- Department of Cardiology, Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: To evaluate the efficacy and safety of lower ablation indexes (AI) guided pulmonary vein isolation (PVI) in treating paroxysmal atrial fibrillation (AF).
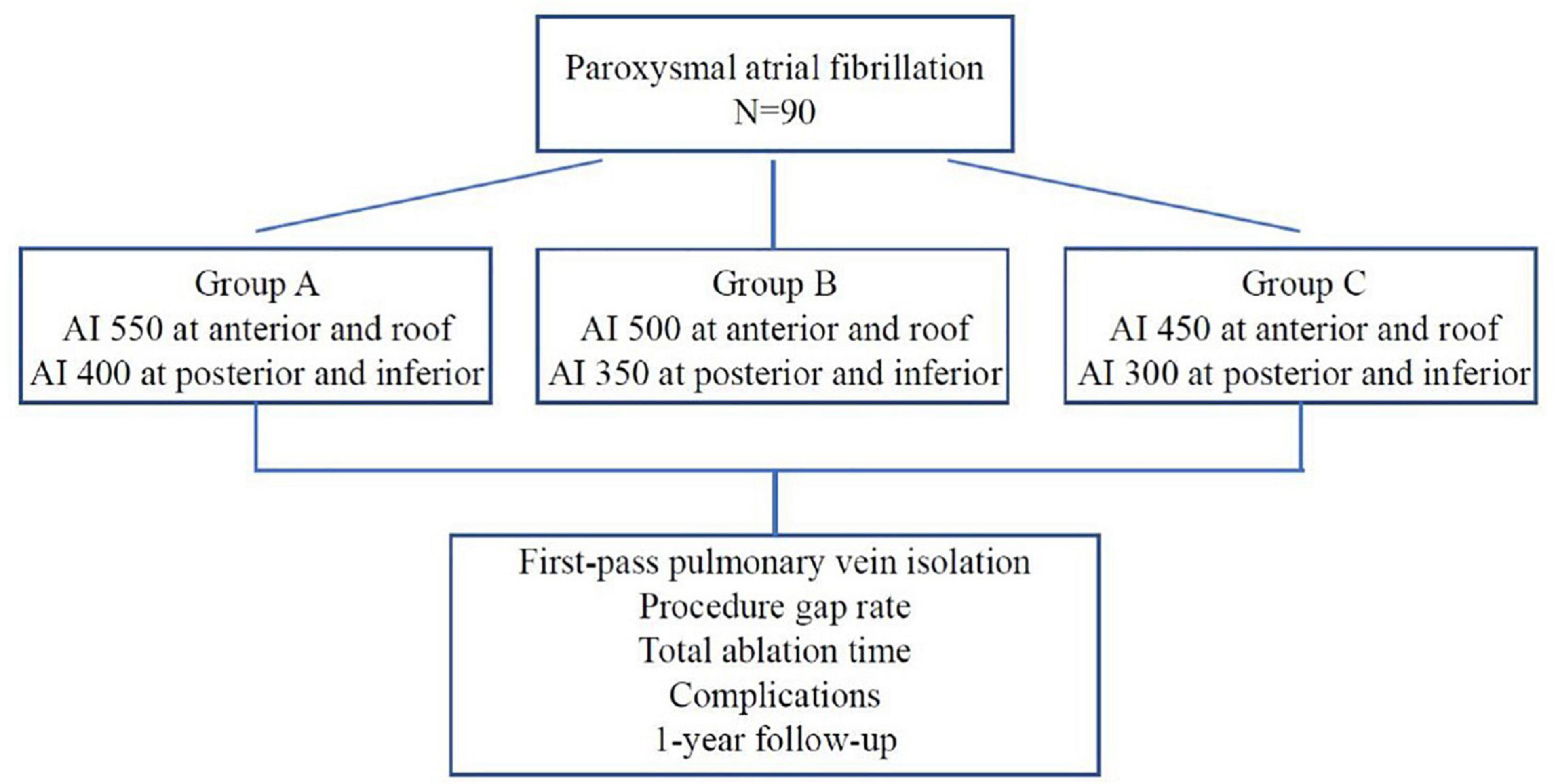
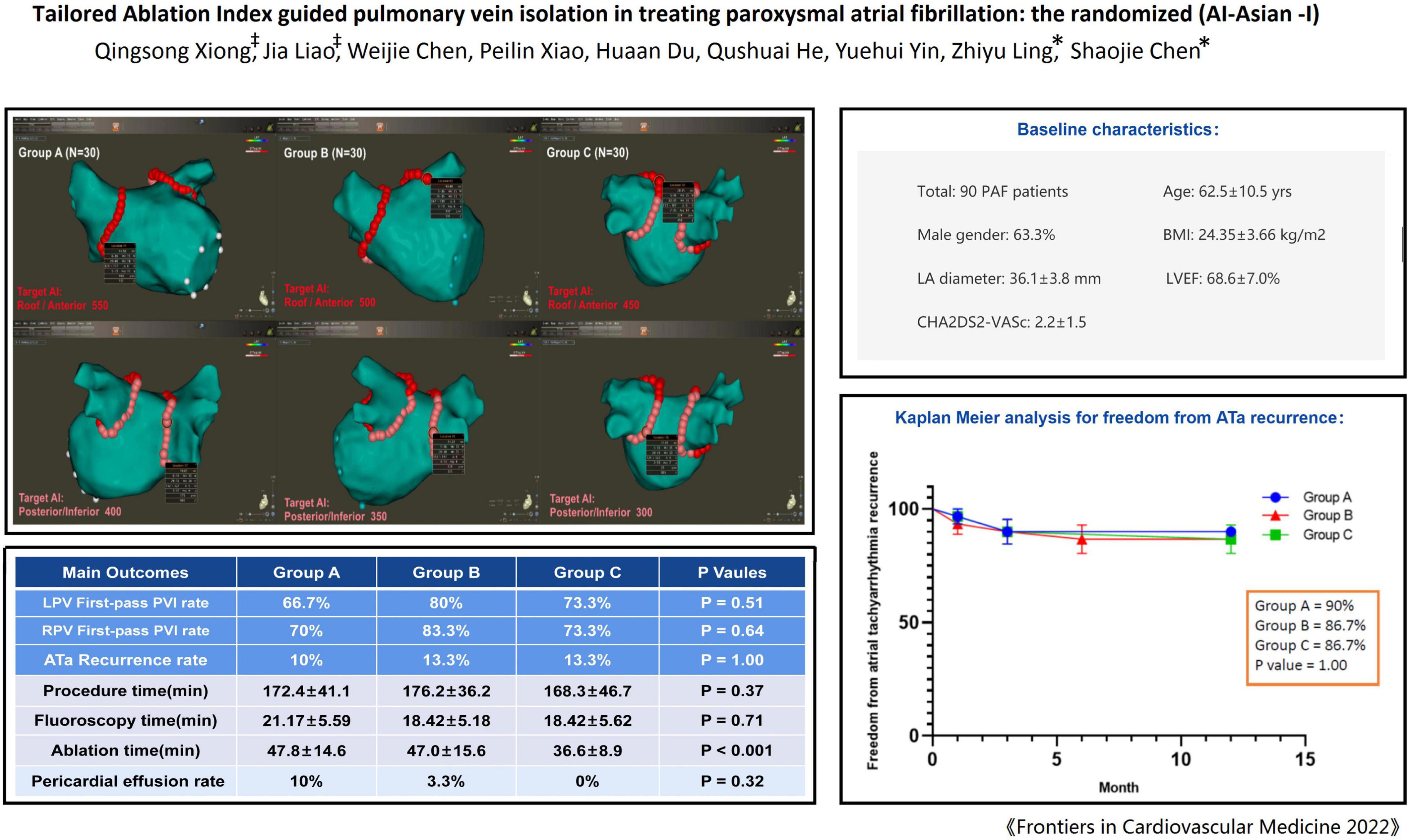
Methods: Ninety patients with paroxysmal AF scheduled for radiofrequency ablation were randomly divided into three groups. The AI targets for PVI were as follows: In group A/B/C, 550/500/450 for roof and anterior wall, and 400/350/300 for posterior/inferior wall. The first-pass PVI rate, ablation time, complications and recurrence of atrial tachyarrhythmia (ATa) were compared.
