
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 25 July 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.950389
 Chien-Yi Hsu1,2,3
Chien-Yi Hsu1,2,3 Hung-Yu Chang4,5
Hung-Yu Chang4,5 Chieh-Ju Chao6
Chieh-Ju Chao6 Wei-Ru Chiou7,8,9
Wei-Ru Chiou7,8,9 Po-Lin Lin7,8,10
Po-Lin Lin7,8,10 Fa-Po Chung5,11
Fa-Po Chung5,11 Wen-Yu Lin12
Wen-Yu Lin12 Jin-Long Huang5,13,14
Jin-Long Huang5,13,14 Huai-Wen Liang15
Huai-Wen Liang15 Chia-Te Liao16
Chia-Te Liao16 Ying-Hsiang Lee7,17,18*
Ying-Hsiang Lee7,17,18*Objective: The aim of this study was to investigate the application of sacubitril/valsartan in clinical practice and the utility of PREDICT-HF score for outcome prediction in Asian heart failure patients with difference risk profiles.
Methods: The TAROT-HF study was a multicenter, single-arm, observational study. Totally 1,187 outpatients with HFrEF treated with sacubitril/valsartan were enrolled and categorized by: (1) high-risk group with ≥1 of the following three risk factors: old age (≥80 years), low baseline systolic blood pressure (<100 mmHg), and renal impairment (eGFR <30 ml/min/1.73 m2), and (2) standard-risk group, those who did not have any risk factors. Clinical outcomes were assessed using the PREDICT-HF risk model.
Results: A total of 305 (25.7%) patients matched the criteria for the high-risk group. The event rates of cardiovascular death or first unplanned heart failure hospitalization (HFH) among the overall population, high-risk, and standard-risk groups were 13.7, 24.9, and 10.8 events per 100 patient-years, respectively. The C statistics for the PREDICT-HF model in the overall cohort and high-risk group for cardiovascular death or first unplanned HFH at 2 years were 0.73 (95% CI 0.70–0.76) and 0.71 (95% CI 0.65–0.76), respectively. The permanent discontinuation rate among the high-risk patients was significantly higher than that among the standard-risk patients (8.3 vs. 2.5 per 100 patient-years, p < 0.001).
Conclusions: Real-world outcomes of the TAROT-HF study demonstrated that the PREDICT-HF model performed well in Asian HFrEF patients. Three easily detected clinical profiles of age, renal function, and systolic BP could help to identify patients at risk before initiating sacubitril/valsartan.
Heart failure (HF) is associated with high morbidity, mortality, and prolonged and frequent hospitalizations, leading to a significant burden on health care systems worldwide (1). Evidence-based medical therapy, including renin-angiotensin system inhibitors (RASis), mineralocorticoid receptor antagonists (MRAs) and cardio-selective beta-blockers, is the most effective way to reduce mortality and morbidity of patients with heart failure and reduced ejection fraction (HFrEF) (2–8). The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial established the beneficial effect of using sacubitril/valsartan (Sac/Val) over RASis for HFrEF patients (9). Among Asian population, the Prospective comparison of ARNI with ACEi to determine the noveL beneficiaL trEatment vaLue in Japanese Heart Failure patients (PARALLEL-HF) study applied similar inclusion and exclusion criteria as the PARADIGM-HF study, and demonstrated that Sac/Val was well-tolerated in Japanese HFrEF patients (10, 11).
However, several limitations have been raised regarding the generalizability of the PARADIGM-HF trial and study population with regards to its representativeness of real-world HFrEF patients (12). For example, patients only qualified for randomization if they were normotensive, had an estimated glomerular filtration rate (eGFR) ≥30 ml/min/1.73 m2, and were able to tolerate a run-in period of enalapril 20 mg daily and a target dose of Sac/Val. Real-world patients are usually older, more fragile, and have extensive comorbidities (13). In addition, in real-world practice, hypotensive and renally impaired patients generally have higher risks than patients with normal blood pressure and renal function. However, these patient groups were under-represented in the PARADIGM-HF and PARALLEL-HF studies. Currently, long-term safety and effectiveness data from unselected Asian patients are limited.
Recently, a new prognostic prediction tool for patients with HFrEF, the PREDICT-HF (Risk of Events and Death in the Contemporary Treatment of Heart Failure) model (14), was developed using the PARADIGM-HF trial cohort and validated in other large data sets to predict mortality and morbidity. Sac/Val has been reimbursed by the Taiwanese National Health Insurance system for the treatment of HFrEF since March 2017. Therefore, the aim of this study was to investigate the application of Sac/Val in clinical practice and the utility of PREDICT-HF model for outcome prediction in Asian heart failure patients with difference risk profiles.
