- 1Department of Medical Biotechnologies, Division of Cardiology, University of Siena, Siena, Italy
- 2Department of Cardiovascular Diseases—Azienda Unitá Sanitaria Locale (AUSL) Romagna, Division of Cardiology, Ospedale Santa Maria delle Croci, Ravenna, Italy
- 3Cardiology Unit and Laboratorio per le Tecnologie delle Terapie Avanzate (LTTA) Centre, University of Ferrara, Ferrara, Italy
- 4Interventional Cardiology, Azienda Ospedaliera Universitaria Senese, Policlinico Le Scotte, Siena, Italy
- 5Department of Cardiac Surgery, University of Siena, Siena, Italy
Objectives: This study aimed to explore the correlation between left ventricular (LV) myocardial work (MW) indices and invasively-derived LV stroke work index (SWI) in a cohort of patients with advanced heart failure (AHF) considered for heart transplantation.
Background: Left ventricular MW has emerged as a promising tool for diagnostic and prognostic purposes in heart failure (HF) but its relationship with hemodynamic data derived from right heart catheterization (RHC) has not been assessed in patients with advanced heart failure yet.
Materials and methods: Consecutive patients with AHF considered for heart transplantation from 2016 to 2021 performing RHC and echocardiography as part of the workup were included. Conventional LV functional parameters and LV MW indices, including LV global work index (GWI), LV global constructive work (GCW), LV global wasted work (GWW), LV global work efficiency (GWE), and other were calculated and compared with invasively-measured LV SWI.
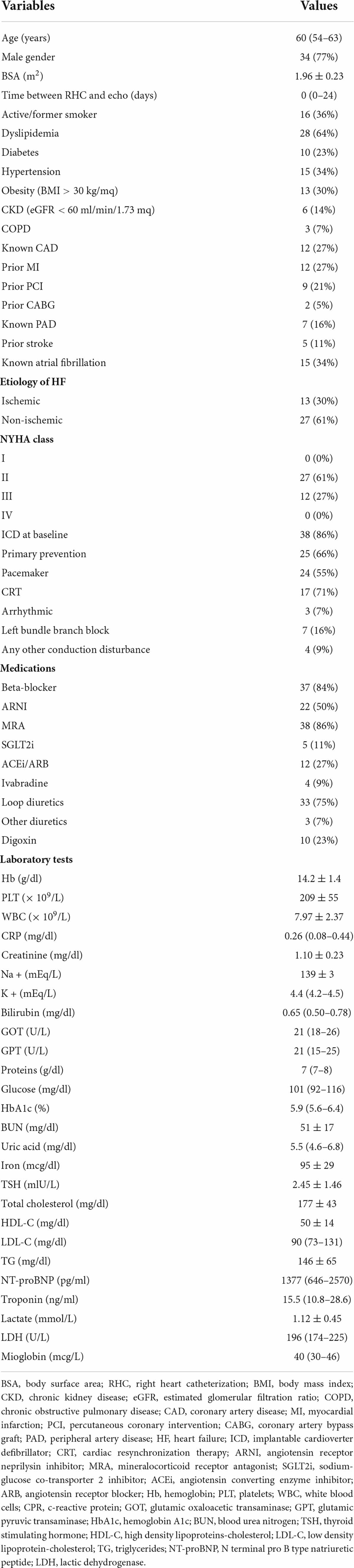
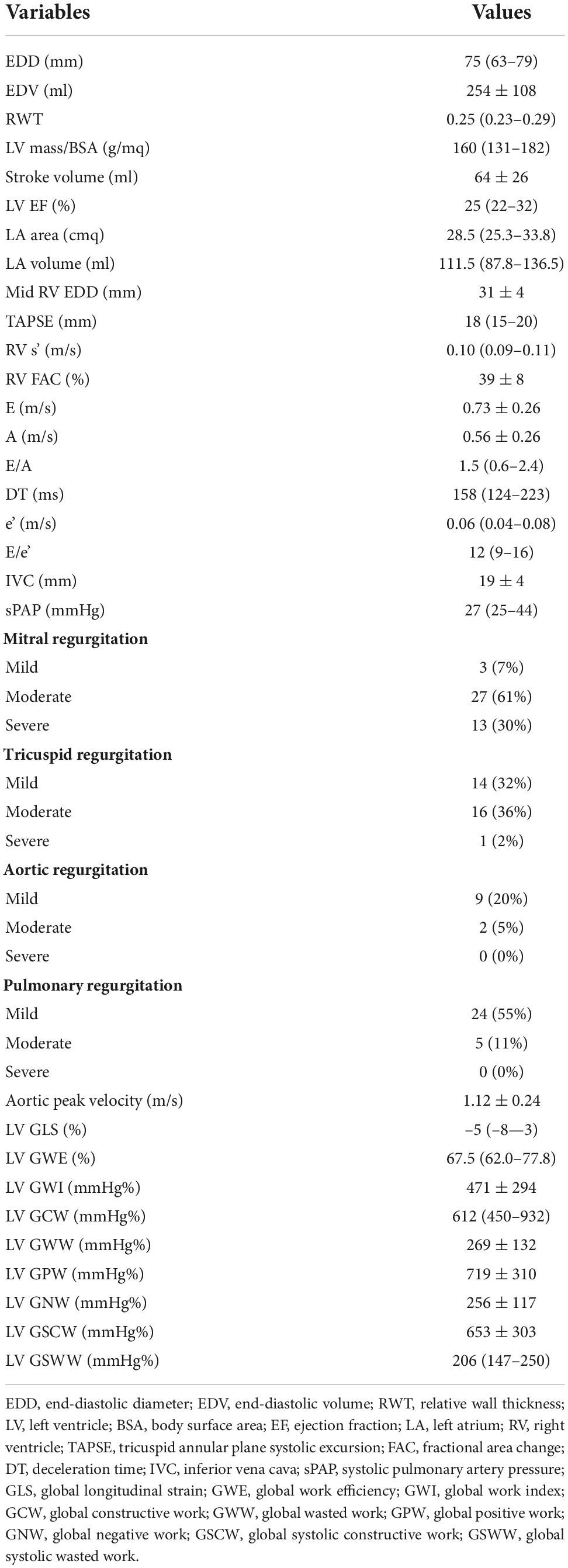
Results: The population included 44 patients. Median time between RHC and echocardiography was 0 days (IQR: 0–24). Median age was 60 years (IQR: 54–63). For the most part, etiology of HF was non-ischemic (61.4%) and all patients were either on class NYHA II (61.4%) or III (27.3%). Median left ventricular ejection fraction was 25% (IQR: 22.3–32.3), median NT-proBNP 1,377 pg/ml (IQR: 646–2570). LV global longitudinal strain (GLS) significantly correlated with LV SWI (r = –0.337; p = 0.031), whereas, LV ejection fraction (EF) did not (r = 0.308; p = 0.050). With regard to LV MW indices, some of them demonstrated correlation with LV SWI, particularly LV GWI (r = 0.425; p = 0.006), LV GCW (r = 0.506; p = 0.001), LV global positive work (LV GPW; r = 0.464; p = 0.003) and LV global systolic constructive work (GSCW; r = 0.471; p = 0.002).
