
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 09 August 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.949538
This article is part of the Research Topic Abdominal Aortic Aneurysms: Advancements in diagnosis, biomarkers, drug therapeutics, surgical and endovascular treatment View all 21 articles
 Can Chen1†
Can Chen1† Benjamin Patterson2†
Benjamin Patterson2† Ruan Simpson3
Ruan Simpson3 Yanli Li1
Yanli Li1 Zhangzhang Chen1
Zhangzhang Chen1 Qianzhou Lv1
Qianzhou Lv1 Daqiao Guo4,5
Daqiao Guo4,5 Xiaoyu Li1*
Xiaoyu Li1* Weiguo Fu4,5*
Weiguo Fu4,5* Baolei Guo4,5,6*
Baolei Guo4,5,6*Objective: The aim of this study was to determine the association between fluoroquinolones (FQs) use, the risk of de novo aortic aneurysm or dissection (AAD), and the prognosis of patients with pre-existing AAD.
Materials and methods: We searched PubMed, EMBASE, CENTRAL, Scopus, and Web of Science on 31 March 2022. Observational studies that evaluated the association of FQs with AAD risk in the general population or FQs with the prognosis of patients with preexisting AAD and presented adjusted effect estimates were included. Two reviewers assessed study eligibility, extracted data, and assessed the risk of bias and certainty of evidence using GRADE.
Results: Of the 13 included studies, 11 focused on the association of FQs with de novo AAD incidence, and only one study investigated the association of FQs with the patient with AAD prognosis. FQ use was associated with an increased risk of de novo AAD within 30 days (RR: 1.42; 95% CI: 1.11–1.81; very low certainty) and 60 days (RR: 1.44; 95% CI: 1.26–1.64; low certainty). Specifically, the association was significant when compared with amoxicillin, azithromycin, doxycycline, or no antibiotic use. Furthermore, patients with preexisting AAD exposure to FQ had an increased risk of all-cause mortality (RR: 1.61; 95% CI: 1.50–1.73; moderate certainty) and aortic-specific mortality (RR: 1.80; 95% CI: 1.50–2.15; moderate certainty), compared to the non-exposed FQ group within a 60-day risk period.
Conclusion: FQs were associated with an increased incidence of AAD in the general population and a higher risk of adverse outcomes in patients with preexisting AAD. Nevertheless, the results may be affected by unmeasured confounding factors. This should be considered by physicians contemplating using FQs in patients with aortic dilation and those at high risk of AAD.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42021230171].
Fluoroquinolones (FQs) are one of the most commonly used classes of antibiotics, partly due to their wide activity spectrum, excellent bioavailability, extensive penetration of tissue, and successful microbiological outcomes (1, 2). Although FQs are well tolerated (3, 4), it has been suggested that they might exacerbate collagen-associated diseases owing to collagen loss and tissue degeneration (5–8).
Type I and type III collagen comprise the majority (80–90%) of collagen in the aorta (9), and FQs may contribute to the development of aortic disease (10, 11). Despite this plausible link, studies to characterize this relationship have yielded conflicting results. Several studies (12–17) using large administrative datasets found that recent FQs exposure was strongly associated with an increased risk of AAD, but after adjusting for the comparator antibiotics, two recent studies (18, 19) did not support this finding. Although a consensus on whether FQ causes de novo aortic disease has not yet been reached (20, 21), this potential association has raised several other important clinical questions, particularly regarding whether FQs can precipitate aortic complications in patients with existing aortic disease. It has been shown that FQ exposure could increase the risk of acute aortic dissection or rupture for patients with underlying aortopathy (10, 11, 22). Subsequently, Chen et al. (23) found that FQ exposure in patients with preexisting AAD was associated with a higher risk of adverse outcomes relative to non-exposure.
Although aortic events associated with FQs are thought to be rare (15), the United States alone has approximately 14 million annual FQ prescriptions (24, 25), and 20% of patients with AAD receive FQs during their hospitalization (26). Considering the serious adverse outcomes associated with aortopathy, including death, FQ-associated aortopathy constitutes a major health problem worldwide. We analyzed the association between FQs and the incidence and prognosis of AAD by systematic review and meta-analysis.
This systematic review and meta-analysis followed the recommendations (27) and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (28). The review was registered through PROSPERO with the registration number CRD42021230171.
We searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, Scopus, and Web of Science on 31 March 2022, for studies on the association between FQs and the risk of AAD incidence, all-cause mortality, and aortic-specific mortality in patients with AAD. In addition, the reference lists of relevant publications were reviewed to identify any additional studies that met the eligibility criteria. The electronic search strategy is presented in the ESM.
Studies were included if the following criteria were met: (1) population: participants who were initially free of AAD and did not have an AAD history when they entered a cohort that was subsequently followed to determine the risk of AAD incidence, or patients with AAD who were enrolled in a cohort that was followed to determine the risk of aortic-specific mortality or all-cause mortality; (2) definition of FQ exposure: participant received at least one prescription or a reimbursement for FQs; (3) studies reported the risk estimates of AAD incidence or mortality exposed to FQs vs. no FQs. Studies that did not report multivariable-adjusted estimates for at least one of the outcomes of interest, reported as abstracts only, presented without any data on relevant outcomes, or included data from Vigibase or the US Food and Drug Administration Adverse Event Reporting System (FAERS) database were excluded. Two reviewers (C.C. and B.G.) independently and in duplicate screened titles and abstracts, followed by full-text screening of potentially eligible studies. Discrepancies were solved through consensus or by the involvement of a third reviewer (W.F.).
The outcomes were the risk of AAD incidence in general populations and the risk of aortic-specific mortality, or all-cause mortality, in patients with AAD, within 30-, 60-, and 90-day risk periods following FQ exposure.
We extracted the following data from eligible studies: (1) study characteristics (first author, publication year, country, data source, definition of FQs exposure, and comparators); (2) population characteristics (age, gender, and number of participants); and (3) outcomes (number of events, exposure to FQs risk period, and the variables used for adjustment).
We assessed the risk of bias in the studies using the Quality in Prognosis Studies (QUIPS) tool (29). We categorized studies as having an overall low risk of bias if they had 5 or 6 low risk of bias domains, an overall high risk of bias if they had 2 or more high risk of bias domains, and an overall moderate risk of bias if they had all other studies (30).
The certainty of the evidence was assessed as high, moderate, low, or very low using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach adapted to prognosis studies (31). This assessment was based on risk of bias, consistency, precision, directness, and other concerns including publication bias (32), where two reviewers (C.C. and B.G.) independently, and in duplicate, extracted data, assessed risk of bias, and assessed certainty of evidence using GRADE. Any discrepancies were resolved by consensus or through the involvement of a third reviewer (W.F.).