The TAROT-HF (Treatment with Angiotensin Receptor neprilysin inhibitor fOr Taiwan Heart Failure patients) study was a principal investigator-initiated, multicenter, real-world observational study of HFrEF patients in Taiwan. Outpatients with HFrEF who began treatment with Sac/Val were enrolled from March 2017 to December 2018. According to the Food and Drug Administration label in the given country, Sac/Val is indicated for symptomatic chronic heart failure (New York Heart Association Class II-IV symptoms) and reduced ejection fraction [left ventricular ejection fraction (LVEF) ≤ 40%]. The study design, purpose, and rationale have been described in detail in the protocol paper and previous studies (15–17). Each hospital's institutional ethics committee approved this study, which complied with the ethical principles of the Declaration of Helsinki.
In brief, a total of 1,772 HFrEF patients who initiated Sac/Val treatment at 10 hospitals between March 2017 and December 2018 were consecutively enrolled and analyzed. The eligibility criteria were: (1) age >20 years, (2) a diagnosis of symptomatic HF with NYHA Fc II-IV symptoms, (3) with documented echocardiographic LVEF ≤ 40%, and (4) first treatment with any dose of Sac/Val. Among the 1,772 patients, the clinical outcomes of 585 patients who initiated Sac/Val treatment during an acute decompensated HF hospitalization (TAROT-AHF arm) have been reported (18). In the present study, we analyzed the other 1,187 patients who initiated Sac/Val at an outpatient department (TAROT-CHF arm).
The patients were further classified into high-risk and standard-risk groups. High-risk patients were defined as those who had one or more of the following three risk factors: old age (≥80 years), low systolic blood pressure (<100 mmHg), and renal impairment (eGFR <30 ml/min/1.73 m2). Outcomes and clinical data, including cardiovascular death, all-cause mortality, HF hospitalization (HFH), and permanent discontinuation of Sac/Val were collected from the date of initiating Sac/Val to March 31, 2021.
The details of the PREDICT-HF risk model have been published previously (14). In brief, Simpson et al. performed multivariate analysis of the PARADIGM-HF study cohort, and identified several variables that could predict cardiovascular death, all-cause mortality, and the composite of cardiovascular death or HFH at both 1 and 2 years. Data from the TAROT-HF study were entered into the online calculator (http://www.predict-hf.com) to calculate the risk for each patient. Estimated outcomes at 1 and 2 years were examined and compared with observed clinical outcomes.
Continuous variables were expressed as mean value ± standard deviation, and categorical variables were reported as percentages. Differences in baseline characteristics and clinical parameters in the high-risk and standard-risk groups were tested using the chi-square test for categorical variables. The student's t-test or the Wilcoxon rank-sum test was used for comparisons of continuous data. The risks of cardiovascular death, all-cause mortality, and composite of cardiovascular death or HFH were analyzed using survival analysis with the Kaplan–Meier method and log–rank test. Multivariate Cox regression analysis was performed to assess the factors associated with clinical outcomes. The predicted vs. actual outcomes at 1 and 2 years were compared in quartiles of the PREDICT-HF risk scores. The C statistic was used to assess the discriminative ability of the PREDICT-HF model when applied to the TAROT-HF cohort to estimate outcomes at 1 and 2 years. A p-value of <0.05 was considered to be statistically significant. The statistical analyses were performed using IBM SPSS Statistics software version 24.0 (IBM SPSS, IBM Corp, Armonk, NY, USA).
A total of 1,187 HFrEF patients (mean age 61.7 ± 14.3 years, 76.3% male, mean LVEF 29.4 ± 7.1%) who initiated Sac/Val at an outpatient department from 10 hospitals between 2017 and 2018 were included in this study. Among these patients, 305 (25.7%) fulfilled the criteria for the high-risk group (253 had only one risk factor; 52 had two or three risk factors), and 882 (74.3%) patients did not match the high-risk criteria (standard-risk group). Table 1 shows detailed baseline characteristics of the study cohort. The high-risk patients were significantly older, more predominantly female, had lower body mass index, eGFR and systolic blood pressure and more severe HF symptoms, and they tended to have a history of hypertension, atrial fibrillation, HF hospitalization, peripheral arterial disease, chronic obstructive pulmonary disease, kidney disease, thyroid disease, malignancy, and hyperuricemia than the standard-risk patients.