Conclusion: Among LV MW indices, LV GCW correlated better with invasively-derived SWI, potentially representing a powerful tool for a more comprehensive evaluation of myocardial function.
Introduction
The prevalence of people being affected by heart failure (HF) worldwide is incessantly increasing and is now over 60 millions (1). Consensually, the ranks of those in advanced stages of the disease are expanding. Heart transplantation (HTX) is recognized as the most effective destination therapy since median survival time after transplantation exceeds 10 years nowadays (2). In a growing donor organs shortage era, potential receiver patients must undergo a fine selection after comprehensive multi-organ evaluation. Particularly, right heart catheterization (RHC) is routinely performed in order to evaluate pulmonary hemodynamic, since pulmonary hypertension is generally considered a contraindication to HTX.
Right heart catheterization is a tremendously informative exam whose results far exceed a mere pressures measurement. Indeed, RHC provides data regarding flow and resistances, which give additional details about cardiopulmonary physiology. Moreover, measures of stroke work, representative of the area inscribed in the pressure-volume (PV) loop whether of the right or the left ventricle, can be easily derived from other data, bringing deeper insight into myocardial functioning, thus helping the clinician to better characterize the failing heart.
The PV diagram is a well-known tool describing cardiac mechanics and energetics. However fascinating on a theoretical level, its applicability has long remained confined to research setting. A novel echocardiographic method derived from speckle tracking echocardiography (STE) analysis, called “Myocardial Work” (MW), has recently been introduced as a non-invasive derivative of PV curve (3). MW analysis produces a pressure-strain (PS) loop using STE as a surrogate of volume and brachial cuff blood pressure to estimate left ventricular pressures during cardiac cycle.
Myocardial work has already emerged as a promising tool for various pathological conditions (4–16), both for diagnosis and prognosis, but it has only been compared with a completely invasive strategy involving micromanometer and sonomicometry use in animal models to date (3). Therefore, this study aimed to explore the correlation between left ventricular (LV) MW indices and RHC-derived LV stroke work index (SWI) in patients with advanced heart failure considered for heart transplantation.
Materials and methods
Patient population
All consecutive patients with advanced heart failure (AHF) considered for HTX from 2016 to 2021 were retrospectively reviewed. Inclusion criteria were: RHC and echocardiography availability, informed consent from the patient. Exclusion criteria were: time between echocardiographic exam and RHC > 3 months, previous left ventricular assist device (LVAD) implantation, previous heart valve surgery/interventions, single chamber ventricular pacing and poor echocardiographic windows. The study was performed in accordance with the Declaration of Helsinki. Local ethical committee approved the study protocol.
Data collection and standard echocardiography
Patients’ baseline characteristics, vital signs, laboratory tests, medications, echocardiographic data and RHC parameters were retrospectively collected.
All echocardiographic examinations were performed by experienced operators using GE Vivid E80/E95 equipped with an adult 1.5–4.3 MHz phased array transducer, and with an ECG continuously traced, according to the American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations (17, 18).
Speckle tracking analysis
For STE analysis, endocardial borders and myocardium of all segments from the apical views (four chambers, two chambers, and apical long axis) had to be clearly visualized throughout the whole cardiac circle and ECG track had to be present in each echocardiographic exam. Left ventricular speckle tracking strain is semi-automatically performed by the software in the three apical views and adjusted by the operator, in terms of region of interest (ROI) width and positioning, to optimize endomyocardial tracking. The software warns the operator whether a specific wall segment is not automatically recognized and they may manually adapt ROI and force analysis.
For subsequent MW analysis, markers for aortic, and mitral valves opening and closure are required to set the beginning and the end of each main phase of cardiac cycle (isovolumetric contraction, ejection, isovolumetric relaxation, filling) and they were visually set from the apical long axis view. Moreover, brachial cuff blood pressure are needed to warp in time and amplitude the reference curve for left ventricular pressures estimation and the one detected at the time of echocardiography was used in order to conclude the analysis.
Finally, the software’s output is a series of indices which depict the PS loop from various perspectives (19, 20). In addition, it is provided a graphic representation of the PS loop. The main MW indices are Global Work Index (GWI), which is the total work performed by the heart from mitral valve closure to mitral valve opening, Global Constructive Work (GCW), which is the work performed during shortening in systole adding work during lengthening in isovolumetric relaxation, Global Wasted Work (GWW), which is the work performed during lengthening in systole adding work performed during shortening in isovolumetric relaxation and Global Work Efficiency (GWE), which is constructive work divided by the sum of constructive and wasted work. Additional MW indices are provided: Global Positive Work (GPW), which is the work performed during shortening in systole adding work performed during isovolumetric ventricular contraction, Global Negative Work (GNW), which is the work performed during lengthening in diastole adding work performed during isovolumetric ventricular relaxation, Global Systolic Constructive Work (GSCW), which is the work performed during shortening in systole and Global Systolic Wasted Work (GSWW), which is the work performed during lengthening in systole. MW analysis was performed using EchoPAC software v204 (GE Healthcare).
Right heart catheterization
Vascular access for RHC examination was obtained with ultrasound guidance through the internal jugular vein under local anesthesia. Adequate zero level was searched using the medium-thoracic line of the supine patient as a reference. Pulmonary artery catheters, also known as Swan-Ganz catheters, were used to measure central venous pressure (CVP), diastolic, and systolic right ventricular pressures, diastolic, systolic, and mean pulmonary artery pressures (PAP) and pulmonary capillary wedge pressure (PCWP). The height of PAP waves was manually measured. Cardiac output was derived by the thermodilution technique (average of five cardiac cycles with < 10% variation) and by the Fick equation. Cardiac index, stroke volume, and stroke volume index were derived indexing cardiac output for body surface area and dividing cardiac output and cardiac index for heart frequency, respectively. Vascular resistances were calculated by the following equations: [(mean pulmonary artery pressure–pulmonary capillary wedge pressure) × 80/cardiac output] for pulmonary vascular resistance and [(mean arterial pressure–right atrial pressure) × 80/cardiac output] for systemic vascular resistance. LV stroke work index (SWI) was retrospectively calculated through the following formula: (mean systemic arterial pressure–mean pulmonary artery wedge pressure) × stroke volume index × 0.0136. All the other parameters were already calculated at the time of catheterization.
Statistical analysis
Continuous data are presented as mean and standard deviation or as median and interquartile range, as appropriate. Kolmogorov–Smirnov test was used to verify normal distribution of variables. Categorical data are summarized as absolute and relative frequencies. Correlation was calculated using Pearson’s and Spearman’s correlation coefficients, as appropriate. Receiver operating characteristic curves were generated to assess predictive performance of STE-derivate indices for LV SWI. A p-value < 0,05 was considered statistically significant. Analysis was performed using SPSS, version 26 (SPSS, Chicago, IL, USA).
