- 1Department of Medicine, Hualien Armed Forces General Hospital, Hualien City, Taiwan
- 2Department of Medicine, Tri-Service General Hospital and National Defense Medical Center, Taipei, Taiwan
- 3Department of Stomatology of Periodontology, Mackay Memorial Hospital, Taipei, Taiwan
- 4College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 5Department of Critical Care Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 6John Ochsner Heart and Vascular Institute, Ochsner Clinical School, The University of Queensland School of Medicine, New Orleans, LA, United States
Aim: This study was aimed to investigate the association of cardiometabolic and ECG markers with left ventricular diastolic dysfunction (LVDD) in physically active Asian young adults, which has not been clarified in prior studies.
Methods and results: A total of 2,019 men aged 18–43 years were included from the military in Taiwan. All the subjects underwent anthropometric, hemodynamic, and blood metabolic marker measurements. Physical fitness was investigated by time for a 3,000-m run. LVDD was defined by presence of either one of the three echocardiographic criteria: (1) mitral inflow E/A ratio < 0.8 with a peak E velocity of > 50 cm/s, (2) tissue Doppler lateral mitral annulus e′ <10 cm/s, and (3) E/e′ ratio > 14. Multiple logistic regressions with adjustments for age, physical fitness, and pulse rate were conducted to determine the association of cardiometabolic and ECG markers with LVDD. The prevalence of LVDD was estimated to be 4.16% (N = 84). Of the cardiometabolic markers, central obesity, defined as waist circumference ≥ 90 cm, was the only independent marker of LVDD [odds ratio (OR) and 95% confidence interval: 2.97 (1.63–5.41)]. There were no association for hypertension, prediabetes, and dyslipidemia. Of the ECG markers, left atrial enlargement and incomplete right bundle branch block/intraventricular conduction delay were the independent ECG markers of LVDD [OR: 2.98 (1.28–6.94) and 1.94 (1.09–3.47), respectively]. There was borderline association for Cornell-based left ventricular hypertrophy and inferior T wave inversion [OR: 1.94 (0.97–3.63) and 2.44 (0.98–6.08), respectively].
Conclusion: In the physically active Asian young male adults, central obesity and some ECG markers for left heart abnormalities were useful to identify LVDD.
Introduction
Left ventricular diastolic dysfunction (LVDD) is common in the early stage of heart failure (HF) (1). LVDD develops mostly due to stiff and reduced compliant left ventricle which impairs the relaxation or diastolic function and thus raises LV end-diastolic pressure as in rigorous exercise or stress (2). In addition, LVDD has been associated with greater risk of morbidity and mortality even in those with preserved LV ejection fraction (3, 4). The prevalence of LVDD in middle- or old-aged individuals is estimated to be 11–35% (4–6) when several cardiometabolic risk factors, e.g., obesity, diabetes, dyslipidemia, arterial hypertension, and coronary artery disease, commonly develop in midlife (7–9). In contrast, the Coronary Artery Risk Development in Young Adults (CARDIA) study revealed that the prevalence of LVDD in young adults with severe diastolic dysfunction was estimated only 1.1% and those with abnormal relaxation was estimated 9.3% (10).
The CARDIA study revealed that the LV diastolic filling of White and Black young adults is related to sex, age, body weight, systolic blood pressure (BP), heart rate, lung function, cardiac systolic function, and physical fitness but not related to electrocardiographic (ECG) LV mass index (10, 11). In addition, prolongation of corrected QT interval (QTc), on the basis of the Bazett's formula (12) representing relaxation of the LV and diastolic phase of electrical repolarization, has been identified as an independent ECG marker to predict the presence of LVDD in middle- and old-aged individuals (13, 14). Since the influences of physical fitness on cardiometabolic risk factors modifications, ECG changes and LVDD were rarely examined in Asian young adults, the aim of this study was to investigate the association of ECG and cardiometabolic markers with LVDD in a physically active military population in Taiwan.
Methods
Study population
The cross-sectional study included 2,688 military personnel aged 18–43 years from the cardiorespiratory fitness and health in eastern armed forces (CHIEF)-heart study in eastern Taiwan of ROC from 2018 to 2021 (15–17). Of them, 2,386 were men and 302 were women receiving daily physical training at their base. Each participant underwent annual health examinations for routine laboratory tests and self-reported their behavior, e.g., tobacco smoking and alcohol intake (active vs. former or never) in a questionnaire in the Hualien Armed Forces General Hospital. All the subjects participated in annual fitness exams for a 3,000-m run test to investigate their endurance capacity in the Hualien Military Physical Training and Testing Center (18, 19). After the exercise test, a 12-lead surface ECG and a transthoracic echocardiography were performed to assess each subject's cardiac structure and LV diastolic function before the end of the same year (20, 21). Participants were excluded if BP ≥ 140/90 mmHg (N = 346), body mass index (BMI) ≥ 35 kg/m2 (N = 32), or serum triglycerides ≥ 400 mg/dl (N =38), leaving a sample of 2,272 participants. Since there were merely 4 cases in 253 women fulfilling the criteria of LVDD in echocardiography, the group of women was further excluded from the analysis, leaving a sample of 2,019 men for the analysis.
