
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 August 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.954704
This article is part of the Research Topic Precision Medicine for Antithrombotic Therapy in Patients after Percutaneous Coronary Interventions View all 10 articles
 Seung-Jun Lee1†
Seung-Jun Lee1† Dong-Woo Choi2,3†
Dong-Woo Choi2,3† Choongki Kim4
Choongki Kim4 Yongsung Suh5
Yongsung Suh5 Sung-Jin Hong1
Sung-Jin Hong1 Chul-Min Ahn1
Chul-Min Ahn1 Jung-Sun Kim1
Jung-Sun Kim1 Byeong-Keuk Kim1
Byeong-Keuk Kim1 Young-Guk Ko1
Young-Guk Ko1 Donghoon Choi1
Donghoon Choi1 Eun-Cheol Park2
Eun-Cheol Park2 Yangsoo Jang6
Yangsoo Jang6 Chung-Mo Nam2*
Chung-Mo Nam2* Myeong-Ki Hong1*
Myeong-Ki Hong1*Background: Optimal duration of dual antiplatelet therapy (DAPT) in patients with diabetes mellitus (DM) who have undergone drug-eluting stent (DES) implantation is not clearly established. This study sought to impact of DAPT duration on real-world clinical outcome in patients with or without DM.
Methods: Using a nationwide cohort database, we investigate the association between DAPT duration and clinical outcome between 1 and 3 years after percutaneous coronary intervention (PCI). Primary outcome was all-cause death. Secondary outcomes were cardiovascular death, myocardial infarction, and composite bleeding events. After weighting, 90,100 DES-treated patients were included; 29,544 patients with DM and 60,556 without DM; 31,233 patients with standard DAPT (6–12 months) and 58,867 with prolonged DAPT (12–24 months).
Results: The incidence of all-cause death was significantly lower in patients with prolonged DAPT [8.3% vs. 10.5% in those with standard DAPT, hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.72–0.84] in diabetic patients and non-diabetic patients (4.5% vs. 5.0% in those with standard DAPT, HR 0.89, 95% CI 0.83–0.96). The incidence of composite bleeding events was 5.7% vs. 5.4%, respectively, (HR 1.07, 95% CI 0.96–1.18) in diabetic patients and 5.6% vs. 5.0%, respectively, in non-diabetic patients (HR 1.13, 95% CI 1.05–1.21). There was a significant interaction between the presence of DM and DAPT duration for all-cause death (p for interaction, pint = 0.01) that further favored prolonged DAPT in diabetic patients. However, there was no significant interaction between the presence of DM and DAPT duration for composite bleeding events (pint = 0.38).
Conclusions: This study showed that prolonged rather than standard DAPT might be clinically beneficial in diabetic patients with DES implantation.
Trial registration: ClinicalTrial.gov (NCT04715594).
Diabetes mellitus (DM) is a major risk factor for atherosclerotic cardiovascular disease including coronary artery disease (CAD). Therefore, diabetic patients have a greater prevalence of CAD and account for a substantial proportion of percutaneous coronary intervention (PCI) with drug-eluting stent (DES) in daily clinical practice (1). Even though PCI was performed successfully, the prognosis of diabetic patients showed worse clinical outcomes with higher rates of mortality, cardiovascular events and stent thrombosis during long-term follow-up (2). High platelet reactivity and activation, hypercoagulability (prothrombotic state) and suboptimal response to standard antiplatelet agents might be related to a high rate of adverse cardiovascular events in patients with DM (3). Prolonged dual antiplatelet therapy (DAPT) for more than 1 year was proposed to reduce the occurrence of adverse cardiovascular events in diabetic patients in the past (4, 5). However, use of next-generation DESs has markedly improved clinical outcome after PCI in high-risk patients including those with DM (6). A minimum duration of DAPT is currently advocated in professional guideline documents and adopted worldwide for management of patients receiving DES (7–9). The current guideline suggests that DM should not be the only appraised patient-specific feature when deciding upon the type or duration of DAPT (7). Despite the increased risk of adverse clinical events after PCI in patients with DM compared to those without, the data to support the need for prolonged DAPT (>12 months) are not sufficient in the era of next-generation DES. Therefore, we sought to investigate real-world clinical outcomes according to duration of DAPT in diabetic patients who underwent next-generation DES implantation in a large-volume nationwide cohort that covers the entire population who received first- and next-generation DES implantation for CAD in Korea (CONNECT DES cohort registry).