Results
Patient population
One hundred and eighty-two patients with AHF who were evaluated for HTX in our center between 2016 and 2021 were reviewed (n = 182). Among them, 86 patients were excluded because they had not performed RHC yet, whereas, five were excluded because of unavailable echo. Moreover, 46 patients were excluded according to exclusion criteria (Figure 1). Therefore, LV MW analysis was performed in 44 patients. Of note, median time between echocardiographic exam and RHC was 0 days (IQR: 0–24).
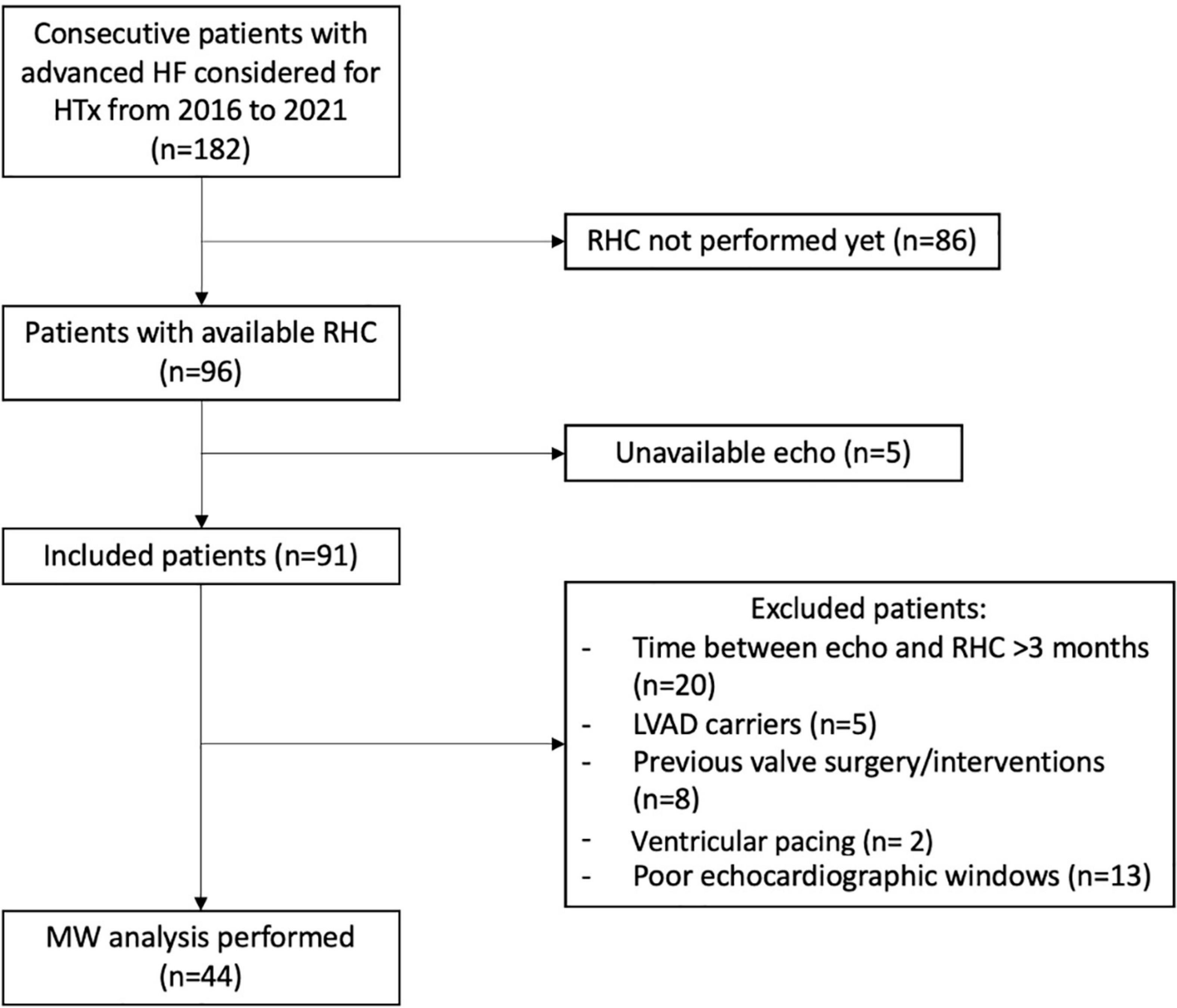
Figure 1. Study flow chart. Inclusion and exclusion criteria as well as patients eventually included in the analysis are reported. HF, heart failure; HTx, heart transplant; RHC, right heart catheterization; LVAD, left ventricular assist device; MW, myocardial work.
Median age was 60 years (IQR: 54–62.8) and most of the patients were men (77.3%). Etiology of HF was predominantly non-ischemic (61.4%) and all patients were either on NYHA class II (61.4%) or III (27.3%). HF therapy is described in Table 1. Median NT-proBNP was 1,377 pg/ml (IQR: 646–2,570). For complete baseline characteristics, see Table 1.
Echocardiography
Median left ventricular end-diastolic diameter was 75 mm (IQR: 63–79) and median left ventricular ejection fraction (EF) was 25% (IQR: 22–32). Mean right ventricular mid end-diastolic diameter was 31 ± 4 mm and mean fractional area chance was 39 ± 7%. Diastolic function was impaired, with a median E/e’ of 12 (IQR: 9–16). Median systolic pulmonary artery pressure was 27 mmHg (IQR: 25–44). Only 9.1% of patients had no or mild mitral regurgitation, whereas, only 4.6% of patients had at least moderate aortic regurgitation. Regarding STE parameters, global longitudinal strain (GLS) was impaired, with a median of –5% (IQR: –8—3) [normal range < –19.7% (21)], as well LV MW indices. In particular, mean GWI was 471 ± 294 mmHg% [normal range 1292–2505 mmHg% (22)], median GCW was 612 mmHg% (IQR: 450–932) [normal range 1582–2881 mmHg% (22)], mean GWW was 269 ± 132 mmHg% [normal range 226 ± 28 mmHg% (22)] and median GWE was 67.5% (IQR: 62.0–77.8) [normal range 91 ± 0.8 mmHg% (22)]. For complete echocardiography, parameters see Table 2.
