- 1Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
- 2Department of Cardiology, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Background: Anthracyclines are commonly used chemotherapeutic agents to treat malignant tumors. However, cardiotoxicity is a potentially serious adverse effect of anthracyclines. Beta-blockers may be effective in preventing anthracycline-induced cardiotoxicity (AIC). However, the lack of direct comparisons of various beta-blockers interferes with clinical decision-making. Network meta-analysis (NMA) was performed to assess the effectiveness of beta-blockers for AIC.
Methods: We searched PubMed, Embase, Web of Science, and the Cochrane Central Register of Clinical Trials. The last update was in May 2022. Randomized controlled trials (RCT) of beta-blockers for AIC were included. Four beta-blockers were selected for comparison based on the number of studies. NMA was conducted with STATA 14.0 software.
Results: A total of 10 RCTs (875 patients) met the selection criteria. NMA results showed that carvedilol was superior to bisoprolol [SMD = −0.50, 95% CI (−0.91, −0.10)] and nebivolol [SMD = −1.46, 95%CI (−2.82, −0.11)] in a delay of LVEF. The results of the cumulative probability ordering are as follows: carvedilol (83.8%) > metoprolol (71.8%) > bisoprolol (43.9%) > placebo (40.9%) > nebivolol (9.5%).
Conclusion: Based on the available evidence, carvedilol is the best beta-blocker for AIC, followed by metoprolol. However, additional studies with large samples should be conducted to confirm our findings.
Introduction
Anthracyclines are anticancer drugs, including Adriamycin, erythromycin, and epi-amycin. They can be used to treat various types of cancer, including breast cancer, lymphoma, and leukemia (1, 2). Although their anticancer effects are notable, numerous clinical studies have found these drugs have serious adverse effects, with cardiotoxicity particularly prominent (3, 4). Anthracycline-induced cardiotoxicity (AIC) is a dose-limiting and possibly fatal complication of anthracycline administration that can arise during any period of chemotherapy (5). The main representative features are arrhythmias, pericardial effusion, and myocardial ischemia. AIC can contribute to cardiac failure and decrease survival (6). Mechanisms of AIC are complex and include free radicals, calcium overload, impaired energy metabolism, and apoptosis (7–12). A liposome-encapsulated formulation, doxorubicin liposome, was developed to limit anthracycline exposure in the myocardium. Liposomal doxorubicin improves the therapeutic index of conventional anthracyclines (13). However, AIC is still a pressing issue.
Beta-blockers are effective in treating hypertension, heart disease, and cardiac failure (14–16). Beta-blockers improve ventricular remodeling and reduce arrhythmias mainly by altering the status of adrenergic receptors (17, 18). Beta-blockers can delay the progression to heart failure in patients who develop cardiotoxicity (19, 20). Several beta-blockers are already available for clinical use, such as metoprolol, atenolol, nebivolol, bisoprolol, and carvedilol. Studies have shown that beta-blockers could be effective in preventing AIC (21). However, previous meta-analyses that evaluated the efficacy of beta-blockers to ameliorate AIC in terms of changes in left ventricular ejection function (LVEF) showed inconsistent results (19, 22–26). Furthermore, due to the small sample size of most studies, there is a lack of direct comparison between beta-blockers to determine which beta-blocker is most effective in preventing AIC.
Network meta-analysis (NMA) synthesizes evidence from direct and indirect comparisons to rank treatment interventions and guides drug selection (27). To provide additional evidence on beta-blocker treatment against AIC, a comprehensive systematic evaluation and NMA of relevant randomized controlled trials (RCTs) were conducted to assess which beta-blockers provided the best cardioprotective effect.
Methods
Network meta-analysis was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statements for Network Meta-Analysis (PRISMA-NMA) (28).
Inclusion and exclusion criteria
The inclusion criteria were: (1) participants: patients were diagnosed with tumors by pathology or imaging and were older than 18 years. All patients received anthracyclines, including epirubicin, pirarubicin, or doxorubicin. The dose and duration of drug treatment were unlimited; (2) type of study: randomized clinical trials (RCTs); (3) interventions: the experimental group began using beta-blockers before chemotherapy to counteract the cardiotoxicity of anthracycline-based chemotherapy. In the control group, a placebo was used; and (4) outcomes: LVEF at baseline and after chemotherapy, mortality, and adverse events. The exclusion criteria were: (1) non-RCT; (2) studies with insufficient data, duplicate data, or data that could not be extracted; (3) cardiotoxicity due to non-anthracycline chemotherapy; and (4) reviews, conference abstracts, or meta-analysis.
Search strategy
Randomized clinical trial studies on beta-blockers for the prevention of AIC were searched in PubMed, Embase, Web of Science, and the Cochrane Central Register of Clinical Trials. The last search was in May 2022. References from included studies were checked to identify additional studies. Search terms included anthracycline, chemotherapy, cardiotoxicity, doxorubicin, atenolol, carvedilol, metoprolol, nebivolol, bisoprolol, arotinolol, adrenergic beta-antagonists, randomized controlled trial, and random*. The search was conducted using a combination of medical subject headings and free-text words (Supplementary Table S1).
Data extraction
Data extracted included: (1) basic information: title, source, author, year; (2) baseline characteristics of the study population: number of trial participants, age, disease type; (3) intervention details, follow-up time; (4) key elements of the risk of bias evaluation; and (5) data on outcome indicators and outcome measures: LVEF, adverse events, and mortality.
Quality assessment
Two investigators independently assessed the risk of bias in the included studies and cross-checked the results. A bias assessment tool recommended in the Cochrane Handbook 5.1.0 was used to evaluate the risk of bias in RCT (29).
