
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 28 September 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.948347
This article is part of the Research Topic Metabolic Alterations in Cardiopulmonary Vascular Dysfunction View all 8 articles
 Jing Chen1†
Jing Chen1† Yina Wang1†
Yina Wang1† Jingyi Wang1
Jingyi Wang1 Lie Chen1
Lie Chen1 Qiushi Luo1
Qiushi Luo1 Bei Wang2
Bei Wang2 Xingwei He1
Xingwei He1 Xuefei Li3
Xuefei Li3 Huakun Zuo3
Huakun Zuo3 Ping Zuo1*
Ping Zuo1* Xiaoyun Yang1*
Xiaoyun Yang1*Background: Electrocardiography (ECG) plays a very important role in various cardiovascular diseases and elevated D-dimer in serum associated with thrombosis. In patients with coronavirus disease 2019 (COVID-19), immense pieces of evidence showed that ECG abnormalities or elevated D-dimer in serum occurred frequently. However, it remains unclear whether ECG abnormalities combined with elevated D-dimer could be a new risk predictor in patients with COVID-19.
Methods and results: This retrospective cohort study enrolled 416 patients with COVID-19 at Wuhan Tongji Hospital from 1 February to 20 March 2020. ECG manifestations, D-dimer levels, and in-hospital deaths were recorded for all patients. Logistic regression analysis was performed to examine the association between ECG manifestations and in-hospital mortality in patients with elevated D-dimer levels. In patients hospitalized for COVID-19, ST-T abnormalities (34.3%) were the most frequent ECG manifestations, whereas sinus tachycardia (ST) (13.3%) and atrial arrhythmias with rapid rhythms (8.5%) were the two most common cardiac arrhythmias. Compared to severely ill patients with COVID-19, ST-T abnormalities, ST and atrial arrhythmias (p<0.001) with rapid rhythms, D-dimer levels, and in-hospital deaths were significantly more frequent in critically ill patients with COVID-19. Moreover, elevated D-dimer levels were observed in all the patients who died. In the subgroup of 303 patients with elevated serum D-dimer levels, the patient's age, the incidence of ST-T abnormalities, ST, atrial fibrillation (AF), and atrial premature beat were significantly higher than those in the non-elevated D-dimer subgroup. Multivariate logistic regression analysis further revealed that ST and AF were risk factors for in-hospital mortality in COVID-19 patients with elevated D-dimer levels.
Conclusions: ECG abnormalities and elevated D-dimer levels were associated with a higher risk of critical illness and death in patients hospitalized for COVID-19. ECG abnormalities, including ST and AF, combined with elevated D-dimer levels, can be used to predict death in COVID-19.
The novel coronavirus disease 2019 (COVID-19) infection has potential effects on the cardiovascular system, with excessive inflammation and immune responses that could induce coronary vasculitis (1), cardiac injury, cardiac arrhythmias (2), and heart failure (3). Electrocardiography (ECG) is an important examination for the diagnosis of heart rhythm disturbances and other myocardial diseases in patients with COVID-19 (4). The electrocardiographic features of patients with COVID-19 pneumonia have been defined in several studies (5, 6). ECG abnormalities in patients with COVID-19 mainly include ST-T abnormalities caused by acute coronary syndromes, myocarditis, acute pericarditis, electrolyte disturbances, rhythm disorders, and pulmonary embolism (PE)-related manifestations (6, 7). A previous study demonstrated that right ventricular strain patterns on ECG are tightly associated with increased mortality in PE (8). On ECG, right ventricular strain pattern (9), fragmented QRS (10), prolonged QT (11), abnormal axis (12), and left bundle branch block (12) have all been associated with poor outcomes and a higher risk of mortality in COVID-19. As ECG is an easy and accessible method, ECG monitoring of patients with COVID-19 can be used as an essential screening tool for identifying high-risk patients.
D-dimer is a product of fibrin degradation and is a marker of fibrinolysis activation. The combination pre-test probability of venous thromboembolism with D-dimer levels <1000 μg/L can be used to evaluate the risk of venous thromboembolism and PE (13). Some studies demonstrated that elevated D-dimer was closely associated with mortality in patients with COVID-19 (14, 15). Furthermore, COVID-19 patients with elevated D-dimer levels exhibited a high prevalence of PE (16). ECG abnormalities in acute PE have been characterized by mounting evidence and play a valuable role in the diagnosis of PE (17). However, ECG abnormalities combined with elevated D-dimer in mortality prediction of COVID-19 remain unexplored.
Hence, we assessed the ECG abnormalities as well as elevated D-dimer levels in COVID-19. Specifically, we investigated whether the abnormalities in ECG together with elevated D-dimer levels were potential risk predictors for mortality in COVID-19.
This was a retrospective, observational study performed on patients diagnosed with COVID-19 admitted to Optical Valley Campus and the Sino-French New Campus of Tongji Hospital of Huazhong University of Science and Technology in Wuhan from 1 February 2020 to 20 March 2020. All consecutive patients who underwent ECG examination during this time frame were included. Optical Valley Campus and the Sino-French New Campus of Tongji Hospital were urgently reconstructed and specially designated hospitals to treat severely or critically ill patients with COVID-19. According to the Guidance for Corona Virus Disease 2019 (7th edition), patients with COVID-19 were diagnosed and divided into the critically ill and severely ill groups (18). This research was approved by the Institutional Review Board for Human Studies, Tongji Hospital, Huazhong University of Science and Technology (TJ-20200140).
