
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 25 July 2022
Sec. Cardiovascular Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.961141
This article is part of the Research Topic Novel Biomarkers and Risk Factors Associated with Cardiometabolic Dysfunction in Heart Failure View all 16 articles
Background and aims: Malnutrition is very common in patients with heart failure (HF) and is associated with a worse clinical outcome. The Controlling Nutritional Status (CONUT) score is an easily derived index for the evaluation of malnutrition. This study aimed to evaluate the association between the CONUT score and the prognosis in patients with HF.
Methods and results: Electronic databases were searched for potential studies from inception up to February 15, 2022. Observational cohort studies included adult participants with HF, and reported the associations between the CONUT score and the adjusted relative risk (RR) of all-cause mortality, and patients with composite major adverse cardiac outcomes (MACEs) were included. We finally included 18 studies comprising 12,532 participants with HF for analysis. The median age of the patients was 70.5 years old, and 35.4% were women. After a median follow-up duration of 32.5 months, patients with HF with a higher CONUT score were associated with a higher risk of all-cause mortality (per 1 increment of the CONUT score: RR, 1.21, 95% CI, 1.13–1.29, I2 = 68%, P for heterogeneity = 0.002) and MACEs (per 1 increment of the CONUT score: RR, 1.14, 95% CI, 1.06–1.23, I2 = 81%, P for heterogeneity <0.0001) after adjusting for other prognostic factors. When the CONUT score was divided into the normal nutritional status and malnourished status, malnourished patients with HF were associated with increased risks of all-cause death (RR, 1.61, 95% CI, 1.40–1.85, I2 = 17%, P for heterogeneity = 0.29) and MACEs (RR, 2.12, 95% CI, 1.49–3.02, I2 = 87%, P for heterogeneity <0.0001), compared with those with normal nutritional status.
Conclusions: The CONUT score is associated with the clinical outcomes in patients with HF, and can be used as a screening tool of nutritional status in HF to improve prognosis.
Heart failure (HF) is a complex clinical syndrome that results from any structural or functional impairment of the heart. Accompanied by the aging of society and a decrease in mortality of multiple cardiovascular diseases, the prevalence of HF has increased rapidly, which contributed to a growing health burden worldwide (1, 2). Although guideline-directed medical therapy (GDMT) had made great progress in the management of HF, it was still associated with high morbidity and mortality. It had been reported that, in patients hospitalized due to the exacerbation of HF, the composite outcomes (including 1-year mortality and re-hospitalization) were >20% (3, 4). Therefore, new risk stratification markers and treatment methods are still needed to improve the prognosis of HF.
Malnutrition is very common in patients with HF and is associated with a higher risk of mortality and re-hospitalization (5, 6). Early detection of malnutrition in HF would be useful for identifying patients at high risk of poor clinical outcomes and recommending nutritional interventions to improve prognosis (7). Many tools and indexes had been proposed for screening malnutrition; however, no consensus had been made on which to use in patients with HF (5, 8–10).
The Controlling Nutritional Status (CONUT) score, developed by Ignacio et al., (11) had been reported to be one of the most robust markers of nutritional status. It is calculated from a patient's serum albumin, total cholesterol level, and total peripheral lymphocyte count. Therefore, The CONUT score is an immune-nutritional index, which can evaluate the protein reserve, lipid metabolism, and immunocompetence. Recently, studies have shown that malnourished status determined by the CONUT score is associated with worse outcomes in patients with HF (12–16). However, these studies were with small sample size and different patient characteristics, which resulted in inconsistent results in the association between the CONUT score and the clinical outcomes in patients with HF. Based on the inconsistency of previous studies, we conducted a meta-analysis of observational cohort studies to evaluate the association between the CONUT score and the prognosis in HF.
We performed the systematic review and meta-analysis according to the recommendations of the MOOSE (Meta-analysis of Observational Studies in Epidemiology) Group (17). Electronic databases, including PubMed, Embase, Google Scholar, the Cochrane Library, and Wanfang, were searched for related studies from inception until February 15, 2022. We developed the search strategies using the terms “Controlling Nutritional Status,” “CONUT,” or “malnutrition” and “heart failure,” “cardiac dysfunction,” or “myocardial dysfunction” and “prognosis,” or “death” or “MACE.” We limited our search to human studies and writing in Chinese or English, and further read the reference lists of the included studies or other systematic reviews to identify potential missing related articles.
