
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 02 August 2022
Sec. Cardiovascular Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.937952
This article is part of the Research Topic Novel Biomarkers and Risk Factors Associated with Cardiometabolic Dysfunction in Heart Failure View all 16 articles
 Yan Lin1,2†
Yan Lin1,2† Xiangming Hu2,3†
Xiangming Hu2,3† Weimian Wang2,3
Weimian Wang2,3 Bingyan Yu2,4
Bingyan Yu2,4 Langping Zhou2
Langping Zhou2 Yingling Zhou2
Yingling Zhou2 Guang Li2*
Guang Li2* Haojian Dong2*
Haojian Dong2*Background: Coronary microvascular dysfunction (CMVD), an important etiology of ischemic heart disease, has been widely studied. D-dimer is a simple indicator of microthrombosis and inflammation. However, whether an increase in D-dimer is related to CMVD is still unclear.
Materials and Methods: This retrospective study consecutively enrolled patients with myocardial ischemia and excluded those with obstructive coronary artery. D-dimer was measured at admission and the TIMI myocardial perfusion grade (TMPG) was used to distinguish CMVD. Patients were divided into the two groups according to whether the D-dimer was elevated (>500 ng/ml). Logistic models and restricted cubic splines were used to explore the relationship between elevated D-dimer and CMVD.
Results: A total of 377 patients were eventually enrolled in this study. Of these, 94 (24.9%) patients with CMVD had older age and higher D-dimer levels than those without CMVD. After full adjustment for other potential clinical risk factors, patients with high D-dimer levels (>500 ng/ml) had a 1.89-times (95% CI: 1.09–3.27) higher risk of CMVD than patients with low D-dimer levels. A non-linear relationship was found between concentrations of D-dimer and CMVD. With increased D-dimer level, the incidence of CMVD increased and then remained at a high level. Stratified analysis was performed and showed similar results.
Conclusion: Elevated D-dimer level is associated with the incidence of CMVD and potentially serves as a simple biomarker to facilitate the diagnosis of CMVD for patients with angina.
Although clinical practice strategies have optimized the prevention and treatment of ischemic heart disease over the past few years, ischemic heart disease has a complex pathophysiology that goes beyond the traditional role of obstructive coronary artery disease (CAD). Coronary microvascular dysfunction (CMVD), a phenotype of ischemic heart disease, is defined as the clinical syndrome of angina without obstructive CAD (1). CMVD may contribute to angina by reducing coronary blood flow, which is prevalent and associated with an increased risk of future adverse cardiovascular outcomes (2, 3). Studies have shown that CMVD is mediated by risk factors traditionally recognized as related to cardiovascular disease, although these factors account for a small sample, leaving a large proportion that cannot be explained (4, 5). Potential pathophysiological mechanisms of ischemia involved in CMVD with non-obstructive CAD have been previously proposed such as microvascular thrombosis, inflammation, and edema (6), indicating a changed myocardial microcirculation environment. However, there is currently a lack of simple tools to identify CMVD and more predictors are still desperately needed.
D-dimer, a degradation product of cross-linked fibrin, is widely recognized as a marker of thrombosis (7). Elevated D-dimer has a prognostic value for adverse cardiovascular events in healthy people and patients with CAD (8, 9). Zhang et al. found that D-dimer in admission could be used to identify the no-reflow phenomenon in patients with ST-segment elevation myocardial infarction, highlighting the advantage of D-dimer in identifying microvascular embolism (10). Previous studies have shown that elevated D-dimer levels are related to microvascular thrombosis (11), inflammation (12), and endothelial injury (13), which contribute to CMVD. The TIMI myocardial perfusion grade (TMPG) is a simple indicator derived from coronary angiography to evaluate coronary microcirculation and identify CMVD (14–16). Although D-dimer is a simple indicator of the microcirculatory environment, the relationship between D-dimer level and CMVD has only rarely been studied.
