
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 05 October 2022
Sec. Cardiovascular Genetics and Systems Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.973338
Background: Extensive epidemiological studies have highlighted the correlation between serum phosphate and cardiovascular diseases. The present study aims to determine whether genetically predicted serum phosphate is causally associated with the distinct subtypes of cardiovascular events through the use of Mendelian randomization (MR) analysis.
Methods: Independent and strongly correlated single-nucleotide polymorphisms (SNPs) for serum phosphate were extracted from publicly available genome-wide association studies. Summary statistics of cardiovascular diseases were derived from large-scale consortiums, including HERMES and FinnGen biobank. MR-Egger, weighted median, inverse variance weighted, pleiotropy residual sum and outlier (MR-PRESSO) methods and MR using robust adjusted profile score (MR-RAPS) were employed to analyze causality. The sensitivity analyses comprised heterogeneity, horizontal pleiotropy, and leave-one-out approaches; these were used to ensure the stability of the results.
Results: Our study demonstrated that increased genetically predicted serum phosphate is causally associated with a higher risk of valvular heart disease (VHD) [For VHD including rheumatic fever: odds ratio (OR) = 2.45; 95% confidence interval (CI), 1.52–3.94; p = 0.0002; for non-rheumatic VHD: OR = 6.58; 95% CI, 2.50–17.32; p = 0.0001]. However, no causal association was detected between serum phosphate and other common cardiovascular diseases (including coronary heart disease, heart failure, atrial fibrillation, and essential hypertension).
Conclusions: The results indicate strong causality between serum phosphate and valvular heart disease. Serum phosphate-lowering therapy within the physiological range may represent a novel therapeutic method for valvular heart disease.
Cardiovascular disease (CVD), a predominant cause of death worldwide, largely contributes to the global burden of disease (1–3). Despite advancements in diagnosis and treatment, further exploration of causative factors is required (4, 5).
Phosphate plays an essential role in various physiological and pathological processes involved in energy metabolism, cellular structure, and signal transduction (6–8). Extensive studies have discussed the epidemiological link between serum phosphate and cardiovascular events, including atherosclerosis (9–11), ischemic heart disease (12, 13), hypertension (14), heart failure (15), and valvular heart disease (VHD) (16–18). However, according to the IMPROVE-CKD study and the LANDMARK randomized clinical trial, treatment with lanthanum carbonate, an intestinal phosphate binder, does not result in a significant difference in the occurrence of composite cardiovascular events in chronic kidney disease with normophosphatemia or hyperphosphatemia (19, 20). The paradoxical role of phosphate in CVDs is a pressing issue that must be addressed, and additional evidence is needed to demonstrate that phosphate clearly precedes CVDs. Furthermore, traditional epidemiology is subject to reverse causation and residual biases. It is unable to ascertain whether serum phosphate is an important preventable cause of CVDs.
Numerous studies have attempted to determine the causality between exposures and outcomes through the utilization of Mendelian randomization (MR) analysis (21, 22). With the natural and random distribution of genetic variants, MR is known to be less vulnerable to confounding and reverse causation (23). Using the Mendelian randomization approach, we intend to explore the potential causal relationship between phosphate and five CVDs: coronary heart disease (CHD), heart failure (HF), atrial fibrillation (AF), essential hypertension (EH), and valvular heart disease (VHD).
The data used in the two-sample MR analysis are publicly available from the Genome-Wide Association Study (GWAS) Catalog (https://www.ebi.ac.uk/gwas). Ethical approval for the studies and the informed consent of all participants were obtained. An overview of the study design is shown in Figure 1.
