- 1Biomedical and Mobile Health Technology Lab, ETH Zürich, Zürich, Switzerland
- 2Department of Physics, ETH Zürich, Zürich, Switzerland
An acute hypotensive episode (AHE) can lead to severe consequences and complications that threaten patients' lives within a short period of time. How to accurately and non-invasively predict AHE in advance has become a hot clinical topic that has attracted a lot of attention in the medical and engineering communities. In the last 20 years, with rapid advancements in machine learning methodology, this topic has been viewed from a different perspective. This review paper examines studies published from 2008 to 2021 that evaluated the performance of various machine learning algorithms developed to predict AHE. A total of 437 articles were found in four databases that were searched, and 35 full-text articles were included in this review. Fourteen machine learning algorithms were assessed in these 35 articles; the Support Vector Machine algorithm was studied in 12 articles, followed by Logistic Regression (six articles) and Artificial Neural Network (six articles). The accuracy of the algorithms ranged from 70 to 96%. The size of the study sample varied from small (12 subjects) to very large (3,825 subjects). Recommendations for future work are also discussed in this review.
Introduction
It is widely accepted that hypotension is defined as absolute mean arterial pressure (MAP) below 60–65 mmHg (1). The incidence of hypotension is estimated to affect around half of the population worldwide (2, 3). While chronic low blood pressure without symptoms is usually not concerning, health problems may occur when blood pressure drops suddenly.
An acute hypotensive episodes (AHE) is defined as lasting for 30 min to 1 h or longer during which at least 90% of the MAP is at or below 60 mmHg. While this definition is widely used, it is not based on consensus or is not part of a medical guideline; rather, it is from the 10th PhysioNet/CinC Challenge (2009) (4). It has also been noted that intra-operative hypotension should be defined as a relative difference from baseline MAP (5, 6). AHE often happens in the intensive care unit (ICU) or operation rooms, commonly caused by sepsis, myocardial infarction, cardiac arrhythmia, pulmonary embolism, hemorrhage, dehydration, and anaphylaxis (4). Hypotension reduces the oxygen supply, resulting in cell and tissue injury and loss of function. Therefore, AHE requires an immediate and appropriate intervention. Without this, patients are at an increased risk of irreversible organ damage and even death.
Currently, several scoring systems are used to predict critical medical events; however, these systems have not been specifically developed for AHE (7, 8). The symptoms of AHE may not be noticeable, and they might last only a few seconds. Hence, an adequately early prediction or warning system is desired to give nurses and physicians enough time to administer preventive care. This is especially important in an ICU setting, as often there is a shortage of nurses.
The importance of predicting AHE was first noted in the European AVERT-IT (Advanced Arterial Hypotension Adverse Event prediction through a Novel Bayesian Neural Network) project in 2008, which was funded by the European Commission to develop a novel bedside monitoring and alerting system to predict AHE (9). In 2009, in the 10th PhysioNet/CinC Challenge, using an automated method, the participants were expected to predict which patients in the challenge dataset (MIMIC II) would experience an AHE (4). Since the challenge, there has been continuous interest in this topic, and more researchers have studied it.
This paper reviews the relevant literature published between 2008 and 2021. Before 2008, there were very few studies in this area, and the methodologies were mainly statistical models rather than machine learning algorithms. Given the advances in machine learning in the last decade, we are re-visiting this topic with a focus on answering the following questions: (1) How well do machine learning algorithms perform in predicting AHE? and (2) What is the potential of these current ML algorithms in the clinical setting?
Methods
Study guidelines
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) (10). A prior review protocol was drafted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (11) for internal use amongst the research team but it was not externally published or registered prospectively.
Search strategy and study eligibility
The PubMed, IEEE database, Embase, and Google Scholar were searched for articles published between January 1, 2008, and January 1, 2022, for all English-language papers using the following keywords: (hypotensive or hypotension or low blood pressure) and (ECG or electrocardiogram or MIMIC) and (automatic detection or machine learning or artificial intelligence or deep learning or prediction) were used. The detailed strategy was discussed in Supplementary material. Gray literature was not included in this review in an attempt to only include peer-reviewed studies. This timeframe was chosen to reflect advances in artificial intelligence technologies and applications in medicine. The search for this review was completed in May 2022.
Inclusion and exclusion criteria
Articles were excluded (a) if the focus was not on hypotension, (b) if ECG data were not used, (c) if a machine learning algorithm was not used, (d) if the article was a review, a book chapter, or a thesis, and (e) if the article did not address the topic (predicting hypotension). One reviewer (AZ) conducted the literature search and two reviewers (AZ and ME) screened the titles, abstracts and full-texts independently for potentially eligible studies. Reference lists of eligible studies were also hand-searched but no additional studies were included on this basis.