Results: The mean age was 62.5 years (male: 63.0%), mean body mass index (BMI): 24.35 ± 3.66 kg/m2. The baseline characteristics were comparable. There was no significant difference in the first-pass PVI rate among the three groups (left-sided-PV: 66.7% vs. 80% vs. 73.3%, P = 0.51; right-sided-PV: 70% vs. 83.3% vs. 73.3%, P = 0.64), also with similar gap rate during the procedural waiting time. At 1-year follow-up there was no significant difference in the recurrence rate of ATa among the three groups (10% vs. 13.3% vs. 13.3%, P = 1.00). The ablation time in the Group C was significantly less than that in the other two groups (47.8 min. vs. 47.0 min. vs. 36.6 min, P < 0.001). Higher AI seemed to link a non-significant trend toward higher rate of pericardial effusion (group A + B vs. group C:6.7% vs. 0%, P = 0.30), although the rate of overall complications was not different among the three groups.
Conclusion: This randomized study shows that, a relatively lower target AI guided ablation may be similarly effective to achieve PVI with significantly reduced ablation time and obtain similar clinical outcome in treating paroxysmal AF in Asian population.
Clinical Trial Registration: [www.ClinicalTrials.gov], identifier [NCT:04549714].
Introduction
Catheter ablation is an effective treatment option for patients with symptomatic atrial fibrillation (AF) (1–5). Electrical reconnection between the pulmonary veins and left atrium has been recognized as an important factor responsible for AF recurrence after ablation (6, 7). Radiofrequency ablation is conventionally performed with or without contact-force technology, usually not guided by standardized quantitative criteria. Lower ablation energy delivery may result in non-transmural damage, but over-shooting increases ablation time and may increase risk of complications (8, 9).
The ablation index (AI) which integrates catheter contact force, ablation power, and ablation duration is an algorithm to guide radiofrequency ablation, and the AI can be used to quantify the ablation energy and predict lesion formation (10–12). Previous studies have shown the efficacy of AI guided ablation for AF, and the target AI values adopted are generally 550 for the anterior wall and the roof and 400 for the inferior/posterior wall (13, 14).
However, (1) the target AI 550/400 was mainly obtained from European-American population; (2) The lesion depth caused by ablation guided by target AI of 550/400 can be deeper than the atrial thickness per se and may therefore potentially increase the risk of complications, particularly among Asian population, an ethnic group normally with smaller atrial size (15, 16). The aim of this randomized study was to investigate the feasibility, safety, efficacy and clinical outcome of catheter ablation guided by predefined lower target AI for pulmonary veins isolation (PVI) in treating paroxysmal AF in Asian population.
Materials and Methods
Study Design
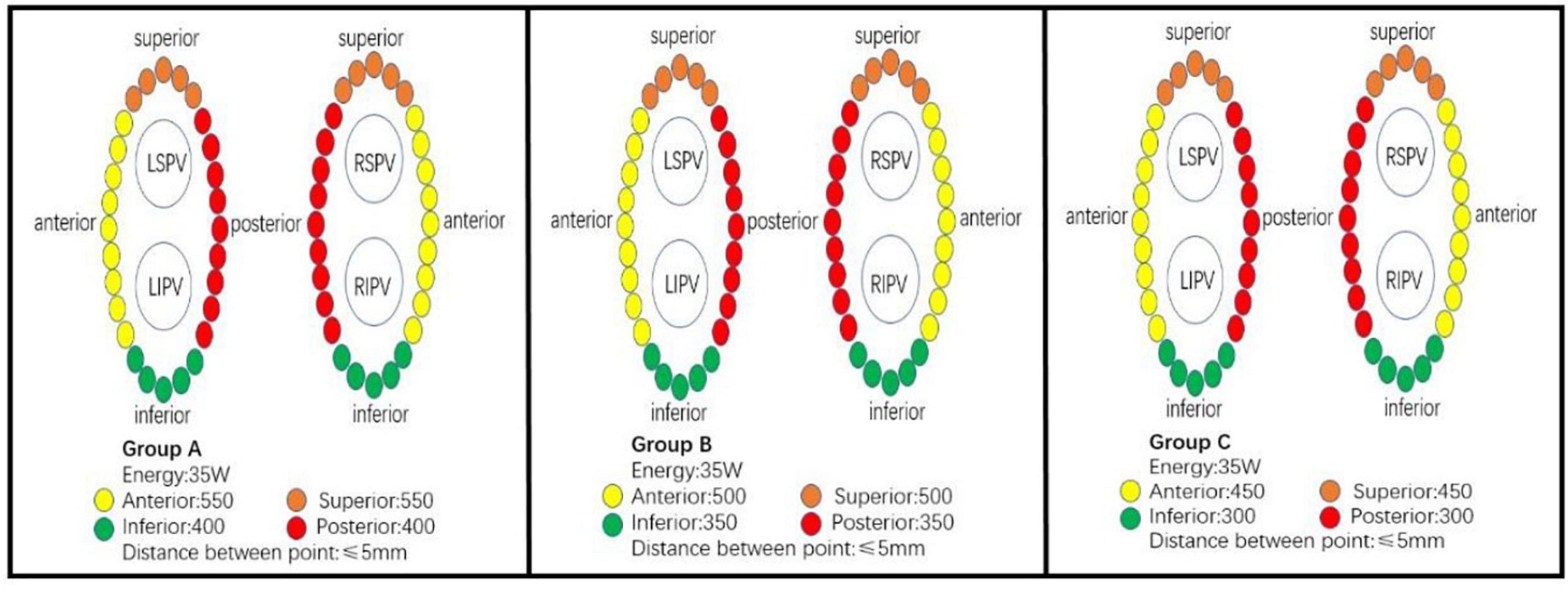
The study was designed as a prospective randomized, single blind, non-placebo controlled clinical study with the primary objective of assessing the optimal ablation index to achieve effective pulmonary vein isolation. A total of 90 patients with paroxysmal AF were randomly assigned to group A, B, and C by using a randomization envelope. Group A was designed with target AI value of 550 at the roof and anterior wall and 400 at the posterior and inferior wall; Group B was designed with target AI value of 500 at the roof and anterior wall and 350 at the posterior and inferior wall; Group C was designed with target AI value of 450 at the roof and anterior wall, and 300 at the posterior and inferior wall (Figure 1). The study was approved by ethics committee of Second Affiliated Hospital of Chongqing Medical University and complied with the declaration of Helsinki. Written informed consent was obtained from all patients.
