
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 09 December 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.961579
Parts of this article's content have been modified or rectified in:
Erratum: The LAUsanne STAPHylococcus aureus ENdocarditis (LAUSTAPHEN) score: A prediction score to estimate initial risk for infective endocarditis in patients with S. aureus bacteremia
Introduction: Infective endocarditis (IE) is a common complication of Staphylococcus aureus bacteremia (SAB). The study aimed to develop and validate a prediction score to determine IE risk among SAB.
Methods: This retrospective study included adults with SAB (2015–2021) and divided them into derivation and validation cohorts. Using the modified 2015 European Society of Cardiology modified Duke Criteria for definite IE, the LAUSTAPHEN score was compared to previous scores.
Results: Among 821 SAB episodes, 419 and 402 were divided into derivation and validation cohorts, respectively. Transthoracic and transoesophageal echocardiography (TOE) were performed in 77.5 and 42.1% of episodes, respectively. Definite IE was diagnosed in 118 episodes (14.4%). Derivation cohort established that cardiac predisposing factors, such as cardiac implantable electronic devices, prolonged bacteremia ≥48 h, and vascular phenomena were independently associated with IE. In addition to those parameters, native bone and joint infections were used to constitute the LAUSTAPHEN score. LAUSTAPHEN and VIRSTA scores misclassified <4% of IE cases as low risk. Misclassification using POSITIVE and PREDICT scores was >10%. The number of TOEs required to safely exclude IE were 66.9 and 51.6% with VIRSTA and LAUSTAPHEN, respectively.
Discussion: LAUSTAPHEN and VIRSTA scores exhibited the lowest misclassification rate of IE cases to the low-risk group. However, the number of patients requiring TOE was higher for VIRSTA than for LAUSTAPHEN.
Staphylococcus aureus is one of the leading causes of bacteremia in both community and nosocomial-acquired infections. S. aureus bacteremia (SAB) is associated with increased mortality which is influenced by the presence of metastatic foci, such as infective endocarditis (IE) (1–6). IE is estimated to complicate 10–20% of SAB (7–11).
According to the guidelines of IE management (12, 13), echocardiography should be performed in all episodes of SAB to exclude IE. Transoesophageal echocardiography (TOE) is preferred to transthoracic (TTE), due to a higher sensitivity. Nevertheless, being an invasive procedure, TOE cannot be performed in all patients (12, 14). Indeed, the development of scores to identify patients in low- and high-risk groups for IE is warranted to avoid unnecessary echocardiograms (15–17). Among several clinical prediction rules proposed so far, VIRSTA, Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT), and POSITIVE scores were recently developed, with VIRSTA showing the best diagnostic accuracy (8, 10, 15, 18).
VIRSTA score included 10 parameters (cerebral or peripheral emboli, meningitis, vertebral osteomyelitis, permanent intracardiac device or previous IE, pre-existing native valve disease, intravenous drug use, prolonged bacteremia for 48 h, community or non-nosocomial healthcare-associated bacteremia, severe sepsis or shock, and C-reactive protein >190 mg/L) with each parameter ranging from 1 to 5 points (8). PREDICT score included three parameters (implantable cardioverter defibrillator or permanent pacemaker, community or healthcare acquisition, and prolonged bacteremia for 72 h) with each parameter ranging from 1 to 3 points (19). Finally, the POSITIVE score included four parameters (time to blood culture positivity, intravenous drug use, cerebral or peripheral emboli, and predisposing heart disease) with each parameter ranging from 2 to 6 points (10). The main drawbacks of the aforementioned scores were the need for complex calculations (multiple parameters with different ranges of each parameter), the fact that all scores contain several variables included in the Duke criteria, and that all scores were calculated and validated in populations that not all patients benefited from echocardiograms (8–11, 19, 20).
This study aimed to develop and validate a new simple prediction rule to stratify the risk of IE within 72 h from SAB onset and to compare it with other existing scores (PREDICT, POSITIVE, and VIRSTA).
This retrospective study was conducted at the Lausanne University Hospital, Lausanne, Switzerland, with an 1100-bed primary and tertiary care hospital, during a 7-year period (2015–2021). The study was approved by the ethics committee of the Canton of Vaud (CER-VD 2021-02516) that waived the need for informed consent.
Inclusion criteria were adult patients (≥18 years old) and the presence of at least one positive blood culture for S. aureus (extracted from the database of the microbiology laboratory). Exclusion criteria were patients' written refusal of the use of their data, incomplete medical files, and death within 48 h from bacteremia onset.
Data regarding demographics (age, sex), comorbidities, laboratory results (white blood cells, C-reactive protein), presence of sepsis or septic shock, foci of infection, individual components of 2015 European Society of Cardiology (ESC) modified Duke Criteria (12), cardiac imaging results, cardiac surgery or ablation of cardiac implantable electronic devices (CIEDs), autopsy results, and persistent bacteremia were retrieved from patients' electronic health records. Study data were collected and managed using REDCap by an infectious diseases specialist. REDCap electronic data capture tools are hosted at Lausanne University Hospital. Research Electronic Data Capture (REDCap) is a secure, web-based software platform designed to support data capture for research studies (18, 21).
According to the internal guidelines, an infectious diseases consultation was performed on a mandatory basis within the same day of blood culture positivity for S. aureus. According to the published evidence (12, 14, 17), our internal policy recommended TTE and TOE in patients with community-acquired bacteremia (Group 1). TTE and TOE were also suggested in the case of nosocomial bacteremia with risk factors for IE such as prior IE, presence of CIED or prosthetic valve, persistent BSI for 72 h, or embolic event (Group 2). For patients with nosocomial not catheter-related bacteremia without the aforementioned risk factors, only TTE was proposed (Group 3). Finally for nosocomial catheter-related bacteremia without risk factors, no further investigation was warranted (Group 4). Follow-up blood cultures at 48 h intervals were recommended until negativization.
