
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 22 July 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.947809
This article is part of the Research Topic Abdominal Aortic Aneurysms: Advancements in diagnosis, biomarkers, drug therapeutics, surgical and endovascular treatment View all 21 articles
Objective: Previous reports have revealed a high incidence of type II endoleak (T2EL) after endovascular aneurysm repair (EVAR). The incidence of T2EL after EVAR is reduced by pre-emptive embolization of aneurysm sac side branches (ASSB) and aneurysm sac coil embolization (ASCE). This study aimed to investigate whether different preventive interventions for T2EL were correlated with suppression of aneurysm sac expansion and reduction of the re-intervention rate.
Methods: The PubMed, Web of Science, MEDLINE and Embase databases, and conference proceedings were searched to identify articles on EVAR with or without embolization. The study was developed in line with the Participants, Interventions, Comparisons, Outcomes, and Study design principles and was conducted and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. We used network meta-analysis based on multivariate random-effects meta-analysis to indirectly compare outcomes of different strategies for embolization during EVAR.
Results: A total of 31 studies met all inclusion criteria and were included in the qualitative and quantitative syntheses. The included studies were published between 2001 and 2022 and analyzed a total of 18,542 patients, including 1,882 patients who received prophylactic embolization treatment during EVAR (experimental group) and 16,660 who did not receive prophylactic embolization during EVAR (control group). The effect of pre-emptive embolization of the inferior mesenteric artery (IMA) (IMA-ASSB) in preventing T2EL was similar (relative risk [RR] 1.01, 95% confidence interval [CI] 0.38–2.63) to the effects of non-selective embolization of ASSB (NS-ASSB) and ASCE (RR 0.88, 95% CI 0.40–1.96). IMA-ASSB showed a better clinical effect in suppressing the aneurysm sac expansion (RR 0.27, 95% CI 0.09–2.25 compared with NS-ASSB; RR 0.93, 95% CI 0.16–5.56 compared with ASCE) and reducing the re-intervention rate (RR 0.34, 95% CI 0.08–1.53 compared with NS-ASSB; RR 0.66, 95% CI 0.19–2.22 compared with ASCE). All prophylactic embolization strategies improved the clinical outcomes of EVAR.
Conclusion: Prophylactic embolization during EVAR effectively prevents T2EL, suppresses the aneurysm sac expansion, and reduces the re-intervention rate. IMA embolization demonstrated benefits in achieving long-term aneurysm sac stability and lowering the risk of secondary surgery. NS-ASSB more effectively reduces the incidence of T2EL, while IMA embolization alone or in combination with ASCE enhances the clinical benefits of EVAR. In addition, as network meta-analysis is still an indirect method based on a refinement of existing data, more studies and evidence are still needed in the future to establish more credible conclusions.
Endovascular aneurysm repair (EVAR) has become the accepted standard therapy for abdominal aortic aneurysm (AAA) because of its low perioperative mortality and minimal invasiveness (1). However, the long-term outcomes of EVAR have not been fully elucidated and remain controversial. Recent clinical trials have shown that EVAR no longer achieves an early survival benefit compared with open surgery, because of a significant increase in death from secondary aneurysm rupture mostly caused by endoleaks (2) and a higher rate of secondary intervention (3).
Among several types of endoleaks, the most common is type II endoleak (T2EL) (4, 5). T2EL occurs because of retrograde perfusion of the AAA sac from the inferior mesenteric artery (IMA), lumbar arteries (LAs), middle sacral artery, or aberrant renal arteries (6). Most T2ELs resolve spontaneously with time and it remains debatable whether T2ELs requires aggressive therapy that may be associated with adverse late outcomes (7–11). A previous study demonstrated that 9.8% of aortic ruptures after EVAR were caused by T2EL (12), while a meta-analysis of 32 studies and 21,744 patients showed that 0.9% of patients with an isolated T2EL had a ruptured AAA (13). A reduction in the incidence of T2EL may improve the prognosis and decrease the psychological and economic burden of patients undergoing EVAR (14).
The role of pre-emptive embolization of aneurysm sac side branches (ASSB) in preventing T2EL has been debated for the past two decades. Early evidence in 2001 suggested that additional ASSB is unnecessary because of the uncertain incidence of T2EL during follow-up (15). However, recent progress in preoperative optimization, medical devices, and high-quality supportive care has led to significantly improved clinical outcomes of ASSB. Despite the lack of high-quality evidence, the latest meta-analysis suggests that ASSB aids in preventing T2EL, aneurysm sac enlargement, and re-intervention (16). The other prophylactic embolization treatment implemented during EVAR is aneurysm sac coil embolization (ASCE). To date, there is still no generalized and commonly accepted standard method of ASCE (11). However, studies have shown that ASCE effectively prevents T2EL, particularly during the mid-term follow-up of high-risk patients (17–20). A meta-analysis of 17 studies with 2,084 participants also demonstrated the safety and effectiveness of ASCE in preventing T2EL (21).
The most common sources of T2EL are the IMA and LAs. However, there is still a lack of clinical trials directly comparing the efficacy of non-selective embolization of patent aortic side branches versus embolization of the IMA alone. Furthermore, few studies have directly compared ASSB and ASCE. There was no recording and follow-up of other arteries such as accessory renal arteries in the studies that we consulted and incorporated. In the present study, we aimed to perform a network meta-analysis to compare the efficacy and basic outcomes of different prophylactic embolization treatments in IMA, Las, and sac embolization during EVAR.
The study was developed in line with the Participants, Interventions, Comparisons, Outcomes, Study design principles and was conducted and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (22).
The participants were patients of any age with AAA who underwent EVAR with or without prophylactic embolization comprising either ASSB or ASCE. ASSB was divided into preoperative pre-emptive embolization of the IMA (IMA-ASSB) and non-selective preoperative embolization of the IMA and LAs (NS-ASSB). Few studies reported embolization of the median sacral artery or accessory renal artery.
EVAR with IMA-ASSB, NS-ASSB, or ASCE.
EVAR without prophylactic embolization treatment.
a. Incidence of T2EL during follow-up. The presence of T2EL was defined as the temporary or permanent appearance of T2EL during postoperative follow-up examination.
b. Incidence of enlargement of the diameter or size of the aneurysm sac during follow-up.
a. Late all-cause-related mortality.
b. Rate of re-intervention during follow-up.
One thing to note, only a few studies mentioned their observation and follow-up of complications which was not sufficient for statistical analysis.
1. Inclusion criteria: retrospective or prospective studies evaluating the effect of ASSB or ASCE during EVAR compared with a control group or not that underwent no preventive intervention measures and had the patent collateral arteries retained; no date or limit on patients or publications; follow-up period of not less than 6 months; outcome indicators included the occurrence of T2EL diagnosed by contrast-enhanced CT, CTA, MRA, or other appropriate imaging examination.
2. Clinical exclusion criteria: flawed study design, implementation process, or statistical methods; funding organization influenced the study design or the implementation, analysis, or interpretation of data; incomplete data in original articles that could not be refined; case report or studies about embolization of other arteries, such as the median sacral artery or accessory renal artery which without intervention to IMA or LAs; prophylactic embolization in animals; fewer than 10 patients per group; Embolization performed in second operations.
3. Non-clinical exclusion criteria: overlapping series (only the latest publication of serial reports of a certain cohort was included); non-original article (i.e., review, case report, editorial).
Multiple electronic health databases (MEDLINE, Embase, PubMed, and Web of Science) were searched to identify relevant articles published from 1 October 2001 to 11 May 2022.
