
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 25 October 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.947197
Background: Transcatheter aortic valve implantation (TAVI) is the preferred treatment option for severe aortic stenosis in the elderly and in patients with comorbidities. We sought to compare outcomes after TAVI and surgical aortic valve replacement (SAVR) in octogenarians.
Methods: In this retrospective cohort study conducted at our tertiary center, clinical data were gathered before and after TAVI and SAVR procedures performed from January 2013 to May 2019; follow-up completed in March 2021. The primary outcome was 1-year mortality. Patients were stratified according to Society of Thoracic Surgeons (STS) score and procedure type. Propensity score-based matching was also performed.
Results: Of 542 patients who matched the inclusion criteria, 273 underwent TAVI and 269 SAVR. TAVI patients were older (85.8 ± 3.0 vs. 82.2 ± 2.2 years; P < 0.001) and had a higher mean STS score (5.0 ± 4.0 vs. 2.8 ± 1.3; P < 0.001) and EuroSCORE II (5.3 ± 4.1 vs. 2.8 ± 6.0; P < 0.001). Rates of postoperative permanent pacemaker insertion (15.0% vs. 9.3%; P = 0.040) and paravalvular leak (9.9% vs. 0.8%; P < 0.001) were higher and acute kidney injury lower (8.8% vs. 32.7%; P < 0.001) after TAVI, with no difference between treatment groups for major bleeding (11.0% vs. 6.7%; P = 0.130) or 30-day mortality (5.5% vs. 3.7%; P = 0.315). A statistically significant difference was found between TAVI and SAVR in low- and intermediate-risk groups when it came to occurrence of paravalvular leak, acute kidney injury, and new onset AF (all P < 0.001).
Conclusion: This analysis of an octogenarian “real-life” population undergoing TAVI or SAVR (with a biological valve) showed similar outcomes regarding clinical endpoints in low- and medium-risk (STS score) groups.
Aortic stenosis (AS) is a common, progressive valvular lesion with a poor prognosis when left untreated (1). Octogenarian patients with AS have a high prevalence of coexisting conditions and may be at increased risk of periprocedural morbidity and mortality when undergoing surgery (2).
Surgical aortic valve replacement (SAVR), first performed in the 1960s, became the gold standard for treating AS (3), but surgeons were initially reluctant to operate on older patients (2). Minimally invasive SAVR, a modification of the original technique, was developed with the purpose of minimizing operative trauma and reducing postoperative mortality and morbidity (4). Balloon valvotomy was the first procedure to emerge as a possible endovascular treatment (3). Aspiration, an even less-invasive method—suitable for patients with little or no chance of surviving surgery—led to implantation of the first transcatheter aortic valve implantation (TAVI), in 2002. Since then, TAVI for AS has been increasingly adopted in clinical practice (5).
With well-established surgical methods it was not easy to prove the non-inferiority of TAVI (6). However, with meticulously designed studies conducted over the past decade, advocates of TAVI not only demonstrated its non-inferiority in high-risk patients, they proved its benefits, and expanded its indication for use in intermediate- and low-risk patients (7–11).
Advances in treatment techniques have led to different options to consider for our patients. The first mention of TAVI was in the 2008 guidelines on the treatment of AS. More recently, evidence from clinical trials has now positioned TAVI as a suitable treatment option for AS (12–14).
The objective of this study was to compare clinical outcomes among octogenarian patients undergoing TAVI or SAVR treated at our facility during the past decade.
In this retrospective cohort study, data from two local registries of patients who had undergone TAVI or SAVR for severe AS at the University Clinical Centre in Ljubljana between January 2013 and May 2019 were gathered. During that period, 3,384 patients had undergone surgical treatment and 513 had undergone TAVI. Our search was restricted to patients older than 80 years at the time of the procedure who underwent isolated treatment of AS. The surgical group included patients who had a biological valve implanted. Additional exclusion criteria were active infective endocarditis, reoperation, and valve-in-valve TAVI. Follow-up data were retrieved by reviewing hospital records and national registries.
The National Ethical Committee approved the study design. Informed consent was waived due to the retrospective nature of the study.
In the TAVI group, the Sapien XT (Edwards Lifesciences, Irvine, CA), Sapien 3 (Edwards Lifesciences), Evolut R (Medtronic, Minneapolis, MN, USA), and Portico (St Jude Medical, Austin, TX) valves were used. The decision on the type of procedure was made by the interdisciplinary heart team. In the SAVR group, the choice of procedure and type of valve were at the surgeon’s discretion. All patients received sutureless or stented biological valves [Trifecta (Abbott, St. Paul, Minnesota, USA), Magna (Edwards Lifesciences), Mitroflow (Sorin Group, Inc., Milan, Italy), Epic (Abbott), Freedom solo (Sorin Group, Inc.), Enable (ATS Medical, Minneapolis, MN), Intuity (Edwards Lifesciences), Perceval (Sorin Group, Inc.), Crown (Sorin Group, Inc.)].
