- 1Department of Cardiology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Peripheral Vascular Diseases, Honghui Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Department of Cardiovascular Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Background: Base excess (BE) represents an increase or decrease of alkali reserves in plasma to diagnose acid-base disorders, independent of respiratory factors. Current findings about the prognostic value of BE on mortality of patients with acute myocardial infarction (AMI) are still unclear. The purpose of this study was to explore the prognostic significance of BE for short-term all-cause mortality in patients with AMI.
Methods: A total of 2,465 patients diagnosed with AMI in the intensive care unit from the Medical Information Mart for Intensive Care III (MIMIC-III) database were included in our study, and we explored the association of BE with 28-day and 90-day all-cause mortality using Cox regression analysis. We also used restricted cubic splines (RCS) to evaluate the relationship between BE and hazard ratio (HR). The primary outcomes were 28-day and 90-day all-cause mortality.
Results: When stratified according to quantiles, low BE levels at admission were strongly associated with higher 28-day and 90-day all-cause mortality. Multivariable Cox proportional hazard models revealed that low BE was an independent risk factor of 28-day all-cause mortality [HR 4.158, 95% CI 3.203–5.398 (low vs. normal BE) and HR 1.354, 95% CI 0.896–2.049 (high vs. normal BE)] and 90-day all-cause mortality [HR 4.078, 95% CI 3.160–5.263 (low vs. normal BE) and HR 1.369, 95% CI 0.917–2.045 (high vs. normal BE)], even after adjustment for significant prognostic covariates. The results were also consistent in subgroup analysis. RCS revealed an “L-type” relationship between BE and 28-day and 90-day all-cause mortality, as well as adjusting for confounding variables. Meanwhile, Kaplan–Meier survival curves were stratified by combining BE with carbon dioxide partial pressure (PaCO2), and patients had the highest mortality in the group which had low BE (< 3.5 mEq/L) and high PaCO2 (> 45 mmHg) compared with other groups.
Conclusion: Our study revealed that low BE was significantly associated with 28-day and 90-day mortality in patients with AMI and indicated the value of stratifying the mortality risk of patients with AMI by BE.
Introduction
The most common cause of cardiovascular disease (CVD) mortality worldwide is acute myocardial infarction (AMI), which is characterized by myocardial cell death caused by prolonged ischemia. More than 2.4 million people in the USA die annually from it, more than 4 million people in Europe and Northern Asia, and about one-third of people in developed countries die from it (1). Although the mortality of AMI has declined substantially in recent decades due to lifestyle and coronary revascularization, global health is substantially impacted by myocardial infarction (2), and complications after myocardial infarction are still a threat to human health. Therefore, it is very important to continue to find effective biological indicators that can predict patient outcomes, such as the risk of early mortality.
The arterial blood gas (ABG) test is a commonly ordered test in intensive care units (ICUs) that also analyses pH and blood gases in addition to electrolytes (3). According to the current European heart failure guidelines, patients with acute heart failure (AHF) should measure their blood pH, partial pressure of carbon dioxide (PaCO2), and lactate levels (4). Among patients with AMI, HF is the most powerful predictor of death and it has important implications for treatment (5). Thus, several studies explored the predictive value of the index of ABG in AMI and revealed that many indexes of ABG had great sensitivity in predicting the mortality of patients with AMI (6–8). For example, Xu et al. found that the higher anion gap (AG) was significantly associated with an increased risk of mortality, and the measure of AG can be a robust and reliable predictor of AMI mortality during follow-up (7). In a recent study, Zhang et al. concluded that acidemia may increase the 30-day survival rate and 90-day survival rate of patients with AMI admitted to the ICU, while acidemia is typically diagnosed based on the ABG with pH < 7.35 (9). However, Miñana et al. found that arterial PaO2, PaCO2, and pH did not correlate with all-cause long-term mortality in patients diagnosed with AHF (10). It is well known that pH is affected by both respiration and metabolic factors, and it may not fully reflect the true acid-base status. However, base excess (BE) represents an increase or decrease of alkali reserves in plasma, independent of respiratory factors.
In previous research, it was found that high BE, but not low BE, was an independent predictor of long-term mortality in patients with AHF (11), indicating that BE was important for assessing acid-base balance, especially in the context of stratifying the mortality risk in patients with AHF. However, It is unknown whether BE is related to mortality in patients with AMI hospitalized in the hospital. Accordingly, we aimed to understand whether BE may serve as a predictive value for a prognosis for patients with AMI.
Materials and methods
Source of data
Our study is a retrospective analysis, in which data are extracted from a vast critical care database named Medical Information Mart for Intensive Care III (MIMIC III). The MIMIC III database is a free and large public database that comprises de-identified and definitional health-related data, which contains over 40,000 patients admitted to intensive care units (ICU) of the Beth Israel Deaconess Medical Center (Boston, MA, United States) between 2001 and 2012 (12). We extracted data from MIMIC III after passing the National Institutes of Health (NIH) web-based training course for protected human study participants. We also passed the Collaborative Institutional Training Initiative examination and applied for data access. The ethical committee waived informed consent because the patients were de-identified, and the MIMIC database contained no protected information. To protect the privacy of the participants, their identification information was concealed.
Participants
Patients with AMI who were admitted for the first time were included in our study. A total of 3,177 patients with AMI were identified using the International Classification of Diseases, Ninth Revision (ICD-9) codes. There were 192 patients who were under 18 years old or with an incorrect age and 520 patients without the result of BE. Thus, a total of 2,465 patients with AMI were ultimately enrolled in our study.
Variables
All variables were extracted from the MIMIC III database using Structured Query Language (SQL) with PostgreSQL (version 9.6). The variables in our study included (1) physical characters, including age, gender, and body mass index (BMI); (2) types of hospital admission, including elective, emergency, and urgent; (3) past history, including hypertension, diabetes, dyslipidemia, atrial fibrillation (AF), acute kidney injury (AKI), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), acute respiratory distress syndrome (ARDS), and sepsis; (4) vital signs, including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR), respiratory rate (RR), and temperature (T); (5) laboratory data, including pH, SpO2, PaCO2, bicarbonate (HCO3–), BE, AG, lactate, hemoglobin, platelet (PLT), white blood cell (WBC), albumin (ALB), urea nitrogen (BUN), creatinine (Scr), glucose, sodium, potassium, percutaneous coronary intervention (PCI), percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass grafting (CABG), Simplified Acute Physiology Score II (SAPS II), and Sequential Organ Failure Assessment (SOFA) score; and (6) oral medication, including aspirin, clopidogrel, beta blockers, diuretics, digitalis, and statin. All blood biochemical variables were the first measurement after the patient’s admission to the hospital before treatment.