The MEDLINE, Embase, PubMed, and Web of Science databases were searched with an unrestricted search strategy that applied a combination of Medical Subject Headings and keywords combined with the Boolean operators AND or OR to retrieve relevant reports. The following terms were used: [“aortic aneurysm abdominal” (Title/Abstract) OR “abdominal aortic aneurysms” (Title/Abstract) OR “aneurysms abdominal aortic” (Title/Abstract) OR “aortic aneurysms abdominal” (Title/Abstract) OR “abdominal aortic aneurysm” (Title/Abstract) OR “aneurysm abdominal aortic” (Title/Abstract)] AND [“embolization” (Title/Abstract) OR “embolism” (Title/Abstract) OR “embolizations” (Title/Abstract)]. Controlled trials comparing prophylactic embolization with non-intervention during EVAR were eligible for inclusion in the general meta-analysis. Single-arm studies were also retrieved for inclusion in the network meta-analysis. We adapted the terms to meet the specific requirements of the explicit search strategy used for each database. A total of 100 citations were obtained from the databases, of which 67 were excluded after browsing the titles and abstracts, yielding 33 articles for detailed review. After reviewing the full texts of the remaining studies and their cited references to identify additional studies, 32 studies were finally selected.
Eligibility assessment was performed independently in an unblinded standardized manner by two reviewers (Guo and Zhang).
Data were extracted from the primary studies and consolidated into single spreadsheets. One author collected the data from the included articles, while another author explicitly checked the presented information. The data analyzed in the present review were all published in the included studies in case of record form or as alphanumeric text. Information filtering from relevant studies was performed manually. Study quality and risk of bias were assessed using the Newcastle Ottawa Scale (NOS) (23) (selection, comparability, and outcome) and the Cochrane Handbook for Systematic Reviews of Interventions (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias). Two reviewers (Wu and Yin) determined the eligibility and compared the quality of the included studies. A cumulative NOS of 7 or more was considered to indicate high quality.
1. Publication details: first author and year.
2. Study details: number of participating institutions, number of participants, controls and interventions, mean follow-up period, inclusion criteria, exclusion criteria, and country.
3. Participants: number of patients undergoing EVAR with or without prophylactic embolization.
4. Primary outcomes: incidences of T2EL and enlargement of the aneurysm sac during follow-up.
5. Secondary outcomes: late all-cause-related mortality and the rate of re-intervention during follow-up.
Statistical analysis was performed in accordance with the Cochrane Handbook for Systematic Reviews, using Stata® Version 16.0, RStudio (version 1.4.1103 for Windows, Boston, MA, United States), Stata package base, metan, mvmeta, metareg, network, R (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria) and R packages base, ggplot2, metafor, meta and gemtc (24). The gemtc package was used in connection with the Just Another Gibbs Sampler package to generate simulations. Statistical applications were set up to statistically analyze which prophylactic embolization treatment had the greatest effect on the progression of AAA after EVAR. There was no significant methodological heterogeneity between the datasets regarding the study design and risk of bias. The primary and secondary outcomes were analyzed as dichotomous variables by estimating the relative risk (RR) with a 95% confidence interval (CI). The I2 value was calculated to assess the statistical heterogeneity between studies. The heterogeneity among studies was considered significant when I2 was > 50%, and was considered highly significant when I2 was > 75%. Publication bias was assessed through funnel plots and Egger tests. The fixed-effect model based on the Mantel-Haenszel estimator was used when there was no or only slight heterogeneity among the studies. When the heterogeneity was significant, the analysis was performed with the random-effects model based on the DerSimonian-Laird estimator. The results were summarized using forest plots.
The search strategy retrieved a total of 3,345 articles, of which 3,314 were excluded after the title and abstract screening because they were not relevant or because they were comments only. After full-text review and data abstraction, 31 studies met all inclusion criteria and were included in the qualitative and quantitative syntheses (2, 17–20, 25–50).
One study was a prospective randomized controlled trial, while 30 were retrospective studies. All studies were published between 2001 and 2022. A total of 18,542 patients were involved in the included studies, of which 1,882 received prophylactic embolization treatment during EVAR and 16,660 did not. One study based on data from the Vascular Quality Initiative database included 15,060 patients (43). The study characteristics, study quality, and literature quality evaluation based on the NOS are summarized in Table 1. The mean NOS was 7.38 (out of a possible 9 points), suggesting that the included studies were high-quality studies. A lack of representativeness and comparability was the main reason for low NOS scores.
Overall, a total of 31 studies including 18,542 patients were analyzed (15,976 in the NS-ASSB group, 998 in the IMA-ASSB group, and 1,568 in the ASCE group). The prevalence of each embolization treatment was 0%–30% for NS-ASSB, 0%–34% for IMA-ASSB, and 0%–23.0% for ASCE. Patients who received prophylactic embolization had a significantly lower risk of T2EL after EVAR than those who did not receive prophylactic embolization in all studies. In the analysis of 25 controlled studies, the relative risk (RR) of T2EL was 0.30 (95% CI 0.11–0.84) for NS-ASSB, 0.49 (95% CI 0.38–0.65) for IMA-ASSB, and 0.48 (95% CI 0.29–0.79) for ASCE. Single-arm studies were not included in the routine meta-analysis because they contained smaller amounts of data. Network meta-analysis including single-arm studies using a multiple regression model demonstrated that both ASCE and IMA-ASSB were inferior to NS-ASSB in preventing T2EL. The effect of IMA-ASSB in preventing T2EL was similar (RR 1.01, 95% CI 0.38–2.63) to that of NS-ASSB and ASCE (RR 0.88, 95% CI 0.40–1.96). The results suggested that the protective effects of NS-ASSB and IMA-ASSB were not significantly different. The surface under the cumulative ranking (SUCRA) curve analysis showed a similar result (Figures 1, 2).
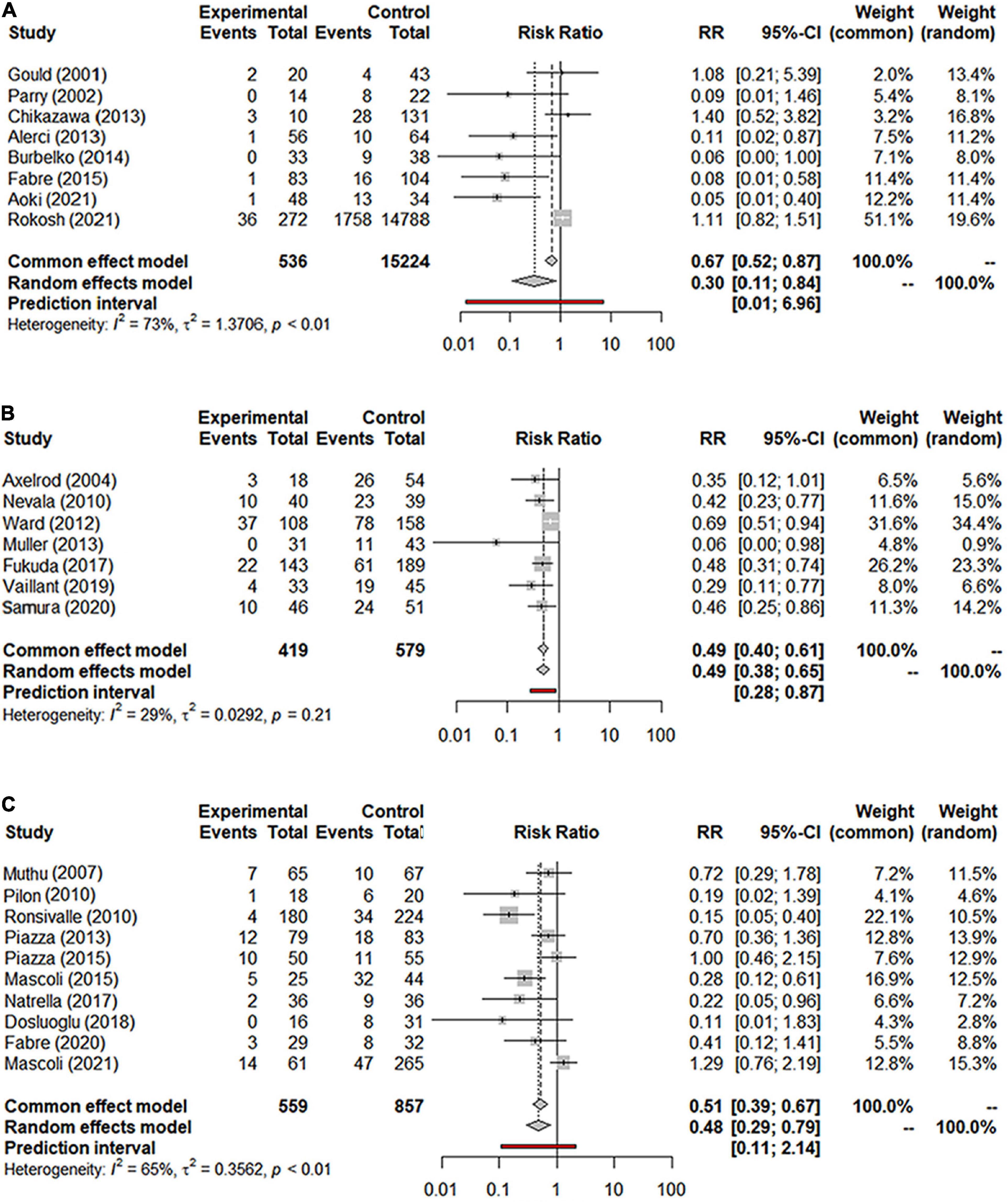
Figure 1. (A) Forest graph of T2EL in NS-ASSB group (RR 0.30 95% Cl 0.11–0.84); (B) forest graph of T2EL in IMA-ASSB group (RR 0.49 95% Cl 0.38–0.65); (C) forest graph of T2EL in ASCE group (RR 0.48 95% Cl 0.29–0.79). NS-ASSB, non-selective embolization of aneurysm sac side branches; IMA-ASSB, embolization of the inferior mesenteric artery; ASCE: aneurysm sac coil embolization.
