
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 29 July 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.949726
This article is part of the Research TopicEffects of Oral Anticoagulant Therapy in Atrial Fibrillation Patients with ComorbiditiesView all 15 articles
Background: Patient prevalence of atrial fibrillation (AF) and heart failure (HF) is increasing, and anticoagulation for patients from heterogeneous backgrounds with both conditions remains controversial. In this meta-analysis, we are aiming to compare the effectiveness and safety of the non-vitamin K antagonist oral anticoagulants (NOACs) and warfarin in AF patients with HF and preserved (HFpEF), mildly reduced (HFmrEF), and reduced (HFrEF) ejection fraction.
Methods and results: We systematically searched the PubMed, Cochrane, and Embase databases until January 2022. The primary effectiveness and safety outcomes were stroke or systemic embolism (SSE) and major bleeding, respectively. We abstracted risk ratios (RR) and 95% confidence intervals (CIs) and compiled them using a random-effects model. We analyzed data of 266,291 patients from 10 studies. By comparing NOACs with warfarin, patients with AF and HF have reduced the risk of SSE (RR: 0.83, 95% CI 0.76–0.91), all-cause mortality (RR: 0.85, 95% CI 0.80–0.91), major bleeding (RR: 0.79, 95% CI 0.69–0.90), and intracranial hemorrhage (RR: 0.54, 95% CI 0.46–0.63). Further analyses based on the HF subtypes showed that NOACs reduced the chances of SSE (RR: 0.71, 95% CI 0.53–0.94) in the HFrEF group and major bleeding (RR: 0.74, 95% CI 0.57–0.95) in HFmrEF and HFpEF groups. There were no differences regarding SSE (RR: 0.91, 95% CI 0.76–1.09) in HFmrEF and HFpEF groups and major bleeding (RR: 0.99, 95% CI 0.79–1.23) in the HFrEF group.
Conclusion: For patients with AF and HF, NOACs have better or similar effectiveness and safety than warfarin, but the stroke prevention superiority of NOACs over warfarin varies in different HF subtypes.
As the most frequent sustained cardio rhythm disorder, atrial fibrillation (AF) frequently exists alongside heart failure (HF) and is linked to a higher risk of stroke and all-cause mortality (1). Anticoagulant therapy, an essential component of the integrated Atrial fibrillation Better Care (ABC) pathway in patients with AF, has been demonstrated to reduce the potential adverse outcomes (2). Current guidelines consistently recommend non-vitamin K antagonist oral anticoagulants (NOACs) as a priority of anticoagulants for patients with AF (3, 4). Traditionally, HF was divided into two phenotypes: HF with reduced (HFrEF) or preserved EF (HFpEF) ejection fraction (EF) (5). Recently, the European Society of Cardiology (ESC) recommends three HF subtypes: HF and preserved (HFpEF, EF ≥ 50%), mildly reduced (HFmrEF, EF 41–49%), and reduced (HFrEF, EF ≤ 40%) EF (6, 7). Although HFrEF and HFpEF share some similar clinical manifestations, they represent entirely different diseases in the HF spectrum, and they are studied and treated separately (8).
For patients in conjunction with AF and HF, some randomized controlled trial (RCT) post hoc analyses have shown that NOACs are non-inferior or even better than vitamin-K antagonists (VKAs) in terms of effectiveness and safety (9–12). An earlier meta-analysis by Chen et al. demonstrated that compared to warfarin, NOACs led to significantly fewer stroke or systemic embolism (SSE) and major bleeding risks in patients with concomitant AF and HF (13). The American Heart Association’s scientific statement encouraged a decision-making process for AF and HFrEF including guideline-directed HF treatment therapy, lifestyle, risk factor adjustment, oral anticoagulation based on the CHAD2DS2-VASc score, pharmacological rate control, and cardioversion if necessary (including catheter ablation and antiarrhythmic treatment) (14). As for AF and HFpEF, there is still a lack of corresponding guidelines and clinical evidence. In addition, a comparison of NOACs and VKAs in AF patients with different HF subtypes (HFpEF, HFmrEF, and HFrEF) remain unknown. Therefore, our study evaluated the safety and effectiveness of NOACs against VKAs in patients with AF accompanied by HF, especially in different subtypes of HF.