In this study, we hypothesized that D-dimer levels might be associated with CMVD evaluated by the TMPG and could be used as an available biomarker for early screening for CMVD.
This retrospective study included 1,654 consecutive patients who were admitted for suspected CAD from September 2014 to September 2015 at the Guangdong Provincial People’s Hospital. Suspected CAD is clinically based on symptoms of ischemia and/or electrocardiographic ischemic changes. Patients with obstructive coronary artery stenosis (defined as ≥70% luminal diameter narrowing of an epicardial stenosis or ≥50% luminal diameter narrowing of the left main artery, n = 1,218), with impaired left ventricular ejection fraction (LVEF) (<40%, n = 35) and without coronary angiography data (n = 24) were excluded (Figure 1). Demographic data, risk factors, and coronary angiography results were collected based on the electronic medical records.
This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital and informed verbal consent was obtained from all the patients. This research was conducted in accordance with the Declaration of Helsinki.
All the patients underwent coronary angiography using the Judkins technique. Coronary angiography was performed with a radial approach and the femoral artery was used in a minority of patients, as clinically necessary. We used 5-Fr or 6-Fr Judkins left and right diagnostic catheters for left and right coronary angiography, respectively. The degree of coronary artery stenosis was judged and recorded by two interventional cardiologists. Evaluation of the TMPG flow was performed by two experienced cardiologists who were blinded to patient’s demographic and clinical information. In the case of disagreement, a third cardiologist was consulted and the majority opinion was adopted.
The TMPG was classified into four grades (17) as follows: (1) TMPG 0: failure of dye to enter the microvasculature, indicating a lack of tissue-level perfusion; (2) TMPG 1: dye enters slowly but fails to exit the microvasculature. There is a ground-glass appearance or opacification of the myocardium in the distribution of the vessel that fails to clear from the microvasculature and dye staining is present on the next injection (30 s); (3) TMPG 2: delayed entry and exit of dye from the microvasculature. Dye strongly persists after three cardiac cycles of the washout phase and either does not or only minimally diminishes in intensity during washout; and (4) TMPG 3: normal entry and exit of dye from the microvasculature. Dye is gone or is mildly/moderately persistent after three cardiac cycles of the washout phase and noticeably diminishes in intensity during the washout phase. The blush that is of only mild intensity throughout the washout phase but fades minimally is also classified as grade 3.
The TMPG flow was used to assess coronary microvascular function and CMVD was distinguished with the TMPG flow <3. Hypertension, diabetes, chronic kidney disease (CKD), smoking, and alcohol consumption were diagnosed according to self-report and discharge diagnosis. A blood routine was detected using the Sysmex XE-5000 machine. High-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol, triglyceride, lipoprotein(a), albumin, uric acid, and creatinine were detected using the Beckman AU5800 spectrophotometer via colorimetry or immunoturbidimetry. D-dimer was detected using the Sysmex CA-1500 via immunoturbidimetry.
Transthoracic echocardiography examination was performed by experienced sonographers using the GE Vivid E95 (GE Healthcare, Milwaukee, WI, United States) interfaced with a 2.5–3.5-MHz phased array probe. LVEF was measured using Simpson’s method.
The total procedure for statistical analysis was divided into four steps. First, we used the t-test for normally distributed data, the Mann–Whitney U test for non-normally distributed data, and the Chi-squared test or Fisher’s exact test for categorical variables to identify significant differences between the two groups. Second, we used the logistic regression models simultaneously for unadjusted, minimally adjusted, and fully adjusted analyses to evaluate the associations between D-dimer and CMVD. Considering the potential influence of age on D-dimer concentration, we added the sensitivity analyses to assess the relationship using an age-adjusted cut-off (500 ng/ml, if age is <50 years or age in years ×10 in patients ≥50 years) (18, 19). Third, because D-dimer is a continuous variable, to visually assess the non-linear relationship between D-dimer level and risk of CMVD, a restricted cubic spline curve was used. Fourth, subgroup analyses were performed using stratified Chi-square models; interactions among subgroups were examined using likelihood ratio tests. Comparisons with P < 0.05 (two-sided) were considered to be statistically significant. All of the analyses were performed with Stata 15.0 (StataCorp LLC, College Station, TX, United States), R version 3.4.3 (The R Project for Statistical Computing, Vienna, Austria), and EmpowerStats (X&Y Solutions Incorporation, Boston, MA, United States).