Study selection and data extraction
One author (AZ) conducted the literature search, and two authors (AZ and ME) independently screened the titles and abstracts for potentially eligible studies. Each potential study for inclusion underwent full-text screening and was assessed to extract study-specific information and data. For each of the included articles, we extracted information from the below perspectives: the year the paper was published, author(s), number of subjects, gender split of the subjects, the signal used, sampling frequency, features extracted, machine learning algorithms evaluated in the study, training data window length, prediction window length, data source, evaluation metric(s) of the machine learning algorithms.
Results
Search results
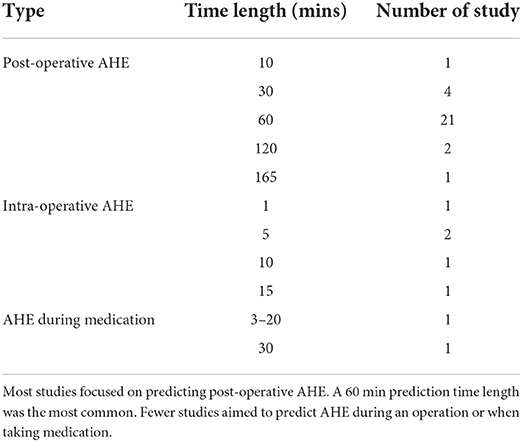
As shown in Figure 1, a total of 485 records were identified with the above-mentioned keywords and year range in the four databases. After comparing the literature titles and authors, 48 duplicates were confirmed and removed, resulted in 437 search records. With the five exclusion criteria mentioned in the Methods section, 354 of the 437 records were excluded after reading the abstract: 40 studies did not use electrocardiogram (ECG) as one of the signals; 161 studies did not investigate hypotension; 63 studies did not aim for blood pressure prediction; 89 were a review article or chapter in a book or a thesis; fiver were excluded because they were either not written in English or the full text was unavailable.
Figure 1. Flow chart of the methodology used to screen the articles. Thirty-five articles published between 2008 and 2021 were included in the review.
The full text was assessed in 83 articles. Of those, 48 articles were further excluded: 11 studies did not use machine learning as a forecasting method; 16 studies focused on blood pressure estimation rather than prediction; six studies forecasted blood pressure in general but not as a way to predict AHE; six studies used blood pressure to forecast other diseases; three studies aimed to determine which feature has the greatest predictive power, rather than to assess an algorithm; one study used an animal model, one focused on developing a new sensor, one aimed to detect artifacts, and one focused on photoplethysmography rather than ECG. Ultimately, 35 articles were included in this systematic review.
Characteristics of included reviews
We read and summarized the included articles based on (a) the year in which the article was published, (b) how many subjects were included in the study and the gender info, (c) what signal(s) was/were used, and the sampling frequency used to obtain the signal, (d) what features were extracted, (e) what machine learning algorithm(s) was/were evaluated in the study, (f) the evaluation metrics of the machine learning algorithm, (g) the duration of the observation window and prediction window, and (h) what data source the authors referred to, as shown in Table 1.
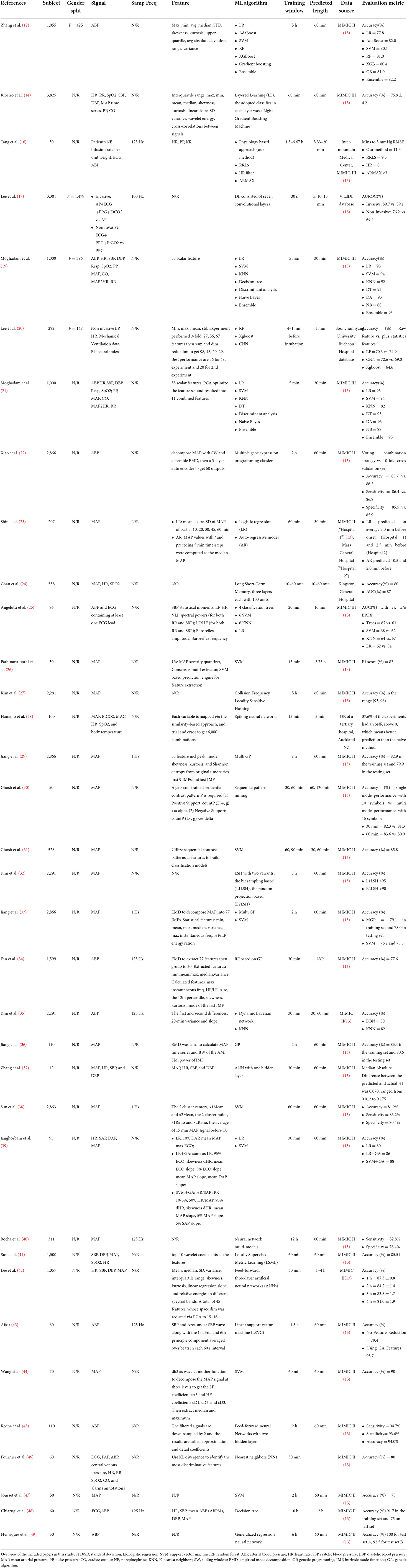
Overall, as shown in Figure 2, the articles were published during the years of the search range; most of the articles were published in 2010 (n = 5), followed by 2009, 2016, 2017, 2021 (n = 4, each). In 2019 and 2020, three articles were published each year. In 2011, 2013, and 2014, only two papers were published on this topic. In 2015 and 2018, only one article was published each year.