Physical examinations and blood biochemistry measurements
Each subject's anthropometric variables of body height, body weight, and waist circumference (WC) were measured in a standing position. BMI was calculated as the ratio of body weight (kg) to the square of body height (m2). The BP and pulse rate of each subject were measured once over the right arm in a sitting position at rest using an automatic BP monitor machine (FT201; Parama-Tech Co., Ltd., Fukuoka, Japan) on the basis of the oscillometric method. Routine blood tests, including serum uric acid (SUA), total cholesterol, serum triglycerides, high-density lipoprotein cholesterol (HDL-C), and fasting plasma glucose, were measured with an auto-analyzer (Olympus AU640; Kobe, Japan). Each subject's blood sample was obtained after an overnight 12-h fast in the same station.
Cardiometabolic risk markers for LVDD
Metabolic syndrome was defined according to the latest criteria of International Diabetes Federation for Chinese (22) as having three or more clinical features: (1) fasting glucose ≥ 100 mg/dl or on anti-diabetic therapy, (2) HDL-C <40 mg/dl for men, (3) serum triglycerides ≥ 150 mg/dl or on lipid-lowering therapy, (4) systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 85 mmHg or on antihypertensive therapy, and (5) central obesity: WC ≥ 90 cm for men. Hyperuricemia was defined as SUA ≥ 7 mg/dl for men (23).
ECG and echocardiographic measurements
The ECG reports generated via the CARDIOVIT MS-2015 machine (Schiller AG, Baar, Switzerland) were reviewed and approved by a certificated cardiologist. Cornell ECG based-LVH in Asian young male adults was fulfilled if (R–aVL + S–V3) ≥ 18 mm (24), and Sokolow-Lyon ECG based-LVH was diagnosed if (S–V1 or S–V2 + R–V5 or R–V6) ≥ 35 mm (25). Sokolow-Lyon ECG based-RVH was defined if (R–V1 + S–V5 or S–V6) >10.5 mm (25), and Myers et al. ECG-based RVH was defined if (1) the R/S ratio of V1 > 1 or the R/S ratio of V5 or V6 < 1 or (2) R–V1 > 6 mm (25). ECG-based left atrial enlargement (LAE) was defined as a notched P wave in lead II ≥ 0.12 s or a notch of P wave ≥ 0.04 s (21). Inferior T wave inversion (TWI) was defined as one or more negative T wave axes in limb leads II, III, or aVF. First-degree atrioventricular block was diagnosed as a PR interval ≥ 200 ms. Complete and incomplete right bundle branch block (RBBB) should fulfill specific ECG patterns, e.g., Rsr of lead V1. The QRS duration of complete RBBB was ≥ 120 ms, and that of incomplete RBBB and intraventricular conduction delay (IVCD) ranged from 100 to 119 ms. A QTc interval ≥ 480 ms was defined as prolongation of QTc (26).
The transthoracic echocardiographic reports generated via the iE33 machine (Philips Medical Systems, Andover, MA, United States) were reviewed by a qualified cardiologist at the Hualien-Armed Forces General Hospital. According to the recommendations of the American Society of Echocardiography (27), LV mass was calculated using the corrected formula proposed by Devereux et al. (28): 0.8 × {1.04 × [(LV internal diameter (LVIDd) + posterior wall thickness + inter-ventricular septal thickness]3 – LVIDd3} + 0.6. The echocardiographic LVH index for body height (m2.7) was determined as ≥ 49 g/m2.7 for male adults (24). LVDD was defined as either one of the three criteria in echocardiography being fulfilled (29): (1) mitral inflow Doppler E/A ratio < 0.8 along with a peak E velocity of > 50 cm/s, (2) velocity of the lateral mitral annulus tissue Doppler, e′ < 10 cm/s, and (3) ratio of E/e′ > 14. Since our participants were physically active young men who might have a greater cardiac structure and function adaptations to exercise (20), the other two echocardiographic criteria for LVDD (29), (4) tricuspid regurgitation jet velocity > 2.8 m/s, and (5) left atrial volume index > 34 ml/m2, were still within the normal limits suggestive for athletes (30, 31) and not appropriate as inclusion criteria for LVDD in our military participants.
Statistical analysis
The clinical characteristics and cardiac features of the military men were presented as mean ± standard deviation (SD) for continuous variables and numbers (%) for categorical variables. Univariate linear regression was conducted to determine the correlation of each cardiometabolic risk marker and ECG marker with LVDD. Multiple linear regression was conducted to determine the independent cardiometabolic risk biomarkers and abnormal ECG surrogates of LVDD. In addition, multivariable logistic regression was carried out to determine the odds ratio (ORs) of all cardiometabolic risk factors and all ECG markers separately, with adjustments for smoking, alcohol intake status, age, pulse rate, and physical fitness. A receiver operating characteristic (ROC) curve for each significant predictor of LVDD was used to determine their cut-off point, positive predictive value (PPV), and negative predictive value (NPV). A value of P < 0.05 was regarded significant. All the analyses were performed using SPSS version 25.0 for Windows (IBM Corp., Armonk, NY, United States). This study was carried out in compliance with the Declaration of Helsinki principles, and was approved by the Institutional Review Board of the Mennonite Christian Hospital (No. 16-05-008) in Hualien, Taiwan. Written informed consent was obtained from all the participants.