Study Population and Exclusion
Study population: (1) Patients with symptomatic paroxysmal AF; (2) Age 18–80 years; (3) Without contraindication to anticoagulant therapy; (4) Transoesophageal ultrasonography excluded atrial/left atrial appendage thrombi. Exclusion criteria: (1) Patients who had undergone prior catheter ablation for AF; (2) Left ventricular ejection fraction (LVEF) < 35%; (3) Pregnant, prepared pregnant or lactating women; (4) Left atrial appendage thrombus detected by transesophageal or intracardiac ultrasonography; (5) Severe abnormalities of hematologic or hepatic and renal function; (6) Combined with other heart disease (congenital heart disease, valvular heart disease, dilated cardiomyopathy, hypertrophic cardiomyopathy); (7) Acute myocardial infarction or patients who had undergone PCI or CABG within 1 year; (8) Pulmonary vein stenosis, occlusion, or thrombus.
Pre-procedure
Pre-operative Transesophageal ultrasonography was completed to exclude intracardiac thrombi. Cardiac Computed tomography (CT) or magnetic resonance imaging (MRI) was performed to assess the anatomy. All patients received anticoagulant therapy according to the recommendation (17). To reduce the risk of bleeding during ablation, oral anticoagulant drugs were suspended on the day of ablation. All patients discontinued antiarrhythmic drugs 5 half-life period.
Ablation
Procedures were performed by two operators; both of them have had more than 5 years of ablation experience and more than 50 procedures per year. All ablation were under local anesthesia, and fentanyl was used to reduce pain during ablation. Heparin was administered at 100–120 U/kg and activated prothrombin time (ACT) was maintained at 300–400 S. After the puncture of the bilateral femoral veins, a 7F vascular sheath was placed through the left femoral vein and a diagnostic electrode catheter (SinusFlex™, APT Medical, China) was placed in the coronary sinus. Two 8.5F Swartz transseptal sheaths (St. Jude) were advanced through the right femoral vein, and transseptal puncture was performed under the guidance of fluoroscopy. A spiral diagnostic mapping catheter (Lasso, Biosense-Webster Inc., Diamond Bar, CA) was positioned in left atrium via the transseptal sheath to document the pulmonary vein ostia potential. A 3.5 mm irrigated tip ablation catheter (Thermocool SmartTouch; Biosense-Webster Inc., Diamond Bar, CA) was advanced in the left atrium via the other transseptal sheath. Anatomical model of left atrial, pulmonary vein, and mitral annulus was constructed under the guidance of the 3D mapping system (CARTO 3 V6; Biosense-Webster, Inc., Diamond Bar, CA). Wide-area circumferential pulmonary vein antrum isolation (PVI) was performed under the guidance of carto mapping system and automated lesion tagging (VisiTag™, Biosense Webster Inc., DiamondBar, CA, United States). The following Visitag settings were used: 3 mm stability for 3 s. Inter-lesion distance (ILD) targets ≤ 5 mm. Ablation power was set at 35 W, target contact force between 5 and 15 g, irrigation rate of 25 ml/min. The target AI for ablation was determined according to randomization (Figure 2). The ablation endpoint was reached if disappearance (or dissociation) of all pulmonary vein potential after completion of the ablation circles. Otherwise, the gaps were searched and ablated until successful PVI. If patient remained in AF after PVI, electrical cardioversion was performed to restore sinus rhythm. 30 min waiting time was required for all patients after PVI, each pulmonary vein was re-examined to confirm procedural success.
Follow-Up
All patients returned to the hospital for follow-up at 1, 3, 6, and 12 months after discharge. The main contents included: 12-channel electrocardiography(ECG), 24-h Holter ECGs, and clinical symptoms. Three-month after the procedure was defined as a blank period. Oral antiarrhythmic drugs remained discontinued after the procedure, cardioversion or AADs were allowed if early recurrence during the blanking period. In case of any symptoms suggestive of an arrhythmia recurrence, 24 h Holter ECGs were performed or patient received an external event monitor. Arrhythmia recurrence was defined as ECG documented atrial arrhythmias longer than 30 s after 3 months without AADs. Oral preventive PPI inhibitors were used for 1 month after the ablation. The primary outcomes were the rate of first-pass PVI and clinical recurrence, and secondary outcomes referred to procedural data, including ablation time, procedure time, rate/location of gaps and procedural complications.
Statistics
The sample size calculation was primarily based on the rates of first-pass PVI guided by different AIs reported in previous literatures, i.e., the rate of first-pass PVI ranged from ca. 70% guided by lower target AI to ca. 95% guided by higher target AI. At α level of 0.05, the sample size of 64 patients (32 in each group) could provide 80% statistical power to estimate the differences between two groups.
Normally distributed continuous data were expressed as mean ± standard deviation. Non-normally distributed data were presented as median with interquartile range. Categorical data were presented as frequencies and percentages. The normality of variable distribution was tested using the Shapiro–Wilk test. The t-test was used to compare continuous variables with normal distribution; otherwise the Mann–Whitney test was used. For categorical variables, comparisons between groups were performed using the chi square test or Fisher’s exact test. Kaplan – Meier analysis and log rank test were used for event free survival analysis. For all calculations, two tailed tests were used, and the level of significance was set at a p-value of 0.05. All calculations were performed using SPSS 25 (IBM Corp., Armonk, NY, United States).