Two researchers (X-WH. and J-JL) independently searched the databases and screened the retrieved items. Potentially related studies were reviewed in full text, and the studies' information was extracted into a pre-defined form. We included studies for meta-analysis if there were: (1) observational cohort studies included adult participants (age ≥18 years old); (2) all the participants were diagnosed with HF; (3) the CONUT score was evaluated at baseline status, which was based on serum albumin, lymphocyte count, and total cholesterol measures (range from 0 to 12); (4) the association between the CONUT score (as a continuous or category metric) and the prognostic outcomes of HF were reported in an adjusted model, which was controlling the other related prognostic factors. We excluded those studies if they were: (1) cross-sectional studies; (2) the follow-up evaluation was <3 months; (3) the relative risk (RR) was not adjusted for other confounders, and (4) duplicated publications from identical cohort studies with the same outcomes.
The CONUT score was calculated based on the patients' serum albumin, total cholesterol, and total peripheral lymphocyte levels (Table 1). The range of the CONUT scores is 0 to 12, and a higher score indicated that the patient was with worse nutritional status (11–16). The quality of the included studies was accessed by the NOS (the Newcastle–Ottawa Quality Assessment Scale for cohort studies), which evaluates the selection (four items with one point in each item), comparability (one item with up to two points), and exposure/outcome (three items with one point in each item), respectively (18, 19). Therefore, up to a highest of 9 points can be awarded in NOS. According to previous reports, the included studies were graded as low quality (<4 points), moderate quality (4–6 points) or high quality (≥7 points), respectively (20, 21).
In this meta-analysis, the primary outcome interested was all-cause mortality in patients with HF. The secondary outcome was composite major adverse cardiac outcomes (MACEs), including all-cause mortality and HF hospitalization. We pooled the association between the exposure (CONUT score) and outcomes in multivariable-adjusted statistical models. If multiple statistical models were reported, we used the data that adjusted the most comprehensive confounders for analysis. As the associations between the CONUT score and the interested outcomes were reported in different ways in the included studies (e.g., per 1 increment as a continuous metric; or as normal nutritional/malnourished status in the category trait), we pooled the RRs for per 1 increment in the CONUT score, as well as malnourished vs. normal nutritional status, respectively. The RRs (logarithmically transformed) and their corresponding standard errors (SEs) were pooled by the inverse variance approach. In case outcomes were presented as odds ratios (ORs) or hazard ratios (HRs), they were regarded as an approximate RR and used in the meta-analysis (22).
Heterogeneity among studies was evaluated with the I2 statistic, an I2 value of <50% or P for heterogeneity <0.1 was considered an indication of no-significant heterogeneity observed among the studies. However, even when no-significant heterogeneity was shown, we combined the results using the DerSimonian and Laird random-effects models over the fixed effects model, considering that, to some extent, both clinically and methodologically were unavoidable (for example, cohort design, the definition of HF, and adjustment of potential confounders) (23). In case of no heterogeneity, the results of fixed and random effects models are the same, while, if there was significant heterogeneity among the included studies, the random-effects model would be more conservative. To further test the stability of the results, we conducted sensitivity analyses by changing the statistical models from random-effects models to fixed-effects models. We also performed sensitivity analyses by deleting one study each time and recalculating the pooled results. The Publication bias was accessed by inspecting the funnel plot for the outcomes. All the statistical analyses were performed with RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). A P value <0.05 is considered statistically significant.
After searching the electronic databases, we retrieved 5,423 potentially related article items. The duplicate items with identical titles, authors, publication journals, and years were deleted. Two investigators (XH and YH) independently screened the titles and abstracts. Then, 62 potentially related full articles were reviewed, and 18 studies were finally included in the pooled analysis according to the pre-defined criteria (Figure 1) (12–16, 24–36). There were 12,532 participants with HF in the included studies, with a median follow-up duration of 32.5 months. The median age of the patients was 70.5 years old, and 35.4% were women. The baseline characteristics of the participants are presented in Table 2. According to the NOS assessment of observational studies, five included studies were graded as fair quality and 13 studies were as good quality (Supplementary File 1). The adjusted confounders in the included studies are summarized in Supplementary File 2.