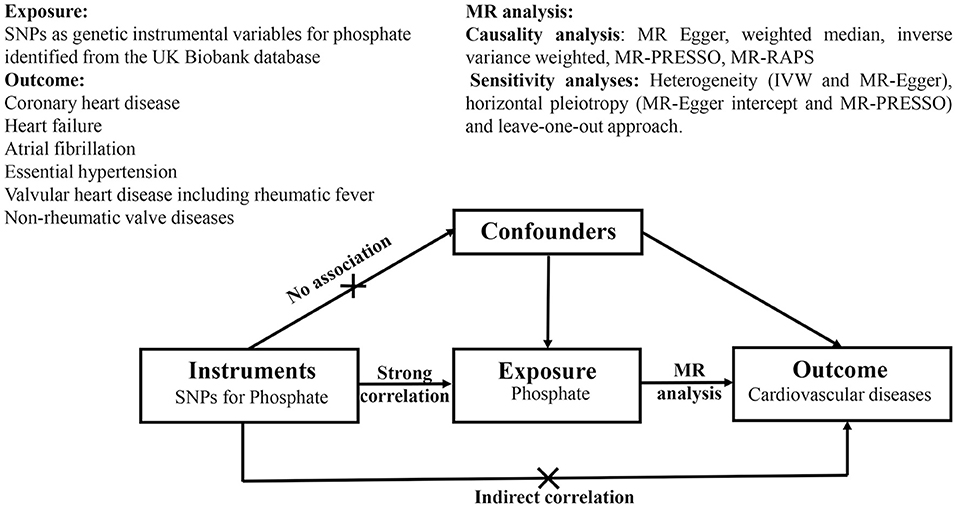
Figure 1. Schematic diagram of Mendelian randomization analyses. SNPs as genetic instruments are used to estimate the causal relationship between phosphate and cardiovascular disease. The genetic variables are not associated with potential confounders. Furthermore, there is no existence of a direct correlation between genetic instruments and outcomes. SNPs, single-nucleotide polymorphisms; MR-PRESSO, MR pleiotropy residual sum and outlier; MR-RAPS, MR using robust adjusted profile score; IVW, inverse variance weighted.
We extracted the single-nucleotide polymorphisms (SNPs) from the GWAS data according to two criteria for strong correlation and independence as follows: genome-wide level of statistical significance (5 × 10−8) and linkage disequilibrium (LD) with r2 < 0.001 and clump window > 10,000 kb. This study collected 159 SNPs as genetic instrumental variables for serum phosphate from the UK Biobank database at (https://gwas.mrcieu.ac.uk/datasets/ukb-d-30810_raw/), which included 431,448 participants of European ancestry. Furthermore, all SNPs were cross-referenced with the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/) to identify associations with confounders and outcomes (24).
The CVDs-associated SNPs were derived from HERMES, the FinnGen biobank, and other large-scale consortiums. The detailed characterization of each CVDs is shown in Table 1.
We performed two-sample MR, which harmonized the SNPs of phosphate and the common CVDs in independent datasets and removed all palindromic SNPs from the analysis. F-statistics was used to assess the strength of genetic variants (25). We estimated the causal effects using five methods: MR Egger, weighted median, inverse variance weighted (IVW), pleiotropy residual sum and outlier (MR-PRESSO) (26), and MR using robust adjusted profile score (MR-RAPS) (27). IVW was regarded as the principal approach (28). Results were presented as odds ratios (ORs) and 95% confidence intervals (CIs) on phosphate risk for common CVDs. The sensitivity analyses comprised three approaches: heterogeneity (IVW and MR-Egger), horizontal pleiotropy (MR-Egger intercept and MR-PRESSO), and leave-one-out. Heterogeneity was measured through Cochran's Q test (29), and outliers were detected via MR-PRESSO analysis (26). The intercept in the MR-Egger regression showed evidence for pleiotropic bias and was visualized using funnel plots (30). The leave-one-out SNP analysis was applied to examine the sensitivity of each genetic variant, which was generally used in MR (31, 32). The MR analyses were conducted in R version 4.1.3 (http://www.r-project.org) using the TwoSampleMR package (33).
