
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 August 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.938902
 Xinyu Wang1†
Xinyu Wang1† Si Cheng1†
Si Cheng1† Jun Lv1,2,3
Jun Lv1,2,3 Canqing Yu1,2
Canqing Yu1,2 Yu Guo4
Yu Guo4 Pei Pei4
Pei Pei4 Ling Yang5,6
Ling Yang5,6 Iona Y. Millwood5,6
Iona Y. Millwood5,6 Robin Walters5,6
Robin Walters5,6 Yiping Chen5,6
Yiping Chen5,6 Huaidong Du5,6
Huaidong Du5,6 Haiping Duan7
Haiping Duan7 Simon Gilbert5
Simon Gilbert5 Daniel Avery5
Daniel Avery5 Junshi Chen8
Junshi Chen8 Yuanjie Pang1*
Yuanjie Pang1* Zhengming Chen5,6
Zhengming Chen5,6 Liming Li1,2* On behalf of the China Kadoorie Biobank Collaborative Group‡
Liming Li1,2* On behalf of the China Kadoorie Biobank Collaborative Group‡Background and aims: Liver biomarkers and metabolic associated fatty liver disease (MAFLD) have been shown to be associated with cardiovascular disease (CVD). However, there is limited evidence on CVD subtypes [myocardial infarction (MI), ischemic stroke (IS), and intracerebral hemorrhage (ICH)], especially in the Chinese population. We examined these associations overall, by genetic predisposition to non-alcoholic fatty liver disease (NAFLD), and by lifestyle risk factors.
Approach and results: This is a nested case-control study of CVD (10,298 cases and 5,388 controls) within the China Kadoorie Biobank. Cox regression was used to estimate adjusted hazard ratios (HRs) for CVD associated with liver biomarkers and MAFLD and by stratum of genetic risk and a combined high-risk lifestyle score. For liver enzymes, there were positive associations with MI and IS, but no associations with ICH or carotid plaque. There were positive associations of NAFLD with risks of MI, IS, and ICH (HR 1.43 [95% CI 1.30–1.57], 1.25 [1.16–1.35], and 1.12 [1.02–1.23]) as well as carotid plaque (odds ratio 2.36 [1.12–4.96]). The associations of NAFLD with CVD and carotid plaque were stronger among individuals with a high genetic risk (ICH: p-interaction < 0.05), while the associations with stroke were stronger among those with a favorable lifestyle (p-interaction < 0.05). The results for MAFLD mirrored those for NAFLD.
Conclusion: In Chinese adults, liver biomarkers and MAFLD were associated with risk of CVD, with different magnitudes of associations by CVD subtypes. Genetic predisposition to NAFLD and lifestyle factors modified the associations of fatty liver with stroke.
Cardiovascular disease (CVD) is one of the leading causes of morbidity and mortality worldwide, especially in people over 50 years old (1). In China, there were 5.09 million deaths from CVD in 2019, with stroke and ischemic heart disease (IHD) ranking the top two causes of death (2). According to the Global Burden of Disease in 2019, the age-standardized incidence rate (ASIR) of stroke in China was much higher than that in Western countries, so was the proportion of intracerebral hemorrhage (ICH). Moreover, ICH and IS accounted for a similar number of deaths in China, despite a three-fold higher incidence of IS.
Several liver biomarkers, including liver enzymes and fatty liver index (FLI), have been reported to be associated with CVD (3–11). Serum levels of liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase (GGT), are important indicators reflecting liver function and thus extensively measured in both clinical settings and population-based studies. FLI, calculated from triglycerides (TC), body mass index (BMI), GGT, and waist circumference (WC), is widely used as a non-invasive test for diagnosis of NAFLD in large-scale epidemiological studies (12). A recent meta-analysis reported that NAFLD was associated with increased risks of MI and IS (13). However, there is limited evidence on the associations of liver biomarkers with CVD subtypes (i.e., ischemic and hemorrhagic).
Previous prospective studies have shown a positive association between metabolic associated fatty liver disease (MAFLD), a newly suggested term to replace “non-alcoholic fatty liver disease (NAFLD)” (14), and CVD, both in Caucasians and East Asians (15–19). However, it is not clear whether the associations with CVD differ for NAFLD and MAFLD. Moreover, there is limited evidence whether the associations differ by genetic predisposition to NAFLD or lifestyle factors. Although a recent study in the UK Biobank (UKB) reported that fatty liver disease-related genetic variants amplified the health impact of MAFLD (16), the study was conducted in the Western population and may not be generalizable to the Chinese population, where substantial differences exist in genetics, lifestyles, and CVD subtypes. Assessing the associations between liver biomarkers and CVD may provide a better understanding of disease etiology and inform disease prognosis, while examining the interactions by genetic factors or lifestyles helps identify individuals who may benefit most from liver disease screening.
Therefore, we aimed to examine the associations of liver biomarkers (i.e., liver enzymes and FLI) and MAFLD with CVD subtypes and to assess whether the associations differ by genetic predisposition to NAFLD or lifestyle factors.
Details of the CKB (China Kadoorie Biobank) prospective cohort study design, survey methods, population characteristics, and long-term follow-up have been described elsewhere (20). Briefly, 512,715 participants (male 41%) aged 30–79 years were recruited into the study from 10 (5 urban and 5 rural) diverse areas in China during 2004–2008. Central ethics approvals were obtained from the Chinese Center for Disease Control (CDC) and the University of Oxford as well as institutional research boards at the local CDCs in the 10 regions. Written informed consent were obtained from all participants. At local study assessment clinics, participants completed an interviewer-administered, laptop-based questionnaire on socio-demographic characteristics, smoking, alcohol consumption, diet, physical activity, personal and family medical histories, and current medication. A range of physical measurements were recorded by trained technicians, including height, weight, hip and WC, bio-impedance, lung function, blood pressure, and heart rate, using calibrated instruments with standard protocols. Detailed descriptions of data collection on lifestyle risk factors were shown in Supplementary Methods.
The present study excluded individuals with a prior history of cancer (n = 8), cirrhosis or hepatitis (n = 203), positive hepatitis B surface antigen (HBsAg) tests (n = 534), missing values of any liver biomarkers (n = 1,136), and without genotyping data (n = 616), leaving 15,686 individuals for the main analysis (Supplementary Figure 1).
The current analysis was based in a nested case-control study of CVD with clinical chemistry measurements among 18,183 participants. Cases were identified as those that had an incident fatal or non-fatal event coded as International Classification of Diseases, 10th Revision (ICD-10): I21-23 for myocardial infarction (MI, n = 1,273); I63 and I69.3 for ischemic stroke (IS, n = 5,447); I61 for ICH (n = 5,150) at the censoring date of 1 January 2015. Common controls (n = 6,313) were frequency matched to cases by age, sex, and region. Cases and controls were free of prior vascular disease (including absence of statin therapy) and cancer. Both MI and IS were atherosclerotic CVD, with etiologies differing from ICH. ICH cases were included to compare and contrast the associations between liver biomarkers and CVD subtypes.