All patients were preoperatively assessed and evaluated with using validated scoring systems, keeping in mind patient frailty and comorbid conditions. High-risk patients were initially defined as those with a logistic EuroSCORE ≥ 20 or a EuroSCORE II ≥ 7, and later as a Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score ≥ 8.0, or using other criteria not included in the scoring systems (patient frailty, porcelain aorta, movement impairment, patient request etc.). At first only patients declined for SAVR were selected for TAVI. From 2017, with the revision of guidelines, the criteria were adjusted according to the new findings, and the multidisciplinary heart team (interventional cardiologist, cardiac surgeon, cardiologist) was involved in decision making process for each patient with severe aortic stenosis requiring a form of treatment.
The primary outcome was 1-year mortality. Secondary outcomes were post-procedural acute kidney injury, new onset atrial fibrillation (AF) (≤30 days), permanent pacemaker implantation (PPI), cerebrovascular stroke or transient ischemic attack (TIA), paravalvular leak, major or life-threatening bleeding, 30-day mortality, in-hospital mortality, and length of hospital stay. All outcomes were defined according to the Valve Academic Research Consortium-2 criteria (15).
Categorical variables are presented as frequencies with percentages, and continuous variables as mean values with standard deviations (SD). Patient death was evaluated as the dependent variable. Normally distributed quantitative variables were analyzed using the one-way ANOVA test, and abnormally distributed variables using the non-parametric Kruskal-Wallis test. Qualitative data were compared using Pearson’s χ2-test, where the statistical difference between the independent variables and the variable was determined. Cox regression and Kaplan-Meier survival curves were used to assess the probability for death. A two-sided P-value less than 0.05 was considered to indicate statistical significance. Nearest neighbor propensity score matching (PSM) with ratio of 1:1 and caliper width set at 0.2 of the standard deviation of logit of propensity score was performed using the MatchIt package in R (Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). Balance between the two groups was maintained by keeping standardized mean difference less than 0.15 and variance ratios between 0.5 and 2. All analyses were performed using SPSS 21 software (IBM, New York, USA).
The study population included detailed information on 542 patients: 273 (50.4%) underwent TAVI and 269 (49.6%) SAVR (Figure 1). Follow-up was completed in March 2021. Patients in the TAVI group were older than those in the SAVR group and had a higher mean EuroSCORE II and STS score (all P < 0.001) (Table 1).
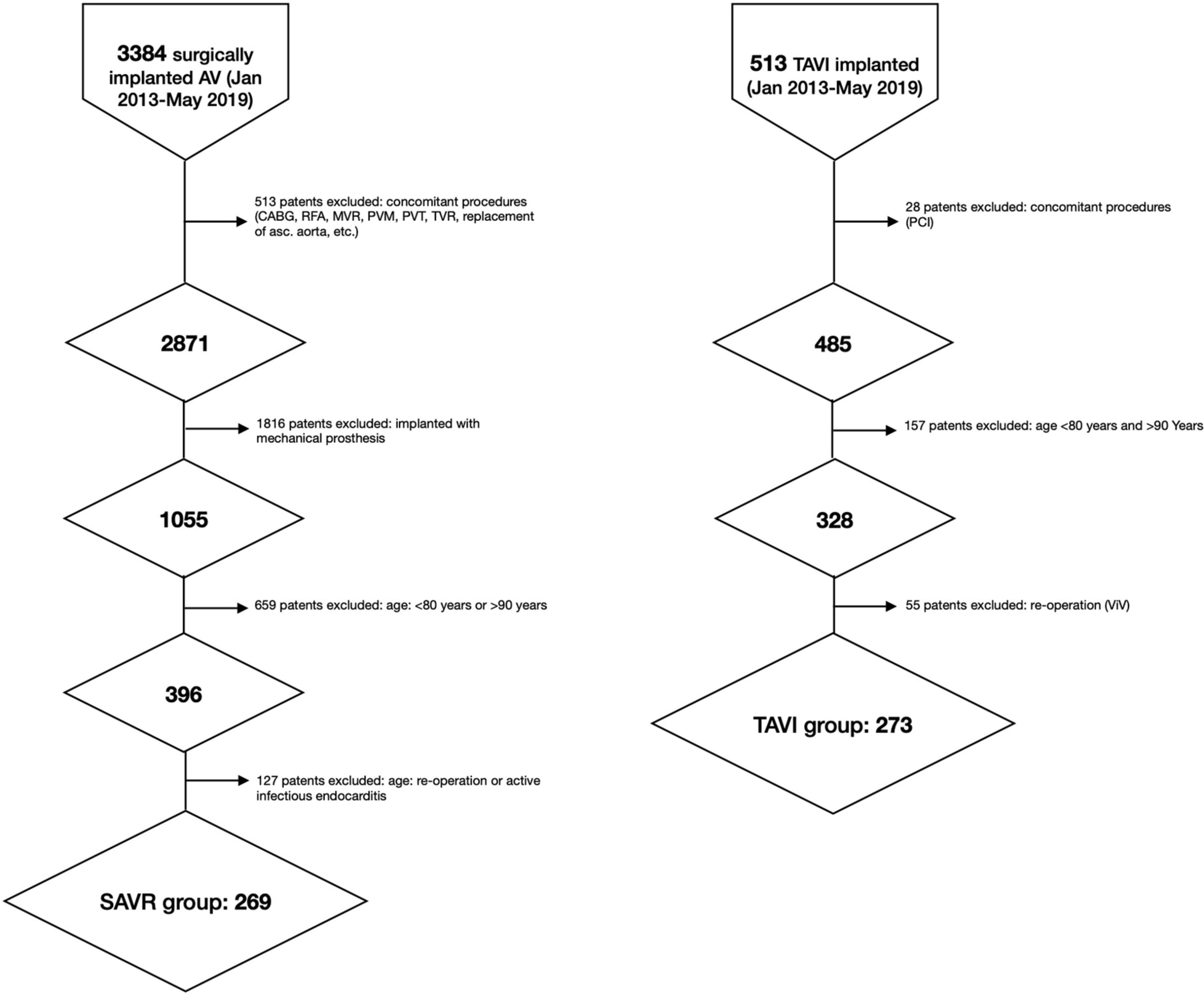
