- 1Department of Cardiology and Vascular Medicine, Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
- 2Division of Cardiology, Department of Internal Medicine, Korea University Medical Center, Seoul, South Korea
- 3R. Syamsudin, SH Regional Public Hospital, Sukabumi, West Java, Indonesia
- 4Division of Physiology, Department of Biomedical Sciences, Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
- 5Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
Background: Recent investigations suggest that premature ventricular complexes (PVCs) during an exercise test are associated with an elevated risk of mortality in asymptomatic individuals. However, given the small number of studies included, the association between these two entities in the asymptomatic population remains obscure. Our aim was to evaluate this matter.
Methods: A comprehensive literature search was conducted utilizing several online databases up to April 2022. The study comprised cohort studies examining the relationship between exercise-induced premature ventricular complexes (EI-PVCs) and all-cause mortality (ACM) as well as cardiovascular mortality (CVM) in asymptomatic populations. To provide diagnostic values across the statistically significant parameters, we additionally calculated sensitivity, specificity, and area under the curve (AUC).
Results: A total of 13 studies consisting of 82,161 patients with a mean age of 49.3 years were included. EI-PVCs were linked to an increased risk of ACM (risk ratio (RR) = 1.30 (95% confidence interval (CI) = 1.18–1.42); p < 0.001; I2 = 59.6%, p-heterogeneity < 0.001) and CVM (RR = 1.67 (95% CI = 1.40–1.99); p < 0.001; I2 = 7.5%, p-heterogeneity = 0.373). Subgroup analysis based on the frequency of PVCs revealed that frequent PVCs were similarly related to a higher risk of ACM and CVM, but not infrequent PVCs. Moreover, diagnostic test accuracy meta-analysis showed that recovery phase EI-PVCs have a higher overall specificity than exercise phase EI-PVCs regarding our outcomes of interest.
Conclusion: EI-PVCs are correlated with a higher risk of ACM and CVM. When compared to the exercise phase, the specificity of PVCs generated during the recovery period in predicting interest outcomes is higher. As a result, we propose that the exercise ECG be utilized on a regular basis in middle-aged asymptomatic individuals to measure the frequency of PVCs and stratify the risk of mortality.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=328852], identifier [CRD42022328852].
Introduction
According to the 2019 European Society of Cardiology (ESC) guideline, an exercise electrocardiogram (ECG) is a valuable tool for confirming the diagnosis of coronary artery disease (CAD) in suspected patients, but it is not indicated in the asymptomatic population without suspicion toward CAD (1). The interpretation of this test to detect CAD is commonly known to rely substantially on the development of ST-segment deviation (2). However, premature ventricular complexes (PVCs), another ECG sign, can also develop during exercise ECG (3–5). After years of examining the predictive significance of PVCs during exercise ECG in CAD patients, researchers have discovered that this ECG marker is substantially linked with all-cause mortality (ACM) and cardiovascular mortality (CVM) (6).
Interestingly, PVCs were also prevalent during exercise ECG in the asymptomatic group without a pre-existing CAD, with a frequency ranging from 3.8 to 27.5% (7, 8). Previously, exercise-induced premature ventricular complexes (EI-PVCs) in the asymptomatic population were thought to be a harmless condition that did not increase the risk of mortality. Nonetheless, three meta-analyses published in 2016 by Lee et al., Kim et al., and Peng et al. demonstrated that EI-PVCs in an asymptomatic population significantly increased the risk of mortality compared to those with normal exercise ECG (6, 9, 10). Furthermore, subgroup analysis in Kim et al. study revealed that recovery-induced PVCs were substantially correlated with a higher risk of ACM (10). However, due to the limited number of studies included in the subgroup analysis and the fact that this meta-analysis was performed in 2016, the association between recovery-induced PVCs with mortality in the asymptomatic population remains equivocal. Therefore, the present meta-analysis has two primary objectives. Firstly, to evaluate the prognostic value of exercise and recovery-induced PVCs in increasing the risk of ACM and CVM in the asymptomatic population. Secondly, compare these two parameters to determine which one is the better predictor of similar outcomes.
Methods
Protocol and registration
This meta-analysis was recorded in the PROSPERO (International Prospective Register of Systematic Reviews) database under registration number CRD42022328852 and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards.
Search strategy
This systematic review and meta-analysis coveted to investigate whether EI-PVCs throughout exercise stress testing prognosticate mortality in both ACM and CVM in asymptomatic individuals. In favor of this aim, two independent investigators searched MEDLINE (via PubMed), Europe PMC, and ScienceDirect databases from inception to April 2022 with the search terms (“ventricular premature complexes” [MeSH Terms]) AND (“all-cause mortality” OR “cardiovascular mortality”). We modified every search term to match the restrictions of each database, and the full-text articles were rigorously evaluated by both authors in case there was disagreement about whether research should be included based on its title or abstract.
Eligibility criteria and outcomes of interest
This study included both prospective and retrospective observational studies providing the occurrence of EI-PVCs in asymptomatic adult participants (age ≥ 18 years) who underwent exercise stress ECG testing, in conjunction with our primary outcomes of interest on clinically validated definitions of mortality, both prompted by any causes (ACM) and cardiovascular (CVM) origin. The studies must report the incidence of our primary outcomes in a comparative demeanor between patients with EI-PVCs to those without these arrhythmias (either during exercise or recovery phase of an exercise stress test). We excluded review articles, editorials, commentaries, case reports/series, meta-analyses, conference abstracts, and studies in languages other than English. The adjudicator would be the third investigator, and any discrepancies would be resolved through discussion.
Study selection and data collection
The preliminary search and duplication elimination were carried out by two separate investigators. Titles and abstracts were reviewed for eligibility, and the full text of potentially eligible papers was evaluated using predetermined inclusion and exclusion criteria. Data extraction from the suitable articles was conducted using a prebuilt table comprising the first author’s name, the origin of the study, study design, total subjects, and population of the study. For meta-regression purposes, several confounding factors such as age, sex, hypertension, diabetes mellitus, dyslipidemia, smoking status, and follow-up time were also included in the data gathering procedure.
Risk of bias assessment
The risk of bias assessment for each and every study was performed independently by two authors using the Newcastle–Ottawa Scale (NOS) (11). A study with a total score of seven or above is considered to have a minimal risk of bias. Otherwise, a study with a total score of six or less was found to have a considerable risk of bias and was removed from the study selection process. Quality rating disagreements were resolved by discussion with a third reviewer.
Data analysis
Software for Statistics and Data Science (STATA) software version 17.0 was utilized to conduct statistical analysis. This meta-analysis’ effect size was expressed as risk ratios (RRs) with 95% confidence intervals (CIs). Any studies that report hazard ratios (HRs) as their effect size were recast into RRs for the purposes of this analysis. The restricted maximum-likelihood method and random-effects models were used to pool the overall effect size despite their heterogeneity. All statistical analyses were two-sided, with statistical significance indicated by a p-value <0.05. The I2 method was used to assess inter-study heterogeneity, and an I2 statistic >50% or p-value <0.10 indicated substantial heterogeneity.