Data from patients, including age, sex, medical history, ECG and laboratory examinations, clinical classification, and outcomes during hospital admission, were collected and analyzed. Clinical data were monitored until 20 March 2020.
The 12-lead routine ECG was recorded at an amplification of 10 mm/mV and a paper speed of 25 mm/s. All ECG examinations were recorded by one investigator who was double-blinded to all other clinical processes. The following abnormal signs in ECG were especially focused on: (1) sinus tachycardia (ST) (heart rate >100 bpm). (2) ST-T abnormalities: the elevation of ST segment was defined as ST 0.06 s after QRS complex in V2 and V3 leads and ≥0.1 mV in ≥2 other contiguous leads. The ST segment was elevated by ≥0.2 mV in men over 40 years and by ≥0.25 mV in men below 40 years. In women, the elevation of the ST segment was ≥0.15 mV (19). The evaluation of depression on the ST segment was defined as horizontal depression >0.05 mV or downsloping depression >0.05 mV from the isoelectric line, 0.06 s after QRS complex ending, and negative T waves were considered negative depth >0.10 mV in 2 contiguous leads (19). (3) incomplete (IRBBB) or complete right bundle branch block (CRBBB). (4) atrial flutter (AFL). (5) atrial fibrillation (AF). (6) abnormal Q wave. (7) left ventricular high voltage; (8) degree I atrioventricular block; (9) atrial premature beat; (10) ventricular premature beat; (11) atrial tachycardia; and (12) sinus bradycardia. ECG abnormalities in the Daniel score system include ST (heart rate >100 bpm), IRBBB or CRBBB, T-wave inversion, and the S1Q3T3 complex (8). The ST (heart rate >100 bpm) and IRBBB scores were recorded at two points. The CRBBB score was recorded as three points. According to the magnitude of the T-wave inversion in leads V1, V2, and V3, the score was recorded as 0–3 points. The score of S1Q3T3 components indicated that the S wave in lead I was 0 points, and the Q wave and T-wave inversion in lead III were both one point.
Serum D-dimer levels were examined in a Tongji Hospital laboratory at the time of admission. D-dimer levels >500 μg/L were considered abnormal.
The ECG distributions in patients with COVID-19 were further analyzed using a descriptive computing program with an SPSS multiple response set. Continuous variables with normal distributions are shown as mean ± SD. Additionally, comparisons between the two groups were analyzed using Student's t-test. Moreover, continuous variables with skewed distributions are represented by the median (quartile range), and the significance between groups was examined using the Mann-Whitney test. Categorical data are expressed as numbers or percentages and were compared using the χ2 test. A two-sided significance level of 0.05 was applied in all hypothesis tests. Logistic regression analyses were used to determine baseline characteristics and ECG signs of mortality in the subgroups of elevated and non-elevated D-dimer levels. For univariate and multivariate analyses, variables with a 2-sided p-value <0.05 were included. All statistical analyses were performed using SPSS (version 18, Chicago, NY, USA).
In this study, we analyzed 416 consecutive patients hospitalized for COVID-19. The frequencies of the ECG characteristics were evaluated. According to the descriptive computing program using an SPSS-multiple response set (Figure 1), ST-T abnormalities (34.3%) were the most frequent abnormality in these hospitalized patients. ST (heart rate >100 bpm) (13.3%) was the most prevalent cardiac arrhythmia encountered. In addition, the RBBB, AF, and AFL increased by 11.9 and 8.5%, respectively. Of all the ECG characteristics, the least occurring was ventricular premature beats (1.7%).
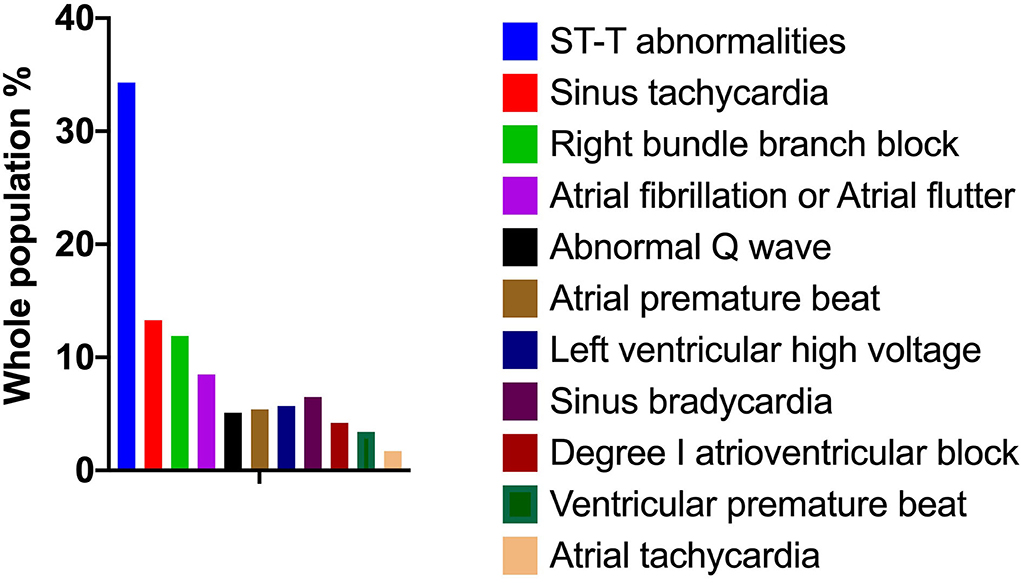
Figure 1. Electrocardiogram characteristics of 416 patients hospitalized for COVID-19. The frequencies of ECG characteristics in patients with COVID-19 were evaluated by a descriptive computing program using an SPSS multiple response set. COVID-19, coronavirus disease 2019; ECG, electrocardiography.