Figure 1. Flow-chart. asc, ascending; AV, aortic valve; CABG, coronary artery bypass graft; RFA, radiofrequency ablation; MVR, mitral valve replacement; PCI, percutaneous coronary intervention; PVT, plasty of tricuspid valve, PVM, plasty of mitral valve; TVR, tricuspid valve replacement; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Operative characteristics are summarized in Table 2. In the TAVI group, 87.9% of patients had transfemoral TAVI, 6.2% had transapical TAVI, and 5.9% had transaortic TAVI. Of the patients undergoing SAVR, 76.6% had minimally invasive SAVR and 23.4% underwent full sternotomy.
After performing propensity score analysis for our population, demographic, and clinical characteristics became well balanced between 170 matched patients. The matched population clinical and procedural characteristics are shown in Tables 1, 2.
Clinical outcomes according to TAVI or SAVR are detailed in Table 3. Mean follow-up was 45.2 ± 25.6 months; 3 patients (0.5%) were lost to survival follow-up.
TAVI was associated with a lower rate of new-onset AF and acute kidney injury (both P < 0.001). More than 40% of patients had acute kidney injury after transapical TAVI compared with only 6.7% among those who had transfemoral TAVI. In the SAVR group, patients with an aortic cross-clamp time exceeding 50 min had a 50% higher occurrence of acute kidney injury.
The rate of PPI (P = 0.040) and the number of patients with mild-to-severe paravalvular leak (P < 0.001) were higher in the TAVI group. Cerebrovascular stroke or TIA was reported in 2 patients (0.7%) in the TAVI group and in 8 patients (3.0%) in the SAVR group (P = 0.053). Major bleeding occurred in 11.0% of patients following TAVI and in 6.7% after SAVR, but the difference was not statistically significant (P = 0.130). There were no differences between TAVI and SAVR groups for length of hospital stay, in-hospital mortality, 30-day mortality, or 1-year mortality.
Propensity score analysis for our population showed similar clinical outcomes with the exception of length of hospital stay (P = 0.032), in hospital mortality (P = 0.016) and 30-day mortality (P = 0.009), all in favor of TAVI group (Table 3).
The results of a Cox regression analysis predicting mortality at 30 days and 1 year are shown in Table 4. Recent myocardial infarction (within 1 month before the procedure) and STS-PROM score were identified as significant predictors of death at 30 days in both groups. In the TAVI group, STS-PROM score, recent myocardial infarction, and creatine concentration were predictive of death at 1 year. In the SAVR group, only recent myocardial infarction was a predictor of death at 1 year.
Stratification of postoperative results by STS score and procedure (TAVI vs. SAVR: low risk: 48.3% vs. 85.5%; intermediate risk: 39.6% vs. 13.4%; high risk: 12.1% vs. 0.7%) is detailed in Table 5. When comparing groups, a statistically significant difference was found between TAVI and SAVR in low- and intermediate-risk groups when it came to occurrence of paravalvular leak, acute kidney injury, and new onset AF (all P < 0.001). Kaplan-Meier survival curves showed no differences between treatment groups (30 days: 3.7% vs. 5.5%, P = 0.315; 1 year: 6.7% vs. 10.2%, P = 0.126; respectively) (Figure 2).
