- 1Department of Nephrology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2Chansn Hospital, Taoyuan, Taiwan
- 3Department of Nephrology, Kidney Research Center, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 4Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Department of Anesthesiology, Mackay Memorial Hospital, Taipei, Taiwan
- 6Department of Cardiothoracic and Vascular Surgery, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
- 7Department of Nephrology, New Taipei Municipal TuCheng Hospital, New Taipei City, Taiwan
- 8Department of Cardiology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
Objects: Cardiac surgery is associated with acute kidney injury (AKI). However, the effects of various pharmacological and non-pharmacological strategies for AKI prevention have not been thoroughly investigated, and their effectiveness in preventing AKI-related adverse outcomes has not been systematically evaluated.
Methods: Studies from PubMed, Embase, and Medline and registered trials from published through December 2021 that evaluated strategies for preventing post–cardiac surgery AKI were identified. The effectiveness of these strategies was assessed through a network meta-analysis (NMA). The secondary outcomes were prevention of dialysis-requiring AKI, mortality, intensive care unit (ICU) length of stay (LOS), and hospital LOS. The interventions were ranked using the P-score method. Confidence in the results of the NMA was assessed using the Confidence in NMA (CINeMA) framework.
Results: A total of 161 trials (involving 46,619 participants) and 53 strategies were identified. Eight pharmacological strategies {natriuretic peptides [odds ratio (OR): 0.30, 95% confidence interval (CI): 0.19–0.47], nitroprusside [OR: 0.29, 95% CI: 0.12–0.68], fenoldopam [OR: 0.36, 95% CI: 0.17–0.76], tolvaptan [OR: 0.35, 95% CI: 0.14–0.90], N-acetyl cysteine with carvedilol [OR: 0.37, 95% CI: 0.16–0.85], dexmedetomidine [OR: 0.49, 95% CI: 0.32–0.76;], levosimendan [OR: 0.56, 95% CI: 0.37–0.84], and erythropoietin [OR: 0.62, 95% CI: 0.41–0.94]} and one non-pharmacological intervention (remote ischemic preconditioning, OR: 0.76, 95% CI: 0.63–0.92) were associated with a lower incidence of post–cardiac surgery AKI with moderate to low confidence. Among these nine strategies, five (fenoldopam, erythropoietin, natriuretic peptides, levosimendan, and remote ischemic preconditioning) were associated with a shorter ICU LOS, and two (natriuretic peptides [OR: 0.30, 95% CI: 0.15–0.60] and levosimendan [OR: 0.68, 95% CI: 0.49–0.95]) were associated with a lower incidence of dialysis-requiring AKI. Natriuretic peptides were also associated with a lower risk of mortality (OR: 0.50, 95% CI: 0.29–0.86). The results of a sensitivity analysis support the robustness and effectiveness of natriuretic peptides and dexmedetomidine.
Conclusion: Nine potentially effective strategies were identified. Natriuretic peptide therapy was the most effective pharmacological strategy, and remote ischemic preconditioning was the only effective non-pharmacological strategy. Preventive strategies might also help prevent AKI-related adverse outcomes. Additional studies are required to explore the optimal dosages and protocols for potentially effective AKI prevention strategies.
Introduction
Acute kidney injury (AKI) is a common complication in hospitalized patients, with an incidence of 7–18% among inpatients (1). Among patients who have undergone surgery, those who have undergone cardiac surgery are at the highest risk of postoperative AKI (2–4). The incidence of AKI among patients who have undergone cardiac surgery ranges from 20 to 70% depending on the type of cardiovascular surgery and the definition of AKI used (5, 6). Post–cardiac surgery AKI is associated with increased risks of short-term and long-term mortality, increased incidence of chronic kidney disease, and higher medical resource utilization (6–10).
The mechanisms underlying post–cardiac surgery AKI are complex and may include intrarenal hemodynamic perturbation, iron metabolism, an increase in the production of reactive oxygen species and an increase in free hemoglobin levels, the activation of an inflammatory and immunological cascade, and factors specific to cardiovascular operations (cardiopulmonary bypass [CPB], aortic cross-clamping, ischemic or reperfusion injury, and frequent exogenous blood transfusion) (6, 10–14). Both perioperative pharmacological and non-pharmacological AKI prevention strategies have been proposed (4, 6). In 2018, the Acute Disease Quality Initiative (ADQI) Group recommended the avoidance of glucose variability; the use of balanced crystalloid solutions, remote ischemic preconditioning (RIPC), dexmedetomidine, and the KDIGO AKI prevention bundle; and limited use of blood transfusions as strategies to prevent post–cardiac surgery AKI (6). However, several new preventive strategies were not thoroughly discussed in the aforementioned ADQI consensus statement. Furthermore, numerous researchers have adopted their own definitions of AKI or have used criteria that are no longer widely accepted. Moreover, non-pharmacological interventions (other than RIPC) have not been systematically examined in meta-analyses (15–17). The most recent network meta-analysis (NMA), which was published in 2018, only enrolled studies published before 2016 that focused on pharmacological strategies, and the study did not evaluate AKI-related adverse outcomes (extended ICU and hospital length of stay [LOS] and mortality) (18).
To address the aforementioned shortcomings of previous relevant studies, we conducted this updated NMA analyzing the effectiveness of various pharmacological and non-pharmacological AKI prevention strategies as well as the effectiveness of such strategies in preventing dialysis-requiring AKI and AKI-related adverse outcomes, including extended hospital length of stay (LOS), ICU LOS, and mortality.
Materials and methods
Literature search strategy
We performed this NMA in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for NMAs (Supplementary Table 1) and registered our study design and protocol in PROSPERO (CRD42021278036).
Two authors (J-JC and TL) independently searched for studies published prior to November 3, 2021, in PubMed, Medline, and Embase. The search strategies targeted published clinical trials that compared the efficacy of different interventions in preventing post–surgery AKI in patients who had undergone cardiac surgery. A detailed description of the search strategy and the results of the search process are provided in Supplementary Table 2. Review articles and meta-analyses were excluded from our analysis, but their references were screened for relevant studies. Two authors also searched ClinicalTrials.gov to identify completed but unpublished trials by using the keywords “acute kidney injury” and “cardiac surgery,” limiting our search to clinical trials involving adult populations. A third reviewer (G. Kou) helped resolve disagreements between the two aforementioned authors (J-JC and TL) and reviewed the search strategy.
Study eligibility criteria
The two authors who conducted the search (J-JC and TL) independently examined the titles and abstracts of the identified studies, and articles were excluded upon initial screening if their titles or abstracts indicated that they were clearly irrelevant to the objective of the current study. The full texts of the relevant articles were reviewed to assess the eligibility of the studies. A study was included if it (1) enrolled adults who had undergone cardiac surgery, including coronary artery bypass grafting (CABG), heart valve surgery, or aortic surgery; (2) was a randomized controlled trial (RCT) with a parallel design; (3) assigned patients to at least two intervention arms to compare the efficacy of preventive strategies; and (4) reported either outcomes related to AKI (or dialysis-requiring AKI) or acute renal failure (diagnosed according to the Kidney Disease: Improving Global Outcomes [KDIGO]; Acute Kidney Injury Network [AKIN]; and Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease [RIFLE] criteria and others). The third reviewer (G. Kou) was consulted to help establish a consensus in case of any disagreement regarding a study’s eligibility. Surgical techniques and procedures (on-pump or off-pump CABG, cerebral protection devices, and CPB-related techniques) were not evaluated in the current study. The study population of interest comprised patients who had undergone open cardiac surgery; therefore, studies that enrolled patients who had undergone endovascular procedures or who had received percutaneous interventions were excluded.
Data extraction and outcomes
Two investigators (J-JC and TL) independently classified the interventions and used a standardized data abstraction form to extract data related to the study design (randomization and double-blind or non-blinded designs), participant demographics (age and gender), interventions, types of cardiac surgery (CABG, heart valve surgery, or aortic surgery), and definitions of AKI (KDIGO, AKIN, RIFLE, or others). Data related to the primary outcome, namely post–cardiac surgery AKI, were also extracted. Data on the secondary outcomes, namely dialysis-requiring AKI, and AKI-related adverse outcomes [mortality and intensive care unit (ICU) and hospital LOS (in days)], were also extracted.
Data synthesis and analysis
The characteristics of the enrolled participants (mean age, percentage of female participants, and cardiac operation type), definition of AKI, and interventions in the included studies were extracted from the corresponding articles (Supplementary Table 3). We identified and classified 54 interventions. For three-arm studies evaluating different doses of erythropoietin (EPO) (19) or dexmedetomidine (20), four-arm studies evaluating different doses of THR-184 (21) or ABT-719 (α-melanocyte-stimulating hormone analog), and five-arm studies evaluating different durations of RIPC (22), we combined the results from intervention arms with different doses/durations into a single arm. Various synthetic replacement fluids (6% hydroxyethyl starch [HES; 130/0.4], 6% HES [7.2% NaCl, HES 200/0.5], and succinylated gelatin [4%]) were categorized as synthetic colloids. Various chloride-poor crystalloid fluids (e.g., Ringer’s lactate solution, Plasma-Lyte, and Hartmann’s solution) were categorized as balanced fluids. One RCT compared two interventions: chloride-liberal and chloride-restrictive intravenous fluids (23). In the chloride-restrictive fluid arm, multiple colloid and crystalloid fluids were used (HES [130/0.4] in balanced colloid solution [Volulyte] and balanced salt crystalloid solution [PlasmaLyte A]). Therefore, we considered this study as the control arm compared with a chloride-restrictive fluid strategy. Normal saline as a replacement fluid and a chloride-liberal fluid strategy were considered as control arms.
To evaluate the effectiveness of various strategies in preventing AKI, dialysis-requiring AKI, and mortality, we calculated odds ratios (ORs) to pool binary outcomes. To analyze ICU and hospital LOS, we extracted the mean and the standard deviation of hospital and ICU LOS within each intervention arm. For studies that reported ICU and hospital LOS in terms of medians and interquartile ranges, we extracted the relevant data and translated them into means and standard deviations according to the Cochrane guidelines (24). Heterogeneity was examined using I2 values (with a value of ≥ 50% indicating substantial heterogeneity). We used the statistical package netmeta in R (version 4.0.2) to perform a frequentist NMA with a random-effects model (25). All pairwise comparisons were summarized in a league table. The P-score method is used to measure the probability that an intervention is superior to a competing intervention in terms of a particular outcome (26, 27). Each intervention was assigned a P-score ranging from 0 to 1; an effective intervention with a higher P score was determined to be more effective than an intervention with a lower P score. We performed a sensitivity analysis to assess the robustness of our results, in which studies that did not use international AKI criteria (those other than the RIFLE, AKIN, or KDIGO criteria) were excluded. We also performed a sensitivity analysis in which studies with some or high risk of bias were excluded and another sensitivity analysis in which studies with small numbers of participants (<50) were excluded. In addition, we performed a subgroup analysis to explore heterogeneity in the outcomes of interest among patients who underwent (1) heart surgery (CABG or valve surgery or combination of both) and (2) aortic surgery. The subgroup analysis only included studies that used international AKI criteria. Additional subgroup analyses were performed to explore heterogeneity in the outcomes of interest among participants with (1) preserved renal function (serum creatinine ≤ 1.2 mg/dL or an estimated glomerular filtration rate ≥ 60 mL/min/1.73 m2) and (2) impaired renal function (serum creatinine > 1.2 mg/dL or an estimated glomerular filtration rate < 60 mL/min/1.73 m2). Studies without baseline mean or median renal function test data were excluded from this subgroup analysis. A two-tailed p-value of < 0.05 was considered statistically significant.
Risk-of-bias and quality assessments
We used the revised Cochrane Risk-of-Bias tool for randomized trials (RoB 2) (28) to assess the quality of the included RCTs. Any disagreements regarding the risk of bias between the two main investigators (J-JC and TL) were resolved through discussion with the third investigator (G. Kou). Inconsistency of current NMA was evaluated using a design-by-treatment interaction model and node-splitting model (29, 30). Small-study effect bias was examined using a funnel plot, and publication bias was measured using the Egger test. The results of the bias assessment were visualized using a risk-of-bias plot (31). The overall confidence in and quality of the results of NMA were evaluated using the Confidence in NMA (CINeMA) framework (30).
Results
Search results
The flowchart and search strategy used in the literature search are detailed in Supplementary Figure 1 and Supplementary Table 2. We identified 490, 656, and 281 studies from PubMed, Embase, and Medline, respectively, as well as two meta-analyses from the Cochrane Library and 45 completed trials registered on ClinicalTrials.gov. After duplicates and registered trials without available data for the outcomes of interest were removed, 1382 studies met the search criteria and were screened by title or abstract. After screening, the full texts of 210 potentially eligible studies were examined. Moreover, we identified 42 additional eligible studies by screening references in other meta-analyses and review articles. Therefore, we reviewed the full texts of a total of 252 potentially eligible studies. Ultimately, 161 studies were included in our study (19–23, 32–187). Some studies were excluded because the enrolled participants had already developed AKI (n = 5), had already received renal replacement therapy (n = 3), had undergone non-cardiac surgery (n = 5), or were not adults (n = 8). Studies that were not RCTs (n = 6), focused on surgical techniques or procedures (n = 23), or did not report the incidence of AKI-related outcomes or events (n = 28) were also excluded. Eight two-arm studies that could not connect to other studies in the network were also excluded (188–194). One study on the management of postoperative cardiogenic shock that compared vasopressin and norepinephrine, which was identified from a review article (6), was excluded for the same reason (195).