Results of studies
Most of the studies (37%, 13 out of 35) were large-scaled (1,110–4,000 patients were included). However, small-sized studies were also common: seven studies included 60–100 patients, six studies included 12–50 patients, and five studies included 110–500 patients. Medium-sized studies were relatively rare; only three studies had 510–1,000 patients. Of the 35 articles, 31 did not report the gender of the subjects; of those that did report on gender, the percentage of females was 40% (n = 2), 44.8% (n = 1), and 52.5% (n = 1). More than half of the articles (n = 18) used the arterial blood pressure (ABP) signal to focus on MAP, while some (n = 10) used more than three data sources. A few studies (n = 7) used ABP+ECG as the signal inputs. The majority of the articles (n = 25) did not mention what sampling frequency was used to acquire the signals. Of those that did report on this factor, 125 Hz was most frequently used (n = 6), followed by 1 Hz (n = 3) and 100 Hz (n = 1). Some (n = 8) of the 35 articles did not mention the features extracted because they simply used the raw data. Five articles described the methodology used to extract the features, but did not mention the exact number of features. Seven articles described the methodology and provided the final number of features, but they did not mention what the features were. Fifteen articles provided a list of the features that were extracted.
Statistics of the raw signal (e.g., maximum, minimum, mean) were the most common features extracted; this was adopted by 13 out of the 27 studies that described the methodology to extract the features, followed by clinical equations that were calculated based on the raw signal (n = 7), Empirical Mode Decomposition (n = 5), wavelet transform (n =3), and contrast sequential pattern and sliding window (n = 2 each). Only five of the 27 studies also conducted feature reduction, and Principal Component Analysis was the only methodology used by more than one study (n = 2).
Most of the studies (n = 24) only investigated one machine learning algorithm. A few of the studies (n = 6) evaluated three to seven machine learning algorithms and a few (n = 5) compared two machine learning algorithms. Of the type of machine learning algorithms used, Support Vector Machine (SVM) was the most studied (n = 12), followed by Logistic Regression (LR) and Artificial Neural Network (ANN) (n = 6 each). Other common machine learning algorithms include Nearest Neighbors (KNN) (n = 5), Genetic Programming (GP) (n = 4), Random Forest (RF), Gradient Boosting Machine, Decision Tree, Naive Bayes (NB), or Dynamic Bayesian Network (n = 3, each), and Locality Sensitive Hashing (LSH) (n = 2). The least examined algorithms were Deep Learning, Spiking Neural Network, Sequential Pattern Mining, and Long-Short-Term Memory (n = 1, each).
Most of the articles (n = 27) reported accuracy as the evaluation metric for the machine learning algorithm(s) that were studied. Eight other articles had their own way of measuring performance without assessing accuracy; those methods included F1 score, area under the curve (AUC), sensitivity (SE), and specificity (SP), prediction time, and the absolute difference between the prediction and the actual hypotension index.
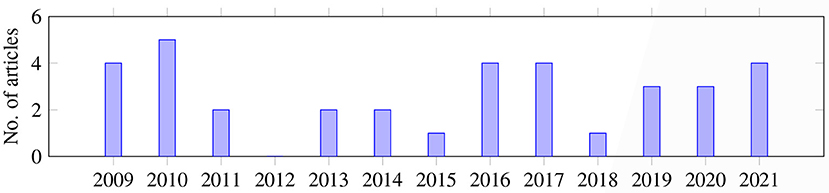
The length of the prediction window depends on the time the AHE is expected to happen. Among the 35 articles, most of the studies (n = 30) focused on predicting AHE in the ICU, where the most common prediction window was 60 min prior to the onset of the event (n = 21), followed by 30 min (n = 4), 120 min (n = 2), or 165 and 10 min (n = 1, each). Some articles looked at a time range, for example 10–60 min or 1–4 h (n = 1, each). Two studies did not report prediction window, assuming that the prediction window occurred right after the observation window.