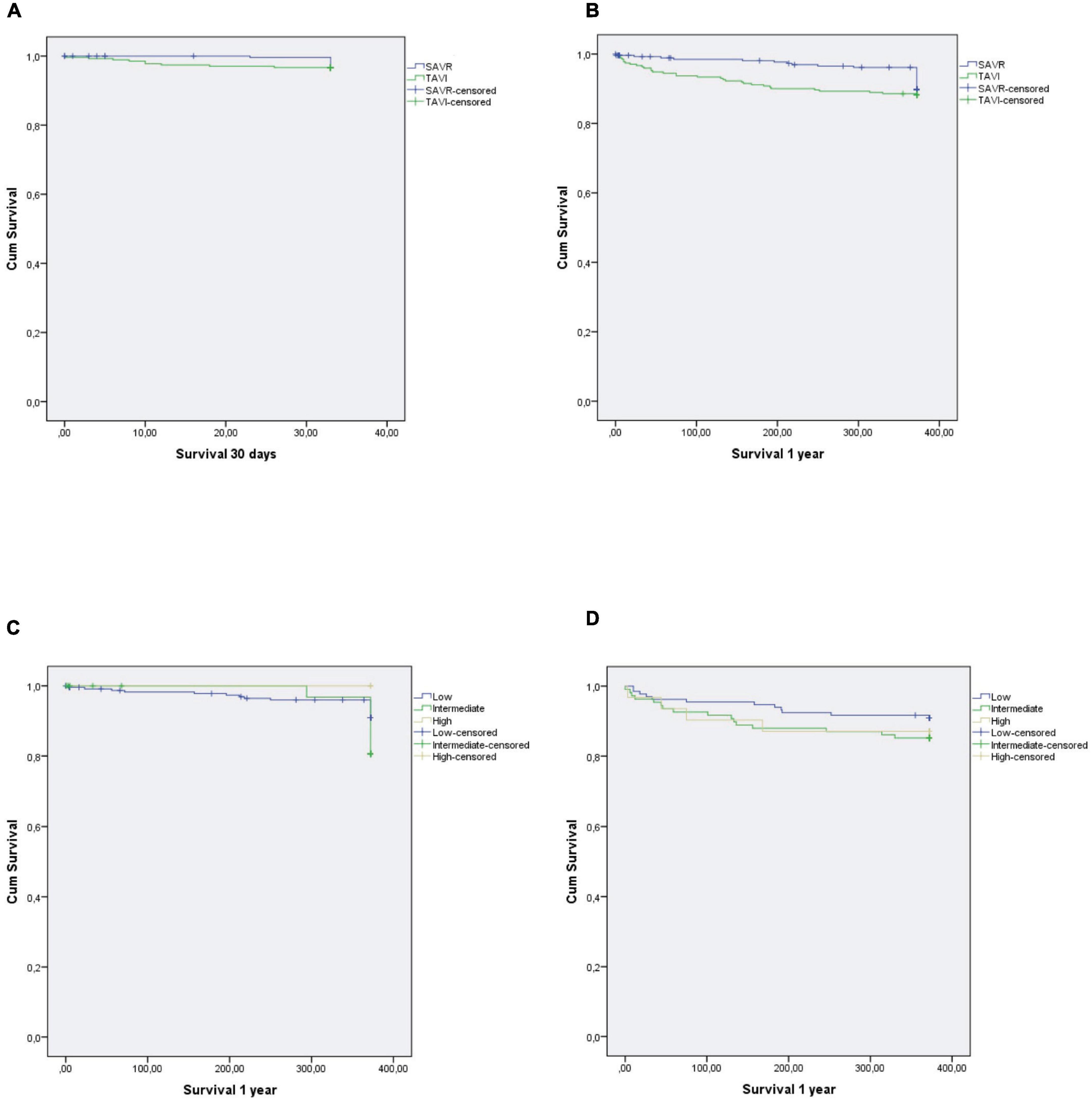
Figure 2. Kaplan-Meier survival curves according to TAVI or SAVR: (A) SAVR vs. TAVI—30-day survival, (B) SAVR vs. TAVI—1-year survival, (C) SAVR—1-year survival stratified by STS score, (D) TAVI—1-year survival stratified by STS score; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
The results of this single-center, retrospective cohort study showed that the type of procedural approach for AS—whether TAVI or SAVR—did not affect in-hospital, 30-day, or 1-year mortality. Therefore, the anticipated advantages of using a less invasive technique did not appear to influence early or mid-term outcome. Rates of PPI and paravalvular leak were more frequent in the TAVI group, whereas new onset AF and acute kidney injury were more common after SAVR. After stratification by STS score, there were no differences in survival between groups, whereas a statistically significant difference was found in low-risk and intermediate-risk groups in terms of moderate or severe paravalvular leak, in favor of SAVR, and in acute kidney injury and new-onset AF, in favor of TAVI.
