Corrigendum: Cost-effectiveness of adding empagliflozin to the standard therapy for Heart Failure with Preserved Ejection Fraction from the perspective of healthcare systems in China
- Department of Cardiology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
Background: The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) is the first randomized controlled trial to provide promising evidence on the efficacy of adding empagliflozin to the standard therapy in patients with Heart Failure with Preserved Ejection Fraction (HFpEF), but the cost-effectiveness of add-on empagliflozin treatment remains unclear.
Method: A Markov model using data from the EMPEROR-Preserved trial and national database was constructed to assess lifetime costs and utility from a China healthcare system perspective. The time horizon was 10 years and a 5% discount rate was applied. Incremental cost-effectiveness ratio (ICER) against willingness to pay (WTP) threshold was performed to evaluate the cost-effectiveness. A series of sensitivity analyses was applied to ensure the robustness of the results.
Results: Compared to standard therapy, the increased cost of adding empagliflozin from $4,645.23 to $5,916.50 was associated with a quality-adjusted life years (QALYs) gain from 4.70 to 4.81, projecting an ICER of $11,292.06, which was lower than a WTP threshold of $12,652.5. Univariate sensitivity analysis revealed that the parameters with the largest impact on ICER were cardiovascular mortality in both groups, followed by the cost of empagliflozin and the cost of hospitalization for heart failure. Probabilistic sensitivity analysis indicated that when the WTP threshold was $12,652.5 and $37,957.5, the probability of being cost-effective for adding empagliflozin was 52.7% and 67.6%, respectively. Scenario analysis demonstrated that the cost of empagliflozin, the cost of hospitalization for heart failure, NYHA functional classes, and time horizon had a greater impact on the ICER.
Conclusion: At a WTP threshold of $12,652.5, the add-on empagliflozin treatment for HFpEF was cost-effective in healthcare systems in China, which promoted the rational use of empagliflozin for HFpEF.
Introduction
Heart failure (HF) has become a growing global public health problem which is a severe clinical manifestation and/or end stage of various cardiac diseases and is a leading cause of hospitalization and death (1). HF is divided into heart failure with mildly reduced ejection fraction (HFmrEF), Heart Failure with Preserved Ejection Fraction (HFpEF), and heart failure with reduced ejection fraction (HFrEF). The clinical characteristics of HFmrEF are more similar to HFpEF. The rate of HF in the United States and Europe ranges from 1 to 14%, of which nearly half are HFpEF (2, 3). The prevalence of HF among adults >35 years old in China is about 1.3%, of which 0.3% is HFpEF (3). The associated HF global economic burden is estimated at $108 billion every year with the most costs of HF deriving from hospitalizations (4). With the aging population in China and the increase in risk factors such as hypertension and coronary heart disease (CHD), the prevalence of HFpEF is on the rise, and the direct and indirect costs of HFpEF will increase significantly in the future.
We have been trying to find a treatment strategy for HFpEF, but compared with the breakthroughs in the treatment of HFrEF, the CHARM-Preserved trial, the TOPCAT trial, and the PARAGON-HF trial showed that candesartan, spironolactone, and SAC/VAL did not reduce the risk of CV or hospitalization for HF among patients with HFpEF which were not satisfactory results (5–7). This reflected the different pathophysiological processes between HFpEF and HFrEF, the mechanism except for the activation of renin-angiotensin aldosterone system (RASS) has not been explored and new therapeutic targets have not been found. Sodium-glucose cotransporter 2 (SGLT-2) has been used as a novel therapy for type 2 diabetes mellitus (T2DM), inhibiting the proximal renal tubular SGLT protein family reabsorption of glucose (8). The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) could reduce the risk of cardiovascular (CV) death or hospitalization for HF in patients with HFpEF (9). Some animal experiments found that there were benefits of HF independently of blood glucose reduction, including higher circulating ketone levels, natriuresis, anti-inflammatory effects, and inhibiting RASS (10, 11). It was noteworthy that the United States Food and Drug Administration (FDA) has approved that empagliflozin could be treated for HFpEF.
However, guidelines emphasized sacubitril/valsartan (SAC/VAL), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta-blockers, and spironolactone for comorbidities for HFpEF (12), and the cost of HFpEF will increase significantly. Previous cost-effective studies focused on HFrEF or HF as a homogeneous group, lacking cost-effective evaluations for HFpEF, financial burden and life quality of HFpEF differed from HFrEF, and higher comorbidity in HFpEF contributed to higher CV-related costs (13). To fill the gap, we are the first to evaluate the cost-effectiveness of the add-on empagliflozin treatment for HFpEF, our study aims to help clinicians and decision-makers judge the economic value of this new therapy.