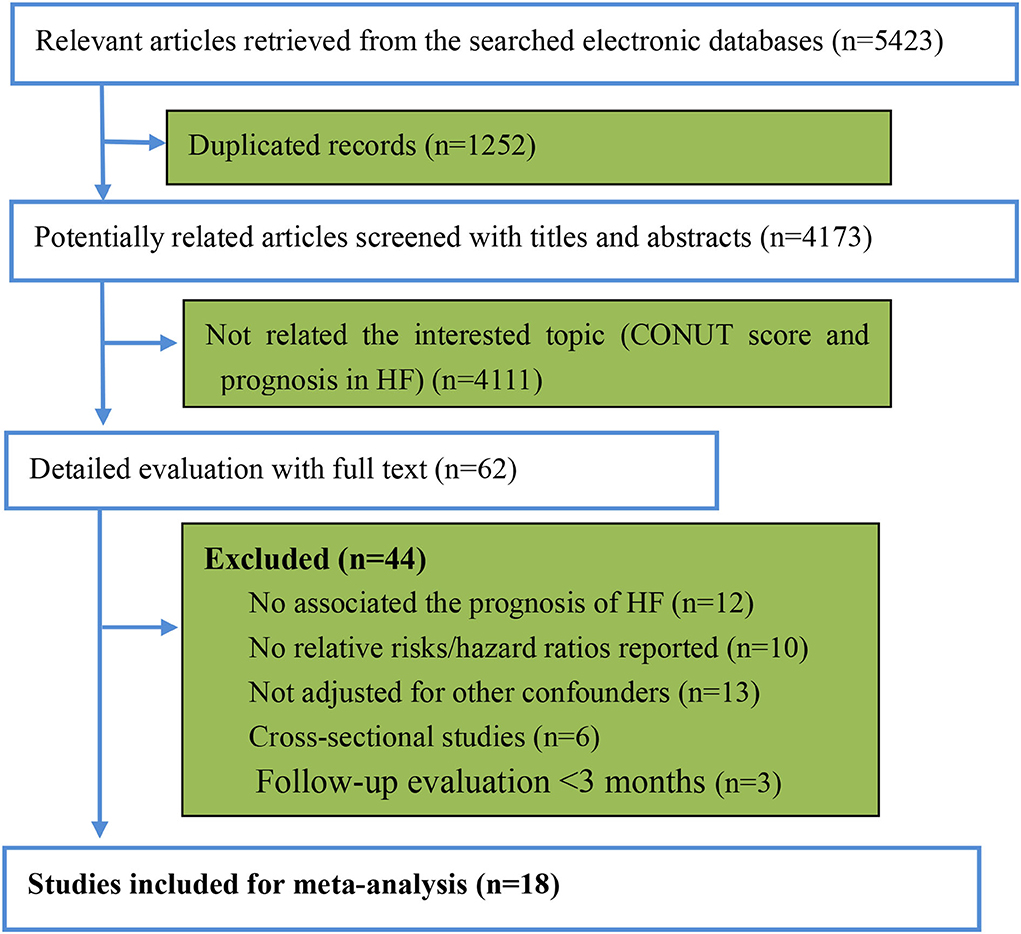
Figure 1. The review flow of the retrieved studies. CONUT, controlling nutritional status; HF: heart failure.
When the CONUT score was reported as a continuous index, we observed that a higher CONUT score was associated with a higher risk of all-cause mortality in patients with HF after adjusting for multiple prognostic factors (per 1 increment of the CONUT score: RR, 1.21, 95% CI, 1.13–1.29, Figure 2). However, significant heterogeneity was observed in the included studies (I2 = 68%, P = 0.002).
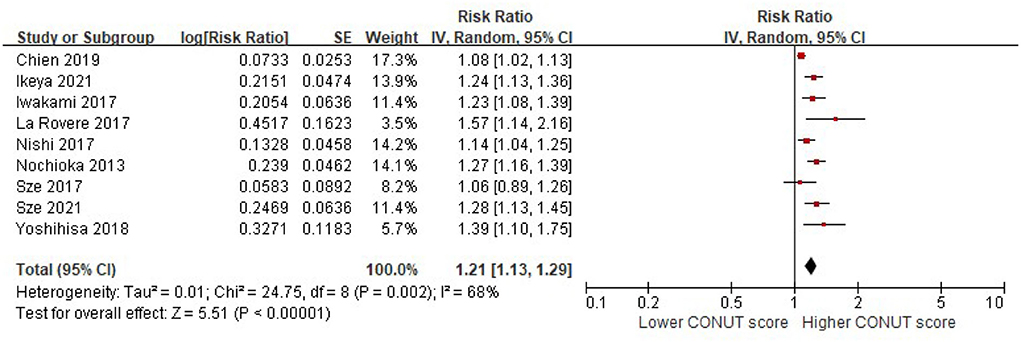
Figure 2. A forest plot of comparison: All-cause mortality in patients with HF associated with per-1 increase of the CONUT score. CONUT, controlling nutritional status; HF, heart failure.