Study characteristics
The 161 studies involved a total of 46,619 participants with a mean age of 66.9 years (range: 43–78.2 years), and 31.7% of the participants (excluding those in one study that did not report baseline demographic characteristics) were female (77). The studies were published between 2000 and 2021 and had sample sizes ranging from 10 to 3647. Of the included studies, 142, 13, and 7 enrolled participants who had undergone pure heart surgery (CABG or cardiac valve surgery), pure aortic surgery (thoracic or abdominal), and a combination of heart and aortic surgery, respectively. A total of 90 studies defined AKI according to international AKI criteria (AKIN, RIFLE, or KDIGO); the remaining 71 studies used other definitions or did not report the definition of AKI or renal failure employed therein. From the 161 RCTs (which comprised 151 two-arm studies, 5 three-arm studies, 4 four-arm studies, and 1 five-arm study), a total of 54 strategies (including controls) were identified: ABT-719 (AP214 acetate), acetaminophen, albumin, amustaline-treated red blood cells, antioxidants (a combination of omega 3 polyunsaturated fatty acids, vitamin C, and vitamin E), aprotinin, autologous platelet-rich plasma, balanced solutions, bicarbonate, chloride-restrictive fluids, calorie-restricted diets, carvedilol, control, curcumin, cyclosporin, dexmedetomidine (low and high doses), dobutamine, dopamine, EA-230, EPO (low and high doses), ethyl pyruvate, fenoldopam, fenoldopam with N-acetyl cysteine (NAC), furosemide, forced diuresis (RenalGuard system), intensive insulin therapy, KDIGO AKI prevention bundles, L-amino acids, levosimendan, mannitol, methylxanthines, minocycline, NAC, NAC with carvedilol, natriuretic peptides, nifedipine, nitroprusside, prophylactic transfusions, restrictive transfusions, rasburicase, RIPC (different durations and preoperative or postoperative), selenium, small interfering RNA (siRNA, teprasiran), spironolactone, statins, steroids, stroke volume variation (SVV)–guided fluid therapy, synthetic colloids, THR-184 (a bone morphogenetic protein-7 agonist), tolvaptan, vitamin C, vitamin D, vitamin E with allopurinol, and volume replacement therapy. The characteristics and study designs of the selected RCTs are summarized in Supplementary Table 3.
Network meta-analysis outcome: Acute kidney injury prevention
Figure 1A presents a network plot of the 52 interventions (nodes), 43,786 participants, and 149 direct comparisons of interventions for preventing post–cardiac surgery AKI from the 141 selected studies. Eight pharmacological interventions (ordered according to the P score: natriuretic peptide [OR: 0.30, 95% CI: 0.19–0.47], nitroprusside [OR: 0.29, 95% CI: 0.12–0.68], fenoldopam [OR: 0.36, 95% CI: 0.17–0.76], tolvaptan [OR: 0.35, 95% CI: 0.14–0.90], NAC with carvedilol [OR: 0.37, 95% CI: 0.16–0.85], dexmedetomidine [OR: 0.49, 95% CI: 0.32–0.76], levosimendan [OR: 0.56, 95% CI: 0.37–0.84], and EPO [OR: 0.62, 95% CI: 0.41–0.94]) and one non-pharmacological intervention (RIPC, OR: 0.76, 95% CI: 0.63–0.92) were associated with a lower incidence of post–cardiac surgery AKI (Figure 1B). One intervention was associated with an increased incidence of AKI (cyclosporin [OR: 4.55, 95% CI: 1.55–13.37]). The results of the NMA and direct pairwise comparisons are summarized in the league table in Supplementary Document 1. Natriuretic peptides were more effective than levosimendan, EPO, and RIPC (Supplementary Document 1). We noted mild heterogeneity among the included studies (I2 = 41.4%).
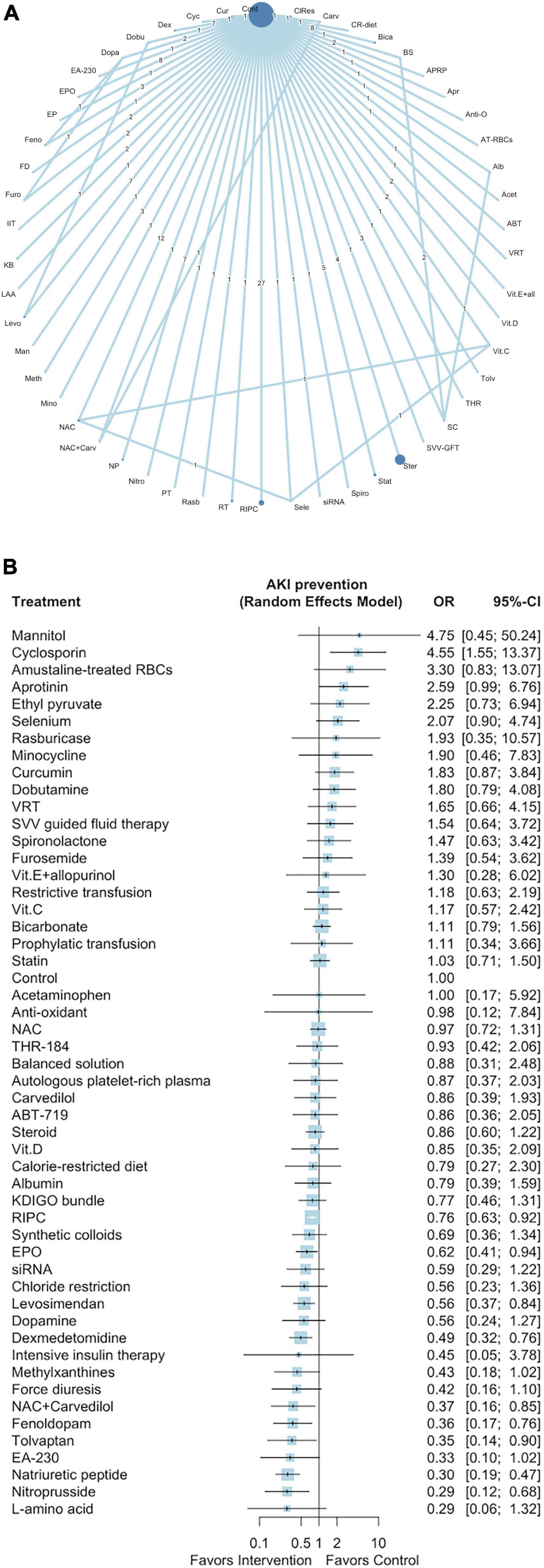
Figure 1. Network plot of eligible comparisons among interventions for AKI prevention (A), Forest plot of network meta-analysis for AKI prevention (B). In the network plot, the size of each node indicates the number of randomized allocated participants. ABT, ABT-719; Acet, acetaminophen; Alb, albumin; AT-RBCs, amustaline-treated RBCs; Anti-O, anti-oxadants; Apr, aprotinin; APRP, autologous platelet-rich plasma; BS, balanced solution; Bica, bicarbonate; CR-diet, calorie-restricted diet; Carv, carvedilol; ClRes, chloride restriction; Cont, control; Cur, curcumin; Cyc, cyclosporin; Dex, dexmedetomidine; Dobu, dobutamine; Dopa, dopamine; EP, ethyl pyruvate; EPO, erythropoietin; Feno, fenoldopam; FD, forced diuresis; Furo, furosemide; IIT, intensive insulin therapy; KB, KDIGO bundle; LAA, L-amino acid; Levo, levosimendan; Man, mannitol; Meth, methylxanthines; Mino, minocycline; NAC, N-acetyl cysteine; NP, natriuretic peptide; Nitro, nitroprusside; PT, prophylactic transfusion; Rasb, rasburicase; RT, restrictive transfusion; RIPC, remote ischemic preconditioning; Sele, selenium; Spiro, spironolactone; Stat, statin; Ster, steroid; SVV-GFT, stroke volume variation guided fluid therapy; SC, synthetic colloids; THR, THR-184; Vit.C, vitamin C; Vit.D, vitamin D; Vit.E + all, vitamin E + allopurinol; VRT, volume replacement therapy.
We used the P-score method to rank the 52 interventions (Supplementary Table 4). The results of the Egger test and the funnel plot revealed small-study bias (Egger test p < 0.01; Supplementary Figure 2). The full design-by-treatment interaction random-effects model revealed inconsistency among the designs of the included studies (Q = 17.47 and p = 0.03). The node-splitting model revealed potential loop inconsistency (Supplementary Figure 3).
Network meta-analysis outcome: Dialysis-requiring acute kidney injury and mortality
Supplementary Figure 4A presents the network plot of the 42 interventions and 31,143 participants from the 119 selected studies that evaluated the effectiveness of the interventions in preventing dialysis-requiring AKI. Three pharmacological interventions (ordered according to the P-score: ABT-719 [OR: 0.22, 95% CI: 0.06–0.87], natriuretic peptides [OR: 0.30, 95% CI: 0.15–0.60], and levosimendan [OR: 0.68, 95% CI: 0.49–0.95]), but no non-pharmacological interventions, were associated with the reduced incidence of post–cardiac surgery dialysis-requiring AKI (Figure 2A and Supplementary Document 2). The P scores of the 42 interventions are listed in Supplementary Table 5. We noted low heterogeneity among the studies (I2 = 0.0%) and no significant publication bias (Egger test p = 0.17; Supplementary Figure 4B).
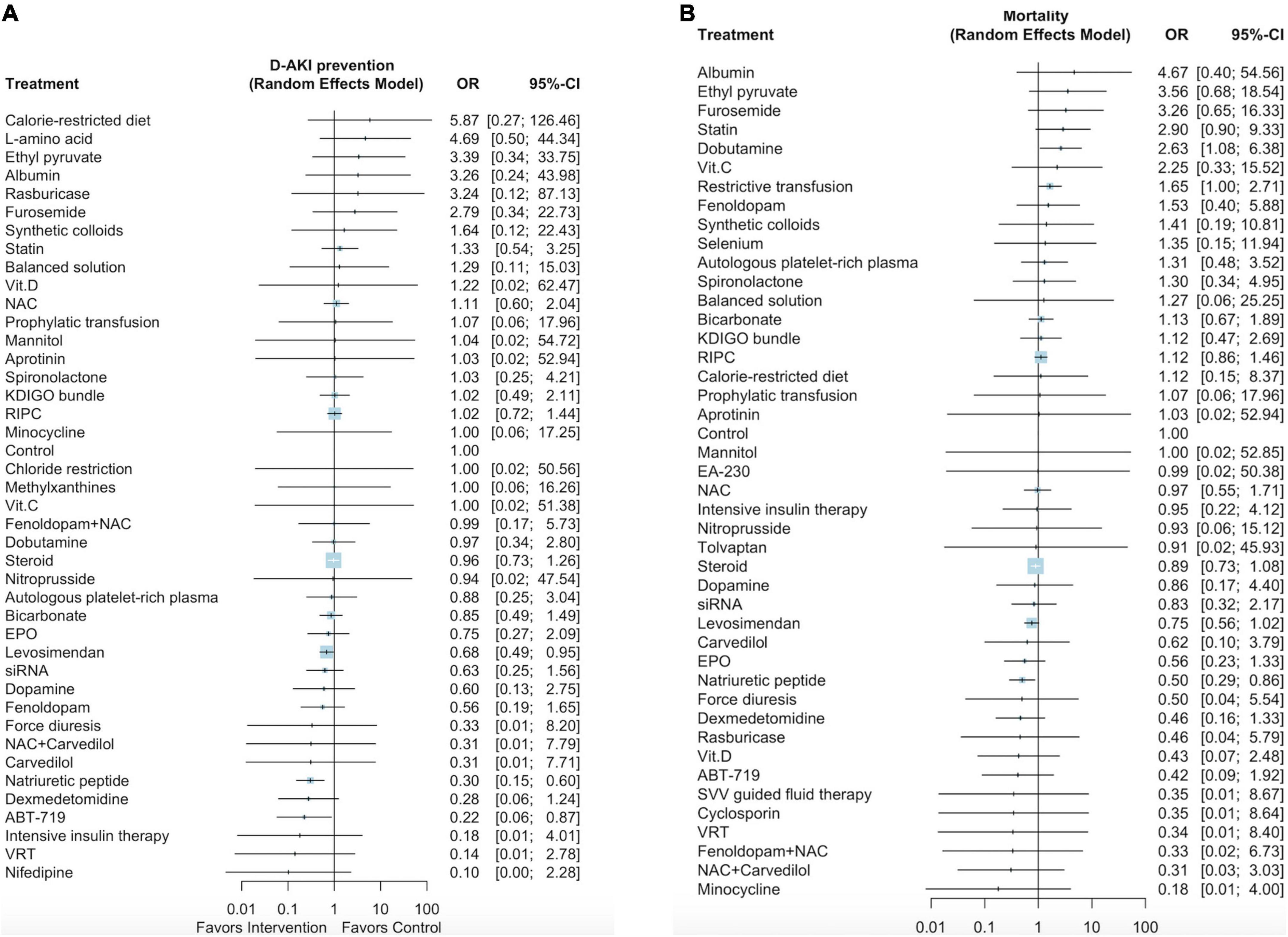
Figure 2. Forest plot of network meta-analysis for dialysis-requiring AKI prevention (A), Forest plot for mortality (B).
Supplementary Figure 5A presents a network plot of the 44 interventions and 42,374 participants from the 126 selected studies that evaluated mortality after cardiac surgery. Natriuretic peptides and dobutamine were associated with lower mortality rates (OR: 0.50, 95% CI: 0.29–0.86; Figure 2B, Supplementary Document 3) and higher mortality rates (OR: 2.63, 95% CI: 1.08–6.38), respectively. The P-scores of the 44 interventions are listed in Supplementary Table 6. We noted low heterogeneity among the studies (I2 = 0.0%) and no significant publication bias (Egger test p = 0.68; Supplementary Figure 5B).
