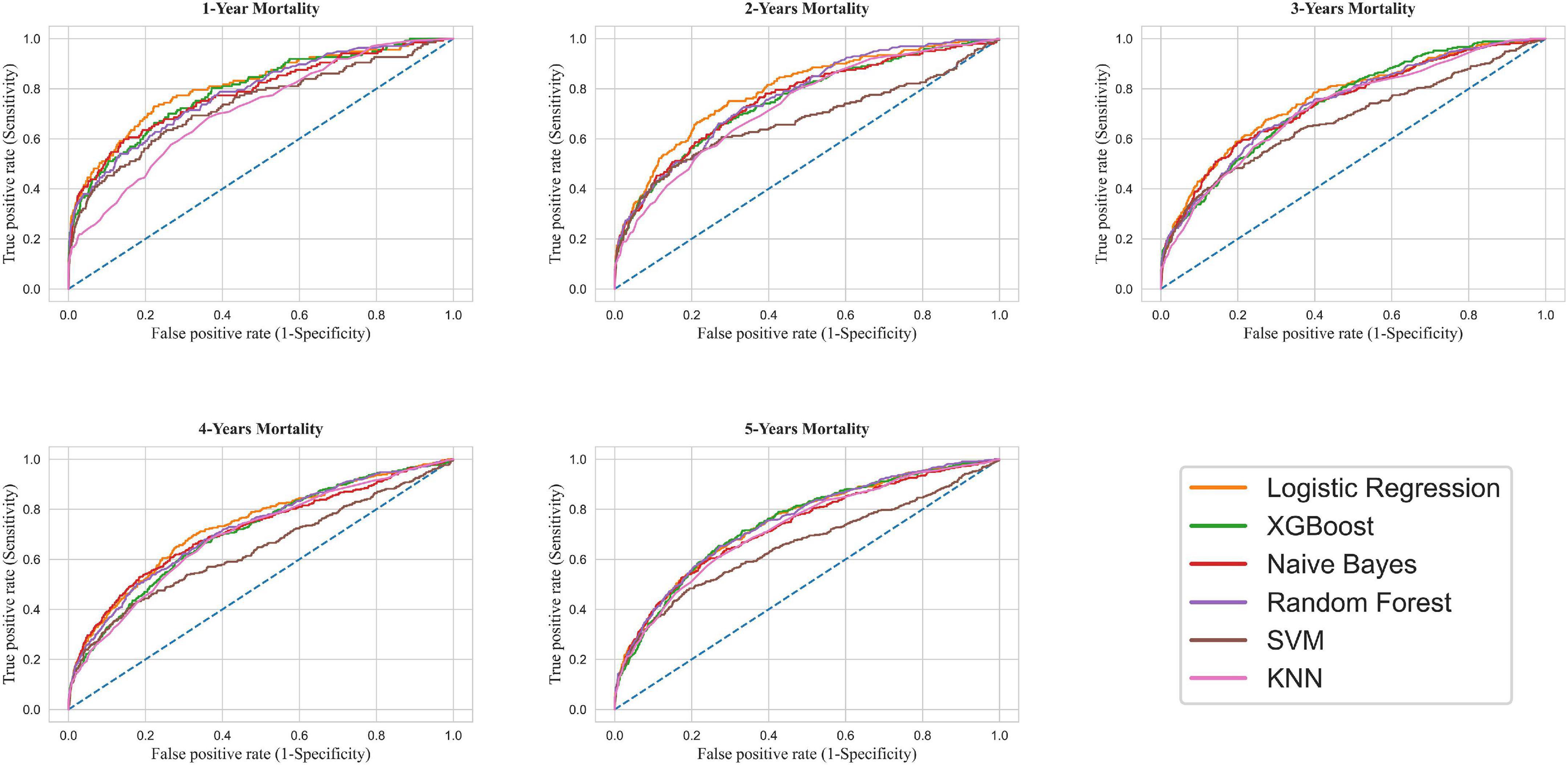
Figure 4. Receiver operating characteristic curve for mortality prediction. XGBoost, Extreme Gradient Boosting; SVM, Support Vector Machine; KNN, K-Nearest Neighbors.
