
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 14 September 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.947639
 Shuangshuang Zhu1,2,3†
Shuangshuang Zhu1,2,3† Yixia Lin1,2,3†
Yixia Lin1,2,3† Yanting Zhang1,2,3†
Yanting Zhang1,2,3† Guohua Wang4†
Guohua Wang4† Mingzhu Qian1,2,3
Mingzhu Qian1,2,3 Lang Gao1,2,3
Lang Gao1,2,3 Mengmeng Ji1,2,3
Mengmeng Ji1,2,3 Mingxing Xie1,2,3*
Mingxing Xie1,2,3* Yuman Li1,2,3*
Yuman Li1,2,3* Li Zhang1,2,3*
Li Zhang1,2,3*Background: Although the left atrium (LA) plays a key role in the pathophysiology and disease progression of heart failure with preserved ejection fraction (HFpEF), the impact of type 2 diabetes mellitus (T2DM) on LA function and stiffness in HFpEF patients remains unclear. Furthermore, the prognostic value of different phases of LA function and stiffness is less well-established in HFpEF patients.
Methods: This study prospectively enrolled 164 HFpEF patients who were in sinus rhythm at the time of echocardiography, including 61 (37%) HFpEF patients with T2DM. LA reservoir, conduit, and pump function were assessed using two-dimensional volume indices and speckle tracking echocardiography. The LA stiffness was calculated as the ratio of early mitral inflow velocity-to-early annular tissue velocity (E/e’) and LA reservoir function. The primary end point was a combined outcome of heart failure hospitalization or death.
Results: Left atrium reservoir function [measured by peak LA strain (LAS-peak)] and LA pump function (measured by LAS-active) remained significantly lower in the HFpEF patients with T2DM compared with those without T2DM, even after adjustment for potential confounders. In addition, the LA stiffness of HFpEF patients with T2DM was higher than those without T2DM. After a median follow-up of 13.7 months, 46 patients (28.1%) reached the composite end point. LAS-peak (hazard ratios: 0.88; 95% confidence interval: 0.81–0.95; P = 0.001) was significantly associated with the risk of heart failure hospitalization or death after adjusting for demographic and clinical characteristics, LV global longitudinal strain, E/e’, and LA volume index. In contrast, other LA function and stiffness parameters did not independently predict the risk of adverse events. Kaplan-Meier analysis showed that HFpEF patients with T2DM and low LAS-peak (<27.2%) had a significantly increased risk of heart failure-related hospitalization or death (log-rank P < 0.001).
Conclusion: Left atrium reservoir and pump function are impaired, whereas LA stiffness is increased in HFpEF patients with T2DM compared with those without T2DM. LAS-peak is a powerful predictor of adverse clinical outcomes and may be crucial for risk stratification in HFpEF patients with and without T2DM.
Heart failure with preserved ejection fraction (HFpEF) is increasingly becoming a global health problem (1, 2). Type 2 diabetes mellitus (T2DM) is a frequent comorbidity of HFpEF (approximately 33–40%) (3–5). Moreover, T2DM significantly increases the risk of hospitalizations and mortality in HFpEF patients (6–8). It has been suggested that T2DM plays a central pathophysiological role in the development of HFpEF. Although the underlying mechanisms of this relationship are unclear, HFpEF patients with diabetic are more likely to have left ventricular (LV) hypertrophy, LV systolic and diastolic dysfunction, and left atrial (LA) enlargement (9, 10). The influence of T2DM on LA remodeling has been previously recognized in patients without symptomatic cardiovascular disease (11). However, the impact of T2DM on LA function and stiffness in HFpEF patients remains unclear (12).
The LA plays an integral role in the pathophysiology and disease progression of HFpEF (13–21). The LA size is independently associated with an increased risk of morbidity and mortality in HFpEF (13, 14). The different phases of LA function can be obtained using two-dimensional (2D) speckle tracking echocardiography (STE) which is a helpful tool for direct measurement of intrinsic LA myocardial deformation. 2D-STE is less dependent on loading conditions and geometric assumptions and has high feasibility and reproducibility (22, 23). Previous studies demonstrated the impairment of LA function in HFpEF compared with normal individuals (15–21, 24). However, LA function in HFpEF patients with and without T2DM has not been well-investigated. Furthermore, there is limited data on the prognostic relevance of LA function in HFpEF patients (25, 26).
The LA stiffness has been recognized as a novel method that estimates LA compliance (27). In addition, studies reported that LA stiffness is a strong independent predictor of recurrence after ablation (28) and is associated with poor clinical outcomes in patients with heart failure and reduced ejection fraction (29, 30). However, LA stiffness in HFpEF with and without T2DM has not been described, and its predictive value has also been unknown.
Accordingly, we aimed to (1) compare LA function and stiffness between HFpEF patients with and without T2DM and further investigate the impact of T2DM on LA function and stiffness in HFpEF patients and (2) determine the independent prognostic significance of LA function and stiffness in HFpEF patients, after adjustment for demographic and clinical characteristics, LV parameters, and LA size.
We prospectively enrolled 164 consecutive HFpEF patients between January 2021 and May 2021 at Union Hospital in Wuhan, China. HFpEF was defined according to the guidelines of the European Heart Journal (2016): (1) typical symptoms and/or signs of HF; (2) elevated B-type natriuretic peptide (BNP) > 35 pg/mL and/or N-terminal pro-B-type natriuretic peptide (NT-proBNP) > 125 pg/mL; and (3) echocardiographic LV ejection fraction (LVEF) ≥50% accompanied by either (a) diastolic dysfunction (ratio of peak early diastolic filling velocity (E) to early diastolic mitral annular velocity (e’) > 15) or (b) LA enlargement (left atrial volume index (LAVI) > 34 ml/m2) (31). Patients were excluded if they had atrial fibrillation, severe valvular disease, chronic obstructive pulmonary disease, congenital heart disease, acute coronary syndrome, pericardial disease, or poor image quality. This study was approved by the Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and was performed in accordance with the Declaration of Helsinki (Ethics No. 0650-01). Furthermore, we obtained written informed consent from all participants.
We collected the following data from all study participants: demographics, New York Heart Association (NYHA) functional class, comorbidities, medications, vital signs, body mass index (BMI), and laboratory data, including creatinine, BNP, NT-proBNP, total cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein. T2DM was defined as the presence of the clinical diagnosis (fasting plasma glucose ≥ 7 mmol/L or glycated hemoglobin ≥ 6.5%) or a self-reported history of diabetes mellitus and/or receiving antidiabetic therapy (32).
A comprehensive echocardiographic examination was acquired using the commercially available system (EPIC 7C, Philips Medical Systems, Andover, United States) with S5-1 and X5-1 transducers. All echocardiographic images were recorded in a native DICOM format. All echocardiographic measurements were performed according to the recommendation of the American Society of Echocardiography (33). Conventional echocardiographic measurements mainly included LV end-diastolic and end-systolic volume, mitral inflow propagation, mitral annular relaxation velocities, LA volume, and LVEF. LVEF was calculated using the biplane Simpson’s method in the apical 2-and 4-chamber views.