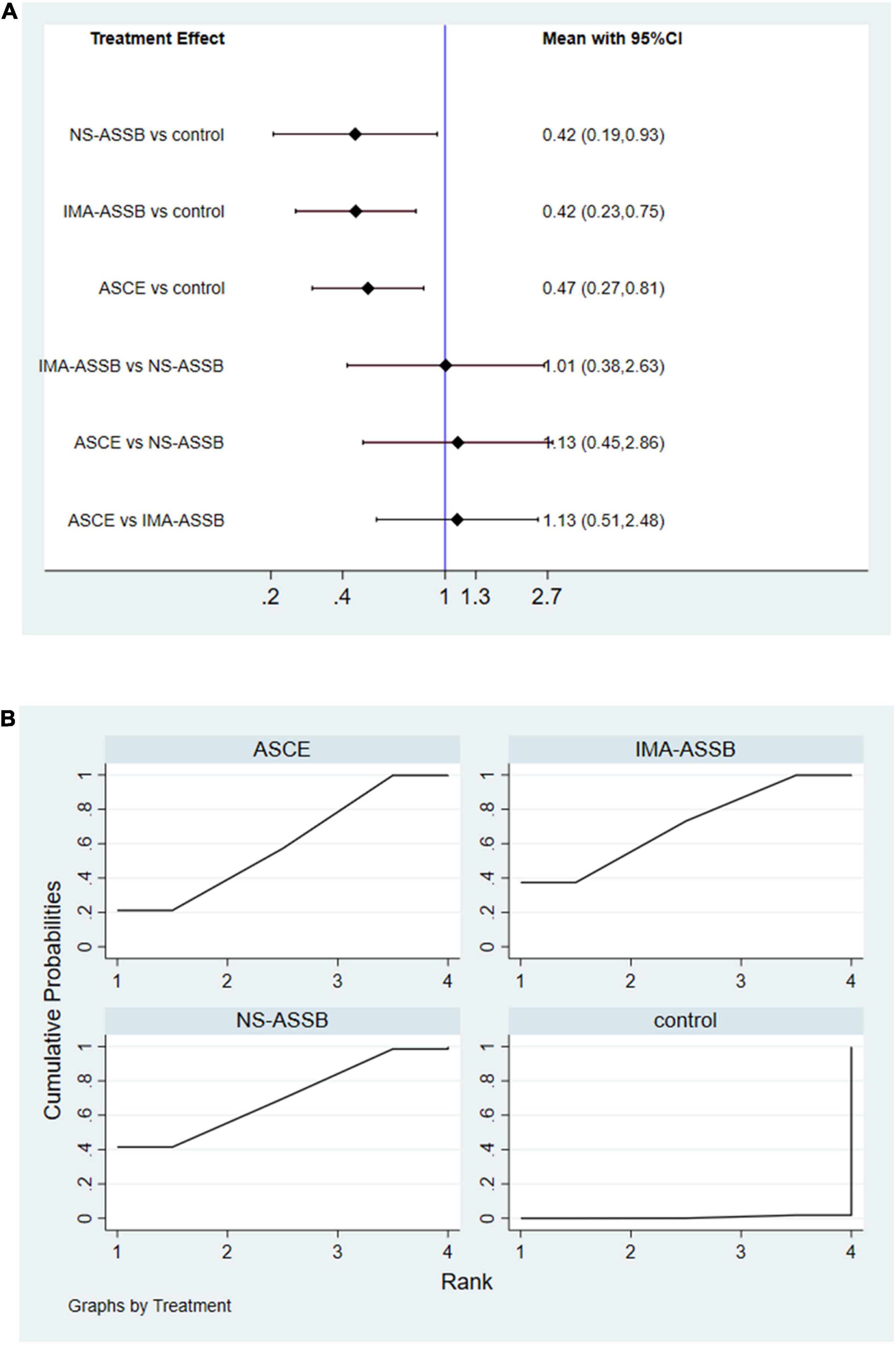
Figure 2. (A) Network meta-analysis forest graph of T2EL in NS-ASSB, IMA-ASSB and ASCE groups; (B) SUCRA curve of T2ELin NS-ASSB, IMA-ASSB and ASCE groups; NS-ASSB, non-selective embolization of aneurysm sac side branches; IMA-ASSB, embolization of the inferior mesenteric artery; ASCE, aneurysm sac coil embolization.
Data on the rate of re-intervention during follow-up were presented in 21 studies with a total of 17,405 patients (15,335 in the NS-ASSB group, 924 in the IMA-ASSB group, and 1,146 in the ASCE group). The rate of re-intervention ranged from 0% to 15.1% (0%–13.6% for NS-ASSB, 0%–15.1% for IMA-ASSB, and 0%–7.78% for ASCE). Prophylactic embolization resulted in a reduction in the incidence of re-intervention after EVAR. The RR of re-intervention in all controlled studies was 0.49 (95% CI 0.16–1.53) for NS-ASSB, 0.26 (95% CI 0.13–0.52) for IMA-ASSB, and 0.44 (95% CI 0.25–0.77) for ASCE. The single-arm studies were not analyzed because of a shortage of data. Network meta-analysis showed that IMA-ASSB was the best in preventing re-intervention after EVAR when all studies were incorporated in the analysis (RR 0.24, 95% CI 0.09–0.61) and was superior to NS-ASSB (RR 0.34, 95% CI 0.08–1.53) and ASCE (RR 0.66, 95% CI 0.19–2.22). The SUCRA curves were analyzed (Figures 3, 4).