Results
Clinical characteristics and cardiovascular features
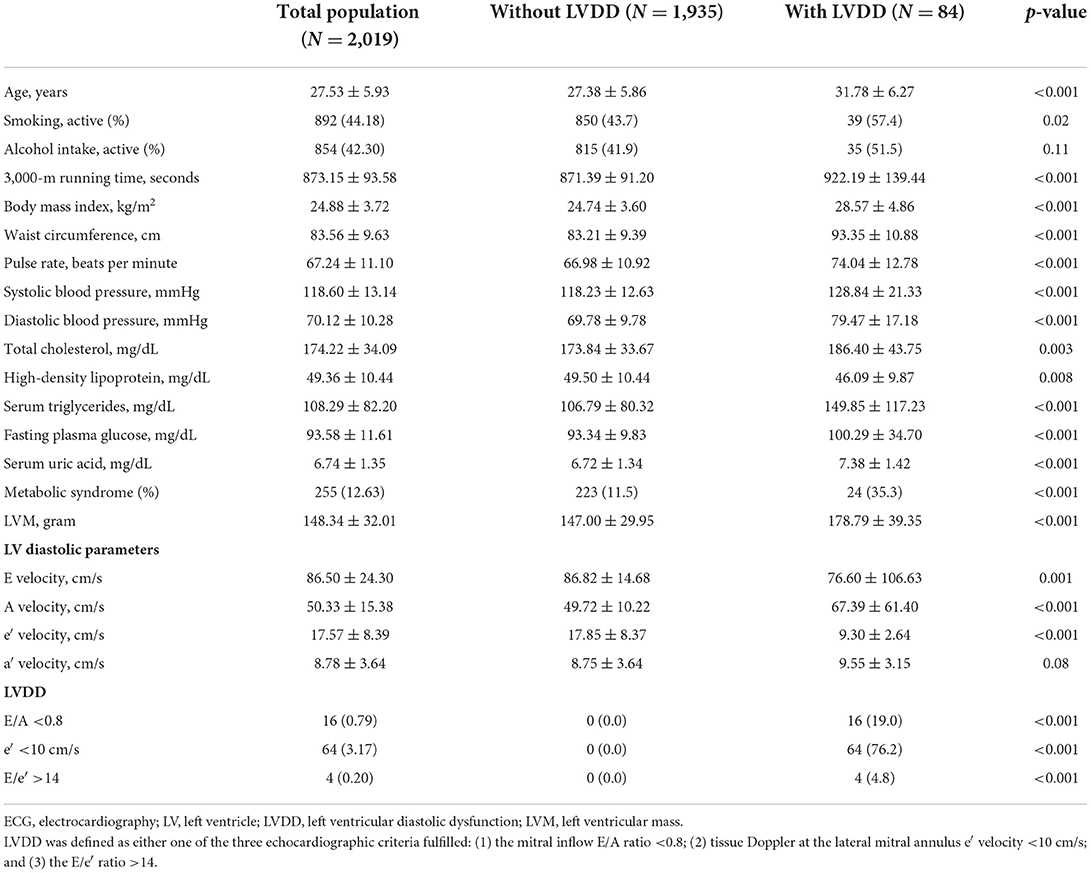
The clinical characteristics and CV features of the study population are shown in Table 1. The average age of the physically active men was 27.5 years. The mean pulse rate was 67.2 beats per min. In the study population, the prevalence of metabolic syndrome was ~12.6%. In total, there were 892 (44.2%) active smokers, 854 (42.3%) active alcohol consumers; 84 of the participants (4.16%) fulfilled the criteria for LVDD. In general, those with LVDD were older and had greater prevalence of cardiometabolic risk factors.
Correlations of cardiometabolic and ECG markers with LV diastolic function markers of LV in young men
The univariate and multivariable linear regression results of cardiometabolic and ECG markers with LV diastolic function markers are shown in Table 2. In the univariate analyses, all of the cardiometabolic markers were significantly correlated with E/A ratio, e′ velocity, and E/e′ ratio. In the multiple linear regression models for E/A ratio and E/e′ ratio, WC was the only independent cardiometabolic marker of E/A and E/e′ {β: −0.3 [95% confidence interval (CI): −0.41, −0.19] and 0.015 (95% CI: 0.005, 0.025), respectively}. With regard to e′, diastolic BP and WC were the independent predictors of e′ [β: −0.069 (95% CI: −0.116, −0.021) and −0.093 (95% CI: −0.138, −0.047), respectively].