Based on previous validated studies and guidelines of the American Society of echocardiography/EACVI, LA, and LV deformation was performed using commercially available VIS (2D Cardiac Performance Analysis, TomTec Imaging Systems, Unterschleissheim, Germany) (34). Analyses of LA strain were performed in the apical four- and two-chamber views, and LV strain was obtained from the apical four-, three-, and two-chamber views. The most suitable cardiac cycle was chosen for each view. The reference point was set at the beginning of the QRS complex. The LA and LV endocardial borders were traced at the end-diastolic frame. The accuracy of tracking was visually confirmed throughout the cardiac cycle and confirmed from the morphology of the strain curves. If necessary, the region of interest was readjusted. Speckles were tracked by the software frame using the frame during the course of 1 cardiac cycle. LV global longitudinal strain was calculated as the average LV longitudinal strain across the 16 segments obtained using the apical four-, three-, and two-chamber views.
From LA speckle tracking analysis, the LA function was estimated using volumes and strain indices calculated as the average across the apical four- and two-chamber views. LA reservoir function was assessed using the peak LA strain (LAS-peak) and the total LA emptying fraction (LAEF-total). LA pump function was evaluated using LAS-active: LA strain at the onset time of the P wave, and LAEF-active: (LA volume at the onset time of the P wave–LA minimum volume)/LA volume at the onset time of the P wave. Left atrial conduit function was estimated using LAS-passive: LAS-peak–LAS-active, and LAEF-passive: (LA maximum volume- LA volume at the onset time of the P wave)/LA maximum volume. The LA stiffness was calculated as the ratio of early diastolic mitral inflow velocity-to-early diastolic mitral annular tissue velocity (E/e’) and LA reservoir function (LAS-peak and LAEF-total) (35).
Intraobserver and interobserver variability of LA strain was estimated in 20 randomly selected subjects and evaluated by intra-class correlation coefficient (ICC) and Bland-Altman analysis. Intraobserver variability was assessed by having one observer remeasure after 4–8 weeks. Interobserver variability was evaluated by a second observer who was blinded to the first observer’s measurements.
Patients were followed until 15 March 2022. The follow-up on outcomes started the day after the comprehensive baseline echocardiography measurements. Follow-up data were collected through hospital visits or telephone contacts by the investigator who was blinded to clinical details and echocardiography data. The primary end point was a combined outcome of heart failure-related hospitalization or death.
All continuous variables are presented as means with 95% confidence intervals (CIs), and categorical variables are presented as numbers and percentages. Comparisons of clinical and echocardiographic characteristics between HFpEF patients with and without T2DM were performed using chi-square tests for categorical data and two-sample t-test or a Mann-Whitney U test for continuous variables. The prognostic value of LA function parameters was assessed using univariate and multivariate Cox regression to obtain hazard ratio (HR) and 95% CI. Survival curves were obtained using the Kaplan-Meier analysis and compared using the log-rank test. All statistical analyses were performed using SPSS version 24 (IBM SPSS Statistics, Chicago, IL). P-value < 0.05 was considered to indicate statistical significance.
The baseline clinical and echocardiographic characteristics of the 164 HFpEF patients are summarized in Table 1. T2DM was identified in 61 patients (37%), and the remaining 103 patients (63%) were classified as non-T2DM patients. There were no significant differences in age, gender, BMI, systolic blood pressure, diastolic blood pressure, heart rate, medications use, and laboratory findings between the two groups, except for more SGLT-2i use, increased BNP or NT-proBNP, and reduced high-density lipoprotein levels in HFpEF patients with T2DM. HFpEF patients with T2DM suffered more frequently from NYHA functional class III or IV symptoms (41% vs. 17%, P = 0.001). However, frequencies of comorbidities including hypertension, hypercholesterolemia, and coronary artery disease were similarly distributed between the two groups (all P > 0.05). In addition, LV global longitudinal strain in HFpEF patients with T2DM was significantly impaired (-18.6 [17.8–19.4]% vs. -19.7 [19.2–20.2]%, P = 0.018), and E/e’ was higher (14.7 [13.7–15.7] vs. 12.5 [11.7–13.2], P = 0.001) than of those without T2DM. In contrast, E/A, LVEF, LV, and LA volumes for the two groups were similar (all P > 0.05).
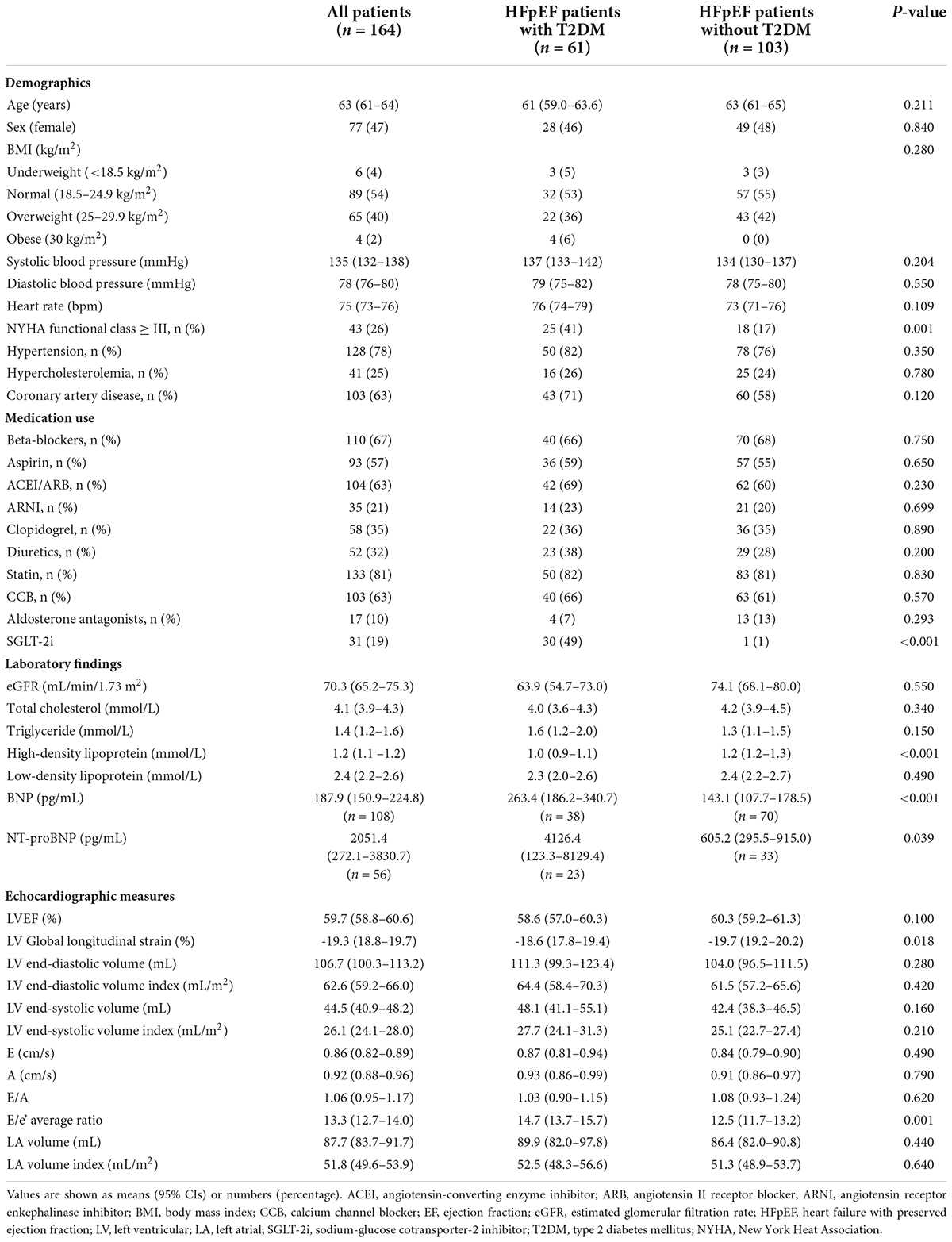
Table 1. Demographic and echocardiographic parameters in HFpEF patients according to diabetes status.