We performed a meta-analysis by extracting and pooling adjusted relative effect estimates (27, 33). When merging data from studies that reported only an odds ratio (OR), we treated the OR as an RR (34) because the AA annual incidence is low (0.4–0.67%) (35). Studies comparing FQs to various controls, including other, or no antibiotics were included in the meta-analysis by making multiple pair-wise comparisons between all possible intervention group pairs (33). Statistical heterogeneity was addressed through the consistency of point estimates and the extent of CIs overlapped (36). We conducted subgroup analyzes according to age, sex, study type, comparators, ruptured or unruptured AA/AD, type of aortic disease, or anatomical site. In the sensitivity analyzes, we restricted analysis to AAD patients with baseline imaging to minimize potential surveillance bias and to individuals with infections to reduce selection bias. For outcomes reported by two or more studies, sensitivity analyzes were performed using a fixed effects model and individually excluded studies to explore the impact of each study on the overall results. For studies that gathered data from the same database and reported similar outcomes, recent publications were selected for primary analysis, and early publications were selected for sensitivity analysis. All meta-analyzes were conducted using Review Manager 5.4. The probability of publication bias was tested by means of Egger’s test using Stata 14.0 software. Two-sided P-values < 0.05 and 95% CIs, not including 1.00, were considered as statistically significant.
We screened 1,434 abstracts and 38 full-text papers and included 13 studies (12–19, 23, 37–40) (Figure 1 and Supplementary Table 1). A total of 11 studies (12–19, 37–40) focused on the association of FQs with de novo AAD incidence, and only one study (23) investigated the association of FQs with all-cause mortality and aortic-specific mortality of patients with existing AAD. Six studies (12, 15, 17, 19, 23, 40) were cohort studies, three (13, 18, 39) were nest case-control studies, and four (14, 16, 37, 38) were self-controlled studies, including two case-time-control studies (14, 16), one case-crossover studies (38), and one self-controlled case series studies (37). Five studies (13, 14, 18, 23, 40) were from Taiwan, three (17, 19, 37) from the United States, and the others were from Canada (12), Sweden (15), France (16), Denmark (38), and Korea (39). Data used in all studies were obtained from various administrative healthcare databases across countries. Seven studies (13, 14, 17, 18, 23, 37, 40) limited inclusion to patients aged 18 years or older of which two (18, 23) applied an age limit of 20 years, four (15, 19, 38, 39) included 40 years or older, and one (12) included 65 years or older. The definition of FQ exposure in all studies relied on the occurrence of a FQ prescription or reimbursement. The identification of AAD in all studies was based on the ICD-9 or ICD-10 codes with, or without, advanced imaging. There was substantial variation in the analytic strategies used, including the adjusted variables.
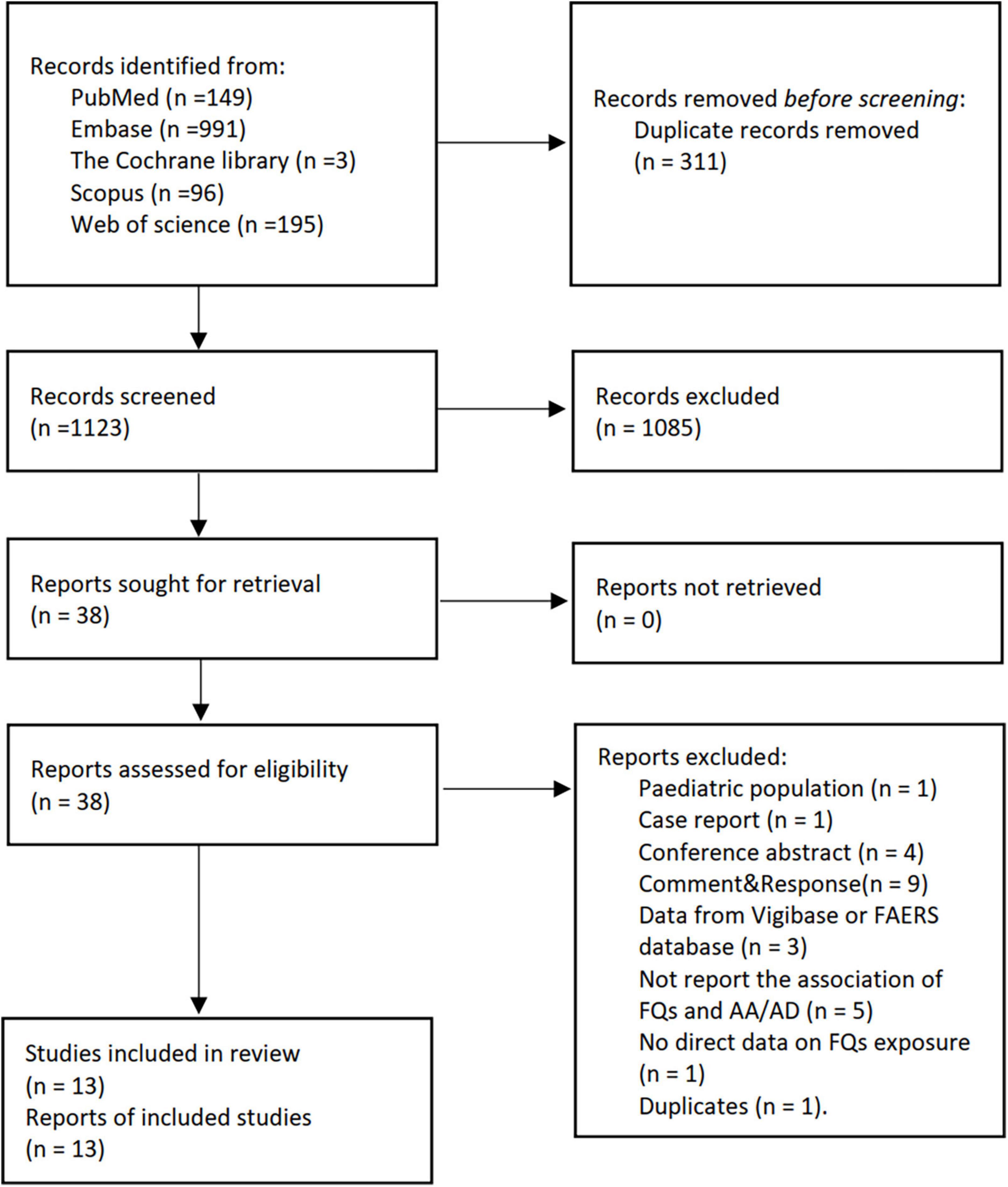
Figure 1. The PRISMA flow diagram for literature screening. FQs, fluoroquinolones; AA, aortic aneurysm; AD, aortic dissection.
The risk of bias was low in 4 studies and moderate in 9 studies. The bias mainly came from the outcome measurement and study of confounding domains (Supplementary Tables 1–13 in the ESM). Additional study characteristics are shown in Table 1 and Supplementary Tables 1–13 in the ESM.
There were four (12, 18, 37, 38), eight (13, 15, 16, 18, 19, 37–39), and four (16, 37, 38, 40) studies that linked the association of FQ use with the incidence of de novo AAD within 30-, 60-, and 90-day risk windows, respectively. The meta-analysis indicated that FQs were associated with an increased de novo AAD risk at 30-day (RR: 1.42; 95% CI: 1.11–1.81; very low certainty) and 60-day (RR: 1.44; 95% CI: 1.26–1.64; low certainty) risk window. AAD incidence showed a trend to increase after exposure to FQs within 90 days, but the difference did not reach statistical significance (RR: 1.45; 95% CI: 1.00–2.10; low certainty). When stratified by comparators, the association of FQs with AAD risk was significantly different across various comparators at 30-day (P = 0.006), 60-day (P < 0.001), and 90-day (P < 0.001) risk periods. Within any risk period, FQs were only associated with higher AAD risk when compared with amoxicillin, azithromycin, or no antibiotics and had a higher AAD rate compared with doxycycline in a 60-day risk period. The forest plots of the meta-analyzes are displayed in Figures 2–4, with different comparator subgroups also shown separately. The summary estimates of the FQ association with AAD risk are presented in Table 15.