Results
Population Baseline Characteristics
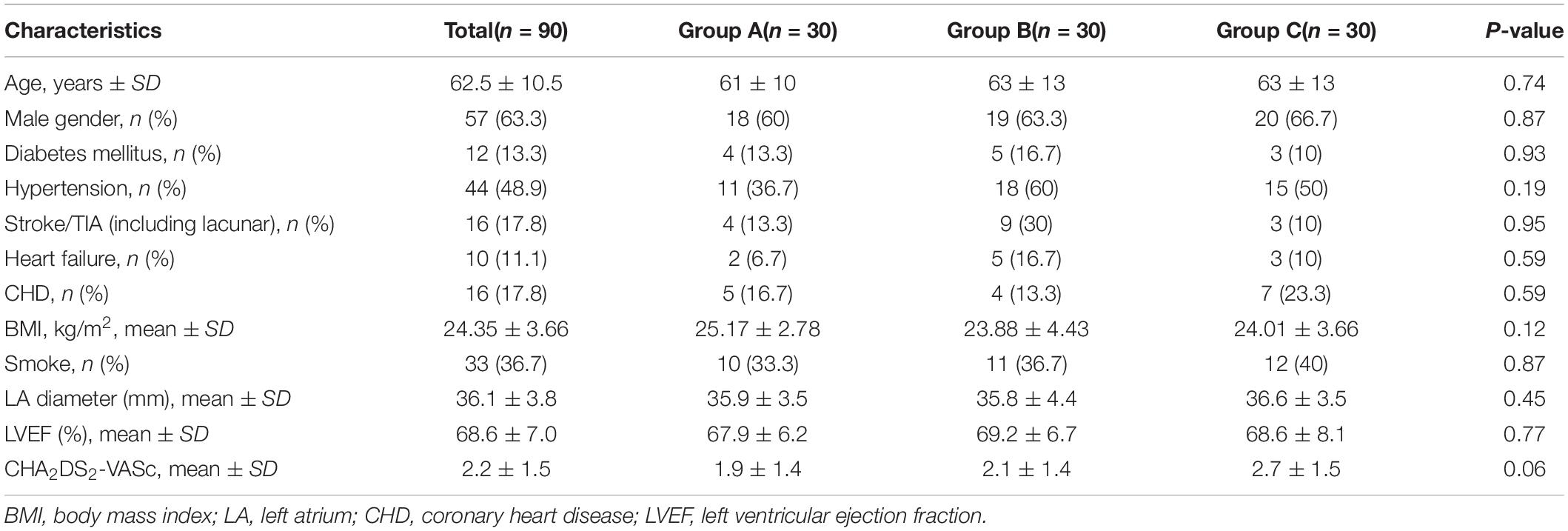
The study recruited the first patient on June 18, 2019 and the last patient on June 29, 2020. All 90 patients completed 1 year follow-up and were included in the data analysis. Table 1 reports the demographic information of all included patients. The three groups were balanced in terms of basic characteristics, mean age 62.5 years, 63.3% male, mean left atrial diameter 36.1 mm by TTE, and left ventricular ejection fraction 68.6%.
First Pass Pulmonary Vein Isolation
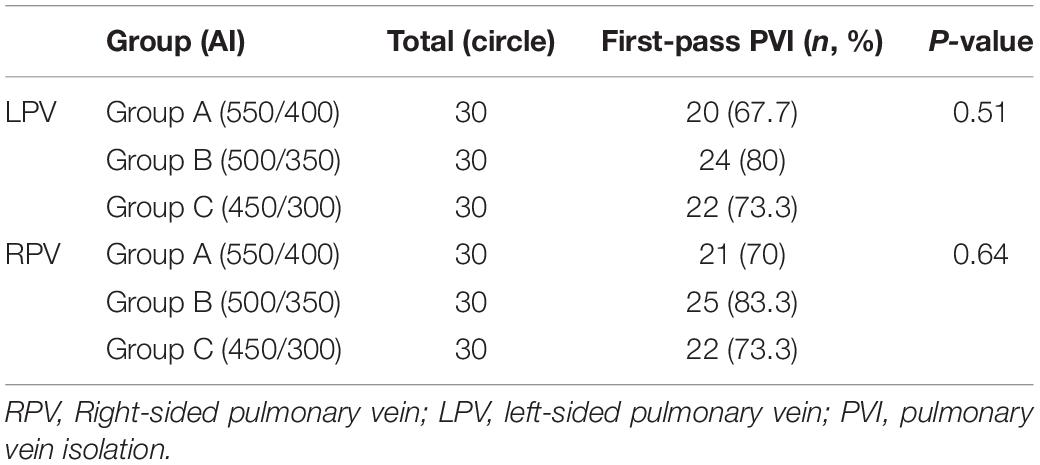
As shown in Table 2, The first-pass isolation rate of left-sided pulmonary vein was 73.3% in 90 patients, and there was no significant statistical difference among the three groups (67.7% vs. 80% vs. 73.3, P = 0.51). The first-pass isolation rate of the right-sided pulmonary vein was 75.6%, with no significant difference between the three groups (70% vs. 83.3% vs. 73.3, P = 0.64) (Table 2).