Liver enzymes included ALT, AST, and GGT. Plasma concentrations of TC was measured by Beckman-Coulter AU680 clinical chemistry analyzers using manufacturers’ reagents, calibrators and settings (Beckman-Coulter, United Kingdom). FLI for each participant was calculated according to a previously published formula involving TC, BMI, GGT, and WC (12). FLI ≥60, without excessive drinking (≥30 g/day in men, ≥20 g/day in women) or concomitant liver diseases (i.e., viral hepatitis, alcoholic liver disease, toxic liver disease, biliary cholangitis, autoimmune hepatitis, Wilson’s disease, or hemochromatosis), was used as an indicator of NAFLD (12).
Metabolic associated fatty liver disease was diagnosed based on the FLI-defined hepatic steatosis (FLI ≥60) and the presence of any of the following three conditions: (1) overweight or obesity (BMI ≥23 kg/m2), (2) presence of type 2 diabetes mellitus (T2D), and (3) presence of at least 2 metabolic abnormalities, including increased WC (≥90/80 cm for men/women), arterial hypertension (≥130/85 mmHg or specific drug treatment), hypertriglyceridemia (≥150 mg/dl or ≥1.70 mmol/L or specific drug treatment), low high-density lipoprotein cholesterol (<40/50 mg/dl or <1.0/1.3 mmol/L for men/women or specific drug treatment), prediabetes (fasting glucose levels 100–125 mg/dl), and subclinical inflammation [plasma high-sensitivity C-reactive protein (CRP) level >2 mg/L]. Insulin resistance was not measured in CKB and thus excluded from our definition compared with the international expert consensus (21).
Detailed assessment of subclinical atherosclerosis was described in Supplementary Methods. In the current study, carotid plaque burden was used as the outcome measure of subclinical atherosclerosis. Carotid plaque burden was derived by standardizing the plaque number and maximum size [i.e., dividing each by its standard deviation (SD)] and estimating the average, then multiplying the average value by the SD of the maximum plaque thickness to provide a plaque burden recorded in millimeter units. Presence of carotid plaque was defined as carotid plaque burden ≥2.
A custom-designed 800K-single-nucleotide variation (SNV) array (Axiom, Affymetrix) with imputation to 1000 Genomes Phase 3 was utilized to conduct genotyping. Genotype data were available for samples from 100,408 participants passing quality controls (overall call rate >99.97% across all variants), which included a population-based sample of 75,736 participants randomly selected from the total CKB cohort and 24,672 participants genotyped as part of nested case-control studies. For the nested case-control study of clinical chemistry, 17,567 participants were genotyped.
PNPLA3 p.I148M (rs738409), a well-established genetic variant for NAFLD in both Caucasians and East Asians (22), was selected as the target locus. The genotype was coded as 0, 1, and 2 for non-carriers, heterozygous carriers, and homozygous carriers of the risk-increasing allele, respectively.
We selected smoking, alcohol, physical activity, and central adiposity to construct a combined high-risk lifestyle score. These factors were selected because of their associations with risk of chronic liver disease in the Chinese population (23–25). To investigate the combined effects of high-risk lifestyle, we grouped each participant into 1 of 5 categories according to the number of healthy lifestyle factors (0–4), including smoking (current or former regular smokers), alcohol (weekly alcohol consumption ≥210 g, ex-regular or reduced-intake drinkers), physical inactivity [total physical activity <14.7 MET-h/day (the bottom 50%)], and central obesity [WC ≥90 cm (men) or 80 cm (women)]. The cut-off points were selected for each lifestyle factor based on a priori knowledge of the risk factors for chronic liver disease and are considered achievable at the population level.
The primary outcome was CVD, and the secondary outcome was subclinical atherosclerosis. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) of specific disease incidence and 95% confidence intervals (CIs), adjusted for age, sex, region, education, smoking, and alcohol. The underlying time scale was time since birth, and participants entered the study at their baseline age. We first examined the dose-response associations between liver biomarkers and incident CVD, using quintiles as cut-off points. Then, we examined the associations of genetic and high-risk lifestyle factors with incident CVD. To further examine whether the associations between liver biomarkers and CVD differed by genetic or lifestyle risk factors, we performed analyses stratified by genetic risk of NAFLD and the number of high-risk lifestyle factors, separately. Participants were divided into two groups according to their genetic predisposition (low risk, 0 alleles; high risk: 1–2 alleles) or the number of high-risk lifestyle factors (low risk, 0–1; high risk, 2–4). For carotid plaques, a logistic regression model was used to calculate odds ratios (ORs) and 95% CIs associated with liver biomarkers, adjusted for the same variables in the Cox regression.
To assess potentially non-linear associations between liver enzymes and CVD risk, restricted cubic splines were calculated using three fixed knots at the 10, 50, and 90% quintiles. Non-linearity was evaluated using the likelihood ratio rest to compare the fit of linear and non-linear models. Subgroup analyses were conducted by age (<60 vs. ≥60 years) and sex. In sensitivity analysis, we additionally adjusted for BMI and physical activity, potential risk factors for NAFLD.
For analyses involving more than two categories, all HRs are presented, with 95% CIs calculated using “floating” standard errors to facilitate comparisons between any two groups rather than just with the reference group (26). Instead of selecting 1 level of the risk factor as the reference group, a “floated” variance is assigned to each level, which describes the uncertainty in risk without reference to another level. All analyses were performed using R program (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria).
Among the 15,686 participants, the mean age of ICH cases was similar to control subjects (Table 1), but the mean age of MI and IS cases was younger. Likewise, the proportion of women among ICH cases was similar to control subjects, but the proportion was lower among MI cases and higher among IS cases. Cases had higher SBP than control subjects, and they were more likely to have diabetes at baseline. Levels of physical activity, overall and central adiposity, and height were similar between cases and control subjects (Table 1).
Overall, the proportions of NAFLD and MAFLD were similar in each group, and cases were more likely to meet the MAFLD criteria (MI 19.1%; IS 17.9%; ICH 16.2%; control subjects 2.6%). For liver enzymes, MI and ICH cases had higher mean concentrations of AST and ALT than control subjects, while IS cases had lower concentrations of AST than control subjects. Cases had higher concentrations of GGT than control subjects.
The average follow-up period of this study is 10 years.