Meta-regression for possible confounders mentioned in the previous subsection was also performed to ensure the true direct effect regarding the aforementioned exposure. Subgroup analyses were also conducted based on the timing and frequency of PVCs to produce an ideal, more robust summary across the relevant studies. The timing of PVCs was divided based on when the PVCs appeared throughout the exercise stress test (either exercise or recovery phase). Furthermore, frequent PVCs were classified as those occurring more than 5 beats per min or accounting for more than 10% of total ventricular depolarizations during the electrocardiographic recordings. We also employed diagnostic test accuracy (DTA) meta-analysis to generate diagnostic values across the statistically significant effect sizes and subgroups, which consisted of sensitivity, specificity, and area under the curve (AUC). Begg’s funnel plot and Egger’s test were used to assessing the likelihood of publication bias in both qualitative and quantitative terms, respectively.
Results
Baseline characteristics
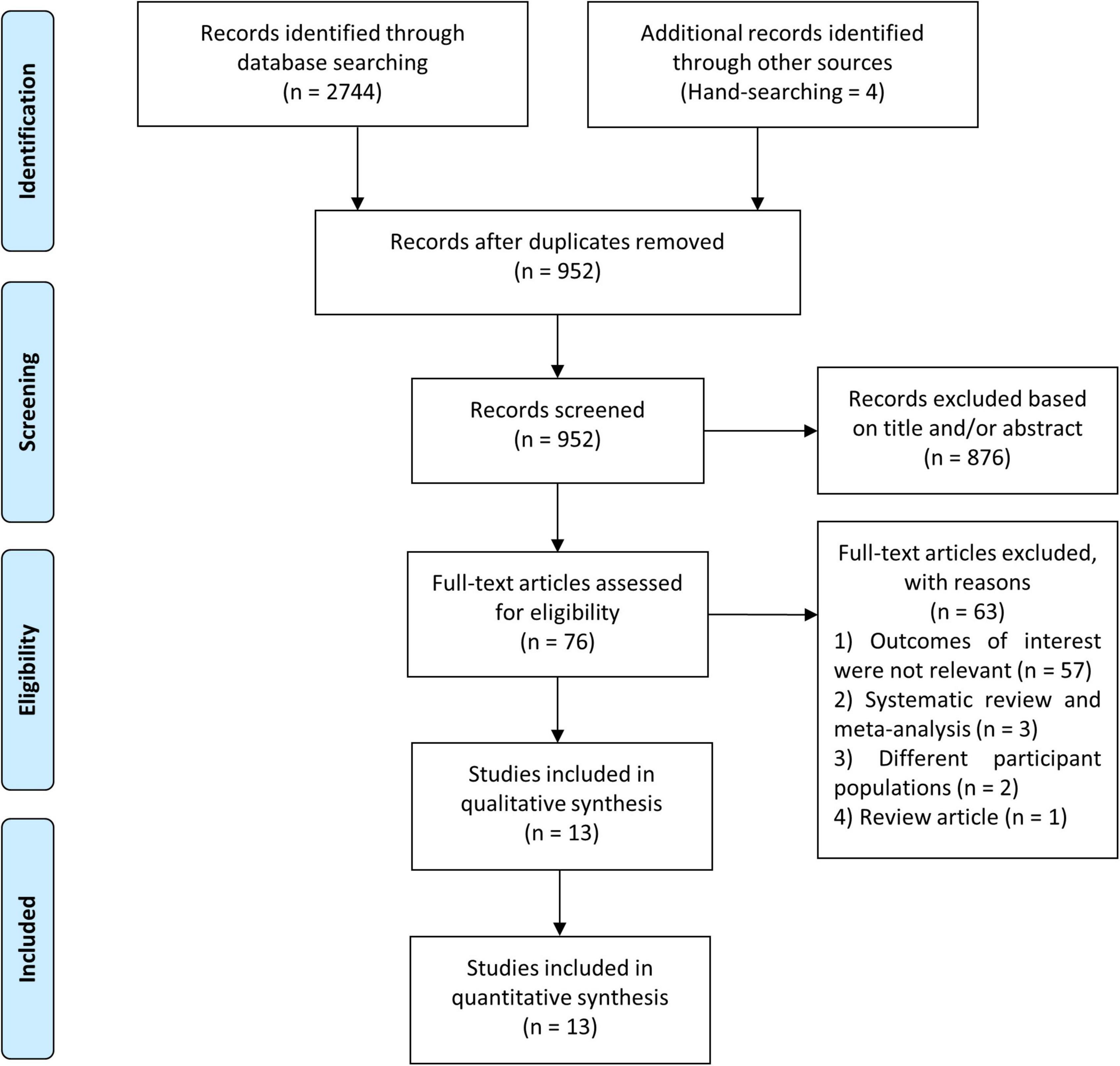
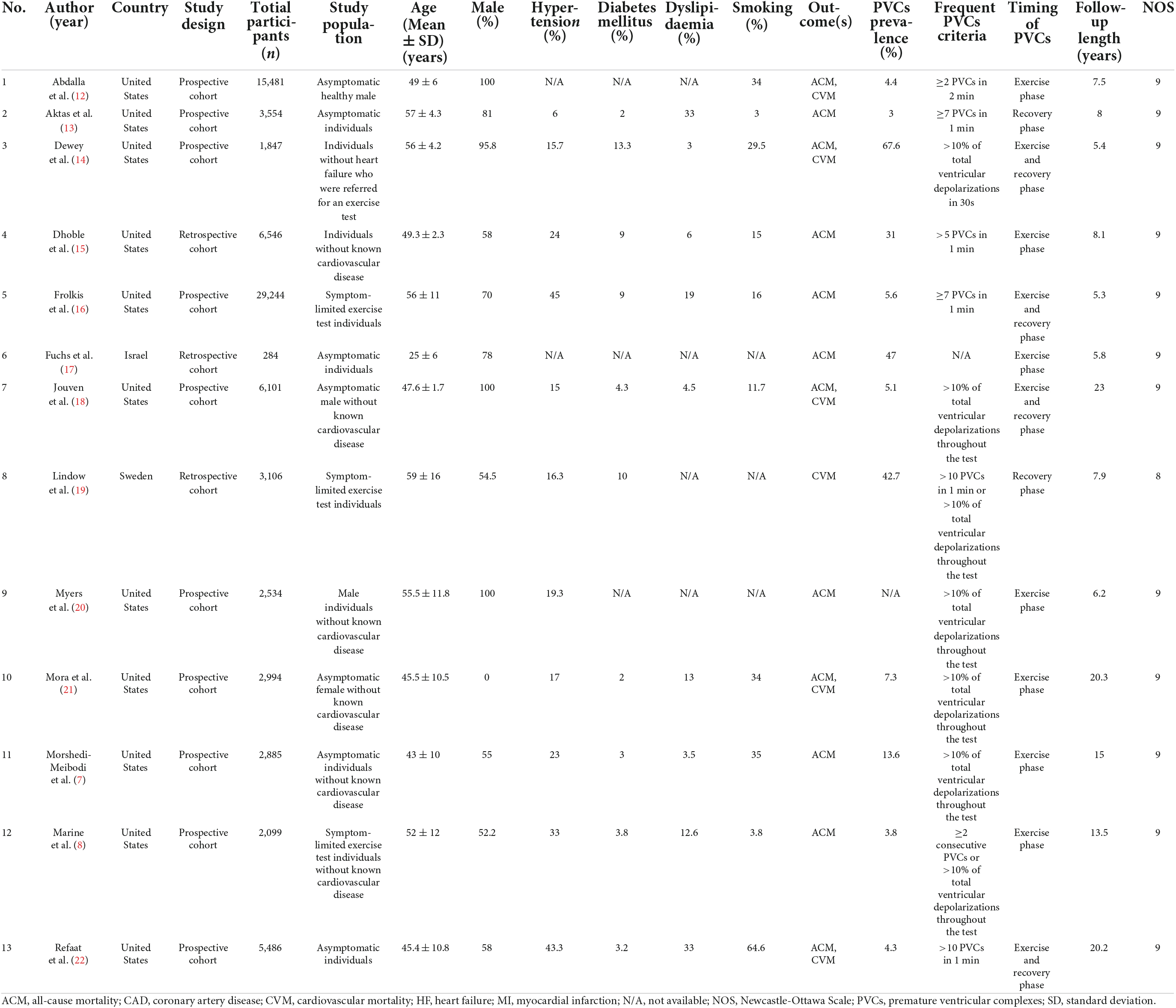
We identified 2,748 studies in our initial search, four of which were discovered via the hand-searching procedure, and 952 studies remained after duplicates were removed. Due to the fact that we used the minimum keywords in order to maximize the search results, the number of research dropped dramatically from 952 studies to 76 studies. During this pre-inclusion screening, the majority of the eliminated studies comprised PVCs that did not occur during the exercise stress test, pharmacological trials, diverse study populations, and animal studies. Conclusively, following a screening procedure based on pre-determined inclusion criteria, thirteen