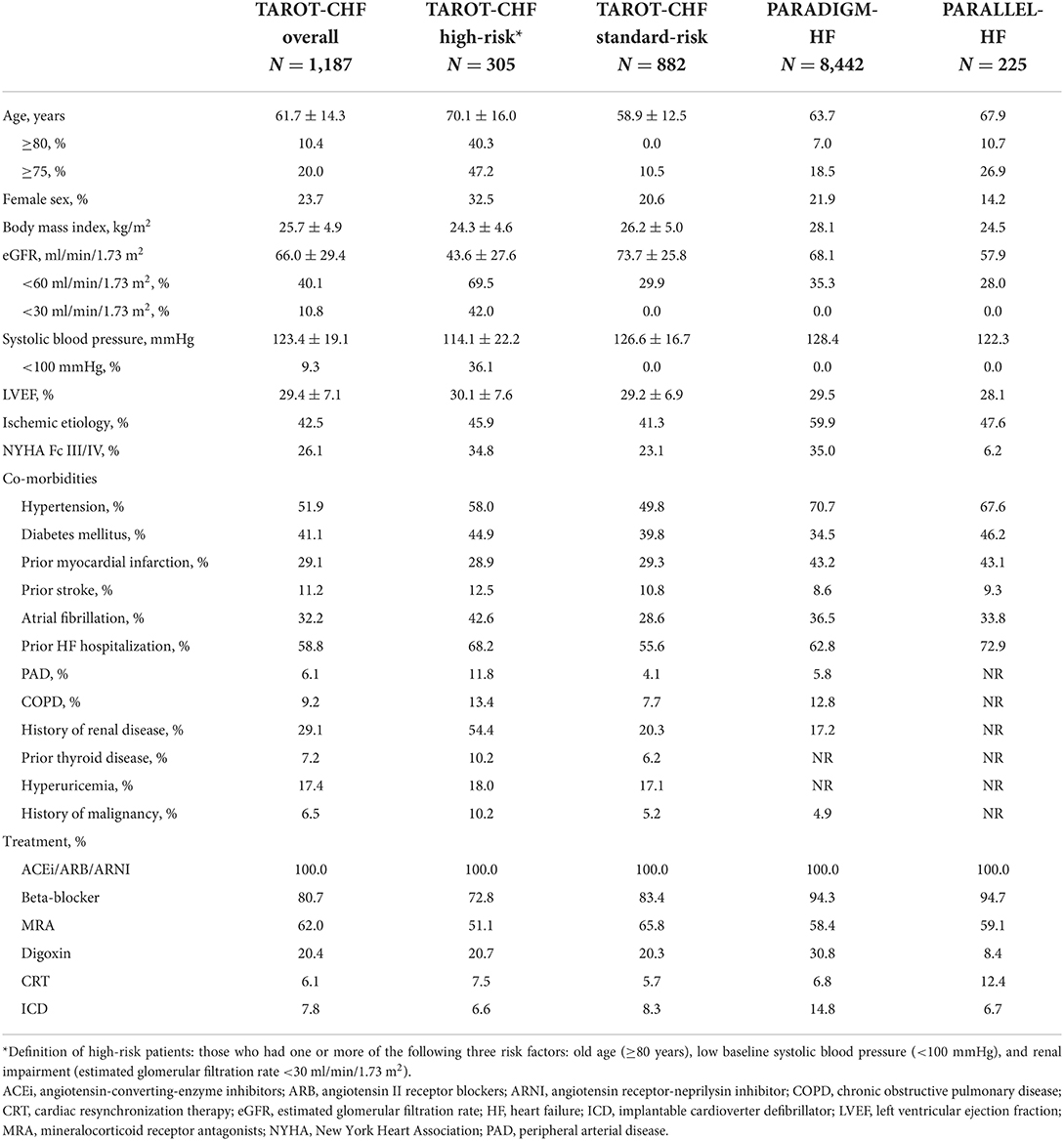
Table 1. Demographics, clinical characteristics and treatment of the current study and clinical trials of sacubitril/valsartan.
Table 1 also shows the baseline characteristics of two randomized trials of Sac/Val (PARADIGM-HF and PARALLEL-HF trials). Among the high-risk TAROT-CHF patients, 42.0% had an eGFR <30 ml/min/1.73 m2 and 36.1% had systolic blood pressure <100 mmHg. These patients were generally excluded from the PARADIGM-HF and PARALLEL-HF trials. The proportions of elderly and female patients were significantly higher in the high-risk TAROT-CHF cohort than the PARADIGM-HF and PARALLEL-HF trials.
Figure 1 shows Kaplan–Meier survival curves for clinical outcomes among the study population. Table 2 shows the event rates of the current study and other recently published randomized trials. During a median follow-up period of 36.7 months (IQR 27.5–43.3 months), the event rate of cardiovascular death or first unplanned HFH among the overall population was 13.7 events per 100 patient-years, including 10.8 and 24.9 per 100 patient-years in the standard-risk and high-risk groups, respectively. Among the high-risk patients, the event rates of those with only one risk factor and those with two or more risk factors were 21.4 and 51.6 per 100 patient-years, respectively (Figure 1A, p < 0.001). The incidence rates of all-cause mortality and cardiovascular death among the overall TAROT-CHF cohort were 5.5 and 4.0 per 100-person years, respectively (Figures 1B,C).