The 416 patients with COVID-19 were divided into critically and severely ill groups. As seen in Table 1, the average age of critically ill patients (67.4 ± 14.4 years) was older than that of severely ill patients (62.1 ± 14.0 years). The proportion of men among severely ill patients (178, 59.1%) was higher than that among critically ill patients (35, 30.4%). Hypertension, coronary heart disease, arrhythmia, and stroke were much more frequent among critically ill patients [60 (52.2%), 21 (18.3%), 6 (5.2%), and 9 (7.8%), respectively] than among severely ill patients [108 (35.9%), 29 (9.6%), 3 (0.9%), and 6 (2.0%), respectively] (Table 1). D-dimer levels in critically ill patients (111, 96.5%) were significantly higher than those in the severely ill group (192, 63.8%) (Table 1).
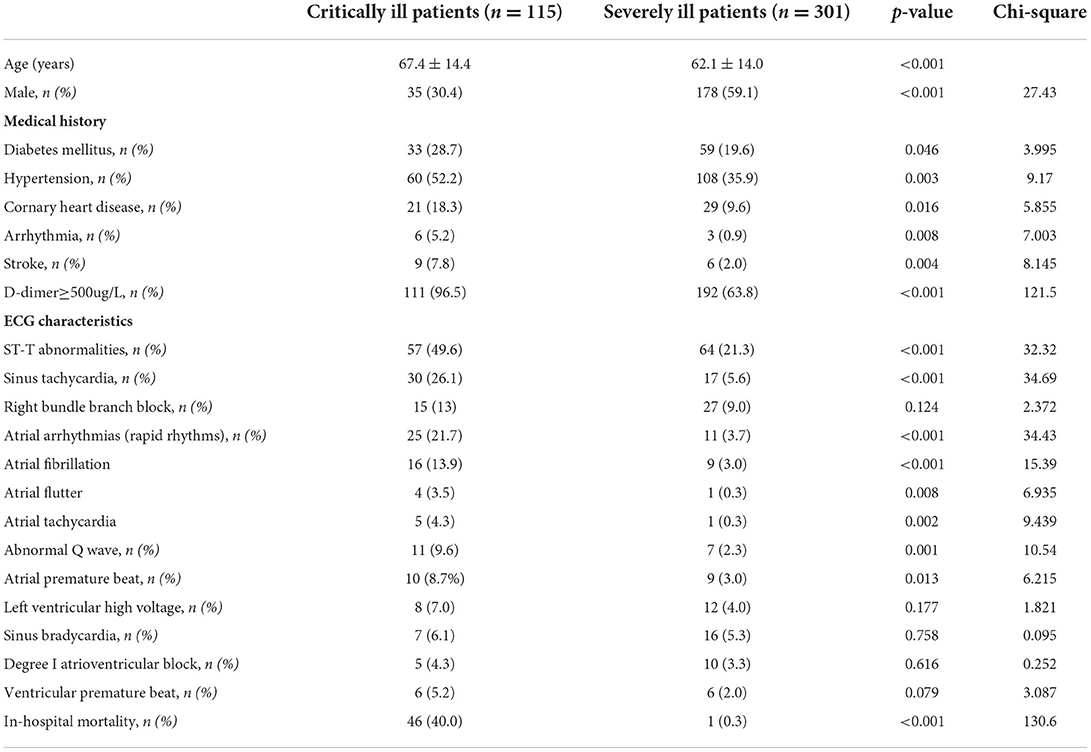
Table 1. Comparison of the characteristics of critically ill patients with COVID-19 and severely ill patients with COVID-19.
ST-T abnormalities (57, 49.6%) were the most prevalent ECG abnormalities, and ST (heart rate >100 bpm) (30, 26.1%) was the most frequent cardiac arrhythmia in the critically ill group. The proportions of these ECG characteristics in critically ill patients were significantly more prevalent when compared to those in the severely ill group [64 (21.3%) and 17 (5.6%), respectively] (Table 1). The second most prevalent cardiac arrhythmia in critically ill patients was atrial arrhythmia, which was characterized by rapid rhythms, including AF, AFL, and atrial tachycardia. Atrial arrhythmias with rapid rhythms were markedly more prevalent in critically ill patients (25, 21.7 %) than in severely ill patients (11, 3.7%). Other prevalent ECG characteristics in critically ill patients include RBBB, abnormal Q wave, atrial premature beat, left ventricular high voltage, sinus bradycardia, degree I atrioventricular block, and ventricular premature beat. In addition, abnormal Q waves and atrial premature beats were significantly more frequent in critically ill patients [11 (9.6%) and 10 (8.7%), respectively] than in severely ill patients [7 (2.3%) and 9 (3.0%), respectively]. In-hospital mortality was significantly higher in critically ill patients (46, 40.0%) than in severely ill patients (1, 0.3%) (Table 1).