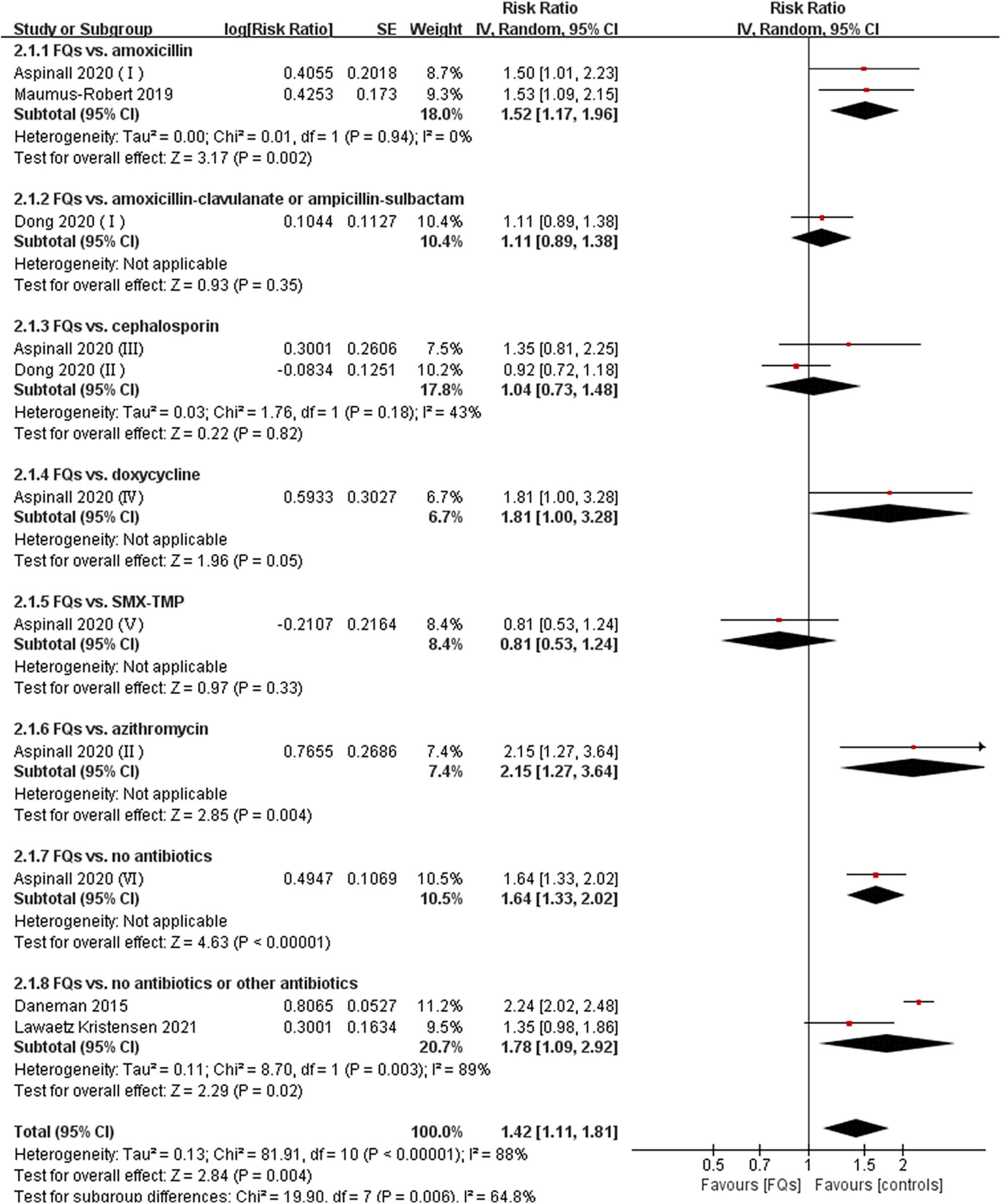
Figure 2. Forest plot of the risk of aortic aneurysm or dissection (AAD) in the comparison of fluoroquinolones (FQs) vs. controls within a 30-day risk period. FQs, fluoroquinolones; SMX-TMP, combined trimethoprim and sulfamethoxazole; IV, inverse variance; CI, confidence interval.
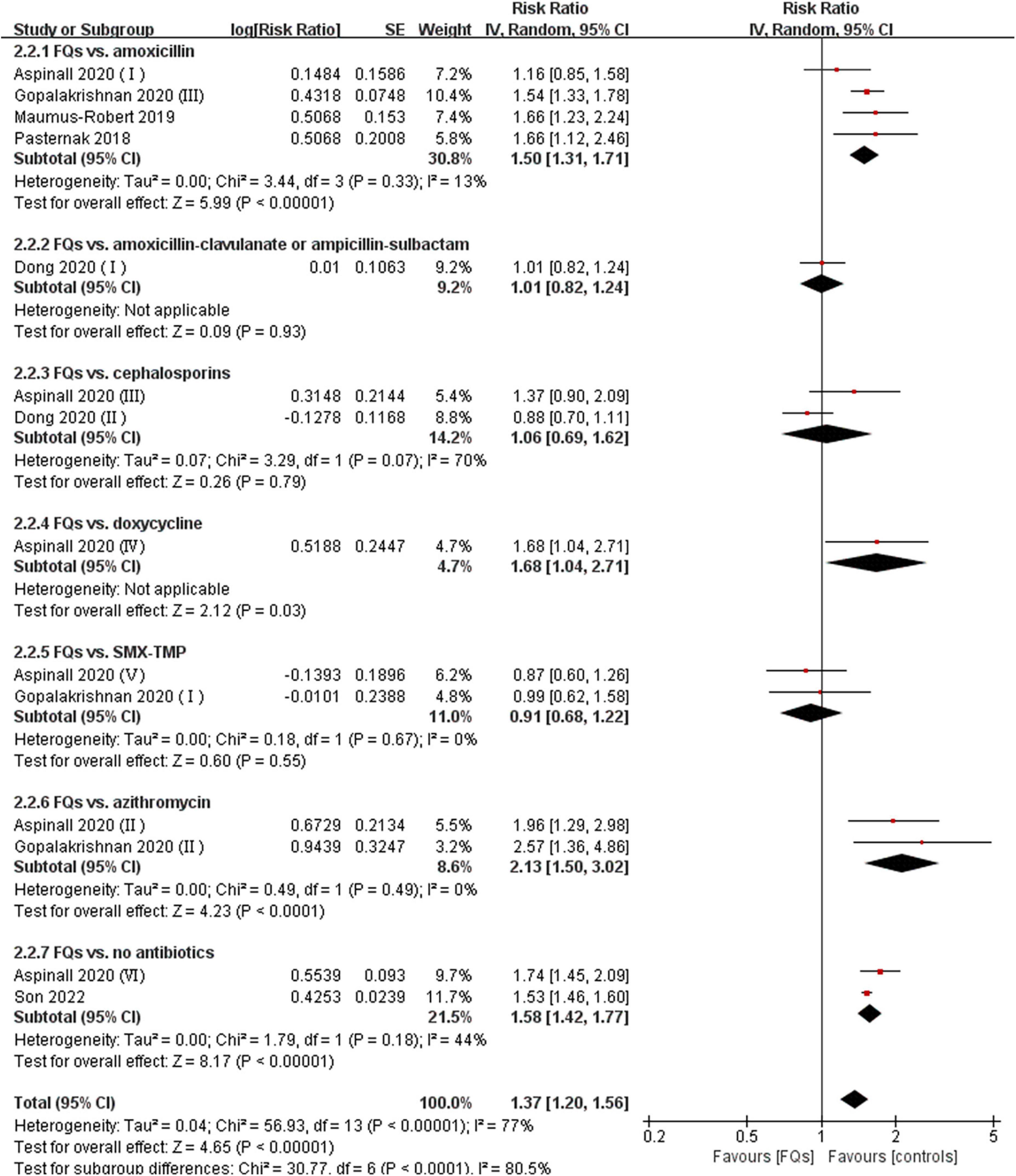
Figure 3. Forest plot of the risk of aortic aneurysm or dissection (AAD) in the comparison of fluoroquinolones (FQs) vs. controls within a 60-day risk period. FQs, fluoroquinolones; SMX-TMP, combined trimethoprim and sulfamethoxazole; IV, inverse variance; CI, confidence interval.