This study was a nationwide retrospective analysis of the national health claims database established by the National Health Insurance Service (NHIS) of Korea, which contains claimed medical costs, drug prescription and adherence, use of medical devices including types of DES, and medical history presented as International Classification of Diseases, Tenth Revision (ICD-10) codes of nearly all inhabitants. Most of the Korean population (97.1%) must subscribe to the NHIS, which is a sole insurer managed by the Korean government. Given that NHIS also covers information for the remaining population (2.9%) categorized as medical aid subjects, this cohort is considered to represent the entire Korean population (10). We were also provided the death certificates and ICD-10 codes from the National Institute of Statistics of Korea. This study was approved by the Institutional Review Board of our institute. Informed consent was waived because personal information was masked after cohort generation according to the strict confidentiality guidelines of the Korean Health Insurance Review and Assessment Service. This study is registered at ClinicalTrial.gov (NCT04715594).
The flow of this study is depicted in Figure 1. From the 52 million inhabitants included in the Korean NHIS database, this study included 273,670 patients (≥20 years old) who were treated with DES between January 2005 and December 2016 in Korea (CONNECT DES cohort registry). The list of included or excluded next-generation DES is presented in Supplementary Table 1. The both types of DESs with biodegradable polymer and durable polymers were included, however, polymer-free DESs were not included in this study. First-generation DES implantation was more frequently performed between 2005 and 2009. Next-generation DESs were more frequently implanted between 2010 and 2016. Following government policy, all information including patients' medical history, drug prescription, and use of medical devices including DES were provided after encryption to protect personal information.
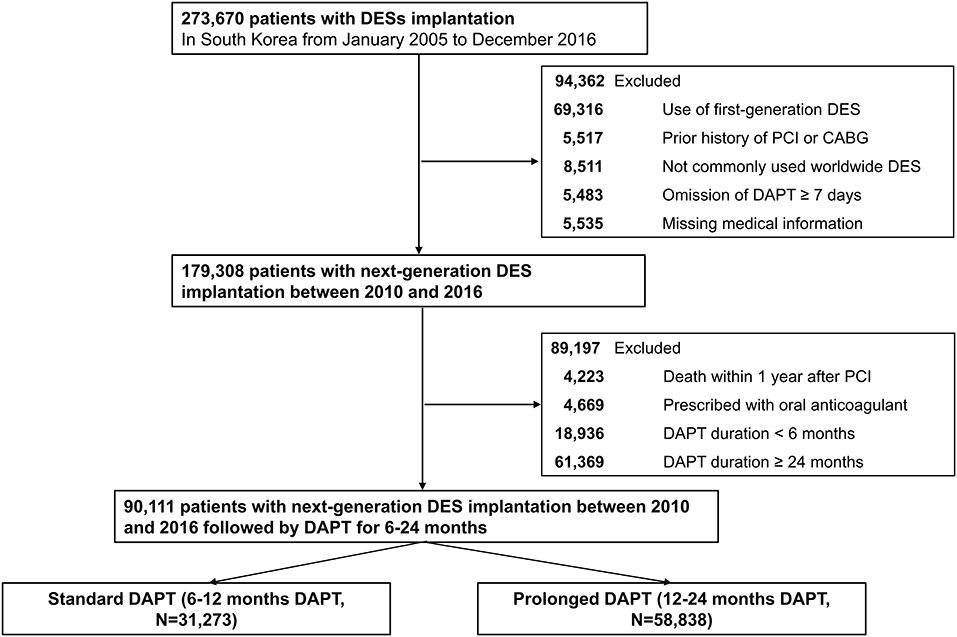
Figure 1. A flowchart of the study population. DES, drug-eluting stent; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; DAPT, dual antiplatelet therapy; Standard DAPT, DAPT between 6 and 12 months; Prolonged DAPT, DAPT between 12 and 24 months.
Of the 273,690 patients who underwent DES implantation between 2005 and 2016, 94,362 were excluded for implanted with first-generation DES (n = 69,316); previous history of PCI or coronary artery bypass surgery (n = 5,517) because clinical events during follow-up cannot be discriminated whether those were caused by a prior PCI (coronary artery bypass surgery) or index PCI; implanted with DES that are not commonly used worldwide (n = 8,511); omitted DAPT ≥ 7 days (n = 5,483); and missing medical information (n = 5,535). Then, 179,308 patients who were treated with next-generation DES remained. To minimize immortal time bias, we excluded those who died within 1 year after PCI from the analyses (n = 4,223). Patients who were prescribed with oral anticoagulant (n = 4,669), DAPT for <6 months (n = 18,936) or DAPT for ≥24 months (n = 61,369) were further excluded to minimize selection bias. Consequently, the remaining 90,111 patients who received next-generation DES implantation with DAPT for 6–24 months (standard DAPT, DAPT between 6–12 months, n = 31,273; prolonged DAPT, DAPT between 12–24 months, n = 58,838, Figure 1) were included in the analyses.