Clinical outcomes
Records from the Social Security Death Index provided information on survivorship (including survival outcome and death date). Notably, 28-day and 90-day all-cause mortality after the date of ICU admission were analyzed in our study.
Statistical analysis
Variables in the categorical form are presented as numbers (percentages) and variables in continuous form as mean ± standard deviation if the distribution is normally distributed and median (interquartile range) otherwise. Continuous variables were analyzed using analysis of variance or Kruskal-Wallis tests to determine baseline differences among groups stratified by BE, and the chi-squared test or Fisher’s exact test was used for categorical variables. We also examined the relationship between BE and the outcomes (with the normal BE group as the reference group) using both univariate and multivariate Cox proportional-hazards models. Variables with P < 0.05 in the univariate Cox proportional-hazards model were entered into separate multivariate models for 28-day and 90-day all-cause mortality: model 1, crude; model 2, included age, gender, SBP, and DBP; model 3, included variables in model 2 and hypertension, AF, COPD, AKI, sepsis, and CHF; model 4, included variables in model 3 and aspirin, clopidogrel, beta-blockers, diuretics, digitalis, statin, PCI, and CABG; model 5, included variables in model 4 and sodium, potassium, ALB, BUN, Scr, AG, and SpO2. Meanwhile, restricted cubic splines (RCS) were used to separately explore the relationship between BE and hazard ratio (HR) of 28-day and 90-day mortality. The cumulative incidence of 28-day and 90-day all-cause mortality was presented using the Kaplan-Meier curve. Additionally, Kaplan-Meier curves were also generated for BE combined with PaCO2 as the respiratory factor. BE cutoff values (–3.5 mEq/L) were determined by receiver operating characteristic (ROC) analysis using the Youden index for 28-day all-cause mortality. To explore the link between admission BE values (modeled as continuous variables) and the risk of 28 and 90-day all-cause mortality, RCS was used. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Baseline characters
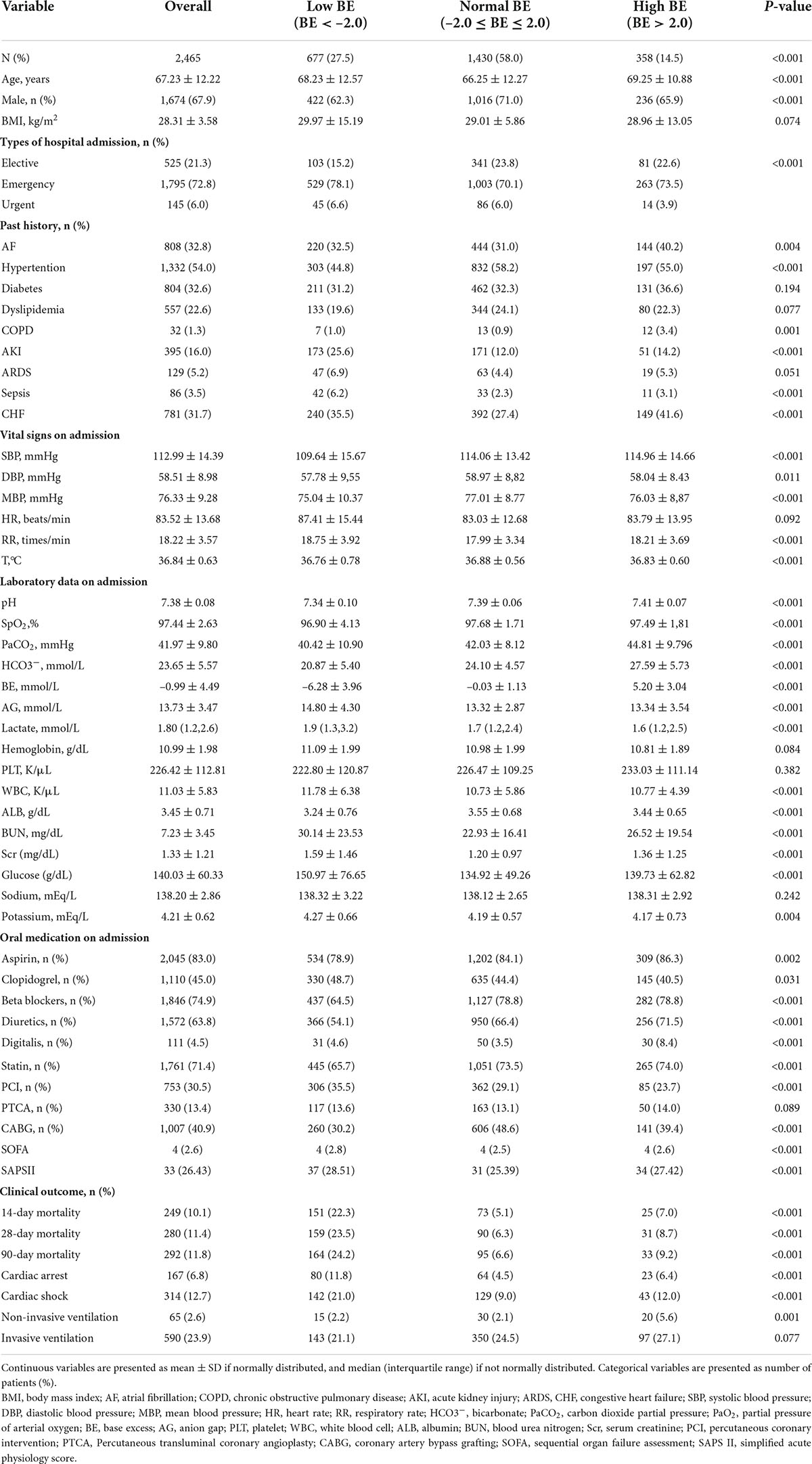
In total, 2,465 patients with AMI were included in our study, among which 1,430 (58.0%), 677 (27.5%), and 358 (14.5%) patients were classified as having normal BE, low BE, and high BE, respectively. The median age was 67 years and 1647 (67.9%) patients were male. Baseline clinical characteristics of patients stratified by BE are shown in Table 1. Patents with low BE were younger with an average age of 68.23. Meanwhile, patients with low BE were more likely to have higher HR, RR, AG, hemoglobin, WBC, BUN, Scr, glucose, and potassium. Moreover, as BE declined, the rate of AKI, ARDS, sepsis, SAPS II score, PCI, CABG, 28-day mortality, 90-day mortality, cardiac arrest, and cardiac shock was increasing. However, patients with low BE had lower SBP, DBP, MBP, T, pH, SpO2, PaCO2, HCO3–, PLT, and ALB as well as the rate of hypertension, diabetes, and invasive ventilation.