Statistical analysis
Stata 14.0 was used to analyze the data. Standardized mean difference (SMD) was used as the effect analysis statistic for the measurement data. Dichotomous variables were analyzed using the risk ratio (RR) as the effect analysis statistic. Each effect size was provided with its 95% confidence interval (95%CI). The χ2 test was used to assess statistical heterogeneity between the results of the studies, while the magnitude of heterogeneity was determined by combining I2 quantification. Fixed effects were used if there was no heterogeneity between studies (I2 <50%, P > 0.1). If there was heterogeneity (I2 > 50%, P < 0.1), the source of heterogeneity was analyzed, and a meta-analysis was performed with random effects after excluding the influence of heterogeneity.
Due to the limited data available in the literature, subgroup analyses were only performed for doses of carvedilol (6.25 mg vs. 12.5 mg vs. 25 mg). Sensitivity analysis was used to test the stability of the meta-analysis results. The mvmeta package was used for data preprocessing in the NMA. When network relationship diagrams were drawn, inconsistency tests should be performed to determine if there were closed loops in the network relationship diagrams. In the present study, no closed loop was formed for each outcome indicator. Therefore, no inconsistency test was performed. The outcome indicators for each intervention were ranked by plotting the surface under the cumulative ranking curve (SUCRA). Comparison-adjusted funnel plots were used to assess publication bias and the effects of the small sample in included studies.
Results
Search results
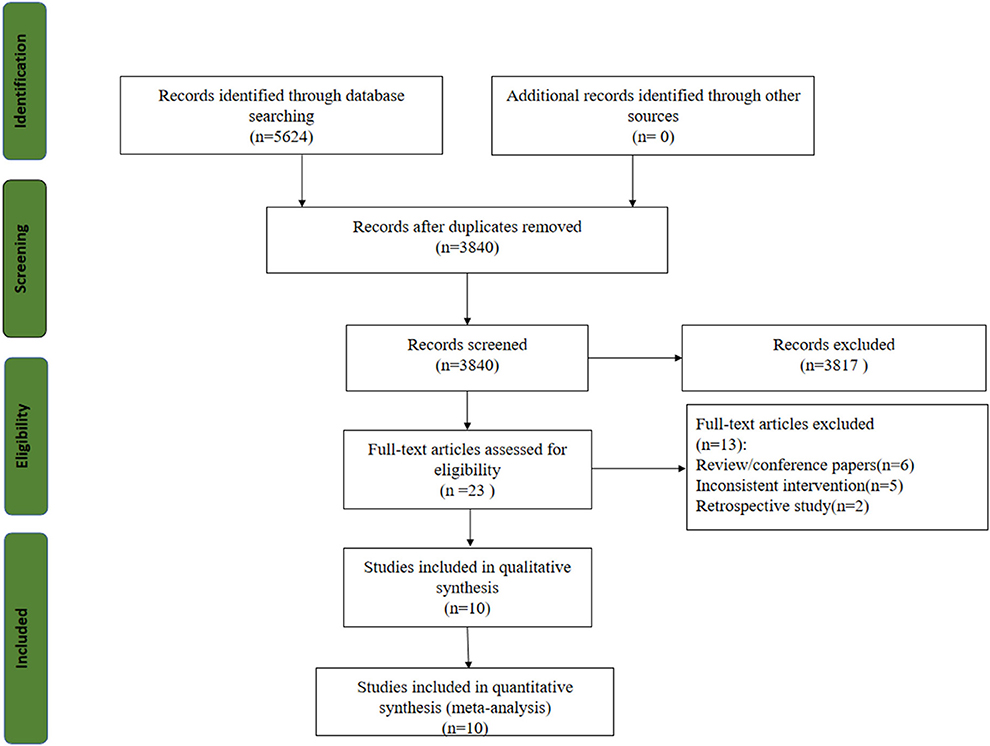
Five thousand six hundred twenty-four studies were obtained from the initial review, and 10 RCTs were included after screening (21, 30–38). The study selection flow chart is shown in Figure 1.
Study and patient characteristics
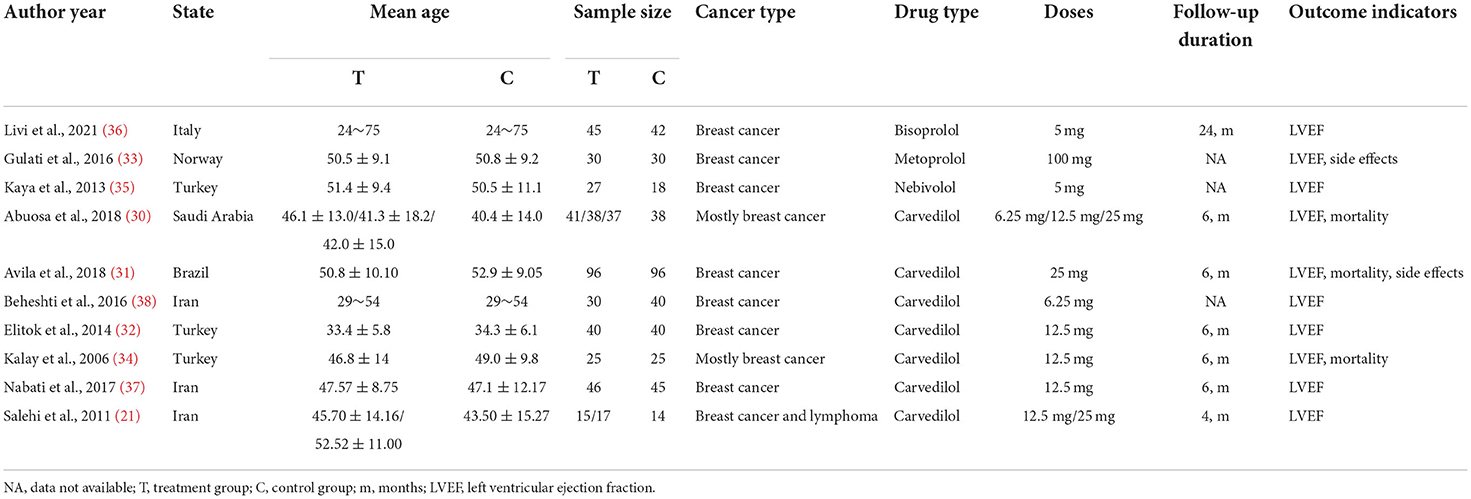
There were 875 patients in the 10 RCTs. Most of the tumor types were breast cancer. Four beta-blockers were included: bisoprolol, metoprolol, nebivolol, and carvedilol. Two studies compared the efficacy of different doses of beta-blockers compared to placebo. The beta-blocker doses ranged from 5 to 100 mg. The experimental groups were comparable to the control groups at baseline in these RCTs. The characteristics of the included studies are shown in Table 1.