We conducted the meta-analysis based on the Cochrane Systematic Review Handbook (15), and the writing followed the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (16) (list of checkpoints displayed in Supplementary Table 1). The included studies were reviewed by the relevant ethics committee before publication, so we did not need ethical approval.
For our analysis, the following criteria were used to select studies: (1) population: adult patients with non-valvular AF complicated with HFpEF, HFmrEF, or HFrEF; (2) outcome measures and intervention: studies assessing at least one effectiveness or safety outcome of NOACs (edoxaban, rivaroxaban, apixaban, or dabigatran) versus VKAs; and (3) study design: RCTs and observational (prospective or retrospective cohort) studies.
We excluded studies where cross-sections, reviews, reports on cases, editorials, letters, or meeting abstracts had insufficient data or study details. For studies that met our inclusion criteria but had overlapping populations, our priority was to study long-term follow-ups or large sample sizes.
We systematically searched all the studies published on electronic databases, such as Cochrane Library, Embase, and PubMed, without linguistic limits (up to January 2022). Supplementary Table 2 presents a listing of the retrieval strategies used: (1) heart failure, AND (2) atrial fibrillation OR atrial flutter, AND (3) non-vitamin K antagonists OR direct oral anticoagulants OR novel oral anticoagulants OR new oral anticoagulants OR edoxaban OR apixaban OR rivaroxaban OR dabigatran, and (4) acenocoumarol OR warfarin OR coumadin OR phenprocoumon OR indandione OR vitamin-K antagonists OR phenindione OR anisindione.
A team of two reviewers reviewed all retrieved studies and abstracted relevant data independently. Based on the qualifications for inclusion, we reviewed the titles and abstracts of the studies and then read the full text in detail to determine the truly eligible studies. In the case of conflict between two reviewers, we reached a consensus by consulting with a third reviewer. We collected the following data from the studies we included: author, publication year, country of the population, data source, study duration, study design, demographics of patients, follow-up period, types of NOACs and dosages, and outcome data (size of sample, count of events in a group, and adjusted effect estimates).
The effectiveness outcomes included SSE, all-cause death, and ischemic stroke, whereas major bleeding, gastrointestinal bleeding, and intracranial bleeding were the safety outcomes. SSE and major bleeding were the primary effectiveness and safety outcomes, whereas others were the secondary outcomes. All the outcomes included in this meta-analysis and definitions of the primary outcomes are shown in Supplementary Table 3.
The Newcastle-Ottawa Scale (NOS) items were used to evaluate observational studies. The RCT post hoc analyses were used as an observational study for quality evaluation. A total of nine points were allocated to the NOS tool’s three domains: cohort selection (0–4 points), cohort comparability (0–2 points), and outcome assessment (0–3 points). NOS scores of 6 or more points were considered medium to high quality, and a score below six points was regarded as low quality (17).
Cochrane Q test and I2 values were used to determine heterogeneity between studies in statistical terms. A p-value of < 0.1 or I2 value > 50% indicated significant heterogeneity across studies. The study effect was estimated with adjusted risk ratios (RRs) and 95% confidence intervals (CIs). The RR natural logarithm and its corresponding standard deviation ((Ln[upper CI]-Ln[lower CI])/3.92) were calculated. Because there were different types and doses of NOACs included in this study, the random-effects model was used in conjunction with the inverse variance method to pool the natural logarithms. Subgroups were performed based on taking 40 and 50% as the left ventricular ejection fraction (LVEF) boundary, study type, renal function, CHA2DS2-VASc score, types of NOACs, New York Heart Association (NYHA) class, and follow-up time. The bias of publication was examined by visually inspecting the funnel plots in which the logRRs were plotted against their standard errors. In addition, Egger’s and Begg’s tests for each outcome were applied to examine publication bias.