Method
Model structure
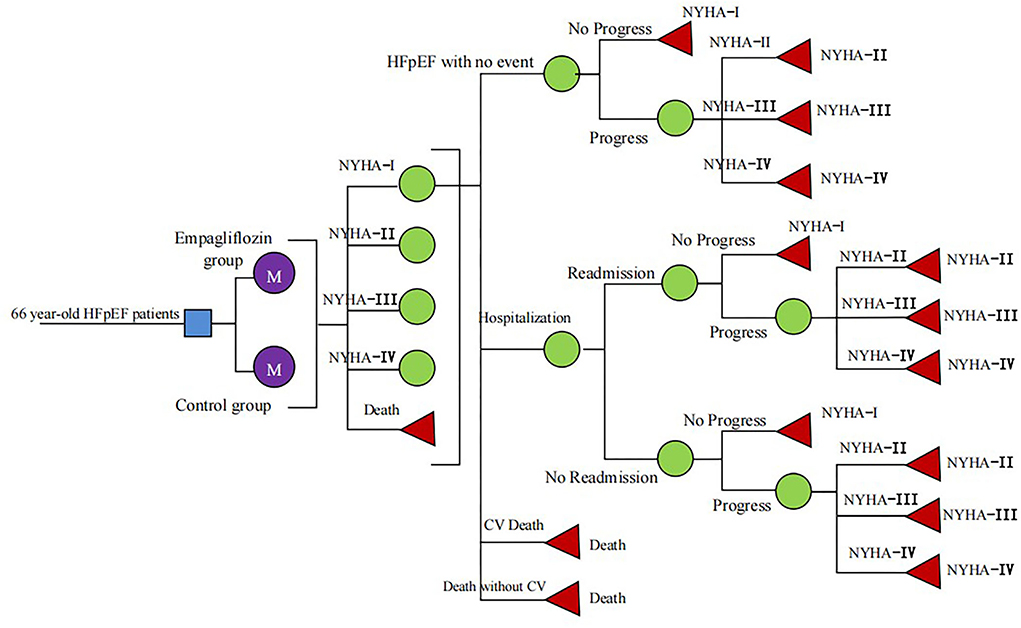
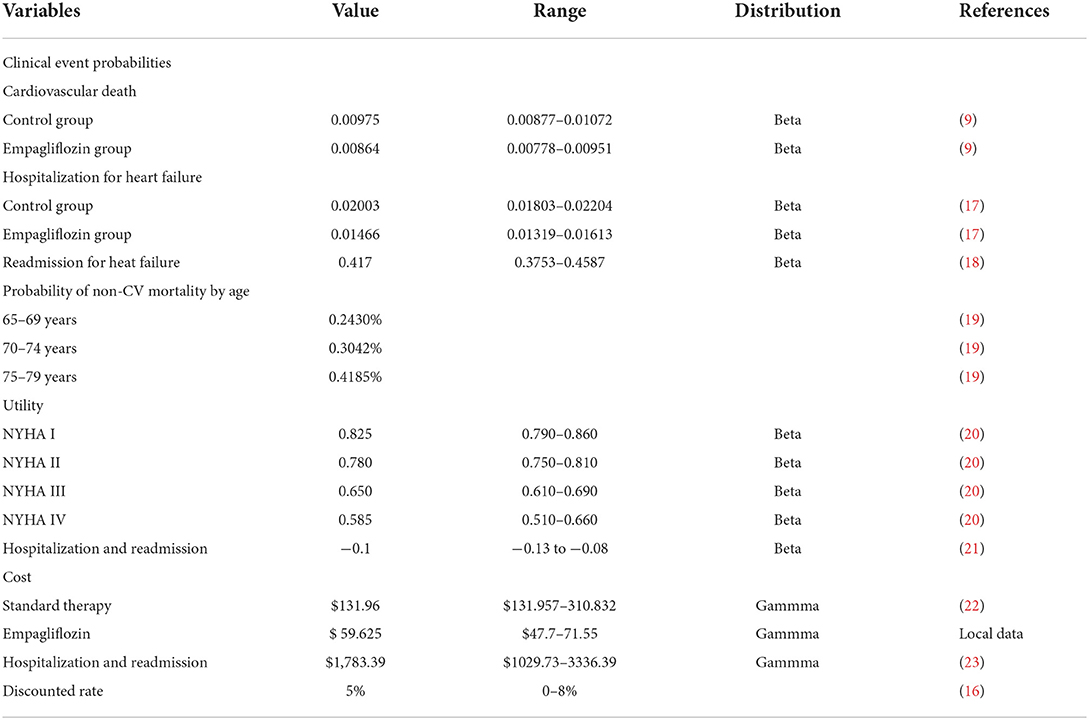
A Markov model was constructed to evaluate the cost-utility of different therapies for HFpEF: standard therapy and standard therapy combined with empagliflozin (10 mg, once daily). According to the history of HFpEF, HFpEF was divided into five states: New York Heart Association (NYHA) function class I, II, III, and IV and death (Figure 1), in which the death was absorption state (14). The patients would be in the same New York Heart Association (NYHA) function classes or they would change the NYHA function classes at the end of each cycle, indicating that the symptom would be improved or worsened. Despite standard HF therapy, the rate of readmission for HF within 3 months of discharge for hospitalized patients approached 30% (15), and we hypothesized that all readmissions related to HF occurred within 3 months in patients who had experienced high-frequency hospitalization. Clinical outcomes included hospitalization for HF, readmission for HF, CV death, and non-CV death. The model was conducted by Excel 2016 software. The simulation time was 10 years and the cycle was set as 3 months (90 days), and the half-cycle correction was applied to prevent overestimation of the expected survival time. A 5% discount rate was also applied according to the recommendations of the Chinese Pharmacoeconomic Evaluation Guide 2019 (16) (Table 1).