Initially, 159 SNPs were identified from the GWAS catalog at the genome-wide significance level (p < 5 × 10−8) and linkage disequilibrium (LD) with r2 < 0.001 and clump window >10,000 kb, as shown in Supplementary Table 1. Based on PhenoScanner, several genetic instrumental variables were removed for their associations with confounders of CVDs (including body mass index, blood pressure, smoking, and lipid levels) and direct connections to outcomes (Supplementary Table 2). After harmonizing the SNPs of phosphate and the common CVDs in independent datasets and removing all palindromic SNPs, the final datasets were obtained (Supplementary Table 3). The F-statistics for phosphate were higher than the threshold of 10, which indicates no evidence of weak instrument bias (Supplementary Table 3).
The main results of the causal analysis are presented in Figure 2. The IVW method showed that genetically predicted serum phosphate levels were positively associated with VHD [For VHD including rheumatic fever: odds ratio (OR) = 2.45; 95% confidence interval (CI), 1.52–3.94; p = 0.0002; for non-rheumatic VHD: OR = 6.58; 95% CI, 2.50–17.32; p = 0.0001]. However, no significant difference was detected in other common CVDs (including CHD, HF, AF, and EH). We utilized a scatter diagram and forest plot to visualize the relationship between each genetic variant and CVDs (Supplementary Figures 1, 2). Cochran's Q statistical and MR-PRESSO analyses revealed the existence of heterogeneity and outliers in our study (Supplementary Tables 4, 5). The analyses using two additional methods (MR-PRESSO and MR-RAPS) highlighted a significant association between serum phosphate and VHD, which is directionally consistent with previous methods (Figure 2). No evidence of horizontal pleiotropy was noted in the MR-Egger regression intercept analysis; the results were visualized through a funnel plot (Supplementary Table 4 and Supplementary Figure 3). Based on the results of the leave-one-out analysis, there was no single genetic variant that altered the causality (Supplementary Figure 4).
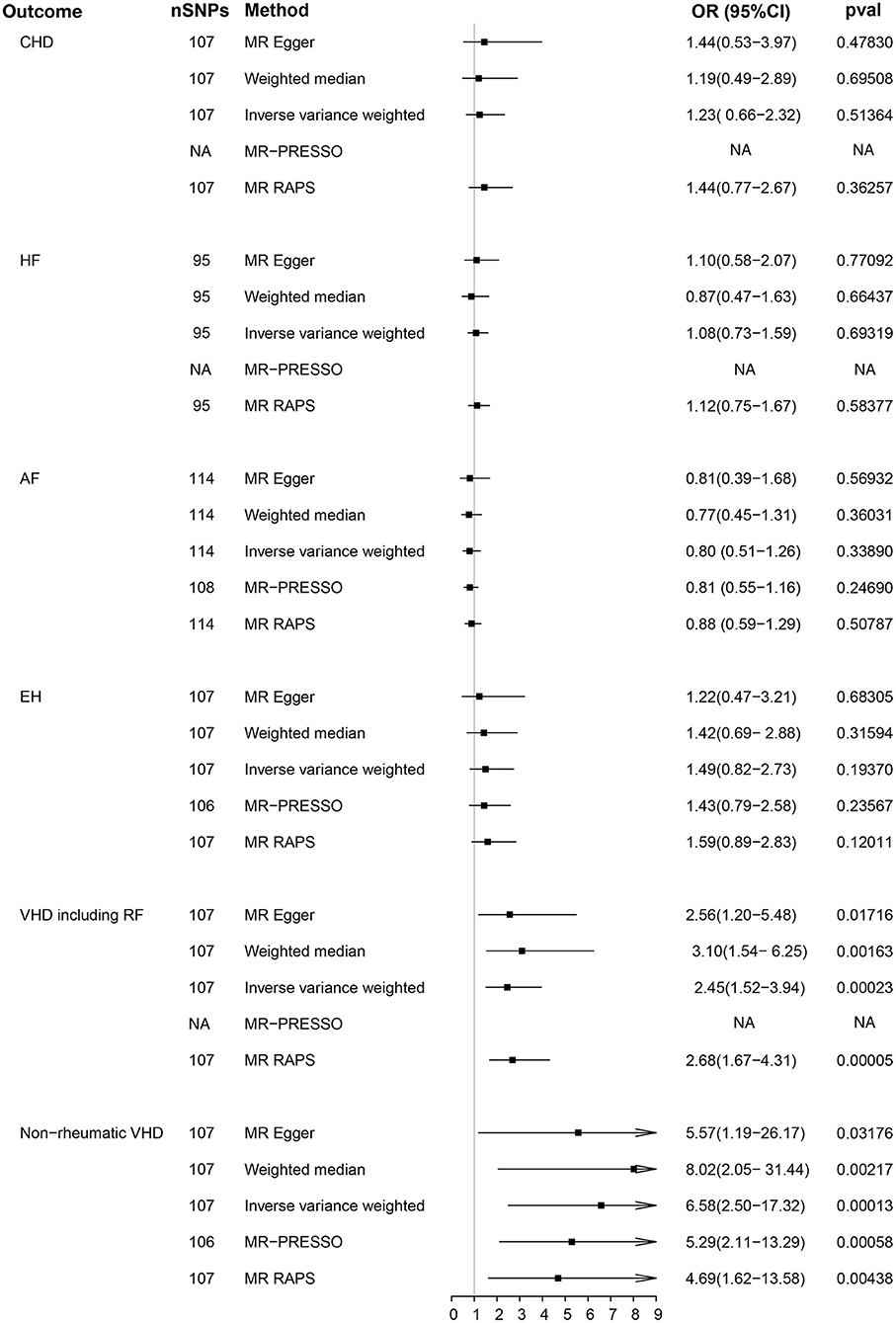
Figure 2. Causality analysis between genetically predicted phosphate and common cardiovascular diseases. P-value, OR and 95% CI of five methods [MR Egger, weighted median, inverse variance weighted (IVW), MR-PRESSO, and MR RAPS] are shown in the results. CHD, coronary heart disease; HF, heart failure; AF, atrial fibrillation; EH, essential hypertension; VDH, valvular heart disease; RF, rheumatic fever; MR-PRESSO, MR pleiotropy residual sum and outlier; MR-RAPS, MR using robust adjusted profile score; OR, odds ratio; CI, confidence interval; NA, not available.