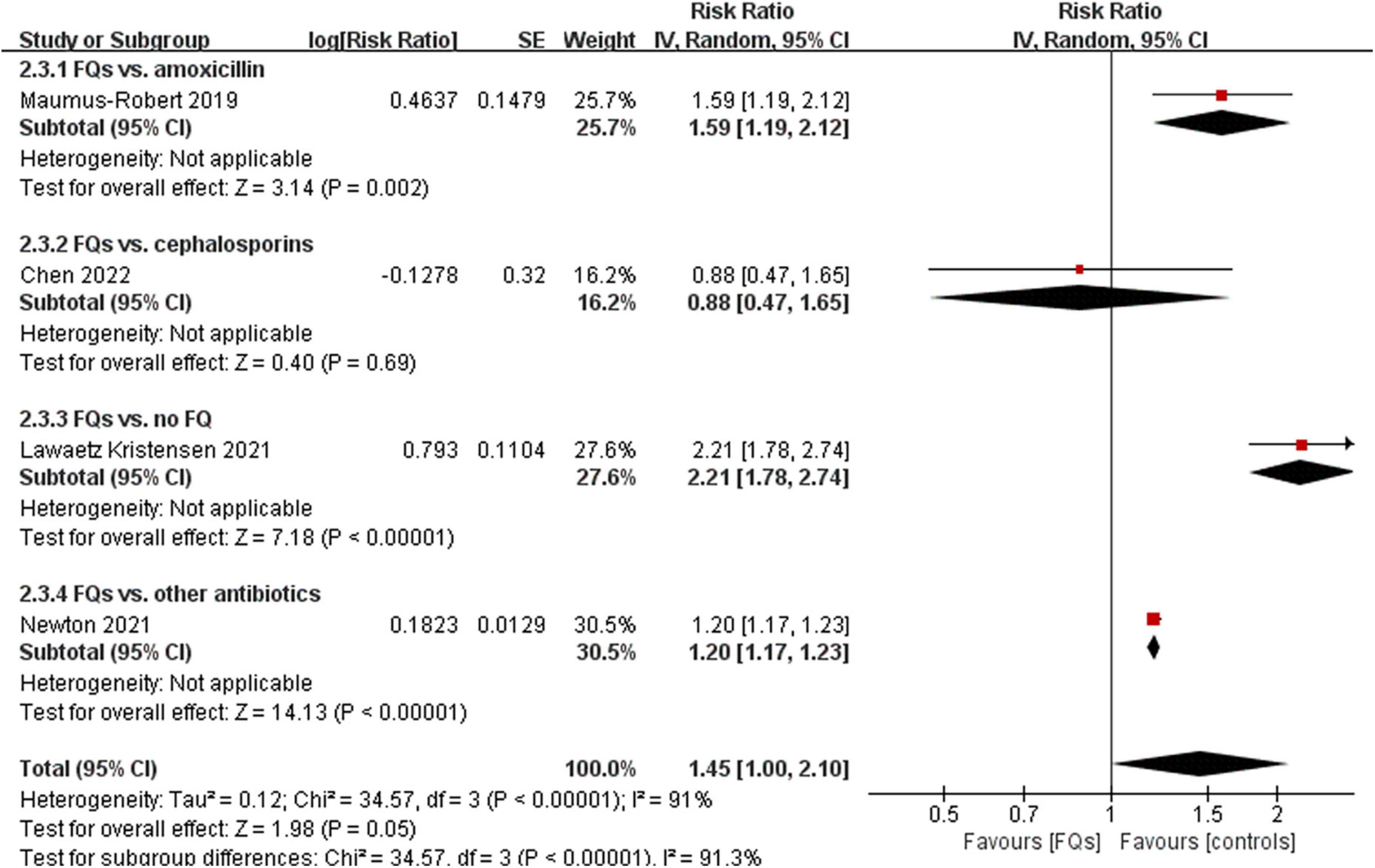
Figure 4. Forest plot of the risk of aortic aneurysm or dissection (AAD) in the comparison of fluoroquinolones (FQs) vs. controls within a 90-day risk period. FQs, fluoroquinolones; IV, inverse variance; CI, confidence interval.
When analyzing the AA and AD patients separately, the effect of FQs on AD and AA risks was not significantly different (Supplementary Figures 1A,B). When stratified by study type, the association of FQs with the incidence of AAD varied significantly across various study designs. Specifically, within a 30-day risk period (self-control studies: RR: 1.46; 95% CI: 1.23–1.73; cohort studies: RR: 2.24; 95% CI: 2.02–2.48; nest case-control studies: RR: 1.02; 95% CI: 0.85–1.22; P < 0.001); within a 90-day risk period (self-control studies: RR: 1.90; 95% CI: 1.38–2.62; cohort studies: RR: 1.20; 95% CI: 1.17–1.23; P = 0.005). However, the association was not significantly different across various study designs within a 60-day risk period (self-control studies: RR: 1.64; 95% CI: 1.30–2.06; cohort studies: RR: 1.56; 95% CI: 0.96–2.56; nest case-control studies: RR: 1.34; 95% CI: 0.95–1.89; P = 0.64). Minimal differences were observed when stratified by age, sex, incidence of ruptured or unruptured AA or AD, or anatomical site (Supplementary Tables 16–18).
In the sensitivity analysis, we explored the comparison of FQ and controls with analysis restricted to patients with AAD with baseline imaging and found that the association was attenuated (RR: 1.05; 95% CI: 0.94–1.18). Results were similar when the analysis was restricted to individuals with infection (Supplementary Table 19). The sensitivity analysis performed via a fixed effects model did not change appreciably when compared to the primary analyzes. Furthermore, we performed sensitivity analyzes by excluding each study individually from the primary analyzes, and the outcomes remained unchanged. The two studies (13, 14) reported using the same database and had the same outcomes within 60 days, and hence the sensitivity analysis results were similar to those of the primary analysis.