When the CONUT score was divided into the normal nutritional status and malnourished status, the patients with a higher CONUT score (malnourished) were associated with a 61% increased risk of all-cause death in HF (RR, 1.61, 95% CI, 1.40–1.85), compared with those with normal nutritional status (a lower CONUT score) (Figure 3) after being adjusted for other prognostic factors. No significant heterogeneity was observed in the included studies (I2 = 17%, P = 0.29). Furthermore, the increased risk of all-cause mortality was only observed in those with moderate to severe malnutrition (the CONUT score ≥5; RR, 1.79; 95% CI, 1.35–2.37), but not in those with mild malnutrition (the CONUT score, 2–4; RR, 1.20; 95% CI,.85–1.71) (Figure 4).
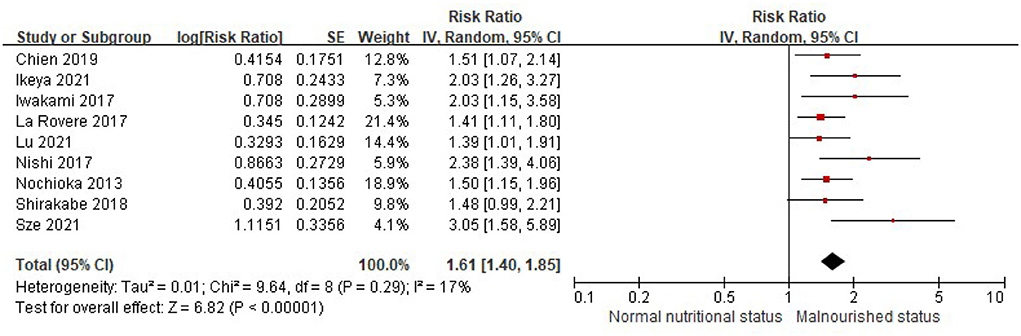
Figure 3. A forest plot of comparison: All-cause mortality in patients with HF associated with malnutrition status defined by the CONUT score. CONUT, controlling nutritional status; HF, heart failure.
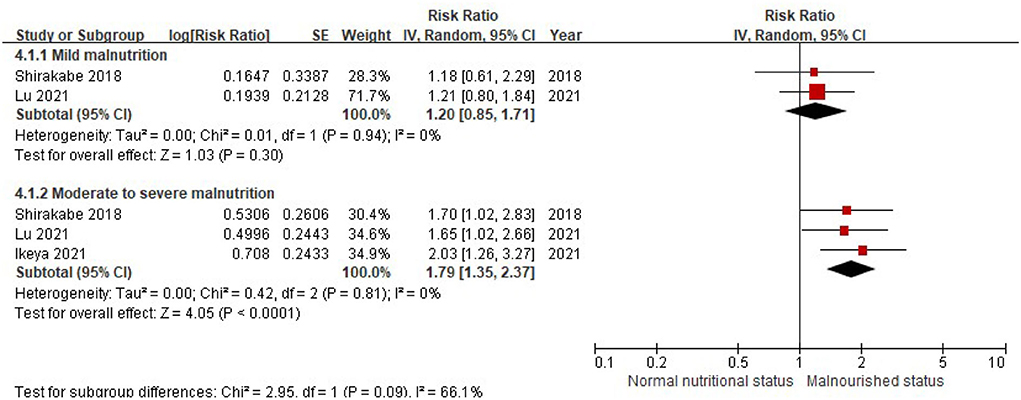
Figure 4. A forest plot of comparison: All-cause mortality in patients with HF associated with different levels of malnourished status defined by the CONUT score. CONUT, controlling nutritional status; HF, heart failure.
The patients with higher CONUT scores were associated with a higher risk of MACEs in the patients with HF after being adjusted for multiple prognostic factors (per 1 increment of the CONUT score: RR, 1.14; 95% CI, 1.06–1.23). Significant heterogeneity was observed in the included studies (I2 = 81%, P < 0.0001) (Figure 5).

Figure 5. A forest plot of comparison: The risk of MACEs in patients with HF associated with per-1 increase of the CONUT score. CONUT, controlling nutritional status; HF, heart failure; MACEs, major adverse cardiac events.
Similarly, when the CONUT score was divided into the normal nutritional status and malnourished status, malnourished patients with HF were associated with a 112% increased risk of MACEs (RR, 2.12; 95% CI, 1.49–3.02), compared with those with normal nutritional status in the multivariable-adjusted model (Figure 6), while significant heterogeneity was observed in the included studies (I2 = 87%, P < 0.0001). Compared with patients with HF, with normal nutritional status, those with mild (RR, 1.63; 95% CI, 1.08–2.46) or moderate to severe malnutrition (RR, 3.96, 95% CI, 1.41–11.13) were associated with a high risk of MACEs (Figure 7).