A total of 377 patients who presented with symptoms of ischemia changes and without obstructive CAD were finally included. Of those, 94 patients had evidence of CMVD assessed using the TMPG flow. The clinical characteristics of patients with CMVD and non-CMVD are shown in Table 1. Patients with CMVD were more likely to be older, have more frequent smoking behavior, and have more hypertension than those without patients without CMVD. D-dimer levels were significantly higher in patients with CMVD than in controls [360.00 (270.00–747.50) vs. 330.00 (270.00–470.00)], while sex, alcohol consumption, diabetes, renal function, blood lipids, and previous medication use presented no difference between the two groups.
Results for the association between D-dimer and CMVD are shown in Table 2. In the unadjusted model, the odds ratio (OR) for CMVD of D-dimer was 2.12 (95% CI: 1.29–3.50). After fully adjusting for other potential clinical risk factors, including age, sex, hypertension, diabetes, smoking, alcohol consumption, and platelets, the risk of CMVD among those with high D-dimer levels (>500 ng/ml) was 1.86-times (95% CI: 1.09–3.19) higher than the risk among patients in low D-dimer levels (P < 0.05). The association between the age-adjusted D-dimer and CMVD remained remarkably significant.
The relationship between D-dimer and the risk of CMVD is given in Figure 2. The restricted cubic spline curve showed a non-linear relationship between the D-dimer level and the prevalence of CMVD (P for linearity <0.05). An elevated D-dimer level was significantly associated with an increased risk of CMVD (P = 0.0241). When the D-dimer was at a margin level (500 ng/ml), the OR for CMVD was 1.30.
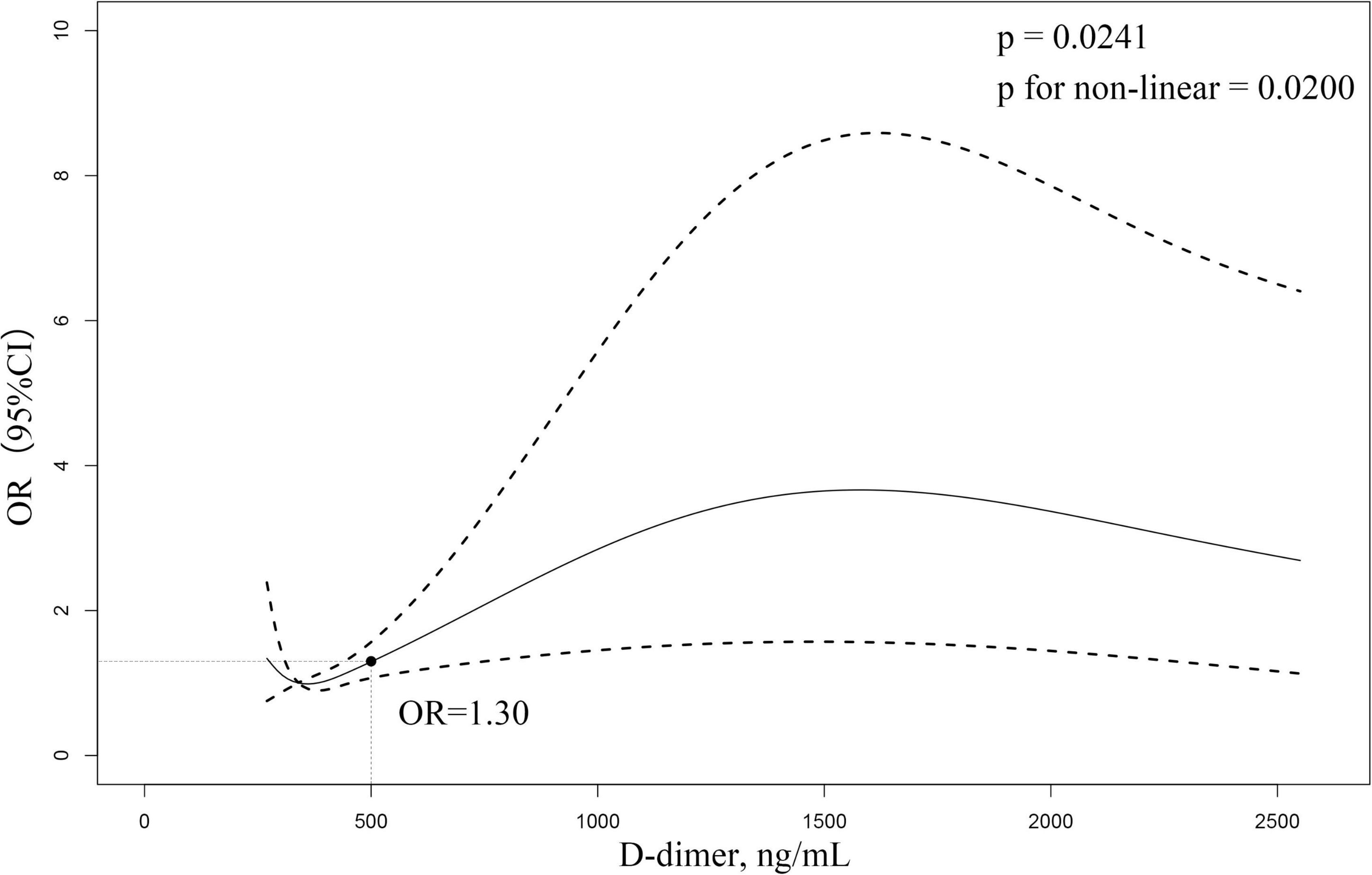
Figure 2. Restricted cubic spline curve to fit the relationship between D-dimer level and CMVD. The model adjusted for age, sex, hypertension, diabetes, smoking, alcohol consumption, and platelets. The middle area of the dash represents 95% confidence interval (CI), and the reference line represents D-dimer margin level. The relationship between D-dimer level and CMVD is not shown for those D-dimer >2,500 ng/mL due to the large (95%) CI.
In subgroup analysis, patients with high D-dimer levels (>500 ng/ml) have CMVD more frequently than those without in most of the strata (Figure 3). No significant interactions for the association between D-dimer and CMVD were found among individuals stratified by age, sex, hypertension, diabetes, smoking, CKD, and LDL-C levels.