Right heart catheterization
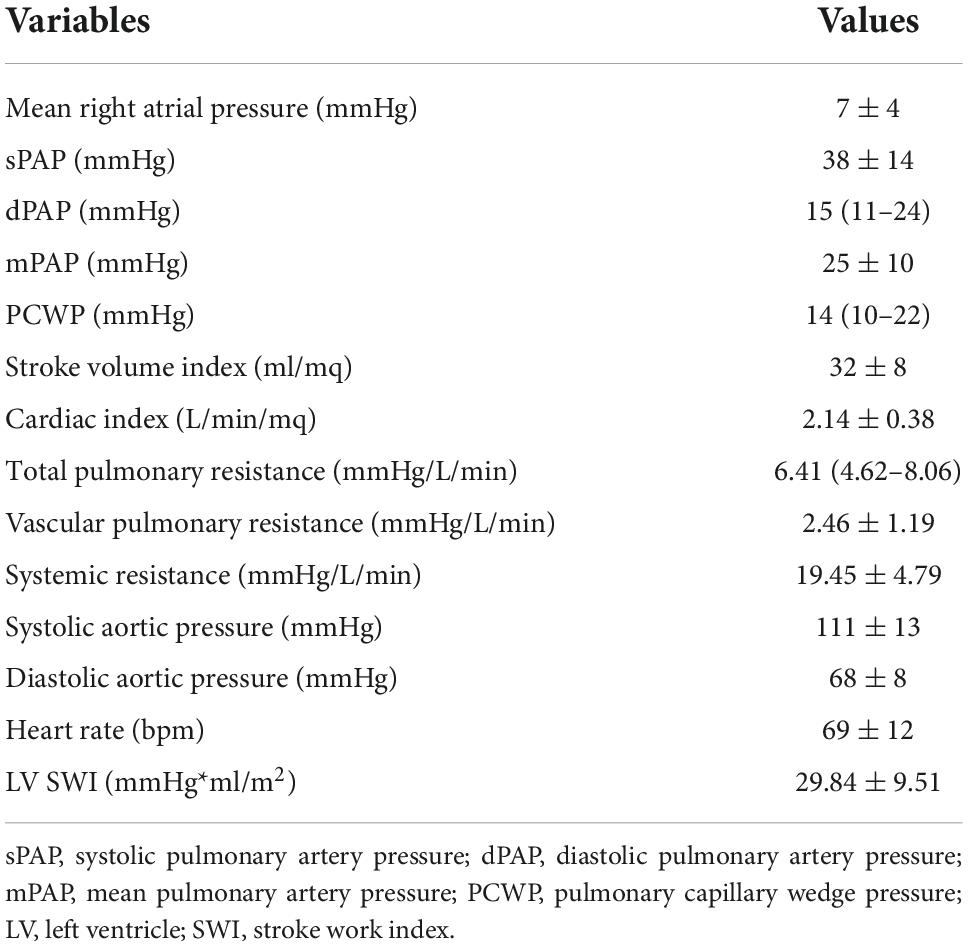
Mean right atrial pressure was 7 ± 4 mmHg. Mean pulmonary artery pressure (mPAP) was increased (25 ± 10 mmHg) and median pulmonary capillary wedge pressure (PCWP) was 14 mmHg (IQR: 10–22). Mean vascular pulmonary resistance was 2.46 ± 1.19 mmHg/l/min. Mean LV SWI was 29.84 ± 9.51 mmHg*ml/m2. RHC parameters are shown in Table 3.
Correlation analysis
Among traditional parameters of LV systolic function, EF did not significantly correlate with SWI (r = 0.308; p = 0.050), whereas, GLS did (r = –0.337; p = 0.031). Among MW indices, GWI, GCW, GPW and GSCW significantly correlated with SWI (r = 0.425, p = 0.006; r = 0.506, p = 0.001; r = 0.464, p = 0.003; r = 0.471, p = 0.002, respectively). For the complete correlation analysis results, see Table 4; Figure 2. Correlation analysis results between combination of STE-derived indices and left ventricular stroke work index are shown in Table 5. Combination of GCW and GWI showed the best correlation with SWI (r = 0.522, p = 0.002).
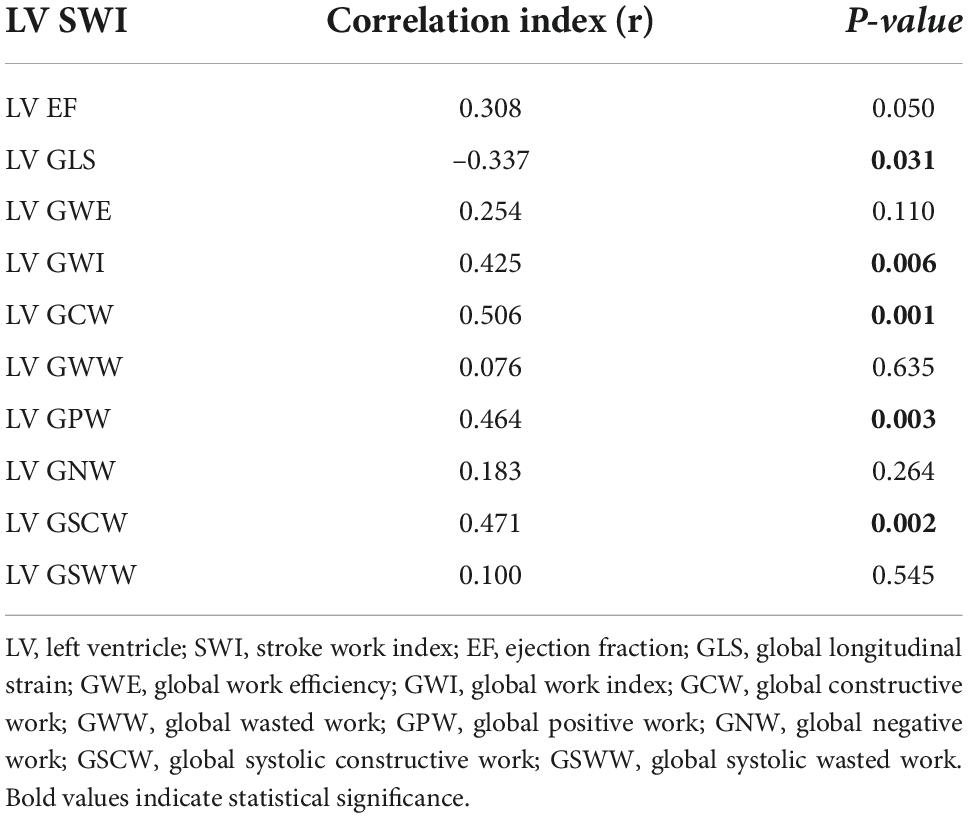
Table 4. Correlation analysis results between some echocardiographic indices and left ventricular stroke work index.