Network meta-analysis outcome: Intensive care unit and hospital length of stay
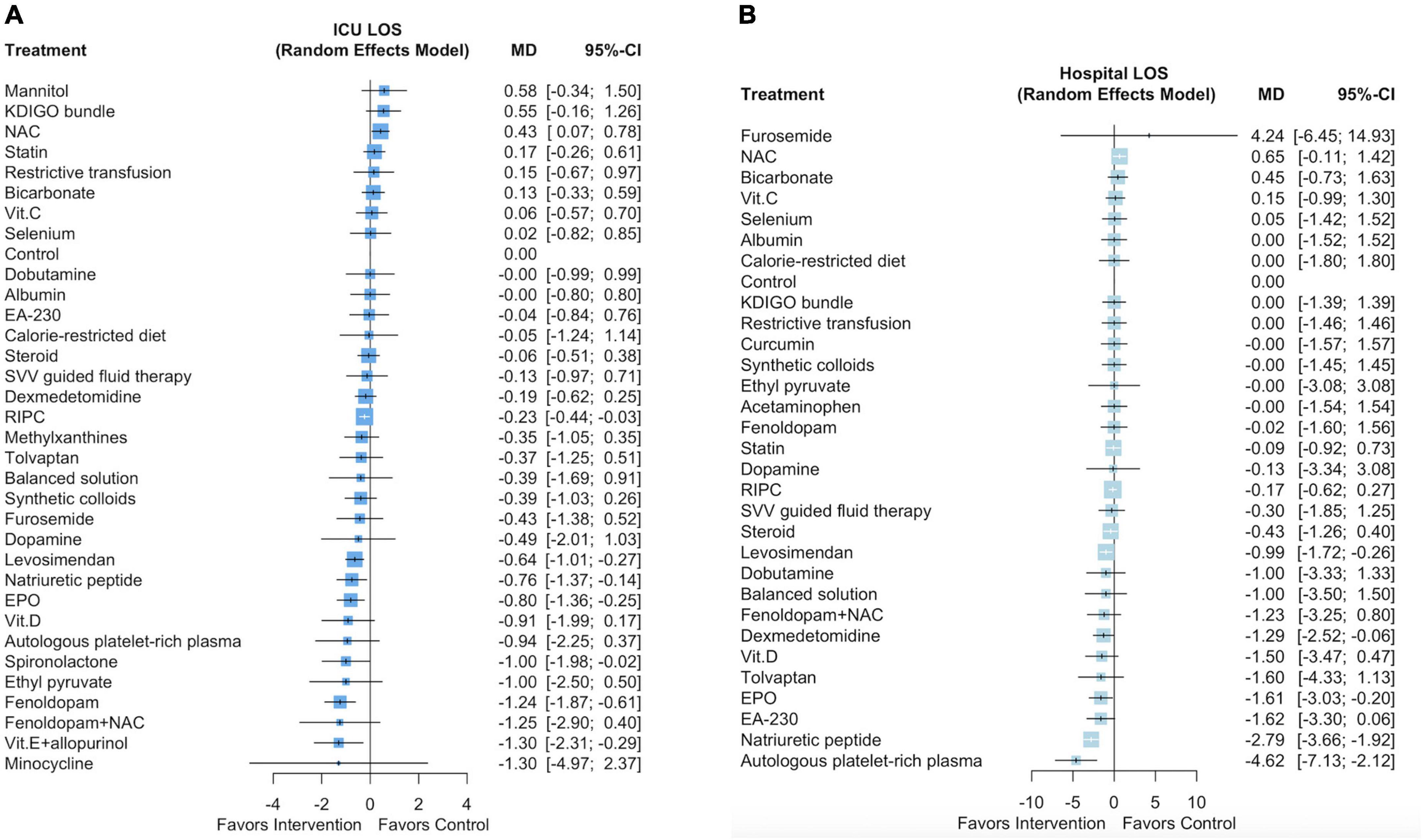
Supplementary Figure 6A presents a network plot of the 34 interventions and 31,785 participants from the 95 selected studies that evaluated ICU LOS. Six pharmacological interventions (ordered according to the P score: fenoldopam [mean difference (MD): –1.24 days, 95% CI: –1.87 to –0.61], vitamin E with allopurinol [MD: –1.3 days, 95% CI: –2.31 to –0.29], spironolactone [MD: –1.0 days, 95% CI: –1.98 to –0.02], EPO [MD: –0.8 days, 95% CI: –1.36 to –0.25], natriuretic peptides [MD: –0.76 days, 95% CI: –1.37 to –0.14], and levosimendan [MD: –0.64 days, 95% CI: –1.01 to –0.27]) and one non-pharmacological intervention (RIPC, MD: –0.23 days, 95% CI: –0.44 to –0.03) were associated with a shorter ICU LOS (Figure 3A and Supplementary Document 4). The P scores of the 34 interventions are listed in Supplementary Table 7. We noted high heterogeneity among the studies (I2 = 86.3%) but no significant publication bias (Egger test p = 0.50; Supplementary Figure 6B).
Supplementary Figure 7A presents a network plot of the 31 interventions and 31,470 participants from the 90 selected studies that evaluated hospital LOS. Five pharmacological interventions (ordered according to the P score: autologous platelet-rich plasma [MD: –4.62 days, 95% CI: –7.13 to –2.12], natriuretic peptides [MD: –2.79 days, 95% CI: –3.66 to –1.92], EPO [MD: –1.61 days, 95% CI: –3.03 to –0.20], dexmedetomidine [MD: –1.29 days, 95% CI: –2.52 to –0.06], and levosimendan [MD: –0.99 days, 95% CI: –1.72 to –0.26]) were associated with a shorter hospital LOS (Figure 3B and Supplementary Document 5). The P scores of the 31 interventions are listed in Supplementary Table 8. We noted substantial heterogeneity among the studies (I2 = 67.6%) but no significant publication bias (Egger test p = 0.99; Supplementary Figure 7B).
Sensitivity analyses
Some of the included studies used non-international AKI criteria (absolute cutoff creatinine levels ranging from 1.5 to 2.0 mg/dL, creatinine elevation of 25–200% of the baseline level, creatinine elevation of > 0.3–0.5 mg/dL from baseline, a decrease in the glomerular filtration rate of > 25% from baseline) or did not report the definition of AKI. We performed a sensitivity analysis excluding these studies to evaluate the robustness of our findings regarding the primary outcome. Supplementary Figure 8A presents a network plot of the 43 interventions and 23,820 participants from the 89 selected studies that evaluated post–cardiac surgery mortality. Four pharmacological interventions (ordered according to the P score: methylxanthines [OR: 0.14, 95% CI: 0.03–0.59], natriuretic peptides [OR: 0.19, 95% CI: 0.08–0.44], tolvaptan [OR: 0.35, 95% CI: 0.13–0.95], and dexmedetomidine [OR: 0.49, 95% CI: 0.31–0.78]) and one non-pharmacological intervention (RIPC, OR: 0.78, 95% CI: 0.62–0.98) were associated with a lower incidence of post–cardiac surgery AKI (Supplementary Figure 8B). We noted substantial heterogeneity among the studies (I2 = 45.8%) but no significant publication bias (Egger test p = 0.63; Supplementary Figure 8C).
We performed an additional sensitivity analysis in which studies with some or high risk of bias were excluded. Supplementary Figure 9A presents a network plot of the 23 interventions and 13,923 participants from the 52 selected studies. Only two pharmacological interventions (ordered according to the P score: natriuretic peptides [OR: 0.23, 95% CI: 0.06–0.84] and dexmedetomidine [OR: 0.43, 95% CI: 0.27–0.71]) were associated with a lower incidence of post–cardiac surgery AKI (Supplementary Figure 9B). We noted substantial heterogeneity among the studies (I2 = 45.9%) but no significant publication bias (Egger test p = 0.15; Supplementary Figure 9C).
Our final sensitivity analysis excluded studies with small numbers of participants (n < 50). Supplementary Figure 10A presents a network plot of the 43,049 participants and 49 interventions from the 123 selected studies. Eight pharmacological interventions (ordered according to the P score: nitroprusside [OR: 0.29, 95% CI: 0.12–0.68], natriuretic peptides [OR: 0.33, 95% CI: 0.20–0.56], fenoldopam [OR: 0.36, 95% CI: 0.17–0.76], tolvaptan [OR: 0.35, 95% CI: 0.14–0.89], NAC with carvedilol [OR: 0.37, 95% CI: 0.16–0.85], dexmedetomidine [OR: 0.44, 95% CI: 0.28–0.70], levosimendan [OR: 0.61, 95% CI: 0.40–0.95], and EPO [OR: 0.62, 95% CI: 0.41–0.94]) and one non-pharmacological intervention (RIPC, OR: 0.78, 95% CI: 0.65–0.95) were associated with a lower incidence of post–cardiac surgery AKI (Supplementary Figure 10B). We noted substantial heterogeneity among the studies (I2 = 43.8%) and possible publication bias (Egger test p < 0.01; Supplementary Figure 10C).
Subgroup analysis: Heart surgery versus aortic surgery
We performed a subgroup analysis including only the 78 studies that enrolled participants who had undergone heart surgery (valve surgery, CABG, or both), which involved a total of 41 interventions and 22,437 participants (Supplementary Figure 11A). Three pharmacological interventions (ordered according to the P score: methylxanthines [OR: 0.14, 95% CI: 0.03–0.60], natriuretic peptides [OR: 0.15, 95% CI: 0.03–0.91], and tolvaptan [OR: 0.35, 95% CI: 0.13–0.96]) and one non-pharmacological intervention (RIPC, OR: 0.77, 95% CI: 0.61–0.98) were associated with a lower incidence of AKI among the participants who had undergone heart surgery (Figure 4A). We noted substantial heterogeneity among the studies (I2 = 50.0%) but no significant publication bias (Egger test p = 0.55; Supplementary Figure 11B).
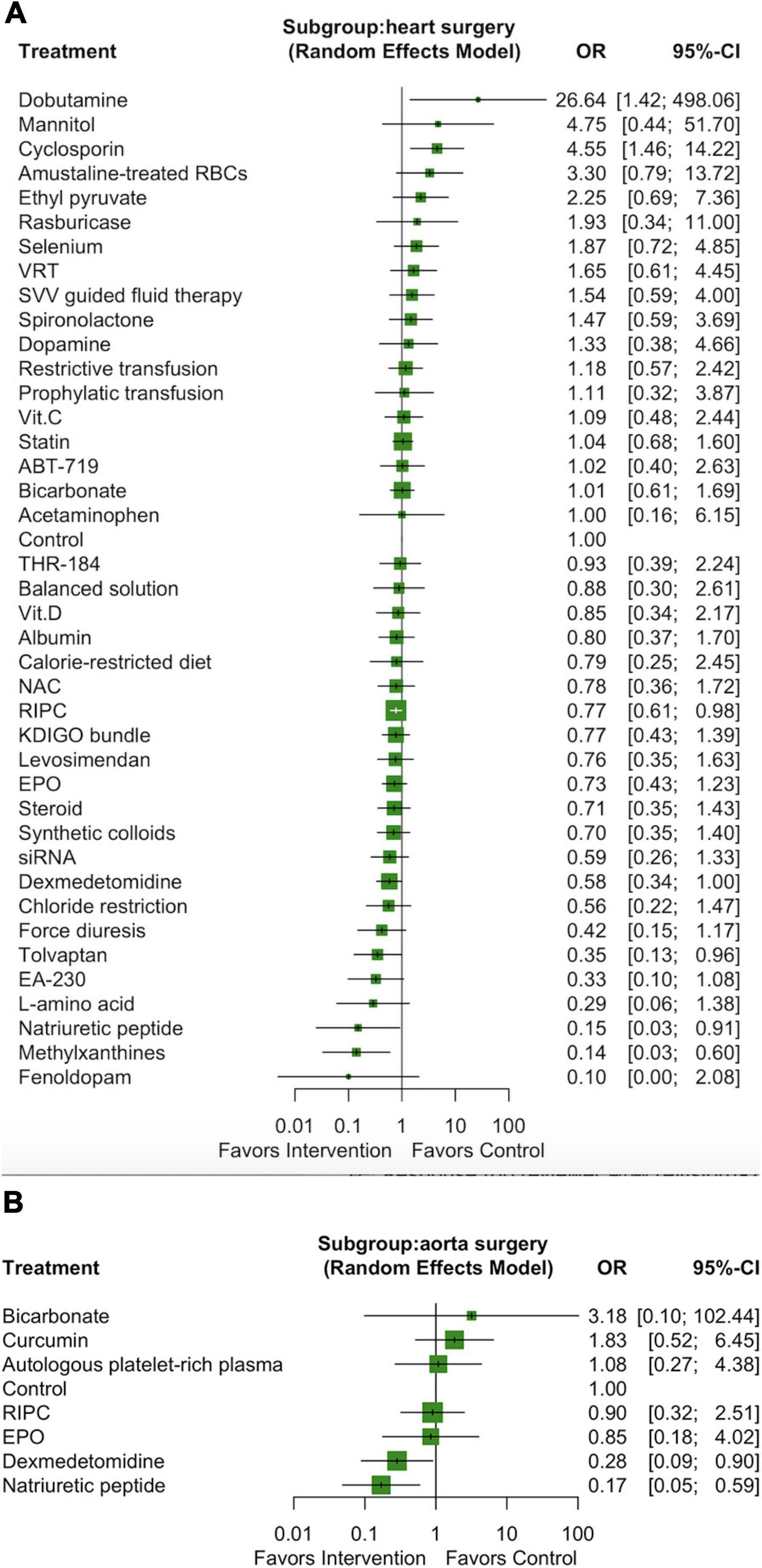
Figure 4. Forest plot of network meta-analysis of subgroup analysis: heart surgery (A) and aorta surgery (B).
We performed another subgroup analysis including only the 10 studies that enrolled participants who had undergone aortic surgery, which involved a total of 8 interventions and 1354 participants (Supplementary Figure 12A). Only two pharmacological interventions (ordered according to the P score: natriuretic peptides [OR: 0.17, 95% CI: 0.05–0.59] and dexmedetomidine [OR: 0.28, 95% CI: 0.09–0.90]) were associated with a lower incidence of AKI among the participants who had undergone aortic surgery (Figure 4B). We noted substantial heterogeneity among the studies (I2 = 53.5%) but no significant publication bias (Egger test p = 0.68; Supplementary Figure 12B).
Subgroup analysis: Preserved versus impaired baseline renal function
We performed a subgroup analysis including only the 89 studies that enrolled participants with preserved renal function (serum creatinine ≤ 1.2 mg/dL or an estimated glomerular filtration rate of ≥ 6), which involved a total of 41 interventions and 33,787 participants (Supplementary Figure 13A). Five pharmacological interventions (ordered according to the P score: natriuretic peptides [OR: 0.30, 95% CI: 0.19–0.49], nitroprusside [OR: 0.29, 95% CI: 0.13–0.67], dexmedetomidine [OR: 0.31, 95% CI: 0.15–0.65], tolvaptan [OR: 0.35, 95% CI: 0.14–0.88], and NAC with carvedilol [OR: 0.37, 95% CI: 0.16–0.84]) and one non-pharmacological intervention (RIPC, OR: 0.76, 95% CI: 0.60–0.96) were associated with a lower incidence of post–cardiac surgery AKI in participants with preserved renal function (Supplementary Figure 13B). We noted substantial heterogeneity among the studies (I2 = 42.9%) and possible publication bias (Egger test p = 0.02; Supplementary Figure 13C).
We performed another subgroup analysis including only the 23 studies that enrolled participants with impaired renal function, which involved a total of 15 interventions and 2669 participants (Supplementary Figure 14A). Only one pharmacological intervention (fenoldopam [OR: 0.26, 95% CI: 0.09–0.70]) was associated with a lower rate of AKI among the participants with impaired renal function (Supplementary Figure 14B). We noted substantial heterogeneity among the studies (I2 = 40.5%) but no significant publication bias (Egger test p = 0.48; Supplementary Figure 14C).