There were positive associations of FLI and GGT with atherosclerosis cardiovascular disease (ASCVD) (i.e., MI and IS) but a weaker positive association with ICH. The adjusted HRs per 1-SD higher FLI were 1.17 (95% CI 1.13–1.21) for MI, 1.16 (1.13–1.19) for IS, and 1.04 (1.01–1.08) for ICH; the corresponding HRs for GGT were 1.08 (1.05, 1.11) for MI, 0.93 (0.90, 0.97) for IS and 1.10 (1.08, 1.12) for ICH (Supplementary Table 1). Of note, the positive association of FLI and GGT with ICH was only observed when comparing the top and the bottom quintile (FLI 1.12 [1.05–1.20] and GGT 1.16 [1.08, 1.24], Figure 1). Similar to FLI, there were positive associations between MAFLD and all CVD subtypes (MI 1.42 [1.30–1.55], IS 1.24 [1.15–1.33], and ICH 1.12 [1.03–1.22], Supplementary Table 3). Participants with high AST had higher risks of MI and ICH, but a lower risk of IS (HR comparing top vs. bottom quintile: MI 1.11 [1.02, 1.21], IS 0.87 [0.81, 0.94], and ICH 1.16 [1.08, 1.23]). For ALT, positive associations were observed with MI, but there were no associations with IS and ICH. Likelihood ratio tests showed evidence of significant non-linear associations of FLI with IS (p-value for non-linearity <0.01), ALT with IS and ICH (p-value for non-linearity ≤0.02), and GGT with MI and IS (p-value for non-linearity < 0.01) (Supplementary Figure 3).
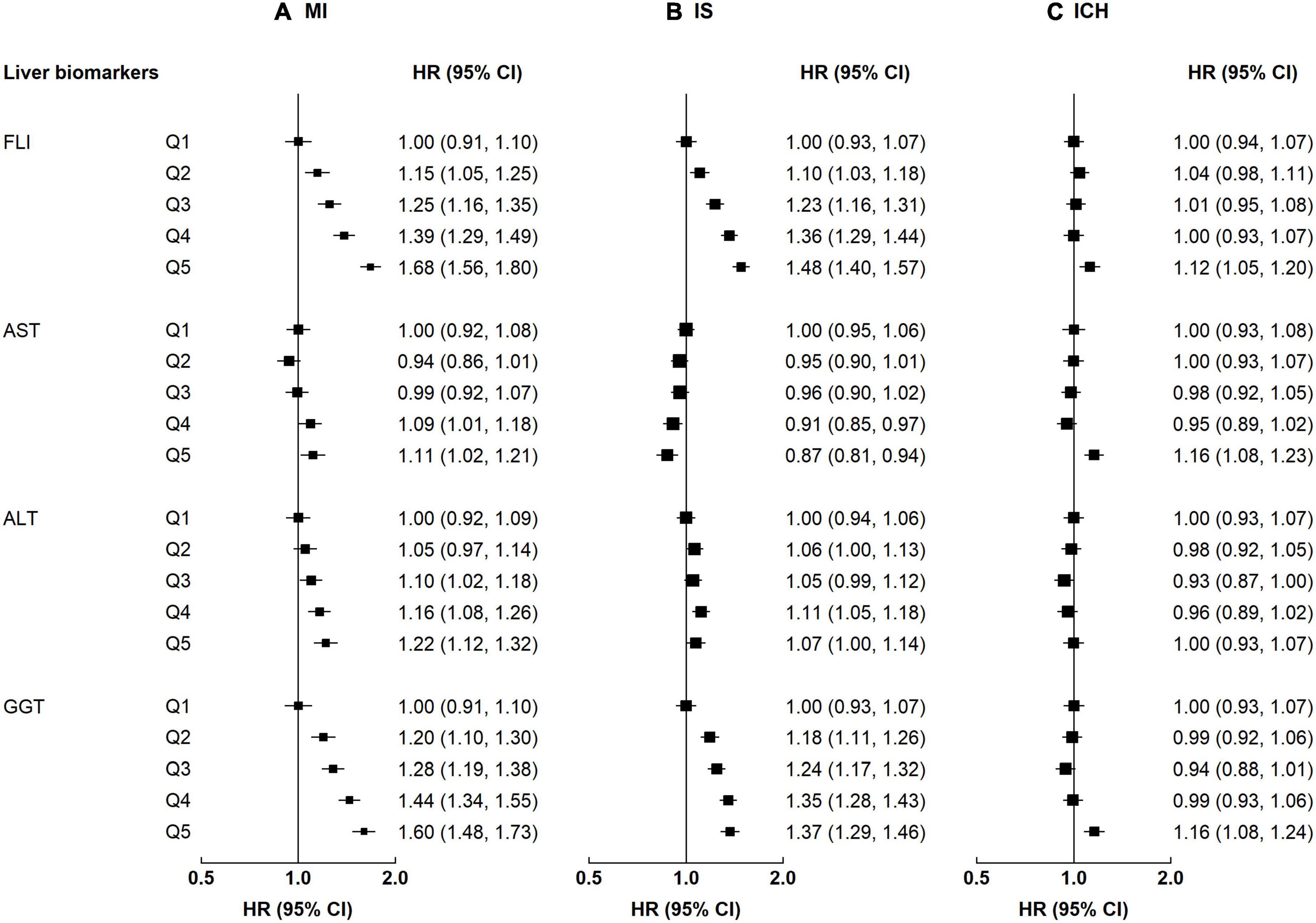
Figure 1. Associations of liver biomarkers with risk of CVD. Columns (A–C) denote the results for MI, IS, and ICH, respectively. Boxes represent the hazard ratios (HRs) of CVD associated with liver biomarkers, with the size of the box inversely proportional to the variance of the logHR. The model is adjusted for age, sex, regions, education, smoking, and alcohol.
When additionally adjusting for BMI and physical activity, the associations of liver biomarkers with CVD slightly attenuated, but the patterns remained (Supplementary Figure 2). The associations of FLI and GGT with ICH differed by sex (p-value for interaction ≤0.02), while the associations of ALT and GGT with MI differed by age (p-value for interaction ≤0.01, Supplementary Table 4). For example, FLI was positively associated with ICH among men, but no such association was observed in women. For MI, the associations with ALT and GGT attenuated among those ≥60 years (Supplementary Figure 4).