Review Manager version 5.4 (the Cochrane Collaboration 2014, Rigshospitalet, Nordic Cochrane Centre Copenhagen, Denmark) was used to perform all the statistical analyses. p-values of < 0.05 were considered statistically significant.
The literature retrieval flowchart is presented in Figure 1. We identified 2,106 articles using the PubMed, Embase, and Cochrane Library databases through our search strategy. A total of 415 studies were duplicated, and 1,691 articles were excluded after screening the title and abstract. The remaining 18 studies were assessed by reading the full text and eight articles were removed for eligibility. Finally, our meta-analysis included 10 studies (4 RCT post hoc analyses and six observational studies) comprising 266,291 patients (9–12, 18–23).
A summary of study characteristics at baseline is shown in Table 1. Among them, four studies were post hoc analyses of RCTs, including RE-LY (dabigatran), ROCKET AF (rivaroxaban), ARISTOTLE (apixaban), and ENGAGE AF-TIMI 48 (edoxaban) trials (9–12). The other 6 studies were observational studies from the United States (n = 4) (18, 19, 21, 22), Japan (n = 1) (23), and Sweden (n = 1) (20), respectively. Sample sizes ranged from 4,904 to 49,448 patients, and the duration of median follow-up time was 0.4–2.8 years. The definition of HF was extracted from the originally included studies and shown in Supplementary Table 3. As a measure of quality, the NOS tool was used to assess the included studies, all of which were judged to be medium-to-high and deemed qualified (Supplementary Table 4).
Among AF with HF patients, in comparison with warfarin (Supplementary Figure 1), the use of NOACs was linked to lower risks of SSE (RR: 0.83, 95% CI 0.76–0.91) and all-cause death (RR: 0.85, 95% CI 0.80–0.91), while a significant difference was not observed in ischemic stroke (RR: 0.88, 95% CI 0.74–1.04). As for the safety outcomes compared to warfarin (Supplementary Figure 2), NOACs in patients with AF and HF were found to reduce major bleeding (RR: 0.79, 95% CI 0.60–0.90) and intracranial bleeding (RR: 0.54, 95% CI 0.46–0.63) risks significantly, but the risk of gastrointestinal bleeding (RR: 1.00, 95% CI 0.76–1.31) was not different between the two groups.
The effectiveness and safety of NOACs and warfarin in AF patients without HF were consistent with those in patients with AF and HF. In AF patients without HF (Supplementary Figure 3), NOACs reduced the risk of SSE (RR: 0.83, 95% CI 0.71–0.97), all-cause mortality (RR: 0.85, 95% CI 0.78–0.92), major bleeding (RR: 0.77, 95% CI 0.68–0.89) and intracranial hemorrhage (RR: 0.46, 95% CI 0.35–0.61) than warfarin, but there was no significant difference in the risks of ischemic stroke (RR: 0.91, 95% CI 0.74–1.12) and gastrointestinal bleeding (RR: 1.08, 95% CI 0.72–1.64).
Effects of NOACs on primary effectiveness and safety outcomes in HFrEF and HFpEF subgroups were analyzed taking 40 and 50% as the LVEF boundary, respectively (Figure 2). Compared with warfarin, the use of NOACs was related to lower SSE risks in patients with HFrEF independent of the LVEF boundary of 40 or 50%.