With the development of TAVI over the past two decades and its introduction into routine clinical practice, it was inevitable to ask whether TAVI is the best treatment for all octogenarians (16, 17). The results of the Placement of Aortic Transcatheter Valves (PARTNER)-1 trial—the first prospective randomized study comparing TAVI, best medical therapy, and surgical therapy in high-risk patients—were published in 2011. Rates of all-cause death at 1-year were similar in the TAVI and SAVR groups, and were superior to best medical therapy (7). PARTNER 2A and Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI), both of which were designed to compare outcomes in intermediate-risk patients, also displayed non-significant differences between TAVI and SAVR in the 1-year non-hierarchical composite of all-cause death or disabling stroke (8, 9). Recent trials focusing on low-risk patients—PARTNER 3 and Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients (EVOLUT LRT)—opened new doors for TAVI and established the role of this approach in all risk groups. However, caution is needed when applying the findings from RCTs, which are subject to strict enrollment criteria, to the spectrum of patients with AS treated in routine clinical practice (18).
Our primary outcome, 1-year survival, was not influenced by the type of procedure. Recent studies focusing solely on octogenarians are scarce and show similar outcomes for death (17). Other reports use different composite outcomes, making direct comparisons difficult (19–22). A meta-analysis by Witberg et al. (23) suggested a trend toward reduced rates of 1-year death with TAVI in RCTs in low-risk populations, whereas observational studies with PSM in the same meta-analysis showed a trend toward increased rates of death with TAVI. In our study, there was no statistically significant difference in hospital or 30-day mortality in the entire cohort or in the low-risk or intermediate-risk groups between TAVI and SAVR. Comparisons in the high-risk group were not possible because of the small number of patients who underwent SAVR. After performing PSM in our population there was a statistically significant difference between the groups in terms of hospital stay, in hospital and 30-day mortality. Mollmann et al. (24) reported lower in-hospital mortality with TAVI in an all-comer AS population treated in Germany. However, a systematic review and meta-analysis by Moss et al. (17) in octogenarians showed no differences in 30-day mortality between TAVI and SAVR.
The rate of PPI in the present study was higher in the TAVI group, in which 15.0% of patients developed postprocedural conduction disturbances. The 9.3% of patients requiring PPI after SAVR is similar to the rate reported in other studies in octogenarians (17, 25) as well as in major TAVI studies (7–11). Interestingly, even though more than half of our patients in the surgical arm had a sutureless self-expanding (Perceval or Enable) or balloon expandable (Intuity) valve implanted, the incidence of PPI was low (26–28). Data regarding the effect of new PPI after TAVI on mortality and morbidity are inconclusive (29). When expanding the indication for TAVI to the younger population, it is important to consider that PPI-related complications are more frequent in younger patients (30).
Postoperative moderate-to-severe paravalvular leak was present in approximately 10% of TAVI patients and in only 0.8% of SAVR patients. Compared to previous RCTs (7–10) our TAVI population presented with relatively high incidence of postoperative moderate-severe paravalvular leak. This could in part be attributed to the fact that we only had access to early echocardiographic findings, with the ultrasound of the heart performed during hospitalization or before the discharge. At least a 30 day follow-up ultrasound would be necessary to properly evaluate our cohort. The PARTNER trial showed that increased severity of paravalvular leak is associated with higher 2-year mortality (31). Hagar et al. (32), however, found no association between paravalvular leak and 1-year mortality in a slightly younger population (mean age 74 years).
Neurological complications in our study were in line with the PARTNER-2 and SURTAVI results for the SAVR group (3% for cerebrovascular stroke or TIA), whereas results in the TAVI group were similar to those of the PARTNER 3 and EVOLUT LRT results, with a much lower incidence (0.7% of cerebrovascular stroke or TIA) despite a higher incidence of patient-related risk factors (33). It is difficult to explain this difference between TAVI and SAVR groups. We could attribute the incidence of neurological complications to unmeasured patient and periprocedural characteristics or operator skill and experience.
New onset AF (within 30 days of the procedure) was significantly more frequent in the SAVR group and is similar to the rate reported in RCTs (7–11) and retrospective studies (34–36). It is important to consider that almost half of the patients in the TAVI group had AF preoperatively. A number of studies evaluated possible risk factors and preventive measures. Older age and moderate-to-severe left atrial enlargement were the only two consistent independent factors of prolonged postoperative AF (34). Mathew et al. (37) showed that each consecutive decade was associated with a 75% increase in the risk of developing postoperative AF. Postoperative pericardial effusion is frequently observed after surgery, and experimental studies indicate that even a small amount of effusion may trigger AF due to mechanical compression of the atria, local inflammation, and oxidative stress (38).