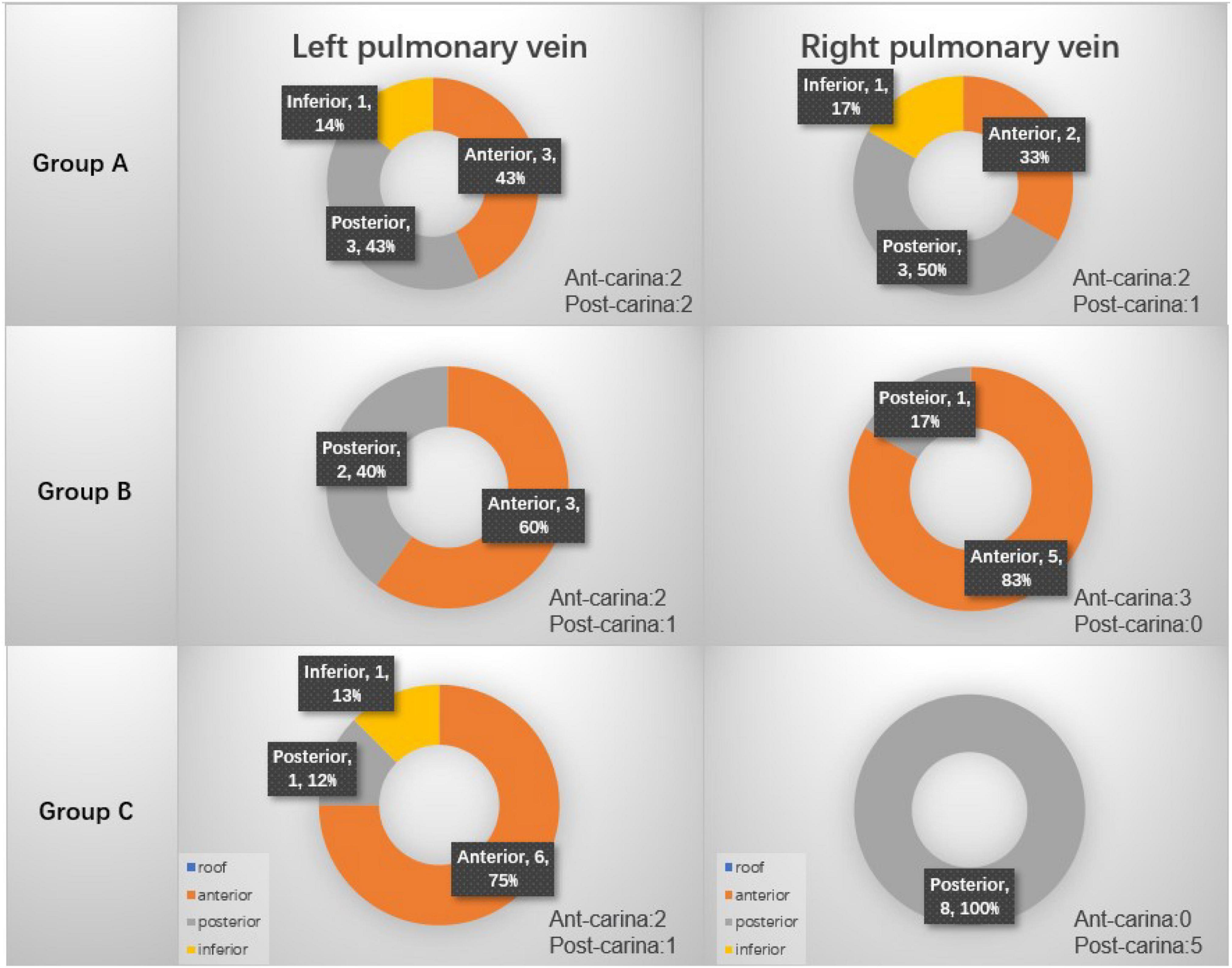
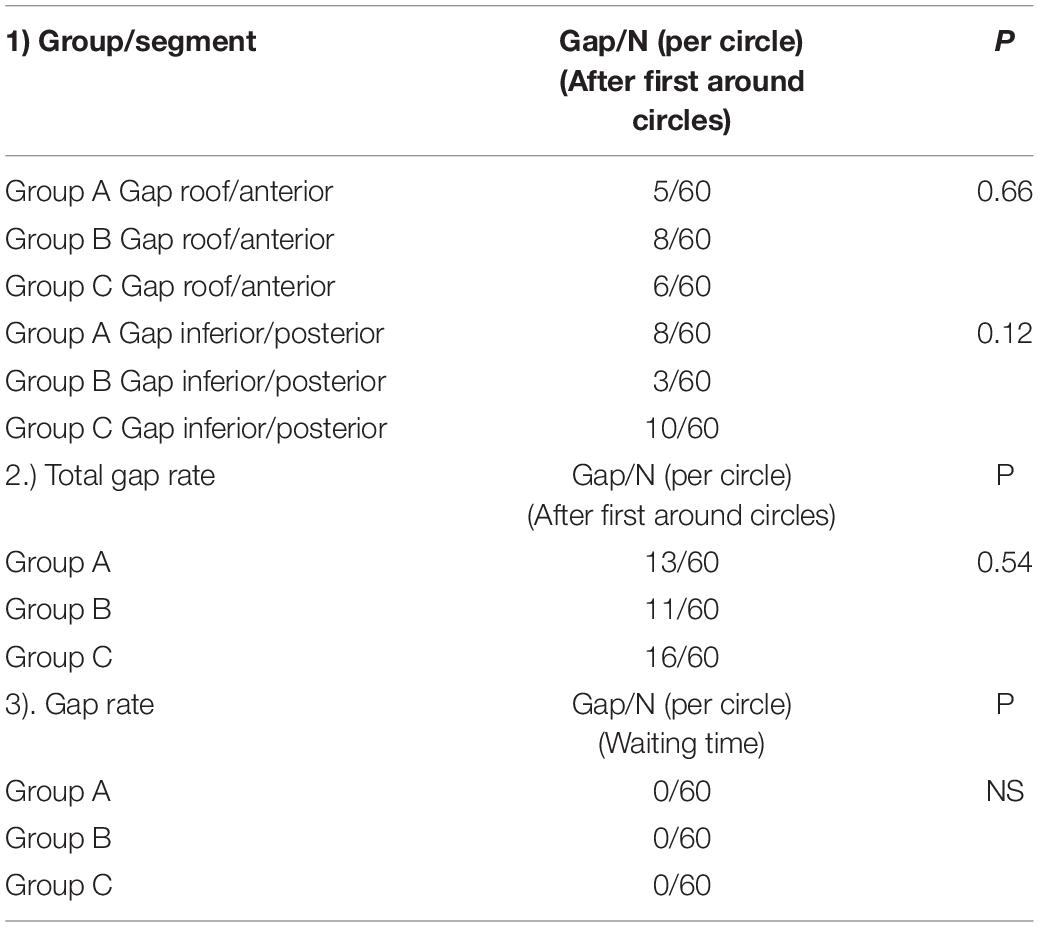
For patients who did not have first-pass isolation, the location of gap was searched. Figure 3 summarizes the procedural gap rate and the distribution of the gaps. In details, for the left pulmonary veins, in group A, 3 gaps were located at the anterior wall, 3 gaps at the posterior wall and 1 gap at the inferior; in Group B, 2 gaps at the posterior wall and 3 gaps at the anterior wall; in group C, there were 6 gaps at anterior wall, 1 gap at inferior wall, and 1 gap at posterior wall. For the right pulmonary veins, in group A, 2 gaps were located at the anterior wall, 3 gaps at the posterior wall, 1 gap at the inferior wall; in Group B, 1 gap at the posterior wall and 5 gaps at the anterior wall; in group C, 8 gaps were at the posterior wall. Table 3 compares the procedural gaps rate (including the 30 min waiting time) among the three groups, and no significant difference was observed.