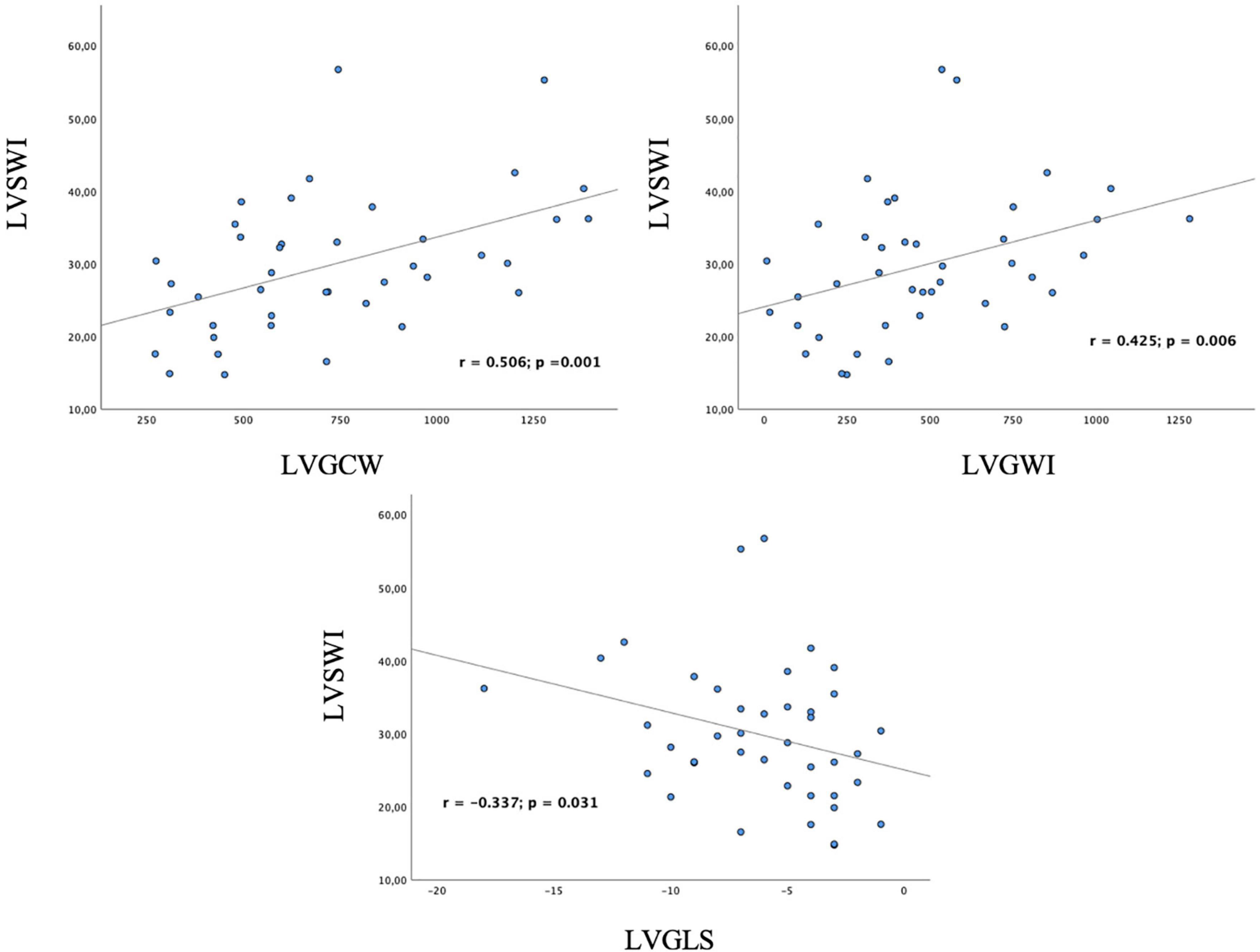
Figure 2. Correlation of left ventricular myocardial work indices and global longitudinal strain with invasive measurement of left ventricular stroke work index. GCW and GWI significantly correlate with invasive measurement of stroke work; GLS significantly correlates as well. LVSWI, left ventricular stroke work index; LVGCW, left ventricular global constructive work; LVGWI, left ventricular global work index; LVGLS, left ventricular global longitudinal strain.
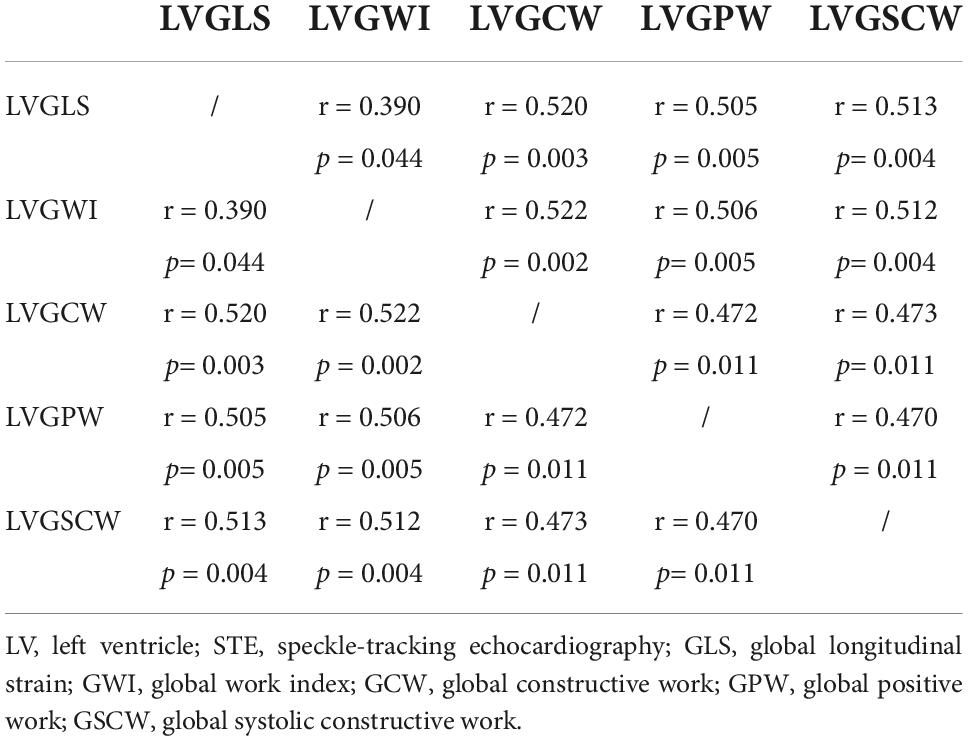
Table 5. Correlation analysis results between combination of STE-derived indices and left ventricular stroke work index.
Receiver operating characteristic curves
Performance for prediction of LV SWI was greatest for LV GCW (AUC 0.824), followed by LV GCSW (AUC 0.818), LV GPW (AUC 0.800), LV GWI (AUC 0.735), and LV GLS (0.714). For ROC curves see Figures 3, 4.