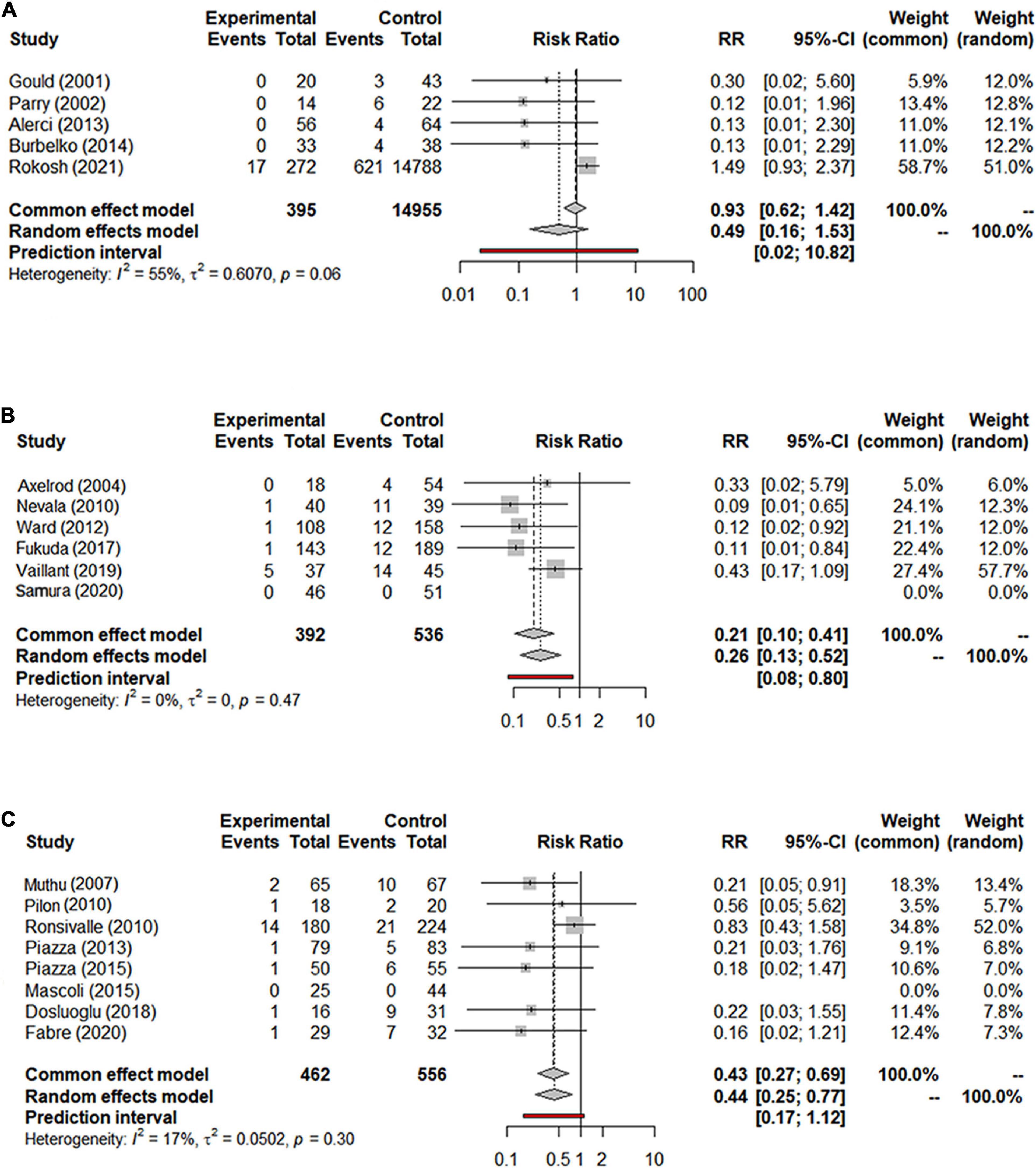
Figure 3. (A) Forest graph of re-intervention in NS-ASSB group (RR 0.49 95% Cl 0.16–1.53); (B) forest graph of re-intervention in IMA-ASSB group (RR 0.26 95% Cl 0.13–0.52); (C) forest graph of re-intervention in ASCE group (RR 0.44 95% Cl 0.25–0.77). NS-ASSB, non-selective embolization of aneurysm sac side branches; IMA-ASSB, embolization of the inferior mesenteric artery; ASCE, aneurysm sac coil embolization.
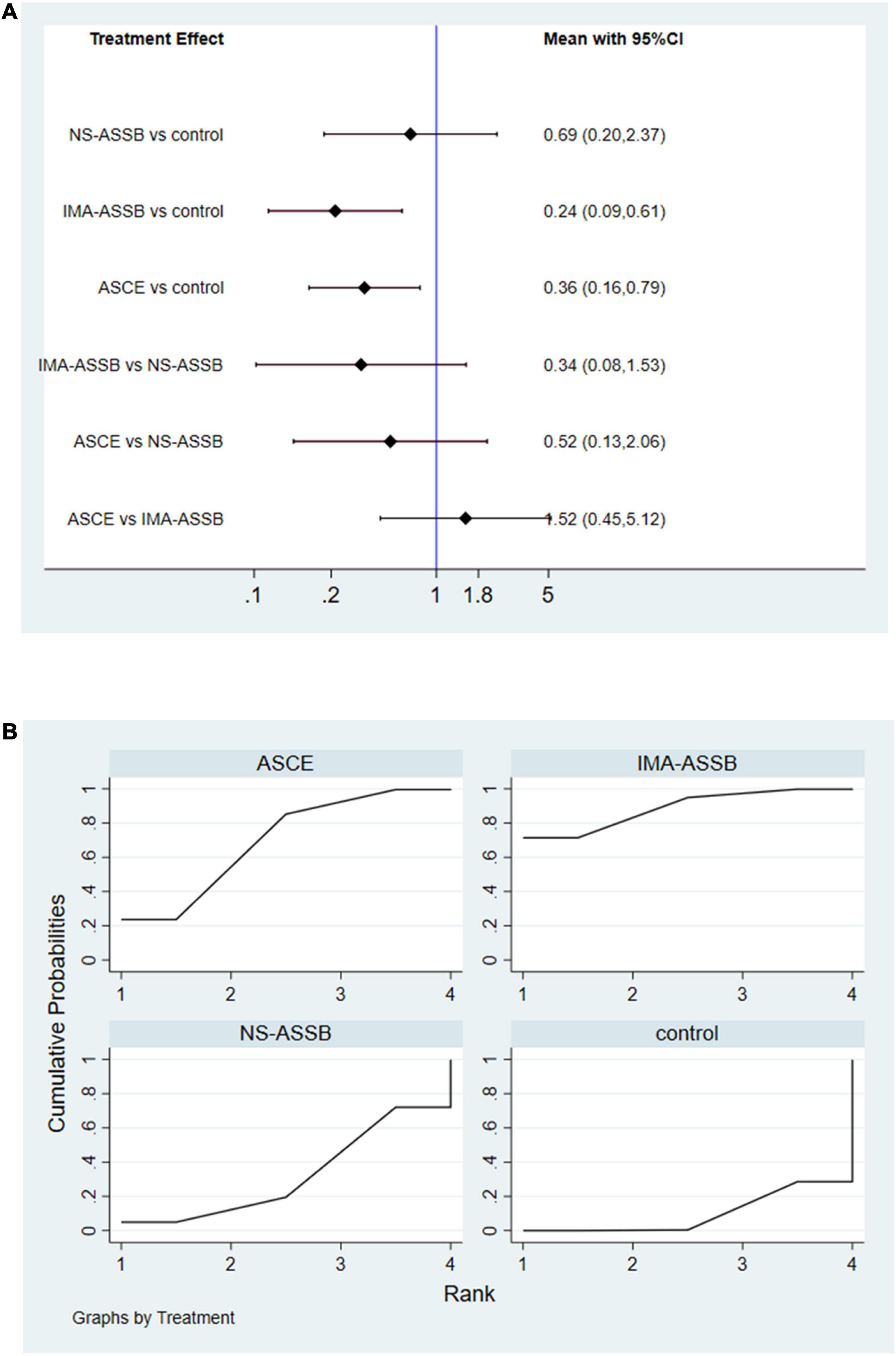
Figure 4. (A) Network meta-analysis forest graph of re-intervention in NS-ASSB, IMA-ASSB and ASCE groups; (B) SUCRA curve of re-intervention in NS-ASSB, IMA-ASSB and ASCE groups; NS-ASSB, non-selective embolization of aneurysm sac side branches; IMA-ASSB, embolization of the inferior mesenteric artery; ASCE, aneurysm sac coil embolization.