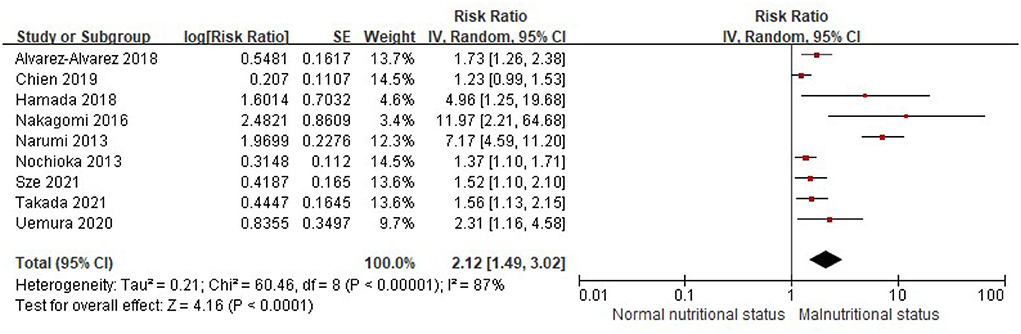
Figure 6. A forest plot of comparison: The risk of MACEs in patients with HF associated with malnutrition status defined by the CONUT score. CONUT, controlling nutritional status; HF, heart failure; MACEs, major adverse cardiac events.
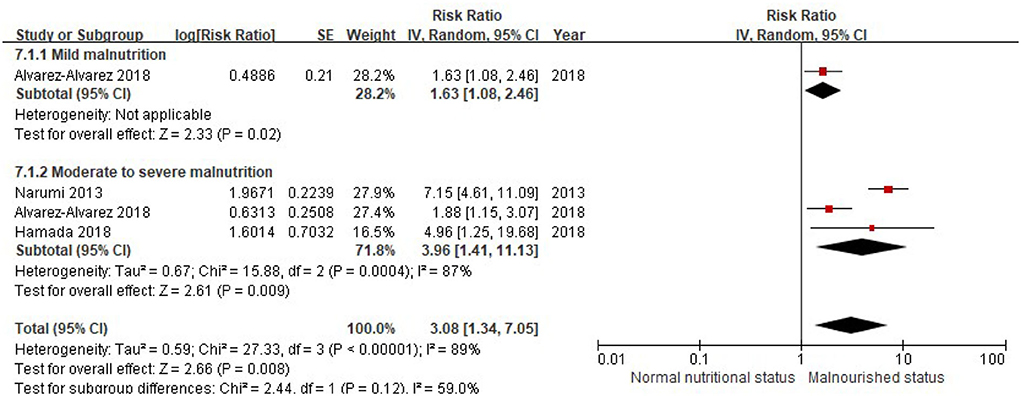
Figure 7. A forest plot of comparison: MACEs in patients with HF associated with different levels of malnourished status defined by the CONUT score. CONUT, controlling nutritional status; HF, heart failure; MACEs, major adverse cardiac events.
The sensitivity analyses confirmed that the association between the CONUT score and the prognosis in the patients with HF did not change with the use of statistical models (fixed-effects models vs. the random-effects models) or recalculation of the RRs by omitting one study at a time. NO significant publication bias was observed for the analyses of all-cause mortality or MACE associated with the CONUT score as a continuous or as a category index by inspection of the funnel plot (Supplementary Files 3–6).
In this meta-analysis, we showed that the CONUT score, which is derived from three commonly detected laboratory biomarkers (e.g., serum albumin, the total cholesterol level, and total peripheral lymphocyte CONUT), is associated with the clinical outcomes in patients with HF. Furthermore, such association was detected when the CONUT score was defined either as a continuous index, or a category divided into the normal nutritional status and malnourished status. These findings support the use of the CONUT score as a screening tool for nutritional status in HF, and guiding the risk stratification, as well as nutritional interventions to improve prognosis in HF.
Similar to our study, a previous meta-analysis by Li et al. (37) included 10 studies involving 5,196 patients with HF, and the results showed that the malnourished patients with HF had an increased risk of follow-up mortality (RR, 2.01; 95% CI, 1.58–2.57). However, the risk of MACEs, including risk of the re-hospitalization, was not evaluated in Li's study. In our meta-analysis, we included a much larger sample size (18 studies with 12,532 participants), which allowed us to perform a much more comprehensive analysis, and our results showed that the risk of MACE in HF was also increased with a higher CONUT score. Furthermore, we found that the worse prognosis (including all-cause mortality and MACEs) was more significant in patients with HF, with moderate to severe malnutrition. Therefore, patients with moderate to severe malnutrition should be emphasized to require more intensive nutritional interventions (e.g., increased protein and energy intake) added to the GDMT, and regular follow-up is needed to improve their prognosis (38).