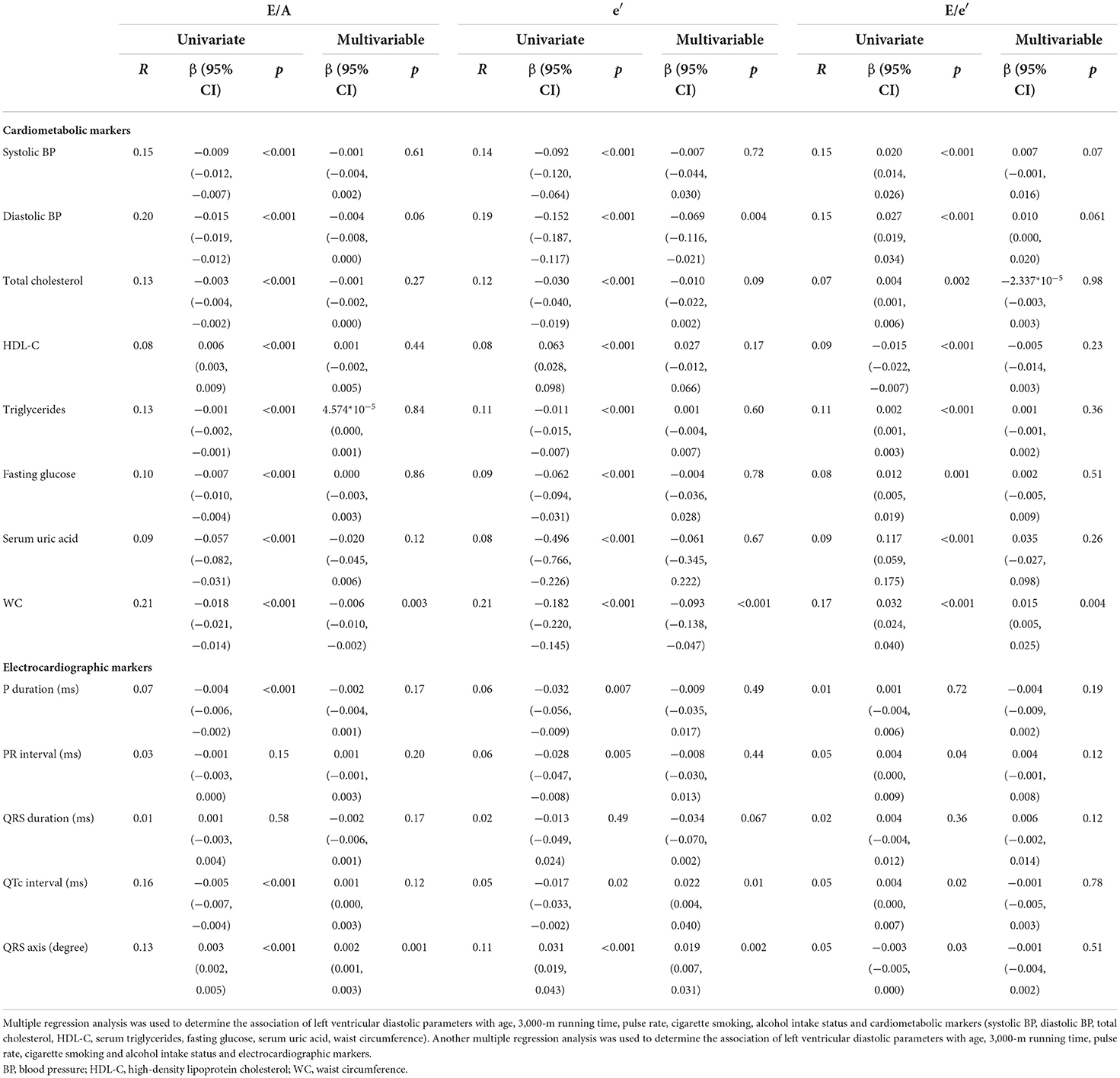
Table 2. Correlations of cardiometabolic and electrocardiographic markers with left ventricular diastolic parameters in physically active young male adults.
In the univariate analyses for the ECG markers, P duration, QTc interval, and QRS axis were significantly correlated with E/A ratio and e′ velocity. PR interval, rather than P duration, was significantly correlated with E/e′ ratio. In the multiple linear regression models for E/A ratio, QRS axis was the only independent ECG marker of E/A [β: 0.002 (95% CI: 0.001, 0.003)]. For e′, QTc interval and QRS axis were the independent ECG markers of e′ [β: −0.095 (95% CI: −0.17, −0.02), 0.072 (95% CI: 0.01, 0.135), and 0.022 (95% CI: 0.004, 0.04), respectively]. With regard to E/e′ ratio, there were no independent ECG makers of E/e′.
Cardiometabolic markers for LVDD in young men
Table 3 shows the results of multiple logistic regression analysis for cardiometabolic markers of LVDD in young adults. Abdominal obesity was the only independent cardiometabolic risk marker of LVDD in model 1 [OR: 3.14 (95% CI: 1.73–5.69)]. An additional adjustment for physical fitness in model 2 did not alter the main results in model 1 [OR: 2.97 (95% CI: 1.63–5.41)]. There were no associations for hyperuricemia, prediabetes, hypertension, and dyslipidemia in models 1 and 2.