Patients with DM were defined as those who received oral hypoglycemic agents and/or injection of insulin. The duration of DAPT was identified using prescription data with Korean Drug and Anatomical Therapeutic Chemical codes (11). If prescription of aspirin along with any P2Y12 inhibitor (clopidogrel, ticagrelor, or prasugrel) has been continued for ≥1 year after index PCI without discontinuation for more than 7 days, we considered the patient to be treated with prolonged DAPT. To minimize immortal time bias, we set the primary follow-up period as 12 to 36 months after index PCI. Patients who experienced ischemic or bleeding events and were alive within 1 year after index PCI were included in the analyses considering the recurrent nature of these clinical events. The history of those clinical events within 1 year after index PCI was adjusted for analyses of primary or secondary outcomes. Primary outcome was all-cause death. Secondary outcomes were composite ischemic events (cardiovascular death, myocardial infarction, or ischemic stroke), composite bleeding events (hemorrhagic stroke, gastrointestinal bleeding, or genitourinary bleeding requiring admission or medical intervention), and each component of an ischemic or bleeding event. Cardiovascular death was ascertained from the National Statistical Office of Korea, which provided death certificates with an accuracy of 92% for the specific cause of death (10, 12). Cardiovascular death was identified by a death certificate with at least one cardiovascular-related diagnosis (acute myocardial infarction, stroke, heart failure, or sudden cardiac death) (13). Myocardial infarction was defined as the simultaneous development of ICD-10 codes corresponding to acute myocardial infarction (11), claims for coronary angiography, admission via the emergency department, and more than four rounds of cardiac biomarker testing. A detailed description for each clinical outcome is presented in Supplementary Table 2. We further included baseline comorbidities and drug prescription status before PCI for propensity score calculation, and inverse probability treatment of weighting (IPTW) was used to account for differences in baseline characteristics, medical history, and confounding bias (11, 13). Details regarding covariates included in the propensity score calculation are described in Supplementary Table 3.
Continuous variables are reported as mean and standard deviation, while dichotomous variables are presented as frequency and percentage. Conformity to the normal distribution was assessed for continuous variables using the Kolmogorov-Smirnov test. To minimize the effect of confounding bias, we calculated the IPTW by the propensity score, which was calculated by logistic regression with covariates including age, sex, history of comorbidities and medications, and year of PCI (Supplementary Table 3). We also stabilized the weights by multiplying IPTW by the marginal probability of receiving treatment. The effect size difference between the two groups for all comorbidities and medications was calculated using the standardized mean difference (SMD) and Kernel density plots. SMD values above 0.2 were regarded as a potential imbalance between the two groups. Cumulative incidence curves and the rate of all-cause death, cardiovascular death, myocardial infarction, and composite bleeding events during follow-up were plotted using the Kaplan–Meier method. The adjusted hazard ratio (HR) for each clinical outcome of interest was calculated using a multivariable Cox proportional hazard regression model. The cause-specific hazard model was used to consider death as a competing risk when comparing the incidences of cardiovascular death and other components of secondary outcomes. A two-sided p-value of <0.05 was considered significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.6 (The R Foundation, www.R-project.org).
After weighting, 90,100 DES-treated patients included 29,544 patients with DM and 60,556 patients without; 31,233 patients with standard DAPT and 58,867 patients with prolonged DAPT. Baseline demographics and medical history of the whole cohort population before and after stabilized IPTW are presented in Supplementary Table 4. After stabilized IPTW, there was no evidence of inequality in the baseline demographic characteristics and medical history between the two groups including the year of PCI and characteristics of DES (all SMD <0.1, Supplementary Figures 1, 2). Furthermore, baseline characteristics were well balanced among the patients receiving standard and prolonged DAPT with or without DM (all SMD <0.1, Table 1). The incidence and relative hazards of all-cause death, cardiovascular death, myocardial infarction, and composite bleeding events at 2 and 3 years after PCI between the two groups in patients with or without DM are presented in Table 2 and Figure 2.