Quality assessment results
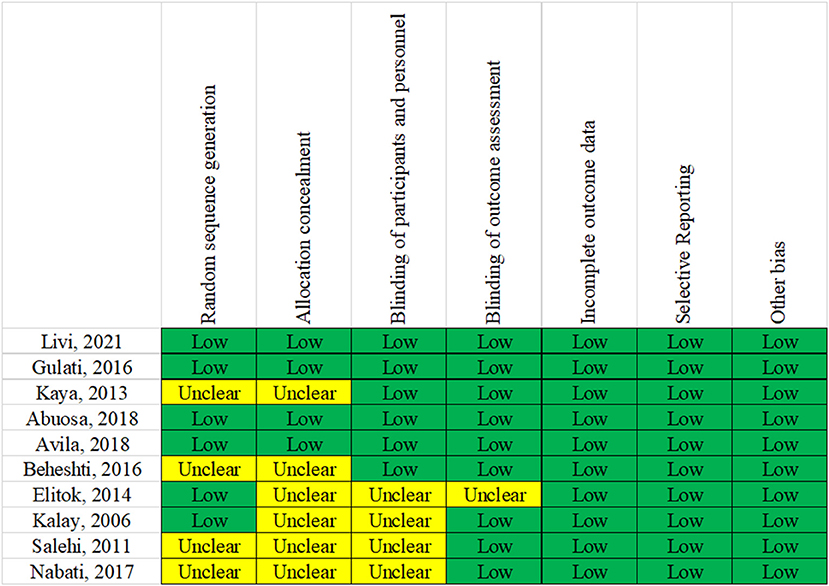
All included RCTs mentioned that the grouping was performed using a random method. Bias risk assessment for randomization showed low-risk bias in six RCTs and unclear in four RCTs. Regarding the concealment of random assignment, four RCTs showed low-risk bias, and six showed unclear results. Placebos were used in all RCTs to implement the blinded method. Regarding data completeness, selective reporting and other aspects showed a low risk of bias. The bias evaluation is shown in Figure 2.
Pairwise comparison of meta-analysis results
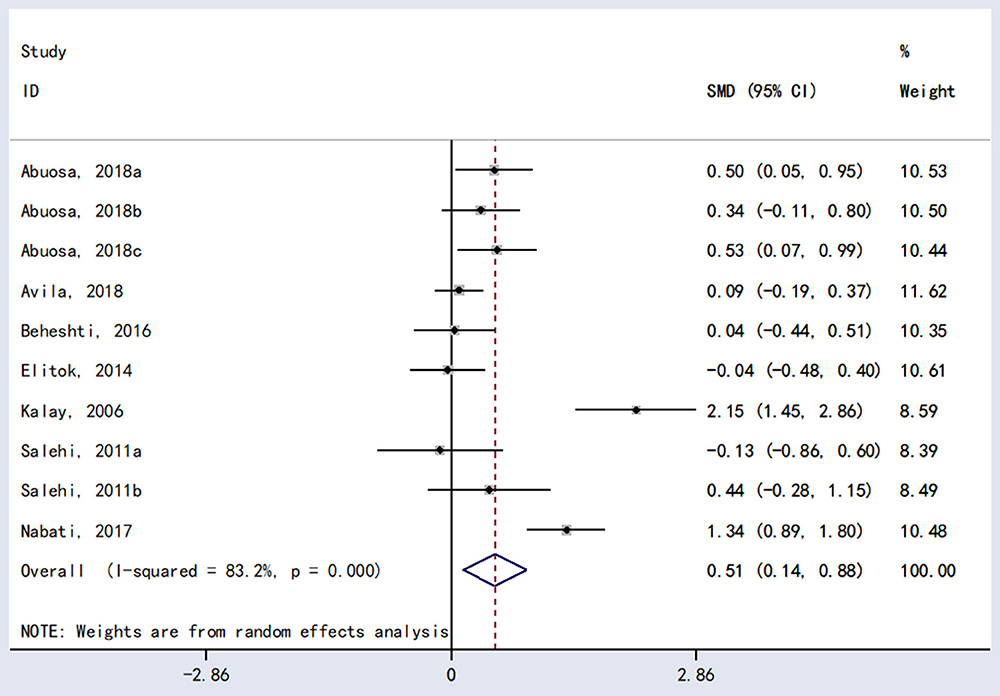
The meta-analysis showed a statistically significant difference between carvedilol and placebo in LVEF [RR = 0.51, 95%CI (0.14, 0.88), P = 0.007; Figure 3].