The association between base excess and the mortality of acute myocardial infarction
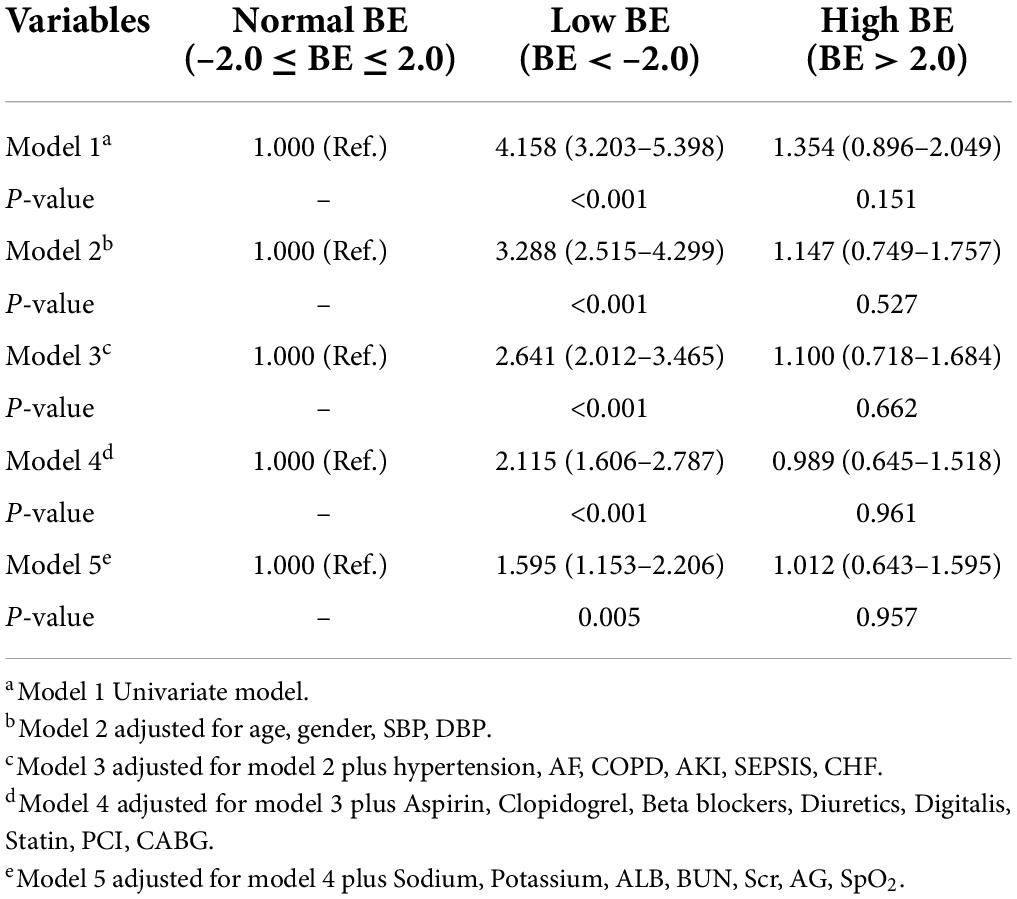
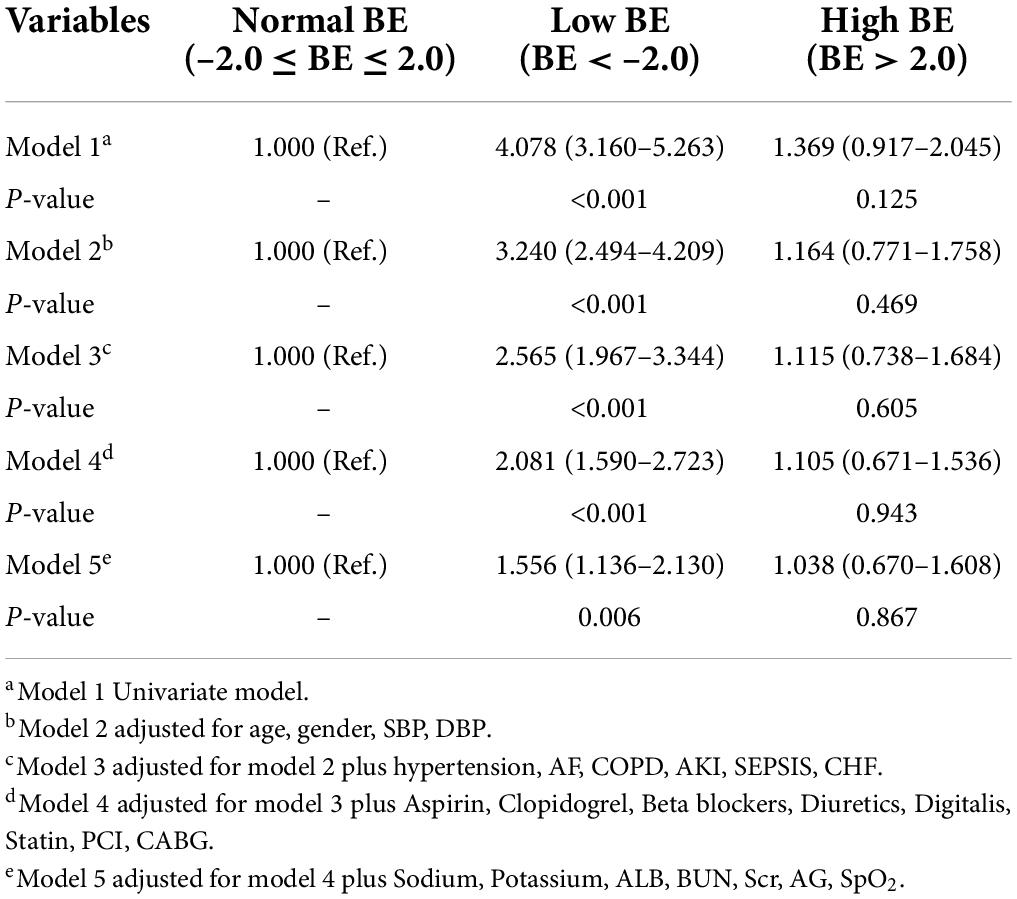
The unadjusted Cox proportional hazard regression model showed that the low BE group, but not a high BE group, was an independent determinant of the risk of 28-day mortality (HR 4.158, 95% CI 3.203–5.398) (Table 2). Despite further adjustments, the associations remained significant with a 1.595-fold increased risk (95% CI 1.153–2.206) in the final model. Meanwhile, the low BE group was still an independent determinant of the risk of 90-day mortality (HR 4.078, 95% CI 3.160–5.263), and the associations were still significant in the model after adjusting the relevant factors (Table 3).