Several underlying mechanisms may be related to the worse prognosis in HF patients with malnutrition. First, gastrointestinal congestion and gut edema can cause appetite loss and malabsorption (39, 40). Second, the chronic inflammatory state in HF would cause metabolic disturbances, activation of the sympathetic nerve system, and anabolic-catabolic imbalance (41, 42). Third, disturbance of cytokine, adipokines, and metabolites may also play a role in the association between malnutrition and clinical outcomes in HF (43, 44).
Except for the CONUT score, some other simple nutritional indexes had also been proposed in patients with HF (8). For example, the prognostic nutritional index (PNI), which was calculated from the serum albumin and total peripheral lymphocyte count, was reported to be associated with a poor prognosis in patients with acute and chronic HF (46, 47). However, the cut-point for malnutrition by the PNI was inconsistent in different studies, which would hamper its wildly clinical use (48). It had been cautious that the total cholesterol level was included as a component in calculating the CONUT score, which would overestimate the prevalence of malnutrition in patients with HF, as most of them may receive statins treatment and resulted in a lower total cholesterol level (5). In the same cohort of patients, it had been shown that the prevalence of malnutrition would be up to 54% when defined by the CONUT, while only 8% when defined by the PNI (45). However, in patients without statins or other lipid-lowing drug treatment, the inclusion of total cholesterol level may be more comprehensive for evaluating the nutritional status, as it also considered the lipid metabolism (9).
Some limitations in our study should be addressed. First, as discussed above, the CONUT score can be significantly affected by the treatment of statins or other lipid-lowering drugs. However, the proportion of statins treatment was unavailable in most of the included studies. Second, most of the included studies only evaluated the nutritional status at enrollment, but not evaluated the change of nutritional status during the follow-up. However, our results support the conclusion that the baseline nutritional status at enrollment is associated with the prognosis in patients with HF. Third, limited studies were available for the analysis of the different levels of malnutrition and the prognosis. Further studies are needed to document whether mild malnutrition was associated with poor clinical outcomes in HF. Fourth, due to the unavailability of individual patients' data, we cannot perform the analysis of risk discrimination (e.g., c-statistic) and reclassification (e.g., net reclassification improvement or an integrated discrimination index).
The CONUT score is an easily available nutritional index associated with the clinical outcomes in patients with HF. Further studies are needed to explore whether the CONUT score can be used as a screening tool for nutritional status in HF and guide the nutritional interventions to improve prognosis in HF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
X-WH and BB: research idea and study design. X-WH and J-JL: data acquisition, data analysis/interpretation, and statistical analysis. BB: supervision and mentorship. All the authors contributed important intellectual content during manuscript drafting or revision and accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.961141/full#supplementary-material
1. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. (2022) 145:e895–1032. doi: 10.1161/CIR.0000000000001073
2. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. (2021) 23:1746–53. doi: 10.1111/dom.14388
3. Taylor CJ, Ordonez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ. (2019) 364:l223. doi: 10.1136/bmj.l223
4. Wu J, Zheng H, Liu X, Chen P, Zhang Y, Luo J, et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ Heart Fail. (2020) 13:e7054. doi: 10.1161/CIRCHEARTFAILURE.120.007054
5. Kinugasa Y, Sota T, Kamitani H, Nakayama N, Nakamura K, Hirai M, et al. Diagnostic performance of nutritional indicators in patients with heart failure. ESC Heart Fail. (2022). doi: 10.1002/ehf2.13886 [Epub ahead of print].