Data on the enlargement of the aneurysm sac were provided in 19 studies with a total of 16,559 patients (15,553 in the NS-ASSB group, 515 in the IMA-ASSB group, and 491 in the ASCE group). Aneurysm sac enlargement occurred in 0% to 12.0% of patients during follow-up (0%–3.7% for NS-ASSB, 0%–12.0% for IMA-ASSB, and 0%–10.1% for ASCE). The cumulative results showed that prophylactic embolization led to a significant decrease in the risk of aneurysm sac enlargement after EVAR. However, the results of individual studies showed the opposite conclusion. The RR of aneurysm sac enlargement in 13 controlled studies was 0.52 (95% CI 0.30–0.92) for NS-ASSB, 0.32 (95% CI 0.14–0.72) for IMA-ASSB, and 0.33 (95% CI 0.17–0.66) for ASCE. The single-arm studies were not analyzed because of a shortage of data. Network meta-analysis using a frequentist model proved that IMA-ASSB had the best performance in preventing enlargement of the aneurysm sac when all studies were incorporated in the analysis (RR 0.29, 95% CI 0.09–1.00), and was superior to NS-ASSB (RR 0.67, 95% CI 0.14–3.34) and similar to ASCE (RR 0.99, 95% CI 0.19–5.26). The same result was shown in the SUCRA curve analysis (Figures 5, 6).
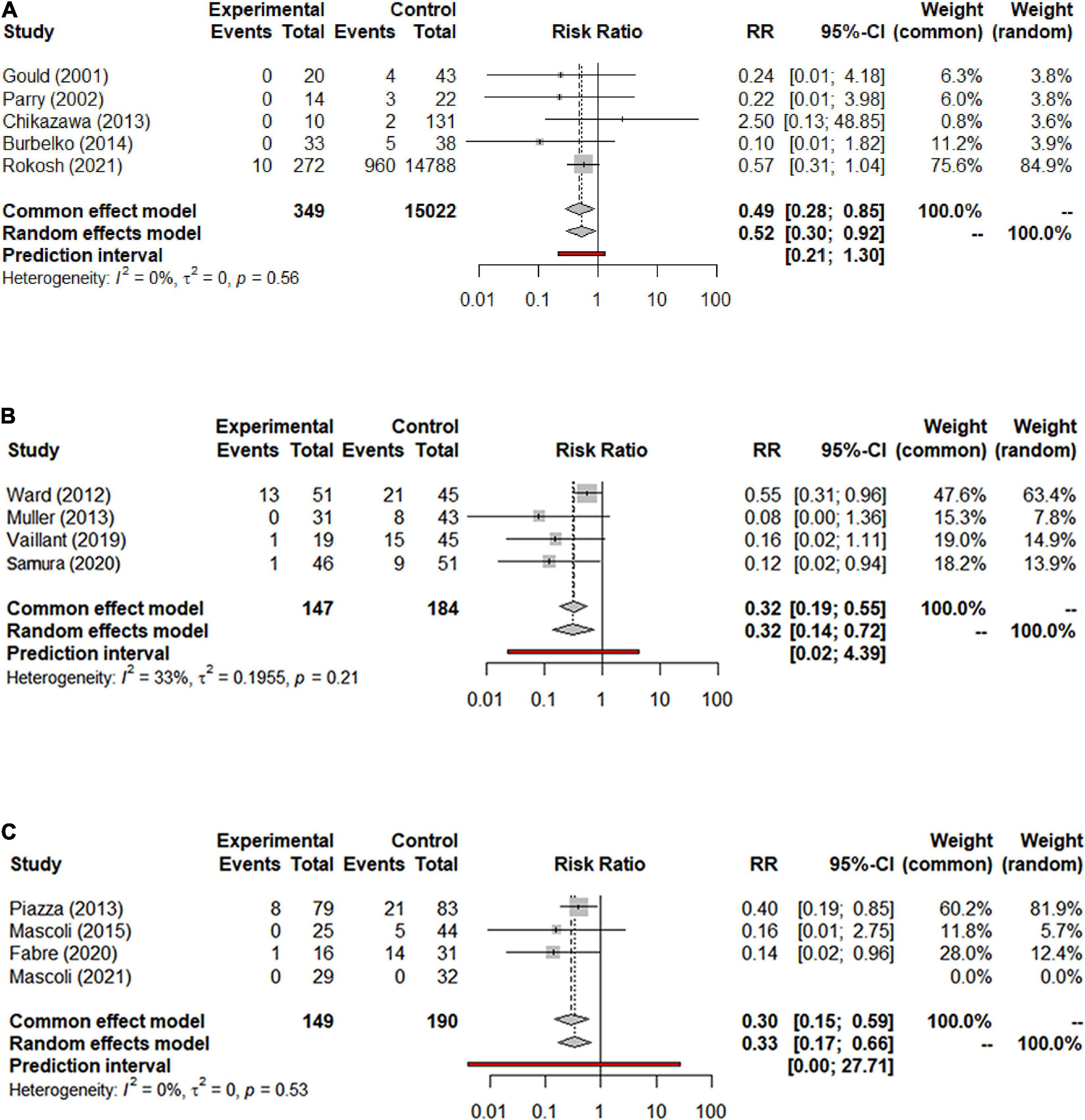
Figure 5. (A) Forest graph of enlargement of the aneurysm sac in NS-ASSB group (RR 0.52 95% Cl 0.30–0.92); (B) forest graph of enlargement of the aneurysm sac in IMA-ASSB group (RR 0.32 95% Cl 0.14–0.72); (C) forest graph of enlargement of the aneurysm sac in ASCE group (RR 0.33 95% Cl 0.17–0.66). NS-ASSB, non-selective embolization of aneurysm sac side branches; IMA-ASSB, embolization of the inferior mesenteric artery; ASCE, aneurysm sac coil embolization.
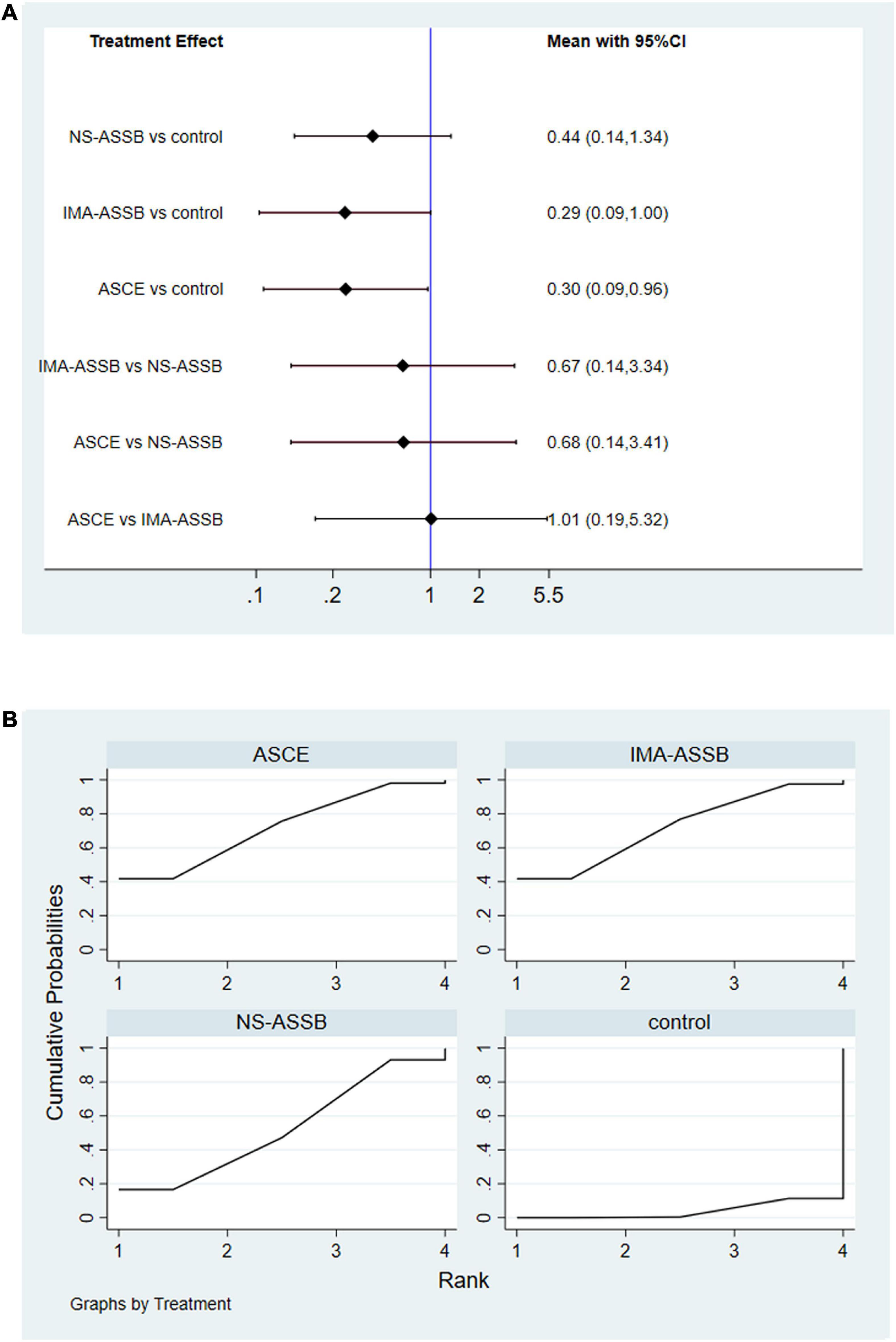
Figure 6. (A) Network meta-analysis forest graph of enlargement of the aneurysm sac in NS-ASSB, IMA-ASSB and ASCE groups; (B) SUCRA curve of enlargement of the aneurysm sac in NS-ASSB, IMA-ASSB and ASCE groups; NS-ASSB, non-selective embolization of aneurysm sac side branches; IMA-ASSB, embolization of the inferior mesenteric artery; ASCE, aneurysm sac coil embolization.