Numerous epidemiological studies have highlighted the correlation between serum phosphate and CVDs. However, few in-depth investigations about the causality of serum phosphate on different subtypes of cardiovascular events have been conducted. Furthermore, the majority of randomized controlled trials have targeted phosphate-related cardiovascular endpoints in chronic kidney diseases rather than independent cardiovascular events (19, 20, 34). Our study demonstrates a causal relationship between genetically predicted serum phosphate and valvular heart disease. The sensitivity analyses (heterogeneity, horizontal pleiotropy, and leave-one-out approaches) proved the stability of the results.
VHD mainly manifests as valve stenosis or incomplete closure, which results in poor quality of life. The epidemiology of VHD presents substantial regional differences: degenerative diseases predominate in high-income countries, while rheumatic heart diseases predominate in low-and middle-income countries (35, 36). Current Mendelian randomization studies of VHD are mainly centered on lipid (37, 38) and blood pressure (39). Our study provides convincing evidence of the causality between genetically predicted serum phosphate and valvular heart disease, which is consistent with prior epidemiological research (18, 40). However, there is no evidence that suggests a causal association of serum phosphate with other common CVDs (including CHD, HF, AF, and EH) in the present MR studies. This discrepancy may suggest the existence of correlation rather than causality between phosphate and these CVDs, which warrants further research to elucidate the underlying relationship.
Mechanistically, increased phosphate promotes hydroxyapatite deposition in the valves and the osteogenic differentiation of vascular smooth muscle and valvular interstitial cells, which accelerates the process of valve calcification (41–43). Furthermore, an extensive number of studies have revealed that phosphate is correlated with inflammation, which provides suggestive evidence for the causality between serum phosphate and VHD (44–46). Taken together, serum phosphate may play a critical role in the pathogenesis of VHD.