The date of collection of the first positive blood culture was defined as infection onset. A new episode was included if more than 30 days had elapsed since the first negative blood culture of the initial episode. Bacteremia was characterized as a community, healthcare, or nosocomial according to Friedman et al. (22) Infection was categorized as sepsis or septic shock according to the definition proposed by the Sepsis-3 International Consensus (23). Definite IE was defined according to the 2015 ESC-modified Duke Criteria (12). Cardiac predisposing factors for IE were defined as cardiac conditions at high or moderate risk for IE (24). Vascular phenomena were defined as arterial, septic lung emboli, renal or splenic emboli, mycotic aneurysm, intracranial ischemia or bleeding, conjunctival bleeding, Janeway lesions, or nail bed bleeding.
The population was divided into a derivation and a validation cohort according to the date of bacteremia onset (derivation cohort: first 6 months of each year; validation cohort: last 6 months of each year). Patients with definite IE were compared to those without (possible IE or rejected IE). Four variables (cardiac predisposing factors, CIED, prolonged bacteremia ≥48 h, vascular phenomena) were preselected for the model according to the clinical practice since their presence is highly associated with IE, leads physicians to suspect IE, and usually triggers further cardiac imaging investigations (15, 17). The primary aim was to reach < 4% of misclassified IE in the low-risk group in conjunction with minimizing the number of cardiac imaging studies needed to be performed. In case the four preselected variables did not suffice to attain the aforementioned threshold, a fifth variable would be selected from other variables known to be associated with IE, namely immunologic phenomena, native bone and joint infections (septic arthritis and vertebral and non-vertebral osteomyelitis) community and non-nosocomial healthcare-associated SAB, time to blood culture positivity < 9 h, meningitis, or septic shock (15). The associations between such variables and IE were measured by univariate analysis. For the identification of the best-performing model, multiple multivariable analyses were performed by including the four preselected variables and each of the aforementioned variables. The best-performing model from the derivation cohort was chosen. LAUSTAPHEN score's diagnostic accuracy was then tested in a separate validation cohort.
We used the predefined cut-offs of the evaluated scores (VIRSTA, POSITIVE, and day 5 PREDICT) for the identification of patients at low or high risk for IE (8, 10, 19). Episodes with VIRSTA score ≥3 (8), POSITIVE score ≥5 (10), and PREDICT score (on day 5) ≥2 (19) were considered at high risk for IE.
The POSITIVE score was not calculated in patients for whom time to positivity of blood cultures was missing (bacteremia onset in other hospitals) or unreliable (polymicrobial bacteremia, blood cultures collected while patient was on antimicrobial treatment). CRP missing values were imputed with the median value.
The primary endpoint was the diagnostic accuracy of LAUSTAPHEN and the aforementioned scores for the diagnosis of IE. Two analyses were performed; for the first one, the reference standard was definite IE according to the 2015 ESC-modified Duke Criteria, while, for the second, the reference standard was the presence of cardiac lesions detected with imaging and pathological examination according to the 2015 ESC-modified Duke Criteria (12). Sensitivity, specificity, positive and negative predictive values (PPV, NPV), and positive and negative likelihood ratios (PLR, NLR), as well as accuracy, were calculated. The number of patients with IE misclassified into low risk was calculated for each score, as well as the number of TOE indications resulting from the high-risk stratification by each score. Receiver operating curves were also generated.
SPSS version 26.0 (SPSS, Chicago, IL, USA) software was used for data analysis. Categorical variables were analyzed using the chi-square or Fisher exact test and continuous variables with Mann–Whitney U-test. Multivariable logistic regression analyses were performed in the derivation cohort by using two dependent variables; definite IE according to the 2015 ESC-modified Duke Criteria and presence of cardiac lesion according to imaging and pathological 2015 ESC-modified Duke Criteria. Variables that did not contribute to multicollinearity were used in multivariable analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association. All statistical tests were two-tailed, and P < 0.05 was considered statistically significant.
Among the 1,060 episodes of SAB, 821 episodes in 762 patients were included in this study (Supplementary Figure 1). The derivation and validation cohorts comprised 419 and 402 patients, respectively. Supplementary Table 1 shows the baseline characteristics of derivation and validation cohorts.
In total, TTE was performed in 636 episodes (77.5%), while TOE was performed in 346 (42.1%). The timing from bacteremia onset to the first echocardiogram (either TTE or TOE) was 2 days (Q1–Q3: 0–4 days). Due to high clinical suspicion of IE, among 606 episodes with negative initial TTE, 79 had a second TTE performed leading to the establishment of the 2015 ESC-modified Duke imaging criterion in four episodes. Furthermore, among 290 episodes with negative initial TEE or TOE, a second TOE was performed due to high clinical suspicion of IE in 20 episodes, five of which fulfilled the 2015 ESC-modified Duke imaging criterion. Among the 606 episodes at high risk according to internal policy for IE (Groups 1 and 2; Supplementary Table 2), TOE was performed in 307 (50.7%) episodes. Although indicated, TOE was not performed in 299 episodes because of the following reasons: a combination of age and comorbidities (100 episodes; 33.4%), contraindication (esophageal varices and severe thrombocytopenia) or non-feasibility (severe obesity, inability to pass the endoscope, death before TOE) (44; 14.7%), and positivity of TTE (10; 3.3%); for the remaining 145 episodes (48.5%), the risk was deemed low by the treating physician or infectious diseases consultant; thus, no further testing was pursued. Other imaging modalities (18-FDG PET-CT, cardiac CT) were performed in 104 episodes (12.7%). In total, at least one cardiac imaging study was performed in 700 episodes (85.3%). According to internal guidelines, at least one cardiac imaging study was performed in 551 episodes (90.9%) among 606 categorized at high risk (Groups 1 and 2) and in 149 (69.3%) among 215 episodes categorized at low risk (Groups 3 and 4) (Supplementary Table 2).