To further explore the relationship between the BE and mortality of AMI, an analysis of Kaplan-Meier data revealed that the low BE group had significantly higher 28-day mortality (Figure 1) and 90-day mortality (Supplementary Figure 1) compared with the normal and high BE groups. Based on admission BE estimates, the RCS corresponding to the risk of 28-day all-cause mortality showed an “L-type” relationship between BE and the risk of mortality (Figure 2A). After adjustment by confounders including gender, age, SBP, DBP, hypertension, AF, COPD, AKI, sepsis, CHF, sodium, potassium, ALB, BUN, Scr, AG, and SpO2, as well as the use of aspirin, clopidogrel, beta-blockers, diuretics, digitalis, statin, PCI, and CABG, there was still an “L-type” relationship between BE and risk of 28-day all-cause mortality (Figure 2B). Similarly, the restricted spline curve also revealed that an “L-type” relationship between BE and 90-day mortality (Supplementary Figure 2A) and “L-type” relationship still exist after adjusting confounders (Supplementary Figure 2B).
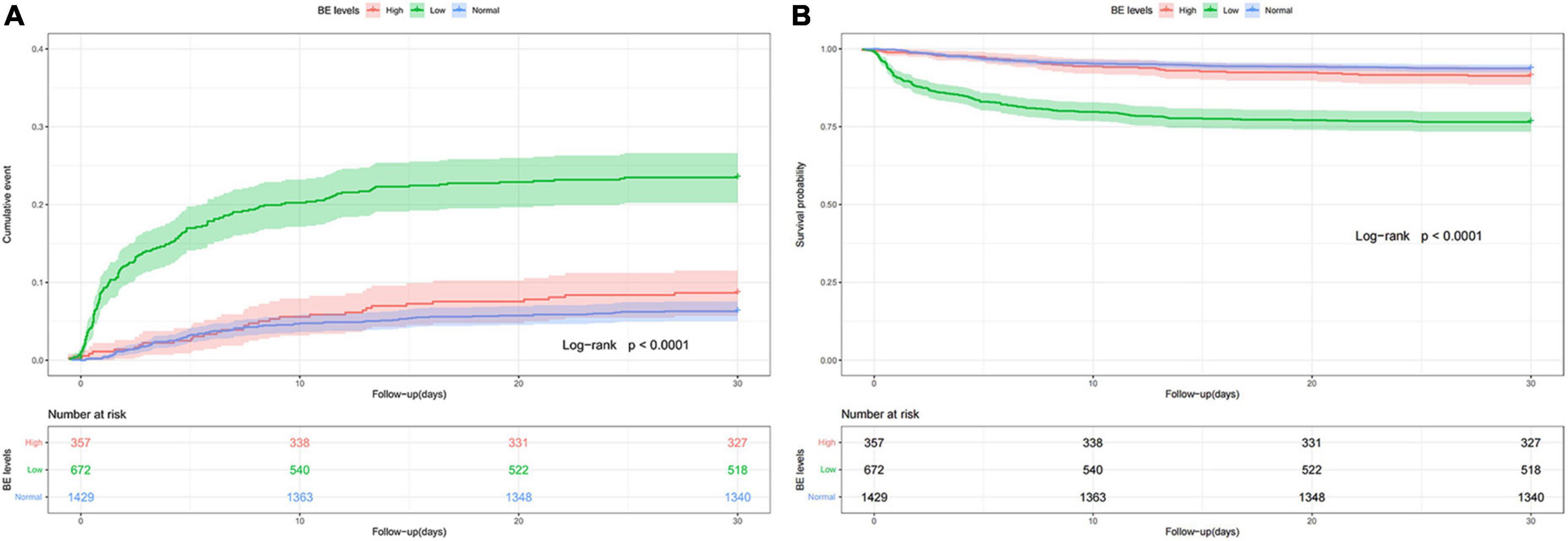
Figure 1. Cumulative incidence (A) and Kaplan-Meier curve (B) of 28-day all-cause mortality stratified by base excess.