A second type of prediction looked at intra-operative AHE; but only three studies focused on this area. Therefore, the prediction window is very short, either 1 min (n = 1), 5 min (n = 1) or 5, 10, or 15 min (n = 1), because intra-operative AHE occurs during anesthesia and only after intubation. The last type of study checked AHE that occurred during medication against septic shock. Data from patients given vasopressor infusion (n = 1) or norepinephrine infusion (n = 1) were studied to predict AHE. The prediction window for this type of forecast was 30 min (n = 1) or 3–20 min (n = 1) before the onset of the AHE.
Similarly, the observation window depends on whether the AHE is post-operation, intra-operative, or occurs when taking medication. For the first type of AHE, the observation window values, ranging from most common to least common, are 30 min (n = 7), 60 min (n = 6), 2 h (n = 6), 5 h (n = 3), 5 min (n = 2), 90 min (n = 2), 15 min (n = 1), 20 min (n = 1), 10 h (n = 1), 12 h (n = 1), 6 h (n = 1), or 10–60 min (n = 1). For intra-operative AHE prediction, the observation windows are 1–4 min before intubation, 30 s and 15 min (n = 1, each). For the AHE prediction during medication, the observation window is either 60 min or 1.3–6.67 h (n = 1, each).
The MIMIC-II database (n = 26) was the most frequently used data source, followed by the MIMIC-III database (n = 4), or a hospital database that is not public (n = 3). The Vital DB was rarely used (n = 1); it is a public database. One study used both the MIMIC-II database and a hospital database.
Discussion
The sample sizes of the studies varied greatly, ranging from a very small-scaled analysis with only a few dozen patients to very large-scaled studies that include several thousand people. Such a large variation in the number of subjects means that a comparison of different studies is not possible or could be strongly biased. Kim et al. (35) demonstrated that the performance of both of the chosen algorithms improved up to a point when the size of the training dataset increased. Patient information, including gender, age, comorbidity, medication, etc., was not reported in most of the reviewed studies, and these factors could have an impact on whether an AHE could occur.
The MIMIC database was mostly often used in the reviewed studies, probably due to its freely accessible nature. There might be data quality concerns regarding this database, for example, missing data. Moreover, the database is continuously updated, meaning that different studies, although all referring to the same database, might not have used the same data.
Since accuracy was the performance measure mostly often reported, we compared the performance of different machine learning algorithms based on this evaluation metric (Figure 3). LSH has the highest average accuracy; however, only two studies used this algorithm, and both had the same first author: Kim and O'Reilly (32). In contrast to some other algorithms that are better established and more widely studied, the performance of LSH needs to be further assessed in future studies. Kim and O'Reilly (32) observed that LSH variants have very different robustness against data irregularities, and noted that further work is needed to develop an effective data representation that can be integrated into the general LSH framework.
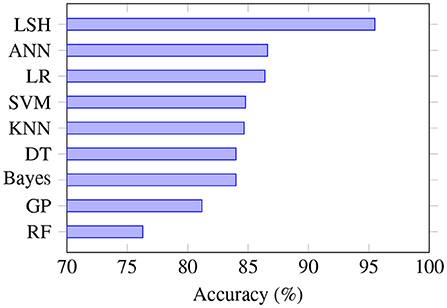
Figure 3. Prediction accuracy based on the type of algorithm. LSH is the most accurate algorithm, although only a few studies used it. SVM is the algorithm that was most often studied, and RF is the least accurate algorithm. RF, random forest; GP, genetic programming; DT, decision tree; KNN, K-nearest neighbors; SVM, support vector machine; LR, logistic regression; ANN, artificial neural network; LSH, locality sensitive hashing.
AHE prediction
When researchers use the same data but different prediction window, as shown in Table 2, they will get different results even with the same machine learning algorithm. In our analysis, we found there is no standard prediction window consensus in this area yet. Thus, the choice of prediction window is mostly subjective. Zhang et al. (12) analyzed the impact of prediction gaps on six machine learning algorithms and concluded that some methodologies are less impacted than others when the prediction gaps change. Lee et al. (42) studied the gap window size ranging from 1 to 4 h and showed that, in general, the overall performance degrades as the gap size increases.
Regarding the training data time length, Lee et al. (20) studied the intra-operative AHE scenario and found 3 min of data performed better than 2 and 1 min. It is not difficult to imagine that a shorter prediction window and a longer training data time would provide better prediction accuracy, but a prediction window that is too short would be clinically less valuable to healthcare providers in terms of providing them with sufficient time to check the patient's situation and decide if an intervention is needed.
Summarization frequency may also impact the accuracy performance. In Pathinarupothi et al. (26) summarization was done once every 5 and 10 min; they found that a 10 min summarization can predict AHE with at least a 10% better F1 score, on average.
Feature extraction
Feature selection is the process of trying to fit the dataset. As mentioned in the previous three sub-sections, the missing patient background information, the diversity in the sample size and the prediction window, and the dynamics of the database can impact the data to be studied, while directly impacting the features to be selected.