Assessing risk of bias, ranking of probabilities, and confidence in the network meta-analysis
The results of the risk-of-bias assessment are presented in Supplementary Figure 15 and Supplementary Table 9. Of the included studies, 63.6, 29.6, and 6.8% had low, moderate, and high risk of bias, respectively (Supplementary Document 6).
Information on the potentially effective interventions, their rankings by the P score of potentially effective interventions, their effects on primary and secondary outcomes, and confidence in the evidence judged by GRADE framework, is summarized in Table 1.
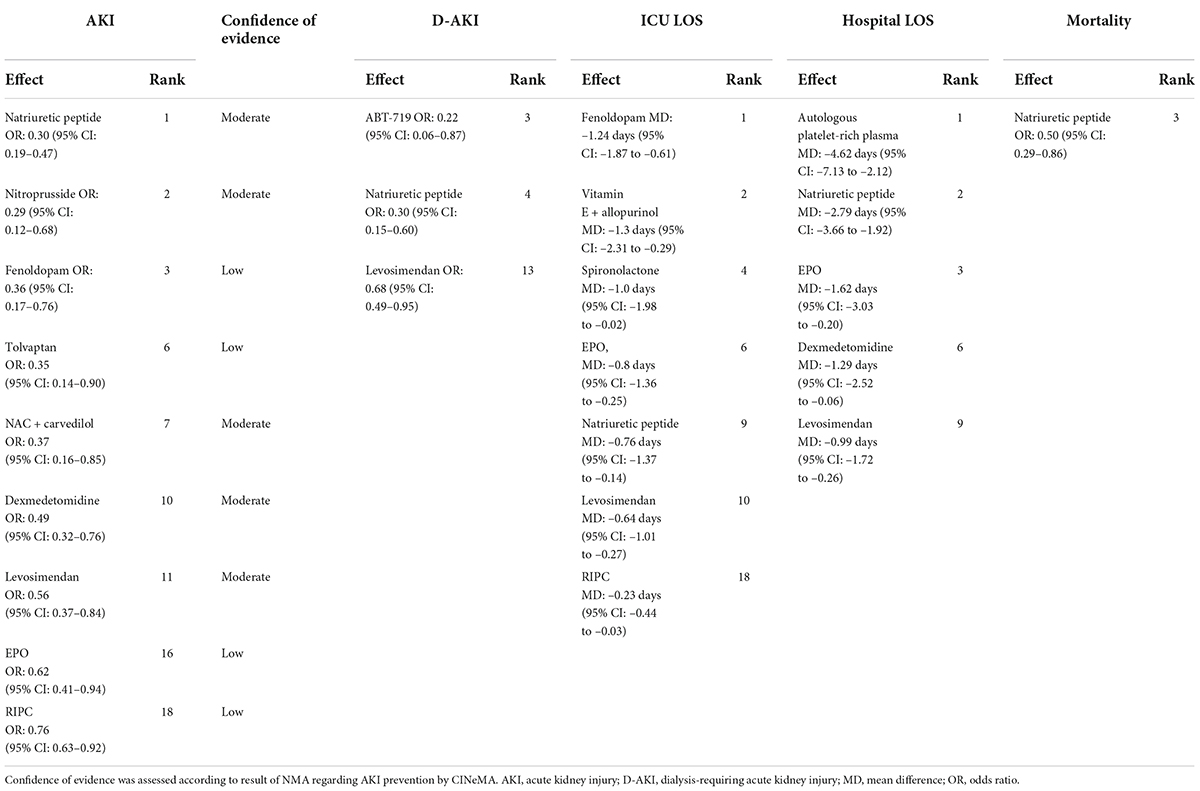
Table 1. Summary of potential effective strategies for primary, secondary outcome and rank probability.
We assessed the degree of confidence in the evidence supporting our findings by using the CINeMA framework. We assessed the confidence of the evidence supporting the effectiveness of each intervention relative to the control group. The results are summarized in Supplementary Table 10. Of the nine strategies determined to be potentially effective in preventing post–cardiac surgery AKI, five (natriuretic peptides, nitroprusside, levosimendan, NAC with carvedilol, and dexmedetomidine) and four (fenoldopam, RIPC, EPO, and tolvaptan) were supported by moderate-confidence and low-confidence evidence, respectively. Detailed descriptions of the bias and confidence assessments are provided in Supplementary Document 7. The trials from ClinicalTrials.gov that were relevant and completed but had no available data for the outcomes of interest are summarized in Supplementary Table 11. We summarized our findings (effect sizes and rankings) regarding the relative effectiveness of the 52 interventions in preventing AKI into findings table (Figure 5), which also includes data regarding confidence in the evidence supporting these interventions (196).
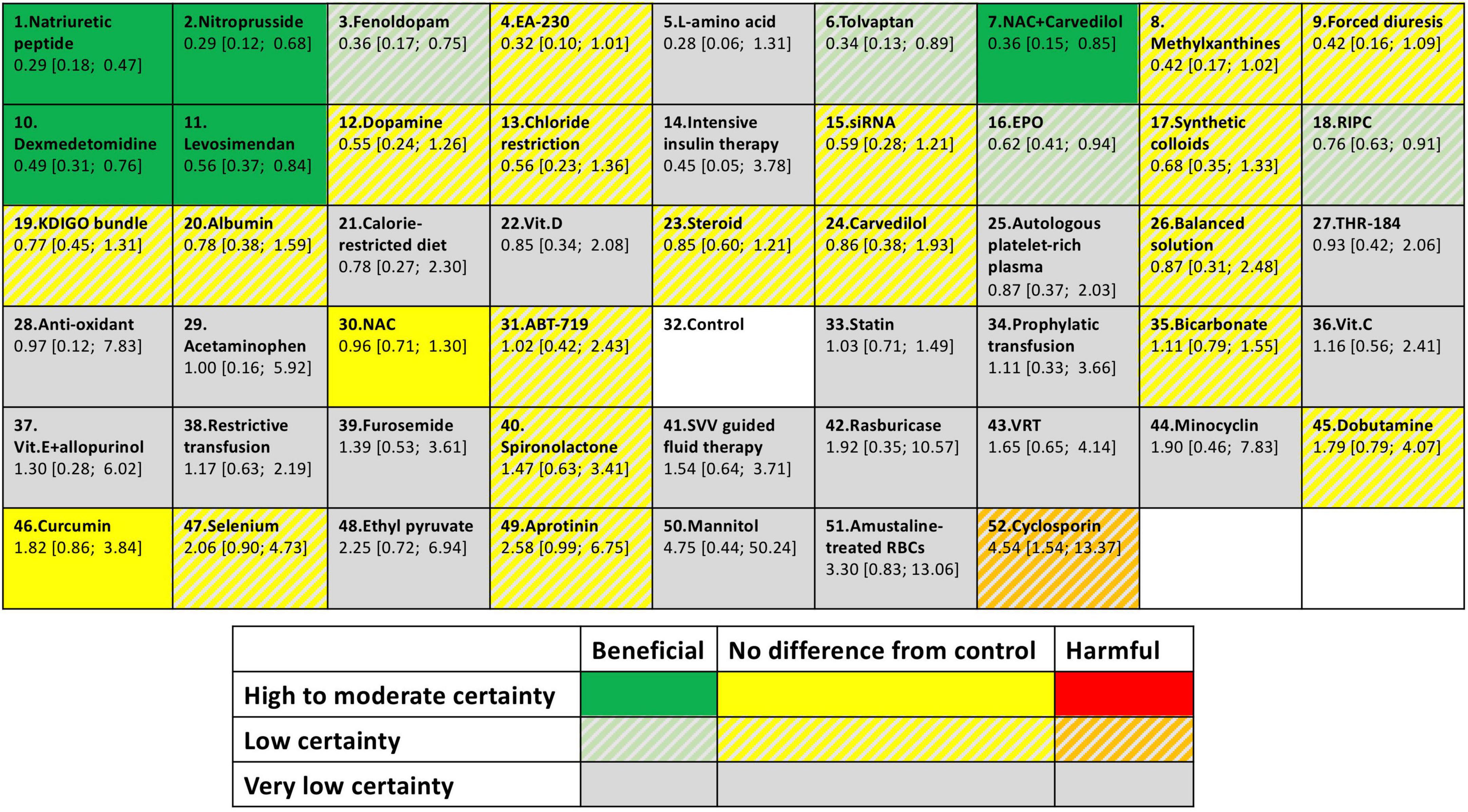
Figure 5. Summary of finding table for AKI prevention. The colors represent in which category of effectiveness and the certainty of evidence of each intervention. Different interventions were ordered according to P-score. Each box provides rank probability and relative estimate effect for AKI prevention (in comparison with the control group).
Discussion
Discussion
The present study obtained several findings. First, natriuretic peptide therapy was identified to be the most effective strategy for preventing post–cardiac surgery AKI and may be associated with a lower incidence of AKI-related adverse outcomes (dialysis-requiring AKI, extended hospital or ICU LOS, and mortality). Second, RIPC was the most effective non-pharmacological strategy and was associated with a shorter ICU LOS. Third, the results of the sensitivity analysis supported the effectiveness of natriuretic peptides, tolvaptan, dexmedetomidine, and RIPC. After studies with some or high risk of bias were excluded, only natriuretic peptides and dexmedetomidine were identified as effective strategies. Fourth, in the patients who had undergone heart surgery, natriuretic peptides, tolvaptan, and RIPC were effective AKI prevention strategies, whereas in the patients who had undergone aortic surgery, only natriuretic peptides and dexmedetomidine were effective. Fifth, dobutamine and cyclosporine were associated with poor outcomes (higher risks of mortality and AKI, respectively).
In the present study, we demonstrated that natriuretic peptide therapy was the most effective pharmacological intervention for preventing post–cardiac surgery AKI, which is consistent with the results of a previous study. (18) Our study also revealed that natriuretic peptides may be effective for preventing post–cardiac surgery adverse outcomes (dialysis-requiring AKI, hospital and ICU LOSs, and mortality). In addition to their natriuretic effects, natriuretic peptides act as renal vasodilators, antagonists to the renin–angiotensin system, and anti-inflammatory and podocyte-protective agents with potential renoprotective effects (197–199). Eleven of the included studies evaluated the efficacy of natriuretic peptides in preventing post–cardiac surgery AKI (42, 68, 118, 122, 123, 149, 152). However, the doses of natriuretic peptides and natriuretic agents (nesiritide: 0.01 μg/kg/min for 2 or 5 days; human atrial natriuretic peptide: 0.02 μg/kg/min before cardiopulmonary bypass and then 0.01 μg/kg/min for 12 h) varied among these studies. Eleven of the included studies (42, 68, 118, 122, 146–150, 152, 153) compared mortality rates among patients who received natriuretic peptides and controls, and two of these reported that natriuretic peptides are beneficial for survival; (118, 153) however, neither of these studies adopted a AKI definition based on standard criteria, and both had high risk of bias (118, 153). Therefore, these findings should be interpreted with caution. In addition, natriuretic peptides did not reduce the need for dialysis among patients with established AKI (200). Additional trials are required to determine the optimal dose, timing, duration, and cost-effectiveness of natriuretic peptide therapy for preventing post–cardiac surgery AKI.
In the present study, RIPC was associated with a lower incidence of post–cardiac surgery AKI and a shorter ICU LOS. To protect against lethal cardiac and renal ischemia events, remote organs were subjected to brief periods of ischemia, which was achieved using a cuff placed around an upper or lower limb at a pressure of 200–300 mmHg for 2–5 min in 2–5 cycles (4, 201). Iatrogenic intermittent hypoxia activates hypoxia-inducible factor 1, Nrf2 transcription factor, and the anti-inflammatory phenotype, which may exert cardio–renoprotective and neuroprotective effects (201–204). Three of the included studies applied not only preoperative RIPC but also postoperative RIPC (83, 84, 93). Deferrari et al. performed a pairwise meta-analysis of 13 studies published before 2016 and reported that RIPC significantly reduced the incidence of post–cardiac surgery AKI only in a subgroup of patients who received volatile anesthetics (16). In the present study, by analyzing 27 studies that evaluated the protective effects of RIPC, we determined that RIPC was effective but exhibited low potency for preventing post–cardiac surgery AKI. Because RIPC is a non-invasive and low-cost treatment strategy without clinically significant known side effects, it may be incorporated into routine clinical practice.
Two agents (tolvaptan and dexmedetomidine) were determined to protect against post–cardiac surgery AKI in the primary analysis and sensitivity analysis. Dexmedetomidine was also associated with a shorter hospital LOS. Dexmedetomidine, a selective α2-adrenergic receptor agonist, increases renal blood flow and reduces inflammation (4, 205, 206). It also has neuroprotective effects and can prevent postoperative delirium (207, 208). The most commonly used dexmedetomidine prescription protocol in the enrolled studies was 0.4 μg/kg/h after induction with tapering to 0.1 μg/kg/h after extubation for 24 h (55, 151, 161). Only one of the included studies evaluated the effectiveness of tolvaptan. Kishimoto et al. reported that oral tolvaptan started on postoperative day 1 with adequate diuresis could restore fluid balance more rapidly and was associated with a lower risk of AKI (98).
Several other pharmacological interventions, including fenoldopam, levosimendan, and EPO, might be associated with a lower risk of post–cardiac surgery AKI according to our NMA and a previous study (18). Fenoldopam is a renal vasodilator and can increase renal perfusion (12), and levosimendan is an inotrope and vasodilator that can increase renal perfusion (12, 209). However, seven of the eight studies on levosimendan and three of four studies on fenoldopam did not use international AKI criteria. Within-study bias was also a concern. In the current study, fenoldopam, levosimendan, and EPO were not associated with a lower risk of AKI in the sensitivity analysis. Methylxanthines were identified as a potentially effective intervention in the sensitivity analysis and subgroup analysis. However, only three studies compared methylxanthines with a control. Two of these three studies did not use international AKI criteria, and in the remaining study, the incidence of AKI in the control group was significantly higher than that in the intervention group (23.6% vs. 4.2%) (155). According to RoB 2, this study had some risk of bias. Therefore, our findings regarding the efficacy of methylxanthines in the subgroup analysis and sensitivity analysis should be interpreted carefully.