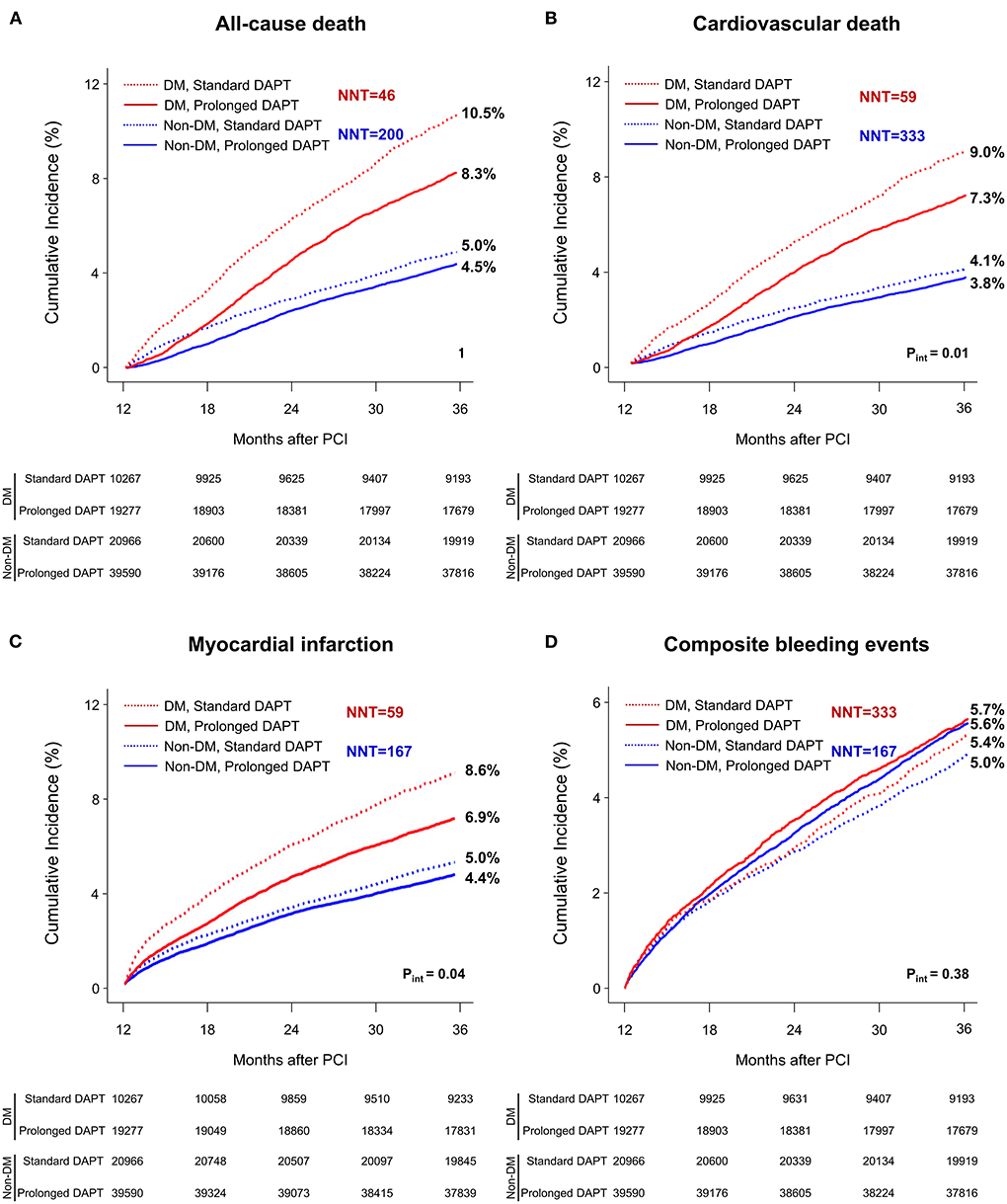
Figure 2. Time-to-event curves for all-cause death, cardiovascular death, myocardial infarction, or composite bleeding events between 1 and 3 years after PCI. The cumulative incidence of (A) all-cause, (B) cardiovascular mortality, (C) myocardial infarction and (D) composite bleeding events between 1 and 3 years after PCI. DAPT, dual antiplatelet therapy; DM, diabetes mellitus; PCI, percutaneous coronary intervention. NNT, number need to treat; NNH, number need to harm.
At 3 years after PCI in patients without DM, the incidence of all-cause death, cardiovascular death and myocardial infarction was significantly lower in patients treated with prolonged DAPT [4.5% vs. 5.0% with standard DAPT, HR 0.89, 95% confidence interval (CI) 0.83–0.96; 3.8% vs. 4.1%, HR 0.91, 95% CI 0.84–0.99; 4.4% vs. 5.0%, HR 0.88, 95% CI 0.81–0.95, respectively].