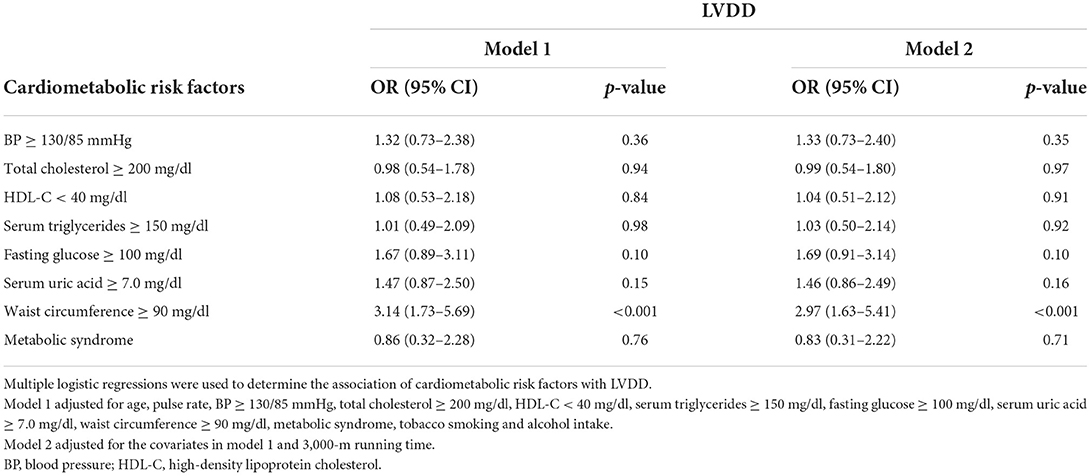
Table 3. Associations between cardiometabolic risk factors and echocardiographic left ventricular diastolic dysfunction in young men.
ECG markers for LVDD in young men
The multiple logistic regression analysis results for the ECG markers of LVDD in men are shown in Table 4. In model 2, ECG-based LAE, incomplete right bundle branch block, and QTc interval prolongation were the independent ECG markers of LVDD [OR: 1.94 (95% CI: 1.09–3.47), 2.98 (95% CI: 1.28–6.94), and 8.87 (95% CI: 1.62–48.6), respectively]. In addition, the association for Cornell ECG-based LVH and inferior TWI was borderline significant [OR: 1.88 (95% CI: 0.97–3.63) and 2.44 (95% CI: 0.98–6.08); p-values = 0.061 and 0.056, respectively]. The additional adjustment for physical fitness in model 2 yielded similar results compared to model 1.
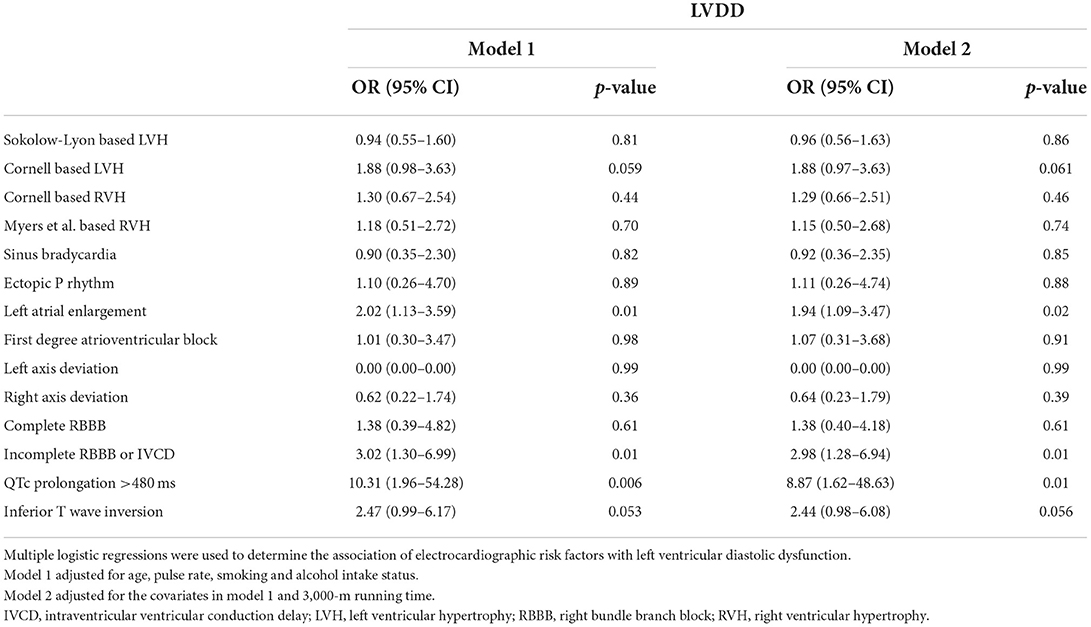
Table 4. Associations between electrocardiographic risk factors and echocardiographic left ventricular diastolic dysfunction in young men.
Cut-off points, PPV, and NPV for the predictors of LVDD by ROC analysis
Table 5 shows the results of cut-off values, PPV, and NPV for the independent predictors of LVDD in the multiple linear regression analysis by ROC analysis. The predictors included age, pulse rate, physical fitness (time for the 3,000-m run test), diastolic BP, WC, QTc interval and QRS axis, and area under curves of ROC for the predictors ranged from 0.62 to 0.76. Using the cut-off value for each predictor for LVDD, the NPV of each predictor was >95%, and the PPV of each predictor was <10%, in which the greatest PPV was observed in WC.