Other non-pharmacological interventions, including the KDIGO AKI prevention bundle, chloride-restrictive fluids, and intensive sugar control, were ineffective in preventing post–cardiac surgery AKI according to our NMA. Notably, the ADQI guidelines suggest the avoidance of glucose variability but not intensive sugar control (6). Cohort studies demonstrated that compared with intensive sugar control, moderate sugar control is associated with lower mortality rates (210), and that high sugar levels (intraoperative glucose concentrations > 150 mg/dL) might be associated with a higher risk of AKI (211). The GLUCO-CABG trial compared intensive sugar control (100–140 mg/dL) with regular sugar control (141–180 mg/dL) and revealed no significant difference in perioperative complications between the two strategies (212). Two of the studies included in our analysis defined the intensive sugar control level as 80–110 and 100–120 mg/dL, respectively (121, 178). Because the results of our NMA revealed that intensive sugar control is ineffective in preventing post–cardiac surgery AKI and is potentially associated with a higher risk of hypoglycemia and mortality, we argue that intensive sugar control should be applied with caution. Other non-pharmacological interventions, namely the KDIGO AKI prevention bundle and chloride-restrictive fluids, are low-cost and are not associated with any clinically significant side effects. Therefore, although they can be safely applied in clinical settings, additional studies are required to further evaluate their efficacy.
Strengths and limitations
Our study has several strengths. First, we assessed the effectiveness of both pharmacological and non-pharmacological interventions in preventing post–cardiac surgery AKI. Second, we conducted a sensitivity analysis to examine the robustness of our findings because many early studies did not use international AKI criteria or did not report the definition of AKI employed and because some of the studies had a risk of bias. Third, we examined other important outcomes related to post–cardiac surgery AKI, namely dialysis-requiring AKI, mortality, and ICU and hospital LOS. Fourth, we assessed the confidence in the results of our NMA by using the CINeMA framework.
Our study also has several limitations. First, we did not analyze the effectiveness of interventions with different dosages or protocols separately. Second, we did not analyze the effectiveness of surgical procedures and CPB-related techniques (on-pump and off-pump CABG, pulsatile CPB, different anticoagulation agents for CPB, and cerebral protection during CPB). Third, we did not analyze the potential renoprotective effect of volatile anesthetics and we did not compare the effectiveness of vasopressin and norepinephrine in preventing postoperative vasoplegia. Bonanni et al. reported that compared with propofol, volatile anesthetics are associated with lower mortality rates among patients who had undergone cardiac surgery (213). The effect of different anesthetics warrant further examination. Fourth, most of the included studies were two-arm studies that compared the effectiveness of a single intervention with a control, and most of the comparisons were drawn on the basis of indirect evidence. Moreover, confidence in the evidence supporting most of the potentially effective strategies was moderate to low (according to the CiNeMA framework).
Conclusion
According to the results of our NMA, we identified nine interventions that are potentially effective in preventing post–cardiac surgery AKI. Among them, natriuretic peptides were the most effective and were determined to be associated with a lower incidence of AKI-related adverse outcomes. RIPC was the only effective non-pharmacological intervention and was associated with a shorter ICU LOS. All nine strategies were supported by only moderate- to low-confidence evidence, and among them, only natriuretic peptides and dexmedetomidine were identified as effective in the sensitivity analysis. Our subgroup analysis revealed heterogeneity in the outcomes between patients who underwent heart (CABG, heart valve surgery) and aortic surgery. The effectiveness of the interventions examined in this study, as well as the optimal dosages and protocols for and the cost-effectiveness of such interventions, must be further explored in additional RCTs in the future.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
J-JC and TL: methodology and writing and writing—original draft preparation. J-JC, GK, and Y-TH: formal analysis. J-JC, TL, and GK: data extraction. C-CL, J-JC, and TL: table and figures formation. P-RC, S-WC, H-YY, H-HH, C-HY, and C-CH: writing—review and editing. Y-CC and C-HC: project administration. All authors have read and approved the final manuscript.
Funding
This study was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG5K0141), and C-HC was supported by the Ministry of Science and Technology (109-2314-B-182A-124).
Acknowledgments
The authors would like to thank the Institute of Epidemiology & Preventive Medicine, College of Public Health, National Taiwan University for its statistical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.960581/full#supplementary-material
References
1. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
2. Hobson C, Singhania G, Bihorac A. Acute kidney injury in the surgical patient. Crit Care Clin. (2015) 31:705–23. doi: 10.1016/j.ccc.2015.06.007
3. Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. (2016) 67:872–80. doi: 10.1053/j.ajkd.2015.07.022
4. Gumbert SD, Kork F, Jackson ML, Vanga N, Ghebremichael SJ, Wang CY, et al. Perioperative acute kidney injury. Anesthesiology. (2020) 132:180–204. doi: 10.1097/ALN.0000000000002968
5. Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and acute kidney injury network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. (2011) 15:R16. doi: 10.1186/cc9960
6. Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th international consensus conference of the ADQI (Acute Disease Quality Initiative) group. J Am Heart Assoc. (2018) 7:e008834. doi: 10.1161/JAHA.118.008834
7. James MT, Bhatt M, Pannu N, Tonelli M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat Rev Nephrol. (2020) 16:193–205. doi: 10.1038/s41581-019-0247-z
8. Chen JJ, Chang CH, Wu VC, Chang SH, Hung KC, Chu PH, et al. Long-term outcomes of acute kidney injury after different types of cardiac surgeries: a population-based study. J Am Heart Assoc. (2021) 10:e019718. doi: 10.1161/JAHA.120.019718
9. Hu L, Gao L, Zhang D, Hou Y, He LL, Zhang H, et al. The incidence, risk factors and outcomes of acute kidney injury in critically ill patients undergoing emergency surgery: a prospective observational study. BMC Nephrol. (2022) 23:42. doi: 10.1186/s12882-022-02675-0
10. Massoth C, Zarbock A. Diagnosis of cardiac surgery-associated acute kidney injury. J Clin Med. (2021) 10:3664. doi: 10.3390/jcm10163664
11. Greenberg JH, Whitlock R, Zhang WR, Thiessen-Philbrook HR, Zappitelli M, Devarajan P, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. (2015) 30:1519–27. doi: 10.1007/s00467-015-3088-4
12. O’Neal JB, Shaw AD, Billings FTT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. (2016) 20:187. doi: 10.1186/s13054-016-1352-z
13. Billings FTT, Ball SK, Roberts LJ II, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. (2011) 50:1480–7. doi: 10.1016/j.freeradbiomed.2011.02.011
14. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. (2017) 13:697–711. doi: 10.1038/nrneph.2017.119
15. Pierce B, Bole I, Patel V, Brown DL. Clinical outcomes of remote ischemic preconditioning prior to cardiac surgery: a meta-analysis of randomized controlled trials. J Am Heart Assoc. (2017) 6:e004666. doi: 10.1161/JAHA.116.004666
16. Deferrari G, Bonanni A, Bruschi M, Alicino C, Signori A. Remote ischaemic preconditioning for renal and cardiac protection in adult patients undergoing cardiac surgery with cardiopulmonary bypass: systematic review and meta-analysis of randomized controlled trials. Nephrol Dial Transplant. (2018) 33:813–24. doi: 10.1093/ndt/gfx210
17. Liu Z, Zhao Y, Lei M, Zhao G, Li D, Sun R, et al. Remote ischemic preconditioning to prevent acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. (2021) 8:601470. doi: 10.3389/fcvm.2021.601470
18. Chen X, Huang T, Cao X, Xu G. Comparative efficacy of drugs for preventing acute kidney injury after cardiac surgery: a network meta-analysis. Am J Cardiovasc Drugs. (2018) 18:49–58. doi: 10.1007/s40256-017-0245-0
19. de Seigneux S, Ponte B, Weiss L, Pugin J, Romand JA, Martin PY, et al. Epoetin administrated after cardiac surgery: effects on renal function and inflammation in a randomized controlled study. BMC Nephrol. (2012) 13:132. doi: 10.1186/1471-2369-13-132
20. Balkanay OO, Goksedef D, Omeroglu SN, Ipek G. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. (2015) 20:209–14. doi: 10.1093/icvts/ivu367
21. Himmelfarb J, Chertow GM, McCullough PA, Mesana T, Shaw AD, Sundt TM, et al. Perioperative THR-184 and AKI after cardiac surgery. J Am Soc Nephrol. (2018) 29:670–9. doi: 10.1681/ASN.2017020217
22. McCullough PA, Bennett-Guerrero E, Chawla LS, Beaver T, Mehta RL, Molitoris BA, et al. ABT-719 for the prevention of acute kidney injury in patients undergoing high-risk cardiac surgery: a randomized phase 2b clinical trial. J Am Heart Assoc. (2016) 5:e003549. doi: 10.1161/JAHA.116.003549
23. Bhaskaran K, Arumugam G, Vinay Kumar PV. A prospective, randomized, comparison study on effect of perioperative use of chloride liberal intravenous fluids versus chloride restricted intravenous fluids on postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass grafting surgeries. Ann Card Anaesth. (2018) 21:413–8. doi: 10.4103/aca.ACA_230_17
24. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. London: Cochrane (2022).
25. R Core Team R: A Language and Environment for Statistical Computing [R, version 4.0.2]. Vienna: R Foundation for Statistical Computing (2022).
26. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. (2015) 15:58. doi: 10.1186/s12874-015-0060-8
27. Mavridis D, Porcher R, Nikolakopoulou A, Salanti G, Ravaud P. Extensions of the probabilistic ranking metrics of competing treatments in network meta-analysis to reflect clinically important relative differences on many outcomes. Biom J. (2020) 62:375–85. doi: 10.1002/bimj.201900026
28. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
29. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3:98–110. doi: 10.1002/jrsm.1044
30. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. (2020) 17:e1003082. doi: 10.1371/journal.pmed.1003082
31. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2020) 12:55–61. doi: 10.1002/jrsm.1411
32. Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. (2008) 155:1143–9. doi: 10.1016/j.ahj.2008.01.013
33. Aldemir M, Koca HB, Dogan Baki E, Carsanba G, Ozturk Kavrut N, Kavakli AS, et al. Effects of N-acetyl cysteine on renal functions evaluated by blood neutrophil gelatinase-associated lipocalin levels in geriatric patients undergoing coronary artery bypass grafting. Anatol J Cardiol. (2016) 16:504–11. doi: 10.5152/AnatolJCardiol.2015.6287
34. Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. (2007) 116:I98–105. doi: 10.1161/circulationaha.106.679167
35. Amini S, Robabi HN, Tashnizi MA, Vakili V. Selenium, vitamin C and N-acetylcysteine do not reduce the risk of acute kidney injury after off-pump CABG: a randomized clinical trial. Braz J Cardiovasc Surg. (2018) 33:129–34. doi: 10.21470/1678-9741-2017-0071
36. Antonic M. Effect of ascorbic acid on postoperative acute kidney injury in coronary artery bypass graft patients: a pilot study. Heart Surg Forum. (2017) 20:E214–8. doi: 10.1532/hsf.1811
37. Bagheri S, Shahbazi S, Shafa M, Borhani-Haghighi A, Kiani M, Sagheb MM. The effect of remote ischemic preconditioning on the incidence of acute kidney injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Iran J Med Sci. (2018) 43:587–95.