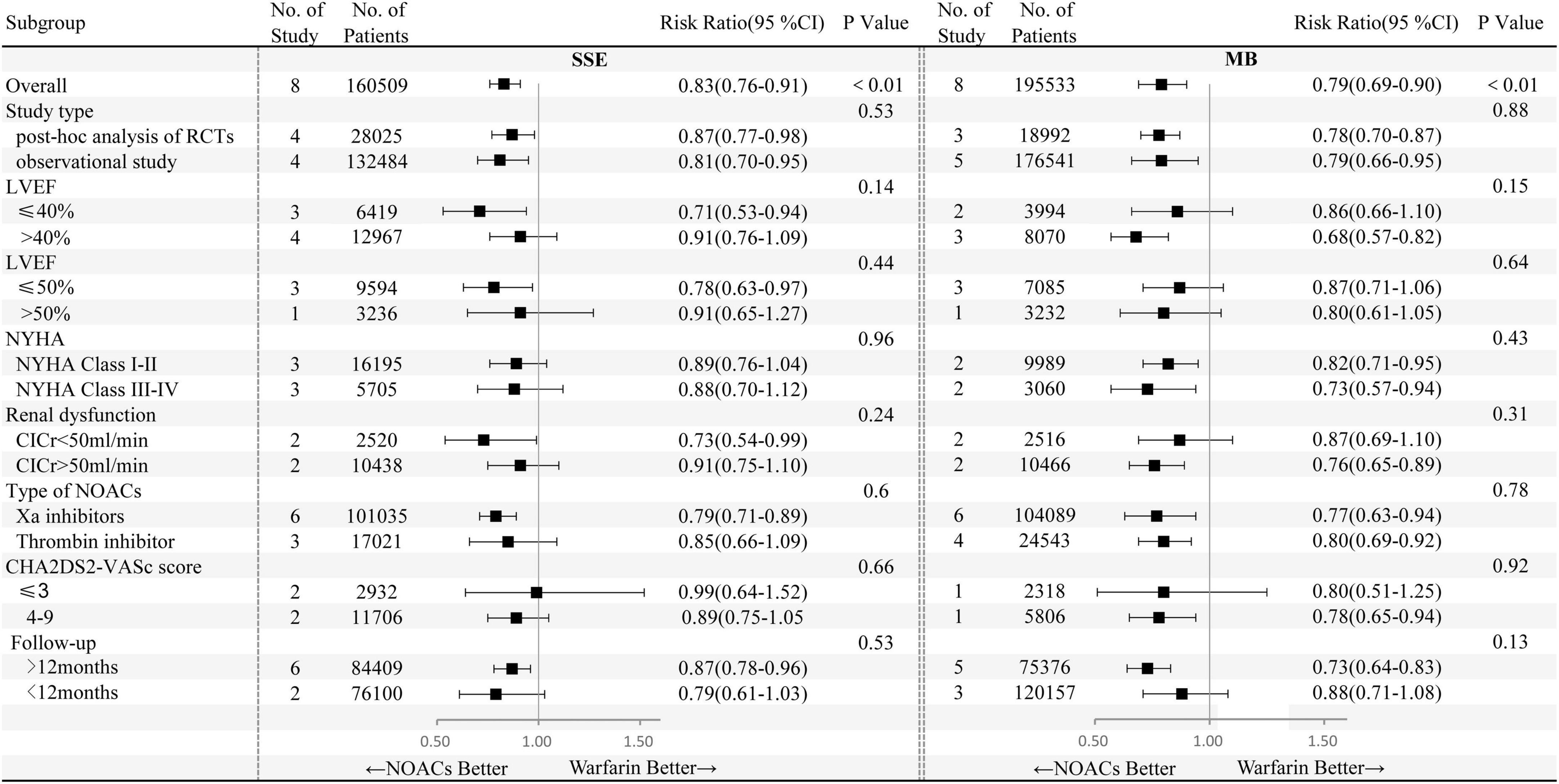
Figure 2. Primary effectiveness and safety outcomes of non-vitamin K antagonist oral anticoagulants (NOACs) versus warfarin according to different subgroups. NYHA, New York Heart Association; ClCr, creatinine clearance; CI, confidence interval.
When categorizing HF into different types of HFpEF, HFmrEF, and HFrEF, NOACs against warfarin significantly decreased SSE (RR: 0.71, 95% CI 0.53–0.94) risks in patients with AF and HFrEF (Figure 3A). However, no significant statistical difference in the risks of SSE (RR: 0.91, 95% CI 0.76–1.09) was indicated in AF patients with HFmrEF or HFpEF. As presented in Figure 3B, in AF patients with concomitant HFmrEF or HFpEF, as compared to warfarin, NOACs reduced the risk of major bleeding (RR: 0.68, 95% CI 0.57–0.82), whereas major bleeding (RR: 0.86, 95% CI 0.66–1.10) risks did not differ in patients with AF and HFrEF.