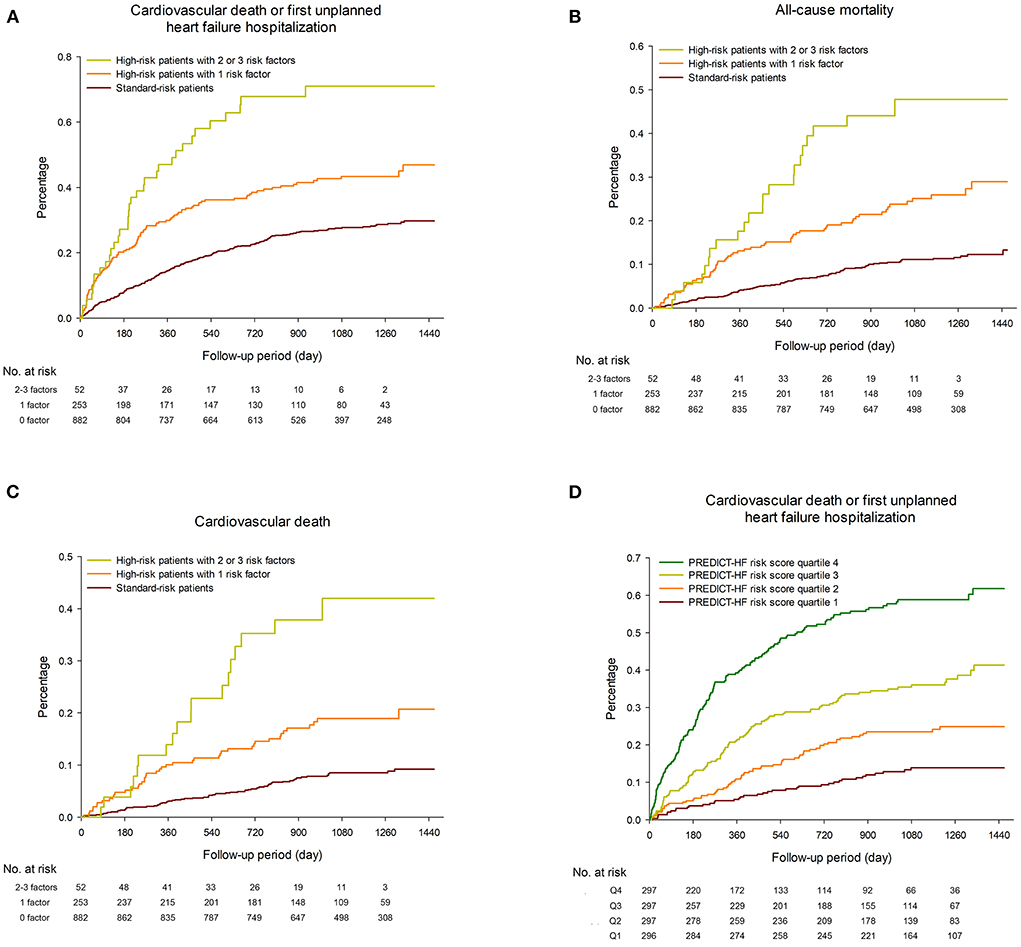
Figure 1. Kaplan–Meier survival curves for (A) cardiovascular death or first unplanned heart failure hospitalization (HFH), (B) all-cause mortality and (C) cardiovascular death among the study population, stratified by different risk groups. (D) Kaplan–Meier plots of the observed event rate in the TAROT-CHF cohort for clinical outcomes, categorized by quartile of PREDICT-HF risk score.
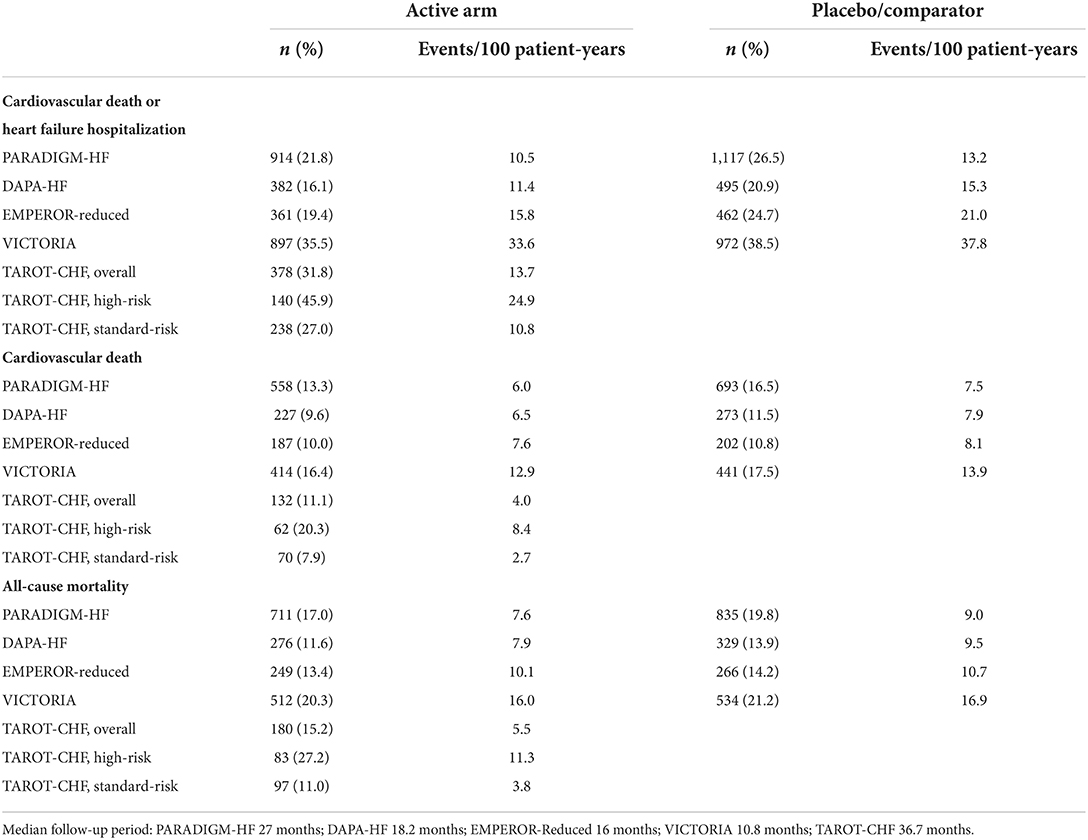
Table 2. Comparisons of outcomes among different randomized controlled trials and the current study.