Unadjusted comparisons of LA function and stiffness between HFpEF patients with and without T2DM are shown in Table 2 and Figure 1. LAS-peak and LAEF-total (reflecting LA reservoir function) and LAS-active (reflecting LA pump function) were significantly lower in HFpEF patients with T2DM than those without T2DM (all P < 0.05). Moreover, E/e’ divided by LAS-peak and E/e’ divided by LAEF-total (reflecting LA stiffness) in HFpEF patients with T2DM were higher than those without T2DM (both P < 0.001). However, LAS-passive and LAEF-passive (measurement of LA conduit function) and LAEF-active (another measurement of LA pump function) had no significant differences between HFpEF patients with and without T2DM.
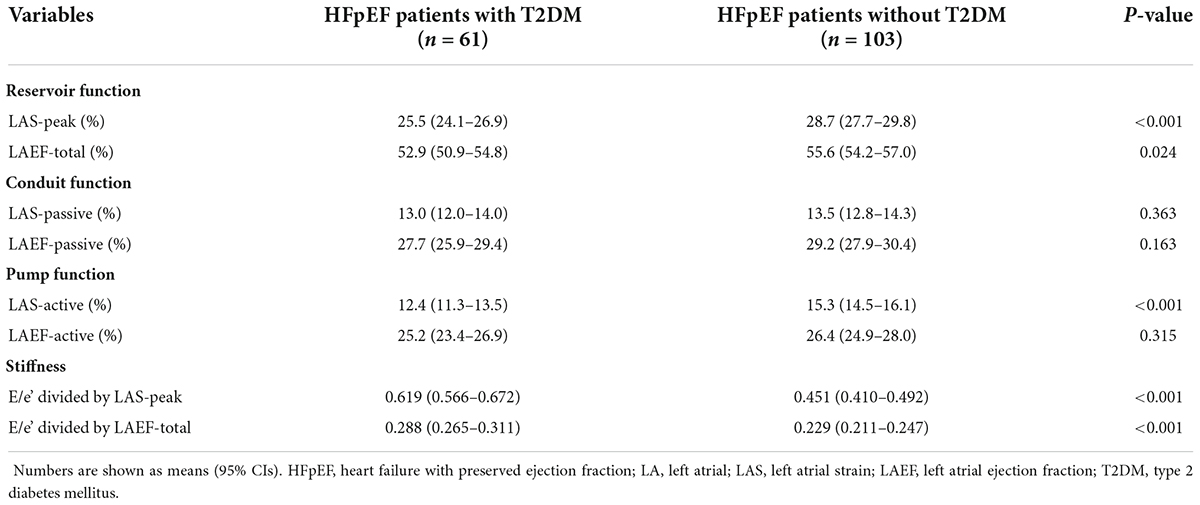
Table 2. Unadjusted comparisons of LA function and stiffness between HFpEF patients with and without T2DM.
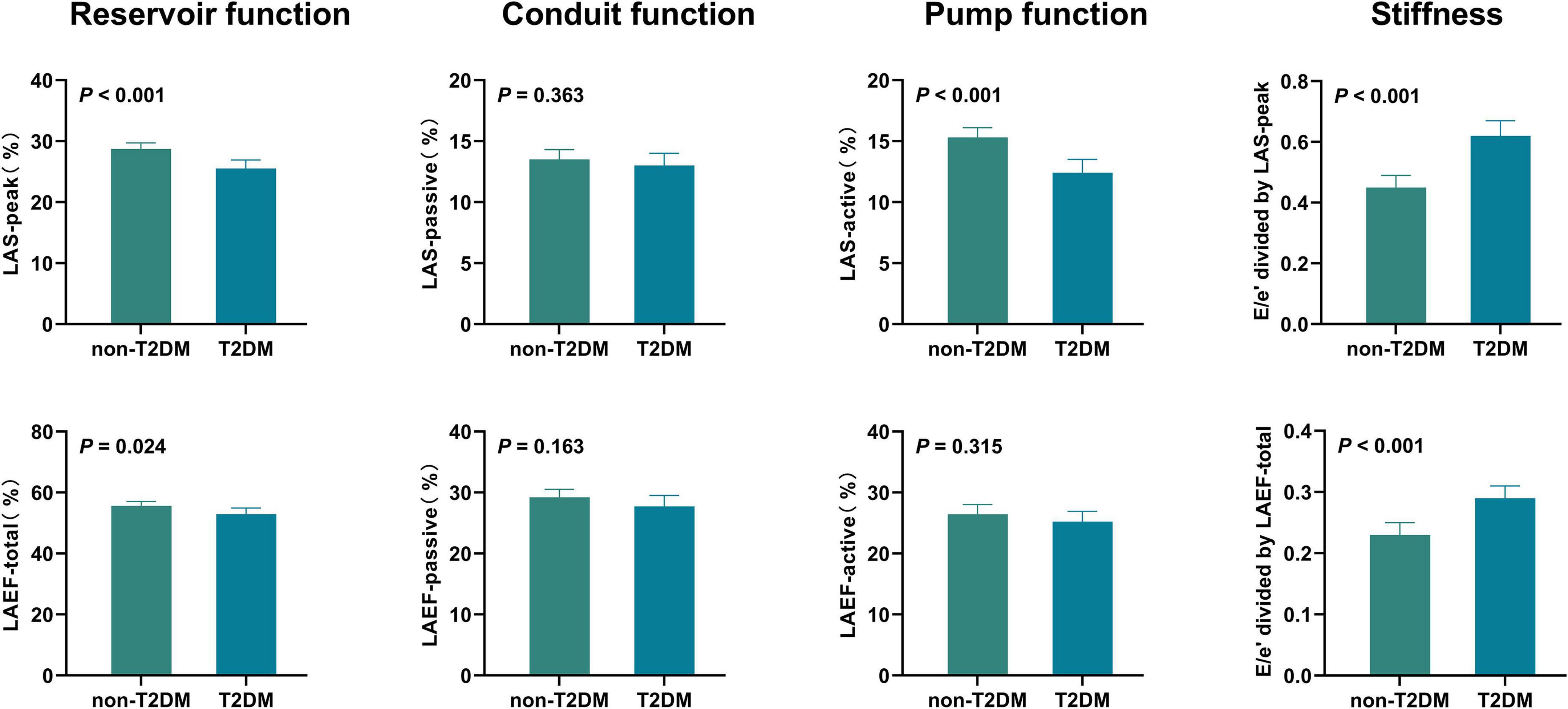
Figure 1. Unadjusted comparisons of LA function and stiffness between HFpEF patients with and without T2DM HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LAS, LA strain; LAEF, LA ejection fraction; T2DM, type 2 diabetes mellitus.