Only one study (23) investigated whether the use of FQs increases the 60-day risk of mortality in the AAD population. The findings suggest that exposure to FQs is associated with a higher risk of all-cause mortality (RR: 1.61; 95% CI: 1.50–1.73; moderate certainty) and aortic-specific mortality (RR: 1.80; 95% CI: 1.50–2.15; moderate certainty) compared with non-FQs (Figure 5, Supplementary Table 20). However, patients with AAD exposed to amoxicillin were not significantly associated with any risk outcomes compared with non-amoxicillin antibiotics (Supplementary Table 21). By comparing the risk of outcomes between the FQ and amoxicillin exposure periods for the same patient, the results demonstrated that the mortality risks were significantly higher during the FQ exposure period than during the amoxicillin exposure period (Supplementary Table 22). The summary estimates for the association of FQs and the AAD patient prognosis are presented in Supplementary Table 23.
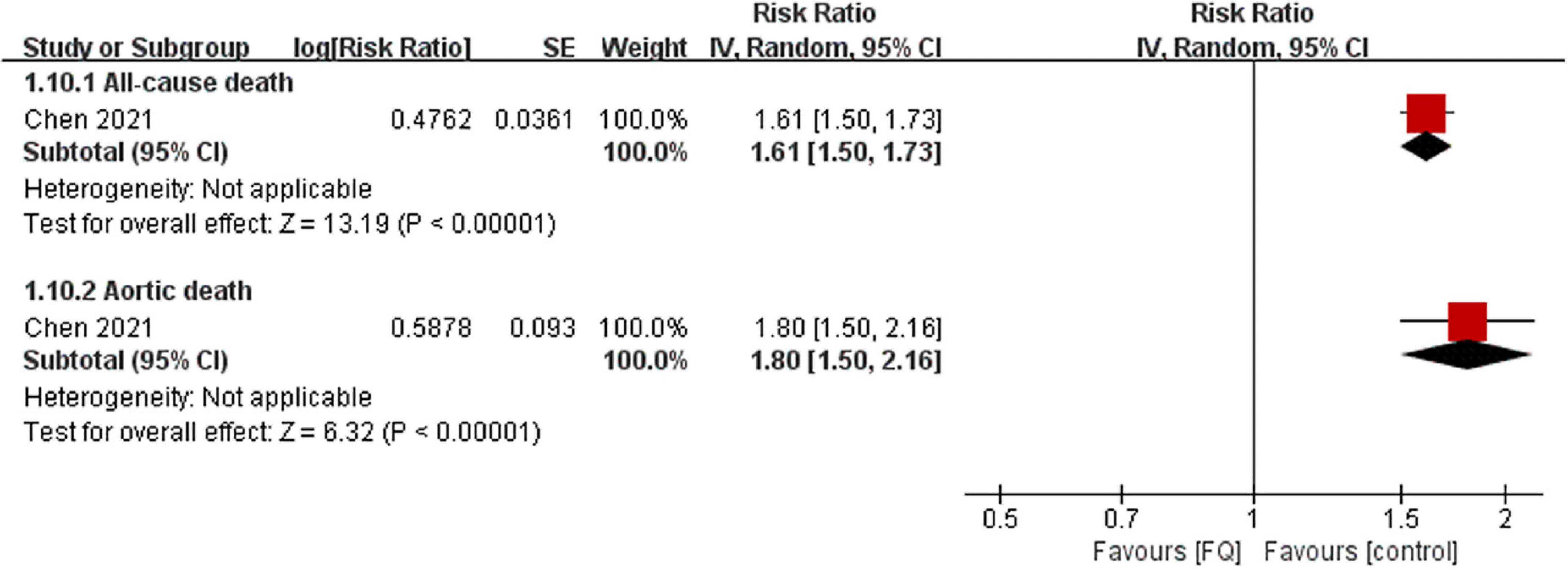
Figure 5. Forest plot of mortality risk of aortic aneurysm or dissection (AAD) patients in the comparison of fluoroquinolones (FQs) vs. control within a 60-day risk period. FQs, fluoroquinolones; IV, inverse variance; CI, confidence interval.
When analyzing the AA and AD patients separately, the effect of FQs did not differ significantly between the AD and AA groups (Supplementary Table 24).
When analyzing the association of FQ use with the de novo AAD incidence within 30-day (P = 0.37), 60-day (P = 0.68), and 90-day (P = 0.51) risk windows, the Egger’s test indicated that no evidence of publication bias was found.
Early studies (12–15) suggesting a possible association between FQs use and the risk of AAD, combined with reasonable evidence of a mechanistic link with other collagen-related diseases disorders (7, 8), led to the US Food and Drug Administration (FDA) (41) issuing a warning against FQ use in patients who were at risk of aortic disease. This study adds to the evidence that FQ use is associated with an increased risk of de novo AAD within a 90-day risk window. Patients with preexisting AAD and exposure to FQ had an increased risk of all-cause mortality and aortic-specific mortality relative to controls within a 60-day risk period, adding further weight to the FDA warning, which was based on inferences from studies designed for the general population rather than specific high-risk groups.
Although the use of FQs appears to lead to a higher risk of AAD incidence than no use of FQs, we found that the associations between FQs and AAD risk may change with various comparator antibiotics. Specifically, the associations were only significant when compared with antibiotics with a broad antimicrobial profile. Patients who are prescribed a broad-spectrum antibiotic potentially have more severe infections than those prescribed narrow-spectrum antibiotics or no antibiotics. Since severe infection may also independently damage the aortic endothelium, the infection may itself increase the risk of AAD (18). Another reason is that patients with more severe infections or indications for FQs therapy are more likely to receive an imaging examination, leading to a higher ascertainment rate (42, 43). Consequently, if the analysis was restricted to patients with prior imaging, then the association was attenuated, probably due to decreased ascertainment of “new” disease. These biases may confound the apparent association between FQs and AAD incidence. Additionally, Newton et al. (17) considered that this inconsistency may be due to the different analytical approaches employed in the studies, such as the inclusion of different populations, different definitions of FQ exposure, different sample sizes, and different ways to adjust confounding factors.
While there is limited evidence for a mechanistic link between antimicrobial use and aortic disease in humans, LeMaire et al. (22) found that wild-type mice given a high-fat diet, angiotensin II infusion, and exposure to ciprofloxacin experienced more severe aortic wall degeneration and a higher incidence of aortic aneurysm, dissection, and rupture, compared with test control mice. Another study (11) revealed that ciprofloxacin-treated mice suffered accelerated aortic enlargement and an increased incidence of aortic dissection and rupture, compared with vehicle-treated mice. This suggests that ciprofloxacin should be avoided in patients with Marfan syndrome. Campana et al. (11, 44) reported a patient who presented fever of unknown origin, elevated systemic inflammation markers, and radiological evidence of aortitis and pneumonia, who was subsequently treated with levofloxacin. Aortic rupture then occurred after 5 days after levofloxacin therapy was commenced. It was postulated that FQs could trigger the activation of ECM remodeling and an increase in MMP activity as a potential mechanism for this effect. Guzzardi et al. (44, 45) published a report suggesting that patients with alpha-1 antitrypsin (A1AT) deficiency and longstanding FQ use (26 months) may have a higher risk of AAD, although patients with A1AT deficiency are not typically considered at risk of aortopathy despite maladaptive connective tissue changes.