Alcohol and central adiposity were each associated with higher risks of all CVD subtypes (Supplementary Table 2). The HRs for alcohol were 1.12 (1.04–1.21) for MI, 1.13 (1.07–1.20) for IS, and 1.18 (1.10–1.26) for ICH. The HRs for central adiposity were 1.38 (1.28–1.48) for MI, 1.34 (1.27–1.42) for IS, and 1.08 (1.00–1.17) for ICH. Smoking was associated with a higher risk of MI (1.27 [1.15–1.41]), and the positive association was borderline significant for IS (1.07 [0.99–1.17]). Physical inactivity was associated with ICH (1.08 [1.00–1.17]). When combining these four high-risk lifestyle factors, there were positive associations between the number of lifestyle factors and risk of all CVD subtypes (Figure 2). Compared with those without high-risk lifestyle factors, the HRs for MI were 1.22 (1.12–1.33), 1.53 (1.45–1.61), and 1.82 (1.72–1.93) among participants with 1, 2, and 3–4 lifestyle factors, respectively. The corresponding HRs for IS were 1.06 (0.99–1.12), 1.25 (1.20–1.30), and 1.47 (1.40–1.55), while the corresponding HRs for ICH were 0.98 (0.91–1.05), 1.12 (1.07–1.17), and 1.22 (1.16–1.28). For rs738409, there was a positive association between the number of rs738409 M allele with ICH, but no association was observed for MI or IS. Compared with those with 0 risk alleles, the HRs for ICH were 1.06 (1.01–1.10) and 1.11 (1.03–1.20) among participants with 1 allele and 2 alleles, respectively.
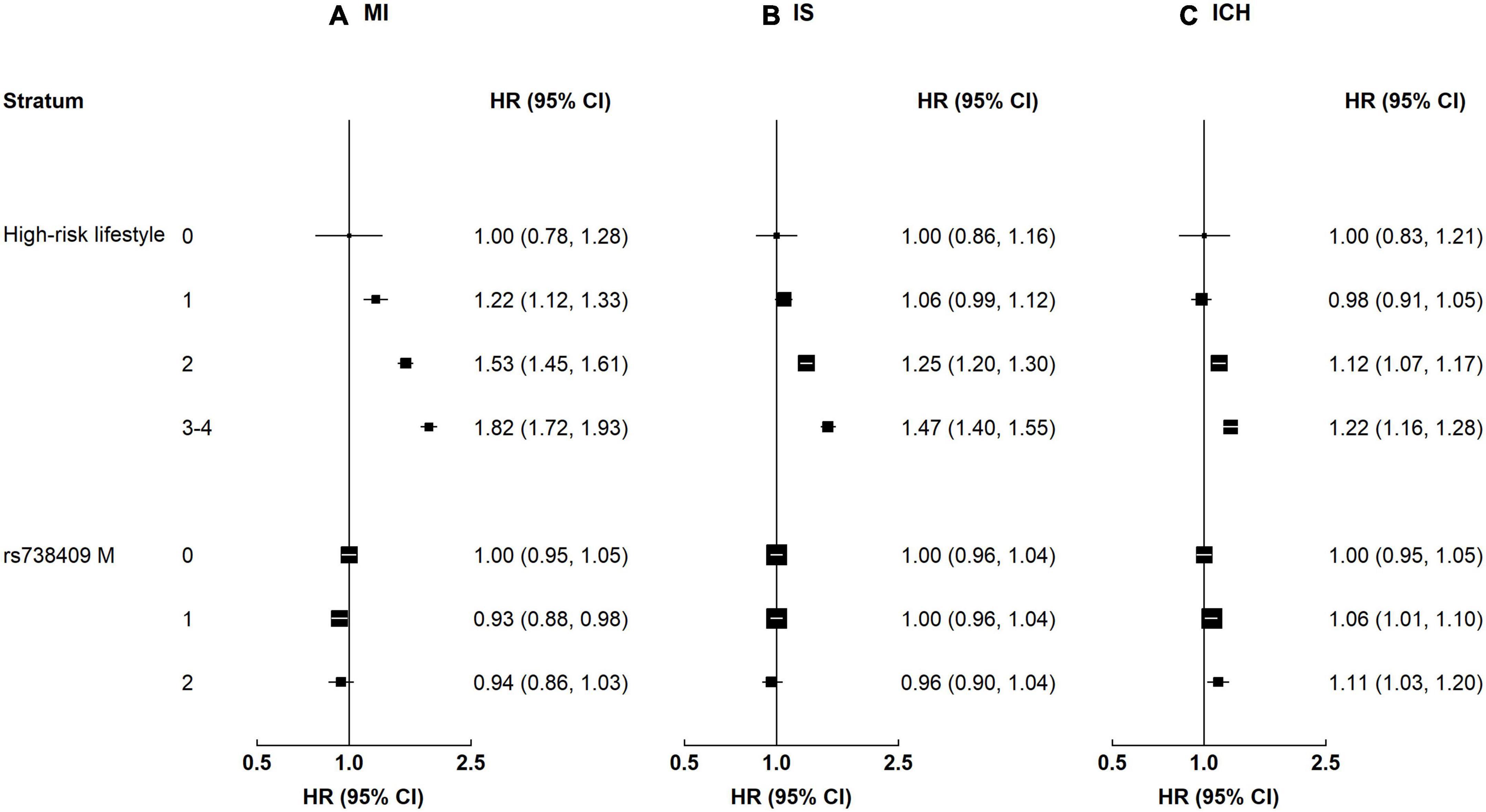
Figure 2. Associations of high-risk lifestyle and genetic risk factors with risk of CVD. Columns (A–C) denote the results for MI, IS, and ICH, respectively. Convention as in Figure 1.
Participants with NAFLD or MAFLD had higher risks of MI and IS, regardless of the genetic risk (p-value for interaction 0.21–0.77, Supplementary Table 3). Although the interaction by genetic predisposition to NAFLD was non-significant, the HRs tended to be stronger among individuals with a high genetic risk (Figure 3). For instance, the HRs associated with MAFLD among participants with a low genetic risk were 1.33 (1.16–1.52) for MI and 1.22 (1.10–1.35) for IS, while the HRs among those with a high genetic risk were 1.48 (1.31–1.66) and 1.25 (1.14–1.37). In contrast, the associations of NAFLD and MAFLD with ICH differed by genetic predisposition to NAFLD, with stronger associations among those with a high genetic risk. For NAFLD, the HRs were 0.96 (0.82–1.12) and 1.24 (1.10–1.39) among those with a low and a high genetic risk, respectively; the corresponding HRs for MAFLD were 0.98 (0.85–1.12) and 1.22 (1.10–1.36) (for both NAFLD and MAFLD, p-value for interaction <0.05). Genetic predisposition to NAFLD did not interact with liver enzymes on risk of CVD (p-value for interaction 0.05–0.78).