The potential effects of T2EL after EVAR remain controversial. Therefore, there are still disputes regarding the management of T2EL and its influence on further outcomes. There is currently no consensus regarding the need for intensive treatment of T2EL. Evidence indicates that T2EL is not an isolated complication and is associated with the occurrence of other types of endoleaks. Despite the controversy over the past two decades around whether prophylactic embolization prevents T2EL, an increasing body of evidence suggests that such treatments may dramatically improve the outcome of EVAR. Studies have reported that prophylactic embolization has potential benefits in decreasing the incidence of T2EL, preventing enlargement of the aneurysm sac, and decreasing the rate of re-intervention (21, 51). The major limitation of these previous studies was a lack of a comparison of different interventions. However, it is difficult to make such comparisons in clinical practice because of the wide variations in the technical difficulty and cost of the interventions.
The clinical benefit of NS-ASSB has been discussed extensively since the first study was published in 2001 (15). The previous meta-analysis has given evidence of the safety and effectiveness of ASCE in preventing T2EL (21). Another meta-analysis shows a different rate of 19.9% vs. 41.4% in patients who accept IMA embolization or not (52). But there is still little information available on the comparison of the re-intervention rate and diameter change in different embolization strategies and no network meta-analysis is available until now. The present systematic review and network meta-analysis investigated the value of prophylactic embolization in preventing adverse outcomes after EVAR and compared different therapeutic regimens. We found that prophylactic embolization had a positive effect on the outcome of EVAR. Non-selective embolization of the IMA and LAs showed the best results in preventing T2EL, but embolization of the IMA alone might provide better benefits in suppressing the expansion of the aneurysm sac and reducing the re-intervention rate. Although all three methods lead to common effects of sac regression and free form re-intervention. The long-term effects of the rate of diameter reduction and the second operation seem to be better when embolization of IMA was carried out in isolation. LAs may play an important role in the outflow tract in sac regression after EVAR. The embolization of LAs may reduce outflow efficiency which decreases the rate of diameter reduction. As mentioned above, the effects of T2EL after EVAR still require verification; however, T2EL may eventually lead to aneurysm sac expansion (53). Aneurysm sac expansion is a predictor of late complications after EVAR (54, 55) and increases the risk of AAA rupture (56). The guidelines of the European Society for Vascular Surgery and prior studies recommend surgical intervention when the diameter of the aneurysm sac enlarges by 10–13 mm during follow-up using the same imaging modality (11, 53, 57). The presence of a T2EL and an increasing aneurysm sac size is likely to lead to a type I endoleak (57), which has a potential correlation with the risk of AAA rupture (58) and requires immediate treatment. There seems to be no consensus on the indications for re-intervention. A previous study assessed type I or III endoleaks, aneurysm sac expansion of more than 10 mm, and the existence of a collateralized IMA feeding vessel as indications for intervention (53). Our results indicated that prophylactic embolization of the IMA reduced the rate of aneurysm sac enlargement and improved the clinical outcomes of EVAR. These results suggest an underlying positive correlation between embolization of the LAs and expansion of the aneurysm sac diameter, which eventually increased the risk of re-intervention. Muthu et al. reported a high incidence of lumbar endoleaks after EVAR (48), which may result in the difference between the outcome of NS-ASSB and the stable optimal outcome of IMA-ASSB and ASCE demonstrated in the present study. More clinical data about the outcome of embolization of the LAs alone during EVAR is needed to confirm whether the hemodynamics are changed by this process or whether the hemodynamics are only changed by occlusion of the IMA. With regards to potential risks of ASSB and ASCE, Ward et (31) reported a 9.3% rate of complications among embolization patients, only one of them died because of multiorgan failure caused by colonic infarction after IMA embolization.
Although there were limited data available regarding the outcomes after prophylactic embolization during EVAR, even fewer data were available regarding the association of specific embolization treatments with aneurysm sac enlargement and re-intervention. Only one of the included studies was a randomized controlled trial, and most of the included studies had small sample sizes while one multicenter retrospective study had an extremely large sample size. Although the network meta-analysis was performed after the exclusion of the data from the large study by Rokosh et al. (43) showed similar results, bias may still exist. Eleven of the included studies were performed in the past 5 years, and most studies were performed over long time intervals that would bring bias caused by technological innovation. In addition, there were limited data on the incidences of aneurysm sac enlargement and re-intervention, and on complications after EVAR with versus without prophylactic embolization treatments. Furthermore, some patients did not receive embolization because they underwent emergency surgery, had unsuitable artery anatomy, or were physically unable to tolerate the procedure, which resulted in bias. Because all the calculated I2 values were less than 75%, publication bias was proved by funnel plots and Egger’s test (p = 0.0027 for the NS-ASSB group, p = 0.0252 for the IMA-ASSB group, and p = 0.0047 for the ASCE group). Besides, network meta-analysis has its advantages and drawbacks. Mvmeta package on Stata platform made based on multivariate regression analysis could obtain outcomes very close to Bayesian model. But only one dummy variable can be set in each operation. Moreover, heterogeneity and transitivity assumptions are still been challenged and stand in the way. To reduce the bias introduced by the performance of retrospective studies at single centers, more randomized controlled trials (preferably multicenter) are needed to verify the safety and efficacy of prophylactic embolization. Studies should be registered and carried out in accordance with the standard instructions for clinical trials. Blinded raters should perform CTA and assess the symptoms and disease progress at admission, every 6 or 12 months, and at discharge. Any CTA imaging data should be measured by independent reviewers using the same software tool. There should also be more precise definitions of aneurysm sac enlargement and indications for re-interventions.
Prophylactic embolization during EVAR effectively prevents T2EL, suppresses the aneurysm sac expansion, and reduces the rate of re-intervention. Non-selective embolization of the IMA and LAs shows the best results in preventing T2EL. IMA embolization demonstrated certain benefits in achieving long-term aneurysm sac stability and lowering the risk of secondary surgery. Embolization of the LAs increases the operation time and medical expenses, but leads to potentially negative effects on the long-term outcome. We recommend conducting prophylactic embolization, especially IMA embolization alone or ASCE, to enhance the clinical benefits. More high-quality studies are needed to confirm the present findings.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
YW and JY conceived the study, designed the method, contributed to the literature searches, provided statistical analysis, and wrote the manuscript. ZH investigated the literatures. GW excluded the documents. All authors interpreted the data, read the manuscript, and approved the final version.