D-dimer levels were evaluated between the surviving and dead groups. Notably, 47 (100%) patients who died of COVID-19 had elevated D-dimer levels (p < 0.001) compared with the surviving group (Table 2).

Table 2. Comparison of in-hospital death in patients with elevated D-dimers and non-elevated D-dimers.
To further investigate the clinical characteristics of these patients with elevated D-dimers, we compared the age, proportion of men, and ECG characteristics of patients with and without elevated D-dimer levels (Table 3). Compared with patients without elevated D-dimers, patients with elevated D-dimers were significantly older (66.8 ± 12.9 vs. 54.9 ± 14.2). In addition, compared with patients with normal D-dimer levels, ECG findings showed that ST-T abnormalities [100 (33%) vs. 21 (18.6%)], ST (sinus rate >100 bpm) [43 (14.2%) vs. 4 (3.5%)], AF [23 (7.6%) vs. 2 (1.8%)], and atrial premature beat [18 (5.9%) vs. 1 (0.8%)] were significantly increased in patients with elevated D-dimer levels (Figure 2, Table 3).
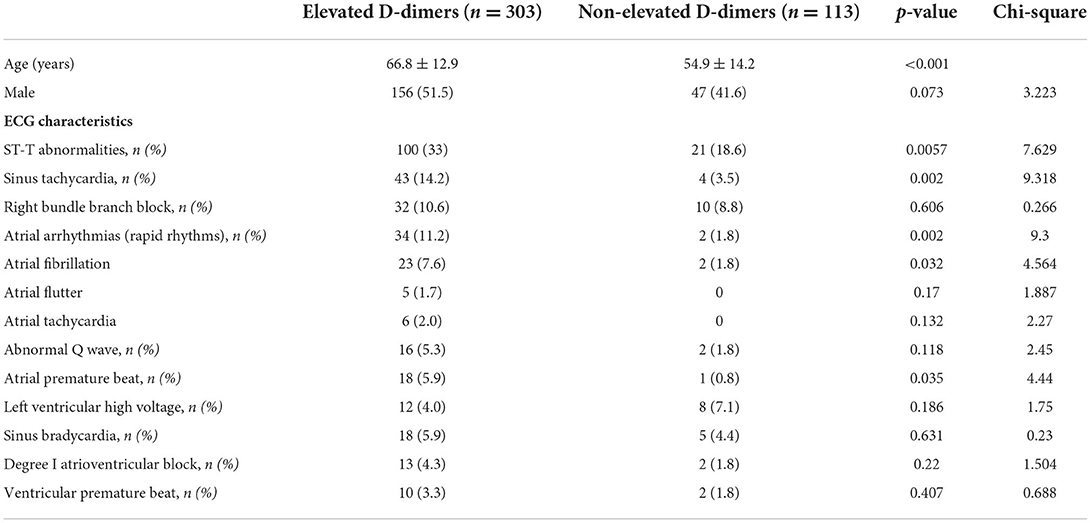
Table 3. Comparison of the characteristics of patients with elevated D-dimers and patients with non-elevated D-dimers.
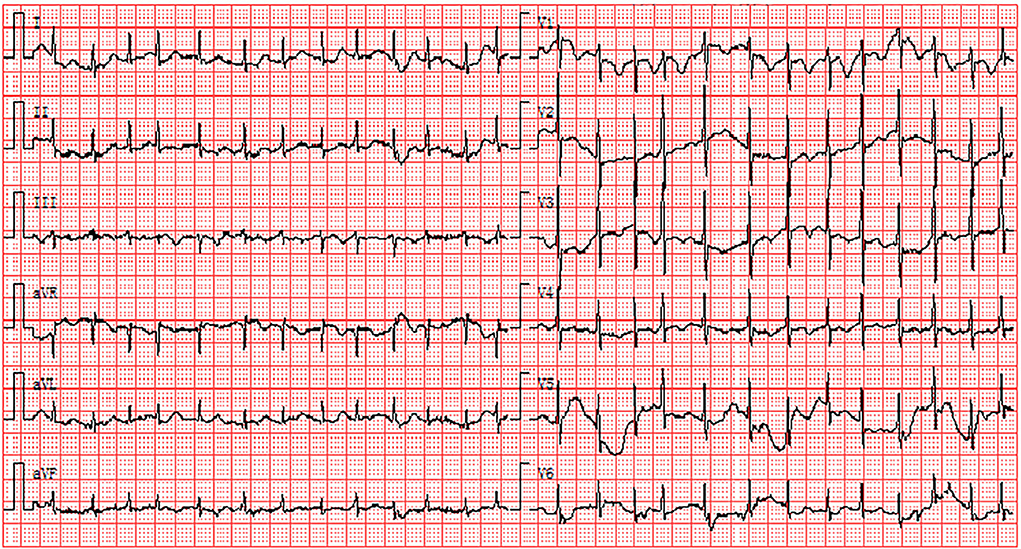
Figure 2. Electrocardiogram abnormalities associated with elevated D-dimers: the existence of AF and ST-T abnormalities. AF, atrial fibrillation.