However, the way in which the features were extracted also varied in the studies. While many studies described what methodology was used to extract the features (27 out of 35 articles), as shown in Figure 4 (left panel), eight articles did not provide details, mostly because raw data were applied and not processed. Kim et al. (35) found that the performance of the models utilizing derived features was worse than the performance of the models simply using the raw time series. In the study of Zhang et al. (12) feature reduction did not impact the accuracy or AUC performance of the selected machine learning algorithms. Afsar (43) reported that using dimensionality reduction effectively improved the prediction accuracy, and only five features were needed for the calculation. Note that the most used number of features is between 2 and 9, as shown in Figure 4 (right panel). Lee et al. (20) compared the use of vital records with the use of vital records plus electronic health records (EHR), and found that for the convolutional neural network model, EHR improves the accuracy by 0.39%; however, for other algorithms, such as RF, Xgboost, and deep neural network, the differences were negligible. Therefore, with these completely different findings, it is difficult to conclude which methodology is the best for extracting the features, what features are universally effective no matter what algorithms are applied, or how feature reduction impacts prediction performance.
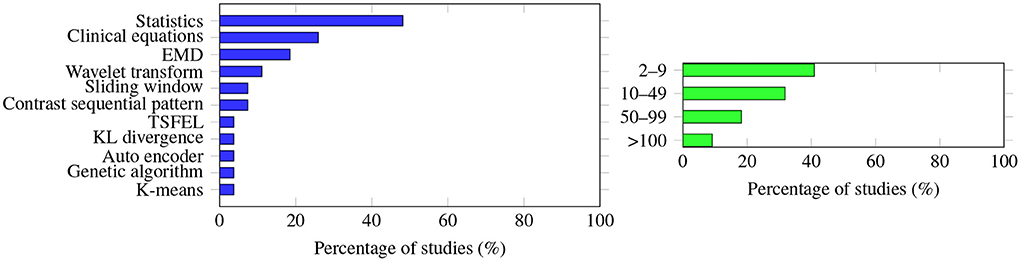
Figure 4. (Left) The methodology used to extract the features was very diverse; no single methodology accounts for more than half of the studies. Statistics (e.g., maximum, minimum, mean values of the raw data) are the extracted features most often studied, followed by clinical equations (apply raw data to the equation to calculate some of the derived information, e.g., cardiac output, MAP to HR ratio). (Right) Graph showing how many features were extracted to predict AHE. The number varies greatly among the studies, with a single feature extraction being the most common.
When considering the different combinations of feature extractions and time windows, the situation could become very complicated. Ghosh et al. (30) studied different combinations of observation windows (30, 60 min), prediction windows (60, 120 min), and feature classification methods (single mode, multi-mode). They found that the prediction accuracy was the highest when both the observation window and prediction window times were 60 min for a single mode extraction mechanism, but the highest prediction accuracy occurred when the observation window was 30 min and the prediction window was 60 min for the multi-mode classification method.
Evaluation metrics
As shown in Figure 5, most of the studies (n = 27) reported accuracy as one of the evaluation metrics, followed by sensitivity (n = 17) and specificity (n = 16).
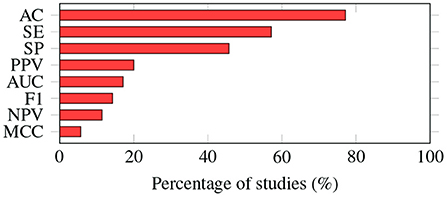
Figure 5. Evaluation metrics. The way in which the performance of machine learning algorithms is evaluated varies from study to study. Accuracy is the most common metric; it was adopted by almost 80% of the studies. AC, accuracy; SE, sensitivity; SP, specificity; PPV, positive predictive value; AUC, area under the ROC curve; F1, F1-score; NPV, negative predictive value; MCC, Matthews correlation coefficient.
However, Ribeiro et al. (14) and Moghadam et al. (21) mentioned that the common ways of measuring performance (including accuracy, sensitivity, specificity, etc.) might not be sufficient to evaluate the performance of an algorithm in predicting AHE, as the data are highly skewed. In Moghadam et al. (21) the Naive Bayes (NB) algorithm raised 17,271 false positive alarms; true positive was only seen in 23,552 cases. However, the accuracy, sensitivity, and specificity of NB were 88, 85, and 88%, respectively. Based on those results, NB is a good machine learning algorithm candidate. However, in clinical practice, such a high false alarm rate means the healthcare providers would gradually lose confidence in the accuracy of the alarm and may not react when a true positive incident occurs. Both authors suggested using positive predictive value (PPV) or the F1 score to evaluate the machine learning algorithm to predict AHE, but PPV and F1 scores are missing in many of the current studies.