The suppression of extracellular matrix (ECM) biosynthesis and stability and the induction of ECM degradation may be key mechanisms underlying the effects of FQ on aortic destruction, dissection, and rupture (22). Consistent with that reported in the cornea (46, 47), tendon cells and tissues (48), and fibroblasts (49), ciprofloxacin exposure significantly increased MMP-2 and MMP-9 expression in the aortic wall, facilitating increased ECM destruction (11, 22). Guzzardi et al. (10) showed that FQ exposure induced a proteolytic MMP-TIMP imbalance driven by decreased TIMP expression, while simultaneously attenuating collagen expression in human aortic myofibroblast mediated ECM dysregulation. Furthermore, ciprofloxacin decreases the expression of Lysyl oxidase (LOX) (22), which is critical in elastic fiber assembly and stabilization and plays an important role in maintaining the integrity of aortic wall. In addition, the activation of stimulator of interferon genes (STING) (50–52), a pro-inflammatory cytosolic DNA sensor that plays a critical role in aortic degeneration, dissection, and rupture (53), is involved in the ciprofloxacin-induced suppression of LOX expression and the induction of MMP expression. Ciprofloxacin may also cause aortic destruction by increasing the number of TUNEL-positive cells in the aortic wall and inducing cell death in cultured aortic SMCs. This finding, corroborated by other studies, shows that ciprofloxacin induces cytotoxicity and death in various types of cells such as tenocytes (50, 51), lens epithelial cells (54), chondrocytes (55), and osteoblasts (56).
There are several potentially important implications of this study. FQs are commonly used in the empirical treatment of infected AAs to cover Salmonella species and sensitive Staphylococcus, which are highly prevalent bacterial species in this setting (57, 58). Alternate antibiotics should be sought as this could potentially increase the risk of aortic rupture in this already high-risk group. Furthermore, a retrospective cohort study (26) revealed that a large number of AAD patients received FQs during hospital admissions in general. Stronger evidence of the association of FQs with aortic-related adverse events may help inform enhanced antimicrobial stewardship around their use, especially given that more than 20% of patients with FQs may not have an appropriate indication for their use (17). Finally, for high-risk patients who have no choice but to use FQs, appropriate surveillance will be needed to minimize the hazard of aortic adverse events.
Despite these associations, additional research is needed to prove a causal link between FQs and aortic events. An in vitro study (10) showed that higher doses for longer periods may increase the susceptibility to FQ-associated acute aortic events. Clinical studies investing in the dose–response relationship between FQ use and the risk of aortic-related adverse events may be warranted. Furthermore, since FQ exposure might have different effects on cells in patients with variable comorbidities, clinical phenotypes, and connective tissue defects (10), clinical studies are needed to clarify differences between these subpopulations. This review only included one study (23) on the association of FQs and the mortality of AAD patients, and from this, it was difficult to determine whether FQ exposure conferred poor prognosis to AAD patients compared with other broad-spectrum antibiotics. Third-generation FQs do not appear to be associated with an increased risk of Achilles tendon rupture compared with first- and second-generation FQs and non-FQs (59), but the potential effect on AAD needs further study.
This systematic review has several strengths. First, the datasets used in this analysis may have contained overlapping patients, and thus complete independence between the databases cannot be ascertained. By reporting the association between FQ use and aortic events grouped by different risk periods (30, 60, or 90 days), this study effectively avoided data merging from studies using the same database. Second, compared with previously published meta-analyses (60–63), this review added to the current literature in studying the relationship between FQs and AAD mortality, and conducted more detailed subgroup analyzes to explore differences in the effects of FQ across different subgroups, and indeed found that differences in the selection of comparators had a substantial effect on the results.
Limitations include that the AAD diagnosis was according to ICD-9/10 codes with or without baseline advanced imaging examination, which is less accurate than clinical adjudication. This implies that some undiagnosed aneurysms were not identified, and aneurysm size or indications for surgical intervention were unavailable. Furthermore, since abdominal imaging was not routinely performed, it is possible that aneurysms classified as incidental may have been preexisting, or FQs simply aggravated a preexisting condition rather than initiated a de novo aneurysm. Second, as with all administrative databases, there is no available information on adherence to prescriptions. Therefore, the possibility of exposure misclassification cannot be excluded. Some included studies suggest that such exposure misclassification was random and did not bias the results (14, 17). Third, systematic reviews and meta-analyzes of observational studies are extremely sensitive to biases resulting from confounding factors. Although adjustment for numerous covariates was conducted, unknown confounders may have caused residual confounding. For instance, several risk factors associated with aneurysm development, such as smoking, were not captured (or reliably captured) in the claims data. Zhang et al. (64) quantified the potential influence of unmeasured confounding by smoking in FQ-AA association using three published approaches, and suggested that confounding by smoking is an unlikely explanation for the apparent association between FQ use and aortic aneurysm. These findings addressed a major limitation of epidemiologic studies regarding confounding by smoking due to an absence of data and strengthened the evidence for a FQ-related AA. Fourth, due to the limited number of included studies and limited data in the reports, we could not establish a safe window for FQ dose or duration, and it seems that higher doses or longer durations of FQ treatment exhibit stronger associations with aortic pathology (10, 13, 14). Finally, due to a lack of pharmacological interaction comparisons, we cannot determine whether FQs combined with other drugs, such as systemic corticosteroids and non-steroidal anti-inflammatory agents, increase the occurrence of aortic diseases (65).
FQ use was associated with an increased short-term risk of developing AAD in the general population and a poor prognosis in patients who have been diagnosed with AAD. There are several important confounding factors from this study, which means that more data are required before this relationship is proven. Regardless, it can help physicians when considering prescribing FQs to patients with aortic dilatation and those at high risk of AAD. We propose considering an alternative antibiotic for patients with aortic pathology and avoiding this class of antibiotics in patients who are prone to AAD.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
WF, DG, and BG proposed the conception and supervised the implementation process. CC and BG conducted the study search and screening, data extraction, and risk of bias assessment. CC, YL, and XL were responsible for data analysis. CC wrote the manuscript. BP, RS, ZC, and QL revised the manuscript. All authors reviewed and approved the final version of the work.