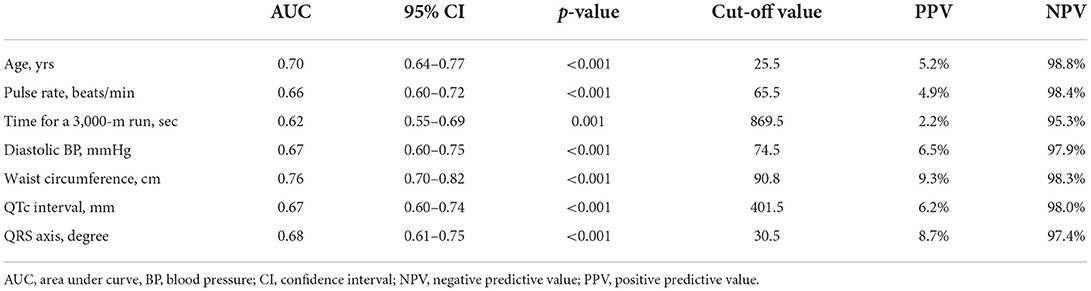
Table 5. Cut-off values, positive predictive values and negative predictive values for the predictors of left ventricular diastolic dysfunction by the receiver operating characteristic curve analysis.
Discussion
The principal findings of this study were that in the physically active men, diastolic BP and WC were the independent cardiometabolic markers, and that the QTc interval and QRS axis were the independent ECG markers correlated with the echocardiographic LV diastolic parameters. In addition, abdominal obesity was the only independent cardiometabolic risk factor, and ECG-based LAE, incomplete RBBB/IVCD, and QTc prolongation were the independent ECG risk factors of LVDD. There might be a suggestion that Cornell ECG-based LVH and inferior TWI were likely the ECG risk factors of LVDD in young men.
Several prior studies have revealed that diastolic BP was stronger than systolic BP in predicting the occurrence of CV disease (CVD) in young adults (32–34). In the CARDIA study, the association of CVD with diastolic BP being stronger than with systolic BP was only presented in White young adults and not in Black ones, indicating a racial difference (35). However, there were rare studies for Asian young adults. In a population study for Korean young adults, the risks of CVD between stage I isolated diastolic and systolic hypertension were similar (36). This study further emphasized that diastolic BP rather than systolic BP was independently and negatively correlated with e′, an LV diastolic parameter that was regarded as an early sign of LVDD and HF in young adults. In addition, central obesity has been well-known as a more potent risk marker than BMI in predicting LVDD (37). In this study, there was also a suggestion of an association between LVDD and inferior TWI, possibly because of leftward and horizontal displacement of the heart base in central obesity (19).
For the ECG markers, IVCD, Cornell-based LVH, and LAE might represent the status of great LV mass and pressure overload in the left heart (20). Incomplete RBBB and IVCD might reflect an increase in pulmonary artery pressure radiating from the left heart in the absence of RVH (38). As observed in prior studies on middle- and old aged individuals, prolongation of QTc, standing for abnormal LV relaxation, was also an independent risk factor of LVDD in physically active young adults in this study. In contrast to the CARDIA study (10, 11), we found that some ECG markers that were highly related to left heart abnormalities were useful to identify LVDD in Asian young adults. As the echocardiographic criteria (29) by tissue Doppler for LVDD were developed after 2016, there were no available data for tissue Doppler-defined LVDD in the CARDIA study. In this study, there were about three-fourths of LVDD cases diagnosed on the basis of the tissue Doppler criteria, indicating that tissue Doppler on the mitral annulus for e′ velocity may be a more sensitive tool for LVDD in young adults.
Study strengths and limitations
The major strength of this study was that the subjects were included from the military in which the training programs and living circumferences were similar, possibly eliminating unrecognized confounders. In contrast, there were some limitations in this study. First, the study included only men and the results could not be applied to women. Second, since this study had a cross-sectional design, temporal associations for the changes in LV diastolic function could not be assessed. Third, some subjects of LVDD might not be taken into account, since the criteria of greater tricuspid regurgitation velocity and left atrial volume index for LVDD (39, 40) were not regarded as inclusion criteria in this study. For instance, there were 384 subjects (19%) with a tricuspid regurgitation velocity > 2.8 m/s, which was possibly due to the effect of athletes' heart. Finally, since this study included physically active military men only, the generalizability might not be applicable to the general population of young adults.
Conclusion
Our study suggested that in physically active young men, central obesity and some ECG markers for left heart abnormalities were useful to identify LVDD. Whether some of the findings for the cardiometabolic and ECG markers were specific to Asian young adults should be verified in other Asian populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the Mennonite Christian Hospital (No. 16-05-008) in Hualien of Taiwan approved access to the data for the CHIEF study, and written informed consent was obtained from all participants. The patients/participants provided their written informed consent to participate in this study.