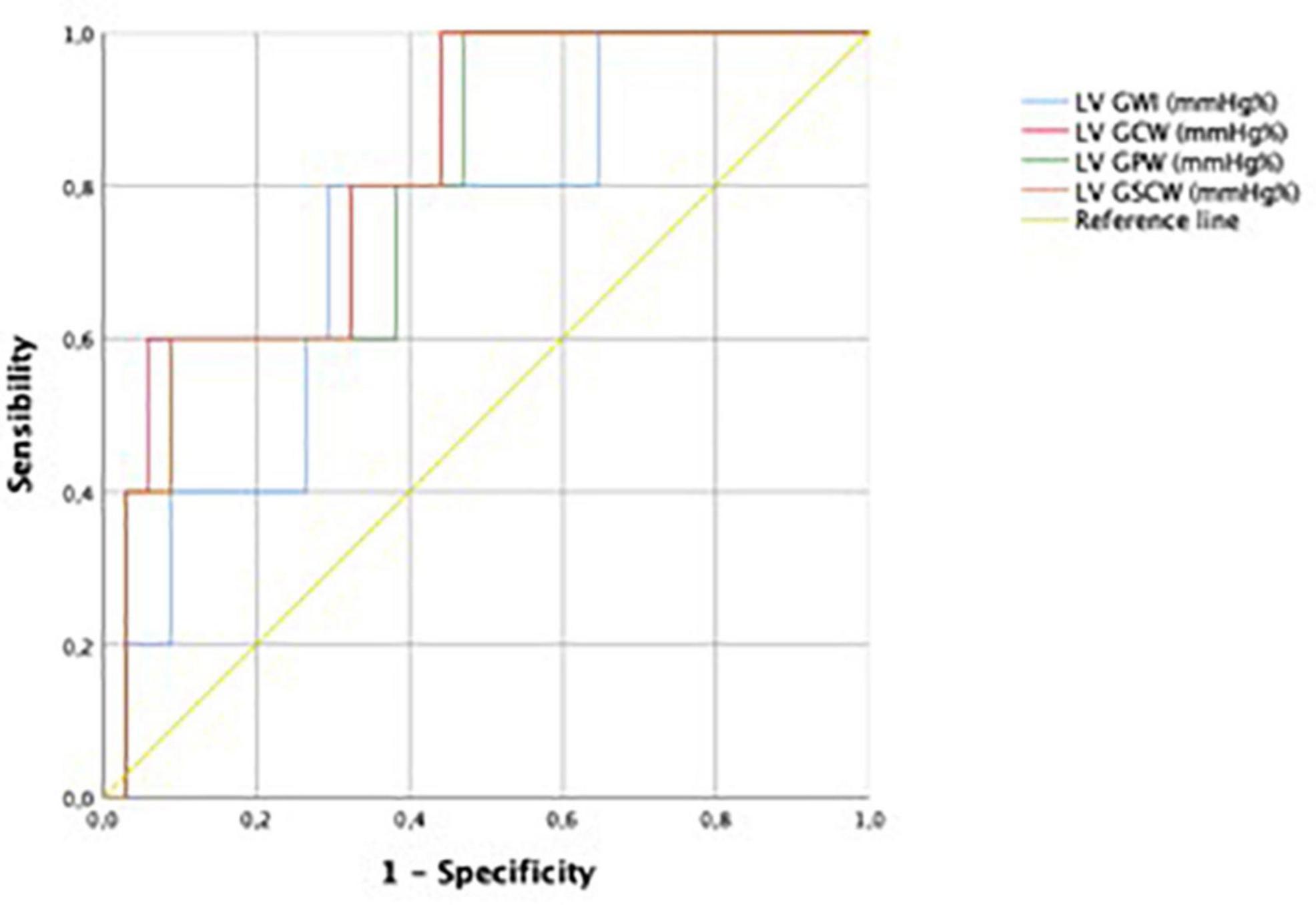
Figure 3. Receiver operating characteristic curves for predictive performance of LVGWI, LVGCW, LVGPW, and LVGSCW for LVSWI. LVGWI, left ventricular global work index; LVGCW, left ventricular global constructive work; LVGPW, left ventricular global positive work; LVGSCW, left ventricular global systolic constructive work; LVSWI, left ventricular stroke work index.
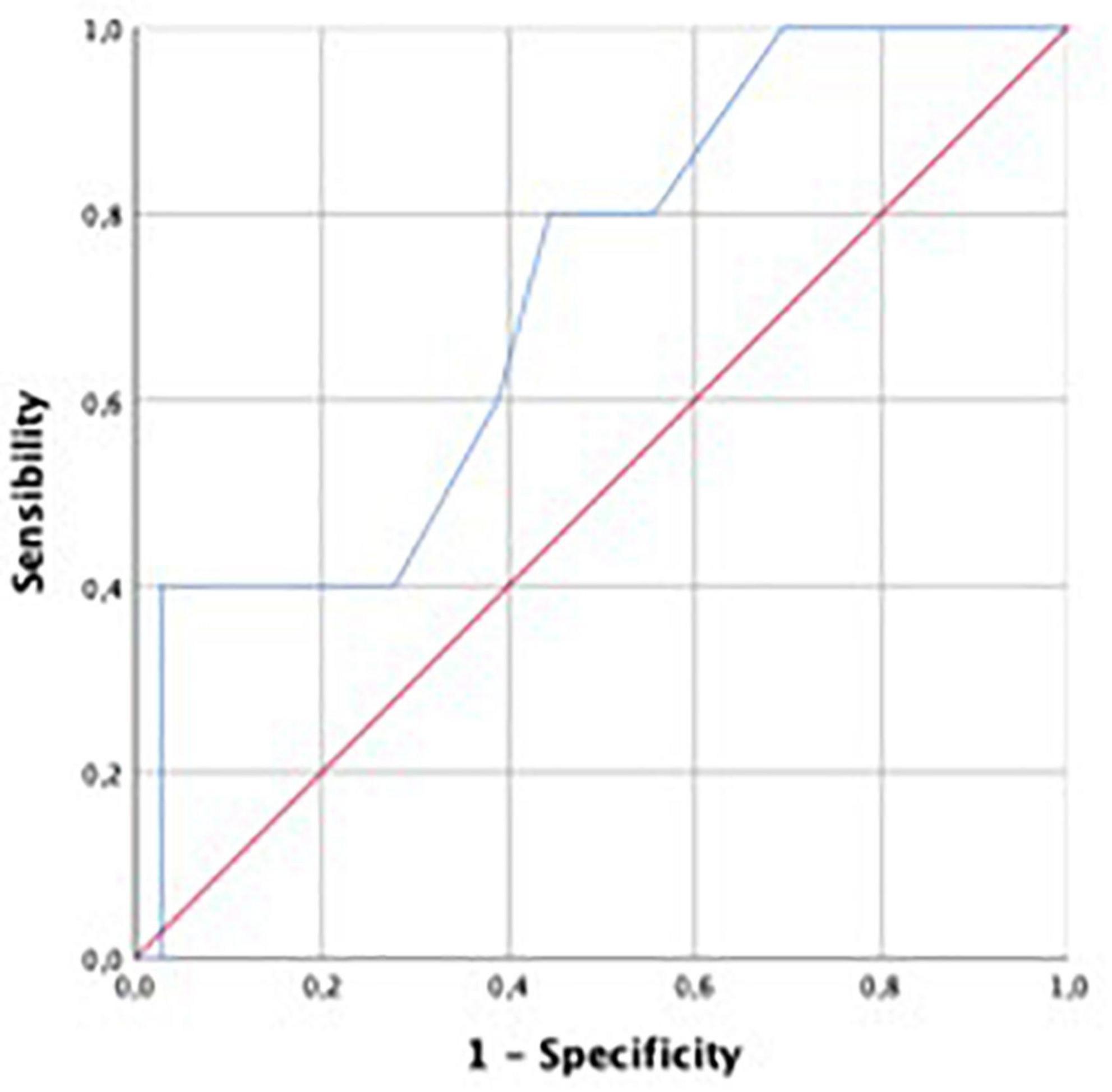
Figure 4. Receiver operating characteristic curve for predictive performance of LVGLS for LVSWI. LVGLS, left ventricular global longitudinal strain; LVSWI, left ventricular stroke work index.