Recurrence
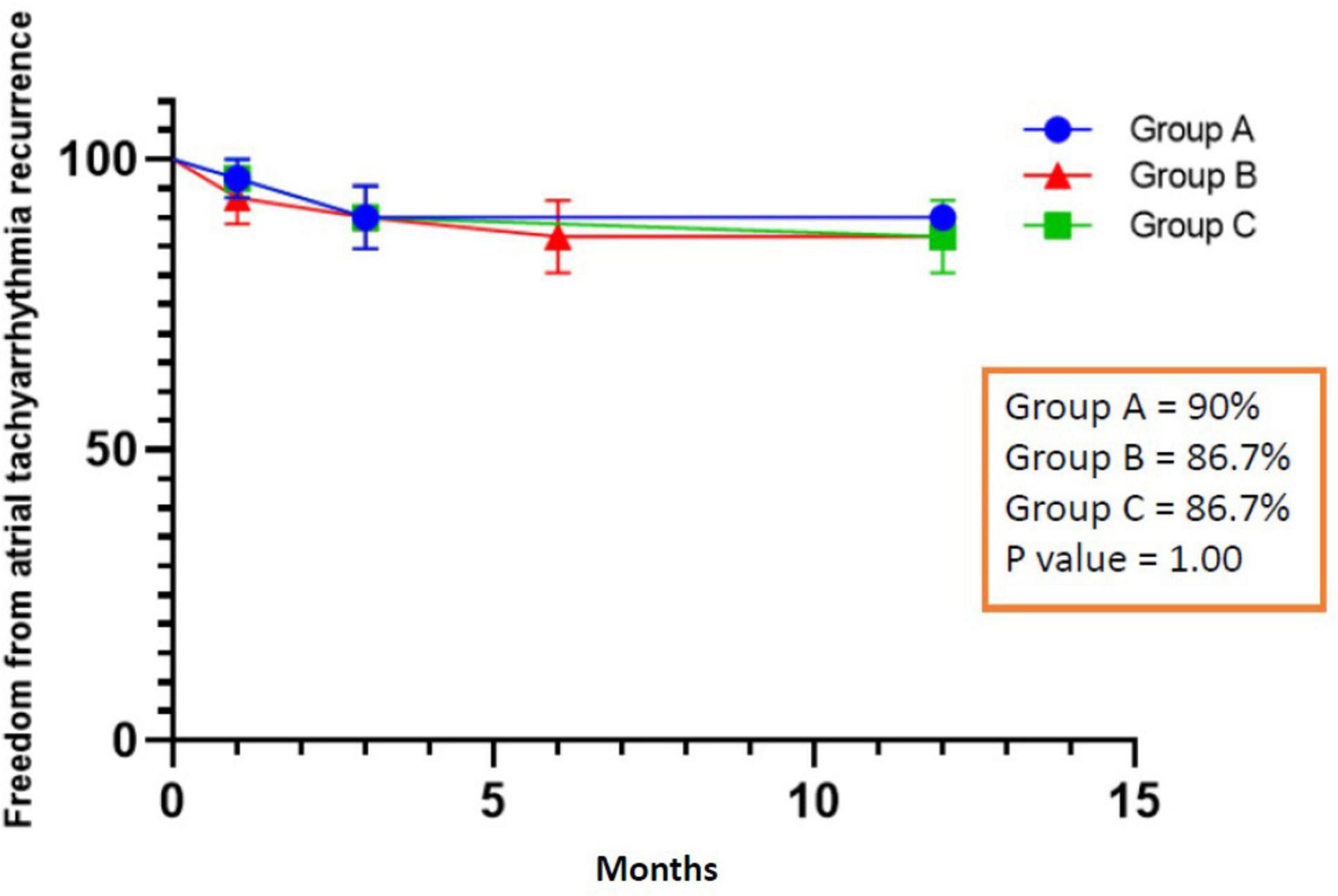
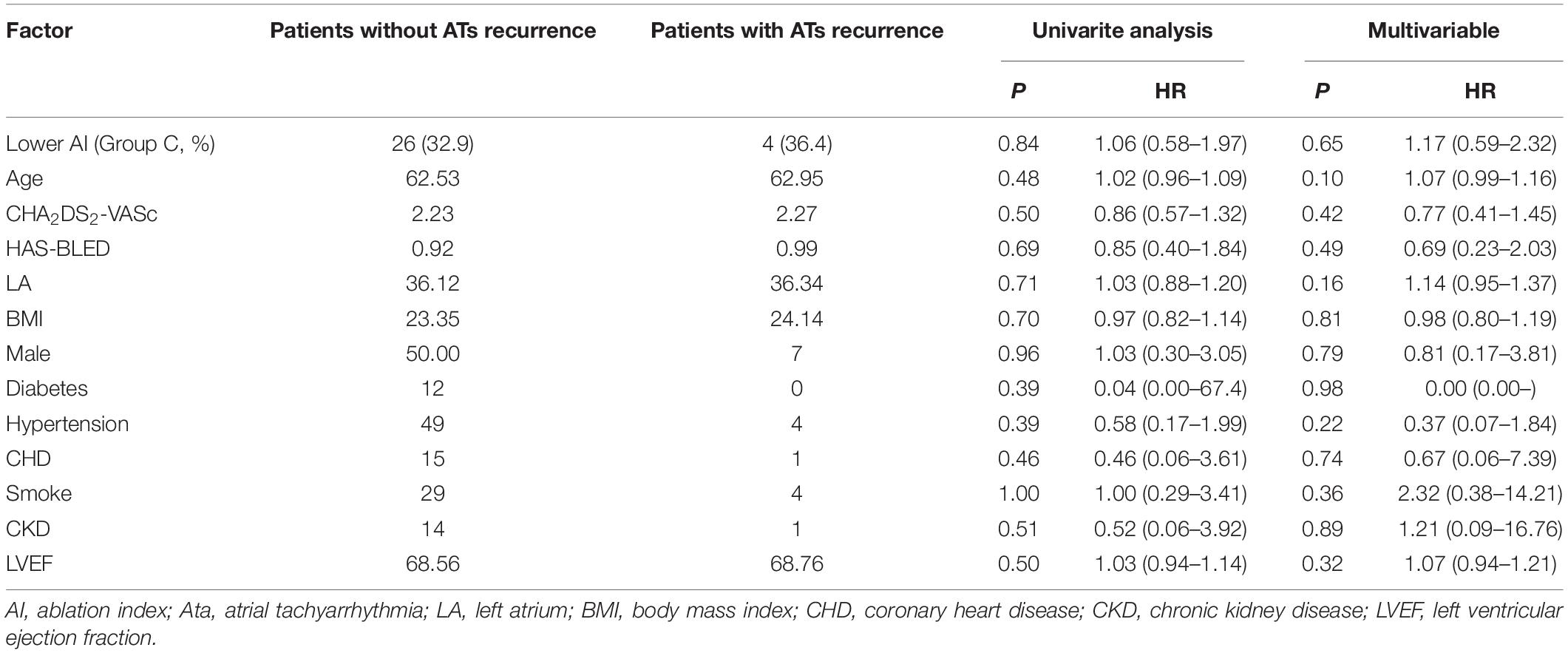
All patients completed 1-year follow-up. The results showed that 3 patients had recurrent AF after ablation in group A, 4 patients in group B (1 patient had recurrent left atrial flutter), and 4 patients recurrent AF in group C. The rate of ATa recurrence was similar among the three groups (10% vs. 13.3% vs. 13.3%, P = 1.00), and Figure 4 shows the Kaplan Meier Curve for freedom from ATa recurrence. In univariate and multivariate regression analysis, no factor related to the recurrence of ATa was found (Table 4).
Ablation Time and Complications
The procedure was successfully completed in all 90 patients, the procedure duration (172.4 ± 41.1 min vs. 176.2 ± 36.2 min vs. 168.3 ± 46.7 min, p = 0.37) and fluoroscopy time (21.17 ± 5.59 min vs. 18.42 ± 5.18 min vs. 18.42 ± 5.62 min, p = 0.71) reached no significant difference, but the ablation time was significantly reduced in the low AI group (group C) compared with the other two groups (47.8 ± 14.6 min. vs. 47.0 ± 15.6 min vs. 36.6 ± 8.9 min, P < 0.001), and there was no statistical difference between groups A and B. Four patients had pericardial effusion, 3 in group A, 1 in group B, and 0 in group C (10% vs. 3.3% vs. 0%, P = 0.32) (group A + B vs. group C: 6.7% vs. 0%, P = 0.30), all pericardial effusions were treated with pericardiocentesis (aspiration of fluid volume: 100–200 ml) without surgery. There was no steam pop in all procedures. One arteriovenous fistula occurred in group A, the patient was asymptomatic and without hematoma at the puncture site. Other complications, such as death, thromboembolic events, major bleeding, PV stenosis, phrenic nerve palsy, or symptoms suggestive of esophageal injury were not observed during the study period.