studies with a total of 82,161 participants were incorporated in this meta-analysis (Figure 1) (7, 8, 12–22). The mean age of participants in this study was 49.3 years, whereas 69.4% of the participants were male. The prevalence of EI-PVCs in our study was 19.6%. Most of our included articles were conducted in the United States, with a mean duration of follow-up of 11.2 years. All studies provided reasonably clear descriptions of participants, operational definitions, and exposures. Detailed information on the included studies was provided in Table 1.
Meta-analysis of exercise-induced premature ventricular complexes and poor prognosis
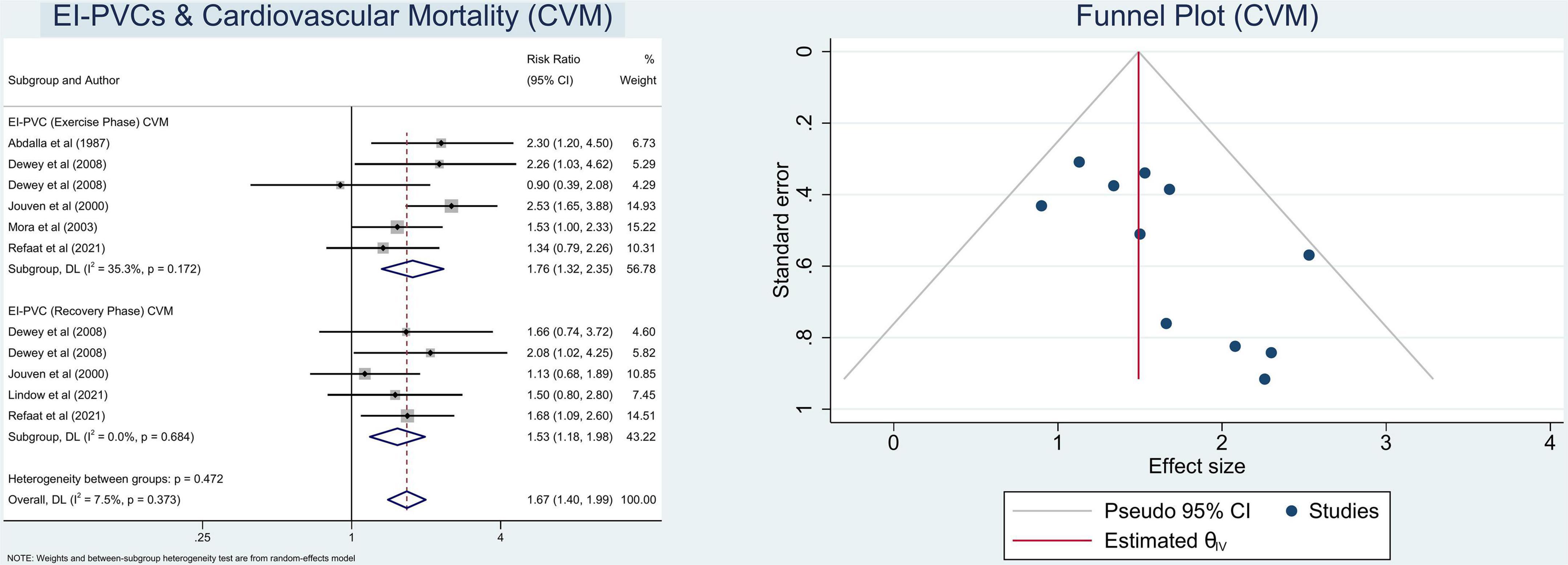
In this meta-analysis, we observed that patients who exhibit EI-PVCs during an exercise stress test have a greater risk of ACM (RR = 1.30 (95% CI = 1.18–1.42); p < 0.001; I2 = 59.6%, p-heterogeneity < 0.001) (Figure 2) and CVM (RR = 1.67 (95% CI = 1.40–1.99); p < 0.001; I2 = 7.5%, p-heterogeneity = 0.373) (Figure 3). The statistically significant overall combined risk ratio of ACM remained unchanged throughout subgroup analyses, as evidenced by 14 studies reporting EI-PVCs during exercise phase (RR = 1.23 (95% CI = 1.10–1.37); p < 0.001; I2 = 46.6%, p-heterogeneity = 0.028), as well as during recovery phase (RR = 1.46 (95% CI = 1.21–1.76); p < 0.001; I2 = 60.9%, p-heterogeneity = 0.026), are related with an elevated risk of ACM. Furthermore, subgroup analyses in the CVM outcome show a substantial increase in the risk of CVM in those who had EI-PVCs during the exercise phase (RR = 1.76 (95% CI = 1.32–2.35); p < 0.001; I2 = 35.3%, p-heterogeneity = 0.172) as well as people who had EI-PVCs during the recovery period (RR = 1.53 (95% CI = 1.18–1.98); p = 0.001; I2 = 0.0%, p-heterogeneity = 0.684).