This study compared six ML models concerning one-to-five-year mortality among CABG patients. Our study revealed that the ventilation time after the surgery and baseline EF were by far the most influential factors for predicting mortality. Furthermore, the LR model had the best predictive ability for one-year mortality with excellent discrimination (AUC = 0.81) (10). Moreover, according to AUC interpretation, all ML models other than LR presented an acceptable performance for predicting one-year mortality (0.7 < AUC < 0.8) (10). The same performance trend was ascertained for two-to-five-year follow-ups.
CABG is one of the most prevalent surgeries worldwide. Several calculators and models have been developed to detect and minimize its main culprits of mortality and morbidity. As the era of big data analytics unfolds, ML algorithms are primed to have a considerable effect and improve contemporary risk calculators and scoring systems. Likewise, the need to have individualized systems to predict outcomes in operation-specific cohorts has highlighted the importance of ML models in recent years (12, 13). Methodologically, ML models allow us to adjust sensitivity and specificity in each clinical setting (14). Several techniques can be used in cases where we face a meager outcome, such as mortality, with the oversampling method being applied frequently. SMOTE oversampling creates synthetic examples for the minority group and is suggested to be better than undersampling methods because of retaining valuable data (15, 16). Lowering the prediction threshold is another common measure to overcome imbalanced data. As the 50% default threshold gives us many missed cases for mortality, we tuned this measure to have the optimum sensitivity and specificity on the ROC curve. This method has been used in several studies (14, 17).
Current risk scores such as STS, EURO-Score I and II were designed to predict short-term mortality and need to be modified to be used for long-term (2–4) as Puskas et al. (18) have proposed. Several models have been developed to predict the prognosis of CABG, with growing attention to ML methods. ML can be a promising tool for improving conventional scoring systems like STS (19, 20). Studies have reported the effectiveness of an ensemble of various ML algorithms concerning in-hospital mortality risk (21). A study investigated five ML methods to predict long-term mortality after CABG. In contrast to our study, this study concluded that Gradient Boosting Machine was the best predictive technique (AUC: 0.767), outperforming the LR technique (22). In agreement with this finding, another study compared various ML models for estimating the long-term mortality risk of the elderly who underwent CABG. Models included LR, RF, Classification And Regression Tree (CART), Multivariate Adaptive Regression Splines (MARS), and XGBoost. Their results showed that the XGBoost model and MARS had the best prediction performance, before and after variable selection, respectively (23).
However, like our findings, a recent meta-analysis concluded that in studies with low risk of bias, LR was as predictive as other ML models, while in studies at high risk of bias, other ML methods had better discrimination ability than LR (24). Despite these controversies, LR is a tried-and-true statistical method. It, therefore, should remain the gold standard until newer approaches can show demonstrably better predictive ability.
Many feature selection methods are available to select the most relevant features in ML models, with RF technique being commonly used in classification models (25). RF models work by constructing several random decision trees with the top features. For the classification, they use major vote among all decision trees to predict the class of the outcome (26).
Total ventilation hours as a peri-operative variable was the most important feature based on our feature selector. The importance of mechanical ventilation time for CABG mortality has also been supported by other studies using LR, RF, and XGBoost modeling (23). Other researchers have also indicated that mechanical ventilation requirement is a predictor of in-hospital and long-term mortality in patients undergoing cardiac surgery (27). In our study, EF was the second most important mortality predictor, followed by TG, age, Hb, Cr, total cholesterol, FBS, LDL-C, HDL-C, and BMI. The importance of these features has been reported in studies using ML methods or otherwise. These factors chime in with a recent study revealing creatinine, EF, and age among the most predictive features for in-hospital and 30-day mortality of cardiac surgery, using the XGBoost model (20). Similarly, a recent study on long-term survival of elderly patients with CABG revealed that age, renal disease, and hyperlipidemia are among the most important survival predictors, using various ML methods (23). The importance of EF on CABG survival has also been repeatedly reported in other studies (28, 29), some of which revealed a dose-response relationship between decreasing EF and overall risk of death (28). This is also the case with the role of our other selected features in estimating long-term CABG survival, including age (30, 31), glucose and lipid profile (32–35), Hb (36, 37), Cr (38, 39), and BMI (31).