Table 3 lists the parameters associated with clinical outcomes. The patients with two or more risk factors had significantly worse outcomes than those with only one risk factor, and the patients without any risk factors had a better prognosis. These differences remained significant after multivariate Cox regression analysis.
During follow-up, a total of 637 HFH events occurred in 330 patients. The incidence of total HFH events was 19.3 per 100-person years, including 75.3, 29.4, and 14.6 per 100-person years for those with two or three risk factors, one risk factor, and the standard-risk patients, respectively (p < 0.001).
An online calculator for the PREDICT-HF model was used to calculate the risks of cardiovascular death or first unplanned HFH, and cardiovascular death alone. Figure 1D shows Kaplan–Meier plots of the observed event rate in the TAROT-CHF cohort for clinical outcomes, categorized by quartile of risk score. Figures 2A,B demonstrate comparisons between the predicted and observed probabilities of cardiovascular death or first unplanned HFH across patient risk quartiles at 1 and 2 years. The C statistics for the PREDICT-HF model when applied to the study cohort for cardiovascular death or first unplanned HFH at 1 and 2 years were 0.74 (95% CI 0.70–0.77) and 0.73 (95% CI 0.70–0.76), respectively. The C statistics for cardiovascular death alone at 1 and 2 years were 0.77 (95% CI 0.72–0.82) and 0.76 (95% CI 0.71–0.80), respectively.
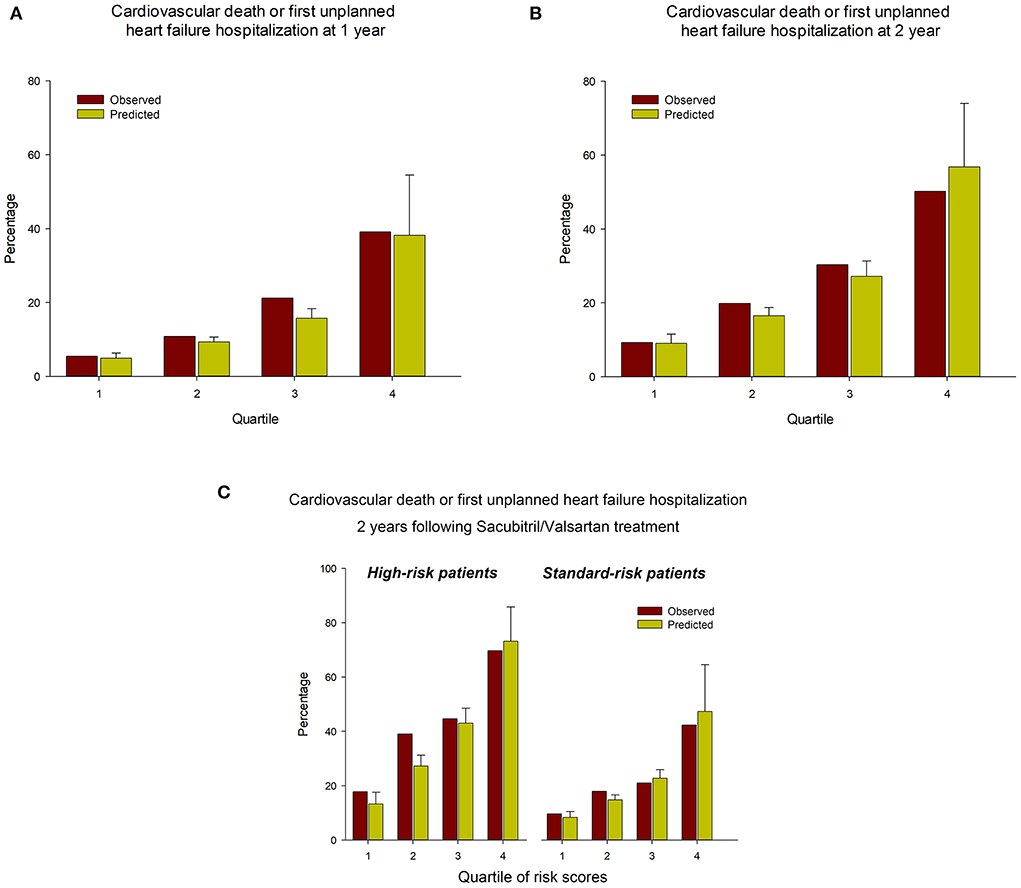
Figure 2. Comparisons between the predicted and observed probabilities of cardiovascular death or first unplanned HFH across patient risk quartiles at (A) 1 year and (B) 2 years. (C) Predicted vs. observed probabilities of cardiovascular death or first unplanned HFH, stratified by high/standard-risk and quartile of PREDICT-HF risk score.