After adjusting for age, sex, BMI, heart rate, systolic blood pressure, LV global longitudinal strain, and (or) E/e’, differences persisted in LA reservoir function (measured by LAS-peak), LA pump function (measured by LAS-active), and LA stiffness between HFpEF patients with and without T2DM (all P < 0.05) (Table 3 and Figure 2).
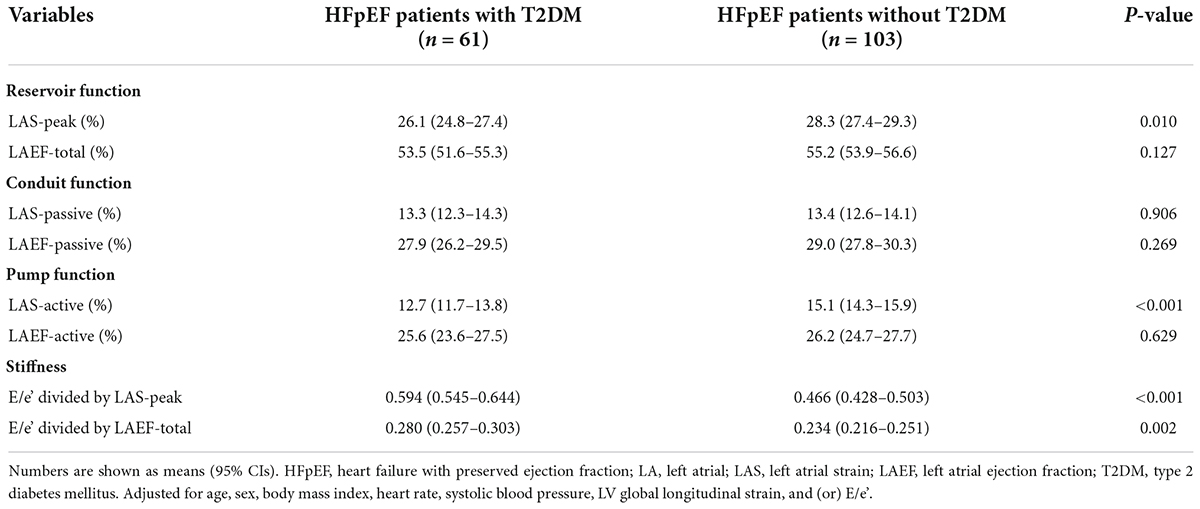
Table 3. Adjusted comparisons of LA function and stiffness between HFpEF patients with and without T2DM.
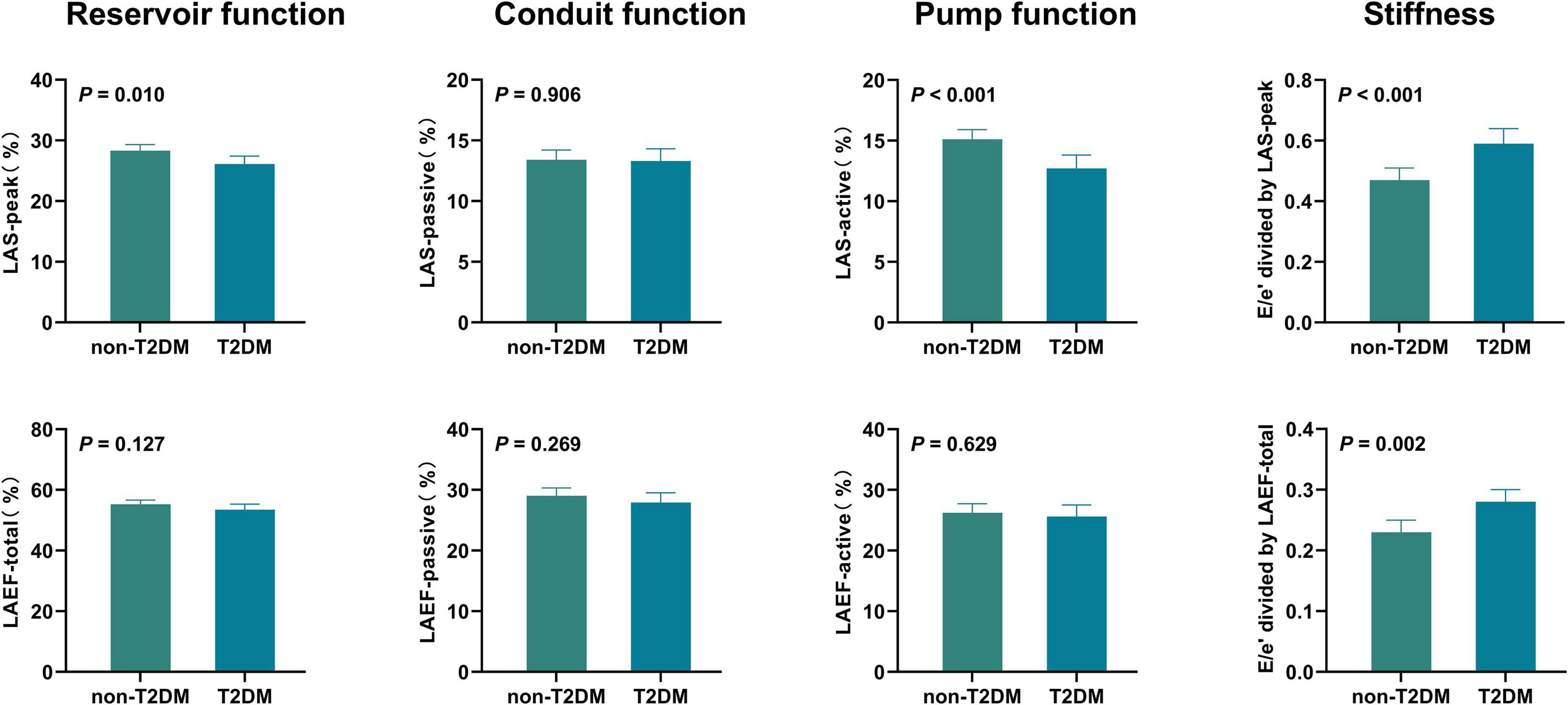
Figure 2. Adjusted comparisons of LA function and stiffness between HFpEF patients with and without T2DM HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LAS, LA strain; LAEF, LA ejection fraction; T2DM, type 2 diabetes mellitus. Adjusted for age, sex, body mass index, heart rate, systolic blood pressure, LV global longitudinal strain, and (or) E/e’.