Definite IE according to the clinical and pathological Duke criteria was diagnosed in 118 episodes (14.4%) (Supplementary Table 3). Surgery was performed in 36 patients (27 with pathological criterion), autopsy in 14 patients (3 with pathological criterion), and CIED removal in 29 patients among 88 with CIED (21 with pathological criterion). In 102 patients with cardiac lesions (imaging and pathological 2015 ESC-modified Duke Criteria), IE involved native valves in 69 episodes (67.6%), prosthetic valves in 25 episodes (24.5%), and CIED in 14 episodes (20.6%). Valvular lesions were detected by imaging studies, cardiac surgery, or autopsy in 84 patients (76 with valvular vegetations, 16 abscesses, 10 perforations, two intracardiac fistulas, and 16 abnormal activities in 18-FDG PET-CT). CIED lesions were detected with imaging studies and CIED removal in 21 patients.
Table 1 shows the univariate and multivariable analyses of IE predictors in the derivation cohort with definite IE according to the 2015 ESC-modified Duke Criteria as the reference standard. In the univariate analysis, among other variables, patients with IE were more likely to have cardiac predisposing factors (P < 0.001), CIED (P < 0.001), prolonged bacteremia ≥48 h (P < 0.001), vascular phenomena (P < 0.001), and native bone and joint infections (septic arthritis and vertebral and non-vertebral osteomyelitis) (P 0.004). Multivariable analysis identified cardiac predisposing factors (P < 0.001; OR 6.4, CI 2.8–14.6), CIED (P < 0.001; OR 8.2, CI 3.2–20.9), prolonged bacteremia ≥48 h (P 0.035; OR 1.3, CI 1.0–1.7), and vascular phenomena (P < 0.001; OR 15.7, CI 7.2–34.4) as independent predictors of IE among patients with SAB.
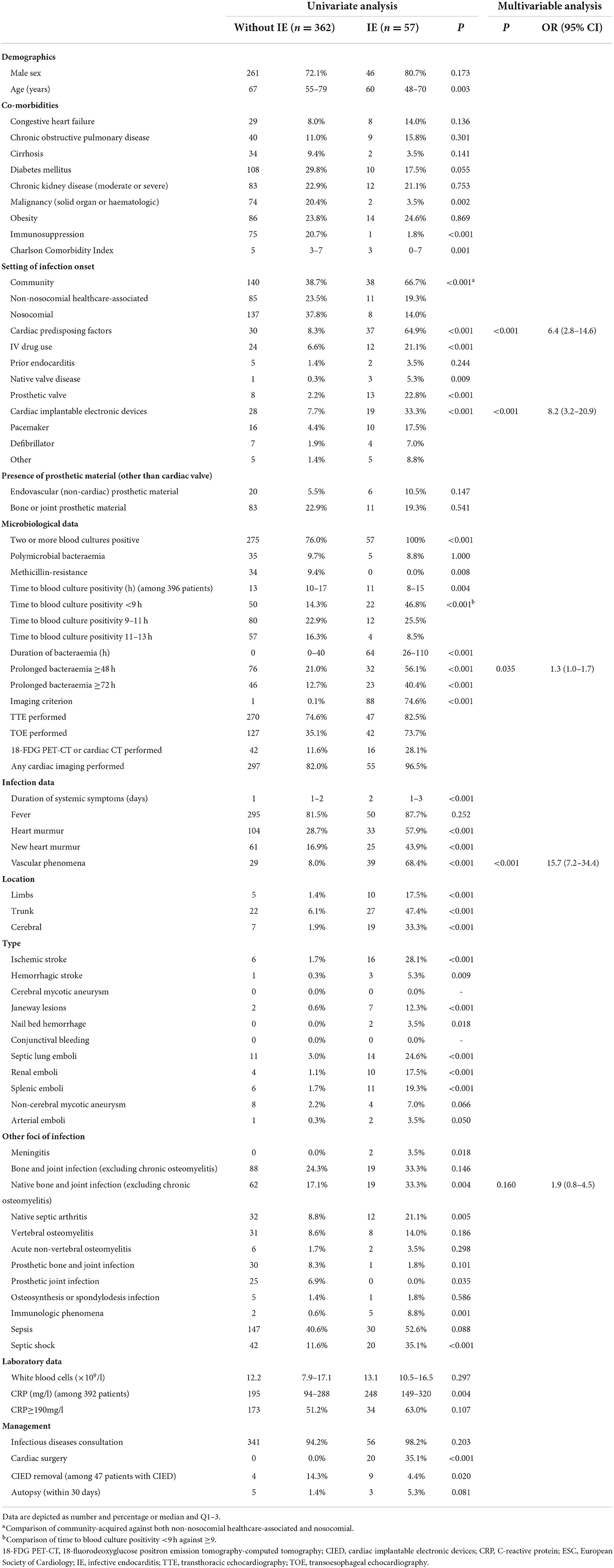
Table 1. Predictors of definite infective endocarditis (according to the 2015 ESC-modified Duke Criteria) in patients with S. aureus bacteremia in the derivation cohort.