Compared to placebo, bisoprolol and nebivolol had an advantage in LVEF, with a statistically significant difference [RR = 0.67, 95%CI (0.23, 1.10), P = 0.002; RR = 1.49, 95% CI (0.82, 2.16), P < 0.0001]. However, the difference between metoprolol and placebo was not statistically significant [RR = 0.06, 95%CI (−0.44, 0.57), P = 0.803].
In terms of mortality, the meta-analysis did not show statistically significant differences between carvedilol and placebo (RR = 1.08, 95% CI (0.51, 2.27), P = 0.889; Figure 4].
Adverse events
There were no significant differences in adverse events between metoprolol and placebo [RR = 4.00, 95% CI (0.47, 33.73), P = 0.203] or carvedilol and placebo [RR = 0.50, 95% CI (0.13, 1.94), P = 0.317].
Subgroup analysis
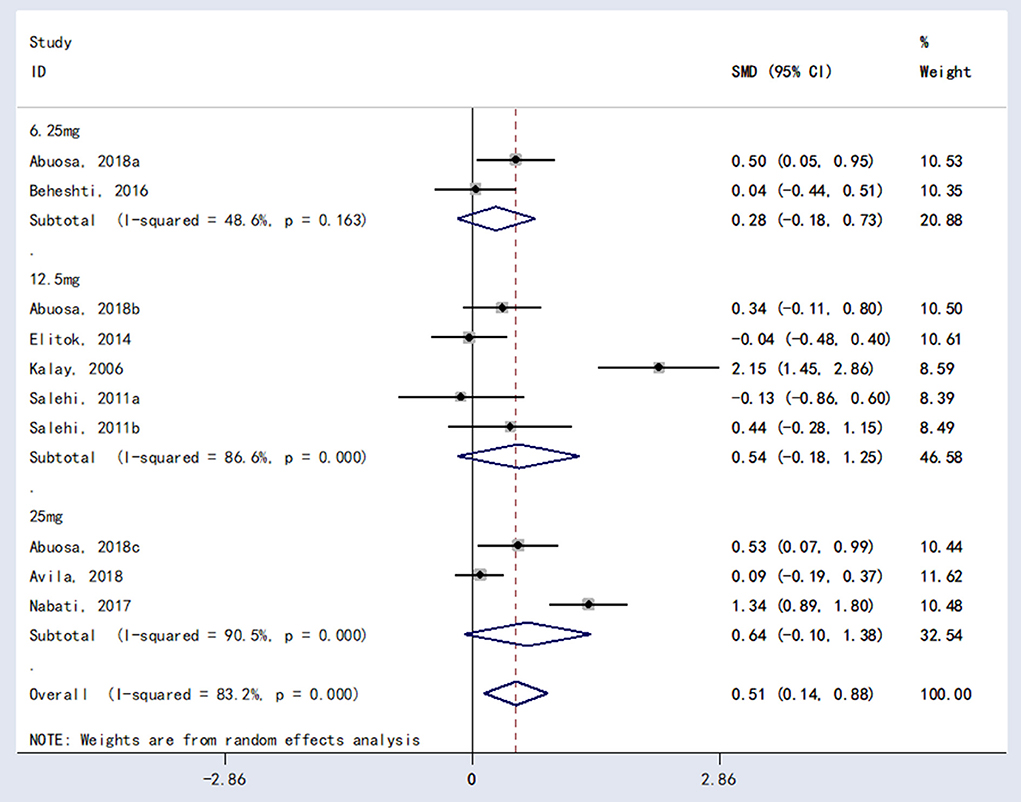
Subgroup analysis was performed for different doses of carvedilol (6.25 mg vs. 12.5 mg vs. 25 mg). There were no statistically significant differences in LVEF performance between 6.25 and 12.5 mg [RR = 0.28, 95%CI (−0.18, 0.73), P = 0.234] or between carvedilol 6.25 mg carvedilol and 25 mg [RR = 0.54, 95%CI (−0.18, 1.25), P = 0.140] or between carvedilol 12.5 mg carvedilol and 25 mg [RR = 0.64, 95%CI (−0.10, 1.38), P = 0.091]. The results are shown in Figure 5.
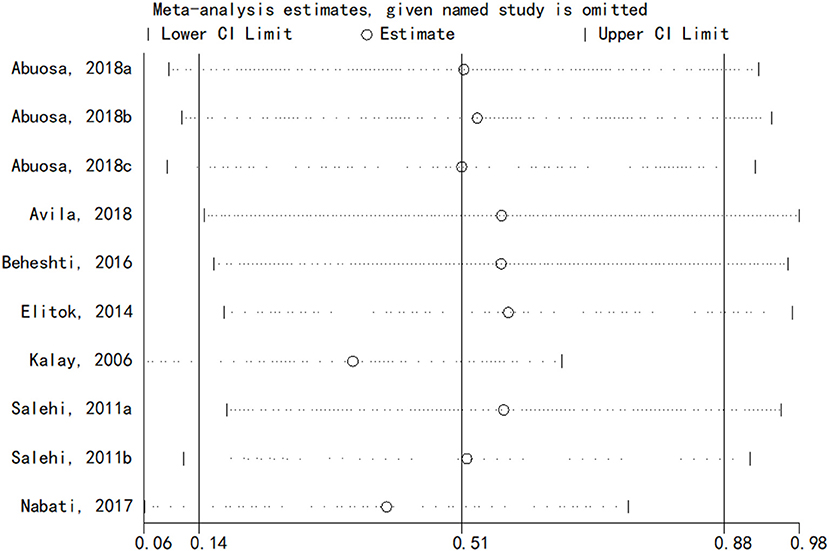
Sensitivity analysis
The sensitivity analysis of the results of LVEF of carvedilol compared to placebo was performed using the one-by-one elimination method. The results showed that the meta-analysis results were stable (Figure 6).