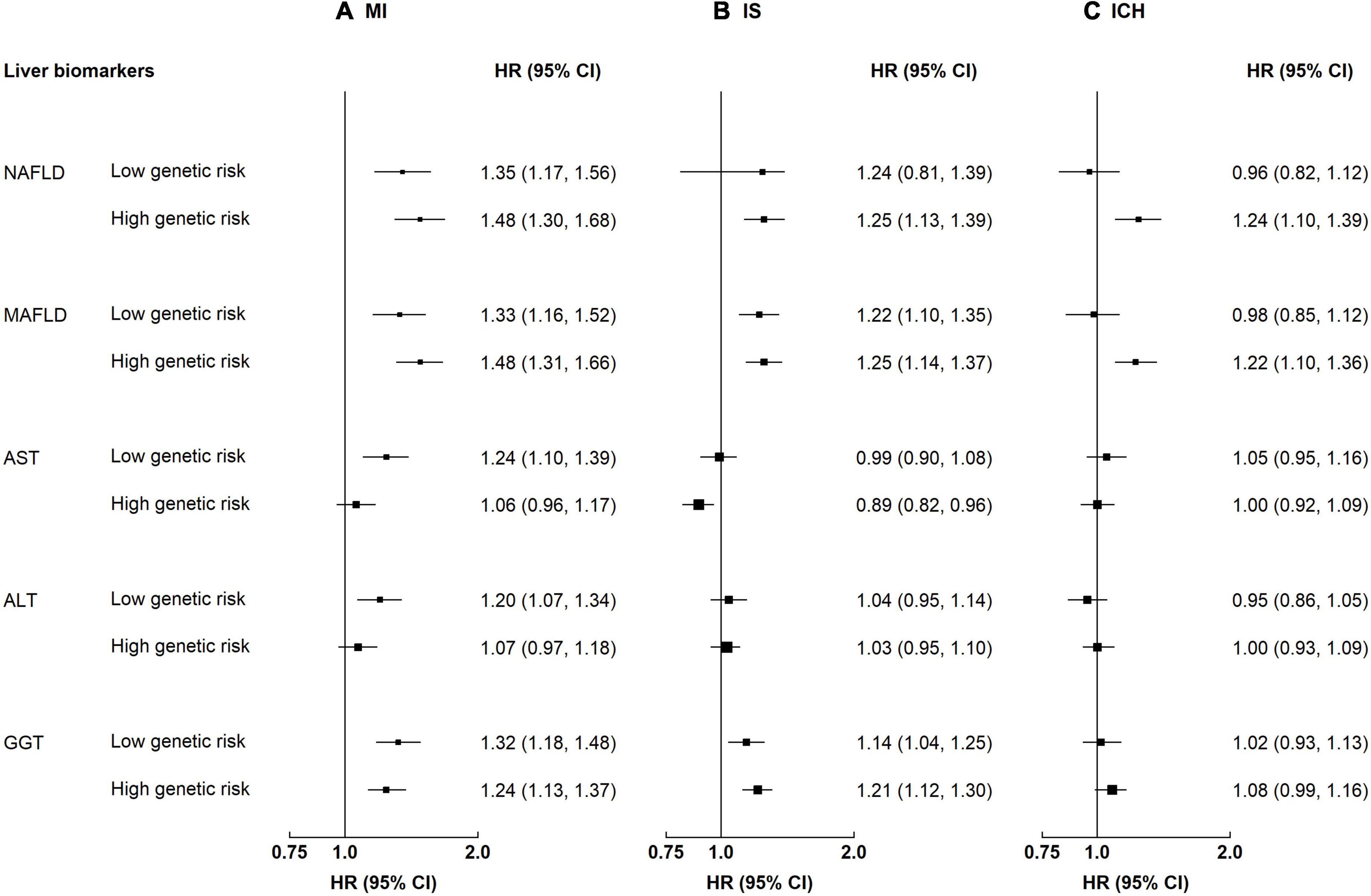
Figure 3. Associations of liver biomarkers with risk of CVD by genetic predisposition to NAFLD. Columns (A–C) denote the results for MI, IS, and ICH, respectively. Boxes represent the hazard ratios (HRs) of CVD associated with high liver biomarkers, with the size of the box inversely proportional to the variance of the logHR. Liver biomarker was each modeled as a dichotomous variable. The cut-off points are: ≥median for AST, ALT, and GGT. High genetic risk denotes 1–2 risk increasing alleles of rs73409. Low genetic risk denotes 0 risk increasing alleles of rs73409. The model is adjusted for age, sex, regions, education, smoking, and alcohol. p-Values for interaction: MI 0.11–0.50, IS 0.05–0.81, and ICH 0.009–0.54. p-Values for interaction <0.05: ICH, FLI 0.009, MAFLD 0.01.
Participants with NAFLD or MAFLD had higher risks for MI, regardless of the lifestyle risk (p-value for interaction 0.28–0.35, Supplementary Table 3). The HR associated with MAFLD among participants with a low lifestyle risk was 1.12 (0.75–1.67) for MI, while the HR among those with a high lifestyle risk was 1.38 (1.26–1.52). In contrast, the associations of NAFLD and MAFLD with IS and ICH differed by lifestyle factors (Figure 4). For NAFLD, the HRs of IS were 1.57 (1.21–2.03) and 1.16 (1.07–1.26) among those with a low and a high lifestyle risk, respectively; the corresponding HRs of ICH were 1.38 (0.98–1.95) and 1.08 (0.98–1.19) (for both NAFLD and MAFLD, p-value for interaction <0.05). Lifestyle risk factors did not interact with liver enzymes on risk of CVD (p-value for interaction 0.19–0.82).