Supplementary Table 4 shows the univariate and multivariable analyses of predictors of cardiac lesions according to imaging and pathological 2015 ESC-modified Duke Criteria in the derivation cohort. In the univariate analysis, among other variables, patients with cardiac lesions were more likely to have cardiac predisposing factors (P < 0.001), CIED (P < 0.001), prolonged bacteremia ≥48 h (P < 0.001), vascular phenomena (P < 0.001), and native bone and joint infections (P 0.002). Multivariable analysis revealed cardiac predisposing factors (P 0.002; OR 3.5, CI 1.6–7.7), CIED (P < 0.001; OR 5.8, CI 2.5–13.7), prolonged bacteremia ≥48 h (P 0.029; OR 1.3, CI 1.0–1.7), and vascular phenomena (P < 0.001; OR 8.9, CI 4.2–18.7) as independent predictors of cardiac lesion among patients with SAB.
Based on the derivation cohort, we developed a new prediction score aiming to reduce the false-negative rate (misclassified IE in the low-risk group) to < 4%, while minimizing the number of cardiac imaging procedures. A model including the four preselected variables (cardiac predisposing factors, CIED, prolonged bacteremia ≥48 h, and vascular phenomena) did not allow for reaching the false-negative cut-off. Thus, to reach the aforementioned threshold, additional models were tested by adding one of the several clinically relevant parameters with a high association with IE (immunologic phenomena, native bone and joint infections, community and non-nosocomial healthcare-associated SAB, time to blood culture positivity < 9 h, and meningitis or septic shock) to the existing four-item model. Among the aforementioned parameters, only the addition of community and non-nosocomial healthcare-associated SAB or native bone and joint infections to the four preselected variables improved the score's performance by achieving a false-negative rate of < 4%. Among the two variables, the presence of native bone and joint infections (septic arthritis, vertebral and non-vertebral osteomyelitis) was then added as a fifth item despite the absence of an independent association with IE in the multivariable model. The decision was based on the fact that the addition of community and non-nosocomial healthcare-associated SAB would have required more cardiac imaging investigations to achieve the same result as native bone and joint infections. The presence of any of the five parameters (cardiac predisposing factors, CIED, prolonged bacteremia ≥48 h, vascular phenomena, and native bone and joint infections) included in the LAUSTAPHEN score at 96 h from the onset of bacteremia classified patients as high-risk; one point was given for the presence of each variable (Table 2).
Table 3 shows the diagnostic accuracies of LAUSTAPHEN (in both, derivation and validation cohorts), PREDICT, VIRSTA, and POSITIVE scores in predicting definite IE. LAUSTAPHEN and VIRSTA scores had a misclassification rate of IE in the low-risk group of < 4% and an NPV of >98%. The NPV of PREDICT and POSITIVE scores was >95%; however, 13.6% and 23.5% of episodes of IE were misclassified in the low-risk group by PREDICT and POSITIVE scores, respectively. If the prediction scores had been applied to the whole study population, VIRSTA would have required more cardiac imaging investigations (TOE in 66.9% episodes with SAB) compared to LAUSTAPHEN (TOE in 51.6%) to achieve the same result (an increase of 29.7% of TOE needed). Table 4 shows the diagnostic accuracies of the aforementioned scores in predicting cardiac lesions. Results were similar, with LAUSTAPHEN and VIRSTA scores having a misclassification rate of IE in the low-risk group of < 4% and an NPV of >98%.
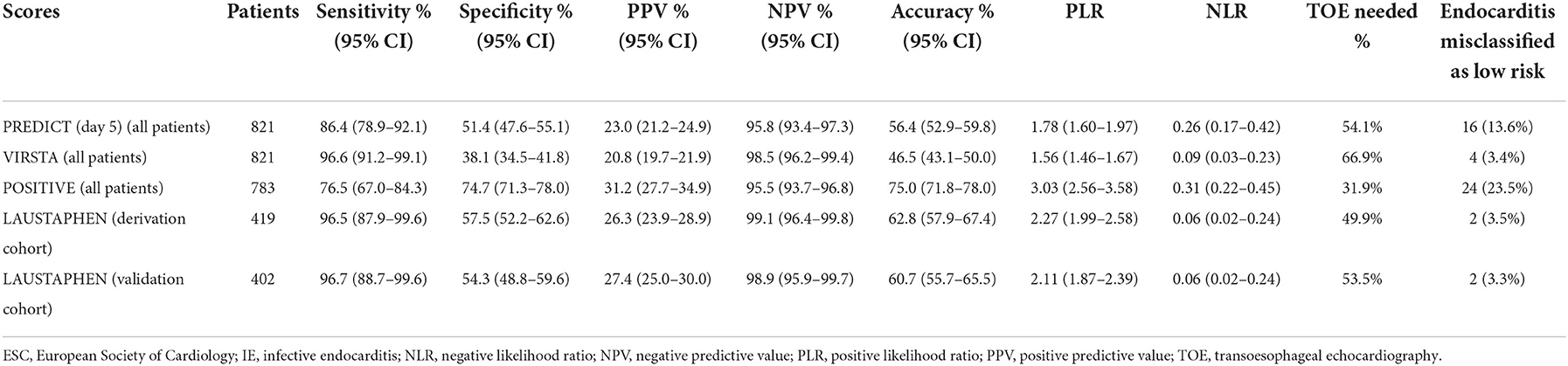
Table 3. Diagnostic accuracies of the fifth day PREDICT, VIRSTA, POSITIVE, and LAUSTAPHEN in predicting definite IE (according to the 2015 ESC-modified Duke Criteria).