Acute kidney injury was present in 32.7% of our SAVR patients. In studies conducted before the use of common diagnostic criteria [RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) or AKIN (Acute Kidney Injury Network)], the reported incidence of acute kidney injury was lower than in our study (39, 40), but later studies showed similar findings, with acute kidney injury present in approximately one-third of patients after SAVR (41–44). An important finding was that even small changes in serum creatinine concentration are associated with adverse outcomes (45). In SAVR, the specific reason is cardiopulmonary bypass that causes inflammation and hemodilution and is accompanied by periods of low pressure and flow rates. The duration of cardiopulmonary bypass and aortic cross-clamp time were linked to the development of postoperative acute kidney injury (46). In our study, the average aortic cross clamp time was 47.0 ± 17.7 min, with a 50% higher occurrence of acute kidney injury in the SAVR group with an aortic cross-clamp time exceeding 50 min. Contrast exposure, embolization, hemodynamic instability, and access route play important roles in acute kidney injury after TAVI. Transapical access, compared with transfemoral access, was shown to be an independent predictor of acute kidney injury following TAVI (45), which was also seen in our study.
Major or life-threatening bleeding occurred in 11.0% of TAVI patients and in 6.7% of surgical patients, but the difference was not statistically significant. Octogenarians are a more vulnerable population because of their advanced age, comorbidities and frailty, independent of the STS score (47, 48), and a higher rate of bleeding complications was expected. The low rate of bleeding in SAVR group could be a consequence of using minimally invasive techniques (76.6% of patients underwent minimally invasive SAVR), and the high rate of bleeding complications in the TAVI group could be due to the large size of the delivery systems used.
Stratification by STS score into three groups showed that quite a sizeable number of patients in TAVI group were low risk. With rapid expansion of TAVI volumes, the risk profile of patients treated in everyday practice has been lower than those included in RCTs, which are the basis for practice guidelines (23). Our results showed no statistically significant differences between specific risk groups (TAVI vs. SAVR) in 30-day or 1-year mortality. Further, the stratification showed the expected significant difference between TAVI and SAVR in low- and intermediate-risk groups in occurrence of acute kidney injury, paravalvular leak, and new onset AF. Analysis of the high-risk group was not possible because it included only 2 SAVR patients.
The promising results from TAVI trials have fundamentally changed the way we treat octogenarians. It is important to take into consideration that not all patients, due to their advanced age, are automatically high risk (49). STS-PROM and STS/American College of Cardiology in-hospital mortality scores are superior to EuroSCORE I, EuroSCORE II, and the German AV Score (50). Despite more than 10 years of clinical experience with TAVI, a reliable risk score model that includes frailty is not yet available. Certain octogenarians with a low STS score may benefit more from a surgical procedure and avoid the possibility of paravalvular leak, which affects overall survival. A reliable risk score would help us recognize such patients and tailor their treatment individually. Treatment options should remain open, and regular revision of “real-life” results should ensure that we can offer patients the best treatment option. Detailed explanation of possible complications and balanced information regarding outcome after each procedure should be given to obtain informed patient consent prior to the choice of procedure.
This single-center study was observational, non-randomized, and retrospective. Some perioperative variables were not recorded, which may explain the differences between groups. Patients in the SAVR group did not routinely have a computed tomography scan before the procedure. The two techniques are not directly comparable, because the decision-making may have taken into consideration variables not included in the scoring system and in our database.
This retrospective study involving an octogenarian “real-life” population treated for isolated severe AS provides insights into our decision-making and shows that similar short- and mid-term results were obtained with both TAVI and SAVR in low- and intermediate-risk (STS score) groups. We showed that the type of procedural approach did not significantly affect 1-year mortality. The expected advantage of a less invasive technique did not influence early outcome, with similar rates of in-hospital mortality and length of hospital stay in both groups. PPI and paravalvular leak were more frequent in the TAVI group, whereas new onset AF and acute kidney injury were more common in the SAVR group. In light of our findings, the treatment strategy in each institution should be adopted according to its own outcome data and facilities.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical committee of Republic of Slovenia. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
TK and MB: conception and design of the study. TK and AK: data collection. TK, NL, AK, DŠ, ZF, and MB: analysis and interpretation. TK, NL, DŠ, ZF, and MB: drafting and critical review of the article. All authors have read and approved the final manuscript.
Sophie Rushton-Smith, Ph.D. (MedLink Healthcare Communications) provided editorial assistance and was funded by authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Varadarajan P, Kapoor N, Bansal RC, Pai RG. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged > or = 80 years. Eur J Cardiothorac Surg. (2006) 30:722–7. doi: 10.1016/j.ejcts.2006.07.028
2. Khosravi A, Wendler O. TAVI 2018: from guidelines to practice. J Cardiol Pract. (2018) 15. Available online at: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/TAVI-2018-from-guidelines-to-practice (accessed April 14, 2022).
