- 1Department of Obstetrics and Gynecology, Southeast University Affiliated Zhongda Hospital, Nanjing, China
- 2Nanjing Maternity and Child Health Care Hospital, Nanjing Maternal and Child Health Institute, Women’s Hospital of Nanjing Medical University, Nanjing, China
Background: The results of randomized controlled studies on aspirin for the prevention of preeclampsia (PE) are conflicting, and some of the related meta-analyses also have limitations or flaws.
Data sources: A search was conducted on PubMed, Embase, and Cochrane Central Register of Controlled Trials databases, with no time or language restrictions.
Study eligibility criteria: Randomized controlled studies comparing aspirin for the prevention of PE were conducted.
Methods: Systematic reviews were performed according to the Cochrane Manual guidelines. A fixed-effects model or a random-effects model was chosen to calculate pooled relative risks with 95% confidence intervals based on the heterogeneity of the included studies. The study aimed to investigate the effect of aspirin on the development of PE in high-risk and general populations of women. Publication bias was assessed by funnel plots. All included studies were assessed for bias by the Cochrane Manual of Bias Assessment. Subgroup analyses were conducted on the aspirin dose, time of initial aspirin intervention, and the region in which the research was conducted, to explore the effective dose of aspirin and time of initial aspirin intervention and to try to find sources of heterogeneity and publication bias.
Results: A total of 39 articles were included, including 29 studies involving pregnant women at high risk for PE (20,133 patients) and 10 studies involving a general population of pregnant women (18,911 patients). Aspirin reduced the incidence of PE by 28% (RR 0.72, 95% CI 0.62–0.83) in women at high risk for PE. Aspirin reduced the incidence of PE by 30% in the general population (RR 0.70, 95% CI 0.52–0.95), but sensitivity analyses found that aspirin in the general population was not robust. A subgroup analysis showed that an aspirin dose of 75 mg/day (RR 0.50, 95% CI 0.32–0.78) had a better protective effect than other doses. Starting aspirin at 12–16 weeks (RR 0.62, 95% CI 0.53–0.74) of gestation or 17–28 weeks (RR 0.62, 95% CI 0.44–0.89) reduced the incidence of PE by 38% in women at high risk for PE, but the results were more reliable for use at 12–16 weeks. Heterogeneity and publication bias of the included studies may be mainly due to the studies completed in Asia.
Conclusion: Aspirin is recommended to be started at 12–16 weeks of pregnancy in women at high risk for PE. The optimal dose of aspirin to use is 75 mg/d.
Systematic review registration: [www.ClinicalTrials.gov], identifier [CRD42022319984].
Introduction
Preeclampsia (PE) is a pregnancy-specific disorder that affects approximately 3–5% of pregnant women worldwide, especially in developing countries (1, 2). PE can cause maternal impairment including kidney damage, liver damage, hemolysis, neurology injuries including seizures (eclampsia), stroke, and death (3, 4). Preterm delivery and fetal growth restriction due to PE often have lifelong consequences for the child. These may include cerebral palsy and neurodevelopmental impediment, respiratory disease, hypertension, renal insufficiency, insulin resistance, obesity, cardiovascular disease, and impaired work capacity (5). In 2014, the International Society for the Study of Hypertension in Pregnancy (ISSHP) defined the diagnostic criteria for PE as new-onset hypertension (≥ 140 mmHg systolic or ≥ 90 mmHg diastolic) after 20-week gestation with the coexistence of either proteinuria (≥ 300 mg/day) or other maternal organ dysfunction such as renal insufficiency, liver involvement, neurological or hematological complications, uteroplacental dysfunction, or fetal growth restriction (6).
Currently, the only treatment for prenatal PE is childbirth. The use of drugs such as aspirin, statins, metformin, and proton pump inhibitors for the prevention and treatment of PE remains controversial (7). Aspirin’s effects on inflammation and platelet aggregation may help prevent or treat PE (8), and several randomized controlled trials have been conducted. The ACOG recommends that women with any high-risk factors (PE in previous pregnancies, multiple pregnancies, kidney disease, autoimmune disease, type 1 or 2 diabetes, and chronic hypertension) and multiple moderate risk factors for PE (First pregnancy, maternal age ≥ 35 years, BMI over 30, family history of PE, sociodemographic characteristics, and personal medical history factors) should receive low-dose (81 mg/day) aspirin to prevent PE. This should begin between 12 and 28 weeks of gestation (preferably before 16 weeks of gestation) and continue until delivery (9).