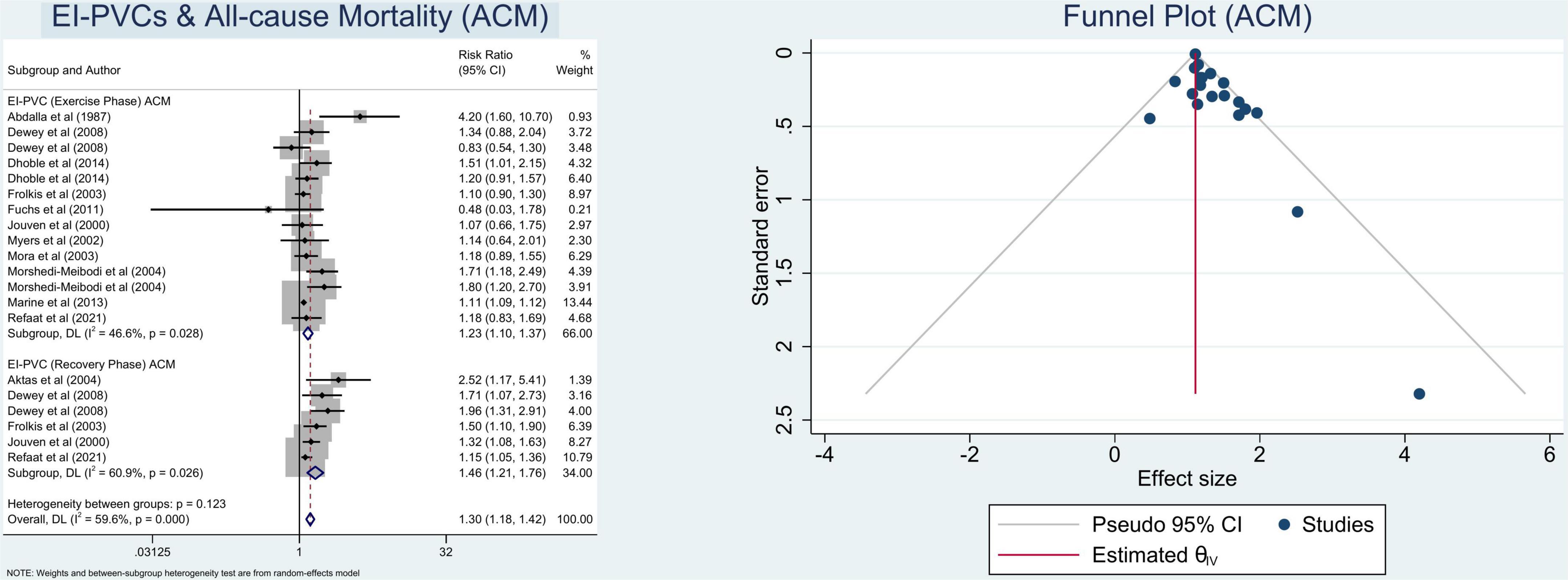
Figure 2. Pooled risk ratios for exercise-induced premature ventricular complexes (EI-PVCs) and all-cause mortality (ACM).
Information on the specific subtypes of the PVCs (frequency of PVCs, period of PVCs) that occurred within the overall included studies was accessible. Thus, we also performed subgroup analyses based on the previously mentioned categorization, which revealed that the overall combined RRs of ACM and CVM regarding frequent PVCs were (RR = 1.27 (95% CI = 1.15–1.39); p < 0.001; I2 = 53.1%, p-heterogeneity = 0.006), and (RR = 1.70 (95% CI = 1.42–2.03); p < 0.001; I2 = 3.8%, p-heterogeneity = 0.403), respectively, whereas the overall RRs of ACM and CVM in relation to infrequent PVCs were (RR = 1.37 (95% CI = 0.96–1.95); p = 0.080; I2 = 72.0%, p-heterogeneity = 0.013), and (RR = 1.41 (95% CI = 0.62–3.20); p = 0.411; I2 = 55.1%, p-heterogeneity = 0.135), respectively, which did not reach statistical significance. Table 2 presents the overall findings as well as the results of other various subsets of subgroup analyses.

Table 2. Results from the subgroup analysis evaluating the mortality risk of exercise-induced premature ventricular complexes (EI-PVCs).
Diagnostic values of exercise-induced premature ventricular complexes
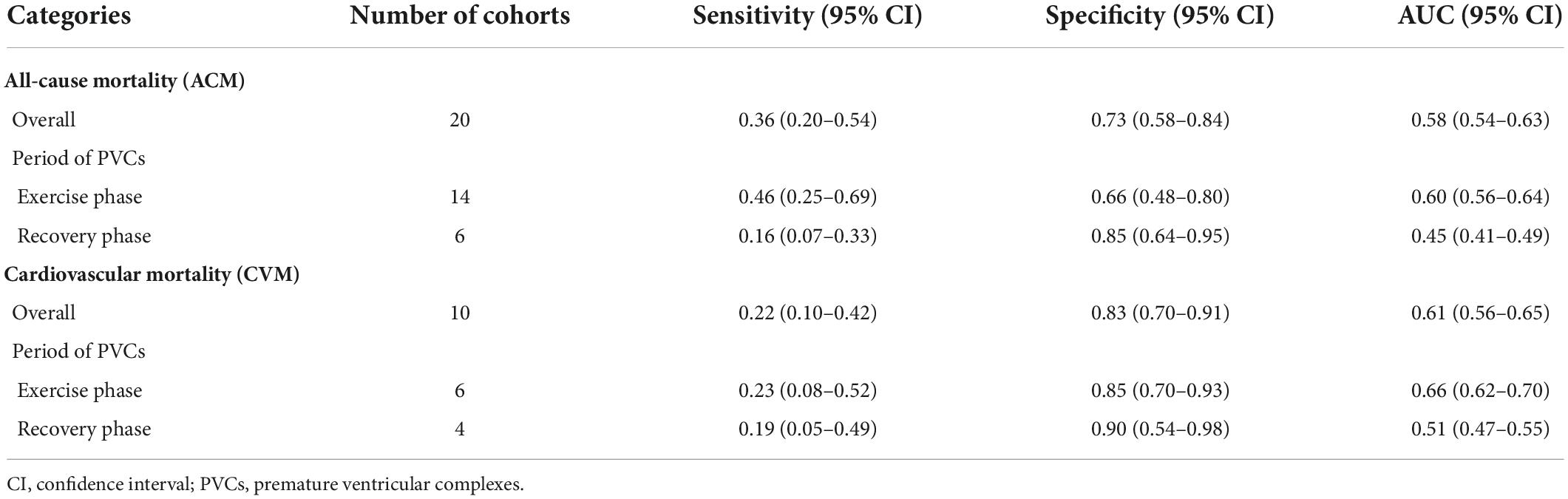
With reference to our previous findings that both exercise and recovery phase EI-PVCs were significantly associated with a higher risk of ACM and CVM, summary receiver operating characteristics (SROC) were drawn, and AUC was calculated to determine the diagnostic value of each phase in the exercise stress test. EI-PVCs in the exercise phase had a sensitivity of 46% (25–69%), specificity of 66% (48–80%), and AUC of 60% (56–64%) for ACM outcome. Moreover, it possesses a sensitivity of 16% (7–33%), specificity of 85% (64–95%), and AUC of 45% (41–49%) during the recovery phase. Besides, pertaining to CVM, it was revealed that EI-PVCs in the exercise phase have a sensitivity of 23% (8–52%), specificity of 85% (70–93%), and AUC of 66% (62–70%). Statistical analysis apropos to the recovery phase in CVM demonstrated a sensitivity of 19% (5–49%), specificity of 90% (54–98%), and AUC of 51% (47–55%). Figures 4, 5 illustrate comparisons of the SROC curve, and Table 3 shows the parameters of diagnostic accuracy of each phase for every outcome.