4. Cosgrove DM III, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg. (1996) 62:596–7.
5. Cribier A. Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis. (2012) 105:146–52. doi: 10.1016/j.acvd.2012.01.005
6. Tamburino C, Valvo R, Crioscione E, Reddavid C, Picci A, Costa G, et al. The path of transcatheter aortic valve implantation: from compassionate to low-risk cases. Eur Heart J Suppl. (2020) 22:L140–5. doi: 10.1093/eurheartj/suaa154
7. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. (2015) 385:2477–84. doi: 10.1016/S0140-6736(15)60308-7
8. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374:1609–20. doi: 10.1056/NEJMoa1514616
9. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376:1321–31. doi: 10.1056/NEJMoa1700456
10. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
11. Thyregod HGH, Ihlemann N, Jorgensen TH, Nissen H, Kjeldsen BJ, Petursson P, et al. Five-year clinical and echocardiographic outcomes from the nordic aortic valve intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation. (2019) 139:2714–23. doi: 10.1161/CIRCULATIONAHA.118.036606
12. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 129:e521–643. doi: 10.1161/CIR.0000000000000031
13. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1093/eurheartj/ehx391
14. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA. 2017 AHA/ACC Focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2017) 135:e1159–95. doi: 10.1161/CIR.0000000000000503
15. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. (2012) 42:S45–60. doi: 10.1093/ejcts/ezs533
16. Hirji SA, Ramirez-Del Val F, Kolkailah AA, Ejiofor JI, McGurk S, Chowdhury R, et al. Outcomes of surgical and transcatheter aortic valve replacement in the octogenarians-surgery still the gold standard? Ann Cardiothorac Surg. (2017) 6:453–62. doi: 10.21037/acs.2017.08.01
17. Moss S, Doyle M, Nagaraja V, Peeceeyen S. A systematic review and meta-analysis of the clinical outcomes of TAVI versus SAVR in the octogenarian population. Indian J Thorac Cardiovasc Surg. (2020) 36:356–64. doi: 10.1007/s12055-019-00912-0
18. Steg PG, Lopez-Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. (2007) 167:68–73. doi: 10.1001/archinte.167.1.68
19. Takeji Y, Taniguchi T, Morimoto T, Saito N, Ando K, Shirai S, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for severe aortic stenosis in real-world clinical practice. Circ J. (2020) 84:806–14. doi: 10.1253/circj.CJ-19-0951
20. Holzhey DM, Shi W, Rastan A, Borger MA, Hänsig M, Mohr FW. Transapical versus conventional aortic valve replacement–a propensity-matched comparison. Heart Surg Forum. (2012) 15:E4–8. doi: 10.1532/HSF98.20111084
21. Muneretto C, Alfieri O, Cesana BM, Bisleri G, De Bonis M, Di Bartolomeo R, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in “real-world” patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg. (2015) 150:1570–7. doi: 10.1016/j.jtcvs.2015.08.052
22. Papadopoulos N, Schiller N, Fichtlscherer S, Lehmann R, Weber CF, Moritz A, et al. Propensity matched analysis of longterm outcomes following transcatheter based aortic valve implantation versus classic aortic valve replacement in patients with previous cardiac surgery. J Cardiothorac Surg. (2014) 9:99. doi: 10.1186/1749-8090-9-99
23. Witberg G, Landes U, Lador A, Yahav D, Kornowski R. Meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients at low surgical risk. EuroIntervention. (2019) 15:e1047–56. doi: 10.4244/EIJ-D-19-00663
24. Mollmann H, Husser O, Blumenstein J, Liebetrau C, Dörr O, Kim WK, et al. Lower mortality in an all-comers aortic stenosis population treated with TAVI in comparison to SAVR. Clin Res Cardiol. (2020) 109:611–5. doi: 10.1007/s00392-019-01548-1
25. Siontis GC, Juni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. (2014) 64:129–40. doi: 10.1016/j.jacc.2014.04.033
26. Aymard T, Kadner A, Walpoth N, Göber V, Englberger L, Stalder M, et al. Clinical experience with the second-generation 3f Enable sutureless aortic valve prosthesis. J Thorac Cardiovasc Surg. (2010) 140:313–6. doi: 10.1016/j.jtcvs.2009.10.041
27. Barnhart GR, Accola KD, Grossi EA, Woo YJ, Mumtaz MA, Sabik JF, et al. TRANSFORM (Multicenter experience with rapid deployment edwards INTUITY valve system for aortic valve replacement) us clinical trial: performance of a rapid deployment aortic valve. J Thorac Cardiovasc Surg. (2017) 153:241–51.e2. doi: 10.1016/j.jtcvs.2016.09.062
28. Powell R, Pelletier MP, Chu MWA, Bouchard D, Melvin KN, Adams C, et al. The perceval sutureless aortic valve: review of outcomes, complications, and future direction. Innovations. (2017) 12:155–73. doi: 10.1097/IMI.0000000000000372
29. Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi N, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2021) 14:115–34. doi: 10.1016/j.jcin.2020.09.063
30. Ozcan KS, Osmonov D, Altay S, Dönmez C, Yıldırım E, Türkkan C, et al. Pacemaker implantation complication rates in elderly and young patients. Clin Interv Aging. (2013) 8:1051–4. doi: 10.2147/CIA.S47121
31. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. (2012) 366:1686–95. doi: 10.1056/NEJMoa1200384
32. Hagar A, Li Y, Wei X, Peng Y, Xu Y, Ou Y, et al. Incidence, predictors, and outcome of paravalvular leak after transcatheter aortic valve implantation. J Interv Cardiol. (2020) 2020:8249497. doi: 10.1155/2020/8249497
33. Aroney N, Patterson T, Allen C, Redwood S, Prendergast B. Neurocognitive status after aortic valve replacement: differences between TAVI and surgery. J Clin Med. (2021) 10:1789. doi: 10.3390/jcm10081789
34. Axtell AL, Moonsamy P, Melnitchouk S, Tolis G, Jassar AS, D’Alessandro DA, et al. Preoperative predictors of new-onset prolonged atrial fibrillation after surgical aortic valve replacement. J Thorac Cardiovasc Surg. (2020) 159:1407–14. doi: 10.1016/j.jtcvs.2019.04.077
35. Paparella D, Santarpino G, Malvindi PG, Moscarelli M, Marchese A, Guida P, et al. Minimally invasive surgical versus transcatheter aortic valve replacement: a multicenter study. Int J Cardiol Heart Vasc. (2019) 23:100362. doi: 10.1016/j.ijcha.2019.100362
36. Parikh K, Dizon J, Biviano A. Revisiting atrial fibrillation in the transcatheter aortic valve replacement era. Interv Cardiol Clin. (2018) 7:459–69. doi: 10.1016/j.iccl.2018.06.001
37. Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. (2004) 291:1720–9. doi: 10.1001/jama.291.14.1720
38. Gaudino M, Sanna T, Ballman KV, Robinson NB, Hameed I, Audisio K, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet. (2021) 398:2075–83. doi: 10.1016/S0140-6736(21)02490-9
39. Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. (2004) 18:442–5. doi: 10.1053/j.jvca.2004.05.021
40. Thakar CV, Liangos O, Yared JP, Nelson DA, Hariachar S, Paganini EP. Predicting acute renal failure after cardiac surgery: validation and re-definition of a risk-stratification algorithm. Hemodial Int. (2003) 7:143–7.
41. D’Onofrio A, Cruz D, Bolgan I, Auriemma S, Cresce GD, Fabbri A, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. (2010) 16(Suppl. 1):S32–6.
42. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. (2009) 119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011
43. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. (2009) 119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913
44. Robert AM, Kramer RS, Dacey LJ, Charlesworth DC, Leavitt BJ, Helm RE, et al. Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. (2010) 90:1939–43. doi: 10.1016/j.athoracsur.2010.08.018
45. Najjar M, Salna M, George I. Acute kidney injury after aortic valve replacement: incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther. (2015) 13:301–16. doi: 10.1586/14779072.2015.1002467
46. Ibrahim KS, Kheirallah KA, Mayyas FA, Alwaqfi NA. Predictors of acute kidney injury following surgical valve replacement. Thorac Cardiovasc Surg. (2021) 69:396–404. doi: 10.1055/s-0040-1710318
47. Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, Brown DL, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. (2015) 116:264–9. doi: 10.1016/j.amjcard.2015.03.061
48. O’Sullivan CJ, Stortecky S, Buellesfeld L, Wenaweser P, Windecker S. Preinterventional screening of the TAVI patient: how to choose the suitable patient and the best procedure. Clin Res Cardiol. (2014) 103:259–74. doi: 10.1007/s00392-014-0676-4
49. Strauch JT, Scherner M, Haldenwang PL, Madershahian N, Pfister R, Kuhn EW, et al. Transapical minimally invasive aortic valve implantation and conventional aortic valve replacement in octogenarians. Thorac Cardiovasc Surg. (2012) 60:335–42. doi: 10.1055/s-0032-1304538
Keywords: TAVI, SAVR, octogenarians, aortic valve stenosis, aortic valve replacement
Citation: Kolar T, Lakič N, Kotnik A, Štubljar D, Fras Z and Bunc M (2022) Similar clinical outcomes with transcatheter aortic valve implantation and surgical aortic valve replacement in octogenarians with aortic stenosis. Front. Cardiovasc. Med. 9:947197. doi: 10.3389/fcvm.2022.947197
Received: 18 May 2022; Accepted: 07 October 2022;
Published: 25 October 2022.
Edited by:
Luca Testa, IRCCS San Donato Polyclinic, ItalyReviewed by:
Shahzad Raja, Harefield Hospital, United KingdomCopyright © 2022 Kolar, Lakič, Kotnik, Štubljar, Fras and Bunc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matjaž Bunc, bWJ1bmNla0B5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.