6. Alataş ÖD, Biteker M, Yildirim B, Acar E, Gökçek K. Comparison of objective nutritional indexes for the prediction of in-hospital mortality among elderly patients with acute heart failure. Eur J Emerg Med. (2020) 27:362–7. doi: 10.1097/MEJ.0000000000000690
7. He M, Fan Q, Zhu Y, Liu D, Liu X, Xu S, et al. The need for nutritional assessment and interventions based on the prognostic nutritional index for patients with femoral fractures: a retrospective study. Perioper Med. (2021) 10:61. doi: 10.1186/s13741-021-00232-1
8. Hu Y, Yang H, Zhou Y, Liu X, Zou C, Ji S, et al. Prediction of all-cause mortality with malnutrition assessed by nutritional screening and assessment tools in patients with heart failure: a systematic review. NutrMetab Cardiovasc Dis. (2022) 32:1361–1374. doi: 10.1016/j.numecd.2022.03.009
9. Kojima I, Tanaka S, Otobe Y, Suzuki M, Koyama S, Kimura Y, et al. What is the optimal nutritional assessment tool for predicting decline in the activity of daily living among older patients with heart failure? Heart Vessels. (2022) 37:1356–1362. doi: 10.1007/s00380-022-02033-y
10. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Agreement and classification performance of malnutrition tools in patients with chronic heart failure. Curr Dev Nutr. (2020) 4:a71. doi: 10.1093/cdn/nzaa071
11. Ignacio DUJ, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
12. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr. (2021) 113:695–705. doi: 10.1093/ajcn/nqaa311
13. Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, et al. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Fail. (2019) 6:953–64. doi: 10.1002/ehf2.12501
14. Shirakabe A, Hata N, Kobayashi N, Okazaki H, Matsushita M, Shibata Y, et al. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the prognostic nutritional index (PNI) and controlling nutritional status (CONUT) score. Heart Vessels. (2018) 33:134–44. doi: 10.1007/s00380-017-1034-z
15. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. (2017) 106:533–41. doi: 10.1007/s00392-017-1082-5
16. Iwakami N, Nagai T, Furukawa TA, Sugano Y, Honda S, Okada A, et al. Prognostic value of malnutrition assessed by controlling nutritional status score for long-term mortality in patients with acute heart failure. Int J Cardiol. (2017) 230:529–36. doi: 10.1016/j.ijcard.2016.12.064
17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
18. GA Wells B, Shea D, O'Connell, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomized Studies in Meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed January 1, 2008).
19. Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Non-alcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. (2020) 40:1594–600. doi: 10.1111/liv.14461
20. Wu J, Qiu M, Sun L, Wen J, Liang DL, Zheng S, et al. Alpha-linolenic acid and risk of heart failure: a meta-analysis. Front Cardiovasc Med. (2021) 8:788452. doi: 10.3389/fcvm.2021.788452
21. Cai X, Sun L, Liu X, Zhu H, Zhang Y, Zheng S, et al. Non-alcoholic fatty liver disease is associated with increased risk of chronic kidney disease. Ther Adv Chronic Dis. (2021) 12:364072937. doi: 10.1177/20406223211024361
22. Zhu H, Zheng H, Xu T, Liu X, Liu X, Sun L, et al. Effects of statins in primary and secondary prevention for venous thromboembolism events: a meta analysis. Vascul Pharmacol. (2022) 142:106931. doi: 10.1016/j.vph.2021.106931
23. Mai L, Wen W, Qiu M, Liu X, Sun L, Zheng H, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. (2021) 23:2476–83. doi: 10.1111/dom.14490
24. Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, et al. Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: a report from the CHART-2 Study. Circ J. (2013) 77:2318–26. doi: 10.1253/circj.CJ-13-0127
25. Narumi T, Arimoto T, Funayama A, Kadowaki S, Otaki Y, Nishiyama S, et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol. (2013) 62:307–13. doi: 10.1016/j.jjcc.2013.05.007
26. Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. (2016) 23:713–27. doi: 10.5551/jat.31526
27. Nishi I, Seo Y, Hamada-Harimura Y, Sato K, Sai S, Yamamoto M, et al. Nutritional screening based on the controlling nutritional status (CONUT) score at the time of admission is useful for long-term prognostic prediction in patients with heart failure requiring hospitalization. Heart Vessels. (2017) 32:1337–49. doi: 10.1007/s00380-017-1001-8
28. La Rovere MT, Maestri R, Olmetti F, Paganini V, Riccardi G, Riccardi R, et al. Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr Metab Cardiovasc Dis. (2017) 27:274–80. doi: 10.1016/j.numecd.2016.09.009
29. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart. (2018) 5:e730. doi: 10.1136/openhrt-2017-000730
