- 1Department of Cardiology, Second Affiliated Hospital of Nantong University, Nantong, China
- 2Department of General Medicine, Second Affiliated Hospital of Nantong University, Nantong, China
- 3Department of Critical Care Medicine, Nantong Third People's Hospital, Nantong University, Nantong, China
Background: Acute myocardial infarction (AMI) is a critical cardiovascular disease (CVD). Laminin (LN) is involved in the process of myocardial fibrosis and ventricular remodeling observed in AMI; however, there are currently no studies on the correlation between LN and AMI prognosis.
Purpose: To explore the predictive value of serum LN levels for major adverse cardiovascular events (MACE) in patients, 6 months after an acute myocardial infarction.
Methods: A total of 202 AMI patients who were hospitalized in the Department of Cardiology of the Second Affiliated Hospital of Nantong University between December 2019 and December 2020 were included. The observation endpoint was the occurrence of MACE. Univariate and multivariate logistic analyses were used to evaluate the relationships between the variables and endpoint. The predictive value of LN for MACE in AMI patients was assessed using receiver operating characteristic (ROC) analysis.
Results: A total of 47 patients developed MACE. Univariate logistic analysis showed that smoking, emergency percutaneous coronary intervention (EPCI), age, cardiac troponin I (c-TNI) levels, N-terminal prohormone brain natriuretic peptide (NT-proBNP) levels, and LN levels were associated with the occurrence of MACE (p < 0.05). Multivariate logistic analysis showed that LN was an independent predictor of MACE (odds ratio [OR] = 1.021, 95%CI: 1.014–1.032, p < 0.001). According to the ROC curve, LN can be used as an effective predictor of MACE (AUC = 0.856, 95%CI: 0.794–0.918, p < 0.001). According to the cutoff value, LN>58.80 ng/ml (sensitivity = 83.00%, specificity = 76.80%) or LN>74.15 ng/ml (sensitivity = 76.6%, specificity = 83.2%) indicate a poor prognosis for AMI. Different cut-off values are selected according to the need for higher sensitivity or specificity in clinical applications.
Conclusions: LN may be a predictor of MACE following AMI in patients and could be utilized as a novel substitute marker for the prevention and treatment of AMI.
Introduction
Acute myocardial infarction (AMI) is one of the most common causes of death worldwide (1). Over the past few decades, improvements in cardiovascular treatments have significantly reduced the early death rate due to AMI. Despite the availability of new therapies, patients who survive an initial ischemic event are at a high risk of future adverse cardiovascular events (2). It is currently believed that ventricular remodeling is a key factor leading to poor prognosis in acute myocardial infarction (3), and the main pathological manifestation of myocardial remodeling is myocardial fibrosis (MF). Increased deposition of the myocardial extracellular matrix (ECM) and changes in collagen composition are closely related to myocardial fibrosis (4, 5). Endomyocardial biopsy is undoubtedly the gold standard for the diagnosis of myocardial fibrosis; however, its clinical application is limited. Therefore, biomarkers are considered an effective method for non-invasive detection of fibrosis. Several previous basic studies (6–8) have shown that laminin (LN) exists on the basement membrane of cardiomyocytes and fibroblasts (FBC), plays a role in connecting type IV, type I, and type III collagen, and participates in myocardial fibrosis. However, no study has reported a correlation between LN and the prognosis of patients with myocardial infarction. This study aimed to investigate the correlation between LN and the prognosis of patients with acute myocardial infarction and to explore the predictive value of LN to facilitate the early detection and intervention of disease progression in patients with myocardial infarction and improve the prognosis of patients.
Methods
Study population
We enrolled 202 patients (152 men and 50 women) with acute myocardial infarction who were admitted to the Department of Cardiology of the Second Affiliated Hospital of Nantong University from December 2019 to December 2020. All included cases were treated with standardized treatment. The patients were followed up for 6 months and then divided into an event group and a non-event group according to the occurrence of MACE. The study complied with the Declaration of Helsinki and was approved by the hospital ethics committee. Informed consent was obtained from all patients. The inclusion criteria were the diagnostic criteria for acute myocardial infarction: patients with troponin I levels above the 99th percentile of the upper limit of the reference value and at least one of the following: chest pain lasting >20 min or diagnostic serial ECG changes, including new pathological Q waves or ST segments and T wave changes (9). Exclusion criteria included fever, acute and chronic infection, malignant tumor, severe liver and kidney insufficiency, autoimmune disease, blood disease, stroke, myogenic disease, valvular heart disease, cardiomyopathy, heart failure, B-mode ultrasound-diagnosed internal carotid artery stenosis, renal artery stenosis, other major diseases, involuntary enrollment, mental and psychological diseases, and long-term bedridden patients who cannot take care of themselves.