Discussion
The main findings are summarized in Figure 5. In this randomized study, we compared different target AI guided PVI in treating paroxysmal AF. The overall rate of first-pass PVI was 73.3%, and the rate of first-pass PVI did not significantly differ between the three groups, also with similar gap rate during the procedural waiting time. Lower AI guided PVI (group C) was associated with significantly less ablation time than that in the other two groups, whereas higher AI guided PVI seemed to link a non-significant trend toward higher rate of pericardial effusion/tamponade, although the rate of overall complications was not different. One-year follow-up showed single-procedural similar freedom from ATa recurrence among the three groups. To the best of our knowledge, this is the first randomized study investigating different target ablation index to guide pulmonary vein isolation in Asian population.
Previous studies showed that AI guided AF ablation may improve the procedural efficacy (18); however, studies to define optimal AI value remains limited. According to the anatomy, the thickness of atrial tissue is about 2–4 mm. In vitro experiments showed that, every 100 increase of the AI caused lesion-depth increase by 1 mm. Thus theoretically, the transmural lesion can be achieved when the AI reaches 200–400 depending on the thickness of atrial wall. From the literature, the mostly adopted target AI was around 500–550/350–400, mainly from European-American population, and such target AI led to a first-pass rate of PVI around 70–90% (19–24).
Notably, the patients enrolled in our study appeared to have significantly lower BMI (24 kg/m2) and smaller LA (36 mm). Consistent with previous studies, the overall rate of first-pass PVI in our study was 73.3%, and interestingly the rate of first-pass PVI did not significantly differ between the three groups, indicating that relatively lower AI guided ablation might be effective to achieve procedural PVI among Asian population. Such assumption seemed to be further supported by the similar procedural gap rate (even during the waiting time) and the similar ATa recurrence rate during follow-up.
Indeed, the 70–80% first-pass PVI observed in our study was not high. A relatively lower power (35W) adopted in our study may play a role since it has been known that high-power ablation may form wider ablation lesion. Second, the target inter-lesion distance of 5 mm was employed in our study, as previous randomized study found that a relatively wider inter-lesion distance (5–6 mm) was associated with significantly lower rate of first-pass PVI as compared with that with closer inter-lesion distance (3–4 mm), when using conventional power ablation for PVI (25).
In general, effective lesion formation with short ablation time indicates an efficient procedure, or even may be safe procedure. Longer ablation time can be potentially associated with increased risk of procedural complication. In our study, four (4/90) patients had pericardial effusion after the AI guided PVI without evidence of notable steam-pop, all pericardial effusion occurred in higher AI groups (3 in group A, 1 in group B), and no pericardial effusion occurred in group C. More specifically, all pericardial effusions were detected after the PVI during routine echocardiography, and all these patients had additional ablation due to no first-pass isolation. For the four patients who had pericardial effusion: three were female, one male patient had known renal dysfunction, all four patients were in older age (mean 70 years old) with relatively low BMI (mean 24 kg/m2), procedural ACTs were between 300 and 350, and no audible steam-pop was noticed during the procedures. The four patients with pericardial effusion were treated with pericardiocentesis (aspiration of fluid volume: 100–200 ml), without needing surgery. These observations may indicate that, (1) pericardial effusion could still occur regardless of steam-pop; (2) repeated or additional ablation to achieve the target AI thereby to close the gap sites due to failure of first-pass isolation appears to be a risk factor of pericardial effusion; (3) the recommended target AI derived from the European-American population might not be necessarily suitable for Asian population, and should be performed with cautions, e.g., in small, older, female patients.
In our study, lower AI guided ablation (group C) was associated with significantly less ablation time. However, we did not count overall procedure and fluoroscopic time for every patient because some patients also had concomitant electrophysiological examination, and some patients had concomitant coronary angiography. Although this was a randomized study, as an initial validation study, the sample size in each group was rather small. We could only investigate the procedural efficacy of the different AI guided PVI, and the long-term durable PVI could not be assessed in the present study. The ATa recurrence was only assessed by Holter-ECG, without continuous heart rhythm monitoring, thus some episodes of ATa recurrences may not be diagnosed in all the three groups. Conventional power instead of high power was used, thus our result cannot be generalized to high power ablation strategy. Due to unavailability our patients could not receive esophageal temperature monitoring during ablation. As preventive strategy, the atrial esophageal anatomical relationship was carefully assessed by pre-procedural cardiac CT or MRI and integrated in the 3D mapping system, energy delivery at those potential adjacent sites were maximally avoided. In addition, all patients received 4 weeks preventive PPI therapy after the ablation, and no patients had symptoms or signs suggestive of esophageal injury during clinical follow-up.
Conclusion
This randomized study shows that, a relatively lower target AI guided AF ablation may be similarly effective to achieve PVI with significantly reduced ablation time and obtain similar clinical outcome in treating paroxysmal atrial fibrillation in Asian population.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The study was approved by Ethics Committee of Second Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZL: study design. QX, JL, WC, PX, HD, QH, YY, and ZL: data collection. QX and JL: data analysis. QX, ZL, and SC: first draft. QX, JL, WC, PX, HD, QH, YY, ZL, and SC: review and approval. ZL and SC were co-mentors for the dissertation. SC dedicated intellectual contribution to the dissertation, interpreted the study results, and made critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was partly supported by Joint Medical Research Project of Chongqing Municipal Health Commission and Chongqing Science and Technology Bureau (Grant No. 2018ZDXM015) (ZL), Future Young Medical innovation Team of Chongqing Medical University (Grant No. W0078) (ZL), and Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China [Grant No. (2020)7] (ZL).