Results of network meta-analysis
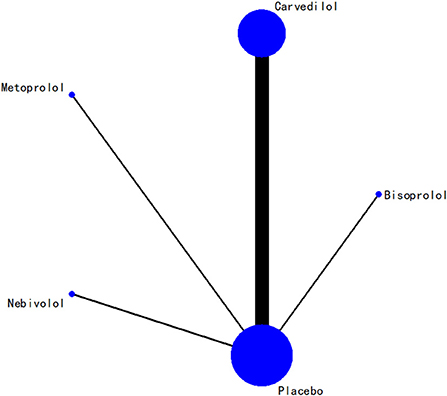
In terms of LVEF, the network relationships for the four beta-blockers are shown in Figure 7. According to the evidence network diagram of NMA comparisons, the width of each edge is proportional to the number of RCTs comparing each pair of treatments, and the size of each treatment node is proportional to the number of randomized participants (sample size).
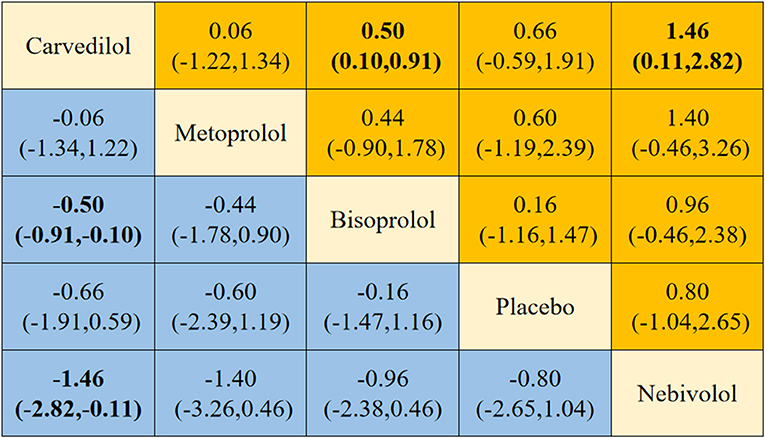
Four different beta-blockers were subjected to NMA, yielding 10 two-by-two comparisons, two of which were statistically significant (Figure 8). NMA results showed that carvedilol was superior to bisoprolol [SMD = −0.50, 95% CI (−0.91, −0.10)] and nebivolol [SMD = −1.46, 95% CI (−2.82, −0.11)] in delaying the reduction in LVEF.
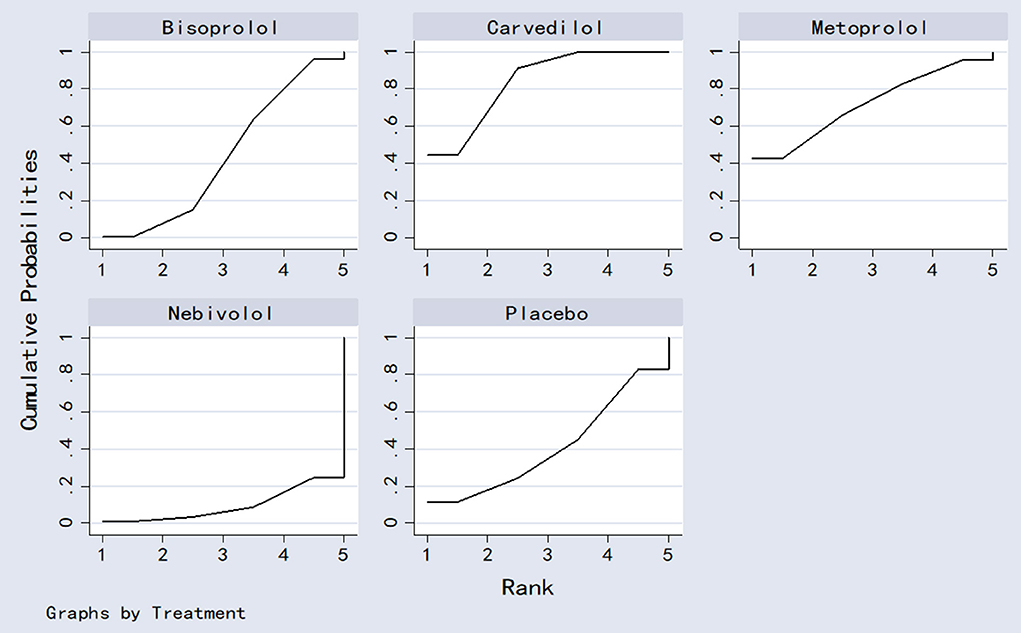
Probability ranking result
The four beta-blockers were ranked based on the SUCRA values (Figure 9). The results of the cumulative probability ordering are as follows: carvedilol (83.8%) > metoprolol (71.8%) > bisoprolol (43.9%) > placebo (40.9%) > nebivolol (9.5%).