Somking and EPCI (emergency percutaneous coronary intervention) will be included as factors in the analysis; According to the regulations of the World Healteh Organization, smoking is defined as those who have smoked continuously or accumulatively for 6 months or more since birth. EPCI refers to emergency percutaneous coronary intervention therapy, that is, coronary catheterization technique implemented within 24 h after the occurrence of acute myocardial infarction to dredge coronary stenosis or even occlusion of coronary vessel lumen, so as to improve myocardial blood perfusion.
Detection of laminin
For patients diagnosed with acute myocardial infarction, 3 ml of venous blood was collected in the morning after 12 h of fasting. The serum was separated and stored at −20°C for future testing. The concentration of LN was detected by chemiluminescence immunosandwich method. One monoclonal antibody against LN was labeled ABEI and the other monoclonal antibody was labeled FITC. Samples, calibration, ABEI labeling monoclonal antibodies, FITC labeled monoclonal antibodies and Magnetic microspheres coated with goat anti-FITC antibody formed immunocomplex. Apply a magnetic field for precipitation, remove the supernatant, wash the precipitated complex with washing solution 3 times, and enter the sample measurement chamber. The instrument (automatic chemiluminescence immunoassay analyzer MAGLUMI X8) was automatically pumped into the automatic immunoassay system with substrates 1 and 2, and the relative light intensity (RLU) emitted within 3 sec was automatically monitored. LN concentration was proportional to RLU, and the detection instrument automatically fitted and calculated LN concentration. The kits were provided by New Industry Biomedical Engineering Co., LTD (Shenzhen, China).
Follow-up
The primary endpoint was MACE, which mainly included composite death, recurrent myocardial infarction, and heart failure hospitalization within 6 months. Endpoint data were obtained from the hospital database and telephone follow-ups, which were validated by reviewing medical records. We completed 100% of the follow-up visits.
Statistical analysis
The measurement data that conformed to the normal distribution are described in the form of mean ± standard deviation, and the independent sample T-test was used for statistical analysis. Data that did not conform to the normal distribution were described using the M-estimator (P25, P75) and the non-parametric test (Kruskal-Wallis test) for statistical analysis. The Chi-square test was used for categorical variables. Independent predictors of myocardial infarction prognosis were screened using logistic regression analysis. Finally, the receiver operating characteristic (ROC) curve was used to judge the predictive value of LN for the occurrence of MACE. All statistical analyses were performed using SPSS version 23 (IBM SPSS Statistics, IBM Corporation Armonk, New York).
Results
Comparison of baseline data between the two groups
Univariate analysis showed that there was no significant difference in gender, Hypertension, Diabetes, BMI, Ccr, ALT, AST, LDL-C between the two groups (p > 0.05). Smoking (%), age, N-terminal prohormone brain natriuretic peptide (NT-proBNP), cardiac troponin I (c-TNI), and LN were significantly higher in the MACE group than in the non-MACE group (p < 0.05). Conversely, emergency percutaneous coronary intervention (%) was significantly lower in the MACE group than in the non-MACE group (p < 0.05) (Table 1).
Multivariate logistic regression of MACE in patients with AMI
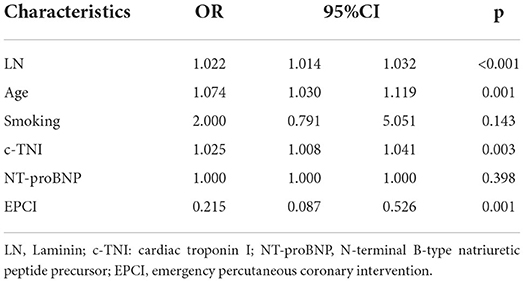
Taking the occurrence of MACE as a dependent variable and the related factors (smoking, EPCI, age, c-TNI, NT-proBNP, LN) as independent variables in univariate analysis, a multivariate logistic regression analysis was performed. Multivariate regression analysis demonstrated that the level of LN was an independent predictor of MACE 6 months after a myocardial infarction [OR = 1.022, 95% CI = (1.014, 1.032), p < 0.001]. In addition, age [OR = 1.074, 95% CI = (1.030, 1.119), p = 0.001] and EPCI [OR = 0.215, 95% CI = (0.087, 0.526), p = 0.001] were independently associated with MACE after 6 months (Table 2).