38. Barba-Navarro R, Tapia-Silva M, Garza-Garcia C, Lopez-Giacoman S, Melgoza-Toral I, Vazquez-Rangel A, et al. The effect of spironolactone on acute kidney injury after cardiac surgery: a randomized, placebo-controlled trial. Am J Kidney Dis. (2017) 69:192–9. doi: 10.1053/j.ajkd.2016.06.013
39. Barkhordari K, Karimi A, Shafiee A, Soltaninia H, Khatami MR, Abbasi K, et al. Effect of pentoxifylline on preventing acute kidney injury after cardiac surgery by measuring urinary neutrophil gelatinase – associated lipocalin. J Cardiothorac Surg. (2011) 6:8. doi: 10.1186/1749-8090-6-8
40. Barr LF, Kolodner K. N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit Care Med. (2008) 36:1427–35. doi: 10.1097/CCM.0b013e31816f48ba
41. Baysal A, Yanartas M, Dogukan M, Gundogus N, Kocak T, Koksal C. Levosimendan improves renal outcome in cardiac surgery: a randomized trial. J Cardiothorac Vasc Anesth. (2014) 28:586–94. doi: 10.1053/j.jvca.2013.09.004
42. Beaver T, Cobb J, Koratala A, Alquadan K, Ejaz A. Nesiritide modulates inflammatory response during cardiac surgery: a pilot study. Res Cardiovasc Med. (2018) 7:137–43. doi: 10.4103/rcm.rcm_15_18
43. Bennett-Guerrero E, Swaminathan M, Grigore AM, Roach GW, Aberle LG, Johnston JM, et al. A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. (2009) 23:324–9. doi: 10.1053/j.jvca.2008.08.005
44. Billings FTT, Petracek MR, Roberts LJ II, Pretorius M. Perioperative intravenous acetaminophen attenuates lipid peroxidation in adults undergoing cardiopulmonary bypass: a randomized clinical trial. PLoS One. (2015) 10:e0117625. doi: 10.1371/journal.pone.0117625
45. Billings FTT, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, et al. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. (2016) 315:877–88. doi: 10.1001/jama.2016.0548
46. Bove T, Landoni G, Calabro MG, Aletti G, Marino G, Cerchierini E, et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: a prospective, double-blind, randomized clinical trial. Circulation. (2005) 111:3230–5. doi: 10.1161/CIRCULATIONAHA.104.509141
47. Brixner V, Kiessling AH, Madlener K, Muller MM, Leibacher J, Dombos S, et al. Red blood cells treated with the amustaline (S-303) pathogen reduction system: a transfusion study in cardiac surgery. Transfusion. (2018) 58:905–16. doi: 10.1111/trf.14528
48. Brulotte V, Leblond FA, Elkouri S, Therasse E, Pichette V, Beaulieu P. Bicarbonates for the prevention of postoperative renal failure in endovascular aortic aneurysm repair: a randomized pilot trial. Anesthesiol Res Pract. (2013) 2013:467326. doi: 10.1155/2013/467326
49. Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, et al. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing cabg surgery: a randomized controlled trial. JAMA. (2005) 294:342–50. doi: 10.1001/jama.294.3.342
50. Caimmi PP, Pagani L, Micalizzi E, Fiume C, Guani S, Bernardi M, et al. Fenoldopam for renal protection in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. (2003) 17:491–4. doi: 10.1016/S1053-0770(03)00155-1
51. Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. (2015) 101:185–92. doi: 10.1136/heartjnl-2014-306178
52. Cao Z, Shen R, Zhang X, Cheng G, Yan Z. Effects of remote ischemic preconditioning on acute myocardial injury in patients undergoing valve replacement. Ir J Med Sci. (2017) 186:889–93. doi: 10.1007/s11845-016-1521-8
53. Carrascal Y, Arnold RJ, De la Fuente L, Revilla A, Sevilla T, Arce N. Efficacy of atorvastatin in prevention of atrial fibrillation after heart valve surgery in the PROFACE trial (PROphylaxis of postoperative atrial fibrillation after cardiac surgEry). J Arrhythm. (2016) 32:191–7. doi: 10.1016/j.joa.2016.01.010
54. Castillo R, Rodrigo R, Perez F, Cereceda M, Asenjo R, Zamorano J, et al. Antioxidant therapy reduces oxidative and inflammatory tissue damage in patients subjected to cardiac surgery with extracorporeal circulation. Basic Clin Pharmacol Toxicol. (2011) 108:256–62. doi: 10.1111/j.1742-7843.2010.00651.x
55. Cho JS, Shim JK, Soh S, Kim MK, Kwak YL. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. (2016) 89:693–700. doi: 10.1038/ki.2015.306
56. Cho JS, Soh S, Shim JK, Kang S, Choi H, Kwak YL. Effect of perioperative sodium bicarbonate administration on renal function following cardiac surgery for infective endocarditis: a randomized, placebo-controlled trial. Crit Care. (2017) 21:3. doi: 10.1186/s13054-016-1591-z
57. Cholley B, Caruba T, Grosjean S, Amour J, Ouattara A, Villacorta J, et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA. (2017) 318:548–56. doi: 10.1001/jama.2017.9973
58. Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, et al. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. (2011) 142:148–54. doi: 10.1016/j.jtcvs.2010.11.018
59. Cogliati AA, Vellutini R, Nardini A, Urovi S, Hamdan M, Landoni G, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: a randomized clinical study. J Cardiothorac Vasc Anesth. (2007) 21:847–50. doi: 10.1053/j.jvca.2007.02.022
60. Coverdale NS, Hamilton A, Petsikas D, McClure RS, Malik P, Milne B, et al. Remote ischemic preconditioning in high-risk cardiovascular surgery patients: a randomized-controlled trial. Semin Thorac Cardiovasc Surg. (2018) 30:26–33. doi: 10.1053/j.semtcvs.2017.09.001
61. Dardashti A, Ederoth P, Algotsson L, Bronden B, Grins E, Larsson M, et al. Erythropoietin and protection of renal function in cardiac surgery (the EPRICS Trial). Anesthesiology. (2014) 121:582–90. doi: 10.1097/ALN.0000000000000321
62. Datzmann T, Hoenicka M, Reinelt H, Liebold A, Gorki H. Influence of 6% hydroxyethyl starch 130/0.4 versus crystalloid solution on structural renal damage markers after coronary artery bypass grafting: a post hoc subgroup analysis of a prospective trial. J Cardiothorac Vasc Anesth. (2018) 32:205–11. doi: 10.1053/j.jvca.2017.05.041
63. Desai PM, Sarkar MS, Umbarkar SR. Prophylactic preoperative levosimendan for off-pump coronary artery bypass grafting in patients with left ventricular dysfunction: single-centered randomized prospective study. Ann Card Anaesth. (2018) 21:123–8. doi: 10.4103/aca.ACA_178_17
64. Dieleman JM, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. (2012) 308:1761–7. doi: 10.1001/jama.2012.14144
65. Duncan AE, Jia Y, Soltesz E, Leung S, Yilmaz HO, Mao G, et al. Effect of 6% hydroxyethyl starch 130/0.4 on kidney and haemostatic function in cardiac surgical patients: a randomised controlled trial. Anaesthesia. (2020) 75:1180–90. doi: 10.1111/anae.14994
66. Ederoth P, Dardashti A, Grins E, Bronden B, Metzsch C, Erdling A, et al. Cyclosporine before coronary artery bypass grafting does not prevent postoperative decreases in renal function: a randomized clinical trial. Anesthesiology. (2018) 128:710–7. doi: 10.1097/ALN.0000000000002104
67. Erb J, Beutlhauser T, Feldheiser A, Schuster B, Treskatsch S, Grubitzsch H, et al. Influence of levosimendan on organ dysfunction in patients with severely reduced left ventricular function undergoing cardiac surgery. J Int Med Res. (2014) 42:750–64. doi: 10.1177/0300060513516293
68. Ejaz AA, Martin TD, Johnson RJ, Winterstein AG, Klodell CT, Hess PJ Jr., et al. Prophylactic nesiritide does not prevent dialysis or all-cause mortality in patients undergoing high-risk cardiac surgery. J Thorac Cardiovasc Surg. (2009) 138:959–64. doi: 10.1016/j.jtcvs.2009.05.014
69. Ejaz AA, Dass B, Lingegowda V, Shimada M, Beaver TM, Ejaz NI, et al. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int Urol Nephrol. (2013) 45:449–58. doi: 10.1007/s11255-012-0192-2
70. Eslami P, Hekmat M, Beheshti M, Baghaei R, Mirhosseini SM, Pourmotahari F, et al. A Randomized, double-blind, placebo-controlled, clinical trial of high-dose, short-term vitamin D administration in the prevention of acute kidney injury after cardiac surgery. Cardiorenal Med. (2021) 11:52–8. doi: 10.1159/000511058
71. Fakhari S, Bavil FM, Bilehjani E, Abolhasani S, Mirinazhad M, Naghipour B. Prophylactic furosemide infusion decreasing early major postoperative renal dysfunction in on-pump adult cardiac surgery: a randomized clinical trial. Res Rep Urol. (2017) 9:5–13. doi: 10.2147/RRU.S126134
72. Franco RA, de Almeida JP, Landoni G, Scheeren TWL, Galas F, Fukushima JT, et al. Dobutamine-sparing versus dobutamine-to-all strategy in cardiac surgery: a randomized noninferiority trial. Ann Intensive Care. (2021) 11:15. doi: 10.1186/s13613-021-00808-6
73. Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, et al. Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int. (2015) 87:473–81. doi: 10.1038/ki.2014.259
74. Garg AX, Devereaux PJ, Hill A, Sood M, Aggarwal B, Dubois L, et al. Oral curcumin in elective abdominal aortic aneurysm repair: a multicentre randomized controlled trial. CMAJ. (2018) 190:E1273–80. doi: 10.1503/cmaj.180510
75. Garg AX, Chan MTV, Cuerden MS, Devereaux PJ, Abbasi SH, Hildebrand A, et al. Effect of methylprednisolone on acute kidney injury in patients undergoing cardiac surgery with a cardiopulmonary bypass pump: a randomized controlled trial. CMAJ. (2019) 191:E247–56. doi: 10.1503/cmaj.181644
76. Golestaneh L, Lindsey K, Malhotra P, Kargoli F, Farkas E, Barner H, et al. Acute kidney injury after cardiac surgery: is minocycline protective? J Nephrol. (2015) 28:193–9. doi: 10.1007/s40620-014-0152-2
77. Grant MC, Kon Z, Joshi A, Christenson E, Kallam S, Burris N, et al. Is aprotinin safe to use in a cohort at increased risk for thrombotic events: results from a randomized, prospective trial in off-pump coronary artery bypass. Ann Thorac Surg. (2008) 86:815–22. doi: 10.1016/j.athoracsur.2008.04.047
78. van Groenendael R, Beunders R, Hemelaar P, Hofland J, Morshuis WJ, van der Hoeven JG, et al. Safety and efficacy of human chorionic gonadotropin hormone-derivative EA-230 in cardiac surgery patients: a randomized double-blind placebo-controlled study. Crit Care Med. (2021) 49:790–803. doi: 10.1097/CCM.0000000000004847
79. Grundmann F, Muller RU, Reppenhorst A, Hulswitt L, Spath MR, Kubacki T, et al. Preoperative short-term calorie restriction for prevention of acute kidney injury after cardiac surgery: a randomized, controlled, open-label, pilot trial. J Am Heart Assoc. (2018) 7:e008181. doi: 10.1161/JAHA.117.008181
80. Haase M, Haase-Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, et al. Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med. (2007) 35:1324–31. doi: 10.1097/01.CCM.0000261887.69976.12
81. Haase M, Haase-Fielitz A, Plass M, Kuppe H, Hetzer R, Hannon C, et al. Prophylactic perioperative sodium bicarbonate to prevent acute kidney injury following open heart surgery: a multicenter double-blinded randomized controlled trial. PLoS Med. (2013) 10:e1001426. doi: 10.1371/journal.pmed.1001426
82. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. (2015) 373:1408–17. doi: 10.1056/NEJMoa1413534
83. Hong DM, Jeon Y, Lee CS, Kim HJ, Lee JM, Bahk JH, et al. Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary artery bypass surgery–randomized controlled trial. Circ J. (2012) 76:884–90. doi: 10.1253/circj.cj-11-1068
84. Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote ischaemic preconditioning with postconditioning outcome trial. Eur Heart J. (2014) 35:176–83. doi: 10.1093/eurheartj/eht346
85. Hu Q, Luo W, Huang L, Huang R, Chen R, Gao Y. Multiorgan protection of remote ischemic perconditioning in valve replacement surgery. J Surg Res. (2016) 200:13–20. doi: 10.1016/j.jss.2015.06.053
86. Hynninen MS, Niemi TT, Poyhia R, Raininko EI, Salmenpera MT, Lepantalo MJ, et al. N-acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: a randomized, double-blind, placebo-controlled trial. Anesth Analg. (2006) 102:1638–45. doi: 10.1213/01.ANE.0000219590.79796.66
87. Jacob KA, Leaf DE, Dieleman JM, van Dijk D, Nierich AP, Rosseel PM, et al. Intraoperative High-Dose Dexamethasone and Severe AKI after Cardiac Surgery. J Am Soc Nephrol. (2015) 26:2947–51. doi: 10.1681/ASN.2014080840
88. Kanchi M, Manjunath R, Massen J, Vincent L, Belani K. Neutrophil gelatinase-associated lipocalin as a biomarker for predicting acute kidney injury during off-pump coronary artery bypass grafting. Ann Card Anaesth. (2017) 20:297–302. doi: 10.4103/aca.ACA_48_17
89. Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology. (2012) 116:613–21. doi: 10.1097/ALN.0b013e3182475e39
90. Kaya K, Oguz M, Akar AR, Durdu S, Aslan A, Erturk S, et al. The effect of sodium nitroprusside infusion on renal function during reperfusion period in patients undergoing coronary artery bypass grafting: a prospective randomized clinical trial. Eur J Cardiothorac Surg. (2007) 31:290–7. doi: 10.1016/j.ejcts.2006.11.015
91. Kramer BK, Preuner J, Ebenburger A, Kaiser M, Bergner U, Eilles C, et al. Lack of renoprotective effect of theophylline during aortocoronary bypass surgery. Nephrol Dial Transplant. (2002) 17:910–5. doi: 10.1093/ndt/17.5.910
92. Kristeller JL, Zavorsky GS, Prior JE, Keating DA, Brady MA, Romaldini TA, et al. Lack of effectiveness of sodium bicarbonate in preventing kidney injury in patients undergoing cardiac surgery: a randomized controlled trial. Pharmacotherapy. (2013) 33:710–7. doi: 10.1002/phar.1262
93. Kim JC, Shim JK, Lee S, Yoo YC, Yang SY, Kwak YL. Effect of combined remote ischemic preconditioning and postconditioning on pulmonary function in valvular heart surgery. Chest. (2012) 142:467–75. doi: 10.1378/chest.11-2246
94. Kim JH, Shim JK, Song JW, Song Y, Kim HB, Kwak YL. Effect of erythropoietin on the incidence of acute kidney injury following complex valvular heart surgery: a double blind, randomized clinical trial of efficacy and safety. Crit Care. (2013) 17:R254. doi: 10.1186/cc13081
95. Kim JE, Song SW, Kim JY, Lee HJ, Chung KH, Shim YH. Effect of a single bolus of erythropoietin on renoprotection in patients undergoing thoracic aortic surgery with moderate hypothermic circulatory arrest. Ann Thorac Surg. (2016) 101:690–6. doi: 10.1016/j.athoracsur.2015.08.007
96. Kim TK, Nam K, Cho YJ, Min JJ, Hong YJ, Park KU, et al. Microvascular reactivity and endothelial glycocalyx degradation when administering hydroxyethyl starch or crystalloid during off-pump coronary artery bypass graft surgery: a randomised trial. Anaesthesia. (2017) 72:204–13. doi: 10.1111/anae.13642
97. Kim TK, Min JJ, Cho YJ, Hausenloy DJ, Ahn H, Kim KH, et al. Effects of delayed remote ischemic preconditioning on peri-operative myocardial injury in patients undergoing cardiac surgery – A randomized controlled trial. Int J Cardiol. (2017) 227:511–5. doi: 10.1016/j.ijcard.2016.10.111
98. Kishimoto Y, Nakamura Y, Harada S, Onohara T, Kishimoto S, Kurashiki T, et al. Can tolvaptan protect renal function in the early postoperative period of cardiac surgery? Results of a single-center randomized controlled study. Circ J. (2018) 82:999–1007. doi: 10.1253/circj.CJ-17-0967
99. Lahtinen P, Pitkanen O, Polonen P, Turpeinen A, Kiviniemi V, Uusaro A. Levosimendan reduces heart failure after cardiac surgery: a prospective, randomized, placebo-controlled trial. Crit Care Med. (2011) 39:2263–70. doi: 10.1097/CCM.0b013e3182227b97
100. Landoni G, Lomivorotov VV, Alvaro G, Lobreglio R, Pisano A, Guarracino F, et al. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med. (2017) 376:2021–31. doi: 10.1056/NEJMoa1616325
101. Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, Hiesmayr M. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. (2000) 11:97–104. doi: 10.1681/ASN.V11197
102. Lee EH, Kim WJ, Kim JY, Chin JH, Choi DK, Sim JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology. (2016) 124:1001–11. doi: 10.1097/ALN.0000000000001051
103. Lee NM, Deriy L, Petersen TR, Shah VO, Hutchens MP, Gerstein NS. Impact of isolyte versus 0.9% saline on postoperative event of acute kidney injury assayed by urinary [TIMP-2]x[IGFBP7] in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. (2019) 33:348–56. doi: 10.1053/j.jvca.2018.07.042
104. Levin RL, Degrange MA, Porcile R, Salvagio F, Blanco N, Botbol AL, et al. [The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome]. Rev Esp Cardiol. (2008) 61:471–9. doi: 10.1016/S1885-5857(08)60160-7
105. Levin R, Degrange M, Del Mazo C, Tanus E, Porcile R. Preoperative levosimendan decreases mortality and the development of low cardiac output in high-risk patients with severe left ventricular dysfunction undergoing coronary artery bypass grafting with cardiopulmonary bypass. Exp Clin Cardiol. (2012) 17:125–30.