Our study has several strengths. Based on the random distribution of genetic variations in the population, our study minimizes reverse causation and residual biases. Similar assessment results across various approaches for causality ensure the credibility of causality. Furthermore, we performed sensitivity analyses through the combined use of diversified approaches. To avoid potential bias from population stratification, our sample was restricted to individuals of European ancestries. The confirmed causality between serum phosphate and VHD suggests a novel therapeutic method for VHD.
This study has several limitations. First, we had no access to comprehensive information regarding the participants (including age, sex, and other influencing factors), thereby causing inevitable heterogeneity. Second, based on the summary-level data, our study was unable to exclude the presence of non-linear relationships. Finally, the causality between serum phosphate and the specific subgroup of VHD remains to be explored due to the absence of classifications in GWAS databases at present.
Our results strongly indicate a causal relationship between serum phosphate and valvular heart disease using the MR method. Targeting the serum phosphate homeostasis as a potential therapeutic approach may provide profound implications for valvular heart disease.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary materials.
The studies involving human participants were reviewed and approved by Local Ethics Committees of consortia in the respective studies. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JH, XX, and JJ designed the study and wrote the manuscript. JH, CZ, QG, YG, XX, and JJ contributed to the data acquisition and revision of the manuscript. All authors approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Grant No. 82170332).
The authors thank various consortiums, including FinnGen biobank, AFGen, HUNT, MGI, deCODE, HERMES, and the UK Biobank study for providing the data publicly. The authors acknowledge all GWAS participants for their contributions to the summary statistics data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.973338/full#supplementary-material
CVD, cardiovascular disease; CHD, coronary heart disease; HF, heart failure; AF, atrial fibrillation; EH, essential hypertension; VHD, valvular heart disease; RF, rheumatic fever; MR, Mendelian randomization; SNP, single-nucleotide polymorphism; GWAS, genome-wide association study; IVW, inverse variance weighted; LD, linkage disequilibrium; OR, odds ratio; CI, confidence interval; MR-PRESSO, MR pleiotropy residual sum and outlier; MR-RAPS, MR using robust adjusted profile score.
1. Townsend N, Kazakiewicz D, Lucy Wright F, Timmis A, Huculeci R, Torbica A, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. (2022) 19:133–43. doi: 10.1038/s41569-021-00607-3
2. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC Asia. (2021) 1:1–13. doi: 10.1016/j.jacasi.2021.04.007
4. Crea F. How epidemiology can improve the understanding of cardiovascular disease: from mechanisms to treatment. Euro Heart J. (2021) 42:4503–7. doi: 10.1093/eurheartj/ehab797
5. Ezzati M, Obermeyer Z, Tzoulaki I, Mayosi BM, Elliott P, Leon DA. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat Rev Cardiol. (2015) 12:508–30. doi: 10.1038/nrcardio.2015.82
6. Bhadada SK, Rao SD. Role of phosphate in biomineralization. Calcified Tissue Int. (2021) 108:32–40. doi: 10.1007/s00223-020-00729-9