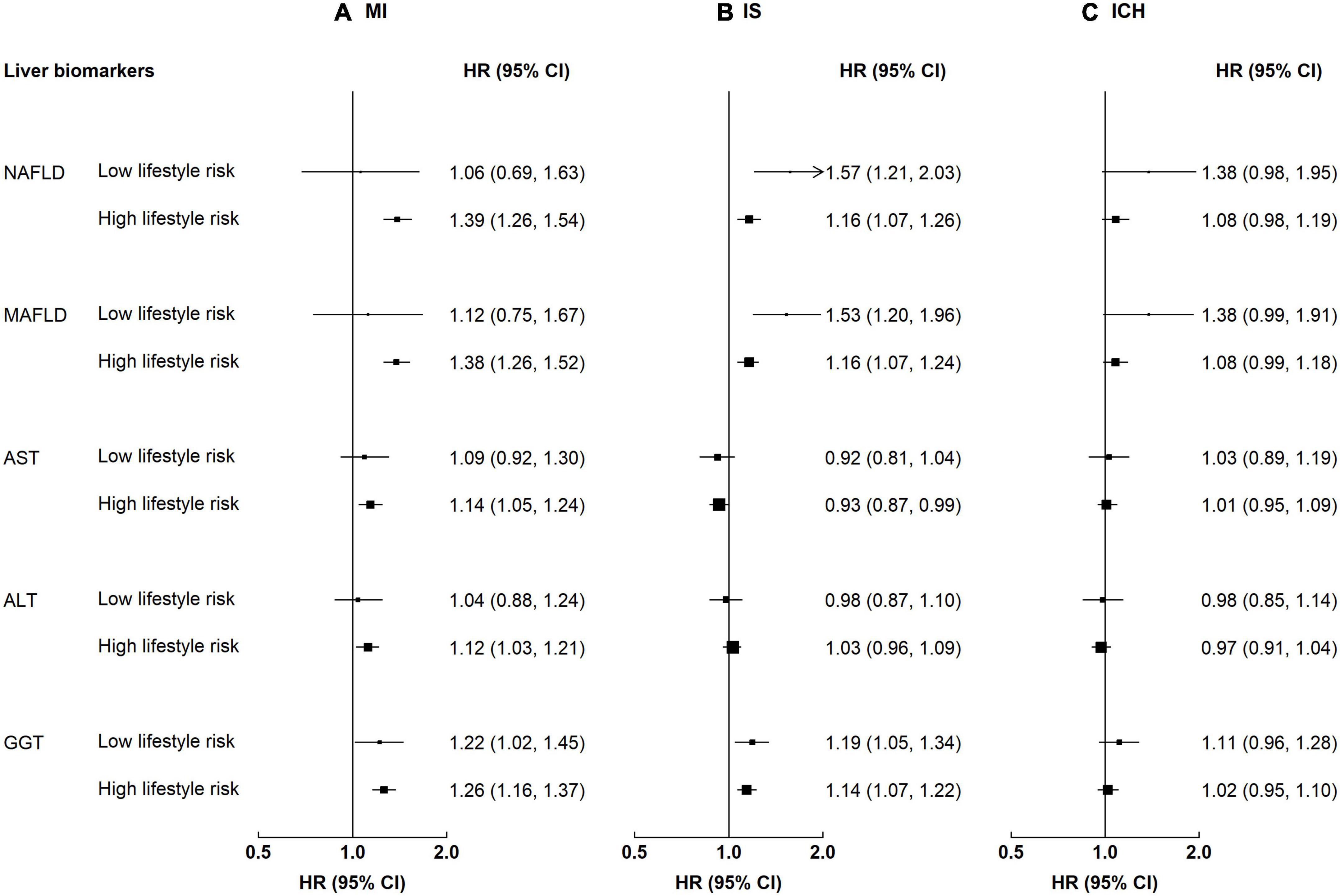
Figure 4. Associations of liver biomarkers with risk of CVD by lifestyle factors. Columns (A–C) denote the results for MI, IS, and ICH, respectively. Convention as in Figure 3. Low lifestyle risk denotes 0–1 high-risk lifestyle factors. High lifestyle risk denotes 2–4 high-risk lifestyle factors. p-Values for interaction: MI 0.28–0.82, IS 0.02–0.74, and ICH 0.04–0.61. p-Values for interaction <0.05: IS, NAFLD 0.03, MAFLD 0.02; ICH, FLI 0.04, MAFLD 0.04.
Overall, there were positive associations of NAFLD and MAFLD with of carotid plaque and no associations were observed for liver enzymes (Supplementary Table 5). For both NAFLD and MAFLD, the associations tended to be stronger among individuals with a high genetic risk, though the interaction was non-significant (p-value for interaction 0.18). Similar associations were observed when stratified by high-risk lifestyle factors.
This study provides a comprehensive examination of the associations of liver biomarkers with risk of CVD by subtypes. The results showed that there were positive associations of liver enzymes with ASCVD (i.e., MI and IS) and weaker associations for ICH, regardless of the genetic predisposition to NAFLD or lifestyle risk factors. Participants with NAFLD or MAFLD had higher risks of all CVD subtypes as well as subclinical atherosclerosis, with stronger associations among individuals with a high genetic risk of NAFLD. For both IS and ICH, the associations of NAFLD and MAFLD were stronger among individuals with a favorable lifestyle.
The current study findings for liver biomarkers were generally consistent with previous studies examining the associations of liver enzymes in relation to CVD (4, 6, 8, 9, 27–29). These studies include a meta-analysis published in 2019 involving 1,067,922 participants and a prospective cohort study involving 416,122 participants in Taiwan (3, 6), both examining CVD mortality. For MI and IS, our findings for ALT and GGT were similar to the associations reported by two large cohort studies involving 6,912,393 participants in Korea (4, 9). In contrast to our null findings for ICH, a cohort of 108,464 Korean men showed positive associations of AST and ALT with ICH (28). For stroke subtypes, two cohorts conducted in Western countries reported positive associations of ALT and AST with ICH (8, 29), while one United States cohort reported a positive association between GGT and IS (8). However, previous studies involved a small number of ICH cases (ranging from 90 to 718) and the associations may be due to chance. Although the current study included a large number of CVD subtypes (5,447 IS cases and 5,150 ICH cases), future studies are still warranted to examine the associations of liver enzymes with CVD subtypes.
Fatty liver index is a commonly used indicator of NAFLD in large population-based studies. Recently, an expert consensus recommended the change from NAFLD to MAFLD, since the latter included more metabolic factors and thus might be more sensitive in demonstrating disease progression (14, 16). Therefore, we explored the associations of MAFLD in relation to CVD as a complement to NAFLD. Notably, our study findings for NAFLD and MAFLD were fairly close, due to the fact that FLI ≥60 captured individuals who met the MAFLD definition in CKB. Previous studies have shown that both NAFLD and MAFLD were associated with higher risk of CVD (7, 10, 11, 13, 16, 18, 19, 30), particularly MI and IS, which was in line with our findings. For intermediate phenotypes, our findings of positive associations of NAFLD and MAFLD with subclinical atherosclerosis were also consistent with previous studies (31, 32). However, only a limited number of studies have examined the association of NAFLD or MAFLD with hemorrhagic CVD and further compared it with the associations with ischemic CVD. Our study filled this gap and showed that compared to ischemic CVD, there were weaker associations of NAFLD and MAFLD with ICH, which indicated that the prognostic role of NAFLD and MAFLD was more important for ischemic CVD.
There is limited evidence whether the associations between liver biomarkers and CVD differ by genetic or lifestyle risk factors. A recent study conducted in the UKB showed that the adverse impact of MAFLD on CVD (mainly ASCVD) was somewhat larger in individuals with a high genetic risk of NAFLD compared with those with a low genetic risk (low 1.37 [1.30–1.44], high 1.42 [1.33–1.50]) (16). This study finding was generally consistent with the results of MI and IS in CKB. In addition to ASCVD, we showed that the associations of MAFLD and NAFLD with ICH were much stronger among individuals with a high genetic risk of NAFLD. The effect modification by genetic risk was only observed for ICH, suggesting that the genetic variants may have little contribution to the development of ischemic CVD compared with hemorrhagic CVD. This is possibly because that high genetic risk amplifies the impact of metabolic factors on the progression of hypertension, a risk factor showing a stronger association with ICH than ischemic CVD in Chinese (33). Regarding lifestyle risk factors, we showed that the associations of MAFLD and NAFLD with both IS and ICH were stronger among individuals with a low-risk lifestyle. A probable explanation was that individuals with a favorable lifestyle differed in other risk factors for stroke (e.g., variability of blood pressure) (34, 35). Future studies in independent cohorts are needed to replicate our findings.