This study was sponsored by the National Natural Science Foundation of China (82000436) and the Science and Technology of Shanghai (201409004800 and 21410710500).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.949538/full#supplementary-material
1. Millanao AR, Mora AY, Villagra NA, Bucarey SA, Hidalgo AA. Biological effects of quinolones: A family of broad-spectrum antimicrobial agents. Molecules. (2021) 26:7153. doi: 10.3390/molecules26237153
2. Chan T, Bunce PE. Fluoroquinolone antimicrobial drugs. CMAJ. (2017) 189:E638. doi: 10.1503/cmaj.160938
3. Owens RC Jr, Ambrose PG. Antimicrobial safety: Focus on fluoroquinolones. Clin Infect Dis. (2005) 41(Suppl. 2):S144–57. doi: 10.1086/428055
4. Liu HH. Safety profile of the fluoroquinolones: Focus on levofloxacin. Drug Saf. (2010) 33:353–69. doi: 10.2165/11536360-000000000-00000
5. Yu X, Jiang DS, Wang J, Wang R, Chen T, Wang K, et al. Fluoroquinolone use and the risk of collagen-associated adverse events: A systematic review and meta-analysis. Drug Saf. (2019) 42:1025–33. doi: 10.1007/s40264-019-00828-z
6. Sendzik J, Shakibaei M, Schäfer-Korting M, Stahlmann R. Fluoroquinolones cause changes in extracellular matrix, signalling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. (2005) 212:24–36. doi: 10.1016/j.tox.2005.04.002
7. Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon injury and fluoroquinolone use: A systematic review. Drug Saf. (2013) 36:709–21. doi: 10.1007/s40264-013-0089-8
8. Chui CS, Wong IC, Wong LY, Chan EW. Association between oral fluoroquinolone use and the development of retinal detachment: A systematic review and meta-analysis of observational studies. J Antimicrob Chemother. (2015) 70:971–8. doi: 10.1093/jac/dku507
9. Berillis P. The role of collagen in the aorta’s structure. Open Circ Vasc J. (2013) 6:1–8. doi: 10.2174/1877382601306010001
10. Guzzardi DG, Teng G, Kang S, Geeraert PJ, Pattar SS, Svystonyuk DA, et al. Induction of human aortic myofibroblast-mediated extracellular matrix dysregulation: A potential mechanism of fluoroquinolone-associated aortopathy. J Thorac Cardiovasc Surg. (2019) 157:109.e–19.e. doi: 10.1016/j.jtcvs.2018.08.079
11. LeMaire SA, Zhang L, Zhang NS, Luo W, Barrish JP, Zhang Q, et al. Ciprofloxacin accelerates aortic enlargement and promotes dissection and rupture in Marfan mice. J Thorac Cardiovasc Surg. (2020) 163:e215–26. doi: 10.1016/j.jtcvs.2020.09.069
12. Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: A longitudinal cohort study. BMJ open. (2015) 5:e010077. doi: 10.1136/bmjopen-2015-010077
13. Lee CC, Gabriel Lee MT, Chen YS, Lee SH, Chen YS, Chen SC, et al. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Internal Med. (2015) 175:1839–47. doi: 10.1001/jamainternmed.2015.5389
14. Lee CC, Lee MTG, Hsieh R, Porta L, Lee WC, Lee SH, et al. Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol. (2018) 72:1369–78. doi: 10.1016/j.jacc.2018.06.067
15. Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: Nationwide cohort study. BMJ. (2018) 360:k678. doi: 10.1136/bmj.k678
16. Maumus-Robert S, Berard X, Mansiaux Y, Tubert-Bitter P, Debette S, Pariente A. Short-term risk of aortoiliac aneurysm or dissection associated with fluoroquinolone use. J Am Coll Cardiol. (2019) 73:875–7. doi: 10.1016/j.jacc.2018.12.012
17. Newton ER, Akerman AW, Strassle PD, Kibbe MR. Association of fluoroquinolone use with short-term risk of development of aortic aneurysm. JAMA Surg. (2021) 156:264–72. doi: 10.1001/jamasurg.2020.6165
18. Dong YH, Chang CH, Wang JL, Wu LC, Lin JW, Toh S. Association of infections and use of fluoroquinolones with the risk of aortic aneurysm or aortic dissection. JAMA Intern Med. (2020) 180:1787–95. doi: 10.1001/jamainternmed.2020.4192
19. Gopalakrishnan C, Bykov K, Fischer MA, Connolly JG, Gagne JJ, Fralick M. Association of fluoroquinolones with the risk of aortic aneurysm or aortic dissection. JAMA Intern Med. (2020) 180:1596–605. doi: 10.1001/jamainternmed.2020.4199
20. DeGette RL, Grant RW, Mph MD. Observational study design challenges-the case of fluoroquinolones and aortic disease. JAMA Intern Med. (2020) 180:1605–6. doi: 10.1001/jamainternmed.2020.4191
21. Lai CC, Lu CT, Kao KC, Lu MC, Ko WC, Hsueh PR. Association of fluoroquinolones use with the risk of aortic aneurysm or aortic dissection: Facts and myths. J Microbiol Immunol Infect. (2021) 54:182–4. doi: 10.1016/j.jmii.2021.03.002
22. LeMaire SA, Zhang L, Luo W, Ren P, Azares AR, Wang Y, et al. Effect of ciprofloxacin on susceptibility to aortic dissection and rupture in mice. JAMA Surg. (2018) 153:e181804. doi: 10.1001/jamasurg.2018.1804
23. Chen SW, Chan YH, Chien-Chia Wu V, Cheng YT, Chen DY, Lin CP, et al. Effects of fluoroquinolones on outcomes of patients with aortic dissection or aneurysm. J Am Coll Cardiol. (2021) 77:1875–87. doi: 10.1016/j.jacc.2021.02.047
24. Buehrle DJ, Wagener MM, Clancy CJ. Outpatient fluoroquinolone prescription fills in the United States, 2014 to 2020: Assessing the impact of food and drug administration safety warnings. Antimicrob Agents Chemother. (2021) 65:e0015121. doi: 10.1128/AAC.00151-21
25. Umarje SP, Alexander CG, Cohen AJ. Ambulatory fluoroquinolone use in the United States, 2015-2019. Open Forum Infect Dis. (2021) 8:ofab538. doi: 10.1093/ofid/ofab538
26. Frankel WC, Trautner BW, Spiegelman A, Grigoryan L, LeMaire SA. Patients at risk for aortic rupture often exposed to fluoroquinolones during hospitalization. Antimicrob Agents Chemother. (2019) 63:e1712–8. doi: 10.1128/AAC.01712-18
27. Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. (2019) 364:k4597. doi: 10.1136/bmj.k4597
28. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
29. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
30. Foroutan F, Guyatt GH, O’Brien K, Bain E, Stein M, Bhagra S, et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: Systematic review of observational studies. BMJ. (2016) 354:i5065. doi: 10.1136/bmj.i5065
31. Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ. (2015) 350:h870. doi: 10.1136/bmj.h870
32. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
33. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of Interventions version 6.2. (2021). Available online at: www.training.cochrane.org/handbook (accessed March 31, 2022).
34. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
35. Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromsø study, 1994-2001. Circulation. (2009) 119:2202–8. doi: 10.1161/CIRCULATIONAHA.108.817619
36. Granholm A, Zeng L, Dionne JC, Perner A, Marker S, Krag M, et al. Predictors of gastrointestinal bleeding in adult ICU patients: A systematic review and meta-analysis. Intensive Care Med. (2019) 45:1347–59. doi: 10.1007/s00134-019-05751-6
37. Aspinall SL, Sylvain NP, Zhao X, Zhang R, Dong D, Echevarria K, et al. Serious cardiovascular adverse events with fluoroquinolones versus other antibiotics: A self-controlled case series analysis. Pharmacol Res Perspect. (2020) 8:e00664. doi: 10.1002/prp2.664
38. Lawaetz Kristensen K, Hallas J, Sanddal Lindholt J. Fluoroquinolones as a trigger for rupture of abdominal aortic aneurysm: A case-crossover analysis. Basic Clin Pharmacol Toxicol. (2021) 129:44–51. doi: 10.1111/bcpt.13591
39. Son N, Choi E, Chung SY, Han SY, Kim B. Risk of aortic aneurysm and aortic dissection with the use of fluoroquinolones in Korea: A nested case-control study. BMC Cardiovasc Disord. (2022) 22:44. doi: 10.1186/s12872-022-02488-x
40. Chen YY, Yang SF, Yeh HW, Yeh YT, Huang JY, Tsao SL, et al. Association between aortic aneurysm and aortic dissection with fluoroquinolones use in patients with urinary tract infections: A population-based cohort study. J Am Heart Assoc. (2022) 11:e023267. doi: 10.1161/JAHA.121.023267
41. United States Food and Drug Administration.FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients. Silver Spring, MD: United States Food and Drug Administration (2022).
42. Sterpetti AV. Concerns about study on fluoroquinolone use and risk of development of aortic aneurysm. JAMA Surg. (2021) 156:1068–9. doi: 10.1001/jamasurg.2021.3026
43. Chen CH, Wang CY, Lai CC. Concerns about study on fluoroquinolone use and risk of development of aortic aneurysm. JAMA Surg. (2021) 156:1068. doi: 10.1001/jamasurg.2021.3023
44. Campana P, Leosco D, Petraglia L, Radice L, Parisi V. Aortic rupture in patient on oral therapy with levofloxacin. Aging Clin Exp Res. (2020) 32:755–7. doi: 10.1007/s40520-019-01267-7
45. Guzzardi DG, Hassanabad AF, Bromley AB, Fedak PWM. Fluoroquinolone-associated type A aortic dissection in alpha-1 anti-trypsin deficiency. Ann Thorac Surg. (2020) 110:e489–91. doi: 10.1016/j.athoracsur.2020.04.044
46. Reviglio VE, Hakim MA, Song JK, O’Brien TP. Effect of topical fluoroquinolones on the expression of matrix metalloproteinases in the cornea. BMC Ophthalmol. (2003) 3:10. doi: 10.1186/1471-2415-3-10
47. Sharma C, Velpandian T, Baskar Singh S, Ranjan Biswas N, Bihari Vajpayee R, Ghose S. Effect of fluoroquinolones on the expression of matrix metalloproteinase in debrided cornea of rats. Toxicol Mech Methods. (2011) 21:6–12. doi: 10.3109/15376516.2010.529183
48. Shakibaei M, Stahlmann R. Ultrastructure of Achilles tendon from rats after treatment with fleroxacin. Arch Toxicol. (2001) 75:97–102. doi: 10.1007/s002040000203
49. Bujor AM, Haines P, Padilla C, Christmann RB, Junie ML, Sampaio-Barros PD, et al. Ciprofloxacin has antifibrotic effects in scleroderma fibroblasts via downregulation of Dnmt1 and upregulation of Fli1. Int J Mol Med. (2012) 30:1473–80. doi: 10.3892/ijmm.2012.1150
50. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. (2013) 339:826–30. doi: 10.1126/science.1229963
51. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. (2013) 498:380–4. doi: 10.1038/nature12306
52. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. (2013) 339:786–91. doi: 10.1126/science.1232458
53. Luo W, Wang Y, Zhang L, Ren P, Zhang C, Li Y, et al. Critical role of cytosolic DNA and its sensing adaptor STING in aortic degeneration, dissection, and rupture. Circulation. (2020) 141:42–66. doi: 10.1161/CIRCULATIONAHA.119.041460
54. Zhao B, Chignell CF, Rammal M, Smith F, Hamilton MG, Andley UP, et al. Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics. Photochem Photobiol. (2010) 86:798–805. doi: 10.1111/j.1751-1097.2010.00755.x
55. Li P, Cheng NN, Chen BY, Wang YM. In vivo and in vitro chondrotoxicity of ciprofloxacin in juvenile rats. Acta Pharmacol Sin. (2004) 25:1262–6.
56. Williams RJ III, Attia E, Wickiewicz TL, Hannafin JA. The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med. (2000) 28:364–9. doi: 10.1177/03635465000280031401
57. Moneta GL, Taylor LM Jr, Yeager RA, Edwards JM, Nicoloff AD, McConnell DB, et al. Surgical treatment of infected aortic aneurysm. Am J Surg. (1998) 175:396–9. doi: 10.1016/S0002-9610(98)00056-7
58. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s choice – European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57:8–93. doi: 10.1016/j.ejvs.2020.09.004
59. Chinen T, Sasabuchi Y, Matsui H, Yasunaga H. Association between third-generation fluoroquinolones and Achilles Tendon rupture: A self-controlled case series analysis. Ann Fam Med. (2021) 19:212–6. doi: 10.1370/afm.2673
60. Wee I, Chin B, Syn N, Lee KS, Ng JJ, Choong A. The association between fluoroquinolones and aortic dissection and aortic aneurysms: A systematic review and meta-analysis. Sci Rep. (2021) 11:11073. doi: 10.1038/s41598-021-90692-8
61. Lai CC, Wang YH, Chen KH, Chen CH, Wang CY. The association between the risk of aortic aneurysm/aortic dissection and the use of fluroquinolones: A systematic review and meta-analysis. Antibiotics (Basel). (2021) 10:697. doi: 10.3390/antibiotics10060697
62. Dai XC, Yang XX, Ma L, Tang GM, Pan YY, Hu HL. Relationship between fluoroquinolones and the risk of aortic diseases: A meta-analysis of observational studies. BMC Cardiovasc Disord. (2020) 20:49. doi: 10.1186/s12872-020-01354-y
63. Singh S, Nautiyal A. Aortic dissection and aortic aneurysms associated with fluoroquinolones: A systematic review and meta-analysis. Am J Med. (2017) 130:1449–57.e9. doi: 10.1016/j.amjmed.2017.06.029
64. Zhang M, Falconer M, Taylor L. A quantitative bias analysis of the confounding effects due to smoking on the association between fluoroquinolones and risk of aortic aneurysm. Pharmacoepidemiol Drug Saf. (2020) 29:958–61. doi: 10.1002/pds.5019
Keywords: fluoroquinolones, aortic aneurysm, aortic dissection, systematic review, meta-analysis
Citation: Chen C, Patterson B, Simpson R, Li Y, Chen Z, Lv Q, Guo D, Li X, Fu W and Guo B (2022) Do fluoroquinolones increase aortic aneurysm or dissection incidence and mortality? A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:949538. doi: 10.3389/fcvm.2022.949538
Received: 21 May 2022; Accepted: 13 July 2022;
Published: 09 August 2022.
Edited by:
Carlos Alves, University of Coimbra, PortugalReviewed by:
Antonino S Rubino, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Chen, Patterson, Simpson, Li, Chen, Lv, Guo, Li, Fu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyu Li, bGkueGlhb3l1QHpzLWhvc3BpdGFsLnNoLmNu; Weiguo Fu, ZnUud2VpZ3VvQHpzLWhvc3BpdGFsLnNoLmNu; Baolei Guo, Z3VvLmJhb2xlaUB6cy1ob3NwaXRhbC5zaC5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.