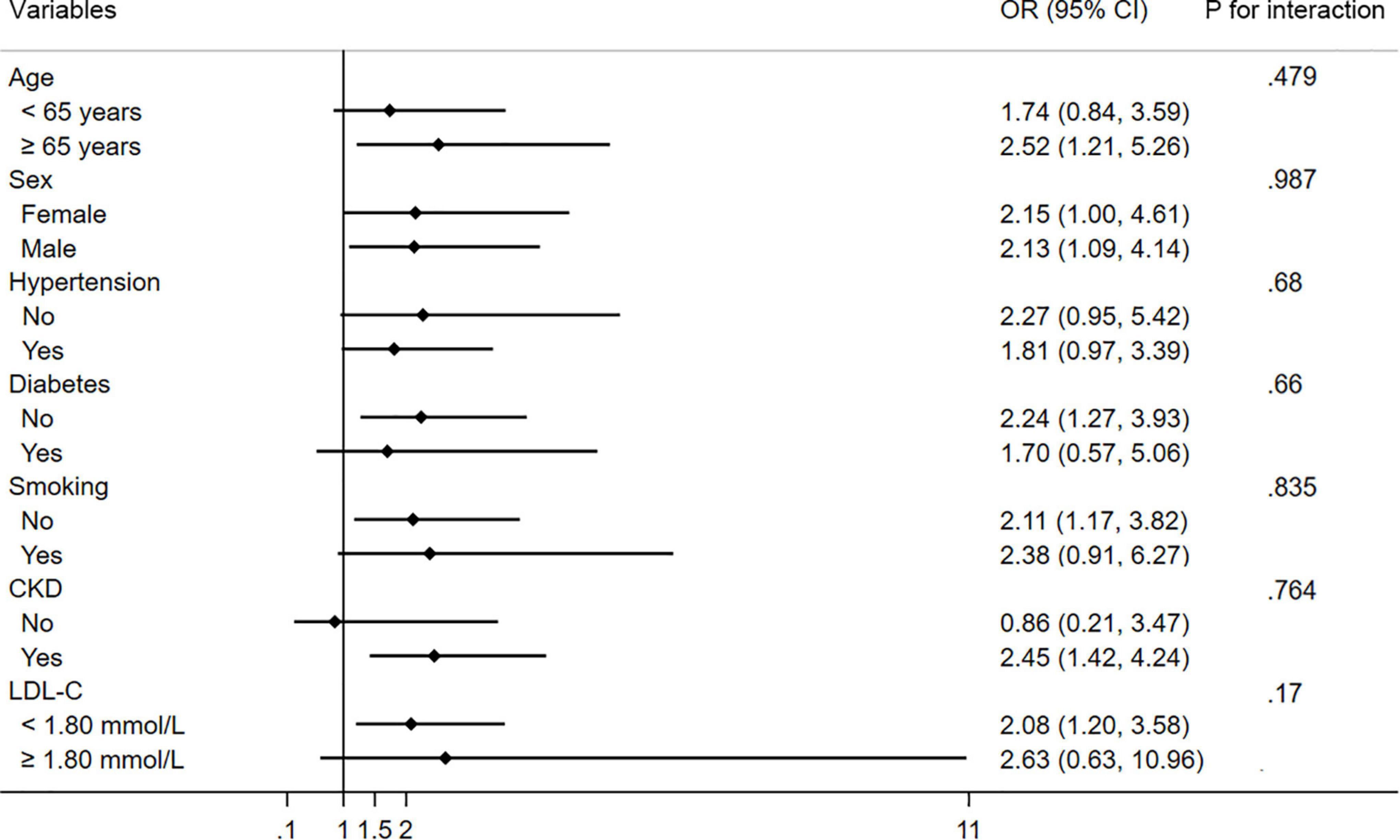
Figure 3. Subgroup analysis of the association between CMVD and D-dimer in prespecified and exploratory subgroups. CI, confidence interval.
In the present study, we found that elevated D-dimer level was strongly associated with the risk of CMVD and the association was independent of traditional risk factors. There was a dose–response relationship between D-dimer concentration and the incidence of CMVD. Elevated D-dimer levels could be used as a biomarker to identify CMVD.
D-dimer, a biomarker of microthrombosis, has been previously reported to reflect microcirculation (11). Previous studies have shown that elevated D-dimer levels are associated with cerebral circulation and microvascular complications in patients with diabetes mellitus, indicating the role of D-dimer in evaluating microvascular function (20, 21). However, there has been little study on the correlation between D-dimer and coronary microcirculation. In our study, the concentration of D-dimer was higher in patients with CMVD than in those without CMVD. Elevated D-dimer levels had a significant relationship with the occurrence of CMVD and the non-linear relationship between them also supports it. In the general population and patients with CAD, elevated baseline D-dimer levels were associated with poor cardiovascular outcomes (8, 9, 22), which may be attributed to the presence of CMVD. In addition, there is also an individual susceptibility to the occurrence of CMVD (23). Acquired risk factors such as older age, hypertension, diabetes, and hyperlipidemia may increase the occurrence of CMVD. In our study, patients with high D-dimer levels were older, had a higher incidence of hypertension, and were more likely to smoke. These comorbidities might contribute to CMVD.