To further clarify the relationship between ECG characteristics and in-hospital death in patients with elevated D-dimer levels, multivariate logistic regression analysis and Forest plots were used. In patients with elevated D-dimer levels, 47 died and 256 survived. Values such as in-hospital death, patients' mean age, the proportion of men, and ECG characteristics were included in the multivariate logistic regression analysis. Odds ratios (ORs) with 95% confidence intervals (CI) are shown in Figure 3. ST (sinus rate >100 bpm) (OR: 6.089, 95% CI: 2.422–15.305, p < 0.001) and AF (OR: 4.166, CI: 1.093–15.879, p = 0.037) were independent risk factors associated with in-hospital mortality in the elevated D-dimer group (Figure 3). Besides the ECG signs, according to the multivariate analysis, the average age (OR: 1.035, CI: 1.00–1.067, p = 0.027) and the proportion of men (OR: 20.076, CI: 4.628–87.094, p < 0.001) were also risk factors associated with mortality in patients hospitalized for COVID-19 with elevated D-dimers.
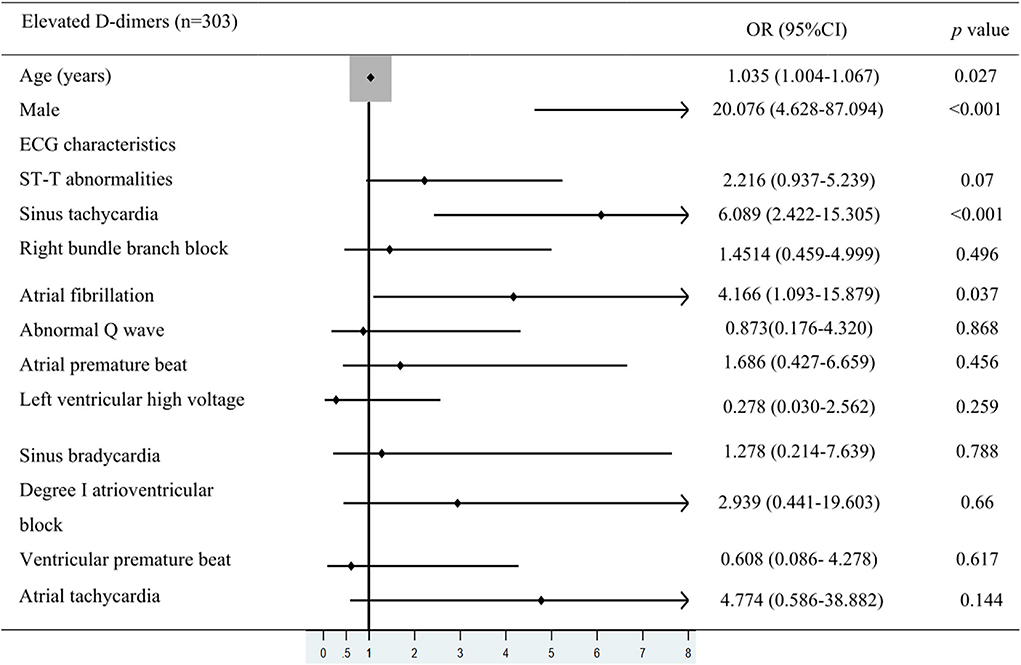
Figure 3. Multivariate logistic regression analysis. The values were included in multivariate logistic regression analysis such as in-hospital death, patients' mean age, the proportion of men, and ECG characteristics in COVID-19 patients with elevated D-dimer levels.
Recent investigations revealed that PE is closely associated with the pathogenesis of severe COVID-19 (20). The absence of anticoagulant therapy and elevation of D-dimer levels were both risk predictors for PE (21). The electrocardiographic Daniel score system has been widely applied to predict the severity of acute PE (22). Right ventricular strain manifested on ECG corresponded to the presence of right ventricular dysfunction with PE (22). The ECG of a 44-year-old man diagnosed with COVID-19 showed the right ventricular strain. The basic rhythm was sinus, accompanied by incomplete RBBB, inversion T waves in leads II and III, and precordial leads V1-V5 (Figure 4). The patient's ECG score was seven points according to the Daniel ECG scoring system. All ECGs of the patients with elevated D-dimer levels were scored according to the Daniel ECG scoring system (Table 4). The average number of deceased patients with COVID-19 was higher than that of recovered patients (2.57 ± 2.093 vs. 0.71 ± 1.28, p < 0.001).
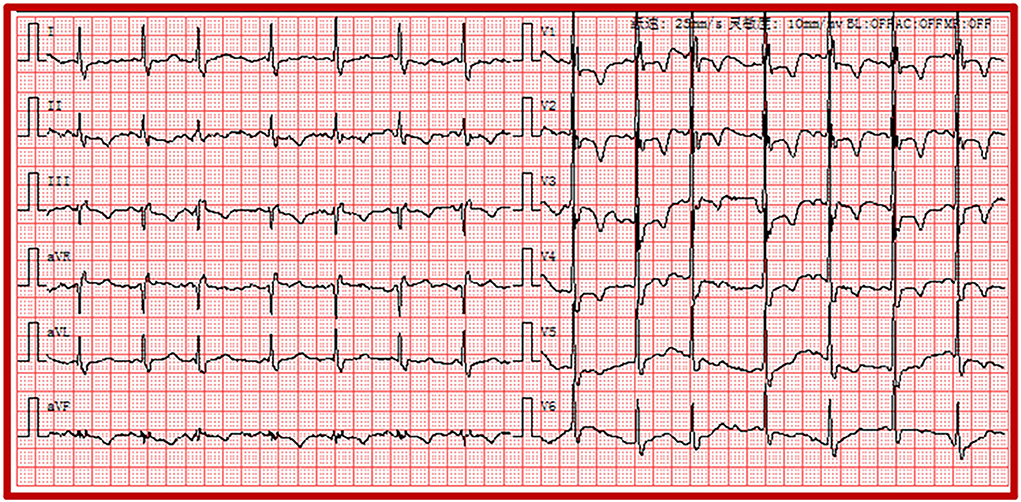
Figure 4. Electrocardiogram of the right ventricular strain including sinus rhythm, atrial premature, IRBBB, and T-wave inversions in the leads II, III and precordial leads V1-V5. IRBBB, incomplete right bundle branch block.