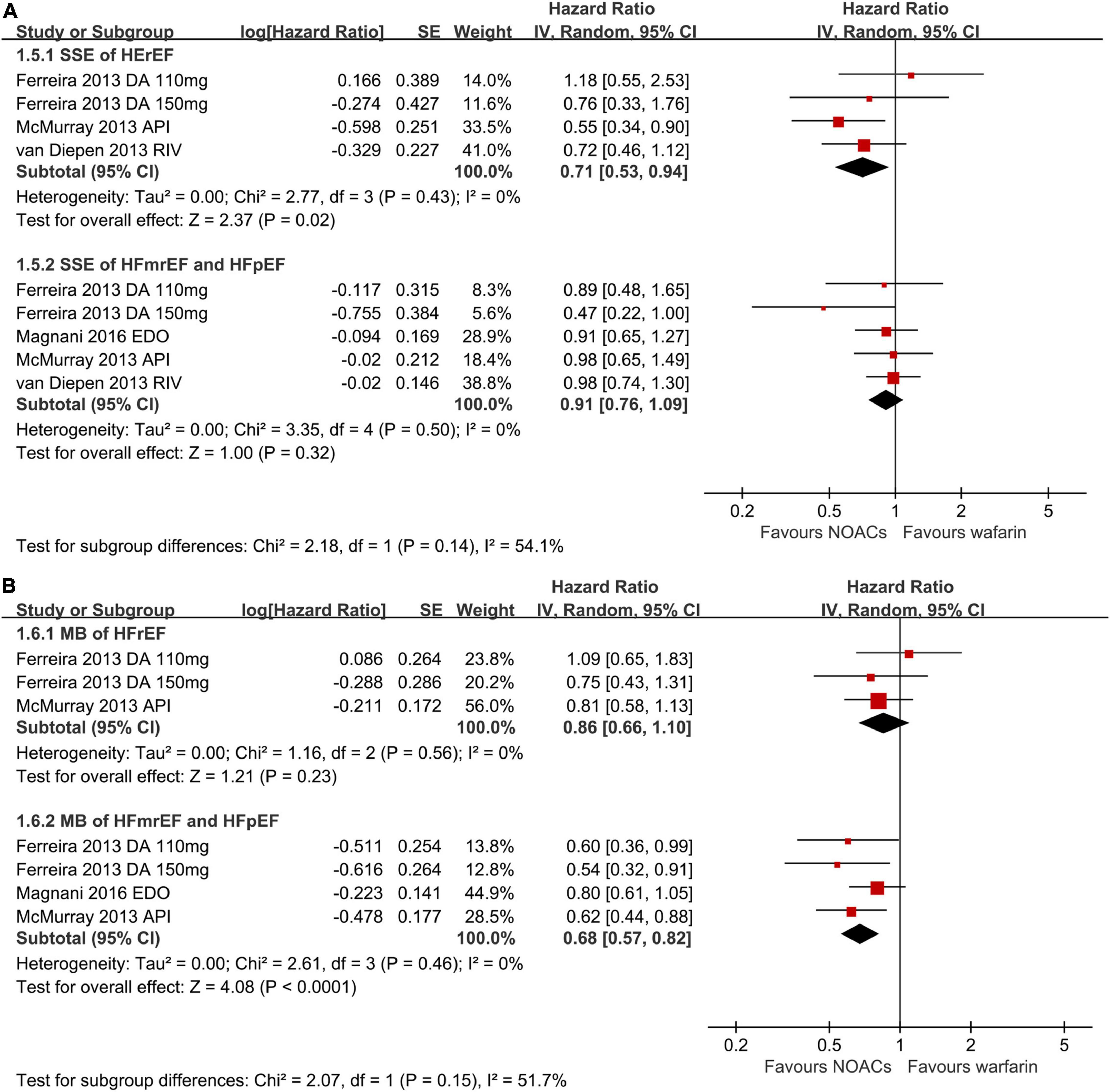
Figure 3. Forest plot for primary effectiveness (A) and safety (B) outcomes in HFrEF, HFmrEF, and HFpEF. HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; CI, confidence interval.