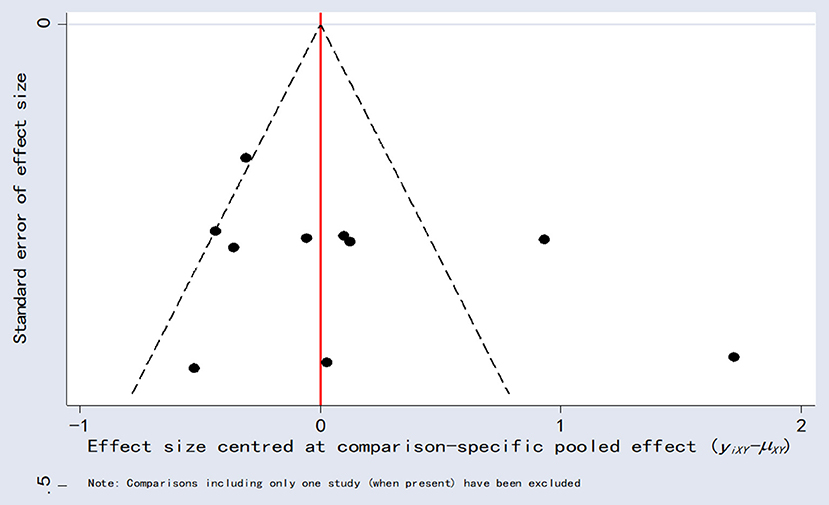
Publication bias analysis
Comparison-adjusted funnel plots for LVEF as an outcome indicator were plotted for publication bias. These results showed poor symmetry, suggesting a possible degree of publication bias (Figure 10).
Discussion
This study evaluated the efficacy of different beta-blockers vs. placebo for preventing AIC by NMA. NMA included 10 RCTs, including 875 patients. Beta-blockers included in the NMA were bisoprolol, metoprolol, nebivolol, and carvedilol. A previous meta-analysis confirmed the carvedilol cardioprotective effects in patients treated with anthracyclines, improving the significant decrease in LVEF and reducing the incidence of cardiovascular events (23). Long-term follow-up studies have shown that LVEF at the end of treatment is an independent predictor of cardiotoxicity (39). Lower LVEF is associated with an increased risk of cardiotoxicity (40). Therefore, a decrease in LVEF is recommended to define chemotherapy-related cardiotoxicity (41, 42).
Anthracyclines are among the most popular chemotherapy drugs due to their broad-spectrum and potent anticancer effects (43). While it provides sound anticancer therapeutic effects, the development of cardiotoxicity limits its application. The cardiotoxic effects become more pronounced as the cumulative dose increases. The risk of congestive heart failure is positively correlated with anthracycline doses (44–46). Early monitoring and timely intervention are essential to avoid the progression to irreversible heart damage (47). Beta-blockers can treat heart failure by stimulating the Gs-AC-cAMP-PKA signaling pathway to produce positive inotropic effects in cardiac myocytes (48).
The results of the direct comparative meta-analysis of this study showed an advantage of carvedilol in causing the delay in the reduction of LVEF compared to placebo. The results are consistent with other studies (22, 23, 25). The result stability was also confirmed by sensitivity analysis ruling out the possibility of false-positive results. In addition, bisoprolol and nebivolol were equally advantageous in mitigating the decline in LVEF. However, more evidence is needed to support the findings due to the size of the included studies. Unlike placebo, metoprolol was not statistically significant in mitigating the LVEF decline. This may be related to the ineffective protective effect against cardiotoxicity due to the absence of antioxidant activity of metoprolol (49).
The NMA results showed that carvedilol was superior to bisoprolol and nebivolol in delaying LVEF reduction. The results of the probability ranking indicated that carvedilol was the best beta-blocker to prevent AIC. Based on direct comparisons, carvedilol and placebo had no statistically significant difference in mortality. Therefore, we recommend carvedilol as the preferred regimen for preventing AIC. Carvedilol is an antioxidant and has more potent antioxidant properties than other types of beta-blockers (34). The metabolites of carvedilol exhibit antioxidant properties. The metabolites are 50 or 100 times more powerful than carvedilol (50). Carvedilol inhibits the lipid peroxidation in cardiac cell membranes and oxygen release from neutrophils. It preserves the body's natural antioxidant system by scavenging peroxides, hypochlorous radicals, and oxygen radicals (51). We attempted to compare the effect of different doses of carvedilol in delaying the reduction of LVEF by subgroup analysis. Unfortunately, no meaningful recommended dose was found. Therefore, future studies with varying doses of carvedilol to prevent AIC should be conducted.
Limitations
First, because of the lack of direct comparisons between different beta-blockers in included studies, the comparisons between other beta-blockers in NMA were obtained by indirect comparisons. Therefore, the results, effectiveness, and safety of the actual drugs may be biased. Second, the included studies were mainly focused on carvedilol (seven studies), while there was only one study for bisoprolol, metoprolol, and nebivolol. Therefore, the results of the studies were prone to bias. Finally, the small sample size of patients included in some of the studies may reduce the credibility of the trial results.