Simulated population
The clinical characteristics of the simulated population in this model were consistent with the EMPEROR-Preserved trial, including ejection fraction >40%, the same NYHA class I-IV distribution, and the N-terminal pro-brain natriuretic peptide >600 pg/mL (1,200 pg/mL for patients with atrial fibrillation). All cohort populations received standard therapy including diuretics, SAC/VAL, ACEI, ARB, beta-blockers, and spironolactone with or without cardiac implants (9). According to the study of China HF outcome registration, the average age of HFpEF patients in the model was 66 years and the majority of HFpEF were the elderly in the real world (24).
Clinical event probabilities
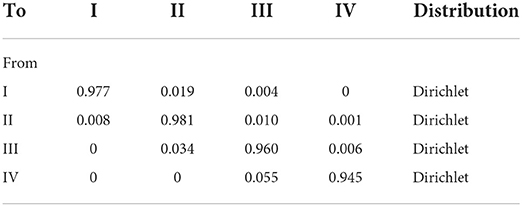
An assumption in our model was proposed that the risk of CV death and hospitalization for HF remained unchanged with age because we could not obtain these data from the EMPEROR-Preserved trial (9). The relevant clinical data for the empagliflozin group and control group were derived from the EMPEROR-Preserved trial, the rate of CV death was 7.2% and 8.3%, and the rate of hospitalization for HF was 12.1% and 16.2%, respectively (9, 17). Some data came from other literature instead of EMPEROR-Preserved trial. The rate of readmission for HF within 30 days was 18%, coming from the I-Preservevd trial (18). Age-dependent non-CV deaths were all from the Report on China's Cause of Death 2018, which was published by the China Center for Disease Control and Prevention (19). The formula r = −1/t ln(S), P = 1-e∧(-r*T) was applied to acquire the clinical event probabilities (S is the rate, t is the time, and P is the clinical event probabilities) (25) (Table 1). The transition between different NYHA function classes at the end of every cycle was assumed to be similar between both groups owning to the lack of the effect of empagliflozin on NYHA function classes. The 3-month transition probability between NYHA function classes was derived from published literature (26) (Table 2).
Cost and utility
The costs in this model were involved with the cost of hospitalization for HF, standard therapy, and empagliflozin. The cost of hospitalization for HF came from the national statistical database which collected the cost of hospitalization all over the country; therefore, the average cost of hospitalization for HF was $1,783.39 (23). Although HFpEF therapy lacked specific drugs, many were already treated with diuretics, SAC/VAL, ACEI, ARB, beta-blockers, and spironolactone. The cost of standard therapy derived from the national claims sampling database was $131.96 in 2022 at a discount rate of 5% (22). According to the latest national negotiation price in 2022, empagliflozin was $0.6625 per 10 mg, enalapril was $0.088 per 10 mg, SAC/VAL was $0.497 per 100 mg, and the cost of SAC/VAL each cycle was $178.875 (target dose 200 mg, twice daily), so we calculated the range of standard therapy (Table 1). All costs of this study were converted into US dollars at the exchange rate of US $1 = 6.4 yuan (27).
HF was graded based on the ability of daily activities. The utility of every NYHA function class was also different, and the quality-adjusted life years (QALYs) were used as a measurement index. The health utility of patients with HFpEF was derived from the study about indirect, direct non-medical costs and QoL by NYHA classification in Chinese heart failure patients (20). Hospitalization for HF tended to produce a negative effect on healthy life quality and so each hospitalization of HF would reduce the utility value by 0.1 (21) (Table 1).
Outcome
This model predicted the cardiovascular mortality and the average survival time of the simulated population. The primary endpoint of this study was incremental QALYs and incremental cost, and the incremental cost-effectiveness ratio (ICER) was calculated by the difference in outcomes and the ICER was used to determine the magnitude of the increased costs for each unit in health improvement. According to the recommendation of the World Health Organization (WHO) on the evaluation of Pharmacoeconomics (16): ICER <1-fold of gross domestic product (GDP) per capita, the increased cost is completely worth it and very cost-effective; 1-fold of GDP per capita < ICER <3-fold of GDP per capita, the increased cost is acceptable and cost-effective; and ICER >3-fold of GDP per capita, the increased cost is not worth it and not cost-effective. Since there was no fixed willingness-to-pay (WTP) to evaluate cost-effectiveness in China, we recommended the WTP threshold of $12,652.5 and $37,687.5, which was associated with the one-time and three-times GDP per capita of China in 2021, to determine whether adding empagliflozin in HFpEF was very cost-effective (ICER ≤ $12,652.5) or acceptable (i.e., ICER ≤ $37,687.5) (28).
Sensitivity analyses
One-way sensitivity analyses were performed to verify the uncertainty of the model by calculating ICER value based on a reasonable range of parameters to make the model more stable according to the recommendations of the Chinese Pharmacoeconomic Evaluation Guide 2019 (16). The 95% confidence intervals of some parameters in the model can be obtained from the published literature. For event probability and medical cost without a specified range, the assuming range was ±10% and ±20%, respectively, and the annual discount rate is in the range of 0–8% (Table 1). The results were presented as a tornado diagram.
Probabilistic sensitivity analysis (PSA) verified the uncertainty of the model in the form of Monte Carlo simulation, which was completed by calculating the random sample results from different input parameter distributions in 1,000 repetitions. The cost parameters adopted gamma distribution, the utility parameters and event probability parameters adopted beta distribution, and the results were represented by cost-effectiveness-acceptability curves (CEACs) and scatter diagram.