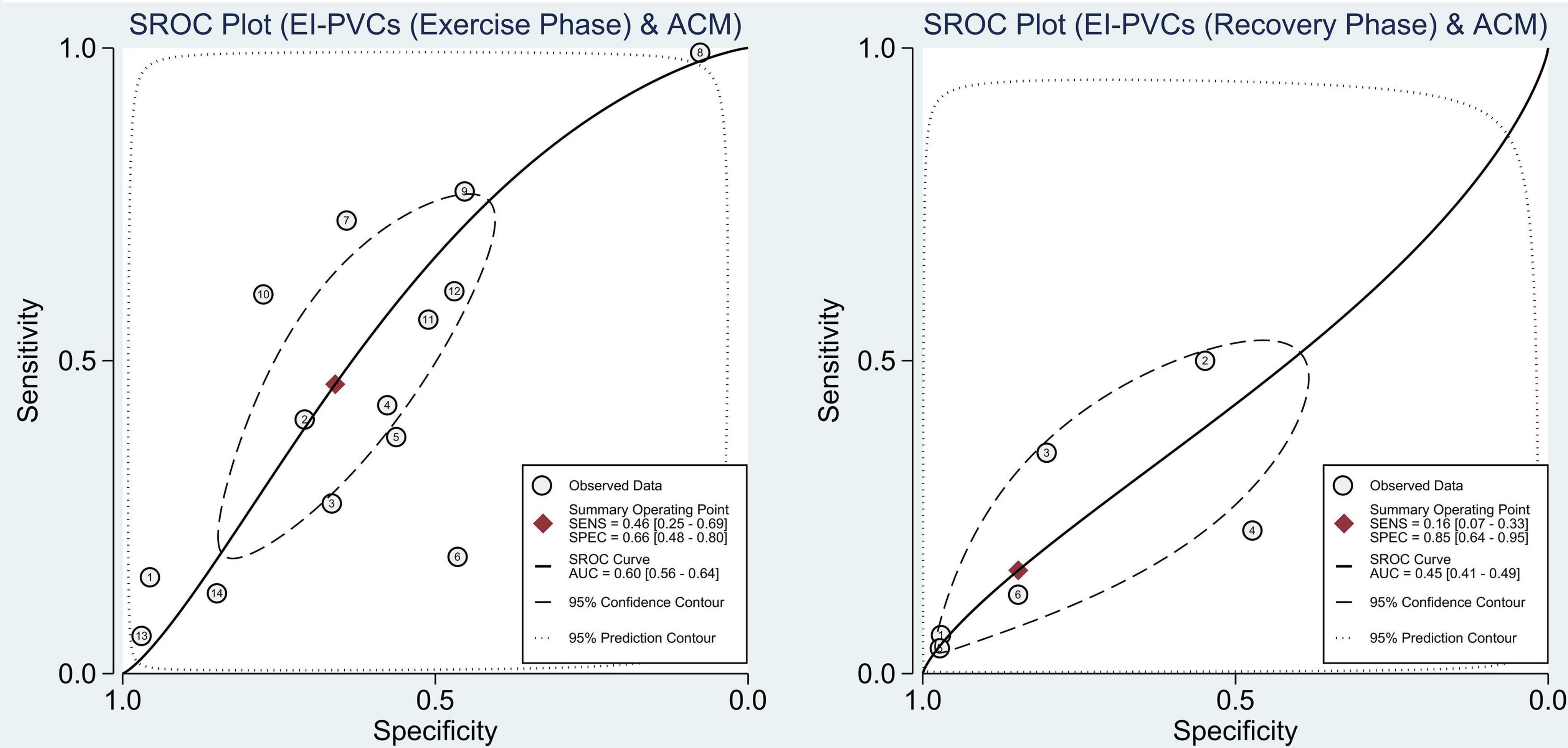
Figure 4. Comparison of SROC curve between two phases regarding ACM. SROC, summary receiver operating characteristics; EI-PVCs, exercise induced premature ventricular complexes; ACM, all-cause mortality; SENS, sensitivity; SPEC, specificity; AUC, area under the curve.