Deep learning models have generated great interest due to breakthroughs in fields like image analysis and speech recognition. However, we noticed that as regards to predicting AHE, deep learning algorithms were not so widely studied, and the performances were not better than other traditional methods (17, 24, 37). One possible explanation could be that deep learning models require a massive dataset for learning, which is usually lacking in the ICU setting; therefore, the interest in exploring deep learning's potential in predicting AHE is less prominent. Another reason might be that deep learning is known to be good at learning from features. At the same time, some research (35) has shown that for predicting AHE, raw data could sometimes be even better, probably because MAP itself is already a good indicator. Thus, a simpler but faster model could be sufficient to fulfill the expectation in AHE prediction.
Recommendation for future work
Summarizing the study findings, we recommend that researchers consider the following aspects when designing future studies:
1. Use a large number of subjects (>100) balanced in gender, age, and ethnicity. Moreover, the health status of the subjects needs to be stated, such as comorbidities.
2. Examine a consistent prediction window, precisely 30, 60 min, or both.
3. Elaborate on the feature selection phase, including the number of features extracted, how the feature extraction was done, and what the features are, since these aspects would impact the algorithm performances.
4. Report different evaluation metrics such as accuracy, sensitivity, specificity, Matthews correlation coefficient (MCC), and F1 score is essential for objective assessment.
Limitations
Due to time constraints, we searched only four databases. It is, therefore, possible that we missed some articles available in other databases. The keyword choice might also lead to the omission of relevant research. Some studies checked applications of machine learning algorithms in multiple areas, which could include but are not limited to AHE prediction.
Conclusion
This review summarizes the application of machine learning algorithms for predicting AHE in articles published from 2008 to 2021. Most of the studies included in the review focused on the prediction of post-operative AHE 30 or 60 min before the onset utilizing ABP signals from the MIMIC database. The machine learning algorithm showed an accuracy between 76.3 and 96.5%. The machine learning algorithms perform well when evaluated with metrics like accuracy, sensitivity, and specificity. However, some researchers (14, 21) reported high false positives in some algorithms, when using metrics like PPV or F1 score. As many of the studies currently do not report MCC or F1 score, it is difficult to say if and which of the machine learning algorithms are ready to be used clinically.
By examining the metrics and machine learning algorithms used in previous studies, this review aimed to enable future researchers to better design experiments and pave the way for the findings to be adopted in a clinical environment. Little evidence is currently available for a meta-analysis due to the variations in the scope and methodologies used in previous studies. Further research is needed to evaluate the technology in real life and examine its impact on patients and healthcare providers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ME designed and led the study. AZ, ME, and CM conceived the study. All authors approved the final manuscript.
Funding
This work was supported by open access funding provided by ETH Zurich.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.937637/full#supplementary-material
References
1. Cooper B. Review and update on inotropes and vasopressors. AACN Adv Crit Care. (2008) 19:5–13. doi: 10.1097/01.AACN.0000310743.32298.1d
2. Schenk J, van der Ven WH, Schuurmans J, Roerhorst S, Cherpanath TGV, Lagrand WK, et al. Definition and incidence of hypotension in intensive care unit patients, an international survey of the European society of intensive care medicine. J Crit Care. (2021) 65:142–8. doi: 10.1016/j.jcrc.2021.05.023
3. Owens P, Lyons S, O'Brien E. Arterial hypotension: prevalence of low blood pressure in the general population using ambulatory blood pressure monitoring. J Hum Hypertens. (2000) 14:243–7. doi: 10.1038/sj.jhh.1000973
4. 10th PhysioNet/Computing in Cardiology Challenge. Predicting Acute Hypotensive Episodes (2009). Available online at: https://physionet.org/content/challenge-2009/1.0.0/
5. Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. (2017) 126:47–65. doi: 10.1097/ALN.0000000000001432
6. Saugel B, Reuter D, Reese P. Intraoperative mean arterial pressure targets: can databases give us a universally valid “magic number” or does physiology still apply for the individual patient? Anesthesiol. (2017) 127:725–6. doi: 10.1097/ALN.0000000000001810
7. Knaus W, Draper E, Wagner D, Zimmerman J. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29.
8. Le Gall J, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA (1993) 270:2957–63.
9. Advanced Arterial Hypotension Adverse Event: Prediction Through a Novel Bayesian Neural Network (2008). Available online at: https://cordis.europa.eu/project/id/217049
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Int J Surg. (2010) 8:336–41.
11. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst Rev. (2015) 4:1–9. doi: 10.1186/2046-4053-4-1
12. Zhang G, Yuan J, Yu M, Wu T, Luo X, Chen F. A machine learning method for acute hypotensive episodes prediction using only non-invasive parameters. Comput Methods Prog Biomed. (2021) 200:105845. doi: 10.1016/j.cmpb.2020.105845
13. MIMIC II Database (2010). Available online at: https://archive.physionet.org/mimic2/
14. Ribeiro B, Cerqueira V, Santos R, Gamboa H. Layered learning for acute hypotensive episode prediction in the ICU: an alternative approach. In: The 9th IEEE International Conference on E-Health and Bioengineering (2021).