Over a median duration of 13.7 (IQR 10.7–14.1) months, 46 patients (28.1%) experienced the primary composite end point, including 40 (24.4%) hospitalized for heart failure and 6 (3.7%) died during follow-up. Table 4, Figures 3 and 4 demonstrate the results of proportional hazards (Cox) models in which LA function and stiffness were assessed as predictors of adverse events.
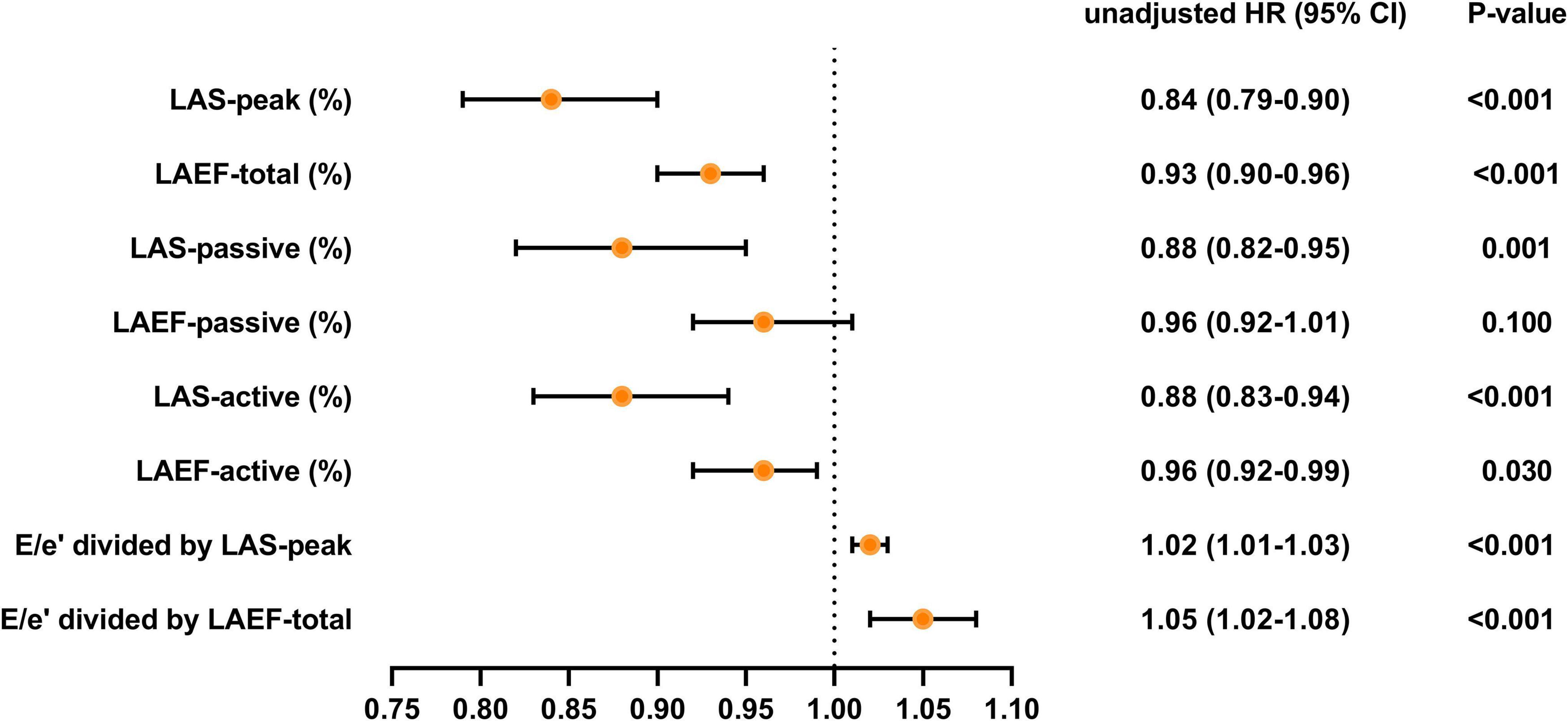
Figure 3. Unadjusted HR for various measures of LA function and stiffness HR, hazard ratio; LA, left atrial; LAS, LA strain; LAEF, LA ejection fraction.
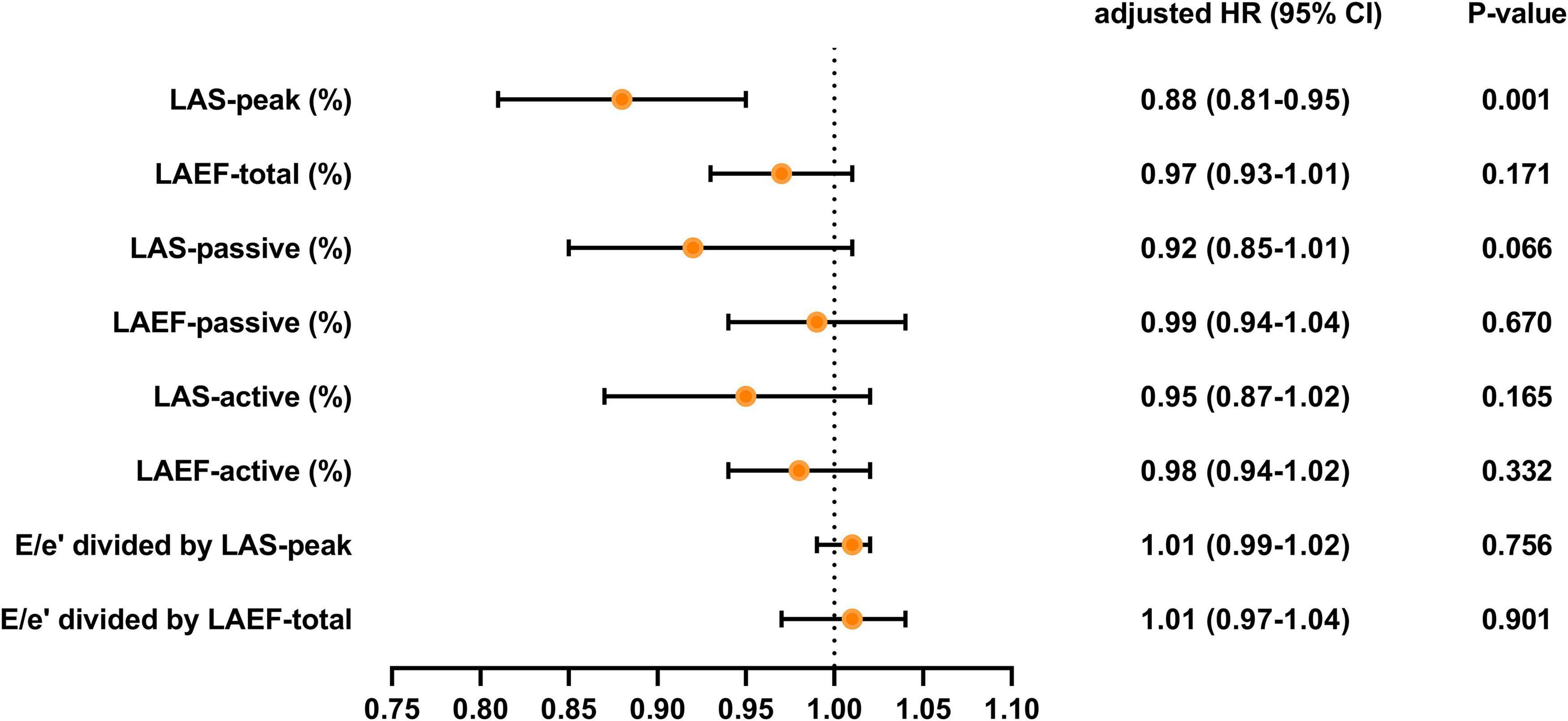
Figure 4. Adjusted HR for various measures of LA function and stiffness HR, hazard ratio; LA, left atrial; LAS, LA strain; LAEF, LA ejection fraction. Models were adjusted for BMI, diabetes, LV global longitudinal strain, LA volume index, and (or) E/e’.