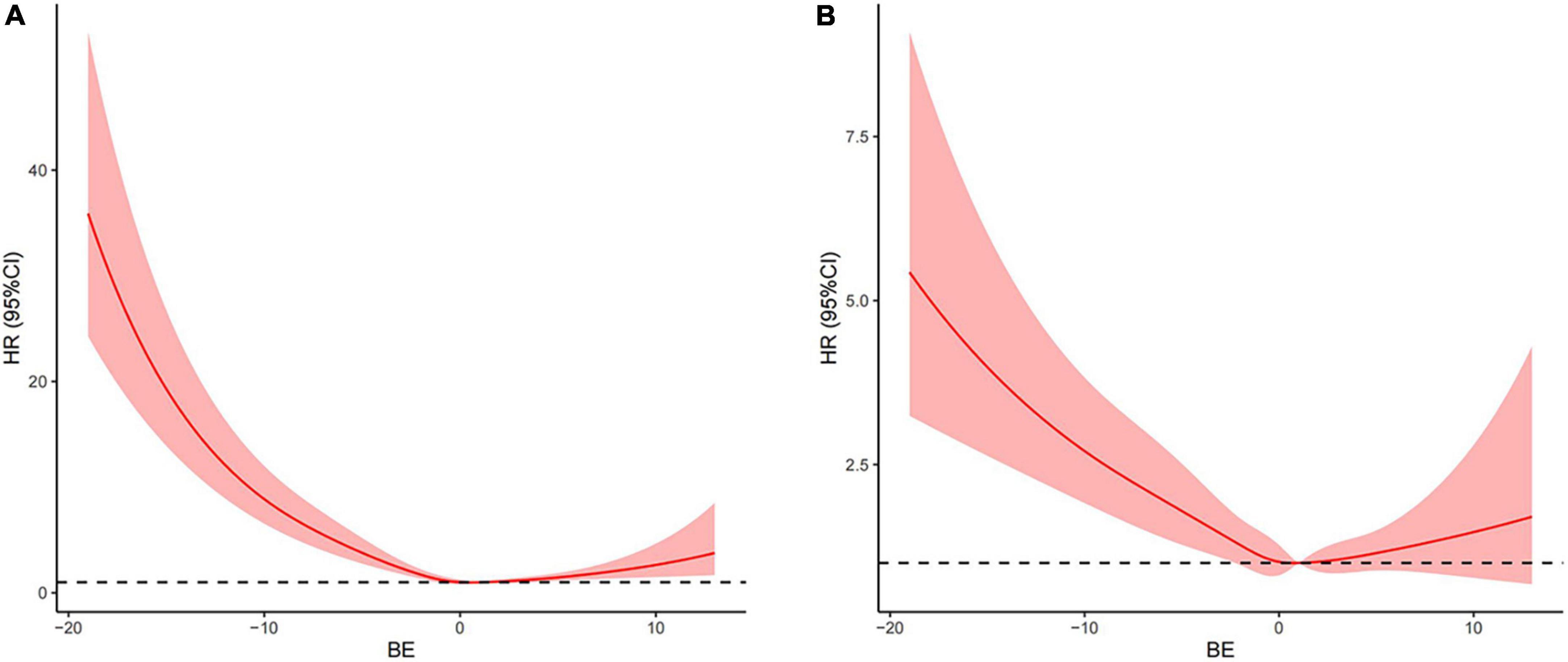
Figure 2. Associations between base excess (BE) on a continuous scale and adjusted risk of 28-day all-cause mortality in patients with AMI. Crude hazard ratio (HR) and 95% CI for BE in 28-day mortality (A). Adjusted HR and 95% CI for BE in 28-day mortality (B). The analyses used a model with restricted cubic splines. Adjusted variables included age, gender, systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), acute kidney injury (AKI), sepsis, congestive heart failure (CHF) aspirin, clopidogrel, beta-blockers, diuretics, digitalis, statin, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), sodium, potassium, albumin (ALB), urea nitrogen (BUN), creatinine (Scr), anion gap (AG), and SpO2, namely, model 5 described above.
Subgroup analysis
To further investigate whether the relationship would be different in various conditions, subgroup analyses were conducted for age, gender, hypertension, diabetes, pH, SpO2, and AKI, and the correlations between low BE and mortality in patients with AMI remained statistically significant in patients with different subgroups. After adjustment for the confounders, the HRs of 28-day mortality were generally increased in patients in the low BE group (Figure 3).
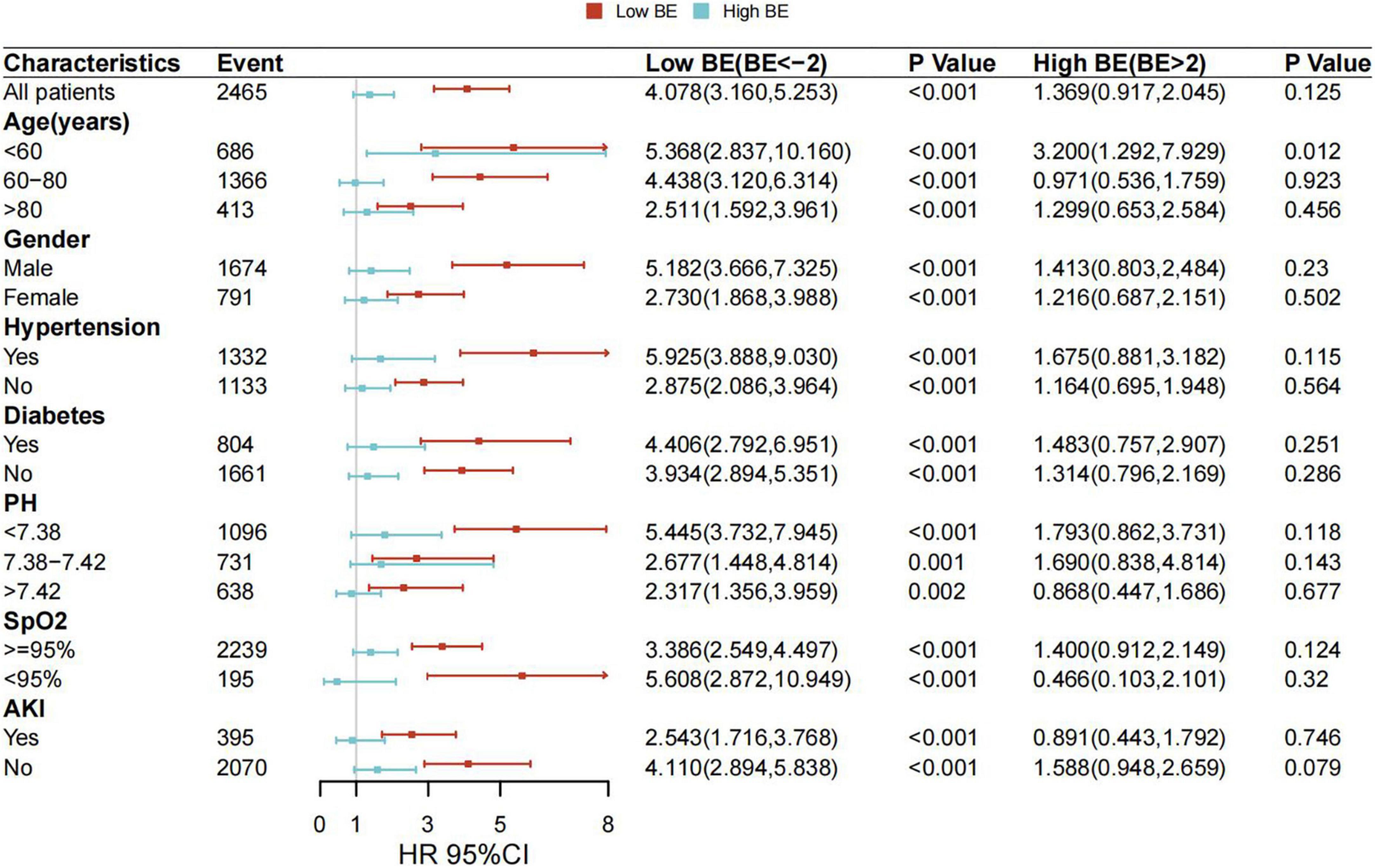
Figure 3. Associations between BE and risk of all-cause mortality in subgroups. Forest plot and adjusted HRs with 95% CI for 28-day all-cause mortality.