Among the high-risk and standard-risk patients, the PREDICT-HF model could still predict outcomes accurately. The C statistics for the PREDICT-HF model in the overall cohort and high-risk group for cardiovascular death or first unplanned HFH at 1 years were 0.74 (95% CI 0.70–0.77) and 0.73 (95% CI 0.67–0.79), and at 2 years were 0.73 (95% CI 0.70–0.76) and 0.71 (95% CI 0.65–0.76), respectively. Figure 2C demonstrates the predicted vs. observed probabilities of cardiovascular death or first unplanned HFH, stratified by high/standard-risk and quartile of PREDICT-HF risk score.
The mean daily dose at the initiation of Sac/Val treatment was 116 ± 56 mg/day. Among the patients taking Sac/Val at the end of follow-up, the mean daily dose was 157 ± 87 mg/day.
A total of 114 patients permanently discontinued Sac/Val during the follow-up period (3.7 discontinuation events per 100 patient-years). The median period from Sac/Val initiation to permanent discontinuation was 169 days (IQR 42–354 days). The permanent discontinuation rate among the high-risk patients was significantly higher than that among the standard-risk patients (8.3 vs. 2.5 discontinuation events per 100 patient-years, p < 0.001, Figure 3). Another 18 patients in the study discontinued Sac/Val transiently. Among these 132 patients who discontinued Sac/Val, the reasons for discontinuation were hypotension in 50 patients (37.9%), renal impairment or hyperkalemia in 26 patients (19.7%), adverse effects or allergy in 18 patients (13.6%), and others in 38 patients (28.8%).
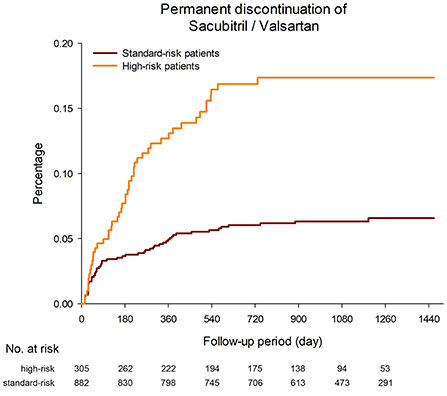
Figure 3. The permanent discontinuation rate among the high-risk patients was significantly higher than that among the standard-risk patients (8.3 vs. 2.5 discontinuation events per 100 patient-years, p < 0.001).
In the present study, 1,187 patients were enrolled during the first 2 years after the introduction of Sac/Val into Taiwan. During a median follow-up period of 36.7 months, the main findings were that the effectiveness and side-effect profile were comparable to those observed in the PARADIGM-HF trial. Although the efficacy and safety of Sac/Val for the treatment of HFrEF patients have been shown in global clinical trials, patient profiles and outcomes vary between different countries (19). This highlights the need of country-based real-world data to better understand local patient and healthcare system's characteristics.
Clinical trials have strict inclusion and exclusion criteria, whereas real-world studies enroll non-selected patients. It is therefore difficult to compare the outcomes of randomized trials and the current study directly. In this single-arm, real-world, multicentric cohort, we classified our patients into high- and standard-risk subgroups. High-risk patients including octogenarians and those with hypotension or severe renal impairment are generally excluded from randomized trials. Our results showed that these high-risk patients had significantly higher risks compared with the PARADIGM-HF trial population. In contrast, among real-world patients younger than 80 years who met the blood pressure and renal function criteria of the PARADIGM-HF trial (standard-risk patients), the observed risks were comparable to those seen in the PARADIGM-HF trial (Table 2).
In this study, we found that 25.7% had a least one of the high risk profile. Considering the beneficial effect but higher cost of using Sac/Val over RASis for HFrEF patients, this real-world prescription pattern implies that in the beginning of Sac/Val introduction, physicians tend to switch traditional RASis to Sac/Val in patients with relatively poor condition. Moreover, we also found that Sac/Val was well-tolerated among our cohort of real-world HFrEF patients. During follow-up, only 9.6% of the TAROT-CHF patients discontinued treatment permanently. Among these patients, more than 50% discontinued Sac/Val within 6 months, indicating that acute hemodynamic change should be closely monitored after the initiation of Sac/Val. Similar to our findings, a previous real-world study reported that 5.5% of their patients discontinued Sac/Val during the first 6 weeks of treatment (20). Symptomatic hypotension developed in 14% of the patients receiving Sac/Val in the PARADIGM-HF trial, but only 0.9% of the patients permanently discontinued Sac/Val during the study period. This could be because the patients who were randomized were already “selected” after the run-in period, and were therefore more likely to tolerate Sac/Val. Several factors were associated with a higher risk of medication discontinuation in the PARADIGM-HF trial, including higher natriuretic peptide levels, lower blood pressure, eGFR < 60 ml/min/1.73 m2, and an ischemic cause (21). In the current study, it is reasonable to expect that the high-risk patients had a significantly higher risk of permanent Sac/Val discontinuation than standard-risk patients due to their old age, low systolic blood pressure or low eGFR. According to post-hoc analysis of the PARADIGM-HF study, patients who received sub-target doses of Sac/Val due to intolerance had similar benefit to those who tolerated higher doses (22), suggesting that even in patients who discontinue this drug, physicians should try to re-initiate Sac/Val at a lower dose in order to obtain clinical benefits. In the current study, 13.6% of the patients who discontinued Sac/Val were able to re-initiate the drug.