Discussion
The main findings of the study can be summarized as follows:
1. Non-invasive measurement of stroke work through myocardial work (MW) calculation significantly correlated with invasively-derived stroke work index in a population of advanced heart failure patients.
2. Among echocardiographic parameters of LV systolic function, correlation with invasive measures increases from classical indices to most recent ones.
3. Ejection fraction is the less reliable tool to evaluate LV systolic function in patients with severely compromised EF, compared to advanced echocardiographic parameters.
The measurement of work performed by the heart has long remained of difficult clinical applicability due to the invasiveness of catheterization. “Myocardial work,” a novel non-invasive echocardiographic method for calculation of work, has already proven its diagnostic and prognostic usefulness in various pathological conditions (5, 8–10, 12, 13). However, it has only been compared with a completely invasive strategy involving micromanometer and sonomicometry use in animal models to date. In fact, in the original paper by Russell et al. (3) the clinical study only compared two groups of patients with invasive vs. non-invasive LV pressure measurements, but both groups involved the use of STE, therefore limiting the comparison to a non-invasive vs. a “hybrid” method. In this study, a correlation analysis between LV MW indices and a completely invasive measurement of work through RHC in patients with advanced heart failure considered for heart transplantation has been performed.
Our results have shown a significant correlation between some indices of myocardial work and invasively-derived stroke work index. More specifically, only the indices, which include the area inscribed in the pressure-strain loop correlated with invasive measurements, that is GWI, GCW, GPW, and GSCW. In fact, each of these indices includes the broader slice of stroke work, namely shortening during the ejection phase. This is not surprising, since invasively calculated work is only stroke work, that is the area inscribed in the pressure-volume loop. Correlation index has reached the highest value with the combination of GWI and GCW, therefore combining two indices could provide more reliable insights into myocardial function. In addition, ROC curves generated to assess predictive performance of significantly correlated STE-derivate indices for LV SWI have shown great areas under the curve, above all GCW, which confirmed itself as the MW index more affine to SWI.
All other myocardial work indices which have not shown a correlation with invasive measurement take into account different aspects of the mechanics and energetics of heart. For instance, GWW and GSWW focus on the work performed by some myocardial segments in the wrong moment of the cardiac cycle, such as lengthening in systole and shortening in diastole. On the contrary, GWE represents an index and for this reason is conceptually different from calculation of work with the invasive method.
However, even though results have shown some degree of correlation between GWI, GCW, GPW, and GSCW with SWI, “r values” are not high enough to support a robust correlation from a statistical point of view. One of the main reasons for which correlation indices were not so high is probably the fact that echocardiographic exams and RHC were not performed on the same day in all cases. It is widely known that these patients suffer from rapid hemodynamic variations indeed.
This study also correlated invasive measurement of work with traditional echocardiographic indices of systolic function, particularly EF and GLS. Even though such indices are conceptually different from work, they also evaluate systolic performance of heart. It is of particular interest the fact that, from EF to MW indices passing through GLS, correlation indices and statistical significance progressively increased. This means that, among the whole range of echocardiographic indices for the evaluation of systolic function available nowadays, newer ones perform better.
Aside from understanding whether these novel indices are reliable or not as compared to invasive measures, one of the key elements toward which future studies should point is how they could be integrated in clinical practice and at what extent they could enhance patients’ management. As a matter of fact, as patients with AHF often need careful clinic visits comprehensive of echocardiogram examination at close time intervals, more sensible advanced parameters of left ventricular systolic function should be implemented to better characterize disease progression and optimize referral for advanced therapies, such as HTX, not to miss the golden window. Since an invasive evaluation strategy with RHC at each time point is inapplicable, a non-invasive evaluation could potentially complement the hemodynamic evaluation at shorter time intervals.
Limitations
This study was a single-center and retrospective analysis. The main limitations of the study include: the enrolled patients are part of a restricted population of subjects with AHF, which limits the extent of the results to the general population. However, since correlation has been performed between measurements with the two methods in the same individual, this should be of limited concern. Time between echocardiographic exam and right heart catheterization was very low, but not null in all cases. Since these particular patients suffer from rapid hemodynamic variations, even few weeks could be potentially relevant. Nonetheless, if this was true, correlation could only be underestimated. The number of patients in which correlation analysis was performed is relatively low. However, considering the invasive nature of catheterization, this was sufficient for the purpose of the study. If it was not, results would not have reached statistical significance.
Conclusion
Speckle tracking echocardiography-derived LV MW allows to non-invasively assess the work performed by the heart during a cardiac cycle. This study demonstrated a significant correlation between some MW indices and invasive measurement of LV stroke work derived from RHC in a cohort of patients with AHF considered for heart transplantation, therefore potentially representing a powerful tool for a more comprehensive evaluation of myocardial function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, with adequate motivation.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato Etico Regione Toscana Area Vasta Sud Est. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
FL, GEM, and MC conceived and designed the study protocol and wrote the manuscript. FL, BC, MB, GM, GD, and CS participated in the acquisition of data. FL performed statistical analysis. ML, FF, FD’A, MFo, MFi, AI, SB, MM, and SV revised the manuscript and participated in the interpretation of results. All authors gave the final approval of the manuscript version to be submitted.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LV, left ventricle; MW, myocardial work; HF, heart failure; AHF, advanced heart failure; RHC, right heart catheterization; SWI, stroke work index; GWI, global work index; GCW, global constructive work; GWW, global wasted work; GWE, global work efficiency; GPW, global positive work; GNW, global negative work; GSWC, global systolic constructive work; GSWW, global systolic wasted work; GLS, global longitudinal strain; EF, ejection fraction; HTX, heart transplantation; PV, pressure-volume; STE, speckle tracking echocardiography; PS, pressure-strain; LVAD, left ventricular assist device; ROI, region of interest; CVP, central venous pressure; PAP, pulmonary artery pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure.