7. Michigami T, Kawai M, Yamazaki M, Ozono K. Phosphate as a signaling molecule and its sensing mechanism. Physiol Rev. (2018) 98:2317–48. doi: 10.1152/physrev.00022.2017
8. Pandol SJ. Serum phosphate levels and alcohol-induced pancreatitis. Gastroenterology. (2022) 162:995–6. doi: 10.1053/j.gastro.2021.11.018
9. Hénaut L, Massy ZA. Phosphate meeting cholesterol-consequences for cardiovascular disease in chronic kidney disease? Kidney Int. (2021) 99:1264–7. doi: 10.1016/j.kint.2021.02.022
10. Shin S, Kim KJ, Chang HJ, Cho I, Kim YJ, Choi BW, et al. Impact of serum calcium and phosphate on coronary atherosclerosis detected by cardiac computed tomography. Euro Heart J. (2012) 33:2873–81. doi: 10.1093/eurheartj/ehs152
11. Park KS, Lee Y, Park GM, Park JH, Kim YG, Yang DH, et al. Association between serum phosphorus and subclinical coronary atherosclerosis in asymptomatic Korean individuals without kidney dysfunction. Am J Clin Nutr. (2020) 112:66–73. doi: 10.1093/ajcn/nqaa091
12. Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. (2005) 112:2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198
13. Jung DH, Park B, Lee YJ. Longitudinal effects of serum calcium and phosphate levels and their ratio on incident ischemic heart disease among Korean adults. Biomolecules. (2022) 12:103. doi: 10.3390/biom12010103
14. Mohammad J, Scanni R, Bestmann L, Hulter HN, Krapf R. A controlled increase in dietary phosphate elevates BP in healthy human subjects. J Am Soc Nephrol. (2018) 29:2089–98. doi: 10.1681/ASN.2017121254
15. Dhingra R, Gona P, Benjamin EJ, Wang TJ, Aragam J, D'Agostino RB, et al. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Euro J Heart Failure. (2010) 12:812–8. doi: 10.1093/eurjhf/hfq106
16. Bortnick AE, Xu S, Kim RS, Kestenbaum B, Ix JH, Jenny NS, et al. Biomarkers of mineral metabolism and progression of aortic valve and mitral annular calcification: the multi-ethnic study of atherosclerosis. Atherosclerosis. (2019) 285:79–86. doi: 10.1016/j.atherosclerosis.2019.04.215
17. Linefsky JP, O'Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol. (2011) 58:291–7. doi: 10.1016/j.jacc.2010.11.073
18. Xia C, Lei W, Hu Y, Yang H, Zeng X, Chen M. Association of serum levels of calcium phosphate and vitamin D with risk of developing aortic stenosis: the UK Biobank cohort. Euro J Prev Cardiol. (2022) 29:1520–8. doi: 10.1093/eurjpc/zwac016
19. Toussaint ND, Pedagogos E, Lioufas NM, Elder GJ, Pascoe EM, Badve SV, et al. A randomized trial on the effect of phosphate reduction on vascular end points in CKD (IMPROVE-CKD). J Am Soc Nephrol. (2020) 31:2653–66. doi: 10.1681/ASN.2020040411
20. Ogata H, Fukagawa M, Hirakata H, Kagimura T, Fukushima M, Akizawa T. Effect of treating hyperphosphatemia with lanthanum carbonate vs calcium carbonate on cardiovascular events in patients with chronic kidney disease undergoing hemodialysis: the LANDMARK randomized clinical trial. JAMA. (2021) 325:1946–54. doi: 10.1001/jama.2021.4807
21. Ai S, Zhang J, Zhao G, Wang N, Li G, So HC, et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear Mendelian randomization analyses in UK Biobank. Euro Heart J. (2021) 42:3349–57. doi: 10.1093/eurheartj/ehab170
22. Ren Z, Simons P, Wesselius A, Stehouwer CDA, Brouwers M. Relationship between non-alcoholic fatty liver disease and coronary artery disease: a Mendelian randomization study. Hepatology. (2022) doi: 10.1002/hep.32534. [Epub ahead of print].
23. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
24. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
25. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
26. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
27. Zhao Q, Wang J, Hemani G, Bowden J, Small DSJTAoS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat. (2020) 48:1742–69. doi: 10.1214/19-AOS1866
28. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
29. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
30. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
31. Ye Z, Zheng J. Verification of the role of ADAMTS13 in the cardiovascular disease using two-sample Mendelian randomization. Front Genet. (2021) 12:660989. doi: 10.3389/fgene.2021.660989
32. Corbin LJ, Richmond RC, Wade KH, Burgess S, Bowden J, Smith GD, et al. BMI as a modifiable risk factor for type 2 diabetes: refining and understanding causal estimates using Mendelian randomization. Diabetes. (2016) 65:3002–7. doi: 10.2337/db16-0418
33. Broadbent JR, Foley CN, Grant AJ, Mason AM, Staley JR, Burgess S. MendelianRandomization v0.5.0: updates to an R package for performing Mendelian randomization analyses using summarized data. Wellcome Open Res. (2020) 5:252. doi: 10.12688/wellcomeopenres.16374.1
34. Isaka Y, Hamano T, Fujii H, Tsujimoto Y, Koiwa F, Sakaguchi Y, et al. Optimal phosphate control related to coronary artery calcification in dialysis patients. J Am Soc Nephrol. (2021) 32:723–35. doi: 10.1681/ASN.2020050598
35. Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. (2021) 18:853–64. doi: 10.1038/s41569-021-00570-z
36. Xu H, Liu Q, Cao K, Ye Y, Zhang B, Li Z, et al. Distribution, characteristics, management of older patients with valvular heart disease in China. JACC Asia. (2022) 2:366–8 doi: 10.1016/j.jacasi.2021.11.013
37. Larsson SC, Gill D, Mason AM, Jiang T, Bäck M, Butterworth AS, et al. Lipoprotein(a) in Alzheimer, atherosclerotic, cerebrovascular, thrombotic, and valvular disease: Mendelian randomization investigation. Circulation. (2020) 141:1826–8. doi: 10.1161/CIRCULATIONAHA.120.045826
38. Jiang Q, Chen M. Assessing causality in associations of lipid levels with aortic valve stenosis. Euro Heart J. (2020) 41:2713. doi: 10.1093/eurheartj/ehaa371
39. Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, Canoy D, Raimondi F, Ayala Solares JR, et al. Systolic blood pressure and risk of valvular heart disease: a Mendelian randomization study. JAMA Cardiol. (2019) 4:788–95. doi: 10.1001/jamacardio.2019.2202
40. Wald DS, Bestwick JP. Association between serum calcium, serum phosphate and aortic stenosis with implications for prevention. Euro J Prev Cardiol. (2018) 25:551–6. doi: 10.1177/2047487318756131
41. Bäck M, Michel JB. From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovasc Res. (2021) 117:2016–29. doi: 10.1093/cvr/cvab038
42. Villa-Bellosta R. Vascular calcification: key roles of phosphate and pyrophosphate. Int J Mol Sci. (2021) 22:13536. doi: 10.3390/ijms222413536
43. Abbasian N. Vascular calcification mechanisms: updates and renewed insight into signaling pathways involved in high phosphate-mediated vascular smooth muscle cell calcification. Biomedicines. (2021) 9:804. doi: 10.3390/biomedicines9070804
44. Voelkl J, Egli-Spichtig D, Alesutan I, Wagner CA. Inflammation: a putative link between phosphate metabolism and cardiovascular disease. Clin Sci. (2021) 135:201–27. doi: 10.1042/CS20190895
45. Voelkl J, Lang F, Eckardt KU, Amann K, Kuro OM, Pasch A, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. (2019) 76:2077–91. doi: 10.1007/s00018-019-03054-z
Keywords: phosphate, cardiovascular disease, Mendelian randomization study, valvular heart disease, causality
Citation: Huang J, Zhang C, Gong Q, Gao Y, Xie X and Jiang J (2022) Genetically predicted phosphate and cardiovascular disease: A Mendelian randomization study. Front. Cardiovasc. Med. 9:973338. doi: 10.3389/fcvm.2022.973338
Received: 20 June 2022; Accepted: 20 September 2022;
Published: 05 October 2022.
Edited by:
Yuling Zhang, Sun Yat-sen Memorial Hospital, ChinaReviewed by:
Longlong Hu, Second Affiliated Hospital of Nanchang University, ChinaCopyright © 2022 Huang, Zhang, Gong, Gao, Xie and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Jiang, amlhbmctanVuQHpqdS5lZHUuY24=; Xiaojie Xie, eGlleGpAemp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.