This work was supported by Capital’s Funds for Health Improvement and Research, 2020-2Z-5014.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.947809/full#supplementary-material
1. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG, EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. (2004) 364:843–8.
2. Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. (2016) 388:2366–74. doi: 10.1016/S0140-6736(16)31135-7
3. De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. (2010) 362:1881–9.
4. Dijkstra ML, Zeebregts CJ, Verhagen HJM, Teijink JAW, Power AH, Bockler D, et al. Incidence, natural course, and outcome of type II endoleaks in infrarenal endovascular aneurysm repair based on the ENGAGE registry data. J Vasc Surg. (2020) 71:780–9. doi: 10.1016/j.jvs.2019.04.486
5. Sidloff DA, Gokani V, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak: conservative management is a safe strategy. Eur J Vasc Endovasc Surg. (2014) 48:391–9. doi: 10.1016/j.ejvs.2014.06.035
6. Rehman ZU. Endoleaks: current concepts and treatments - A narrative review. J Pak Med Assoc. (2021) 71:2224–9. doi: 10.47391/JPMA.03-345
7. Mulay S, Geraedts ACM, Koelemay MJW, Balm R, ODYSSEUS study group. Type 2 endoleak with or without intervention and survival after endovascular aneurysm repair. Eur J Vasc Endovasc Surg. (2021) 61:779–86. doi: 10.1016/j.ejvs.2021.01.017
8. Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. (2007) 46:1–8. doi: 10.1016/j.jvs.2007.02.073
9. Rayt HS, Sandford RM, Salem M, Bown MJ, London NJ, Sayers RD. Conservative management of type 2 endoleaks is not associated with increased risk of aneurysm rupture. Eur J Vasc Endovasc Surg. (2009) 38:718–23. doi: 10.1016/j.ejvs.2009.08.006
10. El Batti S, Cochennec F, Roudot-Thoraval F, Becquemin JP. Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg. (2013) 57:1291–7. doi: 10.1016/j.jvs.2012.10.118
11. Wanhainen A, Verzini F, Van Herzeele I. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57:8–93. Erratum in: Eur J Vasc Endovasc Surg. 2020;59(3):494. doi: 10.1016/j.ejvs.2018.09.020
12. Schlösser FJ, Gusberg RJ, Dardik A, Lin PH, Verhagen HJ, Moll FL, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted? Eur J Vasc Endovasc Surg. (2009) 37:15–22.
13. Sidloff DA, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak after endovascular aneurysm repair. Br J Surg. (2013) 100:1262–70.
14. Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess. (2018) 22:1–132.
15. Gould DA, McWilliams R, Edwards RD, Martin J, White D, Joekes E, et al. Aortic side branch embolization before endovascular aneurysm repair: incidence of type II endoleak. J Vasc Interv Radiol. (2001) 12:337–41.
16. Zhang H, Yang Y, Kou L, Sun H, Chen Z. Effectiveness of collateral arteries embolization before endovascular aneurysm repair to prevent type II endoleaks: a systematic review and meta-analysis. Vascular. (2021):17085381211032764. [Online ahead of print], doi: 10.1177/17085381211032764
17. Natrella M, Rapellino A, Navarretta F, Iob G, Cristoferi M, Castagnola M, et al. Embo-EVAR: a technique to prevent Type II endoleak? A single-center experience. Ann Vasc Surg. (2017) 44:119–27. doi: 10.1016/j.avsg.2017.01.028
18. Dosluoglu HH, Rivero M, Khan SZ, Cherr GS, Harris LM, Dryjski ML. Pre-emptive nonselective perigraft aortic sac embolization with coils to prevent type II endoleak after endovascular aneurysm repair. J Vasc Surg. (2019) 69:1736–46. doi: 10.1016/j.jvs.2018.10.054
19. Pilon F, Tosato F, Danieli D, Campanile F, Zaramella M, Milite D. Intrasac fibrin glue injection after platinum coils placement: the efficacy of a simple intraoperative procedure in preventing type II endoleak after endovascular aneurysm repair. Interact Cardiovasc Thorac Surg. (2010) 11:78–82. doi: 10.1510/icvts.2009.231167
20. Fabre D, Fadel E, Brenot P, Hamdi S, Gomez Caro A, Mussot S, et al. Type II endoleak prevention with coil embolization during endovascular aneurysm repair in high-risk patients. J Vasc Surg. (2015) 62:1–7.
21. Li Q, Hou P. Sac embolization and side branch embolization for preventing Type II endoleaks after endovascular aneurysm repair: a meta-analysis. J Endovasc Ther. (2020) 27:109–16.
22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34.
23. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality Of Nonrandomised Studies In Meta-Analyses. Ottawa: Ottawa Hospital Research Institute (2021).
24. RStudio Team.RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC (2021).
25. Parry DJ, Kessel DO, Robertson I, Denton L, Patel JV, Berridge DC, et al. Type II endoleaks: predictable, preventable, and sometimes treatable? J Vasc Surg. (2002) 36:105–10. doi: 10.1067/mva.2002.125023
26. Axelrod DJ, Lookstein RA, Guller J, Nowakowski FS, Ellozy S, Carroccio A, et al. Inferior mesenteric artery embolization before endovascular aneurysm repair: technique and initial results. J Vasc Interv Radiol. (2004) 15:1263–7. doi: 10.1097/01.RVI.0000141342.42484.90
27. Sheehan MK, Hagino RT, Canby E, Wholey MH, Postoak D, Suri R, et al. Type 2 endoleaks after abdominal aortic aneurysm stent grafting with systematic mesenteric and lumbar coil embolization. Ann Vasc Surg. (2006) 20:458–63. doi: 10.1007/s10016-006-9103-2
28. Nevala T, Biancari F, Manninen H, Matsi P, Mäkinen K, Ylönen K, et al. Inferior mesenteric artery embolization before endovascular repair of an abdominal aortic aneurysm: effect on type II endoleak and aneurysm shrinkage. J Vasc Interv Radiol. (2010) 21:181–5. doi: 10.1016/j.jvir.2009.10.014
29. Chikazawa G, Yoshitaka H, Hiraoka A, Tanaka K, Mouri N, Tamura K, et al. Preoperative coil embolization to aortic branched vessels for prevention of aneurysmal sac enlargement following EVAR: early clinical result. Ann Vasc Dis. (2013) 6:175–9. doi: 10.3400/avd.oa.12.00079
30. Piazza M, Frigatti P, Scrivere P, Bonvini S, Noventa F, Ricotta JJ II, et al. Role of aneurysm sac embolization during endovascular aneurysm repair in the prevention of type II endoleak-related complications. J Vasc Surg. (2013) 57:934–41. doi: 10.1016/j.jvs.2012.10.078
31. Ward TJ, Cohen S, Fischman AM, Kim E, Nowakowski FS, Ellozy SH, et al. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol. (2013) 24:49–55. doi: 10.1016/j.jvir.2012.09.022
32. Burbelko M, Kalinowski M, Heverhagen JT, Piechowiak E, Kiessling A, Figiel J, et al. Prevention of type II endoleak using the AMPLATZER vascular plug before endovascular aneurysm repair. Eur J Vasc Endovasc Surg. (2014) 47:28–36. doi: 10.1016/j.ejvs.2013.10.003
33. Müller-Wille R, Uller W, Gössmann H, Heiss P, Wiggermann P, Dollinger M, et al. Inferior mesenteric artery embolization before endovascular aortic aneurysm repair using amplatzer vascular plug type 4. Cardiovasc Interv Radiol. (2014) 37:928–34. doi: 10.1007/s00270-013-0762-4