Our cohort study was based on the cardiac surgery databank of Tehran Heart Center, one of Iran’s largest observational cardiovascular databases (40). The benefits of using this databank are as follows: (1) it included various demographic, preoperative, intraoperative, and postoperative information; (2) more than 16,000 and 10,000 patients could be tracked for a 1 and 5-year follow-up, respectively; (3) it could present current real-world experiences with CABG patients.
Nevertheless, there were some limitations to our study. As the study was conducted based on single-center data, the generalizability of models is a significant issue since the demographic characteristics of patients are confined to a single center (e.g., male predominance). Some relevant input features were discarded due to high missing data. There is also the potential for confounding variables that were not included in the analysis. No electrocardiogram data and follow-up laboratory results were available.
In this study, we developed different ML models for predicting one-to-five-year mortality in patients undergoing CABG. Feature selection chose eleven features for the prediction, the most vital of which were total ventilation hours and EF. Furthermore, all models demonstrated at least acceptable performance for estimating one-year mortality, with LR demonstrating the highest AUC (0.81). The overall summary of ML models and findings of our study is illustrated in Figure 5. In conclusion, ML algorithms may pave the way for clinicians to select CABG candidates through weighing mortality risks against the merits of receiving the surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tehran Heart Center, Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC.1400.11.23). The patients/participants provided their written informed consent to participate in this study.
AK, AHB, KH, SS, JB, and SA contributed to the conception or design of the work. MP, MJ, AShe, and AF contributed to data acquisition. MP, AK, ASha, and AHB conducted data analysis. All authors contributed to data interpretation, participated in drafting the work or revising it critically for important intellectual content, approved the version for publication, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization [WHO]. Cardiovascular diseases (CVDs) 2021. Geneva: World Health Organization (2021).
2. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. (1999) 16:9–13. doi: 10.1016/S1010-7940(99)00134-7
3. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. (2012) 41:734–44; discussion 44–5. doi: 10.1093/ejcts/ezs043
4. Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1–coronary artery bypass grafting surgery. Ann Thorac Surg. (2009) 88(Suppl. 1):S2–22. doi: 10.1016/j.athoracsur.2009.05.053
5. Karimi A, Ahmadi H, Davoodi S, Movahedi N, Marzban M, Abbasi K, et al. Factors affecting postoperative morbidity and mortality in isolated coronary artery bypass graft surgery. Surg Today. (2008) 38:890–8. doi: 10.1007/s00595-007-3733-z
6. Krittanawong C, Virk HUH, Bangalore S, Wang Z, Johnson KW, Pinotti R, et al. Machine learning prediction in cardiovascular diseases: A meta-analysis. Sci Rep. (2020) 10:16057. doi: 10.1038/s41598-020-72685-1
7. Dimopoulos AC, Nikolaidou M, Caballero FF, Engchuan W, Sanchez-Niubo A, Arndt H, et al. Machine learning methodologies versus cardiovascular risk scores, in predicting disease risk. BMC Med Res Methodol. (2018) 18:179. doi: 10.1186/s12874-018-0644-1
8. Kilic A. Artificial intelligence and machine learning in cardiovascular health care. Ann Thorac Surg. (2020) 109:1323–9. doi: 10.1016/j.athoracsur.2019.09.042
9. Hosseini K, Mortazavi SH, Sadeghian S, Ayati A, Nalini M, Aminorroaya A, et al. Prevalence and trends of coronary artery disease risk factors and their effect on age of diagnosis in patients with established coronary artery disease: Tehran Heart Center (2005–2015). BMC Cardiovasc Disord. (2021) 21:477. doi: 10.1186/s12872-021-02293-y
10. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thoracic Oncol. (2010) 5:1315–6. doi: 10.1097/JTO.0b013e3181ec173d
11. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine learning in python. J Mach Learn Res. (2011) 12:2825–30.