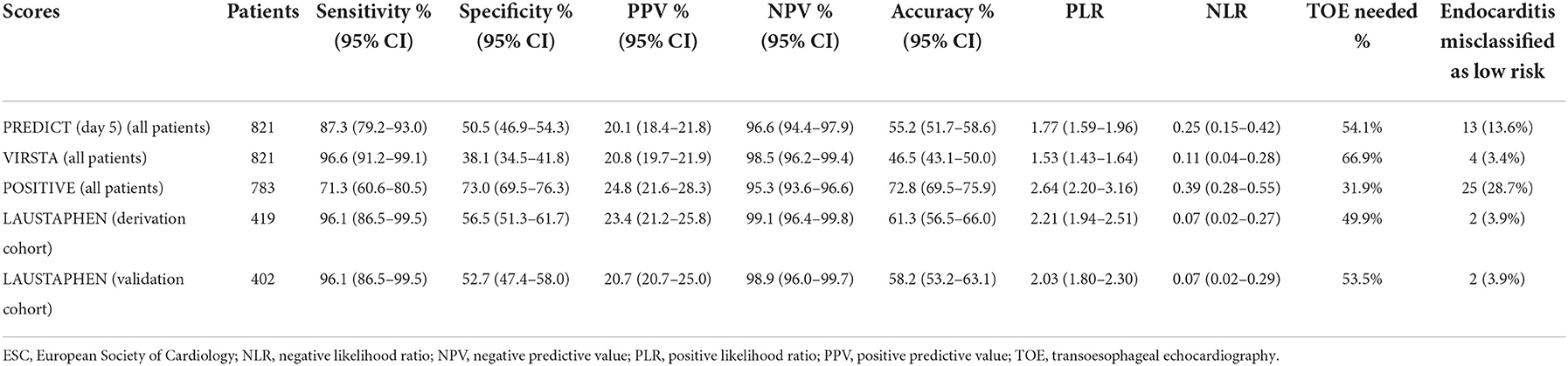
Table 4. Diagnostic accuracies of the fifth day PREDICT, VIRSTA, POSITIVE, and LAUSTAPHEN in predicting cardiac lesions (according to imaging and pathological 2015 ESC-modified Duke Criteria).
Figure 1 shows the ROC of scores in predicting definite IE in the whole study population (1A) and cardiac lesions in patients who had an echocardiogram, cardiac surgery, or autopsy (1B). The area under the curve (AUC) for predicting definite IE by LAUSTAPHEN, VIRSTA, PREDICT, and POSITIVE was 0.87, 0.89, 0.74, and 0.84, respectively. AUC for predicting cardiac lesion by LAUSTAPHEN, VIRSTA, PREDICT, and POSITIVE was 0.83, 0.86, 0.77, and 0.79, respectively.
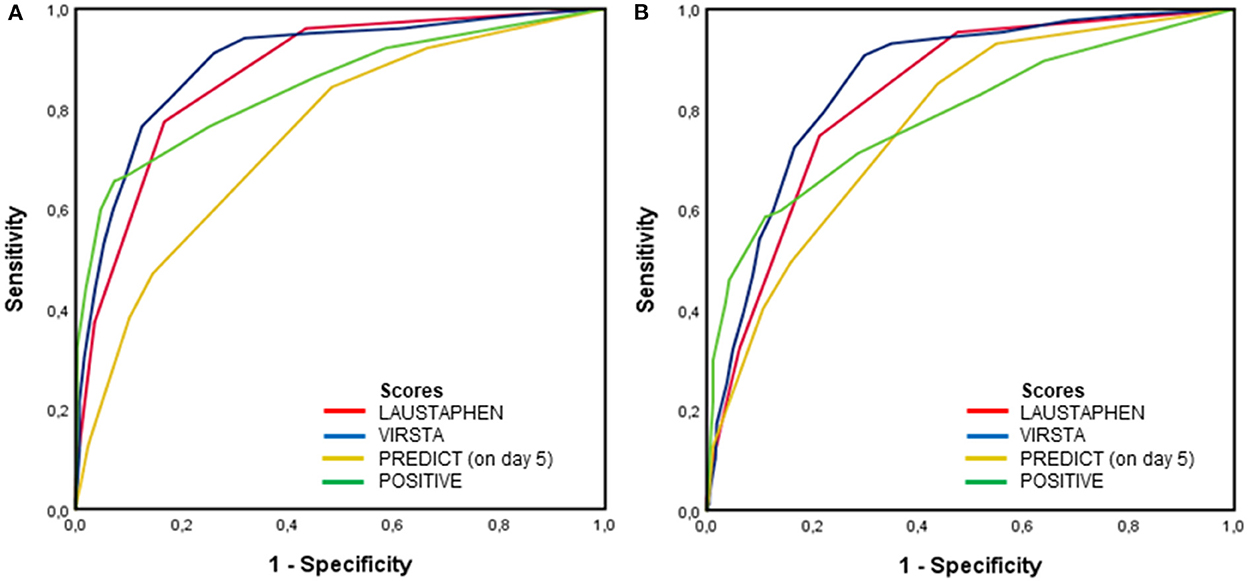
Figure 1. ROC of PREDICT, VIRSTA, POSITIVE, and LAUSTAPHEN scores in predicting (A) definite IE in the whole population and (B) cardiac lesion in patients who had an echocardiogram, cardiac surgery, or autopsy.