In unadjusted analyses (Table 4 and Figure 3), LAS-peak (HR: 0.84; 95% CI: 0.79–0.90; P < 0.001), LAEF-total (HR: 0.93; 95% CI: 0.90–0.96; P < 0.001), LAS-active (HR: 0.88; 95% CI: 0.83–0.94; P < 0.001), LAEF-active (HR: 0.96; 95% CI: 0.92–0.99; P = 0.030), E/e’ divided by LAS-peak (HR: 1.02; 95% CI: 1.01–1.03; P < 0.001), and E/e’ divided by LAEF-total (HR: 1.05; 95% CI: 1.02–1.08; P < 0.001) were significantly predictive of adverse events. Although the LAS-passive (HR: 0.88; 95% CI: 0.82–0.95; P = 0.001) was significantly predictive of adverse events, LAEF-passive was not a predictor of clinical events. The results of univariate analysis evaluating baseline clinical and other echocardiographic variables associated with combined endpoint events are reported in Supplementary Table 1.
In analyses that adjusted for BMI, diabetes, LV global longitudinal strain, LA volume index, and (or) E/e’ (Table 4 and Figure 4), LAEF-total, LAS-passive, LAS-active, LAEF-active, and LA stiffness were not significant predictors of adverse events. LAS-peak (HR: 0.88; 95% CI: 0.81–0.95; P = 0.001) remained significantly predictive of the risk of adverse events.
All HFpEF patients were classified into two groups using the median value of LAS-peak (27.2%). There were 40 HFpEF patients with T2DM and low LAS-peak (<27.2%). This feature was associated with a worse clinical outcome than for the other subgroups (log-rank P = 0.037 vs. HFpEF patients without T2DM and low LAS-peak; log-rank P = 0.002 vs. HFpEF patients with T2DM and high LAS-peak; log-rank P < 0.001 vs. HFpEF patients without T2DM and high LAS-peak; Figure 5).
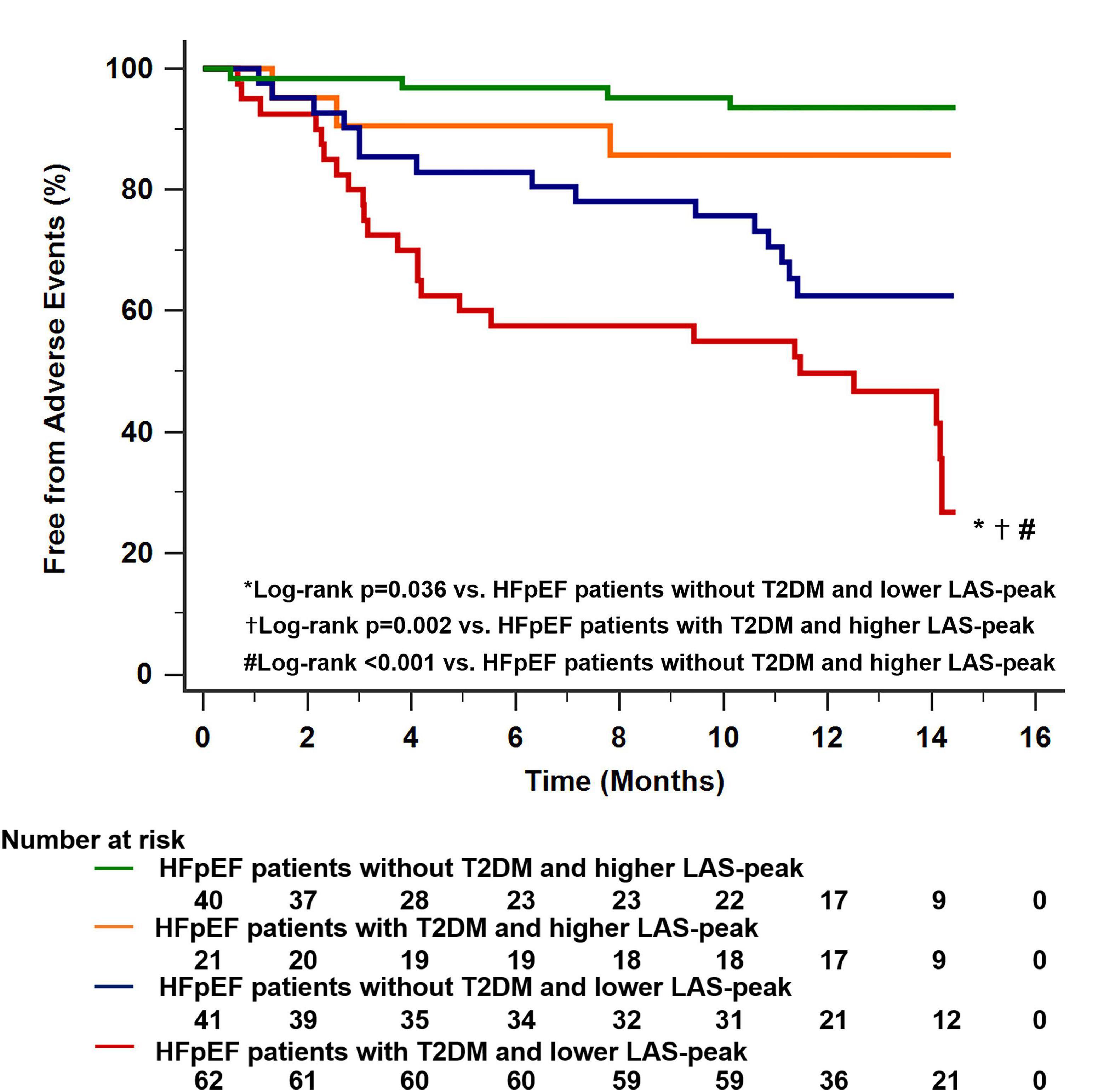
Figure 5. Dividing all HFpEF patients into two main groups using the median value of LAS-peak (27.2%) identified 40 patients with HFpEF with T2DM and low LAS-peak. This characteristic was associated with worse long-term outcomes compared with the other subgroups. HFpEF, heart failure with preserved ejection fraction; LAS, left atrial strain; T2DM, type 2 diabetes mellitus.