Predictive value of LN for the risk of MACE in AMI patients
The area under the ROC curve of LN for predicting the occurrence of MACE was 0.856 [95% CI = (0.800–0.901)], and a cut-off value of 58.80 ng/ml (sensitivity = 83.00%, specificity = 76.80%) or 74.15 ng/ml (sensitivity = 76.6%, specificity = 83.2%) indicate a poor prognosis for AMI (Different cut-off values are selected according to the need for higher sensitivity or specificity in clinical applications). Compared with c-TNI (AUC = 0.655 < 0.856, p = 0.0007) and age (AUC = 0.686 < 0.856, p = 0.0066), LN had a better predictive value for MACE in patients with AMI (Figure 1; Tables 3, 4).
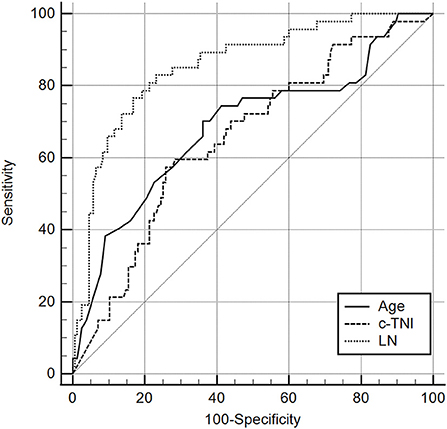
Figure 1. Receiver operating characteristic curves for laminin, age, and c-TNI in the prediction of MACE in AMI patients. The results showed that the predictive value of LN was significantly higher than that of Age (AUC 0.856 > 0.686, P = 0.0066 <0.05). It was also significantly higher than that of C-TNI (AUC 0.856 > 0.0655, P = 0.0007 <0.05).
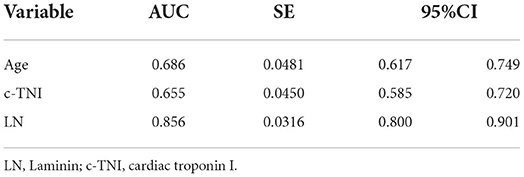
Table 3. Receiver operating characteristic curves for laminin, age, and c-TNI in the prediction of MACE in AMI patients.

Table 4. Pairwise comparison of ROC curves for laminin, age, and c-TNI in the prediction of MACE in AMI patients.
Discussion
The myocardial ECM is composed of collagen, fibronectin (FN), LN, elastin, and proteoglycans. In response to ischemic injury, neurohormonal activation, or changes in hemodynamic load, the ECM undergoes structural remodeling, involving collagen degeneration, production of new collagen, changes in the ratio of collagen phenotypes, and changes in the amount of collagen cross-linking (10). Studies have confirmed that ventricular remodeling is a key factor leading to a poor prognosis in acute myocardial infarction, and that myocardial fibrosis is the main pathological manifestation of myocardial remodeling (11). In the pathological condition of myocardial infarction, myocardial fibroblasts are the main cells tasked to produce ECM (3). Changes in its quantity and function are also associated with extracellular matrix deposition and fibrosis in various tissues and organs (the liver and kidney). Increased deposition of myocardial ECM and changes in collagen composition are closely related to myocardial fibrosis, which in turn is an important factor affecting cardiac function (3, 4, 12). Many studies have suggested that serum levels of extracellular matrix proteins (or their cleavage fragments) may be used to assess the severity and progression of myocardial remodeling (13–15). Changes in the myocardial cytoskeleton and extracellular matrix may affect systolic function because the cytoskeleton organizes the intracellular and intercellular structures. Understanding the basic mechanisms that regulate injury responses is critical for the development of site-specific cell biological intervention strategies to reduce injury and promote repair (10).