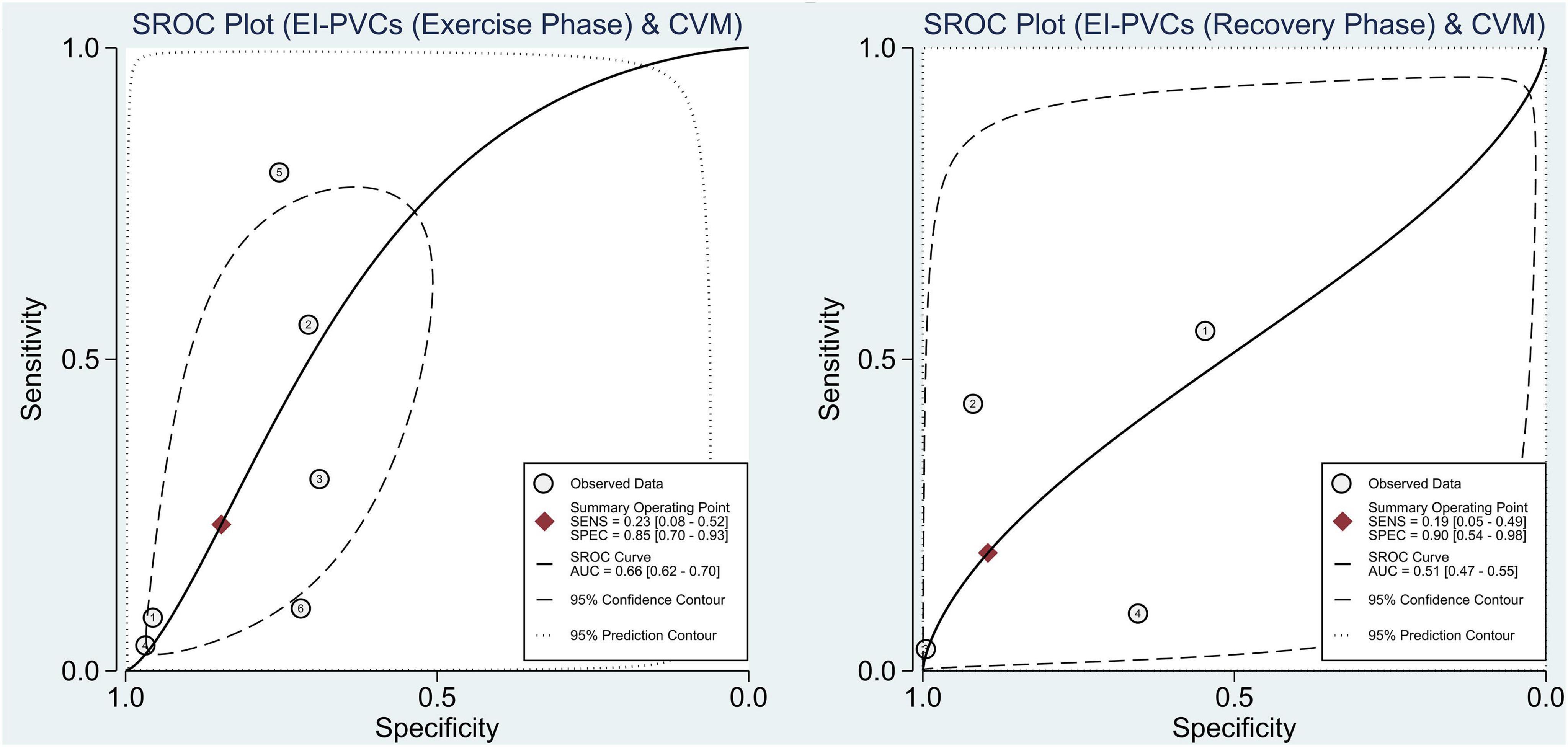
Figure 5. Comparison of SROC curve between two phases regarding CVM. SROC, summary receiver operating characteristics; EI-PVCs, exercise induced premature ventricular complexes; CVM, cardiovascular mortality; SENS, sensitivity; SPEC, specificity; AUC, area under the curve.
Meta-regression analysis
An additional meta-regression analysis was carried out to discover if there was a link between prospective covariates and outcomes of interest in this study. The findings revealed that EI-PVCs occurrence and mortality risk were not affected by age, sex, hypertension, diabetes mellitus, dyslipidemia, smoking status, and follow-up time (p > 0.05).
Heterogeneity and risk of bias assessment
The heterogeneity statistic I2 in this meta-analysis indicates low-to-moderate heterogeneity. There was considerable heterogeneity among the included studies for the ACM subset analysis. Fortunately, after performing a subgroup analysis, the heterogeneity was greatly reduced. With an average NOS score of 8.9 ± 0.3, the Newcastle–Ottawa Scale suggests a minimal likelihood of bias. Eventually, Begg’s funnel plot analysis revealed qualitatively symmetrical funnel plots and there was no indication of small-study effects throughout Egger’s test with regards to the association between EI-PVCs and mortality risk (p > 0.05) (Figures 2, 3).
Discussion
The major findings of this meta-analysis are as follows. Firstly, EI-PVCs increased the risk of ACM and CVM substantially. Secondly, only frequent PVCs are significantly correlated with an elevated risk of ACM and CVM. Thirdly, the specificity of PVCs induced during the recovery phase was higher compared to PVCs that occurred during the exercise phase. Fourthly, EI-PVCs were more significantly associated with ACM and CVM in the short follow-up period (<7.5 years).
Several included cohort studies revealed that patients with EI-PVCs were male, older, smokers, and more likely to have several comorbidities such as hypertension, diabetes mellitus, and dyslipidemia (7, 8, 13, 16, 18, 22, 23). Theoretically, male sex, older age, smoking, and these comorbidities can also contribute to an increase in the risk of mortality in such a population (24–28). Our meta-regression study revealed that the association of EI-PVCs and increased risk of ACM and CVM was not affected by age, sex, hypertension, diabetes mellitus, dyslipidemia, and smoking. Additionally, all included studies performed an adjusted analysis for these covariates. Thus, currently, we can safely infer that EI-PVCs are solely associated with ACM and CVM.
Various hypotheses have been postulated to explain how PVCs induced by exercise testing in an asymptomatic population might enhance the risk of mortality. For starters, the PVC itself can act as the source of ventricular tachyarrhythmia, which increases the risk of sudden cardiac death (SCD) (12). Second, untreated frequent PVCs in the asymptomatic general population can also induce cardiomyopathy, resulting in left ventricular dysfunction and raising the risk of death (29). Our analysis consistently found that, in contrast to infrequent PVCs, frequent PVCs were associated with a considerably elevated risk of ACM and CVM.
As the adage goes “structural heart disease may be silent and go undetected,” it might be one of the foremost inimical risk factors for mortality, given the majority of the included studies did not complete the imaging test at the beginning of the studies (8, 12–15, 18, 20–22). According to two cohort studies conducted by Frolkis et al. (16) and Lindow et al. (19), PVCs were only related to an elevated risk of death when structural heart dysfunctions were present, implying that baseline echocardiography testing may be necessary. However, another large cohort study carried out by Morshedi-Meibodi et al. (7) found that echocardiographic abnormalities were not attributed to an increased risk of mortality in their adjusted-model analysis. As a consequence of these variable results, it is unclear whether structural heart disease raises the risk of mortality in individuals displaying frequent EI-PVCs, suggesting that further studies are required for reassurance. Furthermore, a large cohort study revealed that a group of apparently healthy subjects with no risk factors for CAD had a 24% probability of aberrant stress pattern on an ECG, which is denoted as one of the most critical factors in prognosticating mortality (30). This emphasizes the fact that asymptomatic ischemia is frequent in various clinical populations, which might be a possible source of bias in this investigation. Fortunately, the majority of the studies we considered had already accounted for various potential covariates, including ischemic ECG changes, thereby eliminating this confounding bias (7, 8, 13–16, 18–22).
In the asymptomatic population, PVCs are denoted as a consequence of increasing automaticity in dormant ventricular pacemakers or can be generated by cardiotoxic drugs (e.g., digoxin) or electrolytes disturbances (e.g., hypokalemia and hypomagnesemia) (31). Meanwhile the sympathetic and parasympathetic nervous systems are involved in the underlying mechanism of PVCs created during exercise and recovery phases (32). Exercise-induced PVCs are generated by a surge of catecholamine levels due to sympathetic stimulation during exercise (33). Hayashi et al. study comprising seven patients without structural heart disease discovered that sympathetic predominance can initiate PVCs, resulting in ventricular tachycardia (VT) (34).