Intra-observer and inter-observer reproducibility of LA strain by 2D-STE is shown in Supplementary Table 2. LAS-peak, LAS-active, and LAS-passive exhibited good reproducibility, as reflected by high ICC. Bland-Altman analysis demonstrated high intra-observer and inter-observer agreement, with small bias and narrow limits of agreement.
In this prospective study, we comprehensively compared LA function and stiffness between HFpEF subjects with and without T2DM. We demonstrated that LA reservoir function (measured by LAS-peak) and LA pump function (measured by LAS-active) were significantly impaired in the HFpEF patients with T2DM compared with those without T2DM, even after adjustment for potential confounders. In addition, LA stiffness was higher in HFpEF patients with T2DM than of those without T2DM. More important, LAS-peak was a powerful predictor of heart failure hospitalization or death, independently of other clinical and echocardiographic parameters. In contrast, other LA function and stiffness parameters did not independently predict the risk of adverse events.
Although the LA dysfunction in HFpEF has been demonstrated in previous studies (15–21, 24), the role of all three phases of LA function in HFpEF patients with T2DM is less well-established. To the best of our knowledge, there is only one report on LA function in HFpEF patients with and without T2DM from a single center and small sample study (12). However, in this publication from Ljubica et al., LA function was incompletely assessed (only LA reservoir and pump function were evaluated by strain), and potential confounders were not adjusted. Moreover, they did not investigate the prognostic value of the LA function and stiffness in HFpEF patients with or without diabetes mellitus. In this study, we comprehensively assessed LA function using both volumetric measures (LAEF-total, LAEF-active, and LAEF-passive) and strain-based measures (LAS-peak, LAS-passive, and LAS-active) based on 2D-STE. After adjusting for potential confounders, we found that LAS-peak and LAS-active remained reduced in HFpEF patients with T2DM compared with subjects without T2DM. There were several hypotheses that showed mechanisms of T2DM on LA structure and function (36, 37). First, sustained hyperglycemia induces interstitial fibrosis not only in the LV but also in LA. Second, hyperglycemia is related to enhanced pro-fibrotic signaling molecules that provoke collagen synthesis by cardiac fibroblasts implying that these factors can promote atrial fibrosis in DM. In addition, given that several recent studies suggested that worse LV longitudinal systolic function (38, 39), elevated LV filling pressures (40, 41), and LA size (42) may contribute to LA dysfunction. To avoid the influence of these confounding factors, we adjusted them. In addition, LA stiffness, which is related to LA reservoir function and LV filling pressure, increases with LA remodeling and is recognized as a further indicator of LA performance (27). Nonetheless, LA stiffness in HFpEF patients with and without T2DM has not been described. In our study, we also compared the LA stiffness between the two groups. We found that the LA stiffness was consistently increased in HFpEF subjects with T2DM, both before and after adjustment. Therefore, we thought that T2DM may negatively affect the LA function and stiffness.
The LA reservoir function is a predictor of poor outcomes in various cardiovascular diseases, particularly in heart failure with reduced ejection fraction and stable coronary heart disease (43–46). However, there is less evidence on the prognostic relevance of LA function in HFpEF. Moreover, data on the prognostic value regarding LA function in HFpEF are controversial. Freed et al. assessed LA function measured by strain in HFpEF patients who were followed for a median of 13.8 months, and 115 experienced composite events of hospitalization or death. They revealed that LA reservoir strain and conduit strain remained prognostic after adjustment for atrial fibrillation, LA volume, LV mass, and the MAGGIC risk score (25). In contrast, Santos et al. evaluated LA function measured by volume and strain in symptomatic HFpEF patients who were followed for a median of 31 months (91 composite events). They showed that LA reservoir function was not an independent predictor of mortality in HFpEF (26). In our study, we found that decreased LAS-peak was significantly associated with the risk of composite all-cause mortality or heart failure-related hospitalization, even after adjusting for demographic and clinical characteristics, LV global longitudinal strain, and E/e’. This result is partially consistent with the findings of Santos et al. In addition, T2DM is an independent risk factor for cardiovascular disease and its associated mortality (6–8), and interest in the assessment of risk stratification for HFpEF patients with T2DM has remained strong. In this study, Kaplan-Meier analysis showed that HFpEF patients with T2DM and low LAS-peak (<27.2%) had a significantly increased risk of poor outcomes (log-rank P < 0.001). Therefore, LAS-peak may be essential for risk stratification in HFpEF patients with and without T2DM.
The LA stiffness has been recognized as a novel method that estimates LA diastolic function. Recently, Khurram et al. enrolled 219 patients with AF referred for ablation. After a median follow-up of 10 months, 40 patients had recurrence after AF ablation. Patients with recurrence had higher LA stiffness than those without recurrence. Therefore, Khurram et al. reported that LA stiffness is a strong independent predictor of recurrence after ablation (28). Furthermore, Ibadete et al. revealed that LA stiffness is associated with poor clinical outcomes in patients with heart failure and reduced ejection fraction (29, 30). In our study, LA stiffness was a significant predictor of clinical events in univariate analysis, However, LA stiffness was not a significant predictor of adverse events after adjusting BMI, diabetes, LV global longitudinal strain, and LA volume index. The predictive value of LA stiffness still needed multicenter and large sample data to confirm.
Certain limitations should also be considered. First, this study covered a relatively limited number of patients in a single-center study. Our findings require to be tested in future multicenter studies with larger patient populations. Furthermore, the relatively small number of patients and clinical events limited the power of our ability to adjust for confounders in the Cox model for the combined endpoint. Second, we did not have access to data on the severity or duration of diabetes. Third, subjects with atrial fibrillation and poor echocardiographic image quality were excluded from the analysis. Therefore, there was a risk of selection bias. However, we were able to perform speckle-tracking analysis on the majority of the study participants, and reproducibility was excellent.
Our study comprehensively evaluates the LA function and stiffness and investigates their prognostic significance in HFpEF patients with and without T2DM. We demonstrate that strain-based LA reservoir and pump function are impaired, whereas LA stiffness is increased in HFpEF patients with T2DM compared with those without T2DM. More important, LAS-peak is a powerful predictor of heart failure hospitalization or death, independently of other clinical and echocardiographic parameters. Therefore, a comprehensive assessment of LA function using 2D STE may be essential for risk stratification in HFpEF patients with and without T2DM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and was performed in accordance with the Declaration of Helsinki (Ethics No. 0650-01). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SZ, YiL, YZ, MX, YuL, and LZ conceived the idea and designed this study and drafted the manuscript. SZ, YiL, YZ, MQ, and LG were responsible for the analysis and interpretation of data. SZ, YiL, YZ, GW, MQ, LG, MJ, MX, YuL, and LZ revised the manuscript critically for important intellectual content. SZ, YiL, YZ, GW, MX, YuL, and LZ gave the final approval of the manuscript submitted. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81922033 and 82151316), the Key Research and Development Program of Hubei (2021BCA138, 2021CFA046, and 2020DCD015), and the Fundamental Research Funds for the Central Universities, HUST (2022JYCXJJ044).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.947639/full#supplementary-material
1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9.
2. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. (2013) 128:1085–93. doi: 10.1161/CIRCULATIONAHA.113.001475
3. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. (2014) 64:2281–93.
4. Kristensen SL, Jhund PS, Lee MMY, Køber L, Solomon SD, Granger CB, et al. Prevalence of prediabetes and undiagnosed diabetes in patients with HFpEF and HFrEF and associated clinical outcomes. Cardiovasc Drugs Ther. (2017) 31:545–9. doi: 10.1007/s10557-017-6754-x
5. Yap J, Tay WT, Teng TK, Anand I, Richards AM, Ling LH, et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. (2019) 8:e013114.
6. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. (2010) 55:300–5. doi: 10.1016/j.jacc.2009.12.003
7. Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. (2019) 123:611–7.
8. Tribouilloy C, Rusinaru D, Mahjoub H, Tartière JM, Kesri-Tartière L, Godard S, et al. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction: a prospective five-year study. Heart. (2008) 94:1450–5. doi: 10.1136/hrt.2007.128769
9. Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S, et al. Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the I-preserve trial (Irbesartan in heart failure with preserved ejection fraction). Circulation. (2017) 135:724–35. doi: 10.1161/CIRCULATIONAHA.116.024593
10. Lindman BR, Dávila-Román VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. (2014) 64:541–9.
11. Atas H, Kepez A, Atas DB, Kanar BG, Dervisova R, Kivrak T, et al. Effects of diabetes mellitus on left atrial volume and functions in normotensive patients without symptomatic cardiovascular disease. J Diabetes Complicat. (2014) 28:858–62.
12. Georgievska-Ismail L, Zafirovska P, Hristovski Z. Evaluation of the role of left atrial strain using two-dimensional speckle tracking echocardiography in patients with diabetes mellitus and heart failure with preserved left ventricular ejection fraction. Diab Vasc Dis Res. (2016) 13:384–94. doi: 10.1177/1479164116655558
13. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. (2011) 124:2491–501.
14. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. (2014) 7:740–51.
15. Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. (2014) 16:1096–103.
16. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. (2015) 8:295–303.
17. Sanchis L, Gabrielli L, Andrea R, Falces C, Duchateau N, Perez-Villa F, et al. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. (2015) 16:62–7.
18. Aung SM, Güler A, Güler Y, Huraibat A, Karabay CY, Akdemir I. Left atrial strain in heart failure with preserved ejection fraction. Herz. (2017) 42:194–9.
19. Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart. (2012) 98:1311–7.
20. Freed BH, Shah SJ. Stepping out of the left ventricle’s shadow: time to focus on the left atrium in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. (2017) 10:e00626. doi: 10.1161/CIRCIMAGING.117.006267
21. von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, et al. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. (2017) 10:e00546.
22. Zhang Q, Yip GW, Yu CM. Approaching regional left atrial function by tissue Doppler velocity and strain imaging. Europace. (2008) 10(Suppl 3):iii62–9.
23. Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. (2009) 7:6.
24. Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, et al. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. (2020) 22:472–85.
25. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. (2016) 9:e003754. doi: 10.1161/CIRCIMAGING.115.003754
26. Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. (2016) 9:e002763.
27. Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. (2016) 21:65–76. doi: 10.1007/s10741-015-9520-9
28. Khurram IM, Maqbool F, Berger RD, Marine JE, Spragg DD, Ashikaga H, et al. Association between left atrial stiffness index and atrial fibrillation recurrence in patients undergoing left atrial ablation. Circ Arrhythm Electrophysiol. (2016) 9:e003163. doi: 10.1161/CIRCEP.115.003163
29. Bytyçi I, D’Agostino A, Bajraktari G, Lindqvist P, Dini FL, Henein MY. Left atrial stiffness predicts cardiac events in patients with heart failure and reduced ejection fraction: the impact of diabetes. Circ Arrhythm Electrophysiol. (2016) 9:e003163. doi: 10.1111/cpf.12688
30. Bytyçi I, Dini FL, Bajraktari A, Pugliese NR, D’Agostino A, Bajraktari G, et al. Speckle tracking-derived left atrial stiffness predicts clinical outcome in heart failure patients with reduced to mid-range ejection fraction. J Clin Med. (2020) 9:1244. doi: 10.3390/jcm9051244
31. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975.
32. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
33. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14.
34. Morris DA, Takeuchi M, Krisper M, Köhncke C, Bekfani T, Carstensen T, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. (2015) 16:364–72. doi: 10.1093/ehjci/jeu219
35. Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. (2009) 2:10–5.
36. Kassan M, Choi SK, Galán M, Bishop A, Umezawa K, Trebak M, et al. Enhanced NF-κB activity impairs vascular function through PARP- 1-, SP- 1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes. (2013) 62:2078–87. doi: 10.2337/db12-1374
37. Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Front Physiol. (2019) 10:135. doi: 10.3389/fphys.2019.00135
38. Ersbøll M, Andersen MJ, Valeur N, Mogensen UM, Waziri H, Møller JE, et al. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging. (2013) 6:26–33. doi: 10.1161/CIRCIMAGING.112.978296
39. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. (2012) 98:813–20. doi: 10.1136/heartjnl-2011-301388
40. D’Andrea A, De Corato G, Scarafile R, Romano S, Reigler L, Mita C, et al. Left atrial myocardial function in either physiological or pathological left ventricular hypertrophy: a two-dimensional speckle strain study. Br J Sports Med. (2008) 42:696–702.
41. Guan Z, Zhang D, Huang R, Zhang F, Wang Q, Guo S. Association of left atrial myocardial function with left ventricular diastolic dysfunction in subjects with preserved systolic function: a strain rate imaging study. Clin Cardiol. (2010) 33:643–9.
42. Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L, et al. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. (2018) 19:630–8. doi: 10.1093/ehjci/jey018
43. Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. (2013) 34:278–85.
44. Motoki H, Borowski AG, Shrestha K, Troughton RW, Martin MG, Tang WH, et al. Impact of left ventricular diastolic function on left atrial mechanics in systolic heart failure. Am J Cardiol. (2013) 112:821–6.
45. Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. (2012) 110:264–9.
46. Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. (2012) 59:673–80. doi: 10.1016/j.jacc.2011.11.012
Keywords: heart failure with preserved ejection fraction, type 2 diabetes mellitus, left atrial function, stiffness, prognosis
Citation: Zhu S, Lin Y, Zhang Y, Wang G, Qian M, Gao L, Ji M, Xie M, Li Y and Zhang L (2022) Prognostic relevance of left atrial function and stiffness in heart failure with preserved ejection fraction patients with and without diabetes mellitus. Front. Cardiovasc. Med. 9:947639. doi: 10.3389/fcvm.2022.947639
Received: 19 May 2022; Accepted: 03 August 2022;
Published: 14 September 2022.
Edited by:
Verena Stangl, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Concetta Zito, University of Messina, ItalyCopyright © 2022 Zhu, Lin, Zhang, Wang, Qian, Gao, Ji, Xie, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxing Xie, eGllbXhAaHVzdC5lZHUuY24=; Yuman Li, bGl5bUBodXN0LmVkdS5jbg==; Li Zhang, emxpNDI5QGh1c3QuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.