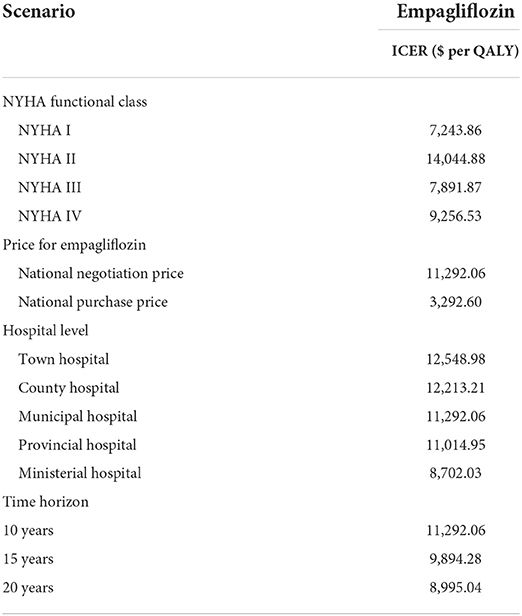
Scenario analysis was used to explain that some scenarios had a significant impact on ICER. First, we explored the effect of the national purchase price of empagliflozin ($0.275 per 10 mg, once daily) in 2022 on ICER; Second, the time horizon should be extended to 15 and 20 years to evaluate the uncertainty caused by the time length; Third, we explored the effect of different NYHA function classes on ICER; Fourth, HFpEF with comorbidity or higher level of the hospital had a higher hospitalization: town-level hospitals ($1,029.73), county-level hospitals ($1,231.06), municipal hospitals ($1,783.39), provincial hospitals ($1,949.55), and ministerial hospitals ($3,336.39) (23), so the cost of hospitalization for HF could affect the ICER.
Results
Model validation and clinical results
At the end of the EMPEROR-Preserved trial, with a mean follow-up of 27.2 months, the rate of CV death was 7.2% and 8.3% in the empagliflozin group and the control group (9). Our model predicted that the cardiovascular mortality of the empagliflozin group and the control group at 27 months was 8.7% and 9.5%, respectively, and the median survival time of the empagliflozin group and the control group was 16.5 and 14.5 years, respectively, indicating that the clinical outcome predicted by our model was relatively reliable and true.
Base-case cost-effectiveness analysis
Table 3 represented the total discounted costs and QALYs of the two therapy strategies during the 10 years. Compared with the control group, the add-on empagliflozin treatment increased by 1,271.27 $ and 0.11 QALYs, and the ICER was $11,292.06 per QALY, which was lower than the one-time GDP in 2021 in China. The add-on empagliflozin treatment for HFpEF was a cost-effective option.
Sensitivity analyses
One-way sensitivity analysis showed that the ICER calculated by changing a reasonable range of parameters was represented as a tornado diagram (Figure 2), in which the CV death of both groups was the main driver for cost-effectiveness, followed by the cost of empagliflozin and the cost of hospitalization for HF which was higher than one-time GDP but lower than three-times GDP. Changes in the quality of life of stable or worsening HF patients had little effect on ICER.
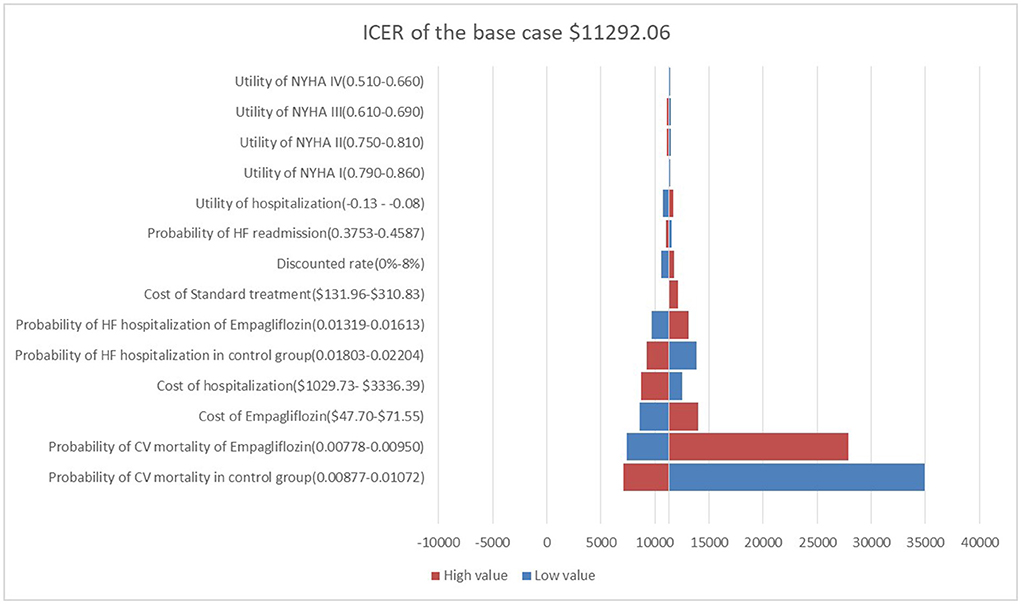
Figure 2. Tornado diagram showing the univariate sensitivity analysis of the Markov model simulation.
With 1,000 iterations, most ICERs fell in the upper-right quadrant, indicating that the add-on empagliflozin treatment usually produced higher costs and improved QALYs (Figure 3). PSA results revealed that when the WTP threshold was $12,652.5 and $37,957.5, the probability of being cost-effective for using add-on empagliflozin was 52.7 and 67.6%, respectively (Figure 4).