106. Li X, Yang J, Nie XL, Zhang Y, Li XY, Li LH, et al. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PLoS One. (2017) 12:e0170757. doi: 10.1371/journal.pone.0170757
107. Liakopoulos OJ, Schmitto JD, Kazmaier S, Brauer A, Quintel M, Schoendube FA, et al. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. (2007) 84:110–8. doi: 10.1016/j.athoracsur.2007.01.003
108. Ljunggren M, Skold A, Dardashti A, Hyllen S. The use of mannitol in cardiopulmonary bypass prime solution-prospective randomized double-blind clinical trial. Acta Anaesthesiol Scand. (2019) 63:1298–305. doi: 10.1111/aas.13445
109. Lomivorotov VV, Fominskiy EV, Efremov SM, Nepomniashchikh VA, Lomivorotov VN, Chernyavskiy AM, et al. Infusion of 7.2% NaCl/6% hydroxyethyl starch 200/0.5 in on-pump coronary artery bypass surgery patients: a randomized, single-blind pilot study. Shock. (2014) 41:193–9. doi: 10.1097/SHK.0000000000000087
110. Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, et al. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. (2012) 116:296–310. doi: 10.1097/ALN.0b013e318242349a
111. Luckraz H, Giri R, Wrigley B, Nagarajan K, Senanayake E, Sharman E, et al. Reduction in acute kidney injury post cardiac surgery using balanced forced diuresis: a randomized, controlled trial. Eur J Cardiothorac Surg. (2021) 59:562–9. doi: 10.1093/ejcts/ezaa395
112. Macedo E, Abdulkader R, Castro I, Sobrinho AC, Yu L, Vieira JM Jr. Lack of protection of N-acetylcysteine (NAC) in acute renal failure related to elective aortic aneurysm repair-a randomized controlled trial. Nephrol Dial Transplant. (2006) 21:1863–9. doi: 10.1093/ndt/gfl079
113. Mannacio VA, Iorio D, De Amicis V, Di Lello F, Musumeci F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trial. J Thorac Cardiovasc Surg. (2008) 136:1541–8. doi: 10.1016/j.jtcvs.2008.06.038
114. McGuinness SP, Parke RL, Bellomo R, Van Haren FM, Bailey M. Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: a phase II multicenter double-blind randomized controlled trial. Crit Care Med. (2013) 41:1599–607. doi: 10.1097/CCM.0b013e31828a3f99
115. Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. (2017) 43:1551–61. doi: 10.1007/s00134-016-4670-3
116. Meersch M, Kullmar M, Pavenstadt H, Rossaint J, Kellum JA, Martens S, et al. Effects of different doses of remote ischemic preconditioning on kidney damage among patients undergoing cardiac surgery: a single-center mechanistic randomized controlled trial. Crit Care Med. (2020) 48:e690–7. doi: 10.1097/CCM.0000000000004415
117. Mehta RH, Leimberger JD, van Diepen S, Meza J, Wang A, Jankowich R, et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med. (2017) 376:2032–42. doi: 10.1056/NEJMoa1616218
118. Mentzer RM Jr., Oz MC, Sladen RN, Graeve AH, Hebeler RF Jr., Luber JM Jr., et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA trial. J Am Coll Cardiol. (2007) 49:716–26. doi: 10.1016/j.jacc.2006.10.048
119. Meybohm P, Renner J, Broch O, Caliebe D, Albrecht M, Cremer J, et al. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double-blind randomized controlled pilot study. PLoS One. (2013) 8:e64743. doi: 10.1371/journal.pone.0064743
120. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. (2015) 373:1397–407. doi: 10.1056/NEJMoa1413579
121. Mohod V, Ganeriwal V, Bhange J. Comparison of intensive insulin therapy and conventional glucose management in patients undergoing coronary artery bypass grafting. J Anaesthesiol Clin Pharmacol. (2019) 35:493–7. doi: 10.4103/joacp.JOACP_61_17
122. Mori Y, Kamada T, Ochiai R. Reduction in the incidence of acute kidney injury after aortic arch surgery with low-dose atrial natriuretic peptide: a randomised controlled trial. Eur J Anaesthesiol. (2014) 31:381–7. doi: 10.1097/EJA.0000000000000035
123. Moriyama T, Hagihara S, Shiramomo T, Nagaoka M, Iwakawa S, Kanmura Y. The protective effect of human atrial natriuretic peptide on renal damage during cardiac surgery. J Anesth. (2017) 31:163–9. doi: 10.1007/s00540-016-2284-0
124. Moscarelli M, Fiorentino F, Suleiman MS, Emanueli C, Reeves BC, Punjabi PP, et al. Remote ischaemic preconditioning in isolated aortic valve and coronary artery bypass surgery: a randomized trialdagger. Eur J Cardiothorac Surg. (2019) 55:905–12. doi: 10.1093/ejcts/ezy404
125. Murphy N, Vijayan A, Frohlich S, O’Farrell F, Barry M, Sheehan S, et al. Remote ischemic preconditioning does not affect the incidence of acute kidney injury after elective abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth. (2014) 28:1285–92. doi: 10.1053/j.jvca.2014.04.018
126. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. (2015) 372:997–1008. doi: 10.1056/NEJMoa1403612
127. Nagpal AD, Cowan A, Li L, Nusca G, Guo L, Novick RJ, et al. Starch or saline after cardiac surgery: a double-blinded randomized controlled trial. Can J Kidney Health Dis. (2020) 7:2054358120940434. doi: 10.1177/2054358120940434
128. Naguib SN, Sabry NA, Farid SF, Alansary AM. Short-term effects of alfacalcidol on hospital length of stay in patients undergoing valve replacement surgery: a randomized clinical trial. Clin Ther. (2021) 43:e1–18. doi: 10.1016/j.clinthera.2020.11.008
129. Nouraei SM, Baradari AG, Jazayeri A. Does remote ischaemic preconditioning protect kidney and cardiomyocytes after coronary revascularization? A double blind controlled clinical trial. Med Arch. (2016) 70:373–8. doi: 10.5455/medarh.2016.70.373-378
130. Nouri-Majalan N, Ardakani EF, Forouzannia K, Moshtaghian H. Effects of allopurinol and vitamin E on renal function in patients with cardiac coronary artery bypass grafts. Vasc Health Risk Manag. (2009) 5:489–94. doi: 10.2147/vhrm.s5761
131. Oh SW, Chin HJ, Chae DW, Na KY. Erythropoietin improves long-term outcomes in patients with acute kidney injury after coronary artery bypass grafting. J Korean Med Sci. (2012) 27:506–11. doi: 10.3346/jkms.2012.27.5.506
132. Ozaydin M, Peker T, Akcay S, Uysal BA, Yucel H, Icli A, et al. Addition of N-acetyl cysteine to carvedilol decreases the incidence of acute renal injury after cardiac surgery. Clin Cardiol. (2014) 37:108–14. doi: 10.1002/clc.22227
133. Paparella D, Parolari A, Rotunno C, Vincent J, Myasoedova V, Guida P, et al. The effects of steroids on coagulation dysfunction induced by cardiopulmonary bypass: a steroids in cardiac surgery (SIRS) trial substudy. Semin Thorac Cardiovasc Surg. (2017) 29:35–44. doi: 10.1053/j.semtcvs.2017.01.007
134. Park JH, Shim JK, Song JW, Soh S, Kwak YL. Effect of atorvastatin on the incidence of acute kidney injury following valvular heart surgery: a randomized, placebo-controlled trial. Intensive Care Med. (2016) 42:1398–407. doi: 10.1007/s00134-016-4358-8
135. Parke RL, McGuinness SP, Gilder E, McCarthy LW, Cowdrey KA. A randomised feasibility study to assess a novel strategy to rationalise fluid in patients after cardiac surgery. Br J Anaesth. (2015) 115:45–52. doi: 10.1093/bja/aev118
136. Prasad A, Banakal S, Muralidhar K. N-acetylcysteine does not prevent renal dysfunction after off-pump coronary artery bypass surgery. Eur J Anaesthesiol. (2010) 27:973–7. doi: 10.1097/EJA.0b013e3283383506
137. Pinaud F, Corbeau JJ, Baufreton C, Binuani JP, De Brux JL, Fouquet O, et al. Remote ischemic preconditioning in aortic valve surgery: results of a randomized controlled study. J Cardiol. (2016) 67:36–41. doi: 10.1016/j.jjcc.2015.06.007
138. Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Haase M, et al. Pilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgery. Nephrology. (2012) 17:215–24. doi: 10.1111/j.1440-1797.2011.01546.x
139. Pu H, Doig GS, Heighes PT, Allingstrup MJ, Wang A, Brereton J, et al. Intravenous amino acid therapy for kidney protection in cardiac surgery patients: a pilot randomized controlled trial. J Thorac Cardiovasc Surg. (2019) 157:2356–66. doi: 10.1016/j.jtcvs.2018.11.097
140. Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation. (2010) 122:S53–9. doi: 10.1161/CIRCULATIONAHA.109.926667
141. Ranucci M, De Benedetti D, Bianchini C, Castelvecchio S, Ballotta A, Frigiola A, et al. Effects of fenoldopam infusion in complex cardiac surgical operations: a prospective, randomized, double-blind, placebo-controlled study. Minerva Anestesiol. (2010) 76:249–59.
142. Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta-Ylinen R, et al. Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. (2006) 97:611–6. doi: 10.1093/bja/ael224
143. Ristikankare A, Poyhia R, Eriksson H, Valtonen M, Leino K, Salmenpera M. Effects of levosimendan on renal function in patients undergoing coronary artery surgery. J Cardiothorac Vasc Anesth. (2012) 26:591–5. doi: 10.1053/j.jvca.2012.01.035
144. Santana-Santos E, Gowdak LH, Gaiotto FA, Puig LB, Hajjar LA, Zeferino SP, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg. (2014) 97:1617–23. doi: 10.1016/j.athoracsur.2014.01.056
145. Sarkar K, Harris RA, Wells S, Harris T, Clout M, Taylor J, et al. Preoperative VolumE Replacement therapy in DIabetic patients undergoing coronary artery bypass grafting surgery: results from an open parallel group randomized Controlled Trial (VeRDiCT). Interact Cardiovasc Thorac Surg. (2020) 30:54–63. doi: 10.1093/icvts/ivz226
146. Sezai A, Shiono M, Hata M, Iida M, Wakui S, Soeda M, et al. Efficacy of continuous low-dose human atrial natriuretic peptide given from the beginning of cardiopulmonary bypass for thoracic aortic surgery. Surg Today. (2006) 36:508–14. doi: 10.1007/s00595-006-3194-9
147. Sezai A, Hata M, Wakui S, Shiono M, Negishi N, Kasamaki Y, et al. Efficacy of low-dose continuous infusion of alpha-human atrial natriuretic peptide (hANP) during cardiac surgery: possibility of postoperative left ventricular remodeling effect. Circ J. (2006) 70:1426–31. doi: 10.1253/circj.70.1426
148. Sezai A, Hata M, Wakui S, Niino T, Takayama T, Hirayama A, et al. Efficacy of continuous low-dose hANP administration in patients undergoing emergent coronary artery bypass grafting for acute coronary syndrome. Circ J. (2007) 71:1401–7. doi: 10.1253/circj.71.1401
149. Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, et al. Influence of continuous infusion of low-dose human atrial natriuretic peptide on renal function during cardiac surgery: a randomized controlled study. J Am Coll Cardiol. (2009) 54:1058–64. doi: 10.1016/j.jacc.2009.05.047
150. Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose Human ANP Infusion Therapy during cardiac surgery) for left ventricular dysfunction. J Am Coll Cardiol. (2010) 55:1844–51. doi: 10.1016/j.jacc.2009.11.085
151. Shan XS, Dai HR, Zhao D, Yang BW, Feng XM, Liu H, et al. Dexmedetomidine reduces acute kidney injury after endovascular aortic repair of Stanford type B aortic dissection: a randomized, double-blind, placebo-controlled pilot study. J Clin Anesth. (2021) 75:110498. doi: 10.1016/j.jclinane.2021.110498
152. Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP Infusion Therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol. (2011) 58:897–903. doi: 10.1016/j.jacc.2011.03.056
153. Sezai A, Nakata K, Iida M, Yoshitake I, Wakui S, Hata H, et al. Results of low-dose carperitide infusion in high-risk patients undergoing coronary artery bypass grafting. Ann Thorac Surg. (2013) 96:119–26. doi: 10.1016/j.athoracsur.2013.03.090
154. Shah B, Sharma P, Brahmbhatt A, Shah R, Rathod B, Shastri N, et al. Study of levosimendan during off-pump coronary artery bypass grafting in patients with LV dysfunction: a double-blind randomized study. Indian J Pharmacol. (2014) 46:29–34. doi: 10.4103/0253-7613.125161
155. Shahbazi S, Alishahi P, Asadpour E. Evaluation of the effect of aminophylline in reducing the incidence of acute kidney injury after cardiac surgery. Anesth Pain Med. (2017) 7:e21740. doi: 10.5812/aapm.21740
156. Sharma P, Malhotra A, Gandhi S, Garg P, Bishnoi A, Gandhi H. Preoperative levosimendan in ischemic mitral valve repair. Asian Cardiovasc Thorac Ann. (2014) 22:539–45. doi: 10.1177/0218492313499352
157. Sisillo E, Ceriani R, Bortone F, Juliano G, Salvi L, Veglia F, et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med. (2008) 36:81–6. doi: 10.1097/01.CCM.0000295305.22281.1D
158. Smart L, Boyd C, Litton E, Pavey W, Vlaskovsky P, Ali U, et al. A randomised controlled trial of succinylated gelatin (4%) fluid on urinary acute kidney injury biomarkers in cardiac surgical patients. Intensive Care Med Exp. (2021) 9:48. doi: 10.1186/s40635-021-00412-9
159. Smith MN, Best D, Sheppard SV, Smith DC. The effect of mannitol on renal function after cardiopulmonary bypass in patients with established renal dysfunction. Anaesthesia. (2008) 63:701–4. doi: 10.1111/j.1365-2044.2007.05408.x