Subgroup analyses were performed based on study type (RCT post hoc analysis and observational study), class of NYHA (NYHA I-II and NYHA III-IV), renal function (creatinine clearance was 50 ml/min as the boundary), CHA2DS2-VASc score (≤3, 4–9), types of NOACs (factor Xa inhibitors, thrombin inhibitor), and follow-up time (12 months as the boundary) (Figure 2).
In comparison with warfarin users, lower SSE and major bleeding risks were associated with factor Xa inhibitor, whereas thrombin inhibitor users had smaller major bleeding risks and similar SSE risks. In addition, during long-term follow-up (>12 months), NOACs versus warfarin significantly decreased the risks of SSE and major bleeding. In other subgroup analyses based on NYHA class, renal dysfunction, study type, LVEF with 50% as the boundary, and the CHA2DS2-VASc score, NOACs and warfarin were at least as safe and effective as each other for the prevention of strokes.
Publication bias was evaluated through a visual check of the asymmetry of the funnel plots (Supplementary Figures 4, 5). No obvious publication biases were found for SSE, ischemic stroke, all-cause mortality, and major bleeding. Egger’s and Begg’s tests did not indicate publication biases for the primary outcomes. However, the funnel plot for intracranial hemorrhage or gastrointestinal bleeding was asymmetrical possibly because only a few studies were included in terms of these outcomes. Therefore, the pooled data should be interpreted cautiously.
We evaluated the adverse outcomes of NOACs across different HF subtypes by performing a meta-analysis in this study. We found that in comparison with warfarin, NOACs use was significantly linked to reduced risks of SSE, all-cause mortality, intracranial bleeding, and major bleeding, whereas risks of ischemic stroke and gastrointestinal bleeding did not differ significantly between the treatment groups. In addition, NOACs outweighed warfarin in decreasing the risks of SSE in the HFrEF group and major bleeding in HFmrEF or HFpEF groups.
The coexistence of AF and HF was common with a patient prevalence of AF in HF exceeding 20% (24). It has been reported that SSE and all-cause mortality risks were increased when both conditions were present (11). As recommended by the current guidelines, NOACs are more effective and safer than warfarin in stroke prevention for AF patients (25). In this meta-analysis, we found that for patients with AF and HF, NOACs were also superior to warfarin in the reduction of SSE, all-cause mortality, intracranial bleeding, and major bleeding. This was consistent with prior meta-analyses which demonstrated that despite the increasing death rate among patients with HF and AF, SSE, major, and intracranial bleeding in AF patients with concomitant HF were significantly reduced by NOACs compared with warfarin (13, 26).
The prevalence of AF and prognosis vary across different HF subtypes. According to the ESC heart failure long-term registry, the prevalence of AF increases with the increase of LVEF (HFrEF: 27%, HFmrEF: 29%, and HFpEF: 39%) (27). Patients with HFpEF are usually older, more likely to be women, and usually have multiple comorbidities, including hypertension, obesity, and diabetes, making the CHA2DS2-VASc score much higher than those with HFrEF (28). However, the annual incidence of stroke was linearly increasing by 0.054% per each 1% of LVEF decrease (29). Indeed, patients with HFrEF had the highest risks of stroke and mortality despite a relatively lower CHA2DS2-VASc score compared with HFpEF (29). In our meta-analysis, NOACs were linked to reduced SSE (RR: 0.71, 95% CI 0.53–0.94) risks significantly in AF patients with HFrEF but not those with HFmrEF or HFpEF. However, limited evidence was available in terms of the superiority of NOACs over warfarin in patients with AF and different phenotypes of HF. Further robust clinical trials were warranted to investigate the safety and efficacy of NOACs in patients with AF among different phenotypes (11, 12).