However, the incidence of composite bleeding events was significantly greater in patients treated with prolonged DAPT (5.6% vs. 5.0% with standard DAPT, HR 1.13, 95% CI 1.05–1.21).
At 3 years after PCI in patients with DM, the incidence of all-cause death, cardiovascular death and myocardial infarction was significantly lower in patients treated with prolonged DAPT (8.3% vs. 10.5% with standard DAPT, HR 0.78, 95% CI 0.72–0.84; 7.3% vs. 9.0% HR 0.79, 95% CI 0.73–0.86; 6.9% vs. 8.6%, HR 0.78, 95% CI 0.71–0.85, respectively). There was no statistically significant difference in incidence of composite bleeding events between patients treated with prolonged DAPT and those treated with standard DAPT (5.7% vs. 5.4%, respectively, HR 1.07, 95% CI 0.96–1.18).
The number need to treat for preventing one case of all-cause death was 46 and 200 for patients with and without DM, respectively. The number need to treat or harm for other clinical outcomes among patients with or without DM are presented in Figure 1.
There was a significant interaction between the presence of DM and DAPT duration for all-cause death (p for interaction, pint = 0.01), cardiovascular death (pint = 0.02) and myocardial infarction (pint = 0.04) that favored prolonged DAPT in patients with DM. However, there was no significant interaction between the presence of DM and DAPT duration for composite bleeding events (pint = 0.38). In a subgroup analysis of diabetic patients, there was no significant interaction between the baseline comorbidities and DAPT duration for all-cause death (Figure 3) or cardiovascular death (Supplementary Figure 3).
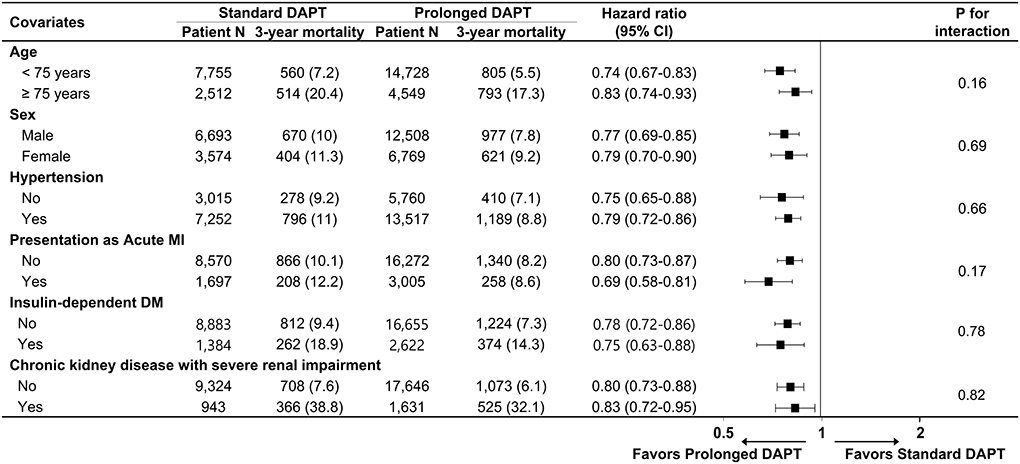
Figure 3. Subgroup analysis for all-cause death in diabetic patients. Numbers and percentages show the number of patients at risk and the all-cause mortality rate between 1 and 3 years after drug-eluting stent implantation. CI, confidence interval; MI, myocardial infarction; DM, diabetes mellitus.
This nationwide cohort analysis assessed mortality and clinical outcomes of standard vs. prolonged DAPT in diabetic patients with next-generation DES implantation. To the best of our knowledge, the results of our analyses were derived from a cohort that included a largest number of diabetic patients who underwent next-generation DES implantation. This study included whole patients who were concurrently encountered in a catheterization laboratory and were very-high-risk (high bleeding risk, end-stage renal disease, and very elderly patients, etc.) who were usually excluded from other randomized studies. The major findings of our study are as follows: (1) in patients with DM, prolonged DAPT (vs. standard DAPT) was associated with lower all-cause mortality, cardiovascular mortality, and myocardial infarction without an increase in composite bleeding events. (2) In patients without DM, prolonged DAPT (vs. standard DAPT) was associated with a decrease in all-cause mortality and cardiovascular mortality and an increase in composite bleeding events.