160. Soh S, Song JW, Shim JK, Kim JH, Kwak YL. Sodium bicarbonate does not prevent postoperative acute kidney injury after off-pump coronary revascularization: a double-blinded randomized controlled trial. Br J Anaesth. (2016) 117:450–7. doi: 10.1093/bja/aew256
161. Soh S, Shim JK, Song JW, Bae JC, Kwak YL. Effect of dexmedetomidine on acute kidney injury after aortic surgery: a single-centre, placebo-controlled, randomised controlled trial. Br J Anaesth (2020). [Epub ahead of print]. doi: 10.1016/j.bja.2019.12.036
162. Song YR, Lee T, You SJ, Chin HJ, Chae DW, Lim C, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. (2009) 30:253–60. doi: 10.1159/000223229
163. Song JW, Shim JK, Soh S, Jang J, Kwak YL. Double-blinded, randomized controlled trial of N-acetylcysteine for prevention of acute kidney injury in high risk patients undergoing off-pump coronary artery bypass. Nephrology. (2015) 20:96–102. doi: 10.1111/nep.12361
164. Song Y, Song JW, Lee S, Jun JH, Kwak YL, Shim JK. Effects of remote ischemic preconditioning in patients with concentric myocardial hypertrophy: a randomized, controlled trial with molecular insights. Int J Cardiol. (2017) 249:36–41. doi: 10.1016/j.ijcard.2017.08.073
165. Song JW, Lee WK, Lee S, Shim JK, Kim HJ, Kwak YL. Remote ischaemic conditioning for prevention of acute kidney injury after valvular heart surgery: a randomised controlled trial. Br J Anaesth. (2018) 121:1034–40. doi: 10.1016/j.bja.2018.07.035
166. Stokfisz K, Ledakowicz-Polak A, Zagorski M, Jander S, Przybylak K, Zielinska M. The clinical utility of remote ischemic preconditioning in protecting against cardiac surgery-associated acute kidney injury: a pilot randomized clinical trial. Adv Clin Exp Med. (2020) 29:189–96. doi: 10.17219/acem/112610
167. Sward K, Valsson F, Odencrants P, Samuelsson O, Ricksten SE. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med. (2004) 32:1310–5. doi: 10.1097/01.ccm.0000128560.57111.cd
168. Tasanarong A, Duangchana S, Sumransurp S, Homvises B, Satdhabudha O. Prophylaxis with erythropoietin versus placebo reduces acute kidney injury and neutrophil gelatinase-associated lipocalin in patients undergoing cardiac surgery: a randomized, double-blind controlled trial. BMC Nephrol. (2013) 14:136. doi: 10.1186/1471-2369-14-136
169. Thielmann M, Corteville D, Szabo G, Swaminathan M, Lamy A, Lehner LJ, et al. Teprasiran, a small interfering RNA, for the prevention of acute kidney injury in high-risk patients undergoing cardiac surgery: a randomized clinical study. Circulation. (2021) 144:1133–44. doi: 10.1161/CIRCULATIONAHA.120.053029
170. Tian WZ, Er JX, Liu L, Chen QL, Han JG. Effects of autologous platelet rich plasma on intraoperative transfusion and short-term outcomes in total arch replacement (Sun’s Procedure): a prospective, randomized trial. J Cardiothorac Vasc Anesth. (2019) 33:2163–9. doi: 10.1053/j.jvca.2019.02.033
171. Tritapepe L, De Santis V, Vitale D, Guarracino F, Pellegrini F, Pietropaoli P, et al. Levosimendan pre-treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth. (2009) 102:198–204. doi: 10.1093/bja/aen367
172. Turner KR, Fisher EC, Hade EM, Houle TT, Rocco MV. The role of perioperative sodium bicarbonate infusion affecting renal function after cardiothoracic surgery. Front Pharmacol. (2014) 5:127. doi: 10.3389/fphar.2014.00127
173. Walsh M, Whitlock R, Garg AX, Legare JF, Duncan AE, Zimmerman R, et al. Effects of remote ischemic preconditioning in high-risk patients undergoing cardiac surgery (Remote IMPACT): a randomized controlled trial. CMAJ. (2016) 188:329–36.
174. Wijeysundera DN, Beattie WS, Rao V, Granton JT, Chan CT. N-acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anaesth. (2007) 54:872–81.
175. Witczak BJ, Hartmann A, Geiran OR, Bugge JF. Renal function after cardiopulmonary bypass surgery in patients with impaired renal function. A randomized study of the effect of nifedipine. Eur J Anaesthesiol. (2008) 25:319–25. doi: 10.1017/S0265021507003158
176. Yoo YC, Shim JK, Kim JC, Jo YY, Lee JH, Kwak YL. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. (2011) 115:929–37. doi: 10.1097/ALN.0b013e318232004b
177. Young PJ, Dalley P, Garden A, Horrocks C, La Flamme A, Mahon B, et al. A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol. (2012) 107:256. doi: 10.1007/s00395-012-0256-6
178. Javaherforoosh Zadeh F, Azemati S. Adjusted tight control blood glucose management in diabetic patients undergoing on pump coronary artery bypass graft. A randomized clinical trial. J Diabetes Metab Disord. (2020) 19:423–30.
179. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. (2015) 313:2133–41.
180. Zarbock A, Kullmar M, Ostermann M, Lucchese G, Baig K, Cennamo A, et al. Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg. (2021) 133:292–302. doi: 10.1213/ANE.0000000000005458
181. Zhai M, Kang F, Han M, Huang X, Li J. The effect of dexmedetomidine on renal function in patients undergoing cardiac valve replacement under cardiopulmonary bypass: a double-blind randomized controlled trial. J Clin Anesth. (2017) 40:33–8. doi: 10.1016/j.jclinane.2017.03.053
182. Zheng Z, Jayaram R, Jiang L, Emberson J, Zhao Y, Li Q, et al. Perioperative Rosuvastatin in Cardiac Surgery. N Engl J Med. (2016) 374: 1744–53.
183. Zhou SF, Estrera AL, Loubser P, Ignacio C, Panthayi S, Miller C III, et al. Autologous platelet-rich plasma reduces transfusions during ascending aortic arch repair: a prospective, randomized, controlled trial. Ann Thorac Surg. (2015) 99:1282–90. doi: 10.1016/j.athoracsur.2014.11.007
184. Zhou H, Yang L, Wang G, Zhang C, Fang Z, Lei G, et al. Remote ischemic preconditioning prevents postoperative acute kidney injury after open total aortic arch replacement: a double-blind, randomized, sham-controlled trial. Anesth Analg. (2019) 129:287–93. doi: 10.1213/ANE.0000000000004127
185. Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, et al. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. (2011) 80:861–7. doi: 10.1038/ki.2011.156
186. University of New Mexico, Fluid Chloride and AKI in Cardiopulmonary Bypass. (2016). Available online at: https://ClinicalTrials.gov/show/NCT02668952 (accessed December, 2021).
187. Wake Forest University Health Sciences. Use of Bicarbonate to Reduce the Incidence of Acute Renal Failure After Cardiac Surgery. (2006). Available online at: https://ClinicalTrials.gov/show/NCT00484354 (accessed December, 2021).
188. Joosten A, Tircoveanu R, Arend S, Wauthy P, Gottignies P, Van der Linden P. Impact of balanced tetrastarch raw material on perioperative blood loss: a randomized double blind controlled trial. Br J Anaesth. (2016) 117:442–9. doi: 10.1093/bja/aew249
189. Callum J, Farkouh ME, Scales DC, Heddle NM, Crowther M, Rao V, et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: the FIBRES randomized clinical trial. JAMA. (2019) 322:1966–76. doi: 10.1001/jama.2019.17312
190. Probst S, Cech C, Haentschel D, Scholz M, Ender J. A specialized post anaesthetic care unit improves fast-track management in cardiac surgery: a prospective randomized trial. Crit Care. (2014) 18:468. doi: 10.1186/s13054-014-0468-2
191. Yoo YC, Shim JK, Song Y, Yang SY, Kwak YL. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. (2014) 86:414–22.
192. Demir A, Yilmaz FM, Ceylan C, Doluoglu OG, Ucar P, Zungun C, et al. A comparison of the effects of ketamine and remifentanil on renal functions in coronary artery bypass graft surgery. Ren Fail. (2015) 37:819–26. doi: 10.3109/0886022X.2015.1015390
193. van Diepen S, Norris CM, Zheng Y, Nagendran J, Graham MM, Gaete Ortega D, et al. Comparison of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker management strategies before cardiac surgery: a pilot randomized controlled registry trial. J Am Heart Assoc. (2018) 7:e009917. doi: 10.1161/JAHA.118.009917
194. Marathias KP, Vassili M, Robola A, Alivizatos PA, Palatianos GM, Geroulanos S, et al. Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artif Organs. (2006) 30:615–21. doi: 10.1111/j.1525-1594.2006.00270.x
195. Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, Rhodes A, Landoni G, Osawa EA. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology. (2017) 126:85–93. doi: 10.1097/ALN.0000000000001434
196. Chu DK, Brignardello-Petersen R, Guyatt GH, Ricci C, Genuneit J. Method’s corner: allergist’s guide to network meta-analysis. Pediatr Allergy Immunol. (2022) 33:e13609. doi: 10.1111/pai.13609
197. Choi MR, Fernandez BE. Protective renal effects of atrial natriuretic peptide: where are we now? Front Physiol. (2021) 12:680213. doi: 10.3389/fphys.2021.680213
198. Zhao J, Pei L. Cardiac endocrinology: heart-derived hormones in physiology and disease. JACC Basic Transl Sci. (2020) 5:949–60.
199. Weber AJ, Kail RE, Stanford LR. Morphology of single, physiologically identified retinogeniculate Y-cell axons in the cat following damage to visual cortex at birth. J Comp Neurol. (1989) 282:446–55. doi: 10.1002/cne.902820310
200. Mitaka C, Ohnuma T, Murayama T, Kunimoto F, Nagashima M, Takei T, et al. Effects of low-dose atrial natriuretic peptide infusion on cardiac surgery-associated acute kidney injury: a multicenter randomized controlled trial. J Crit Care. (2017) 38:253–8. doi: 10.1016/j.jcrc.2016.12.004
201. Zarbock A, Kellum JA. Remote ischemic preconditioning and protection of the kidney–a novel therapeutic option. Crit Care Med. (2016) 44:607–16. doi: 10.1097/CCM.0000000000001381
202. Sprick JD, Mallet RT, Przyklenk K, Rickards CA. Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol. (2019) 104:278–94. doi: 10.1113/EP087122
203. Yang T, Sun Y, Li Q, Li S, Shi Y, Leak RK, et al. Ischemic preconditioning provides long-lasting neuroprotection against ischemic stroke: the role of Nrf2. Exp Neurol. (2020) 325:113142. doi: 10.1016/j.expneurol.2019.113142
204. Johnson ACM, Zager RA. Mechanisms and consequences of oxidant-induced renal preconditioning: an Nrf2-dependent, P21-independent, anti-senescence pathway. Nephrol Dial Transplant. (2018) 33:1927–41. doi: 10.1093/ndt/gfy029
205. Yao H, Chi X, Jin Y, Wang Y, Huang P, Wu S, et al. Dexmedetomidine inhibits TLR4/NF-kappaB activation and reduces acute kidney injury after orthotopic autologous liver transplantation in rats. Sci Rep. (2015) 5:16849. doi: 10.1038/srep16849
206. Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. (2019) 123:777–94.
207. Liu X, Xie G, Zhang K, Song S, Song F, Jin Y, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: a meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care. (2017) 38:190–6. doi: 10.1016/j.jcrc.2016.10.026
208. He H, Peng W, Luan H, Shi C, Tu W. The effect of dexmedetomidine on haemodynamics during intracranial procedures: a meta-analysis. Brain Inj. (2018) 32:1843–8.
209. Toller WG, Stranz C. Levosimendan, a new inotropic and vasodilator agent. Anesthesiology. (2006) 104:556–69.
210. Bhamidipati CM, LaPar DJ, Stukenborg GJ, Morrison CC, Kern JA, Kron IL, et al. Superiority of moderate control of hyperglycemia to tight control in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. (2011) 141:543–51.
211. Song JW, Shim JK, Yoo KJ, Oh SY, Kwak YL. Impact of intraoperative hyperglycaemia on renal dysfunction after off-pump coronary artery bypass. Interact Cardiovasc Thorac Surg. (2013) 17:473–8. doi: 10.1093/icvts/ivt209
212. Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care. (2015) 38:1665–72. doi: 10.2337/dc15-0303
Keywords: acute kidney injury, cardiac surgery, dexmedetomidine, natriuretic peptide, remote ischaemic preconditioning
Citation: Chen J-J, Lee TH, Kuo G, Huang Y-T, Chen P-R, Chen S-W, Yang H-Y, Hsu H-H, Hsiao C-C, Yang C-H, Lee C-C, Chen Y-C and Chang C-H (2022) Strategies for post–cardiac surgery acute kidney injury prevention: A network meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 9:960581. doi: 10.3389/fcvm.2022.960581
Received: 03 June 2022; Accepted: 12 September 2022;
Published: 27 September 2022.
Edited by:
Vincenzo Lionetti, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Alessandro Carrozzo, Istituto Clinico Ligure di Alta Specialità, ItalyGiuseppe Vergaro, Gabriele Monasterio Tuscany Foundation (CNR), Italy
Copyright © 2022 Chen, Lee, Kuo, Huang, Chen, Chen, Yang, Hsu, Hsiao, Yang, Lee, Chen and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Hsiang Chang, ZnJhbndpc2FuZHN1bkBnbWFpbC5jb20=
†These authors share first authorship
 Jia-Jin Chen
Jia-Jin Chen Tao Han Lee
Tao Han Lee George Kuo
George Kuo Yen-Ta Huang
Yen-Ta Huang Pei-Rung Chen5
Pei-Rung Chen5 Huang-Yu Yang
Huang-Yu Yang Ching-Chung Hsiao
Ching-Chung Hsiao Cheng-Chia Lee
Cheng-Chia Lee Chih-Hsiang Chang
Chih-Hsiang Chang