Relationship between base excess combined with carbon dioxide partial pressure and acute myocardial infarction mortality
To further determine the relationship between BE and mortality, we added PaCO2 as a respiratory component to BE. BE cutoff values (–3.5 mEq/L) were determined by ROC analysis for 28-day all-cause mortality using the Youden index (Supplementary Figure 3). Kaplan–Meier survival curves stratified by a combination of admission BE and PaCO2 are shown in Figure 4, which revealed that low BE with high PaCO2 level was associated with the highest 28-day all-cause mortality (Figure 4) and the highest 90-day all-cause mortality (Supplementary Figure 4) among the groups.

Figure 4. Cumulative incidence (A) and Kaplan-Meier curve (B) of 28-day all-cause mortality stratified by BE and PaCO2. BE cutoff values (–3.5 mEq/L) were determined by receiver operating characteristic (ROC) analysis for 28-day all-cause mortality using the Youden index.
Discussion
Our retrospective study of 2,456 patients with AMI showed that low BE was significantly associated with increased 28-day and 90-day all-cause mortality. The correlations between low BE levels and risk of cardiovascular events were independent of other cardiovascular risk factors, and they remained significantly stable in subgroup analyses. In contrast, high BE had no correlation to the mortality of patients with AMI. Moreover, an “L-type” association between BE and 28-day and 90-day all-cause mortality was found. These results indicated meaningful predictive implications in the clinical practice of BE.
Several studies have shown that BE is one of the most important tools for determining the severity of illness in acute care settings (9, 10, 13, 14). Miñana et al. revealed that admission pH, arterial PaO2, and PaCO2 had no association with all-cause mortality in patients with HF (10). However, Park et al. found that the most popular acid-base imbalance was respiratory alkalosis in patients with high-risk AHF, and pH provided additional prognostic value for patients with high-risk AHF and may be helpful for risk stratification and patient care. They also reported that there was no relationship between higher mortality with alkalosis or neutral pH as well as the cause of acidosis had no association with mortality of patients with AHF (15). However, Tang Zhang et al. observed that severe acidemia (non-acidemia, 7.35 ≤ pH ≤ 7.45; mild acidemia, 7.25 ≤ pH ≤ 7.35; severe acidemia, pH < 7.25) could increase the 30-day mortality in patients with AMI (9). Our findings were partly consistent with the results of Tang Zhang et al. and we found that the 28-day and 90-day all-cause mortality was closely related to acidemia in patients with AMI only when BE was low. Meanwhile, we found that the risks of 28-day and 90-day mortality were elevated in patients with AMI when BE was high regardless of whether the pH was acidic, neutral, or alkaline, and after adjusting for potential risk factors and covariates, the association remains significant. Therefore, BE could be a convenient and intuitive index for prognoses of patients with AMI independent of conventional cardiovascular risk factors.
There are several factors affecting BE concentrations and many possible reasons responsible for the difference between our results and others. Previous literature showed that the acid-base balance was first identified by pH. Nevertheless, pH was affected by metabolic and respiratory status. To stratify risk based on alkalemia or acidemia, only patients with those conditions were further investigated (16). Meanwhile, although the pH appeared to be neutral, it might be caused by mixed acid-base disturbances and might mask the actual existence of acid-base imbalance. In addition, some drugs and diseases could also affect the acid-base balance. For example, patients taking diuretics or who had chronic kidney disease or COPD frequently suffered from metabolic alkalosis, metabolic acidosis, or respiratory acidosis. At the same time, BE was considered to be the first accurate measurement of acid-base balance derived from non-respiratory sources (17). Thus, we first stratified the mortality risk by BE, which could separate respiratory factors from acid-base balance, revealing that low BE had the worst outcome of 28-day and 90-day mortality compared with high BE and normal BE. Meanwhile, RCS also showed that BE tended to associate with a worse prognosis with the “L-type” relationship in unadjusted models, and there also existed an “L-type” relationship after adjusting by covariates. Our findings indicated that the short-term prognosis in patients with AMI with metabolic acidosis, as determined by BE level at admission, is significantly worse than that of patients with metabolic alkalosis.