At present, numerous studies have found that many markers of liver fibrosis, such as N-terminal propeptide of procollagen III (PIIINP), 7S domain of the collagen type IV N-terminal propeptide, (P4NP7S) (16), hyaluronic acid (HA) (17) can also be used as indicators of myocardial fibrosis. Laminin is also one of the indicators of liver fibrosis.
Laminin is a non-collagen glycoprotein first discovered by Timple et al. (18). Laminins belong to a family of 16 distinct heterotrimeric proteins. Each laminin isomer is composed of α, β and γ Chain composition, each named after a numerical subtype. Lamellae are widespread but often overlap. Each laminin has a unique phenotype that affects the differentiation and/or maintenance of different tissues from the earliest stages of embryogenesis to adulthood.
Thus far, studies on changes in parameters reflecting the integrity of the basement membrane in patients with acute myocardial infarction have not been sufficient. We can prove that the increase in LN levels after AMI can predict the occurrence of short-term MACE (6 months after an acute myocardial infarction), which may be a substitute marker for the early activation of extracellular myocardial matrix metabolism. In the early stages of AMI, this rapid activation of LN may represent non-specific repair because it is independent of other clinical variables.
The increase in serum LN levels in the early stages of myocardial infarction can be explained by the biological function of the LN family. Laminin is a major structural component of the basement membrane and plays a role in cell proliferation, adhesion, differentiation and migration (10).
Elevated serum LN antigen concentrations have been reported in patients with liver fibrosis and cirrhosis, regardless of the source of the disease (alcohol abuse or chronic viral hepatitis B or C infection) (19–22). Furthermore, LN concentration is associated with the degree of fibrosis and the grade of hepatic fibrosis (23). Animal studies have shown that (24) a large amount of LN is present in the myocardial interstitium of hypertensive rats. Furthermore, LN contributes to extracellular matrix assembly during early healing after myocardial infarction in rats (25). In 2009, Dinh et al. found that the elevation of serum laminin levels in patients with acute myocardial infarction suggested early myocardial remodeling and predicted the progression of myocardial fibrosis (10). A report in 2020 combined with previous basic studies found that laminin-α5 is upregulated transiently in the basement membrane in human and murine muscular dystrophy which is accompanied by cardiomyopathy (26). Laminin can reflect the activity and proliferation of fibroblasts and can be used as an indicator of myocardial fibrosis detection; to a certain extent, it reflects the degree of myocardial tissue damage and the pathological process of secondary myocardial fibrosis (27, 28).
In this study, we noted that laminin levels were significantly higher in patients with AMI who had MACE than in those without MACE. We speculate that laminin reflects early extracellular matrix remodeling and is involved in tissue repair after ischemia. As an indicator of liver fibrosis, laminin could also suggest the occurrence of myocardial fibrosis. More importantly, laminin may be of greater value in the future treatment of AMI patients to improve prognosis.
Our study has some limitations. The progression of remodeling is influenced by many different factors, such as metalloproteinase (MMP) and metalloproteinase tissue inhibitor (TIMP) systems, cytokines, and neuroendocrine activation (29). In addition, the ECM structure varies from patient to patient, in terms of the number of myocytes and fibroblasts. Laminin levels may depend not only on the magnitude of acute injury but also on other pathophysiological changes initiated after AMI. Therefore, the degree of LN elevation does not fully reflect the magnitude of acute myocardial injury or degree of left ventricular remodeling. Rather, it reflects the ability of the collagen matrix to degrade during remodeling, as part of a complex regulatory system.