Our study has several clinical implications. Levels of liver biomarkers not only reflect the status of liver function, but also play an important role in CVD prediction, as demonstrated by our and previous studies. Since liver biomarkers are relatively easy to measure, these can be routinely monitored in clinical practice to identify patients at risk of CVD. Our results showed positive associations for both FLI and MAFLD with CVD risk regardless of the subtypes, suggesting that these two may be better indicators for CVD than liver enzymes. Regarding genetic risk stratification, although effect modification was only observed for ICH, the associations of FLI and MAFLD with CVD were both stronger among individuals with a high genetic risk of NAFLD. This suggests that FLI monitoring and MAFLD screening may be particularly important for those with genetic predisposition to NAFLD to predict risk of CVD.
The strengths of the CKB included a prospective design, a large study population, detailed adjustments for risk factors of CVD, and ascertainment of CVD through linkage to hospital records in addition to death and cancer registries. However, our study also had several limitations. First, we used FLI rather than imaging or biopsy to diagnose NAFLD, which was not the gold standard (36, 37). However, FLI has been externally validated in population-based studies conducted in Western countries and in China and is accepted by clinical practice guidelines as a proxy for imaging or biopsy in large-scale epidemiological studies (38–42). Second, insulin resistance, as a criterion of MAFLD diagnosis, was not assessed in our study due to the lack of serum insulin data in CKB. However, these two limitations might result in non-differential misclassification of NAFLD and MAFLD cases, and thus underestimating the associations between NAFLD/MAFLD and CVD. Third, it is probable that components of genetic predisposition to NAFLD other than rs738409 were not included. However, rs738409 had been recognized as the most important genetic variant of NAFLD across ethnicities (22). A meta-analysis of 12 Asian studies (7 of which were Chinese) with 4,495 cases reported that rs738409 M allele carriers were nearly twice as likely to develop NAFLD as non-carriers (OR 1.92 [1.54–2.39]) (43). Finally, residual confounding due to unmeasured or unknown variables cannot be ruled out (e.g., liver disease medications).
In conclusion, this study in a Chinese population showed that liver biomarkers were associated with risk of CVD, with different magnitudes of associations by CVD subtypes. Genetic predisposition to NAFLD and lifestyle factors modified the associations of fatty liver with stroke, particularly ICH. The current study findings may inform CVD risk stratification using liver biomarkers in Chinese. FLI monitoring and MAFLD screening may be recommended among those with a high genetic risk of NAFLD.
Data described in the article, code book, and analytic code will be made available from the China Kadoorie Biobank upon request (http://www.ckbiobank.org/site/Data+Access), pending application and approval. The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by the Chinese Center for Disease Control (CDC) and the University of Oxford as well as institutional research boards at the local CDCs in the 10 regions. The patients/participants provided their written informed consent to participate in this study.
LL and ZC had full access to the data. XW, SC, YP, and LL conducted data analysis and were responsible for accuracy of the results and the decision to submit for publication. All authors were involved in study design, conduct, long-term follow-up, review and coding of disease events, interpretation of the results, or writing the report, and read and approved the final version of the manuscript.
This work was supported by National Natural Science Foundation of China (82192900, 82192901, 82192904, 81941018, and 91846303). The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants (2016YFC0900500, 2016YFC0900501, and 2016YFC0900504) from the National Key R&D Program of China, and Chinese Ministry of Science and Technology (2011BAI09B01). YP acknowledged support from the Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (BMU2022RCZX022), the Fundamental Research Funds for the Central Universities, and the Peking University Start-up Grant (BMU2022PY014), and the China Postdoctoral Science Foundation (2019TQ0008 and 2020M670071). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
We thank the participants, the project staff, and the China National Centre for Disease Control and Prevention (CDC), and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provides electronic linkage to all hospital admission data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.938902/full#supplementary-material
ALT, alanine aminotransferase; ASCVD, atherosclerosis cardiovascular disease; AST, aspartate aminotransferase; ASIR, age-standardized incidence rate; BMI, body mass index; CDC, Chinese Center for Disease Control; CFR, case fatality rate; CI, confidence interval; CKB, China Kadoorie Biobank; CRP, C-reactive protein; CVD, cardiovascular disease; FLI, fatty liver index; GGT, γ-glutamyltransferase; HBsAg, hepatitis B surface antigen; HR, hazards ratio; ICD-10, International Classification of Diseases, 10th Revision; ICH, intracerebral hemorrhage; IHD, ischemic heart disease; IS, ischemic stroke; MAFLD, metabolic associated fatty liver disease; MI, myocardial infarction; NAFLD, non-alcoholic fatty liver disease; SD, standard deviation; SNV, single-nucleotide variation; OR, odds ratio; TC, triglycerides; T2D, type 2 diabetes mellitus; UKB, UK Biobank; WC, waist circumference
1. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Liu J, Qi J, Yin P, Liu Y, You J, Lin L, et al. Cardiovascular disease mortality - China, 2019. China CDC Wkly. (2021) 3:323–6.
3. Rahmani J, Miri A, Namjoo I, Zamaninour N, Maljaei MB, Zhou K, et al. Elevated liver enzymes and cardiovascular mortality: a systematic review and dose–response meta-analysis of more than one million participants. Eur J Gastroenterol Hepatol. (2019) 31:555–62. doi: 10.1097/MEG.0000000000001353
4. Choi KM, Han K, Park S, Chung HS, Kim NH, Yoo HJ, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: a nationwide population-based cohort study. Sci Rep. (2018) 8:3764. doi: 10.1038/s41598-018-19700-8
5. Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol. (2014) 43:187–201.
6. Xie K, Chen CH, Tsai SP, Lu PJ, Wu H, Zeng Y, et al. Loss of life expectancy by 10 years or more from elevated aspartate aminotransferase: finding aspartate aminotransferase a better mortality predictor for all-cause and liver-related than alanine aminotransferase. Am J Gastroenterol. (2019) 114:1478–87.
7. Zou B, Yeo YH, Cheung R, Ingelsson E, Nguyen MH. Fatty liver index and development of cardiovascular disease: findings from the UK Biobank. Dig Dis Sci. (2021) 66:2092–100.
8. Ruban A, Daya N, Schneider ALC, Gottesman R, Selvin E, Coresh J, et al. Liver enzymes and risk of Stroke: the atherosclerosis risk in communities (ARIC) study. J Stroke. (2020) 22:357–68.