Whereas parasympathetic dysfunction through vagal activity impairment can occur in certain individuals during the recovery phase, creating a pro-arrhythmic substrate that leads to PVCs (35). A meta-analysis conducted by Qiu et al. demonstrated that regardless of the presence of PVCs, parasympathetic dysfunction as manifested by attenuation of heart rate recovery was correlated with an increased risk of ACM (35). In a study of 12 individuals without CAD, Osaka et al. discovered that decreased parasympathetic activity can cause non-sustained ventricular tachycardia (36). Moreover, Fei et al. study evaluating 23 patients without heart disease also revealed that idiopathic ventricular tachycardia occurred mostly by attenuation of parasympathetic activity rather than sympathetic activation (37). This result is aligned with our findings, which revealed that PVCs that occurred in the recovery phase disclosed better specificity compared to the exercise phase in predicting the likelihood of ACM and CVM. In contrast to sympathetic stimulation, it appears that parasympathetic dysfunction plays a more prominent role in the development of idiopathic ventricular tachyarrhythmia produced by PVCs and leads to greater mortality risk. Further to that, autonomic nervous system dysfunction can lead to insulin resistance, oxidative stress, and inflammation, all of which contribute to the pathogenesis of cardiovascular diseases, metabolic dysregulation, and other systemic disorders, ultimately increasing the risk of ACM and CVM (38–40).
This meta-analysis indicates that EI-PVCs have poor sensitivity but good specificity in accordance with the diagnostic test accuracy meta-analysis. Thus, it is better utilized to rule in rather than rule out the probability of the aforementioned outcomes of interest. However, it should be noted that the mean age in the majority of the included studies only varied from 43 to 59 years. Thereby, the association between EI-PVCs and mortality in other age groups remained equivocal. We strongly suggest that exercise ECG, as a convenient and widely available test, be performed as a routine auxiliary test in the middle-aged asymptomatic population in order to evaluate the frequency of PVCs and stratify the risk of mortality.
This study offers various advantages over previous meta-analyses. Firstly, the majority of the included studies (10 of 13 studies) have prospective designs, that diminish the risk of recall and selection bias. Secondly, in comparison to the Kim et al. study, this study contained a sufficient number of studies to perform the analysis between PVCs during the recovery phase and interest outcomes, thus the results were able to answer the cause of the moderately high inter-study heterogeneity in the previous meta-analysis. Lastly, the fact that all of our included studies employed multivariable-adjusted models, specifically ischemic ECG changes, further reinforces the value of this study’s pooled risk estimates, demonstrating that EI-PVCs may be used as a prognosticator in predicting mortality.
However, this study still had several major limitations. First, undetected structural heart disease at the baseline could still partly explain the link between EI-PVCs and mortality. Second, low-to-moderate heterogeneity in the ACM analysis may be attributed to variable criteria to classify PVCs across the included studies. Third, due to the majority of included studies enrolling middle-aged participants, the correlation between EI-PVCs and mortality in the younger and older populations remained unclear. Therefore, future prospective cohort studies consisting of age groups other than this range with fixed frequent PVCs criteria and without structural cardiac disease screened by imaging modalities are needed to address the aforementioned issues.
Conclusion
This study demonstrated that EI-PVCs in both phases (exercise and recovery) substantially raised the risk of ACM and CVM. Nonetheless, only frequent PVCs were shown to be correlated with ACM and CVM. Furthermore, the specificity of PVCs elicited during the recovery phase in predicting the interest outcomes is superior compared to the PVCs in the exercise phase. Hence, we propose that the exercise ECG be employed as an adjunct test in the middle-aged asymptomatic populations to measure the frequency of PVCs and stratify the risk of mortality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MIQ and ICSP conceived and designed the study. ICSP, WK, and RP performed study selection, data extraction, and interpreted the data. MIQ, ICSP, WK, and RP performed extensive search of relevant topics. ICSP, WK, and RP performed the statistical analysis. CA, GK, MP, HG, MRA, ASK, and YHK performed review and extensive editing of the manuscript. All authors contributed significantly to the writing of the manuscript and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European society of cardiology (ESC). Eur Heart J. (2020) 41:407–77.
2. Kharabsheh SM, Al-Sugair A, Al-Buraiki J, Farhan J. Overview of exercise stress testing. Ann Saudi Med. (2006) 26:1–6.
3. Henry RL, Kennedy GT, Crawford MH. Prognostic value of exercise-induced ventricular ectopic activity for mortality after acute myocardial infarction. Am J Cardiol. (1987) 59:1251–5. doi: 10.1016/0002-9149(87)90899-x
4. Sami M, Chaitman B, Fisher L, Holmes D, Fray D, Alderman E. Significance of exercise-induced ventricular arrhythmia in stable coronary artery disease: a coronary artery surgery study project. Am J Cardiol. (1984) 54:1182–8. doi: 10.1016/s0002-9149(84)80064-8
5. Marieb MA, Beller GA, Gibson RS, Lerman BB, Kaul S. Clinical relevance of exercise-induced ventricular arrhythmias in suspected coronary artery disease. Am J Cardiol. (1990) 66:172–8. doi: 10.1016/0002-9149(90)90583-m
6. Peng L, Li S, Tang X, Luo Y, Zhao Y, Dong R, et al. The prognostic value of exercise-induced ventricular arrhythmias in patients with and without coronary artery disease: a meta-analysis. Int J Cardiol. (2016) 218:225–32. doi: 10.1016/j.ijcard.2016.05.052
7. Morshedi-Meibodi A, Evans JC, Levy D, Larson MG, Vasan RS. Clinical correlates and prognostic significance of exercise-induced ventricular premature beats in the community. Circulation. (2004) 109:2417–22. doi: 10.1161/01.CIR.0000129762.41889.41
8. Marine JE, Shetty V, Chow GV, Wright JG, Gerstenblith G, Najjar SS, et al. Prevalence and prognostic significance of exercise-induced nonsustained ventricular tachycardia in asymptomatic volunteers: BLSA (baltimore longitudinal study of aging). J Am Coll Cardiol. (2013) 62:595–600. doi: 10.1016/j.jacc.2013.05.026
9. Lee V, Perera D, Lambiase P. Prognostic significance of exercise-induced premature ventricular complexes: a systematic review and meta-analysis of observational studies. Heart Asia. (2017) 9:14–24. doi: 10.1136/heartasia-2016-010854
10. Kim J, Kwon M, Chang J, Harris D, Gerson MC, Hwang SS, et al. Meta-analysis of prognostic implications of exercise-induced ventricular premature complexes in the general population. Am J Cardiol. (2016) 118:725–32. doi: 10.1016/j.amjcard.2016.06.007
11. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5.