15. MIMIC III Database (2020). Available online at: https://physionet.org/content/mimiciii/1.4/
16. Tang Y, Brown S, Sorensen J, Harley J. Physiology-informed real-time mean arterial blood pressure learning and prediction for septic patients receiving norepinephrine. IEEE Trans Biomed Eng. (2021) 68:181–91. doi: 10.1109/TBME.2020.2997929
17. Lee S, Lee HC, Chu YS, Song SW, Ahn GJ, Lee H, et al. Deep learning models for the prediction of intraoperative hypotension. Br J Anaesth. (2021) 126:808–17. doi: 10.1016/j.bja.2020.12.035
18. VitalDB Database (2020). Available online at: http://vitaldb.net/data-bank
19. Moghadam M, Abad EMK, Bagherzadeh N, Ramsingh D, Li G-P, Kain ZN. A machine-learning approach to predicting hypotensive events in ICU settings. Comput Biol Med. (2020) 118:103626. doi: 10.1016/j.compbiomed.2020.103626
20. Lee J, Woo J, Kang AR, Jeong YS, Jung W, Lee M, et al. Comparative analysis on machine learning and deep learning to predict post-induction hypotension. Sensors. (2020) 20:4575. doi: 10.3390/s20164575
21. Moghadam M, Masoumi E, Bagherzadeh N, Ramsingh D, Kain Z. Supervised machine-learning algorithms in real-time prediction of hypotensive events. Annu Int Conf IEEE Eng Med Biol Soc. (2020) 2020:5468–71. doi: 10.1109/EMBC44109.2020.9175451
22. Xiao G, Garg A, Chen D, Jiang D, Shu W, Xu X, et al. AHE detection with a hybrid intelligence model in smart healthcare. IEEE Access. (2019) 7:37360–70. doi: 10.1109/ACCESS.2019.2905303
23. Shin S, Reisner AT, Yapps B, Bighamian R, Rubin T, Goldstein J, et al. Forecasting hypotension during vasopressor infusion via time series analysis. Annu Int Conf IEEE Eng Med Biol Soc. (2019) 2019:498–501. doi: 10.1109/EMBC.2019.8857084
24. Chan B, Sedghi A, Laird P, Maslove D, Mousavi P. Prediction of patient-specific acute hypotensive episodes in ICU using deep models. Annu Int Conf IEEE Eng Med Biol Soc. (2019) 2019:566–9. doi: 10.1109/EMBC.2019.8856985
25. Angelotti G, Morandini P, Lehman L, Mark R, Barbieri R. The role of baroreflex sensitivity in acute hypotensive episodes prediction in the intensive care unit. Annu Int Conf IEEE Eng Med Biol Soc. (2018) 2018:2784–7. doi: 10.1109/EMBC.2018.8512859
26. Pathinarupothi R, Rangan E. Consensus motifs as adaptive and efficient predictors for acute hypotensive episodes. In: 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Jeju (2017). doi: 10.1109/EMBC.2017.8037166
27. Kim Y, Hemberg E, O'Reilly UM. Collision frequency locality-sensitive hashing for prediction of critical events. In: 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Jeju (2017). doi: 10.1109/EMBC.2017.8037510
28. Hamano G, Lowe A, Cumin D. Design of spiking neural networks for blood pressure prediction during general anesthesia: considerations for optimizing results. Evol Syst. (2017) 8:203–10. doi: 10.1007/s12530-017-9176-x
29. Jiang D, Hu B, Wu Z. Prediction of acute hypotensive episodes using EMD, statistical method and multi GP. Soft Comput. (2017) 21:5123–32. doi: 10.1007/s00500-016-2107-0
30. Ghosh S, Feng M, Nguyen H, & Li J. Hypotension risk prediction via sequential contrast patterns of ICU blood pressure. IEEE J Biomed Health Inform. (2016) 20:1416–26. doi: 10.1109/JBHI.2015.2453478
31. Ghosh S, Nguyen H, Li J. Predicting short-term ICU outcomes using a sequential contrast motif based classification framework. Annu Int Conf IEEE Eng Med Biol Soc. (2016) 2016:5612–5. doi: 10.1109/EMBC.2016.7591999
32. Kim Y, O'Reilly UM. Analysis of locality-sensitive hashing for fast critical event prediction on physiological time series. In: 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Orlando, FL (2016). doi: 10.1109/EMBC.2016.7590818
33. Jiang D, Hu B, Wu Z. Predicting acute hypotensive episodes based on multi GP. In: International Symposium on Computational Intelligence and Intelligent Systems. London (2016).