Metabolic acidosis is divided into high AG metabolic acidosis and hyperchloremic or normal AG metabolic acidosis. In addition to lactic acidosis and ketoacidosis, metabolic acidosis could be caused by ethylene glycol, methanol, and salicylate intoxication. Similarly, hyperchloremic metabolic acidosis is most commonly caused by renal tubular acidosis, gastrointestinal bicarbonate depletion, drug-induced hyperkalemia, early renal failure, and acid infusion. In patients with AMI, falling cardiac output combined with arterial hypoxemia causes tissue hypoxia, metabolic acidosis, and a drop in plasma bicarbonate due to lactic acid accumulation. Acidosis of the metabolism increases with disease severity and is an important cause of death. The build-up of acidic metabolites during cardiac ischemia results in a drop in intracellular and extracellular pH, reaching as low as 6.0–6.5. Ischemic injury is exacerbated by resulting tissue acidosis, which negatively affects cardiac function (18). In an earlier study of 50 patients with AMI, serum pH < 7.35 was significantly correlated with a patient mortality rate of > 60% (19). Bicarbonate can also reflect an acid-base state. In epidemiological studies, lower bicarbonate levels are associated with hypertension, both prevalent and incident (20–22), insulin resistance (23), progression of kidney disease (24), and mortality (25). These findings from previous literature show that metabolic acidosis worsens the prognosis supporting our results. But a cohort study of 6,229 community adults showed opposite results, compared with bicarbonate 23–24 mEq/L, and bicarbonate ≥ 25 mEq/L was associated with 3.0 g of greater LVM (95% CI 0.5–5.0) and 1.0 mm Hg of higher aortic PP (95% CI 0.4–2.0). Increases in bicarbonate concentration were associated with a 13% greater risk of HF (HR 1.13, 95% CI 1.01–2.11) (26), showing that higher serum bicarbonate levels were related to CVD subclinical stages and new HF. Additionally, among a well-characterized but smaller group of diabetics in Australia, higher serum bicarbonate was associated with a lower risk of incident coronary heart disease, but not HF (27). Evidently, although there are few studies that link hypercapnia with AMI, it is crucial to gain a better understanding of how acid-base balance impacts cardiovascular function.
Furthermore, our study revealed that the patients with coexisting metabolic acidosis and respiratory acidosis could have the worst prognosis among the four groups which had different BE levels and PaCO2. Hypercapnia can often be found in patients with AMI requiring intubation, and it is always associated with severe lung diseases or heart disease (28, 29). Notably, BE was a significant independent determinant of 28-day and 90-day all-cause mortality, but PaCO2 was not an independent variable in our study. When we combined the BE with PaCO2 to explore the prognosis of patients with AMI, the predictive efficiency would be much better. Therefore, our results could indicate that PaCO2 would be useful for risk stratification when in conjunction with BE.
Conclusion
Our study revealed that low BE was significantly associated with 28 and 90-day mortality in patients with AMI and indicated the value of stratifying the mortality risk of patients with AMI by BE, especially when combined with PaCO2. BE, as an easily obtained and important marker on admission for critically ill patients, could be used as a reliable predictor of prognosis in patients with AMI.
Limitations
Our study has some limitations. First, our study is an observational study, and we cannot determine the causal relationship between BE and the mortality of patients with AMI, so there needs a cohort study to explore the mechanism. Second, AMI-related risk factors were included as much as possible in our study, such as comorbidities and laboratory examinations. However, due to the limitations of the data, other residual confounding risk factors may not be included in the logistic model. So more detailed clinical cohort studies are still needed to support our study conclusions. Third, this study was limited to short-term outcomes, and data on the relationship between BE level and long-term outcomes of patients with AMI are still missing.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://mimic.mit.edu/docs/gettingstarted/.
Author contributions
CL, ZD, and GT designed the study. CL, ZD, TZ, QL, and DH analyzed and interpreted the data. CL and ZD drafted the manuscript. CL, ZD, TZ, DW, BW, PG, and GT revised the manuscript. All authors gave final approval of the final version to be published.
Funding
This study was supported by the Nature Science Foundation of China (Grant Nos. 81873513, 81600574, and 30871042), the Key Projects of Shaanxi Science and Technology Research and Development Plan (Grant No. 2018ZDXM-SF-049), the Key Project of Clinical Research in the First Affiliated Hospital of Xi’an Jiaotong University (Grant No. XJTU1AF-CRF-2018-005), and the Shaanxi Science and Technology Research and Development Plan of International Science and Technology (Grant Nos. 2012 kw-40-01 and 2014 JM2-8145).
Acknowledgments
We acknowledged the contributions of the MIMIC III (version 1.4) program registry for creating and updating the MIMIC III database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.942485/full#supplementary-material
Supplementary Figure 1 | Cumulative incidence (A) and Kaplan-Meier curve (B) of 90-day all-cause mortality stratified by base excess.
Supplementary Figure 2 | Associations between base excess (BE) on a continuous scale and adjusted risk of 90-day all-cause mortality in patients with AMI. Crude hazard ratio (HR) and 95% CI for BE in 90-day mortality (A). Adjusted HR and 95% CI for BE in 90-day mortality (B). The analyses used a model with restricted cubic splines. Adjusted variables included age, gender, systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), acute kidney injury (AKI), sepsis, congestive heart failure (CHF) aspirin, clopidogrel, beta-blockers, diuretics, digitalis, statin, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), sodium, potassium, albumin (ALB), urea nitrogen (BUN), creatinine (Scr), anion gap (AG), and SpO2, namely, model 5 described above.
Supplementary Figure 3 | Receiver operating characteristic (ROC) analysis for the diagnostic accuracy of BE in 28-day all-cause mortality.
Supplementary Figure 4 | Cumulative incidence (A) and Kaplan-Meier curve (B) of 90-day all-cause mortality stratified by BE and PaCO2.