Conclusions
Carvedilol may be the best beta-blocker for preventing AIC, followed by metoprolol. To confirm and support the findings of this NMA, larger sample sizes and high-quality RCTs are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
DH and JH write this paper and analyze the data. YL and XZ design this study, perform the statistical analysis, and review this paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Scientific Research Project of Chengdu Municipal Health Commission (No. 2021066) and Sichuan Medical Association (Youth Innovation) Scientific Research Project (Q21067).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.968534/full#supplementary-material
References
1. Feher A, Baldassarre LA, Sinusas AJ. Novel cardiac computed tomography methods for the assessment of anthracycline induced cardiotoxicity. Front Cardiovasc Med. (2022) 9:875150. doi: 10.3389/fcvm.2022.875150
2. Al-Otaibi TK, Weitzman B, Tahir UA, Asnani A. Genetics of anthracycline-associated cardiotoxicity. Front Cardiovasc Med. (2022) 9:867873. doi: 10.3389/fcvm.2022.867873
3. Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. (2018) 104:971–7. doi: 10.1136/heartjnl-2017-312103
4. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. (2017) 31:63–75. doi: 10.1007/s10557-016-6711-0
5. Liesse K, Harris J, Chan M, Schmidt ML, Chiu B. Dexrazoxane significantly reduces anthracycline-induced cardiotoxicity in pediatric solid tumor patients: a systematic review. J Pediatr Hematol Oncol. (2018) 40:417–25. doi: 10.1097/mph.0000000000001118
6. Bisoc A, Ciurescu D, Rădoi M, Tântu MM, Rogozea L, Sweidan AJ, et al. Elevations in high-sensitive cardiac troponin t and n-terminal prohormone brain natriuretic peptide levels in the serum can predict the development of anthracycline-induced cardiomyopathy. Am J Ther. (2020) 27:e142–50. doi: 10.1097/mjt.0000000000000930
7. Rocca C, Pasqua T, Cerra MC, Angelone T. Cardiac damage in anthracyclines therapy: focus on oxidative stress and inflammation. Antioxid Redox Signal. (2020) 32:1081–97. doi: 10.1089/ars.2020.8016
8. Kim SH, Park Y, Lim JW, Kim H. Effect of docosahexaenoic acid on Ca(2+) signaling pathways in cerulein-treated pancreatic acinar cells, determined by RNA-sequencing analysis. Nutrients. (2019) 11:1445. doi: 10.3390/nu11071445
9. Palaskas N, Lopez?Attei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
10. Karimi S, Hosseinimehr SJ, Mohammadi HR, Khalatbary AR, Amiri FT. Zataria multiflora ameliorates Cisplatin-induced testicular damage via suppression of oxidative stress and apoptosis in a mice model. Iran J Basic Med Sci. (2018) 21:607–14. doi: 10.22038/IJBMS.2018.26784.6558
11. Alanazi A, Fadda L, Alhusaini A, Ahmad R. Antioxidant, antiapoptotic, and antifibrotic effects of the combination of liposomal resveratrol and carvedilol against doxorubicin-induced cardiomyopathy in rats. J Biochem Mol Toxicol. (2020) 34:e22492. doi: 10.1002/jbt.22492
12. Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. (2021) 280:119760. doi: 10.1016/j.lfs.2021.119760
13. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. (2016) 66:309–25. doi: 10.3322/caac.21341
14. Mancia G, Kjeldsen SE, Kreutz R, Pathak A, Grassi G, Esler M. Individualized beta-blocker treatment for high blood pressure dictated by medical comorbidities: indications beyond the 2018 European Society of Cardiology/European Society of Hypertension guidelines. Hypertension. (2022) 79:1153–66. doi: 10.1161/hypertensionaha.122.19020
15. Bugiardini R, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Miličić D, et al. Prior beta-blocker therapy for hypertension and sex-based differences in heart failure among patients with incident coronary heart disease. Hypertension. (2020) 76:819–26. doi: 10.1161/hypertensionaha.120.15323
16. Al-Gobari M, Al-Aqeel S, Gueyffier F, Burnand B. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: an overview of systematic reviews. BMJ Open. (2018) 8:e021108. doi: 10.1136/bmjopen-2017-021108
17. Kobayashi M, Machida N, Tanaka R, Yamane Y. Effects of beta-blocker on left ventricular remodeling in rats with volume overload cardiac failure. J Vet Med Sci. (2008) 70:1231–7. doi: 10.1292/jvms.70.1231
18. Fang Y, Nicol L, Harouki N, Monteil C, Wecker D, Debunne M, et al. Improvement of left ventricular diastolic function induced by β-blockade: a comparison between nebivolol and metoprolol. J Mol Cell Cardiol. (2011) 51:168–76. doi: 10.1016/j.yjmcc.2011.05.012
19. Xu L, Long Y, Tang X, Zhang N. Cardioprotective effects and duration of beta blocker therapy in anthracycline-treated patients: a systematic review and meta-analysis. Cardiovasc Toxicol. (2020) 20:11–9. doi: 10.1007/s12012-019-09558-1
20. Yun S, Vincelette ND, Abraham I. Cardioprotective role of β-blockers and angiotensin antagonists in early-onset anthracyclines-induced cardiotoxicity in adult patients: a systematic review and meta-analysis. Postgrad Med J. (2015) 91:627–33. doi: 10.1136/postgradmedj-2015-133535
21. Salehi R, Zamani B, Esfehani A, Ghafari S, Abasnezhad M, Goldust M. Protective effect of carvedilol in cardiomyopathy caused by anthracyclines in patients suffering from breast cancer and lymphoma. Am Heart Hosp J. (2011) 9:95–8. doi: 10.15420/ahhj.2011.9.2.95
22. Kheiri B, Abdalla A, Osman M, Haykal T, Chahine A, Ahmed S, et al. Meta-analysis of carvedilol for the prevention of anthracycline-induced cardiotoxicity. Am J Cardiol. (2018) 122:1959–64. doi: 10.1016/j.amjcard.2018.08.039