34. Fan Z, Zuo Y, Jiang D, Cai X. Prediction of acute hypotensive episodes using random forest based on genetic programming. In: IEEE Congress on Evolutionary Computation (CEC). Sendai (2015). doi: 10.1109/CEC.2015.7256957
35. Kim Y, Seo J, O'Reilly UM. Large-scale methodological comparison of acute hypotensive episode forecasting using MIMIC2 physiological waveforms. In: IEEE 27th International Symposium on Computer-Based Medical Systems. New York, NY (2014). doi: 10.1109/CBMS.2014.24
36. Jiang D, Li L, Fan Z. Detection of acute hypotensive episodes via empirical mode decomposition and genetic programming. In: International Conference on Identification, Information and Knowledge in the Internet of Things. Beijing (2014). doi: 10.1109/IIKI.2014.53
37. Zhang Z, Lee J, Scott D, Lehman LW, Mark R. A research infrastructure for real-time evaluation of predictive algorithms for intensive care units. In: International Conference on Complex Medical Engineering. Beijing (2013). doi: 10.1109/ICCME.2013.6548221
38. Sun H, Xie S, Wu Y, Yan M, Zhang C. A method for prediction of acute hypotensive episodes in ICU via PSO and K-means. In: Sixth International Symposium on Computational Intelligence and Design. Hangzhou (2013). doi: 10.1109/ISCID.2013.32
39. Janghorbani A, Arasteh A, Moradi M. Prediction of acute hypotension episodes using logistic regression model and support vector machine: a comparative study. In: 19th Iranian Conference on Electrical Engineering (2011).
40. Rocha T, Paredes S, de Carvalho P, Henriques J. Prediction of acute hypotensive episodes by means of neural network multi-models. Comput Biol Med. (2011) 41: 881–90. doi: 10.1016/j.compbiomed.2011.07.006
41. Sun J, Sow D, Hu J, Ebadollahi S. A system for mining temporal physiological data streams for advanced prognostic decision support. In: IEEE International Conference on Data Mining. Sydney, NSW (2010). doi: 10.1109/ICDM.2010.102
42. Lee J, Mark R. A hypotensive episode predictor for intensive care based on heart rate and blood pressure time series. Comput Cardiol. (2010) 37:81–4.
43. Afsar F. Prediction of acute hypotension episodes in patients taking pressor medication using modeling of arterial blood pressure waveforms. In: 4th International Conference on Bioinformatics and Biomedical Engineering. Chengdu (2010). doi: 10.1109/ICBBE.2010.5516765
44. Wang Z, Lai L, Xiong D, Wu X. Study on predicting method for acute hypotensive episodes based on wavelet transform and support vector machine. In: 3rd International Conference on Biomedical Engineering and Informatics. Yantai (2010). doi: 10.1109/BMEI.2010.5639747
45. Rocha T, Carvalho P, Henriques J, Harris M. Wavelet based time series forecast with application to acute hypotensive episodes prediction. In: 32nd Annual International Conference of the IEEE EMBS. Buenos Aires (2010). doi: 10.1109/IEMBS.2010.5626115
46. Fournier P, Roy J. Acute hypotension episode prediction using information divergence for feature selection, and non-parametric methods for classification. Comput Cardiol. (2009) 36:625–8.
47. Jousset F, Lemay M, Vesin J. Computers in cardiology / physionet challenge 2009: predicting acute hypotensive episodes. In: 36th Annual Computers in Cardiology Conference (CinC). Park City, UT (2009).
48. Chiarugi F, Karatzanis I, Sakkalis V, Tsamardinos I, Dermitzaki Th, Foukarakis M, et al. Predicting the occurrence of acute hypotensive episodes: the physionet challenge. Comput Cardiol. (2009) 36:621–4.
Keywords: digital health, hypotension, hypertension, intensive care unit, anesthesia, obstetric and gynecologic, emergency and critical care, low blood pressure
Citation: Zhao A, Elgendi M and Menon C (2022) Machine learning for predicting acute hypotension: A systematic review. Front. Cardiovasc. Med. 9:937637. doi: 10.3389/fcvm.2022.937637
Received: 06 May 2022; Accepted: 04 August 2022;
Published: 23 August 2022.
Edited by:
Kamal Sharma, B. J. Medical College and Civil hospital, IndiaReviewed by:
Devender Kumar, Technical University of Denmark, DenmarkJung Bin Kim, Korea University, South Korea
Copyright © 2022 Zhao, Elgendi and Menon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Elgendi, bW9lLmVsZ2VuZGlAaGVzdC5ldGh6LmNo
†These authors have contributed equally to this work and share first authorship
 Anxing Zhao
Anxing Zhao Mohamed Elgendi
Mohamed Elgendi Carlo Menon
Carlo Menon