Author contributions
P-YL and G-ML wrote the article. K-ZT conducted the statistical analyses. W-CH interpreted the data. CL raised critical comments and edited the manuscript. G-ML was the principal investigator for the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Medical Affairs Bureau Ministry of National Defense (MND-MAB-D-11115) and Hualien Armed Forces General Hospital (HAFGH-D-111003), where the main place was involved in the study design, data collection, analyses, and writing of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.941912/full#supplementary-material
References
1. von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. (2010) 53:1033–45. doi: 10.1007/s00125-010-1682-3
2. Mascherbauer J, Zotter-Tufaro C, Duca F, Binder C, Koschutnik M, Kammerlander AA, et al. Wedge pressure rather than left ventricular end-diastolic pressure predicts outcome in heart failure with preserved ejection fraction. JACC Heart Fail. (2017) 5:795–801. doi: 10.1016/j.jchf.2017.08.005
3. Achong N, Wahi S, Marwick TH. Evolution and outcome of diastolic dysfunction. Heart. (2009) 95:813–8. doi: 10.1136/hrt.2008.159020
4. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. (2003) 289:194–202. doi: 10.1001/jama.289.2.194
5. Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. (2003) 24:320–8. doi: 10.1016/S0195-668X(02)00428-1
6. Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. (2006) 92:1259–64. doi: 10.1136/hrt.2005.080150
7. Pavlopoulos H, Grapsa J, Stefanadi E, Kamperidis V, Philippou E, Dawson D, et al. The evolution of diastolic dysfunction in the hypertensive disease. Eur J Echocardiogr. (2008) 9:772–8. doi: 10.1093/ejechocard/jen145
8. Zheng C, Chen Z, Zhang L, Wang X, Dong Y, Wang J, et al. Metabolic risk factors and left ventricular diastolic function in middle-aged chinese living in the Tibetan Plateau. J Am Heart Assoc. (2019) 8:e010454. doi: 10.1161/JAHA.118.010454
9. Inoue T, Morooka S, Hayashi T, Takayanagi K, Sakai Y, Takabatake Y. Left ventricular diastolic dysfunction in coronary artery disease: effects of coronary revascularization. Clin Cardiol. (1992) 15:577–81. doi: 10.1002/clc.4960150806
10. Xie X, Gidding SS, Gardin JM, Bild DE, Wong ND, Liu K. Left ventricular diastolic function in young adults: the Coronary Artery Risk Development in Young Adults Study. J Am Soc Echocardiogr. (1995) 8:771–9. doi: 10.1016/S0894-7317(05)80001-X
11. Desai CS, Colangelo LA, Liu K, Jacobs DR Jr, Cook NL, Lloyd-Jones DM, et al. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol. (2013) 177:20–32. doi: 10.1093/aje/kws224
13. Wilcox JE, Rosenberg J, Vallakati A, Gheorghiade M, Shah SJ. Usefulness of electrocardiographic QT interval to predict left ventricular diastolic dysfunction. Am J Cardiol. (2011) 108:1760–6. doi: 10.1016/j.amjcard.2011.07.050
14. Tamer T, Sayed K, Saad M, Samir M. How accurate can electrocardiogram predict left ventricular diastolic dysfunction? Egypit Heart J. (2016) 68:117–23. doi: 10.1016/j.ehj.2015.01.002
15. Lin GM, Lu HH. A 12-Lead ECG-based system with physiological parameters and machine learning to identify right ventricular hypertrophy in young adults. IEEE J Transl Eng Health Med. (2020) 8:1900510. doi: 10.1109/JTEHM.2020.2996370
16. Lin GM, Liu K. An electrocardiographic system with anthropometrics via machine learning to screen left ventricular hypertrophy among young adults. IEEE J Transl Eng Health Med. (2020) 8:1800111. doi: 10.1109/JTEHM.2020.2990073
17. Hsu CY, Liu PY, Liu SH, Kwon Y, Lavie CJ, Lin GM. Machine learning for electrocardiographic features to identify left atrial enlargement in young adults: CHIEF Heart study. Front Cardiovasc Med. (2022) 9:840585. doi: 10.3389/fcvm.2022.840585
18. Lin GM, Li YH, Lee CJ, Shiang JC, Lin KH, Chen KW, et al. Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in Eastern Taiwan. World J Cardiol. (2016) 8:464–71. doi: 10.4330/wjc.v8.i8.464
19. Lin YK, Tsai KZ. Han CL, Lin YP, Lee JT, Lin GM. Obesity phenotypes and electrocardiographic characteristics in physically active males: CHIEF study. Front Cardiovasc Med. (2021) 8:738575. doi: 10.3389/fcvm.2021.738575
20. Liu PY, Tsai KZ, Lima JAC, Lavie CJ, Lin GM. Athlete's heart in Asian military males: the CHIEF heart study. Front Cardiovasc Med. (2021) 8:725852. doi: 10.3389/fcvm.2021.725852
21. Lin YK, Tsai KZ, Han CL, Lee JT, Lin GM. Athlete's heart assessed by sit-up strength exercises in military men and women: the CHIEF heart study. Front Cardiovasc Med. (2022) 8:737607. doi: 10.3389/fcvm.2021.737607
22. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