Compared to bare-metal stents or first-generation DES, the favorable mechanochemical characteristics of next-generation DES have significantly reduced concern for stent thrombosis (6, 14). In this regard, a growing concern for the risk of bleeding according to prolonged DAPT has emerged as an important issue for long-term management of patients who underwent PCI, and attempts to balance ischemic and bleeding events have led to further shortening of the duration of DAPT (15, 16). A recent meta-analysis with 24 randomized trials reported that extended-term (>12 months) DAPT was associated with a reduced risk of myocardial infarction and a higher risk of major bleeding in comparison with short-term (<6 months) or standard (6–12 months) DAPT (17). There was no significant difference in mortality between the patients with extended-term DAPT and those with short-term or standard DAPT (17). In this regard, the bleeding risk of individual patients, as well as ischemic risk, are taken into consideration for deciding the appropriate length of DAPT (18, 19).
DM is a well-recognized key risk factor for CAD and worse prognosis after PCI (8), which is responsible for systemic atherosclerotic change of the entire vascular structure (20). Therefore, management for DM includes multifactorial life style modification together with intensive medical intervention through glucose lowering agents, lipid-lowering agents, and blood pressure-lowering agents. Indeed, adequate control of DM through sodium-glucose cotransporter-2 inhibitor (21) or glucagon-like peptide-1 receptor agonists (22) are known to reduce the risk of ischemic stroke or cardiovascular death as well as recurrent myocardial infarction, implying that systemic treatment (drugs) rather than local treatment (PCI) is essential for management of diabetic patients with cardiovascular complications. Altered systemic metabolism in patients with DM is associated with hypercoagulability, endothelial dysfunction, and platelet activation, together resulting in a prothrombotic state (23) that possibly requires long-term anti-thrombotic therapy or a more potent P2Y12 inhibitor. Furthermore, patients with DM have been reported to have a suboptimal response to aspirin or clopidogrel, probably due to the altered metabolic and pharmacokinetic profile (3, 24).
Despite the theoretical benefit of long-term DAPT in diabetic patients with DES implantation, to date, the clinical benefit of long-term DAPT in the era of next-generation DES has not been clearly demonstrated. At an individual patient-level meta-analysis that compared the clinical outcome between short-term (<6 months) and standard (6–12 months) DAPT in patients with and without DM after DES implantation, standard DAPT resulted in an augmented risk of bleeding without significantly reducing the ischemic events (8). All-cause or cardiovascular mortality within 1 year after PCI were not different among patients treated with short-term or standard DAPT regardless of presence of DM (7). However, in a post-hoc analysis of a randomized DAPT trial that investigated the clinical outcome between 12 and 30 months DAPT after PCI, extended DAPT (30 months) was associated with reduced risk of recurrent myocardial infarction in diabetic patients (25). Another post-hoc analysis for a randomized DAPT trial identified DM as a significant predictor for future coronary thrombotic events, and DM was incorporated as one of the positive predictors that would benefit from extended DAPT (26). Compared to the present study, part of the study population included in previous randomized studies were patients treated with first-generation DES. The previous randomized studies did not include clinically very-high-risk patients who might be expected to show worse prognosis despite successful DES implantation during long-term follow-up (8, 25, 26). Therefore, the findings of previous studies might have difficulty representing the current situation in an era of next-generation DES. Additionally, in contrast to a previous meta-analysis report from randomized trials that have mostly investigated 1-year clinical outcomes after index PCI in diabetic patients (8), our nationwide cohort analysis investigated the clinical outcome between 1 and 3 years after next-generation DES implantation in diabetic patients. Given that DM is a long-lasting risk factor that continuously hampers prognosis after PCI (27), investigating the clinical impact of DAPT in this period could be of noteworthy importance. Furthermore, the DAPT trial investigated the clinical outcomes between 12 and 30 months DAPT after index PCI excluding the patients who experienced ischemic or bleeding events before 12 months after index PCI (28). Whereas, the present study included patients who were alive and had experienced ischemic or bleeding events within 1 year after PCI and were considered to harbor clinical or procedural risk factors for future hard events (all-cause or cardiovascular death) (29).
In general, prolonged DAPT after PCI is related to significantly increased risk of bleeding compared to short or standard DAPT (30). However, the results of this study demonstrated that the significant reduction of ischemic events by prolonged DAPT in diabetic patients led to favorable outcome including reduced mortality, overwhelming the numerically, but statistically not significant, increased bleeding events. Indeed, there is no obvious correlation between the diabetes and augmented bleeding risk by DAPT after PCI (31). Taken together, the findings indicate that, after DES implantation, prolonged DAPT could be further favored in diabetic patients, as compared with non-diabetic patients to alleviate the risk of recurrent ischemic events and consequent cardiovascular or all-cause mortality.