30. Alvarez-Alvarez B, Garcia-Seara J, Rodriguez-Manero M, Iglesias-Alvarez D, Martinez-Sande JL, Agra-Bermejo RM, et al. Prognostic value of nutrition status in the response of cardiac resynchronization therapy. Indian Pacing Electrophysiol J. (2018) 18:133–9. doi: 10.1016/j.ipej.2018.04.001
31. Hamada T, Kubo T, Yamasaki N, Kitaoka H. Predictive factors of rehospitalization for worsening heart failure and cardiac death within 1 year in octogenarians hospitalized for heart failure. Geriatr Gerontol Int. (2018) 18:101–7. doi: 10.1111/ggi.13148
32. Uemura Y, Shibata R, Masuda A, Katsumi Y, Takemoto K, Koyasu M, et al. Utility of the nutritional screening in predicting adverse outcome of patients with overweight/obesity and acute heart failure. J Card Fail. (2020) 26:566–73. doi: 10.1016/j.cardfail.2020.02.005
33. Komorita T, Yamamoto E, Sueta D, Tokitsu T, Fujisue K, Usuku H, et al. The controlling nutritional status score predicts outcomes of cardiovascular events in patients with heart failure with preserved ejection fraction. Int J Cardiol Heart Vasc. (2020) 29:100563. doi: 10.1016/j.ijcha.2020.100563
34. Ikeya Y, Saito Y, Nakai T, Kogawa R, Otsuka N, Wakamatsu Y, et al. Prognostic importance of the controlling nutritional status (CONUT) score in patients undergoing cardiac resynchronisation therapy. Open Heart. (2021) 8:e001740. doi: 10.1136/openhrt-2021-001740
35. Lu XY, Cheang XH, Liao SG, Zhu X, Zhang HF, Zhou YL, et al. [Association between the controlling nutritional status score and long-term outcome in patients with acute heart failure]. Zhonghua Xin Xue Guan Bing ZaZhi. (2021) 49:1220–6. doi: 10.3760/cma.j.cn112148-20211101-00944
36. Takada T, Jujo K, Inagaki K, Abe T, Kishihara M, Shirotani S, et al. Nutritional status during hospitalization is associated with the long-term prognosis of patients with heart failure. ESC Heart Fail. (2021) 8:5372–82. doi: 10.1002/ehf2.13629
37. Li H, Zhou P, Zhao Y, Ni H, Luo X, Li J. Prediction of all-cause mortality with malnutrition assessed by controlling nutritional status score in patients with heart failure: a systematic review and meta-analysis. Public Health Nutr. (2021) 1–8. doi: 10.1017/S1368980021002470 [Epub ahead of print].
38. Abu-Sawwa R, Dunbar SB, Quyyumi AA, Sattler E. Nutrition intervention in heart failure: should consumption of the DASH eating pattern be recommended to improve outcomes? Heart Fail Rev. (2019) 24:565–73. doi: 10.1007/s10741-019-09781-6
39. McKeag NA, McKinley MC, Harbinson MT, McGinty A, Neville CE, Woodside JV, et al. Dietary micronutrient intake and micronutrient status in patients with chronic stable heart failure: an observational study. J Cardiovasc Nurs. (2017) 32:148–55. doi: 10.1097/JCN.0000000000000322
40. Mijan-de-la-Torre A. Recent insights on chronic heart failure, cachexia and nutrition. Curr Opin Clin Nutr Metab Care. (2009) 12:251–7. doi: 10.1097/MCO.0b013e32832a2171
41. Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: its mechanism and clinical significance. Int J Mol Sci. (2021) 22:8491. doi: 10.3390/ijms22168491
42. Corsetti G, Pasini E, Romano C, Chen-Scarabelli C, Scarabelli TM, Flati V, et al. How can malnutrition affect autophagy in chronic heart failure? Focus and perspectives. Int J Mol Sci. (2021) 22:3332. doi: 10.3390/ijms22073332
43. Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, et al. SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev. (2021) 2021:9265016. doi: 10.1155/2021/9265016
44. Li W, Huang A, Zhu H, Liu X, Huang X, Huang Y, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Aust. (2020) 213:374–9. doi: 10.5694/mja2.50781
45. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland J, Wong K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. (2018) 6:476–86. doi: 10.1016/j.jchf.2018.02.018
46. Candeloro M, Di Nisio M, Balducci M, Genova S, Valeriani E, Pierdomenico SD, et al. Prognostic nutritional index in elderly patients hospitalized for acute heart failure. ESC Heart Fail. (2020) 7:2479–84. doi: 10.1002/ehf2.12812
47. Ju C, Zhou J, Lee S, Tan MS, Liu T, Bazoukis G, et al. Derivation of an electronic frailty index for predicting short-term mortality in heart failure: a machine learning approach. ESC Heart Fail. (2021) 8:2837–45. doi: 10.1002/ehf2.13358
Keywords: heart failure, malnutrition, prognosis, risk, the controlling nutritional status
Citation: Huang X-W, Luo J-J and Baldinger B (2022) The controlling nutritional status score and clinical outcomes in patients with heart failure: Pool analysis of observational studies. Front. Cardiovasc. Med. 9:961141. doi: 10.3389/fcvm.2022.961141
Received: 04 June 2022; Accepted: 27 June 2022;
Published: 25 July 2022.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
Yanhua Yang, Southern Medical University, ChinaCopyright © 2022 Huang, Luo and Baldinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Wen Huang, aHh3MTg5Mjc0OTE5MDhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.