The mechanisms by which D-dimer reflects microcirculation mainly include microvascular thrombosis, endothelial dysfunction, and inflammation. Previous studies on CMVD after ST-segment elevation myocardial infarction have shown that elevated D-dimer levels were largely influenced by thrombus burden and distal microvascular thrombosis (10). In addition to distal microvascular embolization, Erkol et al. found that in situ thrombosis may also contribute to poor myocardial perfusion (24). D-dimer, a biomarker that can reflect the severity of hypercoagulability, can be increased by microvascular embolism. Research involving patients with angina, non-obstructive CAD, and normal left ventricular function has shown that endomyocardial biopsy-proven endothelial cell activation occurred more frequently in patients with CMVD (25). Normal coronary blood flow and myocardial perfusion rely on the normally functioning endothelium, which regulates smooth muscle function through the release of vasodilators, such as nitric oxide (26). Inversely, damage to endothelial cells would lead to platelet activation and a coagulation cascade (27). The correlation between elevated D-dimer and endothelial cell dysfunction has been widely reported in previous studies (28–30). The significantly independent association between D-dimer levels and endothelial function determined via flow-mediated dilatation of the brachial artery supports the role of D-dimer as a biomarker for endothelial dysfunction (31), suggesting that an increased D-dimer level indicates the presence of endothelial dysfunction. Another potential mechanism contributing to CMVD is vascular inflammation. Prior studies have suggested that traditional cardiovascular risk factors may be important and they fail to fully account for the increased risk of the development of CMVD (32). In this regard, Klein et al. reported that patients with biopsy-proven myocardial inflammation infiltrate had an apparently reduced coronary flow reserve, indicating an association between inflammation and the occurrence of microvascular dysfunction (33). What is more, high-sensitivity C-reactive protein (CRP) is inversely related to coronary flow reserve in patients with angina and without obstructive CAD, showing a direct relationship between inflammation and CMVD (34). D-dimer, as an acute phase reactant, is increasingly recognized as a marker of inflammatory reaction (27). D-dimer concentrations have been reported to be related to vascular inflammation and microvascular complications in patients with metabolic and infectious diseases, such as diabetes mellitus, chronic obstructive pulmonary disease, and HIV infection (10, 35). Our results indicated that inflammation markers, such as white blood cell count and CRP, were slightly higher in patients with CMVD, although not significantly. In all, the microenvironment of inflammation and endothelial damage reflected by D-dimer plays an important role in the development of CMVD among patients with ischemic heart disease.
This study has several limitations. First, the present study had a retrospective cross-sectional design, so no causal relationship can be inferred between D-dimer levels and CMVD. Second, the research was conducted in a single center and the sample size is relatively small, so the conclusions drawn here cannot be generally extrapolated. Third, other factors that influence the level of D-dimer were not considered in this study, including infectious diseases, pulmonary embolism, and subclinical deep vein thrombosis. However, the effect of these factors can be ruled out because individuals with highly abnormal D-dimer levels were excluded and the vast majority of the study population had D-dimer concentrations of <2,500 ng/ml. Fourth, it is widely recognized that the TMPG is a clinically accessible and practical method to assess CMVD. However, other more accurate quantitative methods could be considered as options for further studies, such as cardiac MR, index of microcirculatory resistance, and fractional flow reserve.
In summary, D-dimer levels are significantly associated with the incidence of CMVD. This biomarker may be useful in identifying patients with CMVD for ischemic heart disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangdong Provincial People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XH, YL, and HD conceived and designed study, and drafted and refined the manuscript. WW, BY, LZ, and XH collected and complied, and analyzed the data. YZ, GL, and HD revised the manuscript critically. All authors have read and agreed to the published version of the manuscript.