Our study is the first to demonstrate that ECG abnormalities combined with elevated D-dimer levels can estimate the risk of mortality in patients with COVID-19.
Our study observed that the rate of critically ill patients hospitalized with COVID-19 was 27.6% and the overall in-hospital mortality rate was 11.3%. Abnormal ECG manifestations were significantly more frequent in critically ill patients with COVID-19. The proportion of COVID-19 patients with elevated D-dimer levels was as high as 72.8%. D-dimer represents activation of the coagulation and fibrinolysis systems. Several studies showed that elevated D-dimer levels are closely associated with a poor prognosis of COVID-19 (21, 23, 24). Our studies similarly observed that elevated D-dimer levels contributed to higher mortality in patients with COVID-19 than in those without elevated D-dimer levels.
A systematic review of ECG findings in patients with COVID-19 revealed that the most prevalent ECG abnormalities were various patterns of ST-T abnormalities (25). Barmen et al. reported that ST-T abnormalities, including ST depression, T-wave inversion, and ST-T changes on admission ECG, were closely associated with the severity of COVID-19 (26). A study of 177 cases demonstrated that the proportion of ST-T changes on ECG was 49.15% in patients with PE (27). ST-T abnormalities are most common in precordial leads V1-V3 on ECG in PE (28). Figure 4 also shows that inversion T-waves occurred in the right precordial leads, which might be associated with the right myocardial strain. Similarly, in our study, ST-T abnormalities were the most prevalent ECG abnormalities in patients with COVID-19. ST-T abnormalities were more frequent in the critically ill group than in the severely ill group. Moreover, ST-T abnormalities were the most common ECG abnormalities in COVID-19 patients with elevated D-dimer levels. However, the relationship between elevated D-dimer levels and ST-T abnormalities in patients with COVID-19 has not been elucidated. We speculate that, in patients with COVID-19, ST-T abnormalities that widely occurred in those with elevated D-dimer were perhaps related to PE.
In those patients with COVID-19, the potential mechanisms of arrhythmogenesis were associated with multiple pathological conditions, such as hypoxia due to viral infection in lung tissues, impairment of host immune response, and myocardial ischemia (29). ST (heart rate >100 bpm) has been recognized as the most frequent supraventricular tachycardia in patients with COVID-19. The occurrence of ST (heart rate >100 bpm) was related to hypoxia, hypotension, hypoperfusion, and elevated body temperature in COVID-19 (7). In a study of 414 patients hospitalized for COVID-19, AF was the more frequent incident of arrhythmia (30). An Italian multicenter study reported that AF or AFL was found in nearly 22% of patients with COVID-19 (6). The occurrence of AF in COVID-19 might be associated with hypoxemia and could be permanent when accompanied by impaired pulmonary function (31). Furthermore, hypoxia and tachycardia have been proved to be the signs of PE (32). Atrial tachycardia has more frequently been associated with pulmonary or structural heart disease and hypomagnesemia (33). In our entire cohort, ST (heart rate >100 bpm) and atrial arrhythmias with rapid rhythms, including AF, AFL, and atrial tachycardia, were the two most common rhythm disturbances in patients with COVID-19. Moreover, the proportions of ST (heart rate >100 bpm) and atrial arrhythmias with rapid rhythms were higher in severely ill patients with COVID-19 than in critically ill patients.
The right myocardial strain has been shown to increase the incidence of circulatory shock and mortality in patients with PE (8). The main ECG signs of the right myocardial strain include ST (heart rate >100 bpm), S1Q3T3 pattern, IRBBB or CRBBB, and T-wave inversions (8). AF has also been found in >10% of patients with PE (34). However, the occurrence of AF was not associated with risk stratification in PE (34). D-dimer levels >1000 μg/L were associated with a high incidence of COVID-19 in patients with PE (16). In our study, a significant and direct association was observed between elevated D-dimer levels and ST (heart rate >100 bpm), AF, and atrial premature beats.