9. Cho EJ, Han K, Lee SP, Shin DW, Yu SJ. Liver enzyme variability and risk of heart disease and mortality: a nationwide population-based study. Liver Int. (2020) 40:1292–302.
10. Alexander KS, Zakai NA, Lidofsky SD, Callas PW, Judd SE, Tracy RP, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: the reasons for geographic and racial differences in stroke cohort. PLoS One. (2018) 13:194153. doi: 10.1371/journal.pone.0194153
11. Kim JH, Moon JS, Byun SJ, Lee JH, Kang DR, Sung KC, et al. Fatty liver index and development of cardiovascular disease in Koreans without pre-existing myocardial infarction and ischemic stroke: a large population-based study. Cardiovasc Diabetol. (2020) 19:51. doi: 10.1186/s12933-020-01025-4
12. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
13. Alon L, Corica B, Raparelli V, Cangemi R, Basili S, Proietti M, et al. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Prev Cardiol. (2021) 29:938–46.
14. Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014. doi: 10.1053/j.gastro.2019.11.312
15. Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. (2021) 75:1284–91. doi: 10.1016/j.jhep.2021.07.035
16. Liu Z, Suo C, Shi O, Lin C, Zhao R, Yuan H, et al. The health impact of MAFLD, a novel disease cluster of NAFLD, is amplified by the integrated effect of fatty liver disease-related genetic variants. Clin Gastroenterol Hepatol. (2020) 30:33. doi: 10.1016/j.cgh.2020.12.033
17. Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. (2021) 19:2172–81.
18. Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. (2021) 19:2138–47. doi: 10.1016/j.cgh.2020.12.022
19. Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab. (2022) 107:88–97. doi: 10.1210/clinem/dgab641
20. Chen ZM, Chen JS, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. (2011) 40:1652–66. doi: 10.1093/ije/dyr120
21. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73: 202–9.
22. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. (2008) 40:1461–5.
23. Pang Y, Kartsonaki C, Guo Y, Chen Y, Yang L, Bian Z, et al. Central adiposity in relation to risk of liver cancer in Chinese adults: a prospective study of 0.5 million people. Int J Cancer. (2019) 145:1245–53. doi: 10.1002/ijc.32148
24. Pang Y, Kartsonaki C, Lv J, Millwood IY, Yu C, Guo Y, et al. Observational and Genetic associations of body mass index and hepatobiliary diseases in a relatively lean Chinese population. JAMA Netw Open. (2020) 3:e2018721. doi: 10.1001/jamanetworkopen.2020.18721
25. Pang Y, Lv J, Kartsonaki C, Yu C, Guo Y, Chen Y, et al. Metabolic risk factors, genetic predisposition, and risk of severe liver disease in Chinese: a prospective study of 0.5 million people. Am J Clin Nutr. (2021) 114:496–504. doi: 10.1093/ajcn/nqab099
26. Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. (1991) 10:1025–35. doi: 10.1002/sim.4780100703
27. Xu L, Jiang CQ, Lam TH, Zhang WS, Zhu F, Jin YL, et al. Mendelian randomization estimates of alanine aminotransferase with cardiovascular disease: Guangzhou Biobank cohort study. Hum Mol Genet. (2017) 26:430–7. doi: 10.1093/hmg/ddw396
28. Kim HC, Kang DR, Nam CM, Hur NW, Shim JS, Jee SH, et al. Elevated serum aminotransferase level as a predictor of intracerebral hemorrhage. Stroke. (2005) 36:1642–7.
29. Weikert C, Drogan D, di Giuseppe R, Fritsche A, Buijsse B, Nothlings U, et al. Liver enzymes and stroke risk in middle-aged German adults. Atherosclerosis. (2013) 228:508–14.
30. Olubamwo OO, Virtanen JK, Voutilainen A, Kauhanen J, Pihlajamaki J, Tuomainen TP. Association of fatty liver index with the risk of incident cardiovascular disease and acute myocardial infarction. Eur J Gastroenterol Hepatol. (2018) 30:1047–54.
31. Kozakova M, Palombo C, Eng MP, Dekker J, Flyvbjerg A, Mitrakou A, et al. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology. (2012) 55:1406–15. doi: 10.1002/hep.25555
32. Pais R, Redheuil A, Cluzel P, Ratziu V, Giral P. Relationship among fatty liver, specific and multiple-site atherosclerosis, and 10-year framingham score. Hepatology. (2019) 69:1453–63. doi: 10.1002/hep.30223
33. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. (2014) 2:901–10. doi: 10.1016/S2213-8587(14)70032-4
34. Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. (2016) 68:1375–86.
35. Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. (1999) 34:685–91.
36. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1264–81.
37. Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. (2018) 67:1348–59. doi: 10.1002/hep.29639
38. Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. (2013) 11:1201–4. doi: 10.1016/j.cgh.2012.12.031
39. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. (2014) 40:1209–22.
40. European Association for the Study of the Liver (EAEL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. (2016) 59:1121–40.
41. Zhang Q, Zhu Y, Yu W, Xu Z, Zhao Z, Liu S, et al. Diagnostic accuracy assessment of molecular prediction model for the risk of NAFLD based on MRI-PDFF diagnosed Chinese Han population. BMC Gastroenterol. (2021) 21:88. doi: 10.1186/s12876-021-01675-y
42. Li C, Guo P, Zhang R, Zhang M, Li Y, Huang M, et al. Both WHR and FLI as better algorithms for both lean and overweight/obese NAFLD in a Chinese population. J Clin Gastroenterol. (2019) 53:e253–60. doi: 10.1097/MCG.0000000000001089
Keywords: liver enzyme, fatty liver disease (FLD), cardiovascular disease, genetics, lifestyle
Citation: Wang X, Cheng S, Lv J, Yu C, Guo Y, Pei P, Yang L, Millwood IY, Walters R, Chen Y, Du H, Duan H, Gilbert S, Avery D, Chen J, Pang Y, Chen Z and Li L (2022) Liver biomarkers, genetic and lifestyle risk factors in relation to risk of cardiovascular disease in Chinese. Front. Cardiovasc. Med. 9:938902. doi: 10.3389/fcvm.2022.938902
Received: 08 May 2022; Accepted: 20 July 2022;
Published: 11 August 2022.
Edited by:
Harry H. X. Wang, Sun Yat-sen University, ChinaReviewed by:
Yu-Jin Kwon, Yonsei University College of Medicine, South KoreaCopyright © 2022 Wang, Cheng, Lv, Yu, Guo, Pei, Yang, Millwood, Walters, Chen, Du, Duan, Gilbert, Avery, Chen, Pang, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanjie Pang, yuanjie_p@163.com; Liming Li, lmleeph@vip.163.com
†These authors share first authorship
‡The members of steering committee and collaborative group are listed in Supplementary material.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.