Predicting the risk in HFrEF patients may allow physicians to make accurate decisions regarding the timing of guideline-recommended medical therapy adjustments and referral for advanced HF treatment. Several models have been established for predicting adverse outcomes in patients with HF (23, 24). However, most of these models did not include natriuretic peptide in the derivation model and were developed before the era of Sac/Val. The PREDICT-HF model was developed according to the PARADIGM-HF cohort and is the most current predictive model (14). This model was externally validated for all-cause mortality by the SwedeHF registry (14, 25). Although the current study enrolled high-risk patients not included in the PARADIGM-HF trial, our results clearly demonstrated that the PREDICT-HF model performed well in real-world Asian HFrEF patients. In addition, our results showed that three easily detected clinical profiles of age, renal function, and systolic blood pressure could help to identify patients at risk before initiating Sac/Val. Nevertheless, the PREDICT-HF score model could distinguish future outcomes more accurately, as shown in Figure 2.
Several limitations inherent in the retrospective design of this study should be mentioned. First, treatment decisions were based on real-world practice by the participating cardiologists. This type of retrospective study may have potential unmeasured bias, however, the objective of this study was to include a broad range of patients reflecting the current reality of real-world practice for Sac/Val rather than the narrowly defined HFrEF population in clinical trials. Second, although the baseline characteristics of the current cohort were complete without missing data for the PREDICT-HF model, some laboratory data such as total bilirubin and natriuretic peptide were not available in 30–40% of the patients.
In conclusion, among a real-world Asian population with chronic HFrEF, the PREDICT-HF score model performed well in this Asian cohort receiving contemporary HF treatment. Three easily detected clinical profiles of age, renal function, and systolic BP could help to identify patients at risk before initiating Sac/Val.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by MacKay Memorial Hospital IRB (reference number: 18MMHIS115e), Taipei Medical University Hospital IRB (reference number: TMU-JIRB_N20204142), Cheng Hsin General Hospital IRB (reference number: (615)106A-23), and Chi-Mei Medical Center IRB (reference number: 10903-010).
Conception, study design, data analysis, and interpretation: C-YH, H-YC, C-JC, and Y-HL. Project administration and data acquisition: C-YH, H-YC, W-RC, P-LL, F-PC, W-YL, J-LH, H-WL, C-TL, and Y-HL. Statistical analysis: H-YC and C-JC. Writing—original draft: C-YH and H-YC. Writing—review editing: C-JC and Y-HL. Funding acquisition: C-YH, H-YC, and Y-HL. All authors contributed to the article and approved the submitted version.
The present work was supported by the following research grants: Taiwan Society of Cardiology (TSOC 107-0505), Cheng Hsin General Hospital (CHGH111-(N)09; CHGH111-(N)10), Taipei Medical University and Taipei Medical University Hospital (109TMU-TMUH-16, 110TMU-TMUH-14, 111TMUH-MOST-21), and the Ministry of Science and Technology (MOST-110-2314-B-038-131). The funding institutions took no part in the study design, data collection or analysis, publication intent, or manuscript preparation.