A multivariate analysis revealed that ECG signs, including ST (heart >100 bpm) and AF, were independent predictors of in-hospital death in patients with elevated D-dimer levels. Furthermore, on ECG, the existence of ST and AF might reveal a poor prognosis in patients with elevated D-dimer levels. In addition, patients' age and proportion of men were significantly associated with in-hospital death in patients with elevated D-dimer levels. According to our study, ECG abnormalities combined with elevated D-dimer levels were able to predict mortality in patients with COVID-19. Furthermore, elevated D-dimer levels were associated with diffused pulmonary intravascular thrombosis in COVID-19 but can occur due to many other conditions (35). Usually, the diagnosis of PE relies on imaging tests (such as computed tomography pulmonary angiography [CTPA]). However, the clinical use of CTPA is limited because of the adverse effects of the contrast medium. Due to the important diagnostic meanings of ECG and elevated D-dimer in PE, the occurrence of abnormal ECG and elevated D-dimer levels may be the potential risk factors for the increased mortality in COVID-19, which had a preference for PE or micro-embolism complications.
The Daniel scoring system has been extensively applied in predicting increased pulmonary arterial pressure by assigning points (0–21) to ECG components (22). We calculated the Daniel score in patients with and without elevated D-dimer levels. Similarly, the mean Daniel score in patients with elevated D-dimer levels was significantly higher than that in those without elevated D-dimer levels. Especially due to the outbreak of the COVID-19 pandemic, which limits critical medical resources, better use of limited CTPA resources seems to be required. Therefore, as an additional option, it seems that the combination of ECG abnormalities with elevated D-dimers is reasonable since it was able to predict the severity and mortality of COVID-19.
Our study has some limitations. First, we performed a retrospective study of patients with COVID-19 and specific laboratory investigations, such as CTPA, were not available. Moreover, CTPA examinations were not widely applied due to the limitation of critical medical resources during the outbreak of COVID-19 in 2020. Second, ECG signs dynamically changed in ECG monitoring during the process of disease duration, and some valuable and repeated manifestations were not possible.
Elevated D-dimer levels are associated with an increased risk of critical illness and in-hospital mortality in patients with COVID-19. Moreover, the existence of ST (heart rate >100 bpm) and AF on the ECG may indicate a poor prognosis in patients with elevated D-dimer levels. ECG abnormalities (including ST [heart rate > 100 bpm] and AFL) in elevated D-dimer COVID-19 patients may indicate a higher risk of mortality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board for human studies and the privacy of Tongji Hospital of Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional Requirements.
JC performed the statistical analysis and drafted the manuscript. YW collected the ECG recordings of patients with COVID-19. JW, LC, and QL collected clinical data from patients with COVID-19. XH and BW helped draft the manuscript. XL and HZ helped analyze data. XY and PZ conceived the study, participated in its design, and helped draft the manuscript. All authors contributed to the article and approved the submitted version.
The supported funding was from the Interdisciplinary program of Wuhan National High Magnetic Field Center (Grant No. WHMFC22021122), Huazhong University of Science and Technology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AF, atrial fibrillation; AFL, atrial flutter; CRBB, complete right bundle branch block; CTPA, computed tomography pulmonary angiography; CI, confidence interval; COVID-19, coronavirus disease 2019; ECG, electrocardiography; IRBBB, incomplete right bundle branch block; OR, odds ratio; PE, pulmonary embolism; ST, sinus tachycardia.
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus I and Research T. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
3. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
4. Angeli F, Spanevello A, De Ponti R, Visca D, Marazzato J, Palmiotto G, et al. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. (2020) 78:101–6. doi: 10.1016/j.ejim.2020.06.015
5. Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias trends. Cardiovasc Med. (2020) 30:451–60. doi: 10.1016/j.tcm.2020.08.002
6. Bertini M, Ferrari R, Guardigli G, Malagu M, Vitali F, Zucchetti O, et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: an insight into the mechanisms of cardiac involvement. Europace. (2020) 22:1848–54. doi: 10.1093/europace/euaa258
7. Long B, Brady WJ, Bridwell RE, Ramzy M, Montrief T, Singh M, et al. Electrocardiographic manifestations of COVID-19. Am J Emerg Med. (2021) 41:96–103. doi: 10.1016/j.ajem.2020.12.060
8. Shopp JD, Stewart LK, Emmett TW, Kline JA. Findings from 12-lead electrocardiography that predict circulatory shock from pulmonary embolism: systematic review and meta-analysis. Acad Emerg Med. (2015) 22:1127–37. doi: 10.1111/acem.12769
9. Barman HA, Atici A, Sahin I, Dogan O, Okur O, Tugrul S, et al. Prognostic value of right ventricular strain pattern on ECG in COVID-19 patients. Am J Emerg Med. (2021) 49:1–5. doi: 10.1016/j.ajem.2021.05.039