The diagnostic accuracies of the aforementioned scores were additionally tested among the subgroup of patients that had all appropriate imaging investigations according to their Group allocation (patients with TOE in Groups 1 and 2, patients with TTE or TOE in Group 3, all patients in Group 4; Supplementary Table 2) are depicted in Supplementary Tables 5, 6. Supplementary Table 7 shows the diagnostic accuracies of the aforementioned scores in patients belonging to Groups 1 and 2 (high risk). In high-risk patients, LAUSTAPHEN and VIRSTA scores had a misclassification rate of IE in the low-risk group of < 1% and an NPV of >98%, but VIRSTA would have required more cardiac imaging investigations (TOE in 81.0% episodes) compared to LAUSTAPHEN (TOE in 64.9%) to achieve the same result. Supplementary Table 8 shows the diagnostic accuracies of the aforementioned scores in patients that had at least one cardiac imaging study (TTE, TOE, PET-CT, or cardiac CT) performed. In high-risk patients, LAUSTAPHEN and VIRSTA scores had a misclassification rate of IE in the low-risk group of 3.4% and an NPV of ≥98%, but VIRSTA would have required more cardiac imaging investigations (TOE in 72.0% episodes) compared to LAUSTAPHEN (TOE in 58.0%) to achieve the same result.
In the present study, the rate of definite IE among SAB (14.4%) was similar to that reported in previous studies (6.7–18.2%) (7–11). We developed and validated LAUSTAPHEN, a new prediction score of IE among patients with SAB. The primary aim of these scores is to reliably identify patients with SAB at low risk for IE, in order to avoid further cardiac imaging studies. Among the evaluated cores, only LAUSTAPHEN and VIRSTA achieved the specified threshold (< 4%) of misclassified episodes with IE in the low-risk group.
van der Vaart et al. (7) used an NPV above 98% as a threshold to consider a score safe for the exclusion of IE. In this study, beyond high NPV, we included a low false-negative rate (< 4%) for the evaluation of the scores. LAUSTAPHEN and VIRSTA scores achieved such criteria and both scores had an NLR of < 0.1, underlying their importance as good “rule-out” scores. These results are in line with previous studies showing that VIRSTA has an NPV above 98% (7–9); although, in a recent study, VIRSTA failed by a little to achieve that threshold (NPV of 97.8%) (20). Day 5 PREDICT score had an NPV of 96% which is comparable to previous studies, including the study that proposed a score of 94.5–97.9% (7, 9, 19, 20). However, our results are in contrast to the study that validated the PREDICT score which showed an NPV of 100% (11). Such difference might be explained by the low number of patients included in that study (n = 199), when compared to our cohort (n = 821) (11). Importantly, the high misclassification rate of IE episodes in the low-risk group of PREDICT (13.6%) and POSITIVE (23.5%) in this study rendered them inapplicable in clinical practice (7, 9, 10, 20).
Predictors included in PREDICT, POSITIVE, and VIRSTA scores were identified by multivariable regression models (19, 24). Although statistically robust, this approach might result in several inconsistencies. As an example, PREDICT does not include the presence of prosthetic valves or vascular phenomena as predictors (19). A previous meta-analysis recognized the presence of embolic events as the most important factor in patients with SAB to predict IE (15). Accordingly, a patient with SAB and an embolic event should be considered as having IE until proven otherwise and TOE is indicated, even if PREDICT score is < 2. Furthermore, the POSITIVE score does not include prolonged bacteremia as a predictor; the absence of that predictor might be explained by the excellent performance of the time to blood culture positivity in that study; all patients with IE in the POSITIVE cohort had a time to positivity inferior to 15 h (10). In this study, only 81.4% reached that threshold. Moreover, the POSITIVE score cannot be applied to all SAB since the calculation of the time to positivity might not be available, for example in patients with bacteremia onset in other hospitals. Time to positivity might also be unreliable in the context of polymicrobial bacteremia or in patients under antimicrobial treatment when initial blood cultures are collected (10). Finally, one of the 10 criteria included in the VIRSTA score is the presence of meningitis, which was also identified by their multivariable regression model. Nevertheless, meningitis accounted for < 2% of SAB, making its impact on clinical decisions minimal (8).
Even though the addition of native bone and joint infections as a fifth item of the LAUSTAPHEN score might seem arbitrary, it was based on the established association between native bone and joint infections with IE. IE prevalence among patients with bacteraemic native bone and joint infections due to S. aureus could reach 33% (25–27); thus, the presence of vertebral and non-vertebral osteomyelitis is part of the previous criteria for classifying patients at high risk for IE (14) or VIRSTA score (7).
Another important aspect of this study was that predictive scores were evaluated not only on their diagnostic accuracy but also on their clinical implications. In previous studies, TOE was performed in 30–50% of the cases, a rate similar to the present study (42.1% in the whole population and 50.7% in the high-risk group according to internal guidelines) (7–10, 20). If the scores were to be implemented in clinical practice, the number of TOEs needed to safely exclude IE would have increased to 66.9% with VIRSTA; this result is consistent with van der Vaart et al. (7) who showed that VIRSTA tended to overestimate the risk of IE. On the contrary, LAUSTAPHEN categorized only 51.6% of the included population into the high-risk group, reducing the number of TOEs needed as compared to VIRSTA.
Another advantage of LAUSTAPHEN is the limited number of variables to assess; only five items known to be associated with IE need to be evaluated, while VIRSTA score is based on 10 different parameters (8). The absence of complex calculations (presence of any criterion of the LAUSTAPHEN score categorizes the patient into the high-risk group) which are necessary for other scores (VIRSTA, POSITIVE, and PREDICT), renders LAUSTAPHEN an easy and practical score (8, 10, 19).