12. Bica I, Alaa AM, Lambert C, van der Schaar M. From real-world patient data to individualized treatment effects using machine learning: Current and future methods to address underlying challenges. Clin Pharmacol Ther. (2021) 109:87–100. doi: 10.1002/cpt.1907
13. MacEachern SJ, Forkert ND. Machine learning for precision medicine. Genome. (2021) 64:416–25. doi: 10.1139/gen-2020-0131
14. Bihorac A, Ozrazgat-Baslanti T, Ebadi A, Motaei A, Madkour M, Pardalos PM, et al. MySurgeryRisk: Development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg. (2019) 269:652. doi: 10.1097/SLA.0000000000002706
15. Karanasiou GS, Tripoliti EE, Papadopoulos TG, Kalatzis FG, Goletsis Y, Naka KK, et al. Predicting adherence of patients with HF through machine learning techniques. Healthc Technol Lett. (2016) 3:165–70. doi: 10.1049/htl.2016.0041
16. Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: Synthetic minority over-sampling technique. J Artificial Intell Res. (2002) 16:321–57. doi: 10.1613/jair.953
17. Thorsen-Meyer H-C, Nielsen AB, Nielsen AP, Kaas-Hansen BS, Toft P, Schierbeck J, et al. Dynamic and explainable machine learning prediction of mortality in patients in the intensive care unit: A retrospective study of high-frequency data in electronic patient records. Lancet Digital Health. (2020) 2:e179–91. doi: 10.1016/S2589-7500(20)30018-2
18. Puskas JD, Kilgo PD, Thourani VH, Lattouf OM, Chen E, Vega JD, et al. The society of thoracic surgeons 30-day predicted risk of mortality score also predicts long-term survival. Ann Thorac Surg. (2012) 93:26–33; discussion 33–5. doi: 10.1016/j.athoracsur.2011.07.086
19. Zea-Vera R, Ryan CT, Havelka J, Corr SJ, Nguyen TC, Chatterjee S, et al. Machine learning to predict outcomes and cost by phase of care after coronary artery bypass grafting. Ann Thorac Surg. (2021): [Epub ahead of print]. doi: 10.1016/j.athoracsur.2021.08.040
20. Kilic A, Goyal A, Miller JK, Gjekmarkaj E, Tam WL, Gleason TG, et al. Predictive utility of a machine learning algorithm in estimating mortality risk in cardiac surgery. Ann Thorac Surg. (2020) 109:1811–9. doi: 10.1016/j.athoracsur.2019.09.049
21. Allyn J, Allou N, Augustin P, Philip I, Martinet O, Belghiti M, et al. A comparison of a machine learning model with EuroSCORE II in predicting mortality after elective cardiac surgery: A decision curve analysis. PLoS One. (2017) 12:e0169772. doi: 10.1371/journal.pone.0169772
22. Forte JC, Wiering MA, Bouma HR, Geus F, Epema AH. Predicting long-term mortality with first week post-operative data after Coronary Artery Bypass Grafting using Machine Learning models. In: Finale D-V, Jim F, David K, Rajesh R, Byron W, Jenna W editors. Proceedings of the 2nd machine learning for healthcare conference. Proceedings of Machine Learning Research: PMLR. Groningen: University of Groningen (2017). p. 39–58.
23. Huang YC, Li SJ, Chen M, Lee TS, Chien YN. Machine-learning techniques for feature selection and prediction of mortality in elderly CABG patients. Healthcare (Basel). (2021) 9:547. doi: 10.3390/healthcare9050547
24. Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. (2019) 110:12–22. doi: 10.1016/j.jclinepi.2019.02.004
25. Chen R-C, Dewi C, Huang S-W, Caraka RE. Selecting critical features for data classification based on machine learning methods. J Big Data. (2020) 7:52. doi: 10.1186/s40537-020-00327-4
27. Pappalardo F, Franco A, Landoni G, Cardano P, Zangrillo A, Alfieri O. Long-term outcome and quality of life of patients requiring prolonged mechanical ventilation after cardiac surgery. Eur J Cardio Thoracic Surg. (2004) 25:548–52. doi: 10.1016/j.ejcts.2003.11.034
28. Omer S, Adeseye A, Jimenez E, Cornwell LD, Massarweh NN. Low left ventricular ejection fraction, complication rescue, and long-term survival after coronary artery bypass grafting. J Thorac Cardiovasc Surg. (2022) 163:111–9.e2. doi: 10.1016/j.jtcvs.2020.03.040