10. Ozdemir IH, Ozlek B, Ozen MB, Gunduz R, Cetin N, Ozlek E, et al. Fragmented QRS is a marker of mortality in patients with severe COVID-19: a retrospective observational study. Anatol J Cardiol. (2021) 25:811–20. doi: 10.5152/AnatolJCardiol.2021.62
11. Akhtar Z, Gallagher MM, Yap YG, Leung LWM, Elbatran AI, Madden B, et al. Prolonged QT predicts prognosis in COVID-19. Pacing Clin Electrophysiol. (2021) 44:875–82. doi: 10.1111/pace.14232
12. De Carvalho H, Leonard-Pons L, Segard J, Goffinet N, Javaudin F, Martinage A, et al. Electrocardiographic abnormalities in COVID-19 patients visiting the emergency department: a multicenter retrospective study. BMC Emerg Med. (2021) 21:141. doi: 10.1186/s12873-021-00539-8
13. Kearon C, de Wit K, Parpia S, Schulman S, Afilalo M, Hirsch A, et al. and Investigators PES. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N Engl J Med. (2019) 381:2125–34. doi: 10.1056/NEJMoa1909159
14. Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, et al. Elevated D-dimer levels are associated with increased risk of mortality in coronavirus disease 2019: a systematic review and meta-analysis. Cardiol Rev. (2020) 28:295–302. doi: 10.1097/CRD.0000000000000330
15. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. (2020) 7:e438–40. doi: 10.1016/S2352-3026(20)30145-9
16. Alonso-Fernandez A, Toledo-Pons N, Cosio BG, Millan A, Calvo N, Ramon L, et al. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: a prospective study. PLoS ONE. (2020) 15:e0238216. doi: 10.1371/journal.pone.0238216
17. Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J. (2005) 25:843–8. doi: 10.1183/09031936.05.00119704
18. New coronavirus pneumonia prevention control program (7th ed). (2020) (in Chinese). Available online at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml
19. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. (2012) 126:2020–35. doi: 10.1161/CIR.0b013e31826e1058
20. O'Donnell JS, Peyvandi F, Martin-Loeches I. Pulmonary immuno-thrombosis in COVID-19 ARDS pathogenesis. Intensive Care Med. (2021) 47:899–902. doi: 10.1007/s00134-021-06419-w
21. Mouhat B, Besutti M, Bouiller K, Grillet F, Monnin C, Ecarnot F, et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J. (2020) 56:2020. doi: 10.1183/13993003.01811-2020
22. Daniel KR, Courtney D, Kline JA. Assessment of cardiac stress from massive pulmonary embolism with 12-lead ECG. Chest. (2001) 120:474–81. doi: 10.1378/chest.120.2.474
23. Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. (2020) 13:1265–75. doi: 10.1080/17474086.2020.1831383
24. Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection a scoping review. Thromb Res. (2020) 192:152–60. doi: 10.1016/j.thromres.2020.05.039
25. Mehraeen E, Seyed Alinaghi SA, Nowroozi A, Dadras O, Alilou S, Shobeiri P, et al. A systematic review of ECG findings in patients with COVID-19. Indian Heart J. (2020) 72:500–7. doi: 10.1016/j.ihj.2020.11.007
26. Barman HA, Atici A, Alici G, Sit O, Tugrul S, Gungor B, et al. The effect of the severity COVID-19 infection on electrocardiography. Am J Emerg Med. (2021) 46:317–22. doi: 10.1016/j.ajem.2020.10.005
27. Gong B, Xu Q, Pang Y, Hu J. [Clinical features of patients with venous thromboembolism: 177 case analysis in 10 years]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2019) 31:453–7. doi: 10.3760/cma.j.issn.2095-4352.2019.04.016
28. Adhikari CM, Bishal KC, Khadka S. Clinical profile, management and outcome of pulmonary embolism in Shahid Gangalal National Heart Centre, Kathmandu, Nepal. Egypt Heart J. (2018) 70:41–3. doi: 10.1016/j.ehj.2017.06.001
29. Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B et al. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol. (2020) 6:1193–204. doi: 10.1016/j.jacep.2020.08.002
30. Russo V, Di Maio M, Mottola FF, Pagnano G, Attena E, Verde N, et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest. (2020) 50:e13387. doi: 10.1111/eci.13387
31. Haseeb S, Gul EE, Cinier G, Bazoukis G, Alvarez-Garcia J, Garcia-Zamora S, et al. International Society of Electrocardiology. Value of electrocardiography in coronavirus disease 2019 (COVID-19). J Electrocardiol. (2020) 62:39–45. doi: 10.1016/j.jelectrocard.2020.08.007
32. Bajaj N, Bozarth AL, Guillot J, Kojokittah J, Appalaneni SR, Cestero C, et al. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. J Thromb Thrombolysis. (2014) 37:287–92. doi: 10.1007/s11239-013-0942-8
33. Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. Evidence Review Committee Chairdouble D. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. (2016) 133:e506–74. doi: 10.1161/CIR.0000000000000311
34. Ebner M, Rogge NIJ, Parwani AS, Sentler C, Lerchbaumer MH, Pieske B, et al. Atrial fibrillation is frequent but does not affect risk stratification in pulmonary embolism. J Intern Med. (2020) 287:100–13. doi: 10.1111/joim.12985
Keywords: COVID-19, electrocardiogram, d-dimer, in hospital, death prediction
Citation: Chen J, Wang Y, Wang J, Chen L, Luo Q, Wang B, He X, Li X, Zuo H, Zuo P and Yang X (2022) Coronavirus disease 2019 death prediction by electrocardiographic abnormalities and elevated D-dimer levels. Front. Cardiovasc. Med. 9:948347. doi: 10.3389/fcvm.2022.948347
Received: 19 May 2022; Accepted: 12 July 2022;
Published: 28 September 2022.
Edited by:
Olga Tura-Ceide, Hospital Clinic of Barcelona, SpainReviewed by:
Tong Liu, Tianjin Medical University, ChinaCopyright © 2022 Chen, Wang, Wang, Chen, Luo, Wang, He, Li, Zuo, Zuo and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zuo, cHp1bzQxMkAxMjYuY29t; Xiaoyun Yang, eWFuZ3hpYW95dW4zMjFAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.