23. Ma Y, Bai F, Qin F, Li J, Liu N, Li D, et al. Beta-blockers for the primary prevention of anthracycline-induced cardiotoxicity: a meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol. (2019) 20:18. doi: 10.1186/s40360-019-0298-6
24. Shah P, Garris R, Abboud R, Vasudev R, Patel H, Doshi R, et al. Meta-analysis comparing usefulness of beta blockers to preserve left ventricular function during anthracycline therapy. Am J Cardiol. (2019) 124:789–94. doi: 10.1016/j.amjcard.2019.05.046
25. Zhan T, Daniyal M, Li J, Mao Y. Preventive use of carvedilol for anthracycline-induced cardiotoxicity: a systematic review and meta-analysis of randomized controlled trials. Herz. (2020) 45:1–14. doi: 10.1007/s00059-018-4779-y
26. Huang S, Zhao Q, Yang ZG, Diao KY, He Y, Shi K, et al. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity-a systematic review and meta-analysis of carvedilol. Heart Fail Rev. (2019) 24:325–33. doi: 10.1007/s10741-018-9755-3
27. Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. (2015) 386:628–30. doi: 10.1016/s0140-6736(15)61478-7
28. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/m14-2385
29. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration (2013). Available online at: www.handbook.cochrane.org
30. Abuosa AM, Elshiekh AH, Qureshi K, Abrar MB, Kholeif MA, Kinsara AJ, et al. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J. (2018) 70:S96–s100. doi: 10.1016/j.ihj.2018.06.011
31. Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. (2018) 71:2281–90. doi: 10.1016/j.jacc.2018.02.049
32. Elitok A, Oz F, Cizgici AY, Kilic L, Ciftci R, Sen F, et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six-month follow-up. Cardiol J. (2014) 21:509–15. doi: 10.5603/CJ.a2013.0150
33. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 ×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. (2016) 37:1671–80. doi: 10.1093/eurheartj/ehw022
34. Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. (2006) 48:2258–62. doi: 10.1016/j.jacc.2006.07.052
35. Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. (2013) 167:2306–10. doi: 10.1016/j.ijcard.2012.06.023
36. Livi L, Barletta G, Martella F, Saieva C, Desideri I, Bacci C, et al. Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol. (2021) 7:1544–9. doi: 10.1001/jamaoncol.2021.3395
37. Nabati M, Janbabai G, Baghyari S, Esmaili K, Yazdani J. Cardioprotective effects of carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. (2017) 69:279–85. doi: 10.1097/fjc.0000000000000470
38. Tashakori Beheshti A, Mostafavi Toroghi H, Hosseini G, Zarifian A, Homaei Shandiz F, Fazlinezhad A. Carvedilol administration can prevent doxorubicin-induced cardiotoxicity: a double-blind randomized trial. Cardiology. (2016) 134:47–53. doi: 10.1159/000442722
39. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. (2015) 131:1981–8. doi: 10.1161/circulationaha.114.013777
40. Upshaw JN, Ruthazer R, Miller KD, Parsons SK, Erban JK, O'Neill AM, et al. Personalized decision making in early stage breast cancer: applying clinical prediction models for anthracycline cardiotoxicity and breast cancer mortality demonstrates substantial heterogeneity of benefit-harm trade-off. Clin Breast Cancer. (2019) 19:259–67.e251. doi: 10.1016/j.clbc.2019.04.012
41. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2014) 27:911–39. doi: 10.1016/j.echo.2014.07.012
42. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. (2020) 31:171–90. doi: 10.1016/j.annonc.2019.10.023
43. Fabiani I, Aimo A, Grigoratos C, Castiglione V, Gentile F, Saccaro LF, et al. Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail Rev. (2021) 26:881–90. doi: 10.1007/s10741-020-10063-9
44. Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2011) 13:1–10. doi: 10.1093/eurjhf/hfq213
45. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. (2003) 97:2869–79. doi: 10.1002/cncr.11407
46. Wihandono A, Azhar Y, Abdurahman M, Hidayat S. The role of lisinopril and bisoprolol to prevent anthracycline induced cardiotoxicity in locally advanced breast cancer patients. Asian Pac J Cancer Prev. (2021) 22:2847–53. doi: 10.31557/apjcp.2021.22.9.2847
47. Vuong JT, Stein-Merlob AF, Cheng RK, Yang EH. Novel therapeutics for anthracycline induced cardiotoxicity. Front Cardiovasc Med. (2022) 9:863314. doi: 10.3389/fcvm.2022.863314
48. Yang J, Liu Y, Fan X, Li Z, Cheng Y. A pathway and network review on beta-adrenoceptor signaling and beta blockers in cardiac remodeling. Heart Fail Rev. (2014) 19:799–814. doi: 10.1007/s10741-013-9417-4
49. Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N, Marinakis T, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol. (2010) 85:894–6. doi: 10.1002/ajh.21840
50. El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail. (2012) 18:607–13. doi: 10.1016/j.cardfail.2012.06.416
Keywords: beta-blockers, anthracycline, cardiotoxicity, systematic review, network meta-analysis
Citation: He D, Hu J, Li Y and Zeng X (2022) Preventive use of beta-blockers for anthracycline-induced cardiotoxicity: A network meta-analysis. Front. Cardiovasc. Med. 9:968534. doi: 10.3389/fcvm.2022.968534
Received: 14 June 2022; Accepted: 19 July 2022;
Published: 11 August 2022.
Edited by:
Shusen Sun, Western New England University, United StatesReviewed by:
Qiang Zhang, Cancer Center, Daping Hospital, ChinaWenjian Yao, Henan Provincial People's Hospital, China
Copyright © 2022 He, Hu, Li and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, bGl5aW5nMDUxMDAzQDE2My5jb20=; Xiaofei Zeng, emVuZ2RvY3RAMTYzLmNvbQ==
†These authors share first authorship
 Dongsheng He1†
Dongsheng He1† Xiaofei Zeng
Xiaofei Zeng