In addition, the definition of HF and the cut-off value of HFpEF, HFmrEF, and HFrEF were also heterogeneous. Therefore, the results derived from the included studies may not reflect the real therapeutic effects of NOACs and should be interpreted cautiously.
Our meta-analysis had several limitations that should be further addressed. First, the choice of drugs for these patients depends on many factors, and it is difficult to directly compare NOACs with each other given the differences in trial design and study population among the four post hoc analyses of RCTs. Second, the definition of HF and the cut-off valve of HFpEF, HFmrEF, and HFrEF differ in the included studies in this meta-analysis, hence the results should be interpreted cautiously. Further robust clinical trials with consistent definitions and categories of HF are warranted.
Our current evidence of this meta-analysis suggested that in patients with AF and HF, NOACs have better or similar effectiveness and safety than warfarin, but the stroke prevention superiority of NOACs over warfarin varies in different HF subtypes.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
JH and ZW designed the study and revised the manuscript. KW and ZX carried out the literature search, article screen, assessing quality, and statistics. KW wrote the manuscript. ZW and YC reformulated the manuscript and revised the English grammar. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (21866019), the Natural Science Foundation of Guangdong Province, China (2018A030313448 and 2021A1515011774), and the Sun Yat-sen University Clinical Research 5010 Program (2017003).
We sincerely thank Wengen Zhu (Department of Cardiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China) for his direction in the whole process of this meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.949726/full#supplementary-material
1. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. (2019) 7:447–56. doi: 10.1016/j.jchf.2019.03.005
2. Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (atrial fibrillation better care) pathway. Am J Med. (2018) 131:1359–66.e6. doi: 10.1016/j.amjmed.2018.06.012
3. Lip GY, Nielsen PB. Should patients with atrial fibrillation and 1 stroke risk factor (CHA2DS2-VASc score 1 in men, 2 in women) be anticoagulated? Yes: even 1 stroke risk factor confers a real risk of stroke. Circulation. (2016) 133:1498–503; discussion 1503. doi: 10.1161/CIRCULATIONAHA.115.016713
4. Fauchier L, Lecoq C, Clementy N, Bernard A, Angoulvant D, Ivanes F, et al. Oral anticoagulation and the risk of stroke or death in patients with atrial fibrillation and one additional stroke risk factor: the Loire Valley atrial fibrillation project. Chest. (2016) 149:960–8. doi: 10.1378/chest.15-1622
5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726.
7. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure: endorsed by the Canadian heart failure society, heart failure association of India, cardiac society of Australia and New Zealand, and Chinese heart failure association. Eur J Heart Fail. (2021) 23:352–80. doi: 10.1002/ejhf.2115
8. Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. (2011) 123:2006–13; discussion 2014. doi: 10.1161/CIRCULATIONAHA.110.954388
9. Ferreira J, Ezekowitz MD, Connolly SJ, Brueckmann M, Fraessdorf M, Reilly PA, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: a subgroup analysis of the RE-LY trial. Eur J Heart Fail. (2013) 15:1053–61. doi: 10.1093/eurjhf/hft111
10. van Diepen S, Hellkamp AS, Patel MR, Becker RC, Breithardt G, Hacke W, et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail. (2013) 6:740–7. doi: 10.1161/CIRCHEARTFAILURE.113.000212
11. McMurray JJ, Ezekowitz JA, Lewis BS, Gersh BJ, van Diepen S, Amerena J, et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail. (2013) 6:451–60. doi: 10.1161/CIRCHEARTFAILURE.112.000143