This study was supported by the National Key Research and Development Program of China (No. 2016YFC1301202).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Accdon (www.accdon.com) for its linguistic assistance during the preparation of this manuscript.
1. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. (2018) 250:16–20. doi: 10.1016/j.ijcard.2017.08.068
2. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. (2015) 8:1445–53. doi: 10.1016/j.jcin.2015.06.017
3. Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. (2012) 33:734–44. doi: 10.1093/eurheartj/ehr331
4. Rubinshtein R, Yang EH, Rihal CS, Prasad A, Lennon RJ, Best PJ, et al. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur Heart J. (2010) 31:936–42. doi: 10.1093/eurheartj/ehp459
5. Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. (2010) 17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x
6. Ford TJ, Rocchiccioli P, Good R, Mcentegart M, Eteiba H, Watkins S, et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J. (2018) 39:4086–97. doi: 10.1093/eurheartj/ehy529
7. Ariëns RA, de Lange M, Snieder H, Boothby M, Spector TD, Grant PJ. Activation markers of coagulation and fibrinolysis in twins: heritability of the prethrombotic state. Lancet. (2002) 359:667–71. doi: 10.1016/S0140-6736(02)07813-3
8. Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. (1994) 90:2236–40. doi: 10.1161/01.cir.90.5.2236
9. Menown IB, Mathew TP, Gracey HM, Nesbitt GS, Murray P, Young IS, et al. Prediction of recurrent events by D-dimer and inflammatory markers in patients with normal cardiac troponin I (PREDICT) study. Am Heart J. (2003) 145:986–92. doi: 10.1016/S0002-8703(03)00169-8
10. Zhang M, Zhang J, Zhang Q, Yang X, Shan H, Ming Z, et al. D-dimer as a potential biomarker for the progression of COPD. Clin Chim Acta. (2016) 455:55–9. doi: 10.1016/j.cca.2016.01.024
11. Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. (2021) 19:1064–70. doi: 10.1111/jth.15267
12. Bruinstroop E, van de Ree MA, Huisman MV. The use of D-dimer in specific clinical conditions: a narrative review. Eur J Intern Med. (2009) 20:441–6. doi: 10.1016/j.ejim.2008.12.004
13. Hileman CO, Longenecker CT, Carman TL, Milne GL, Labbato DE, Storer NJ, et al. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. (2012) 17:1345–9. doi: 10.3851/IMP2297
14. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. (2007) 356:830–40. doi: 10.1056/NEJMra061889
15. Bethke A, Halvorsen S, Bøhmer E, Abdelnoor M, Arnesen H, Hoffmann P. Myocardial perfusion grade predicts final infarct size and left ventricular function in patients with ST-elevation myocardial infarction treated with a pharmaco-invasive strategy (thrombolysis and early angioplasty). Eurointervention. (2015) 11:518–24. doi: 10.4244/EIJY15M04_02
16. Abraham JM, Gibson CM, Pena G, Sanz R, Almahameed A, Murphy SA, et al. Association of angiographic perfusion score following percutaneous coronary intervention for ST-elevation myocardial infarction with left ventricular remodeling at 6 weeks in GRACIA-2. J Thromb Thrombolysis. (2009) 27:253–8. doi: 10.1007/s11239-008-0206-1
17. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. (2000) 101:125–30. doi: 10.1161/01.cir.101.2.125
18. Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. (2014) 311:1117–24. doi: 10.1001/jama.2014.2135
19. Freund Y, Chauvin A, Jimenez S, Philippon AL, Curac S, Fémy F, et al. Effect of a diagnostic strategy using an elevated and age-adjusted D-dimer threshold on thromboembolic events in emergency department patients with suspected pulmonary embolism: a randomized clinical trial. JAMA. (2021) 326:2141–9. doi: 10.1001/jama.2021.20750
20. Chang J, Arani K, Chew S, Frosch MP, Gonzalez RG, Maza N, et al. Susceptibility etching on MRI in patients with microangiopathy. J Neuroimaging. (2017) 27:43–9. doi: 10.1111/jon.12384
21. Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. (2016) 30:738–45. doi: 10.1016/j.jdiacomp.2015.12.018
22. Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, et al. D-dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease: LIPID study. Circulation. (2018) 138:712–23. doi: 10.1161/CIRCULATIONAHA.117.029901
23. Severino P, D’Amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, et al. Ischemic heart disease pathophysiology paradigms overview: from plaque activation to microvascular dysfunction. Int J Mol Sci. (2020) 21:8118. doi: 10.3390/ijms21218118
24. Erkol A, Oduncu V, Turan B, Kılıçgedik A, Sırma D, Gözübüyük G, et al. The value of plasma D-dimer level on admission in predicting no-reflow after primary percutaneous coronary intervention and long-term prognosis in patients with acute ST segment elevation myocardial infarction. J Thromb Thrombolysis. (2014) 38:339–47. doi: 10.1007/s11239-013-1044-3
25. Lindemann H, Petrovic I, Hill S, Athanasiadis A, Mahrholdt H, Schäufele T, et al. Biopsy-confirmed endothelial cell activation in patients with coronary microvascular dysfunction. Coron Artery Dis. (2018) 29:216–22. doi: 10.1097/MCA.0000000000000599
26. Shaw J, Anderson T. Coronary endothelial dysfunction in non-obstructive coronary artery disease: risk, pathogenesis, diagnosis and therapy. Vasc Med. (2016) 21:146–55. doi: 10.1177/1358863X15618268
27. Goldenberg NM, Kuebler WM. Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Compr Physiol. (2015) 5:531–59. doi: 10.1002/cphy.c140024
28. Graham SM, Mwilu R, Liles WC. Clinical utility of biomarkers of endothelial activation and coagulation for prognosis in HIV infection: a systematic review. Virulence. (2013) 4:564–71. doi: 10.4161/viru.25221
29. Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Markers of coagulation activation, endothelial stimulation and inflammation in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. (2005) 29:171–6. doi: 10.1016/j.ejvs.2004.11.001
30. Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care. (2004) 8:R82–90. doi: 10.1186/cc2459
31. Zhao X, Li J, Tang X, Jiang L, Chen J, Qiao S, et al. D-dimer as a thrombus biomarker for predicting 2-year mortality after percutaneous coronary intervention. Ther Adv Chronic Dis. (2020) 11:2040622320904302. doi: 10.1177/2040622320904302
32. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute WISE (women’s ischemia syndrome evaluation) study. J Am Coll Cardiol. (2010) 55:2825–32. doi: 10.1016/j.jacc.2010.01.054
33. Klein RM, Schwartzkopff B, Gabbert HE, Strauer BE. Diminished coronary reserve in patients with biopsy-proven inflammatory infiltrates. Cardiology. (2003) 100:120–8. doi: 10.1159/000073912
34. Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. (2013) 6:660–7. doi: 10.1016/j.jcmg.2012.12.011
Keywords: non-obstructive coronary artery disease, preserved ejection fraction, coronary microvascular dysfunction, TIMI myocardial perfusion grade, D-dimer
Citation: Lin Y, Hu X, Wang W, Yu B, Zhou L, Zhou Y, Li G and Dong H (2022) D-Dimer Is Associated With Coronary Microvascular Dysfunction in Patients With Non-obstructive Coronary Artery Disease and Preserved Ejection Fraction. Front. Cardiovasc. Med. 9:937952. doi: 10.3389/fcvm.2022.937952
Received: 06 May 2022; Accepted: 21 June 2022;
Published: 02 August 2022.
Edited by:
Jie Yu, University of New South Wales, AustraliaReviewed by:
Boyu Li, Capital Medical University, ChinaCopyright © 2022 Lin, Hu, Wang, Yu, Zhou, Zhou, Li and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haojian Dong, ZG9uZ2hhb2ppYW5Ac2luYS5jb20=; Guang Li, ZHJsaWd1YW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.