This study has several limitations. First, observational studies that evaluated the clinical impact of DAPT after PCI are possibly prone to immortal time bias, although we excluded those who died within 1 year after PCI. Second, clinical events that occurred early after PCI or the patient's own characteristics might have influenced the physician's decision for the duration of DAPT. In this regard, there could be persistent residual confounding factors, although we tried to minimize the bias using stabilized IPTW. Third, because the NHIS database does not routinely collect laboratory profiles, the level of glycosylated hemoglobin A1c that represents the severity of DM, was not included as a covariate for the stabilized IPTW model or Cox regression analysis. However, since the Korean health insurance system strictly regulates the use of oral hypoglycemic agent according to the level of glycosylated hemoglobin A1c, it is presumable that the imbalance of DM severity between the two groups would be limited as we defined DM according to the performance of treatment rather than the presence of diagnostic codes. Furthermore, laboratory information regarding platelet reactivity that could give explanation for the suboptimal outcome of diabetic patients after cessation of DAPT was not available. Fourth, contemporary bleeding classification system with prognostic impact [e.g. BARC (Bleeding Academic Research Consortium), TIMI (Thrombolysis in Myocardial Infarction), GUSTO (Global Use of Strategies to Open Occluded Arteries), etc] could not be applied due to limited information. Finally, the occurrence of stent thrombosis was also could not be investigated due to lack of angiographic information. Taken together, the results from this observational study should not be used to establish a causal relationship, until our findings are recapitulated by well-conducted randomized studies.
In this nationwide cohort of patients treated with new-generation DES in Korea, prolonged DAPT rather than standard DAPT might be clinically beneficial in diabetic patients with DES implantation.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated for the analyses are not publicly available because of strict government restrictions. Requests to access these datasets should be directed to bWtob25nNjFAeXVocy5hYw==.
The studies involving human participants were reviewed and approved by Yonsei University Health System. The Ethics Committee waived the requirement of written informed consent for participation.
S-JL, D-WC, C-MN, and M-KH contributed to the conception and design, verified the data, and conducted all analyses. S-JL and M-KH wrote the study protocol. D-WC and C-MN performed the programming to extract the data from the NHIS database. M-KH and C-MN had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have provided a critical review of the manuscript, read, and approved the final publication.
This work was supported by the Cardiovascular Research Center, Seoul, Korea. The funder had no role in the design and conduct of the study, data collection, management, statistical analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.954704/full#supplementary-material
1. Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American heart association. Circulation. (2020) 141:e779–806. doi: 10.1161/CIR.0000000000000766
2. Ritsinger V, Saleh N, Lagerqvist B, Norhammar A. High event rate after a first percutaneous coronary intervention in patients with diabetes mellitus: results from the Swedish coronary angiography and angioplasty registry. Circ Cardiovasc Interv. (2015) 8:e002328. doi: 10.1161/CIRCINTERVENTIONS.114.002328
3. Jung JH, Tantry U, Gurbel PA, Jeong YH. Current antiplatelet treatment strategy in patients with diabetes mellitus. Diabetes Metab J. (2015) 39:95–113. doi: 10.4093/dmj.2015.39.2.95
4. Palmerini T, Stone GW. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: conceptual evolution based on emerging evidence. Eur Heart J. (2016) 37:353–64. doi: 10.1093/eurheartj/ehv712
5. Angiolillo DJ, Galli M, Collet JP, Kastrati A, O'Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. (2022) 17:e1371–96. doi: 10.1253/circj.CJ-21-0751
6. Palmerini T, Benedetto U, Biondi-Zoccai G, Riva DD, Bacchi-Reggiani L, Smits PC, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. (2015) 65:2496–507. doi: 10.1016/j.jacc.2015.04.017
7. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
8. Gargiulo G, Windecker S, da Costa BR, Feres F, Hong MK, Gilard M, et al. Short term vs. long term dual antiplatelet therapy after implantation of drug eluting stent in patients with or without diabetes: systematic review and meta-analysis of individual participant data from randomised trials. BMJ. (2016) 355:i5483. doi: 10.1136/bmj.i5483
9. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2016) 68:1082–115. doi: 10.1016/j.jacc.2016.03.513
10. Choi EK. Cardiovascular research using the Korean national health information database. Korean Circ J. (2020) 50:754–72. doi: 10.4070/kcj.2020.0171
11. Kim J, Kang D, Park H, Kang M, Park TK, Lee JM, et al. Long-term beta-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J. (2020) 41:3521–9. doi: 10.1093/eurheartj/ehaa376