In conclusion, our findings suggest that the development of short-term MACE due to early cardiac remodeling after ischemic injury is reflected by an increase in serum LN levels. Therefore, LN may be a predictor of MACE following AMI. It is also necessary to expand the sample size and extend the follow-up time to further demonstrate the correlation between LN level and AMI prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
L-YX: conceptualization, methodology, formal analysis, data curation, resources, writing—original draft, writing—review and editing, project administration, and funding acquisition. LX: investigation, validation, formal analysis, data curation, writing, and original draft. JW: formal analysis and investigation. H-XC: formal analysis and data curation. H-LC: formal analysis and validation. L-JT and QZ: resources, writing—review and editing, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific Research Project of Nantong Municipal Health Commission (grant MB2021010), Kangda College of Nanjing Medical University (grant KD2021KYJJZD012), and Nantong Science and Technology Bureau Plan Project (grant JC2020054).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the support of the Cardiology and Department of General Medicine of the Second Affiliated Hospital of Nantong University. We would also like to thank all patients and volunteers who participated in this study.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.936983/full#supplementary-material
References
1. Du X, Patel A, Anderson CS, Dong J, Ma C. Epidemiology of cardiovascular disease in China and opportunities for improvement: JACC International. J Am Coll Cardiol. (2019) 73:3135–47. doi: 10.1016/j.jacc.2019.04.036
2. Seropian IM, Cerliani JP, Toldo S, Van Tassell BW, Ilarregui JM, González GE, et al.. Rabinovich, Galectin-1 controls cardiac inflammation and ventricular remodeling during acute myocardial infarction. Am J Pathol. (2012) 182:29–40. doi: 10.1016/j.ajpath.09, 022.
3. Moore L, Fan D, Basu R, Kandalam V, Kassiri Z. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev. (2012) 17:693–706. doi: 10.1007/s10741-011-9266-y
4. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, et al. Effects of Sacubitril/Valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. (2019) 73:795–806. doi: 10.1016/j.jacc.2018.11.042
5. Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol Aspects Med. (2008) 29:58–66. doi: 10.1016/j.mam.2007.09.002
6. Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res. (2014) 114:872–88. doi: 10.1161/CIRCRESAHA.114.302533
7. Franz M, Wolheim A, Richter P, Umbreit C, Dahse R, Driemel O, et al. Stromal laminin chain distribution in normal, hyperplastic and malignant oral mucosa: relation to myofibroblast occurrence and vessel formation. J Oral Pathol Med. (2010) 39:290–8. doi: 10.1111/j.1600-0714.2009.00840.x
8. Lumkwana D, Botha A, Samodien E, Hanser S, Lopes J. Laminin, laminin-entactin and extracellular matrix are equally appropriate adhesive substrates for isolated adult rat cardiomyocyte culture and experimentation. Cell Adh Migr. (2018) 12:503–11. doi: 10.1080/19336918.2017.1387693
9. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
10. Dinh W, Bansemir L, Futh R, Nickl W, Stasch JP, Coll-Barroso M, et al. Increased levels of laminin and collagen type VI may reflect early remodelling in patients with acute myocardial infarction. Acta Cardiol. (2009) 64:329–34. doi: 10.2143/AC.64.3.2038017
11. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. (2004) 291:2727–33. doi: 10.1001/jama.291.22.2727
12. Cloutier G, Sallenbach-Morrissette A, Beaulieu JF. Non-integrin laminin receptors in epithelia. Tissue Cell. (2019) 56:71–8. doi: 10.1016/j.tice.2018.12.005
13. Radovan J, Vaclav P, Petr W, Jan C, Michal A, Richard P, et al. Changes of collagen metabolism predict the left ventricular remodeling after myocardial infarction. Mol Cell Biochem. (2006) 293:71–8. doi: 10.1007/s11010-006-2955-5
14. Kelly PJ, Morrow JD, Ning M, Koroshetz W, Lo EH, Terry E, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. (2008) 39:100–4. doi: 10.1161/STROKEAHA.107.488189
15. Orn S, Manhenke C, Squire IB, Ng L, Anand I, Dickstein K, et al. Plasma MMP-2, MMP-9 and N-BNP in long-term survivors following complicated myocardial infarction: relation to cardiac magnetic resonance imaging measures of left ventricular structure and function. J Card Fail. (2007) 13:843–9. doi: 10.1016/j.cardfail.2007.07.006
16. Nagao K, Tamura A, Sato Y, Hata R, Kawase Y, Kadota K, et al. Utility of collagen-derived peptides as markers of organ injury in patients with acute heart failure. Open Heart. (2020) 7:e001041. doi: 10.1136/openhrt-2019-001041
17. Li G, Yan QB, Wei LM. Serum concentrations of hyaluronic acid, procollagen type III NH2-terminal peptide, and laminin in patients with chronic congestive heart failure. Chin Med Sci J. (2006) 21:175–8.
18. Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR, et al. Laminin–a glycoprotein from basement membranes. J Biol Chem. (1979) 254:9933–7. doi: 10.1016/S0021-9258(19)83607-4
19. Lebensztejn DM, Kaczmarski M, Sobaniec-Lotowska M, Bauer M, Voelker M, Schuppan D, et al. Serum laminin-2 and hyaluronan predict severe liver fibrosis in children with chronic hepatitis B. Hepatology. (2004) 39:868–9. doi: 10.1002/hep.20147
20. Gressner AM, Tittor W. Serum laminin–its concentration increases with portal hypertension in cirrhotic liver disease. Klin Wochenschr. (1986) 64:1240–8. doi: 10.1007/BF01734467
21. Tsutsumi M, Urashima S, Nakase K, Takada A. Changes in laminin content in livers of patients with alcoholic liver disease. Liver. (1995) 15:324–31. doi: 10.1111/j.1600-0676.1995.tb00693.x
22. Neves LB, Catarino RM, Silva MR, Parise ER. [Increased serum levels of laminin in the experimental cirrhosis induced by carbon tetrachloride]. Arq Gastroenterol. (2003) 40:173–6. doi: 10.1590/S0004-28032003000300007
23. Ding XJ, Li SB, Li SZ, Liu HS, Liu B, Xu FM, et al. [A quantitative study of the relationship between levels of liver fibrosis markers in sera and fibrosis stages of liver tissues of patients with chronic hepatic diseases]. Zhonghua Gan Zang Bing Za Zhi. (2005) 13:911–4.
24. Panizo A, Pardo J, Hernández M, Galindo MF, Cenarruzabeitia E, Díez J. Quinapril decreases myocardial accumulation of extracellular matrix components in spontaneously hypertensive rats Am J Hypertens. (1995) 8:815–22. doi: 10.1016/0895-7061(95)00120-E
25. Morishita N, Kusachi S, Yamasaki S, Kondo J, Tsuji T. Sequential changes in laminin and type IV collagen in the infarct zone–immunohistochemical study in rat myocardial infarction. Jpn Circ J. (1996) 60:108–14. doi: 10.1253/jcj.60.108
26. Hochman-Mendez C, Curty E, Taylor DA. Change the laminin, change the cardiomyocyte: improve untreatable heart failure. Int J Mol Sci. (2020) 21:6013. doi: 10.3390/ijms21176013
27. DiGiacomo V, Meruelo D. Looking into laminin receptor: critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol Rev Camb Philos Soc. (2016) 91:288–310. doi: 10.1111/brv.12170
28. Yap L, Wang JW, Moreno-Moral A, Chong LY, Sun Y, Harmston N, et al. In vivo generation of post-infarct human cardiac muscle by laminin-promoted cardiovascular progenitors. Cell Rep. (2019) 26:3231–45e9. doi: 10.101016/jcelrep02.2019.083
Keywords: acute myocardial infarction, cardiovascular disease, laminin, MACE, serum biomarker
Citation: Xu L-Y, Xie L, Wang J, Chen H-X, Cai H-L, Tian L-J and Zhang Q (2022) Correlation between serum laminin levels and prognosis of acute myocardial infarction. Front. Cardiovasc. Med. 9:936983. doi: 10.3389/fcvm.2022.936983
Received: 05 May 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Jun-ichiro Koga, University of Occupational and Environmental Health Japan, JapanReviewed by:
Jianxin Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaDale Hunter, Upstate Medical University, United States
Mayu Nishio, Saiseikaisenri Hospital, Japan
Copyright © 2022 Xu, Xie, Wang, Chen, Cai, Tian and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Jun Tian, YWRhbS0xMjAmI3gwMDA0MDsxNjMuY29t; Qing Zhang, enpoYW5ncWluZzMyJiN4MDAwNDA7c2luYS5jbg==
†These authors have contributed equally to this work and share first authorship
 Lou-Yuan Xu
Lou-Yuan Xu Ling Xie1†
Ling Xie1† Jing Wang
Jing Wang Hai-Xiao Chen
Hai-Xiao Chen Li-Jun Tian
Li-Jun Tian Qing Zhang
Qing Zhang