In 2017, a high-quality randomized controlled study found that daily aspirin of 150 mg from 11 to 14 weeks of gestation until 36 weeks of gestation reduced the incidence of preterm PE in women at high risk for PE (10). However, a 2022 study in China found that daily oral administration of 100 mg of aspirin started at 12–20 weeks of gestation to 34 weeks of gestation in high-risk groups of PE did not reduce the incidence of PE. A recent meta-analysis (11) of the effect of aspirin on the occurrence of preterm PE in women at moderate to high risk of PE incorrectly counted the total number of participants as 3,294 (the correct number is 3,269) when extracting data from the study by Subtil et al. (12). While this error may not affect the results of the analysis, it can make the conclusions less rigorous. Another study investigating low-dose aspirin for the prevention of PE did not differentiate between pregnant women at high risk of PE and those in the general population (13). It should be noted that since the general population of women in this study was not screened for PE risk factors, they may have included some women with PE risk factors.
These contradictory or flawed conclusions make clinicians hesitant to use aspirin for the prevention of PE. Therefore, we performed a meta-analysis of published randomized controlled studies on aspirin for the prevention of PE, to obtain more comprehensive conclusions and to try to explain the reasons for the inconsistency of the conclusions of previous studies.
Methods
This systematic review was conducted as per the Guidelines for Systematic Review and Meta-Analysis (PRISMA) (14). This study only included randomized controlled studies to assess the use of aspirin for the prevention of PE. This study was registered in PROSPERO with the registration number CRD42022319984.
Eligibility criteria, information sources, and search strategy
We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials databases for randomized controlled trials related to aspirin and PE. We used MeSH terms and keywords and followed the Harvard Medical School Library’s recommended search strategy for the search as follows. There were no language restrictions, and the databases were searched for the literature published from the time of inception to 9 March 2022 (Supplementary material 1). The search for relevant literature followed the principles of population, intervention, comparator, outcomes, and study design (PICOS). In addition, we also conducted a screening of the included studies for relevant references.
Study selection
All the articles retrieved from the database were deduplicated by EndNote X9. After browsing the article titles and abstracts, irrelevant articles were excluded and the remaining articles were comprehensively evaluated by two independent reviewers (Yixiao Wang and Xiaojun Guo) to select articles that met the criteria. If there was any disagreement at any time, they were discussed and solved with the corresponding author (Chengqian Wu). The inclusion criteria for this meta-analysis were studies with one group of pregnant women that received aspirin before delivery and the other group that received a placebo or no treatment and studies that documented the incidence of PE. Review articles, editorials, case reports, and conference abstracts were excluded.
Data extraction
Two authors independently reviewed each study and extracted the following data from the articles: first or corresponding author name, date of publication, the location where the study was conducted, aspirin intervention time and cut-off time, aspirin dose used, the incidence of PE, inclusion and exclusion criteria for the included women, and diagnostic criteria for PE.
Risk of bias assessment and sensitivity analysis
Publication bias was assessed using funnel plots, and the Cochrane Bias Assessment Manual was used to assess selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases for all the included studies. We performed sensitivity analyses by excluding individual studies to explore the strength of each included study.
Subgroup analysis
This study conducted a subgroup analysis to explore the time of initial aspirin intervention and prophylactic aspirin dosage used among PE high-risk groups. In addition, we performed subgroup analyses by region (Africa, America, Asia, and Europe, and involving multiple continents) to explore the sources of heterogeneity and bias.
Statistical analysis
All data were analyzed by Review Manager software, version 5.3 (Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark), except for sensitivity analysis which was conducted and plotted using GraphPad Prism 7 software. Forest plots to obtain pooled relative risk (RR) and 95% confidence intervals (95% CI) were drawn. If I2 < 50% and P > 0.10, a fixed-effects model was applied to calculate pooled effect estimates. A random-effects model was applied if I2 ≥ 50% or P ≤ 0.10. Statistical significance was defined as P < 0.05.