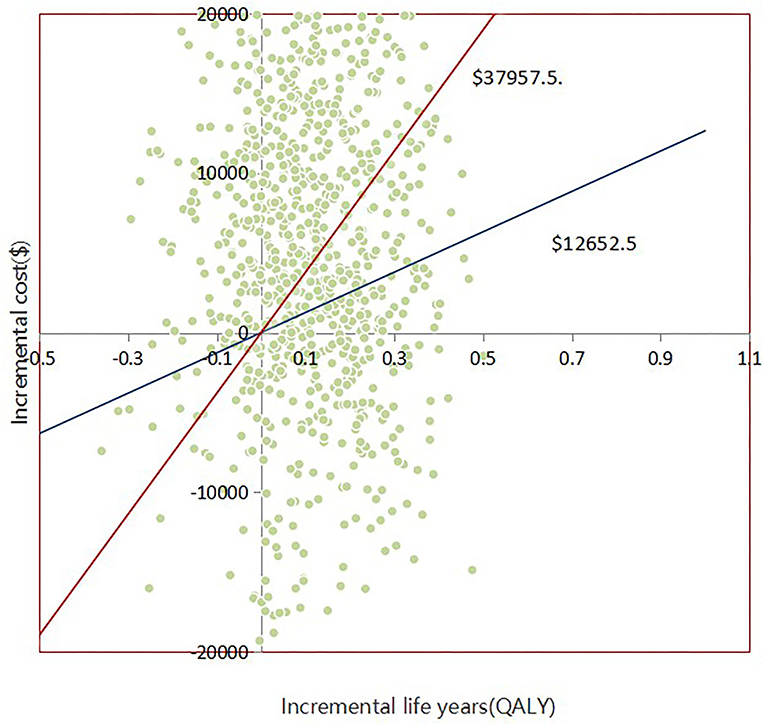
Figure 3. Scatter plot showing the incremental costs and incremental quality-adjusted life-year of a thousand simulations for Empagliflozin group and Control group.
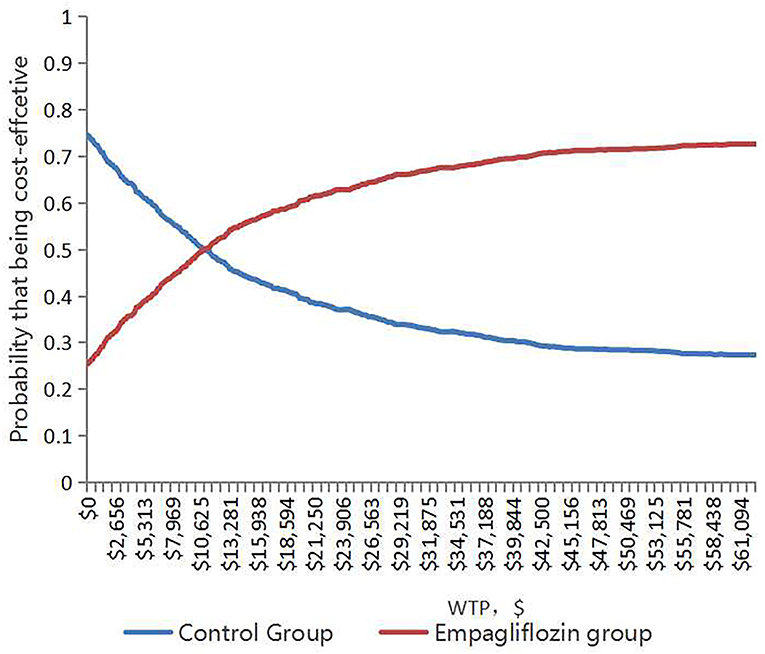
Figure 4. The cost-effectiveness acceptability curve shows the maximum willingness to pay and the corresponding probability of cost-effectiveness for the Empagliflozin group and Control group.
Based on scenario analysis, it was more cost-effective to add empagliflozin treating NYHA I, III, and IV HFpEF patients. The lower national purchase price of empagliflozin, the higher cost of hospitalization for HFpEF with comorbidity, and the longer time horizon contributed to more cost-effectiveness (Table 4).
Discussion
The study explored the cost-effectiveness of adding empagliflozin to standard therapy for patients with HFpEF based on the Markov model. HFpEF patients spent $11,292.06 for each QALY, which was lower than the one-time GDP in China. Adding empagliflozin to standard therapy for patients with HFpEF was cost-effective. One-way sensitivity analysis, PSA, and the scenario analysis were applied to ensure the robustness of the results. The add-on empagliflozin treatment for HFpEF could not only reduce the risk and cost of hospitalization related to HF or outpatient or emergency but also improve the quality of life, mainly because chronic clinical events seriously affected the quality of life (29). Overall, our study provided a valuable quantitative assessment of empagliflozin for medical decision-makers and healthcare payers.
In the sensitivity analysis, it was found that CV death in both groups was the most sensitive to the ICER. Although the risk of CV death was not statistically different between the two groups (hazard ratio, 0.92; 95% CI: 0.75–1.12) (9), comparing outcomes using absolute risk differences provided an alternative picture of therapy benefits, prompting us to explore the cost-effectiveness of adding empagliflozin to standard therapy. If empagliflozin could reduce more CV deaths, the empagliflozin group would produce more QALYs, and the ICER would be lower and more cost-effective.