We are grateful to Dr. Chao-Wen Hsueh, Dr. Wei-Tsung Lai, Dr. Hao-Neng Fu, Ms. Yi-Hua Lin, Ms. I-Ching Liu, Ms. Wei-Ting Huang, Ms. Yu-Ping Lin, Ms. Yu-Ching Lai, Ms. Fang-Hsiu Kou, and Mr. Tzu-Yuan Sung for their efforts in data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Redfield MM. Heart failure—an epidemic of uncertain proportions. N Engl J Med. (2002) 347:1442–4. doi: 10.1056/NEJMe020115
2. The CONSENSUS trial study group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. (1987) 316:1429–35. doi: 10.1056/NEJM198706043162301
3. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. (1991) 325:293–302. doi: 10.1056/NEJM199108013250501
4. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. (1999) 353:9–13. doi: 10.1016/S0140-6736(98)11181-9
5. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. (1999) 353:2001–7. doi: 10.1016/S0140-6736(99)04440-2
6. Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. (2002) 106:2194–9. doi: 10.1161/01.CIR.0000035653.72855.BF
7. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. (1999) 341:709–17. doi: 10.1056/NEJM199909023411001
8. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. (2011) 364:11–21. doi: 10.1056/NEJMoa1009492
9. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371:993–1004. doi: 10.1056/NEJMoa1409077
10. Tsutsui H, Momomura S, Saito Y, Ito H, Yamamoto K, Ohishi T, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) in Japanese patients with chronic heart failure and reduced ejection fraction: rationale for and design of the randomized, double-blind PARALLEL-HF study. J Cardiol. (2017) 70:225–31. doi: 10.1016/j.jjcc.2016.11.011
11. Tsutsui H, Momomura SI, Saito Y, Ito H, Yamamoto K, Sakata Y, et al. Efficacy and safety of sacubitril/valsartan in japanese patients with chronic heart failure and reduced ejection fraction - results from the PARALLEL-HF Study. Circ J. (2021) 85:584–94. doi: 10.1253/circj.CJ-20-0854
12. Yandrapalli S, Aronow WS, Mondal P, Chabbott DR. Limitations of sacubitril/valsartan in the management of heart failure. Am J Ther. (2017) 24:e234–9. doi: 10.1097/MJT.0000000000000473
13. Martens P, Belien H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail. (2018) 5:275–83. doi: 10.1002/ehf2.12258
14. Simpson J, Jhund PS, Lund LH, Padmanabhan S, Claggett BL, Shen L, et al. Prognostic models derived in PARADIGM-HF and validated in ATMOSPHERE and the Swedish heart failure registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol. (2020) 5:432–41. doi: 10.1001/jamacardio.2019.5850
15. Lin WY, Chung FP, Liao CT, Huang JL, Liang HW, Lee YH, et al. Treatment with angiotensin receptor neprilysin inhibitor for Taiwan heart failure patients: rationale and baseline characteristics of the TAROT-HF study. J Chin Med Assoc. (2021) 84:833–41. doi: 10.1097/JCMA.0000000000000578
16. Lee YH, Chiou WR, Hsu CY, Lin PL, Liang HW, Chung FP, et al. Different left ventricular remodeling patterns and clinical outcomes between non-ischemic and ischemic etiologies in heart failure patients receiving sacubitril/valsartan treatment. Eur Heart J Cardiovasc Pharmacother. (2022) 8:118–29. doi: 10.1093/ehjcvp/pvaa125
17. Lee YH, Lin PL, Chiou WR, Huang JL, Lin WY, Liao CT, et al. Combination of ivabradine and sacubitril/valsartan in patients with heart failure and reduced ejection fraction. ESC Heart Fail. (2021) 8:1204–15. doi: 10.1002/ehf2.13182
18. Liang HW, Liao CT, Lin WY, Chung FP, Huang JL, Lee YH, et al. The evolution of guideline-directed medical therapy among decompensated HFrEF patients in sacubitril/valsartan era: medical expenses and clinical effectiveness. J Chin Med Assoc. (2021) 84:588–95. doi: 10.1097/JCMA.0000000000000546
19. Dewan P, Jhund PS, Shen L, Petrie MC, Abraham WT, Atif Ali M, et al. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail. (2019) 21:577–87. doi: 10.1002/ejhf.1347
20. Antol DD, Casebeer AW, DeClue RW, Stemkowski S, Russo PA. An early view of real-world patient response to sacubitril/valsartan: a retrospective study of patients with heart failure with reduced ejection fraction. Adv Ther. (2018) 35:785–95. doi: 10.1007/s12325-018-0710-4
21. Desai AS, Solomon S, Claggett B, McMurray JJ, Rouleau J, Swedberg K, et al. Factors associated with noncompletion during the run-in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM-HF trial. Circ Hear Fail. (2016) 9:e002735. doi: 10.1161/CIRCHEARTFAILURE.115.002735
22. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. (2016) 18:1228–34. doi: 10.1002/ejhf.580
23. Alba AC, Agoritsas T, Jankowski M, Courvoisier D, Walter SD, Guyatt GH, et al. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail. (2013) 6:881–9. doi: 10.1161/CIRCHEARTFAILURE.112.000043
24. Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. (2014) 2:440–6. doi: 10.1016/j.jchf.2014.04.008
Keywords: heart failure, sacubitril/valsartan, PREDICT-HF model, high-risk population, TAROT-HF
Citation: Hsu C-Y, Chang H-Y, Chao C-J, Chiou W-R, Lin P-L, Chung F-P, Lin W-Y, Huang J-L, Liang H-W, Liao C-T and Lee Y-H (2022) Utility of PREDICT-HF score in high-risk Asian heart failure patients receiving sacubitril/valsartan. Front. Cardiovasc. Med. 9:950389. doi: 10.3389/fcvm.2022.950389
Received: 22 May 2022; Accepted: 27 June 2022;
Published: 25 July 2022.
Edited by:
Elisabetta Salvioni, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Giuseppe Vitale, Ospedale Vincenzo Cervello, ItalyCopyright © 2022 Hsu, Chang, Chao, Chiou, Lin, Chung, Lin, Huang, Liang, Liao and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Hsiang Lee, c3BlYWtlcmxlZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.