12. Abdalla IS, Prineas RJ, Neaton JD, Jacobs DR, Crow RS. Relation between ventricular premature complexes and sudden cardiac death in apparently healthy men. Am J Cardiol. (1987) 60:1036–42.
13. Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. (2004) 292:1462–8. doi: 10.1001/jama.292.12.1462
14. Dewey FE, Kapoor JR, Williams RS, Lipinski MJ, Ashley EA, Hadley D, et al. Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch Intern Med. (2008) 168:225–34.
15. Dhoble A, Lahr BD, Allison TG, Kopecky SL. Cardiopulmonary fitness and heart rate recovery as predictors of mortality in a referral population. J Am Heart Assoc. (2014) 3:e000559. doi: 10.1161/JAHA.113.000559
16. Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. (2003) 348:781–90.
17. Fuchs T, Torjman A, Galitzkaya L, Leitman M, Pilz-Burstein R. The clinical significance of ventricular arrhythmias during an exercise test in non-competitive and competitive athletes. Isr Med Assoc. (2011) 13:735–9.
18. Jouven X, Zureik M, Desnos M, Courbon D, Ducimetière P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med. (2000) 343:826–33.
19. Lindow T, Ekström M, Brudin L, Hedman K, Ugander M. Prognostic implications of structural heart disease and premature ventricular contractions in recovery of exercise. medRxiv [Preprint]. (2022). 2021.12.22.21268216. doi: 10.1038/s41598-022-14535-w
20. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. (2002) 346:793–801.
21. Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. (2003) 290:1600–7. doi: 10.1001/jama.290.12.1600
22. Refaat MM, Gharios C, Moorthy MV, Abdulhai F, Blumenthal RS, Jaffa MA, et al. Exercise-induced ventricular ectopy and cardiovascular mortality in asymptomatic individuals. J Am Coll Cardiol. (2021) 78:2267–77.
23. Dzikowicz DJ, Carey MG. Exercise-induced premature ventricular contractions are associated with myocardial ischemia among asymptomatic adult male firefighters: implications for enhanced risk stratification. Biol Res Nurs. (2020) 22:369–77. doi: 10.1177/1099800420921944
24. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086
25. Rethy L, Shah NS, Paparello JJ, Lloyd-Jones DM, Khan SS. Trends in hypertension-related cardiovascular mortality in the United States, 2000 to 2018. Hypertension. (2020) 76:e23–5. doi: 10.1161/HYPERTENSIONAHA.120.15153
26. Yi SW, Yi JJ, Ohrr H. Total cholesterol and all-cause mortality by sex and age: a prospective cohort study among 12.8 million adults. Sci Rep. (2019) 9:1596. doi: 10.1038/s41598-018-38461-y
27. Holipah H, Sulistomo HW, Maharani A. Tobacco smoking and risk of all-cause mortality in Indonesia. PLoS One. (2020) 15:e0242558. doi: 10.1371/journal.pone.0242558
28. Sidney S, Lee C, Liu J, Khan SS, Lloyd-Jones DM, Rana JS. Age-adjusted mortality rates and age and risk–associated contributions to change in heart disease and stroke mortality, 2011-2019 and 2019-2020. JAMA Netw Open. (2022) 5:e223872. doi: 10.1001/jamanetworkopen.2022.3872
29. Panizo JG, Barra S, Mellor G, Heck P, Agarwal S. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. (2018) 7:128–34.
30. Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple risk factor intervention trial research group. JAMA (1982) 248:1465–77.
31. Goldberger AL, Goldberger ZD, Shvilkin A. Goldberger’s Clinical Electrocardiography A Simplified Approach. 9th ed. London: Elsevier (2018).
32. He W, Lu Z, Bao M, Yu L, He B, Zhang Y, et al. Autonomic involvement in idiopathic premature ventricular contractions. Clin Res Cardiol Off J Ger Card Soc. (2013) 102:361–70.
33. Koester C, Ibrahim AM, Cancel M, Labedi MR. The ubiquitous premature ventricular complex. Cureus. (2020) 12:e6585.
34. Hayashi H, Fujiki A, Tani M, Mizumaki K, Shimono M, Inoue H. Role of sympathovagal balance in the initiation of idiopathic ventricular tachycardia originating from right ventricular outflow tract. Pacing Clin Electrophysiol PACE. (1997) 20:2371–7. doi: 10.1111/j.1540-8159.1997.tb06073.x
35. Qiu S, Cai X, Sun Z, Li L, Zuegel M, Steinacker JM, et al. Heart rate recovery and risk of cardiovascular events and all−cause mortality: a meta−analysis of prospective cohort studies. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. (2017) 6:e005505. doi: 10.1161/JAHA.117.005505
36. Osaka M, Saitoh H, Sasabe N, Atarashi H, Katoh T, Hayakawa H, et al. Changes in autonomic activity preceding onset of nonsustained ventricular tachycardia. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc. (1996) 1:3–11.
37. Fei L, Statters DJ, Hnatkova K, Poloniecki J, Malik M, Camm AJ. Change of autonomic influence on the heart immediately before the onset of spontaneous idiopathic ventricular tachycardia. J Am Coll Cardiol. (1994) 24:1515–22. doi: 10.1016/0735-1097(94)90148-1
38. Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 academic conference award from the Japanese society of hypertension. Hypertens Res. (2013) 36:845–51. doi: 10.1038/hr.2013.73
39. Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol. (2011) 300:R818–26. doi: 10.1152/ajpregu.00426.2010
Keywords: electrocardiography, premature ventricular complexes, exercise test, EI-PVCs, arrhythmia, mortality
Citation: Iqbal M, Putra ICS, Kamarullah W, Pranata R, Achmad C, Karwiky G, Pramudyo M, Goenawan H, Akbar MR, Kartasasmita AS and Kim YH (2022) Revisiting exercise-induced premature ventricular complexes as a prognostic factor for mortality in asymptomatic patients: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:949694. doi: 10.3389/fcvm.2022.949694
Received: 21 May 2022; Accepted: 05 September 2022;
Published: 29 September 2022.
Edited by:
Michael Brunner, Artemed Kliniken Freiburg, GermanyReviewed by:
Xiangwei Ding, Nanjing Medical University, ChinaJaakko Tapio Leinonen, Institute for Molecular Medicine Finland, Helsinki Institute of Life Science, Faculty of Agriculture and Forestry, University of Helsinki, Finland
Copyright © 2022 Iqbal, Putra, Kamarullah, Pranata, Achmad, Karwiky, Pramudyo, Goenawan, Akbar, Kartasasmita and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Iqbal, bW9oYW1tYWRpcWJhbDE3OEBnbWFpbC5jb20=
 Mohammad Iqbal
Mohammad Iqbal Iwan Cahyo Santosa Putra
Iwan Cahyo Santosa Putra William Kamarullah
William Kamarullah Raymond Pranata1
Raymond Pranata1 Miftah Pramudyo
Miftah Pramudyo