Data on CV death came from the general population, and the rate of CV death in the Asian population was greater than that in the general population (9), and the ICER was more cost-effective in reality. On the other hand, costs of empagliflozin and hospitalization for HF substantially influenced the pharmacoeconomic benefits, the national purchase price of empagliflozin ($0.275 per 10 mg, once daily) brought more pharmacoeconomic benefits based on the scenario analysis (ICER of $3,292.60 per QALY). The higher the cost of hospitalization, the more cost-effective the adding empagliflozin. For example, the prevalence of HF in China was 1.3%, an increase of 44.0% from 2000 and there are 8.9 million HF patients, and our model predicted that adding empagliflozin to standard therapy could reduce about 17,800 hospitalizations a year and save the cost of hospitalization by $30 million a year. Adding empagliflozin could also reduce the incidence of hospitalizations for HF requiring cardiac care unit/intensive care unit care, emergency or urgent care visits for worsening HF requiring intravenous therapy outpatients, and outpatient intensification of diuretics additionally (17). In the scenario analysis of the time horizon, the longer the time horizon, the more pharmacoeconomic benefits yielded by adding empagliflozin. HF was a chronic disease and required long-term medication. When the simulated horizon was longer than 0.75 and 8 years, respectively, the ICER was lower than the WTP threshold of one-time and three-times GDP. Although NYHA II HFpEF patients spent more cost to gain a QALY due to over 80% of NYHA II HFpEF patients from the EMPEROR-Preserved trial, adding empagliflozin was still cost-effective.
Cost-effectiveness analyses of the add-on empagliflozin in HFrEF had also been proved to be cost-effective in China, the Asia-Pacific Region, and Thailand (30–32), but at the local Thai threshold of 4,773.27 $/QALY, adding empagliflozin to standard therapy yielded a QALY gain of 0.05 at an increased total cost of $622.49 compared to standard therapy alone (ICER: 11,809 $/QALY gained), in which empagliflozin was not a cost-effective add-on treatment for patients with HFpEF (32). We thought the local Thai threshold was much lower than our WTP. Reifsnider et al. reported an economic evaluation of empagliflozin combined with standard therapy for HF patients from healthcare system perspectives in America and the United Kingdom. However, the relevant parameters in that study came from subgroup data from the EMPA-REG OUTCOME trial, which included HFrEF. So the study might not fully reflect the pharmacoeconomic advantages of empagliflozin in HFpEF (33). PSA revealed that when the WTP threshold was $12,652.5 and $37,957.5, the probability of being cost-effective for using add-on empagliflozin was 52.7 and 67.6%, respectively, differing from some published cost-effective study, whose probability was mostly higher than 90% (21, 25), owing to the expensive healthcare expenditure and the higher WTP threshold. Our results were in keeping with basic national conditions and suitable for the healthcare system in China.
To our best knowledge, we are the first to study the pharmacoeconomics of the add-on empagliflozin treatment for HFpEF. Previous studies have focused on HFrEF or HF as a homogeneous group (34). I-Preservevd trial, CHARM-Preserved trial, PARAGON-HF trial, and TOPCAT trial showed that irbesartan, candesartan, SAC/VAL, and spironolactone did not reduce the risk of CV or hospitalization for HF among patients with HFpEF (7, 35, 36). Empagliflozin was the first to be proved to improve HFpEF by a randomized controlled trial with sufficient statistical efficacy. Some molecular mechanisms proposed that empagliflozin could improve the specific circulating microRNA to regulate the endothelial function significantly and empagliflozin could reduce inflammatory and oxidative stress in HFpEF to decrease pathological cardiomyocyte stiffness by inhibiting NHE1 (Na+/H+ exchanger 1), which supported the wide use of empagliflozin in HFpEF (37–39). However, over 86% of HFpEF were using ACE-I/ARBs, 80% were using beta-blockers, and over 24% were using spironolactone in reality (7), which increased the burden of treating HFpEF to some extent. Although there were no head-to-head trials to compare the results of irbesartan, candesartan, SAC/VAL, and spironolactone, because of clinical effects from previous studies or pharmacoeconomic benefits from our cost-effectiveness analysis, empagliflozin was the first choice for HFpEF.
HFpEF patients had many comorbidities including diabetes, half of HFpEF patients in the EMPEROR-Preserved trial had diabetes (9). Although studies have shown that empagliflozin with or without metformin was cost-effective in the treatment of T2DM (40, 41), there was no cost-effective study on the subgroups analysis of HFpEF with diabetes, and clinical outcomes in the diabetes subgroup were similar to that in the general population (9). Adding empagliflozin to standard therapy was a well cost-effective choice for HFpEF with T2DM.
This was a mathematical model combined with national conditions in China, which was suitable for healthcare system in China but may not be suitable for other countries. Hospitalization for non-HF was not considered in our model, but the EMPEROR-Preserved trial showed that empagliflozin could also reduce the risk of all-cause hospitalization by 7% (9). Our results may be more reliable when considering this condition. It was worth mentioning that data on clinical event and utility were derived from other countries, which might cause racial bias to some extent and our study lacked relevant data for subgroup analysis. The fixed parameters such as event probability and utility were also the limiting factors in our model, because these parameters would change with age or interruption of treatment, but the sensitivity analysis explained that our model was robust over a relative wide range of parameters. We assumed that HFpEF in the model could tolerate each drug, ignoring the adverse events but the EMPEROR-Preserved trial showed that the most common adverse events including urinary tract infection, hypovolemia, renal failure, amputation, diabetic ketoacidosis, and gangrene were not significantly different (9). Other possible treatment options in the real world such as drug conversion, drug compliance, or heart transplantation had not been measured. Indirect costs such as productivity loss and disability for HF were not considered in the model because our primary objective was to compare the cost-effectiveness of adding empagliflozin to standard therapy in HFpEF. The model structure in this cost-effectiveness analysis focused on only the CV outcomes of HFpEF patients and other clinically important diseases such as kidney diseases were not considered.