Conflict of Interest
SC has been invited as consultant to Biosense Webster, Boston Scientific, and declares no financial funding with regard to the content of the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. January CT, Wann S, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000719
2. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, et al. The 2020 Canadian cardiovascular society/Canadian heart rhythm society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. (2020) 36:1847–948. doi: 10.1016/j.cjca.2020.09.001
3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Revista Española de Cardiol. (2021) 74:437. doi: 10.1016/j.rec.2021.03.009
4. Chen S, Pürerfellner H, Meyer C, Acou W-J, Schratter A, Ling Z, et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J. (2020) 41:2863–73. doi: 10.1093/eurheartj/ehz443
5. Chen S, Pürerfellner H, Ouyang F, Kiuchi MG, Meyer C, Martinek M, et al. Catheter ablation vs. antiarrhythmic drugs as ‘first-line’ initial therapy for atrial fibrillation: a pooled analysis of randomized data. Europace. (2021) 23:1950–60. doi: 10.1093/europace/euab185
6. Rajappan K, Kistler PM, Earley MJ, Thomas G, Izquierdo M, Sporton SC, et al. Acute and chronic pulmonary vein reconnection after atrial fibrillation ablation: a prospective characterization of anatomical sites. Pacing Clin Electrophysiol. (2008) 31:1598–605. doi: 10.1111/j.1540-8159.2008.01232.x
7. Kuck KH, Hoffman BA, Ernst S, Wegscheider K, Treszl A, Metzner A, et al. Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: results from the gap-atrial fibrillation-german atrial fibrillation competence network 1 trial. Circ Arrhyth Electrophysiol. (2016) 9:e003337. doi: 10.1161/CIRCEP.115.003337
8. Nakagawa H, Jackman WM. The role of contact force in atrial fibrillation ablation. J Atr Fibrillation. (2014) 7:1027.
9. Zhang Z-W, Zhang P, Jiang R-H, Liu Q, Sun Y-X, Yu L, et al. Risk of esophageal thermal injury during catheter ablation for atrial fibrillation guided by different ablation index. Pacing Clin Electrophysiol. (2020) 63:197–205.
10. Pranata R, Vania R, Huang I. Ablation-index guided versus conventional contact-force guided ablation in pulmonary vein isolation – Systematic review and meta-analysis. Indian Pacing Electrophysiol J. (2019) 19:155–60. doi: 10.1016/j.ipej.2019.05.001
11. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A, et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol. (2018) 11:e006576. doi: 10.1161/CIRCEP.118.006576
12. Kiliszek M, Krzyzanowski K, Wierzbowski R, Winkler A, Smalc-Stasiak M. The value of the ablation index in patients undergoing ablation for atrial fibrillation. Kardiologia Polska. (2020) 78:1015–9. doi: 10.33963/KP.15523
13. Chen S, Schmidt B, Seeger A, Bordignon S, Tohoku S, Willems F, et al. Catheter ablation of atrial fibrillation using ablation index-guided high power (50 W) for pulmonary vein isolation with or without esophageal temperature probe (the AI-HP ESO II). Heart Rhythm. (2020) 17:1833–40. doi: 10.1016/j.hrthm.2020.05.029
14. Chen S, Schmidt B, Bordignon S, Tohoku S, Urban VC, Schulte-Hahn B, et al. Catheter ablation of atrial fibrillation using ablation index-guided high-power technique: frankfurt AI high-power 15-month follow-u. J Cardiovasc Electrophysiol. (2021) 32:616–24. doi: 10.1111/jce.14912
15. Lycke M, O’Neill L, Gillis K, Wielandts J-Y, Le Polain De Waroux J-B, Tavernier R, et al. How close are we toward an optimal balance in safety and efficacy in catheter ablation of atrial fibrillation? lessons from the CLOSE protocol. J Clin Med. (2021) 10:4268. doi: 10.3390/jcm10184268
16. The EchoNoRMAL Collaboration. Ethnic-specific normative reference values for echocardiographic LA and LV Size, LV mass, and systolic function: the EchoNoRMAL study. JACC Cardiovasc Imaging. (2015) 8:656–65.
17. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehab648
18. Ioannou A, Papageorgiou N, Lim WY, Wongwarawipat T, Hunter RJ, Dhillion G, et al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: an updated meta-analysis. Europace. (2020) 22:1659–71. doi: 10.1093/europace/euaa224
19. Hussein A, Das M, Chaturvedi V, Asfour IK, Daryanani N, Morgan M, et al. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. (2017) 28:1037–47. doi: 10.1111/jce.13281
20. Taghji P, Phlips T, Wolf M, Demolder A, Choudhury R, Knecht S, et al. Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation. in Heart Rhythm 2016. Circ Arrhythm Electrophysiol. (2016) 10:e004867. doi: 10.1161/CIRCEP.116.004867
21. Lepillier A, Solimene F, De Ruvo E, Scaglione M, Anselmino M, Sebag F, et al. Reproducibility of pulmonary vein isolation guided by the ablation index: one-year outcome of the AIR registry. Arch Cardiovasc Dis Suppl. (2021) 13:89–90. doi: 10.1016/j.acvdsp.2020.10.202
22. Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace. (2018) 20:f419–27. doi: 10.1093/europace/eux376
23. Lee S-R, Choi E-K, Lee E-J, Choe W-S, Cha M-J, Oh S. Efficacy of the optimal ablation index–targeted strategy for pulmonary vein isolation in patients with atrial fibrillation: the OPTIMUM study results. J Intervent Cardiac Electrophysiol. (2019) 55:171–81. doi: 10.1007/s10840-019-00565-4
24. Mulder MJ, Kemme MJB, Hagen AMD, Hopman LHGA, van de Ven PM, Hauer HA, et al. Impact of local left atrial wall thickness on the incidence of acute pulmonary vein reconnection after Ablation Index-guided atrial fibrillation ablation. IJC Heart Vasc. (2020) 29:100574. doi: 10.1016/j.ijcha.2020.100574
Keywords: atrial fibrillation, ablation index, pulmonary vein isolation, randomized, recurrence
Citation: Xiong Q, Liao J, Chen W, Xiao P, Du H, He Q, Yin Y, Ling Z and Chen S (2022) Tailored Target Ablation Index Guided Pulmonary Vein Isolation in Treating Paroxysmal Atrial Fibrillation: A Single Center Randomized Study in Asian Population (AI-Asian-I). Front. Cardiovasc. Med. 9:937913. doi: 10.3389/fcvm.2022.937913
Received: 06 May 2022; Accepted: 20 June 2022;
Published: 07 July 2022.
Edited by:
Carlo de Asmundis, University Hospital Brussels, BelgiumReviewed by:
Grigorii Gromyko, MGUPP, RussiaJin Iwasawa, International University Health and Welfare, Japan
Paul Martin Bansmann, Krankenhaus Porz am Rhein gGmbH, Germany
Copyright © 2022 Xiong, Liao, Chen, Xiao, Du, He, Yin, Ling and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyu Ling, bGluZ3poaXl1QGNxbXUuZWR1LmNu; Shaojie Chen, ZHJzamNoZW5AMTI2LmNvbQ==
†Present address: Shaojie Chen, Cardioangiologisches Centrum Bethanien (CCB), Frankfurt am Main, Germany
‡These authors have contributed equally to this work and share first authorship
 Qingsong Xiong
Qingsong Xiong Jia Liao‡
Jia Liao‡ Weijie Chen
Weijie Chen Peilin Xiao
Peilin Xiao Yuehui Yin
Yuehui Yin Zhiyu Ling
Zhiyu Ling Shaojie Chen
Shaojie Chen