23. Lin JW, Tsai KZ, Chen KW, Su FY, Li YH, Lin YP, et al. Sex-specific association between serum uric acid and elevated alanine aminotransferase in a military cohort: the CHIEF Study. Endocr Metab Immune Disord Drug Targets. (2019) 19:333–40. doi: 10.2174/1871530319666181129163802
24. Su FY, Li YH, Lin YP, Lee CJ, Wang CH, Meng FC, et al. A comparison of Cornell and Sokolow-Lyon electrocardiographic criteria for left ventricular hypertrophy in a military male population in Taiwan: the Cardiorespiratory fitness and HospItalization Events in armed Forces study. Cardiovasc Diagn Ther. (2017) 7:244–51. doi: 10.21037/cdt.2017.01.16
25. Chao WH, Su FY, Lin F, Yu YS, Lin GM. Association of electrocardiographic left and right ventricular hypertrophy with physical fitness of military males: the CHIEF study. Eur J Sport Sci. (2019) 19:1214–20. doi: 10.1080/17461391.2019.1595741
26. Vink AS, Clur SB, Wilde AAM, Blom NA. Effect of age and gender on the QTc-interval in healthy individuals and patients with long-QT syndrome. Trends Cardiovasc Med. (2018) 28:64–75. doi: 10.1016/j.tcm.2017.07.012
27. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
28. Devereux RB, Casale PN, Eisenberg RR, Miller DH, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. (1984) 3:82–7. doi: 10.1016/S0735-1097(84)80433-7
29. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
30. Dawkins TG, Curry BA, Wright SP, Meah VL, Yousef Z, Eves ND, et al. Right ventricular function and region-specific adaptation in athletes engaged in high-dynamic sports: a meta-analysis. Circ Cardiovasc Imaging. (2021) 14:e012315. doi: 10.1161/CIRCIMAGING.120.012315
31. D'Andrea A, Riegler L, Cocchia R, Scarafile R, Salerno G, Gravino R, et al. Left atrial volume index in highly trained athletes. Am Heart J. (2010) 159:1155–61. doi: 10.1016/j.ahj.2010.03.036
32. Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham heart study. Circulation. (2001) 103:1245–9. doi: 10.1161/01.CIR.103.9.1245
33. Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. (2001) 104:783–9. doi: 10.1161/hc3201.094227
34. Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, et al. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. (2014) 130:466–74. doi: 10.1161/CIRCULATIONAHA.113.004876
35. Reis JP, Tedla YG, Goff DC Jr, Jacobs DR Jr, Sidney S, et al. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: coronary artery risk development in young adults (CARDIA) study. JAMA Cardiol. (2017) 2:381–9. doi: 10.1001/jamacardio.2016.5678
36. Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd-Jones DM, et al. Cardiovascular risk of isolated systolic or diastolic hypertension in young adults. Circulation. (2020) 141:1778–86. doi: 10.1161/CIRCULATIONAHA.119.044838
37. Ammar KA, Redfield MM, Mahoney DW, Johnson M, Jacobsen SJ, Rodeheffer RJ. Central obesity: association with left ventricular dysfunction and mortality in the community. Am Heart J. (2008) 156:975–81. doi: 10.1016/j.ahj.2008.06.018
38. Scott RC, Kaplan S, Fowler NO, Helm RA, Westcott RN, Walker IC, et al. The electrocardiographic pattern of right ventricular hypertrophy in chronic cor pulmonale. Circulation. (1955) 11:927–36. doi: 10.1161/01.CIR.11.6.927
39. Ashour K. Early detection of diastolic dysfunction in diabetic patients (single center cross sectional study). J Heart Cardiovasc Res. (2018) 2:3. doi: 10.21767/2576-1455.1000115
Keywords: cardiometabolic risk factors, electrocardiography, left ventricular diastolic dysfunction, physical fitness, young adult
Citation: Liu P-Y, Tsai K-Z, Huang W-C, Lavie CJ and Lin G-M (2022) Electrocardiographic and cardiometabolic risk markers of left ventricular diastolic dysfunction in physically active adults: CHIEF heart study. Front. Cardiovasc. Med. 9:941912. doi: 10.3389/fcvm.2022.941912
Received: 12 May 2022; Accepted: 06 July 2022;
Published: 27 July 2022.
Edited by:
Chayakrit Krittanawong, New York University, United StatesReviewed by:
Ji-Guang Wang, Shanghai Institute of Hypertension, ChinaOxana Rotar, Almazov National Medical Research Centre, Russia
Roxana Mihaela Chiorescu, Iuliu Haţieganu University of Medicine and Pharmacy, Romania
Liwei Lang, Augusta University, United States
Raman Kutty, Amala Cancer Research Centre, India
Marco Matteo Ciccone, University of Bari Aldo Moro, Italy
Galaleldin Nagib Elkilany, Gulf Medical University, United Arab Emirates
Copyright © 2022 Liu, Tsai, Huang, Lavie and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gen-Min Lin, ZmFybWVyNTA3QHlhaG9vLmNvbS50dw==
 Pang-Yen Liu
Pang-Yen Liu Kun-Zhe Tsai1,3
Kun-Zhe Tsai1,3 Wei-Chun Huang
Wei-Chun Huang Carl J. Lavie
Carl J. Lavie Gen-Min Lin
Gen-Min Lin