This study has several limitations. First, it is monocentric and retrospective, even though the number of included patients exceeded that of many previous studies (7, 10, 11). Second, despite internal guidelines, 14.7% of episodes did not undergo any cardiac imaging study (TTE, TOE, PET-CT, or cardiac CT), with 9.1% belonging to the high-risk group and 30.7% belonging to the low-risk group. However, in the 90 days following the initial SAB, only a small proportion of patients (2.1%) had a SAB relapse and only one patient developed S. aureus IE. Considering the aforementioned elements, we decided to include all patients, even those who did not undergo echocardiography and therefore were less likely to have IE. We performed subgroup analyses including only patients who had all imaging investigations considered appropriate according to their group allocation (Supplementary Tables 5, 6) and in patients that had at least one cardiac imaging study independently of their group allocation (Supplementary Table 8), where LAUSTAPHEN confirmed a false-negative rate inferior to 4%. Third, even though a cardiac imaging study was not performed for some patients, others had a second TTE and/or TOE performed due to high clinical suspicion; although this attitude was in accordance with 2015 ESC guidelines, it added to the selection bias created by the internal policy on the management of SAB (12). Fourth, LAUSTAPHEN and other scores included multiple variables (embolic events, cardiac predisposing conditions) that are also part of the reference standard (minor criteria for definite IE according to the 2015 ESC-modified Duke Criteria), possibly increasing the diagnostic accuracy for these predictors (8, 10, 19). To overcome this incorporation bias, we conducted a supplementary analysis using as a reference standard the presence of cardiac lesions representative of IE, which is less dependable from the variables included in the LAUSTAPHEN score. This analysis confirmed the low rate (< 4%) of misclassification of IE in the low-risk group and the high NPV (>98%) of the LAUSTAPHEN score. Fifth, although the LAUSTAPHEN score was validated in a different cohort than the derivation cohort, both originated from Lausanne University Hospital, which has an internal policy for SAB management that proposes that cardiac imaging studies are needed according to a pre-assessment of the IE risk based on the type of SAB (community vs. nosocomial) and common IE risk factors. The internal policy reflected common practice in many institutions as not all patients with SAB are at the same risk of acquiring IE (14, 15, 17). Therefore, it should be tested on an external patient population before being considered for application in clinical practice. Finally, in our hospital, all patients with SAB were examined by an infectious diseases specialist on the day of blood culture positivity, which led to improved clinical detection of embolic lesions not previously described by the treating physician and to a more systematic prescription of additional imaging studies for embolic foci detection. This could explain the high percentage of embolic events found in the present study (16.2%) as compared to previous studies (4.5–6.0%) (8, 9). Thus, the results of the present study cannot be extrapolated to centers in which infectious disease consultation is not mandatory for SAB.
In conclusion, we developed and validated a new reliable and easy-to-assess score including only five variables known to be associated with IE. Compared to POSITIVE and PREDICT scores, LAUSTAPHEN seemed more appropriate for clinical practice, due to its much lower misclassification rate of IE episodes in the low-risk group. Although LAUSTAPHEN and VIRSTA scores exhibited the lowest misclassification rate, VIRSTA significantly overestimated IE risk, leading to a higher number of echocardiograms (an increase of 29.7%) needed to achieve the same result as LAUSTAPHEN. Finally, no score is flawless; they might be used in conjunction with clinical judgment to help physicians to better guide further investigations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Canton of Vaud (CER-VD). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BG and LS conceived the idea. MP-O and PM collected the patients' data, performed the analysis, and interpreted the results. BG supervised the project. MP-O wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Open access funding was provided by the University of Lausanne.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.961579/full#supplementary-material
1. Willekens R, Puig-Asensio M, Suanzes P, Fernandez-Hidalgo N, Larrosa MN, Gonzalez-Lopez JJ, et al. Mortality in staphylococcus aureus bacteraemia remains high despite adherence to quality indicators: secondary analysis of a prospective cohort study. J Infect. (2021) 83:656–63. doi: 10.1016/j.jinf.2021.10.001
2. Marchaim D, Kaye KS, Fowler VG, Anderson DJ, Chawla V, Golan Y, et al. Case-control study to identify factors associated with mortality among patients with methicillin-resistant staphylococcus aureus bacteraemia. Clin Microbiol Infect. (2010) 16:747–52. doi: 10.1111/j.1469-0691.2009.02934.x
3. Kim SH, Park WB, Lee KD, Kang CI, Kim HB, Oh MD, et al. Outcome of staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin Infect Dis. (2003) 37:794–9. doi: 10.1086/377540
4. Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr, Hellmich M, Hopkins S, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect. (2014) 68:242–51. doi: 10.1016/j.jinf.2013.10.015
5. Bassetti M, Peghin M, Trecarichi EM, Carnelutti A, Righi E, Del Giacomo P, et al. Characteristics of staphylococcus aureus bacteraemia and predictors of early and late mortality. PLoS ONE. (2017) 12:e0170236. doi: 10.1371/journal.pone.0170236