References
1. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. (2017) 389:197–210. doi: 10.1016/S0140-6736(16)30677-8
2. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur Heart J. (2014) 35:2929. doi: 10.1093/eurheartj/ehu299
3. Bijapur MB, Kudligi NA, Asma S. Central venous blood gas analysis: An alternative to arterial blood gas analysis for pH, PCO2, bicarbonate, sodium, potassium and chloride in the intensive care unit patients. Indian J Crit Care Med. (2019) 23:258–62. doi: 10.5005/jp-journals-10071-23176
4. Authors/Task Force Members, Mcdonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131.
5. Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Fail. (2018) 6:179–86. doi: 10.1016/j.jchf.2017.09.015
6. Sahu A, Cooper HA, Panza JA. The initial anion gap is a predictor of mortality in acute myocardial infarction. Coron Artery Dis. (2006) 17:409–12. doi: 10.1097/00019501-200608000-00002
7. Xu C, Sun L, Dong M, Ullah H, Ullah H, Zhou J, et al. Serum anion gap is associated with risk of all-cause mortality in critically ill patients with acute myocardial infarction. Int J Gen Med. (2022) 15:223–31. doi: 10.2147/IJGM.S336701
8. Aleksandar J, Vladan P, Markovic-Jovanovic S, Stolic R, Mitic J, Smilic T. Hyperlactatemia and the outcome of Type 2 diabetic patients suffering acute myocardial infarction. J Diabetes Res. (2016) 2016:6901345. doi: 10.1155/2016/6901345
9. Zhang T, Guan YZ, Liu H. Association of acidemia with short-term mortality of acute myocardial infarction: A retrospective study base on MIMIC-III database. Clin Appl Thromb Hemost. (2020) 26:1076029620950837. doi: 10.1177/1076029620950837
10. Miñana G, Núñez J, Bañuls P, Sanchis J, Núñez E, Robles R, et al. Prognostic implications of arterial blood gases in acute decompensated heart failure. Eur J Intern Med. (2011) 22:489–94. doi: 10.1016/j.ejim.2011.01.014
11. Guo W, Peng C, Liu Q, Zhao L, Guo W, Chen X, et al. Association between base excess and mortality in patients with congestive heart failure. ESC Heart Fail. (2021) 8:250–8. doi: 10.1002/ehf2.12939
12. Johnson AE, Pollard TJ, Shen L, Lehman LWH, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
13. Davis JW, Shackford SR, Mackersie RC, Hoyt DB. Base deficit as a guide to volume resuscitation. J Trauma. (1988) 28:1464–7. doi: 10.1097/00005373-198810000-00010
14. Peñasco Y, González-Castro A, Rodrí-guez-Borregán JC, Llorca J. Base excess, a useful marker in the prognosis of chest trauma in the geriatric population. Rev Esp Anestesiol Reanim. (2017) 64:250–6. doi: 10.1016/j.redare.2017.03.003
15. Park JJ, Choi DJ, Yoon CH, Oh IY, Lee JH, Ahn S, et al. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: An analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail. (2015) 17:601–11. doi: 10.1002/ejhf.276
16. Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. (2008) 25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x
17. Berend K. Diagnostic use of base excess in acid-base disorders. N Engl J Med. (2018) 378:1419–28. doi: 10.1056/NEJMra1711860
18. Redd MA, Scheuer SE, Saez NJ, Yoshikawa Y, Chiu HS, Gao L, et al. Therapeutic inhibition of Acid-sensing ion channel 1a recovers heart function after ischemia-reperfusion injury. Circulation. (2021) 144:947–60. doi: 10.1161/CIRCULATIONAHA.121.054360
19. Gandhi A, Akholkar P. Metabolic acidosis in acute myocardial infarction. Int J Adv Med. (2015) 2:260–3. doi: 10.18203/2349-3933.ijam20150556
20. Mandel EI, Forman JP, Curhan GC, Taylor EN. Plasma bicarbonate and odds of incident hypertension. Am J Hypertens. (2013) 26:1405–12. doi: 10.1093/ajh/hpt133
21. Forman JP, Rifas-Shiman SL, Taylor EN, Lane K, Gillman MW. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens. (2008) 22:122–5. doi: 10.1038/sj.jhh.1002286
22. Taylor EN, Forman JP, Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. (2007) 50:320–4. doi: 10.1161/HYPERTENSIONAHA.107.092643
23. Mandel EI, Curhan GC, Hu FB, Taylor EN. Plasma bicarbonate and risk of type 2 diabetes mellitus. CMAJ. (2012) 184:E719–25. doi: 10.1503/cmaj.120438
24. Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. (2009) 24:1232–7. doi: 10.1093/ndt/gfn633
25. Raphael KL, Murphy RA, Shlipak MG, Satterfield S, Huston HK, Sebastian A, et al. Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study. Clin J Am Soc Nephrol. (2016) 11:308–16. doi: 10.2215/CJN.06200615
26. Kendrick JB, Zelnick L, Chonchol MB, Siscovick D, Hoofnagle AN, Ix JH, et al. Serum bicarbonate is associated with heart failure in the multi-ethnic study of atherosclerosis. Am J Nephrol. (2017) 45:118–26. doi: 10.1159/000454783
27. Paul Chubb SA, Davis WA, Peters KE, Davis TME. Serum bicarbonate concentration and the risk of cardiovascular disease and death in type 2 diabetes: The Fremantle Diabetes Study. Cardiovasc Diabetol. (2016) 15:143. doi: 10.1186/s12933-016-0462-x
28. Shigemura M, Lecuona E, Sznajder JI. Effects of hypercapnia on the lung. J. Physiol. (2017) 595:2431–7. doi: 10.1113/JP273781
Keywords: base excess, blood gas analysis, acute myocardial infarction, prognosis, all-cause mortality
Citation: Luo C, Duan Z, Zheng T, Li Q, Wang D, Wang B, Gao P, Han D and Tian G (2022) Base excess is associated with the risk of all-cause mortality in critically ill patients with acute myocardial infarction. Front. Cardiovasc. Med. 9:942485. doi: 10.3389/fcvm.2022.942485
Received: 12 May 2022; Accepted: 13 July 2022;
Published: 09 August 2022.
Edited by:
Nicola Cosentino, Unit of Experimental Cardio-Oncology and Cardiovascular Aging, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Chenyu Fan, Shanghai Jiao Tong University, ChinaJian Hou, Sun Yat-sen University, China
Copyright © 2022 Luo, Duan, Zheng, Li, Wang, Wang, Gao, Han and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Tian, dGlhbmdhbmdAeGp0dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Chaodi Luo1†
Chaodi Luo1† Gang Tian
Gang Tian