Conclusion
Generally, from the perspective of the healthcare system in China, this study found that adding empagliflozin to the standard therapy for HFpEF is regarded as a cost-effective option, which provided new insights for medical decision-makers. We believed that our findings would provide some guidance for cost-effective analysis in other countries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YJ collected the data regarding heart failure, analyzed the data, and developed a Markov model. Both authors have read and approved the final manuscript.
Acknowledgments
We thank Ting Zhou for advice on data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
QALY, Quality-Adjusted Life Year; ACEI, Angiotensin-Converting Enzyme Inhibitor; HFrEF, Heart Failure with Reduced Ejection Fraction; HFpEF, Heart Failure with Preserved Ejection Fraction; EMPEROR-Preserved, The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; T2DM, Type 2 Diabetes Mellitus; Sac/VAL, Sacubitril/Valsartan; ICER, the Incremental Cost-Effectiveness Ratio; WTP, the Willingness to Pay; ARB, Angiotensin Receptor Antagonists; ARNI, Angiotensin Receptor-Neprilysin Enzyme Inhibitor; SGLT-2 Inhibitor, Sodium-Glucose Cotransporter 2 Inhibitor; WHO, the World Health Organization; GDP, Gross Domestic Product; CEACs, Cost-Effectiveness Acceptability Curves; NYHA, New York Heart Association; CV, Cardiovascular.
References
1. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet. (2018) 391:572–80. doi: 10.1016/S0140-6736(17)32520-5
2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail. (2013) 6:606–19. doi: 10.1161/HHF.0b013e318291329a
3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol. (2016) 74:1037–147. doi: 10.5603/KP.2016.0141
4. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. (2014) 171:368–76. doi: 10.1016/j.ijcard.2013.12.028
5. Abdul-Rahim AH, Perez AC, MacIsaac RL, Jhund PS, Claggett BL, Carson PE, et al. Risk of stroke in chronic heart failure patients with preserved ejection fraction, but without atrial fibrillation: Analysis of the CHARM-Preserved and I-Preserve trials. Eur Heart J. (2017) 38:742–50. doi: 10.1093/eurheartj/ehw509
6. Hegde SM, Claggett B, Shah AM, Lewis EF, Anand I, Shah SJ, et al. Physical activity and prognosis in the TOPCAT trial (Treatment of preserved cardiac function heart failure with an aldosterone antagonist). Circulation. (2017) 136:982–92. doi: 10.1161/CIRCULATIONAHA.117.028002
7. Solomon SD, McMurray J, Anand IS, Ge J, Lam C, Maggioni AP, et al. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381:1609–20. doi: 10.1056/NEJMoa1908655
8. Chao EC, Henry RR. SGLT2 inhibition–a novel strategy for diabetes treatment. Nat Rev Drug Discov. (2010) 9:551–9. doi: 10.1038/nrd3180
9. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
10. Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. (2020) 19:91. doi: 10.1186/s12933-020-01066-9
11. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review. JACC Basic Transl Sci. (2020) 5:632–44. doi: 10.1016/j.jacbts.2020.02.004
12. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
13. Stalhammar J, Stern L, Linder R, Sherman S, Parikh R, Ariely R, et al. The burden of preserved ejection fraction heart failure in a real-world Swedish patient population. J Med Econ. (2014) 17:43–51. doi: 10.3111/13696998.2013.848808
14. Wu Y, Tian S, Rong P, Zhang F, Chen Y, Guo X, et al. Sacubitril-Valsartan compared with enalapril for the treatment of heart failure: A Decision-Analytic markov model simulation in china. Front Pharmacol. (2020) 11:1101. doi: 10.3389/fphar.2020.01101
15. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. (2015) 12:220–9. doi: 10.1038/nrcardio.2015.14
16. Research group of China Pharmacoeconomics Evaluation. China Guidelines for Pharmacoeconomic Evaluations (2019). China Pharmacoeconomics. (2019) 7:11–48.
17. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-Preserved trial. Circulation. (2021) 144:1284–94. doi: 10.1161/CIRCULATIONAHA.121.056824
18. Carson PE, Anand IS, Win S, Rector T, Haass M, Lopez-Sendon J, et al. The hospitalization burden and Post-Hospitalization mortality risk in heart failure with preserved ejection fraction: Results from the I-PRESERVE trial (Irbesartan in heart failure and preserved ejection fraction). JACC Heart Fail. (2015) 3:429–41. doi: 10.1016/j.jchf.2014.12.017
19. National Center for Chronic and Noncommunicable Disease Control and Prevention. Chinese Center for Disease Control and Prevention. In China Mortality Surveillance Dataset 2018. Beijing: China Science and Technology Press (2019).