6. Austin ED, Sullivan SS, Macesic N, Mehta M, Miko BA, Nematollahi S, et al. Reduced mortality of staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007-2015. Clin Infect Dis. (2020) 70:1666–74. doi: 10.1093/cid/ciz498
7. van der Vaart TW, Prins JM, Soetekouw R, van Twillert G, Veenstra J, Herpers BL, et al. Prediction rules for ruling out endocarditis in patients with staphylococcus aureus bacteremia. Clin Infect Dis. (2021). doi: 10.1093/cid/ciab632
8. Tubiana S, Duval X, Alla F, Selton-Suty C, Tattevin P, Delahaye F, et al. The virsta score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with staphylococcus aureus bacteremia. J Infect. (2016) 72:544–53. doi: 10.1016/j.jinf.2016.02.003
9. Peinado-Acevedo JS, Hurtado-Guerra JJ, Hincapie C, Mesa-Abad J, Uribe-Delgado JR, Giraldo-Ramirez S, et al. Validation of virsta and predicting risk of endocarditis using a clinical tool (predict) scores to determine the priority of echocardiography in patients with staphylococcus aureus bacteremia. Clin Infect Dis. (2021) 73:e1151–e7. doi: 10.1093/cid/ciaa1844
10. Kahn F, Resman F, Bergmark S, Filiptsev P, Nilson B, Gilje P, et al. Time to blood culture positivity in staphylococcus aureus bacteraemia to determine risk of infective endocarditis. Clin Microbiol Infect. (2021) 27:1345.e7–12. doi: 10.1016/j.cmi.2020.11.007
11. Abu Saleh O, Fida M, Asbury K, Narichania A, Sotello D, Bosch W, et al. Prospective validation of predict and its impact on the transesophageal echocardiography use in management of staphylococcus aureus bacteremia. Clin Infect Dis. (2021) 73:e1745–e53. doi: 10.1093/cid/ciaa844
12. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 Esc guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (Esc). Endorsed By: European Association for Cardio-Thoracic Surgery (Eacts), the European Association of Nuclear Medicine (Eanm). European heart journal. (2015) 36:3075–128. doi: 10.1093/eurheartj/ehv319
13. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children. Clin Infect Dis. (2011) 52:e18–55. doi: 10.1093/cid/ciq146
14. Holland TL, Arnold C, Fowler VG Jr. Clinical management of staphylococcus aureus bacteremia: a review. JAMA. (2014) 312:1330–41. doi: 10.1001/jama.2014.9743
15. Bai AD, Agarwal A, Steinberg M, Showler A, Burry L, Tomlinson GA, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect. (2017) 23:900–6. doi: 10.1016/j.cmi.2017.04.025
16. Heriot GS, Tong SYC, Cheng AC, Liew D. What risk of endocarditis is low enough to justify the omission of transoesophageal echocardiography in staphylococcus aureus bacteraemia? A narrative review. Clin Microbiol Infect. (2018) 24:1251–6. doi: 10.1016/j.cmi.2018.03.027
17. Joseph JP, Meddows TR, Webster DP, Newton JD, Myerson SG, Prendergast B, et al. Prioritizing echocardiography in staphylococcus aureus bacteraemia. J Antimicrob Chemother. (2013) 68:444–9. doi: 10.1093/jac/dks408
18. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The redcap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
19. Palraj BR, Baddour LM, Hess EP, Steckelberg JM, Wilson WR, Lahr BD, et al. Predicting risk of endocarditis using a clinical tool (predict): scoring system to guide use of echocardiography in the management of staphylococcus aureus bacteremia. Clin Infect Dis. (2015) 61:18–28. doi: 10.1093/cid/civ235
20. Calderon-Parra J, Diego-Yague I, Santamarina-Alcantud B, Mingo-Santos S, Mora-Vargas A, Vazquez-Comendador JM, et al. Unreliability of clinical prediction rules to exclude without echocardiography infective endocarditis in Staphylococcus aureus bacteremia. J Clin Med. (2022) 11:1502. doi: 10.3390/jcm11061502
21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
22. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. (2002) 137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007
23. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
24. Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. Clin Infect Dis. (1997) 25:1448–58. doi: 10.1086/516156
25. Murillo O, Grau I, Gomez-Junyent J, Cabrera C, Ribera A, Tubau F, et al. Endocarditis associated with vertebral osteomyelitis and septic arthritis of the axial skeleton. Infection. (2018) 46:245–51. doi: 10.1007/s15010-018-1121-9
26. Lesens O, Hansmann Y, Storck D, Christmann D. Risk factors for metastatic infection in patients with staphylococcus aureus bacteremia with and without endocarditis. Eur J Intern Med. (2003) 14:227–31. doi: 10.1016/S0953-6205(03)00063-3
27. Beaufrere M, Pressat-Laffouilhere T, Marcelli C, Michon J, Lequerre T, Prum-Delepine C, et al. Valvular and infection-associated risk factors as criteria to guide the use of echocardiography in patients with native joint infections. Semin Arthritis Rheum. (2021) 51:1274–81. doi: 10.1016/j.semarthrit.2021.08.008
Keywords: Staphylococcus aureus bacteraemia, infective endocarditis, transoesophageal echocardiography (TOE), bloodstream infection, risk stratification
Citation: Papadimitriou-Olivgeris M, Monney P, Mueller L, Senn L and Guery B (2022) The LAUsanne STAPHylococcus aureus ENdocarditis (LAUSTAPHEN) score: A prediction score to estimate initial risk for infective endocarditis in patients with S. aureus bacteremia. Front. Cardiovasc. Med. 9:961579. doi: 10.3389/fcvm.2022.961579
Received: 04 June 2022; Accepted: 17 November 2022;
Published: 09 December 2022.
Edited by:
Filip Zemrak, Barts Health NHS Trust, United KingdomReviewed by:
Marco Ochs, Theresienkrankenhaus, GermanyCopyright © 2022 Papadimitriou-Olivgeris, Monney, Mueller, Senn and Guery. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthaios Papadimitriou-Olivgeris, bWF0dGhhaW9zLnBhcGFkaW1pdHJpb3Utb2xpdmdlcmlzQGNodXYuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.