29. Talukder S, Dimagli A, Benedetto U, Gray A, Gerry S, Lees B, et al. Prognostic factors of 10-year mortality after coronary artery bypass graft surgery: A secondary analysis of the arterial revascularization trial. Eur J Cardiothorac Surg. (2022) 61:1414–20. doi: 10.1093/ejcts/ezac043
30. Nicolini F, Fortuna D, Contini GA, Pacini D, Gabbieri D, Zussa C, et al. The impact of age on clinical outcomes of coronary artery bypass grafting: Long-term results of a real-world registry. BioMed Res Int. (2017) 2017:9829487. doi: 10.1155/2017/9829487
31. van Straten AH, Bramer S, Soliman Hamad MA, van Zundert AA, Martens EJ, Schönberger JP, et al. Effect of body mass index on early and late mortality after coronary artery bypass grafting. Ann Thorac Surg. (2010) 89:30–7. doi: 10.1016/j.athoracsur.2009.09.050
32. Jahangiry L, Najafi M, Farhangi MA, Jafarabadi MA. Coronary artery bypass graft surgery outcomes following 6.5 years: A nested case-control study. Int J Prev Med. (2017) 8:23. doi: 10.4103/ijpvm.IJPVM_250_16
33. Ram E, Sternik L, Klempfner R, Iakobishvili Z, Fisman EZ, Tenenbaum A, et al. Type 2 diabetes mellitus increases the mortality risk after acute coronary syndrome treated with coronary artery bypass surgery. Cardiovasc Diabetol. (2020) 19:86. doi: 10.1186/s12933-020-01069-6
34. Anderson RE, Klerdal K, Ivert T, Hammar N, Barr G, Owall A. Are even impaired fasting blood glucose levels preoperatively associated with increased mortality after CABG surgery? Eur Heart J. (2005) 26:1513–8. doi: 10.1093/eurheartj/ehi182
35. Sattartabar B, Ajam A, Pashang M, Jalali A, Sadeghian S, Mortazavi H, et al. Sex and age difference in risk factor distribution, trend, and long-term outcome of patients undergoing isolated coronary artery bypass graft surgery. BMC Cardiovasc Disord. (2021) 21:460. doi: 10.1186/s12872-021-02273-2
36. Bell ML, Grunwald GK, Baltz JH, McDonald GO, Bell MR, Grover FL, et al. Does preoperative hemoglobin independently predict short-term outcomes after coronary artery bypass graft surgery? Ann Thorac Surg. (2008) 86:1415–23. doi: 10.1016/j.athoracsur.2008.07.088
37. Miceli A, Romeo F, Glauber M, de Siena PM, Caputo M, Angelini GD. Preoperative anemia increases mortality and postoperative morbidity after cardiac surgery. J Cardiothorac Surg. (2014) 9:137. doi: 10.1186/1749-8090-9-137
38. Hillis GS, Croal BL, Buchan KG, El-Shafei H, Gibson G, Jeffrey RR, et al. Renal function and outcome from coronary artery bypass grafting. Circulation. (2006) 113:1056–62. doi: 10.1161/CIRCULATIONAHA.105.591990
39. Carr BM, Romeiser J, Ruan J, Gupta S, Seifert FC, Zhu W, et al. Long-Term Post-CABG survival: Performance of clinical risk models versus actuarial predictions. J Card Surg. (2016) 31:23–30. doi: 10.1111/jocs.12665
Keywords: machine learning, feature selection, coronary artery bypass, prediction, mortality
Citation: Khalaji A, Behnoush AH, Jameie M, Sharifi A, Sheikhy A, Fallahzadeh A, Sadeghian S, Pashang M, Bagheri J, Ahmadi Tafti SH and Hosseini K (2022) Machine learning algorithms for predicting mortality after coronary artery bypass grafting. Front. Cardiovasc. Med. 9:977747. doi: 10.3389/fcvm.2022.977747
Received: 24 June 2022; Accepted: 02 August 2022;
Published: 24 August 2022.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Ayse Baysal, Pendik Veterinary Control and Research Institute, TurkeyCopyright © 2022 Khalaji, Behnoush, Jameie, Sharifi, Sheikhy, Fallahzadeh, Sadeghian, Pashang, Bagheri, Ahmadi Tafti and Hosseini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaveh Hosseini, a2F2ZWhfaG9zc2VpbmkxMzBAeWFob28uY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.