References
1. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
2. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J Hear Lung Transplant. (2017) 36:1037–46. doi: 10.1016/j.healun.2017.07.019
3. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, et al. A novel clinical method for quantification of regional left ventricular pressurestrain loop area: a non-invasive index of myocardial work. Eur Heart J. (2012) 33:724–33. doi: 10.1093/eurheartj/ehs016
4. Boe E, Russell K, Eek C, Eriksen M, Remme EW, Smiseth OA, et al. Non-invasive myocardial work index identifies acute coronary occlusion in patients with non-STsegment elevation-acute coronary syndrome. Eur Heart J Cardiovasc Imaging. (2015) 16:1247–55. doi: 10.1093/ehjci/jev078
5. Galli E, Leclercq C, Hubert A, Bernard A, Smiseth OA, Mabo P, et al. Role ofmyocardial constructive work in the identification of responders to CRT. Eur Heart J Cardiovasc Imaging. (2018) 19:1010–8. doi: 10.1093/ehjci/jex191
6. Jain R, Bajwa T, Roemer S, Huisheree H, Allaqaband SQ, Kroboth S, et al. Myocardial work assessment in severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Hear J Cardiovasc Imaging. (2020) 22:715–21. doi: 10.1093/ehjci/jeaa257
7. Clemmensen TS, Eiskjær H, Ladefoged B, Mikkelsen F, Sørensen J, Granstam S-O, et al. Prognostic implications of left ventricular myocardial work indices in cardiac amyloidosis. Eur Hear J Cardiovasc Imaging. (2020) 22:695–704. doi: 10.1093/ehjci/jeaa097
8. Wang CL, Chan YH, Wu VCC, Lee HF, Hsiao FC, Chu PH. Incremental prognostic value of global myocardial work over ejection fraction and global longitudinal strain in patients with heart failure and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. (2021) 22:348–56. doi: 10.1093/ehjci/jeaa162
9. Przewlocka-Kosmala M, Marwick TH, Mysiak A, Kosowski W, Kosmala W. Usefulness of myocardial work measurement in the assessment of left ventricular systolic reserve response to spironolactone in heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. (2019) 20:1138–46. doi: 10.1093/ehjci/jez027
10. Chan J, Edwards NFA, Khandheria BK, Shiino K, Sabapathy S, Anderson B, et al. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2019) 20:31–9. doi: 10.1093/ehjci/jey131
11. Edwards NFA, Scalia GM, Shiino K, Sabapathy S, Anderson B, Chamberlain R, et al. Global myocardial work is superior to global longitudinal strain to predict significant coronary artery disease in patients with normal left ventricular function and wall motion. J Am Soc Echocardiogr. (2019) 32:947–57. doi: 10.1016/j.echo.2019.02.014
12. Hedwig F, Nemchyna O, Stein J, Knosalla C, Merke N, Knebel F, et al. Myocardial work assessment for the prediction of prognosis in advanced heart failure. Front Cardiovasc Med. (2021) 8:691611. doi: 10.3389/fcvm.2021.691611
13. Bouali Y, Donal E, Gallard A, Laurin C, Hubert A, Bidaut A, et al. Prognostic usefulness of myocardial work in patients with heart failure and reduced ejection fraction treated by sacubitril/valsartan. Am J Cardiol. (2020) 125:1856–62. doi: 10.1016/j.amjcard.2020.03.031
14. de Paula Lustosa R, van der Bijl P, El Mahdiui M, Cabezas JMM, Kostyukevich MV, Knuuti JM, et al. Global left ventricular myocardial work efficiency and long-term prognosis in patients after ST-segment elevation myocardial infarction. J Am Coll Cardiol. (2020) 75:1754. doi: 10.1016/S0735-1097(20)32381-0
15. Hiemstra YL, van der Bijl P, el Mahdiui M, Bax JJ, Delgado V, Marsan NA. Myocardial work in nonobstructive hypertrophic cardiomyopathy: implications for outcome. J Am Soc Echocardiogr. (2020) 33:1201–8. doi: 10.1016/j.echo.2020.05.010
16. D’Andrea A, Sperlongano S, Formisano T, Tocci G, Cameli M, Tusa M, et al. Stress echocardiography and strain in aortic regurgitation (SESAR protocol): left ventricular contractile reserve and myocardial work in asymptomatic patients with severe aortic regurgitation. Echocardiography. (2020) 37:1213–21. doi: 10.1111/echo.14804
17. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–71.
18. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the american society of echocardiography. J Am Soc Echocardiogr. (2019) 32:1–64. doi: 10.1016/j.echo.2018.06.004
19. Smiseth OA, Donal E, Penicka M, Sletten OJ. How to measure left ventricular myocardial work by pressure-strain loops. Eur Heart J Cardiovasc Imaging. (2021) 22:259–61.
20. van der Bijl P, Kostyukevich M, El Mahdiui M, Hansen G, Samset E, Ajmone Marsan N, et al. A roadmap to assess myocardial work: from theory to clinical practice. JACC Cardiovasc Imaging. (2019) 12:2549–54. doi: 10.1016/j.jcmg.2019.05.028
21. Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. (2013) 26:185–91.
Keywords: myocardial work, stroke work, left ventricle, advanced echocardiography, advanced heart failure, speckle tracking analysis, speckle tracking echocardiography, right heart catheterization
Citation: Landra F, Mandoli GE, Chiantini B, Barilli M, Merello G, De Carli G, Sciaccaluga C, Lisi M, Flamigni F, D’Ascenzi F, Focardi M, Fineschi M, Iadanza A, Bernazzali S, Maccherini M, Valente S and Cameli M (2022) Correlation of left ventricular myocardial work indices with invasive measurement of stroke work in patients with advanced heart failure. Front. Cardiovasc. Med. 9:946540. doi: 10.3389/fcvm.2022.946540
Received: 17 May 2022; Accepted: 26 September 2022;
Published: 17 October 2022.
Edited by:
Stefania Paolillo, University of Naples Federico II, ItalyReviewed by:
Massimo Mapelli, Monzino Cardiology Center (IRCCS), ItalyThomas E. Sharp III, Louisiana State University, United States
Copyright © 2022 Landra, Mandoli, Chiantini, Barilli, Merello, De Carli, Sciaccaluga, Lisi, Flamigni, D’Ascenzi, Focardi, Fineschi, Iadanza, Bernazzali, Maccherini, Valente and Cameli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Landra, Zi5sYW5kcmFAc3R1ZGVudC51bmlzaS5pdA==
†These authors have contributed equally to this work and share first authorship
 Federico Landra
Federico Landra Giulia Elena Mandoli
Giulia Elena Mandoli Benedetta Chiantini1
Benedetta Chiantini1 Carlotta Sciaccaluga
Carlotta Sciaccaluga Matteo Lisi
Matteo Lisi Flavio D’Ascenzi
Flavio D’Ascenzi Alessandro Iadanza
Alessandro Iadanza Matteo Cameli
Matteo Cameli