20. Zhu S, Zhang M, Ni Q, Sun Q, Xuan J. Indirect, direct non-medical cost and qol by New York heart association (NYHA) classification in Chinese heart failure patients. Value Health. (2017) 20:A268. doi: 10.1016/S0735-1097(17)34107-4
21. King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost-Effectiveness of Sacubitril-Valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail. (2016) 4:392–402. doi: 10.1016/j.jchf.2016.02.007
22. Huang J, Yin H, Zhang M, Ni Q, Xuan J. Understanding the economic burden of heart failure in China: Impact on disease management and resource utilization. J Med Econ. (2017) 20:549–53. doi: 10.1080/13696998.2017.1297309
23. Ma XW. China Health Statistics Yearbook 2020. Beijing: China Union Medical University Press (2020).
24. Li L, Liu R, Jiang C, Du X, Huffman MD, Lam C, et al. Assessing the evidence-practice gap for heart failure in China: The heart failure registry of patient outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail. (2020) 22:646–60. doi: 10.1002/ejhf.1630
25. Park SK, Hong SH, Kim H, Kim S, Lee EK. Cost-utility analysis of Sacubitril/Valsartan use compared with standard care in chronic heart failure patients with reduced ejection fraction in south korea. Clin Ther. (2019) 41:1066–79. doi: 10.1016/j.clinthera.2019.04.031
26. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. (2005) 352:1539–49. doi: 10.1056/NEJMoa050496
28. National Bureau of statistics of the people's Republic of China. China Health Statistics Yearbook 2019. Beijing: Peking Union Medical College Press.
29. Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ. (2004) 13:429–36. doi: 10.1002/hec.853
30. Lin X, Lin M, Liu M, Huang W, Nie X, Chen Z, et al. Cost-effectiveness of empagliflozin as a treatment for heart failure with reduced ejection fraction: An analysis from the Chinese healthcare perspective. J Thorac Dis. (2022) 14:1588–97. doi: 10.21037/jtd-22-463
31. Liao CT, Yang CT, Kuo FH, Lee MC, Chang WT, Tang HJ, et al. Cost-Effectiveness evaluation of add-on empagliflozin in patients with heart failure and a reduced ejection fraction from the healthcare system's perspective in the Asia-Pacific region. Front Cardiovasc Med. (2021) 8:750381. doi: 10.3389/fcvm.2021.750381
32. Krittayaphong R, Permsuwan U. Cost-Utility analysis of combination empagliflozin and standard treatment versus standard treatment alone in Thai heart failure patients with reduced or preserved ejection fraction. Am J Cardiovasc Drugs. (2022). doi: 10.1007/s40256-022-00542-9. [Epub ahead of print].
33. Reifsnider OS, Kansal AR, Franke J, Lee J, George JT, Brueckmann M, et al. Cost-effectiveness of empagliflozin in the UK in an EMPA-REG OUTCOME subgroup with type 2 diabetes and heart failure. ESC Heart Fail. (2020) 7:3910–18. doi: 10.1002/ehf2.12985
34. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. doi: 10.1038/nrcardio.2017.65
35. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. (2008) 359:2456–67. doi: 10.1056/NEJMoa0805450
36. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet. (2003) 362:777–81. doi: 10.1016/S0140-6736(03)14285-7
37. Mone P, Lombardi A, Kansakar U, Varzideh F, Jankauskas SS, Pansini A, et al. Empagliflozin improves the microRNA signature of endothelial dysfunction in patients with HFpEF and diabetes. J Pharmacol Exp Ther. (2022). doi: 10.1124/jpet.121.001251. [Epub ahead of print].
38. Kolijn D, Pabel S, Tian Y, Lodi M, Herwig M, Carrizzo A, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Galpha oxidation. Cardiovasc Res. (2021) 117:495–507. doi: 10.1093/cvr/cvaa123
39. Bayes-Genis A, Iborra-Egea O, Spitaleri G, Domingo M, Revuelta-Lopez E, Codina P, et al. Decoding empagliflozin's molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Sci Rep. (2021) 11:12025. doi: 10.1038/s41598-021-91546-z
40. Reifsnider O, Kansal A, Pimple P, Aponte-Ribero V, Brand S, Shetty S. Cost-effectiveness analysis of empagliflozin versus sitagliptin as second-line therapy for treatment in patients with type 2 diabetes in the United States. Diabetes Obes Metab. (2021) 23:791–9. doi: 10.1111/dom.14268
Keywords: empagliflozin, Heart Failure with Preserved Ejection Fraction, China, cost-effectiveness analysis, healthcare systems
Citation: Jiang Y and Xie J (2022) Cost-effectiveness of adding empagliflozin to the standard therapy for Heart Failure with Preserved Ejection Fraction from the perspective of healthcare systems in China. Front. Cardiovasc. Med. 9:946399. doi: 10.3389/fcvm.2022.946399
Received: 17 May 2022; Accepted: 11 August 2022;
Published: 02 September 2022.
Edited by:
Gaurang Vaidya, University of Kentucky, United StatesReviewed by:
Simon Lebek, University of Regensburg, GermanyZiyu Meng, Tianjin Medical University Chu Hsien-I Memorial Hospital, China
Copyright © 2022 Jiang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Xie, eGllanVuMjAxOTIwMjImI3gwMDA0MDsxNjMuY29t
 Yaohui Jiang
Yaohui Jiang Jun Xie*
Jun Xie*