34. Mascoli C, Freyrie A, Gargiulo M, Gallitto E, Pini R, Faggioli G, et al. Selective Intra-procedural AAA sac embolization during EVAR reduces the rate of Type II endoleak. Eur J Vasc Endovasc Surg. (2016) 51:632–9. doi: 10.1016/j.ejvs.2015.12.009
35. Nakai M, Ikoma A, Sato M, Sato H, Nishimura Y, Okamura Y. Prophylactic intraoperative embolization of abdominal aortic aneurysm sacs using N-Butyl cyanoacrylate/lipiodol/ethanol mixture with proximal neck aortic balloon occlusion during endovascular abdominal aortic repair. J Vasc Interv Radiol. (2016) 27:954–60. doi: 10.1016/j.jvir.2016.03.037
36. Piazza M, Squizzato F, Zavatta M, Menegolo M, Ricotta JJ II, Lepidi S, et al. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for Type II endoleak. J Vasc Surg. (2016) 63:32–8. doi: 10.1016/j.jvs.2015.08.049
37. Vaillant M, Barral PA, Mancini J, De Masi M, Bal L, Piquet P, et al. Preoperative inferior mesenteric artery embolization is a cost-effective technique that may reduce the rate of aneurysm sac diameter enlargement and reintervention after EVAR. Ann Vasc Surg. (2019) 60:85–94. doi: 10.1016/j.avsg.2019.03.012
38. Samura M, Morikage N, Otsuka R, Mizoguchi T, Takeuchi Y, Nagase T, et al. Endovascular aneurysm repair with inferior mesenteric artery embolization for preventing Type II endoleak: a prospective randomized controlled trial. Ann Surg. (2020) 271:238–44. doi: 10.1097/SLA.0000000000003299
39. Branzan D, Geisler A, Steiner S, Doss M, Matschuck M, Scheinert D, et al. Type II endoleak and aortic aneurysm sac shrinkage after preemptive embolization of aneurysm sac side branches. J Vasc Surg. (2021) 73:1973–9.e1. doi: 10.1016/j.jvs.2020.11.032
40. Fabre D, Mougin J, Mitilian D, Cochennec F, Garcia Alonso C, Becquemin JP, et al. Prospective, randomised two centre trial of endovascular repair of abdominal aortic aneurysm with or without sac embolisation. Eur J Vasc Endovasc Surg. (2021) 61:201–9. doi: 10.1016/j.ejvs.2020.11.028
41. Mascoli C, Faggioli G, Gallitto E, Pini R, Fenelli C, Cercenelli L, et al. Tailored Sac embolization during EVAR for preventing persistent Type II endoleak. Ann Vasc Surg. (2021) 76:293–301. doi: 10.1016/j.avsg.2021.01.118
42. Mathlouthi A, Guajardo I, Al-Nouri O, Malas M, Barleben A. Prophylactic aneurysm embolization during EVAR is safe, improves sac regression and decreases the incidence of Type II endoleak. Ann Vasc Surg. (2021) 74:36–41. doi: 10.1016/j.avsg.2020.12.060
43. Rokosh RS, Chang H, Butler JR, Rockman CB, Patel VI, Milner R, et al. Prophylactic sac outflow vessel embolization is associated with improved sac regression in patients undergoing endovascular aortic aneurysm repair. J Vasc Surg. (2021) 76:113–21.e8. doi: 10.1016/j.jvs.2021.11.070
44. Aoki A, Maruta K, Omoto T, Masuda T. Midterm outcomes of endovascular abdominal aortic aneurysm repair with prevention of Type 2 endoleak by intraoperative aortic side branch coil embolization. Ann Vasc Surg. (2022) 78:180–9. doi: 10.1016/j.avsg.2021.06.037
45. Alerci M, Giamboni A, Wyttenbach R, Porretta AP, Antonucci F, Bogen M, et al. Endovascular abdominal aneurysm repair and impact of systematic preoperative embolization of collateral arteries: endoleak analysis and long-term follow-up. J Endovasc Ther. (2013) 20:663–71. doi: 10.1583/12-4188MR.1
46. Bonvini R, Alerci M, Antonucci F, Tutta P, Wyttenbach R, Bogen M, et al. Preoperative embolization of collateral side branches: a valid means to reduce type II endoleaks after endovascular AAA repair. J Endovasc Ther. (2003) 10:227–32. doi: 10.1177/152660280301000210
47. Fukuda T, Matsuda H, Tanaka H, Sanda Y, Morita Y, Seike Y. Selective Inferior mesenteric artery embolization during endovascular abdominal aortic aneurysm repair to prevent Type II endoleak. Kobe J Med Sci. (2018) 63:E130–5.
48. Muthu C, Maani J, Plank LD, Holden A, Hill A. Strategies to reduce the rate of type II endoleaks: routine intraoperative embolization of the inferior mesenteric artery and thrombin injection into the aneurysm sac. J Endovasc Ther. (2007) 14:661–8. doi: 10.1177/152660280701400509
49. Ronsivalle S, Faresin F, Franz F, Rettore C, Zanchetta M, Olivieri A. Aneurysm sac “thrombization” and stabilization in EVAR: a technique to reduce the risk of type II endoleak. J Endovasc Ther. (2010) 17:517–24. doi: 10.1583/09-3004.1
50. Zanchetta M, Faresin F, Pedon L, Ronsivalle S. Intraoperative intrasac thrombin injection to prevent type II endoleak after endovascular abdominal aortic aneurysm repair. J Endovasc Ther. (2007) 14:176–83. doi: 10.1177/152660280701400209
51. Yu HYH, Lindström D, Wanhainen A, Tegler G, Hassan B, Mani K. Systematic review and meta-analysis of prophylactic aortic side branch embolization to prevent type II endoleaks. J Vasc Surg. (2020) 72:1783–92.e1. doi: 10.1016/j.jvs.2020.05.020
52. Biancari F, Mäkelä J, Juvonen T, Venermo M. Is inferior mesenteric artery embolization indicated prior to endovascular repair of abdominal aortic aneurysm? Eur J Vasc Endovascular Surg. (2015) 50:671–4. doi: 10.1016/j.ejvs.2015.06.116
53. Ajalat M, Williams R, Wilson SE. The natural history of type 2 endoleaks after endovascular aneurysm repair justifies conservative management. Vascular. (2018) 26:524–30. doi: 10.1177/1708538118766103
54. Bastos Gonçalves F, Baderkhan H, Verhagen HJ, Wanhainen A, Björck M, Stolker RJ, et al. Early sac shrinkage predicts a low risk of late complications after endovascular aortic aneurysm repair. Br J Surg. (2014) 101:802–10. doi: 10.1002/bjs.9516
55. Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. (2011) 123:2848–55. Erratum in: Circulation. 2012;125(2):e266. doi: 10.1161/CIRCULATIONAHA.110.014902
56. Chaikof E, Dalman R, Eskandari M, Jackson B, Lee W, Mansour M, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. (2018) 67:2–77. doi: 10.1016/j.jvs.2017.10.044
57. Eden CL, Long GW, Major M, Studzinski D, Brown OW. Type II endoleak with an enlarging aortic sac after endovascular aneurysm repair predisposes to the development of a type IA endoleak. J Vasc Surg. (2020) 72:1354–9. doi: 10.1016/j.jvs.2020.01.038
Keywords: prophylactic embolization, inferior mesenteric artery, type II endoleak, meta-analysis, abdominal aortic aneurysm
Citation: Wu Y, Yin J, Hongpeng Z and Wei G (2022) Systematic review and network meta-analysis of pre-emptive embolization of the aneurysm sac side branches and aneurysm sac coil embolization to improve the outcomes of endovascular aneurysm repair. Front. Cardiovasc. Med. 9:947809. doi: 10.3389/fcvm.2022.947809
Received: 19 May 2022; Accepted: 29 June 2022;
Published: 22 July 2022.
Edited by:
Wei Wang, Central South University, ChinaReviewed by:
Bao Liu, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2022 Wu, Yin, Hongpeng and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Hongpeng, emhhbmdob25ncGVuZ0B2aXAuc2luYS5jb20=; Guo Wei, Z3Vvd2VpcGxhZ2hAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.