12. Won TY, Kang BS, Im TH, Choi HJ. The study of accuracy of death statistics. Korean J Emerg Med. (2007) 18:256–62.
13. You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. (2020) 324:1640–50. doi: 10.1001/jama.2020.16167
14. Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. (2019) 393:2503–10. doi: 10.1016/S0140-6736(19)30474-X
15. Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2016) 134:e156–78. doi: 10.1016/j.jacc.2016.03.512
16. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs. ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: The TICO randomized clinical trial. JAMA. (2020) 323:2407–16. doi: 10.1001/jama.2020.7580
17. Khan SU, Singh M, Valavoor S, Khan MU, Lone AN, Khan MZ, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation. (2020) 142:1425–36. doi: 10.1161/CIRCULATIONAHA.120.046308
18. Costa F, Van Klaveren D, Feres F, James S, Räber L, Pilgrim T, et al. Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol. (2019) 73:741–54. doi: 10.1016/j.jacc.2018.11.048
19. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy. J Am Coll Cardiol. (2018) 72:2915–31. doi: 10.1016/j.jacc.2018.09.057
20. Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The changing landscape of diabetes therapy for cardiovascular risk reduction. J Am Coll Cardiol. (2018) 72:1856–69. doi: 10.1016/j.jacc.2018.07.071
21. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
22. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
23. Domingueti CP, Dusse LMS, Carvalho MD, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. (2016) 30:738–45. doi: 10.1016/j.jdiacomp.2015.12.018
24. Bhatt DL, Grosser T, Dong JF, Logan D, Jeske W, Angiolillo DJ, et al. Enteric coating and aspirin nonresponsiveness in patients with type 2 diabetes mellitus. J Am Coll Cardiol. (2017) 69:603–12. doi: 10.1016/j.jacc.2016.11.050
25. Meredith IT, Tanguay JF, Kereiakes DJ, Cutlip DE, Yeh RW, Garratt KN, et al. Diabetes mellitus and prevention of late myocardial infarction after coronary stenting in the randomized dual antiplatelet therapy study. Circulation. (2016) 133:1772–82. doi: 10.1161/CIRCULATIONAHA.115.016783
26. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. (2016) 315:1735–49. doi: 10.1001/jama.2016.3775
27. Thukkani AK, Agrawal K, Prince L, Smoot KJ, Dufour AB, Cho K, et al. Long-term outcomes in patients with diabetes mellitus related to prolonging clopidogrel more than 12 months after coronary stenting. J Am Coll Cardiol. (2015) 66:1091–101. doi: 10.1016/j.jacc.2015.06.1339
28. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. (2014) 371:2155–66. doi: 10.1056/NEJMoa1409312
29. D'Ascenzo F, De Filippo O, Gallone G, Mittone G, Deriu MA, Iannaccone M, et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): a modelling study of pooled datasets. Lancet. (2021) 397:199–207. doi: 10.1016/S0140-6736(20)32519-8
30. Benenati S, Galli M, De Marzo V, Pescetelli F, Toma M, Andreotti F, et al. Very short vs. long dual antiplatelet therapy after second generation drug-eluting stents in 35,785 patients undergoing percutaneous coronary interventions: a meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. (2021) 7:86–93. doi: 10.1093/ehjcvp/pvaa001
31. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, et al. PRECISE-DAPT study investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. (2017) 389:1025–34. doi: 10.1016/S0140-6736(17)30397-5
Keywords: drug-eluting stents, diabetes mellitus, dual antiplatelet therapy, coronary artery disease, treatment outcome
Citation: Lee S-J, Choi D-W, Kim C, Suh Y, Hong S-J, Ahn C-M, Kim J-S, Kim B-K, Ko Y-G, Choi D, Park E-C, Jang Y, Nam C-M and Hong M-K (2022) Prolonged dual antiplatelet therapy after drug-eluting stent implantation in patients with diabetes mellitus: A nationwide retrospective cohort study. Front. Cardiovasc. Med. 9:954704. doi: 10.3389/fcvm.2022.954704
Received: 27 May 2022; Accepted: 29 June 2022;
Published: 11 August 2022.
Edited by:
Mattia Galli, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Stefano Benenati, San Martino Hospital (IRCCS), ItalyCopyright © 2022 Lee, Choi, Kim, Suh, Hong, Ahn, Kim, Kim, Ko, Choi, Park, Jang, Nam and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myeong-Ki Hong, bWtob25nNjFAeXVocy5hYw==; Chung-Mo Nam, Y21uYW1AeXVocy5hYw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.