12. Magnani G, Giugliano RP, Ruff CT, Murphy SA, Nordio F, Metra M, et al. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48. Eur J Heart Fail. (2016) 18:1153–61. doi: 10.1002/ejhf.595
13. Chen F, Zhou Y, Wan Q, Yu P, Ma J, Hu J. Effect of non-vitamin K antagonist oral anticoagulants versus warfarin in heart failure patients with atrial fibrillation. Heart Fail Rev. (2021) 26:1391–7. doi: 10.1007/s10741-020-09946-8
14. Gopinathannair R, Chen LY, Chung MK, Cornwell WK, Furie KL, Lakkireddy DR, et al. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American heart association. Circ Arrhythm Electrophysiol. (2021) 14:HAE0000000000000078. doi: 10.1161/HAE.0000000000000080
15. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. doi: 10.1161/STROKEAHA.120.031007
18. Adeboyeje G, Sylwestrzak G, Barron JJ, White J, Rosenberg A, Abarca J, et al. Major bleeding risk during anticoagulation with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. (2017) 23:968–78. doi: 10.18553/jmcp.2017.23.9.968
19. Amin A, Garcia Reeves AB, Li X, Dhamane A, Luo X, Di Fusco M, et al. Effectiveness and safety of oral anticoagulants in older adults with non-valvular atrial fibrillation and heart failure. PLoS One. (2019) 14:e0213614. doi: 10.1371/journal.pone.0213614
20. Friberg L, Oldgren J. Efficacy and safety of non-vitamin K antagonist oral anticoagulants compared with warfarin in patients with atrial fibrillation. Open Heart. (2017) 4:e000682. doi: 10.1136/openhrt-2017-000682
21. Jackevicius CA, Lu L, Ghaznavi Z, Warner AL. Bleeding risk of direct oral anticoagulants in patients with heart failure and atrial fibrillation. Circ Cardiovasc Qual Outcomes. (2021) 14:e007230. doi: 10.1161/CIRCOUTCOMES.120.007230
22. Martinez BK, Bunz TJ, Eriksson D, Meinecke AK, Sood NA, Coleman CI. Effectiveness and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and heart failure. ESC Heart Fail. (2019) 6:10–5. doi: 10.1002/ehf2.12365
23. Yoshihisa A, Sato Y, Sato T, Suzuki S, Oikawa M, Takeishi Y. Better clinical outcome with direct oral anticoagulants in hospitalized heart failure patients with atrial fibrillation. BMC Cardiovasc Disord. (2018) 18:11. doi: 10.1186/s12872-018-0746-z
24. De Ferrari GM, Klersy C, Ferrero P, Fantoni C, Salerno-Uriarte D, Manca L, et al. Atrial fibrillation in heart failure patients: prevalence in daily practice and effect on the severity of symptoms. Data from the ALPHA study registry. Eur J Heart Fail. (2007) 9:502–9. doi: 10.1016/j.ejheart.2006.10.021
25. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehab648
26. Savarese G, Giugliano RP, Rosano GM, McMurray J, Magnani G, Filippatos G, et al. Efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation and heart failure: a meta-analysis. JACC Heart Fail. (2016) 4:870–80. doi: 10.1016/j.jchf.2016.07.012
27. Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European society of cardiology heart failure long-term registry. Eur Heart J. (2018) 39:4277–84. doi: 10.1093/eurheartj/ehy626
28. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. doi: 10.1038/nrcardio.2017.65
Keywords: atrial fibrillation, anticoagulants, heart failure, warfarin, meta
Citation: Wulamiding K, Xu Z, Chen Y, He J and Wu Z (2022) Non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with heart failure and preserved, mildly reduced, and reduced ejection fraction: A systemic review and meta-analysis. Front. Cardiovasc. Med. 9:949726. doi: 10.3389/fcvm.2022.949726
Received: 21 May 2022; Accepted: 01 July 2022;
Published: 29 July 2022.
Edited by:
Jianyong Ma, University of Cincinnati, United StatesReviewed by:
Yidan Wang, Capital Medical University, ChinaCopyright © 2022 Wulamiding, Xu, Chen, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangui He, aGVqaWFuZ3VpQDE2My5jb20=; Zexuan Wu, d3V6eDI3QG1haWwuc3lzdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.