Results
Study selection
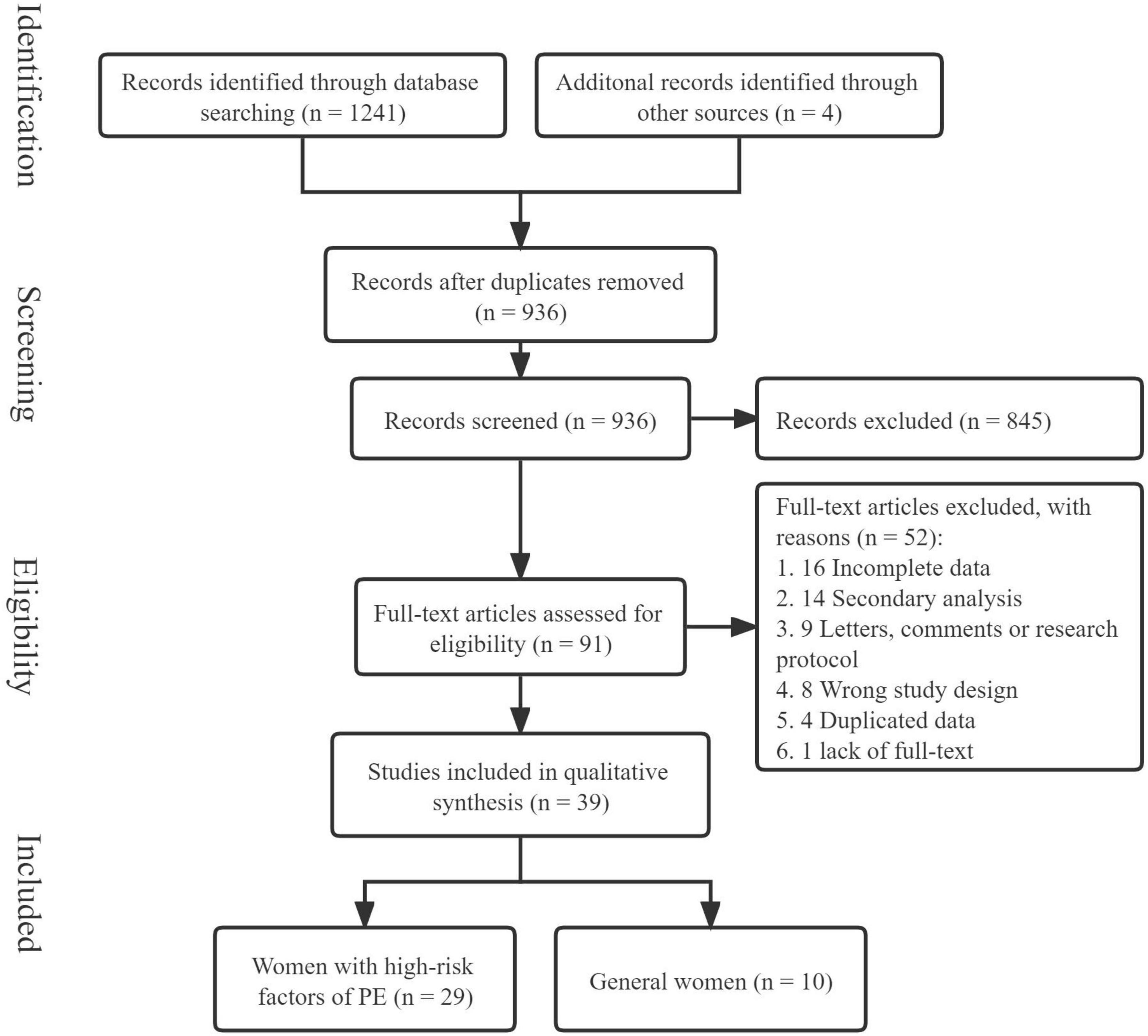
A total of 1,241 relevant articles from the inception of the databases to 9 March 2022, were retrieved, and 4 articles were included from the references of included articles. After removing duplicates, 936 articles remained, and 845 irrelevant articles were excluded after reading the article titles and abstracts. Of the remaining 91 articles, 52 articles were excluded after careful reading of the full text, and ultimately, our meta-analysis included 39 articles (Figure 1).
Study characteristics
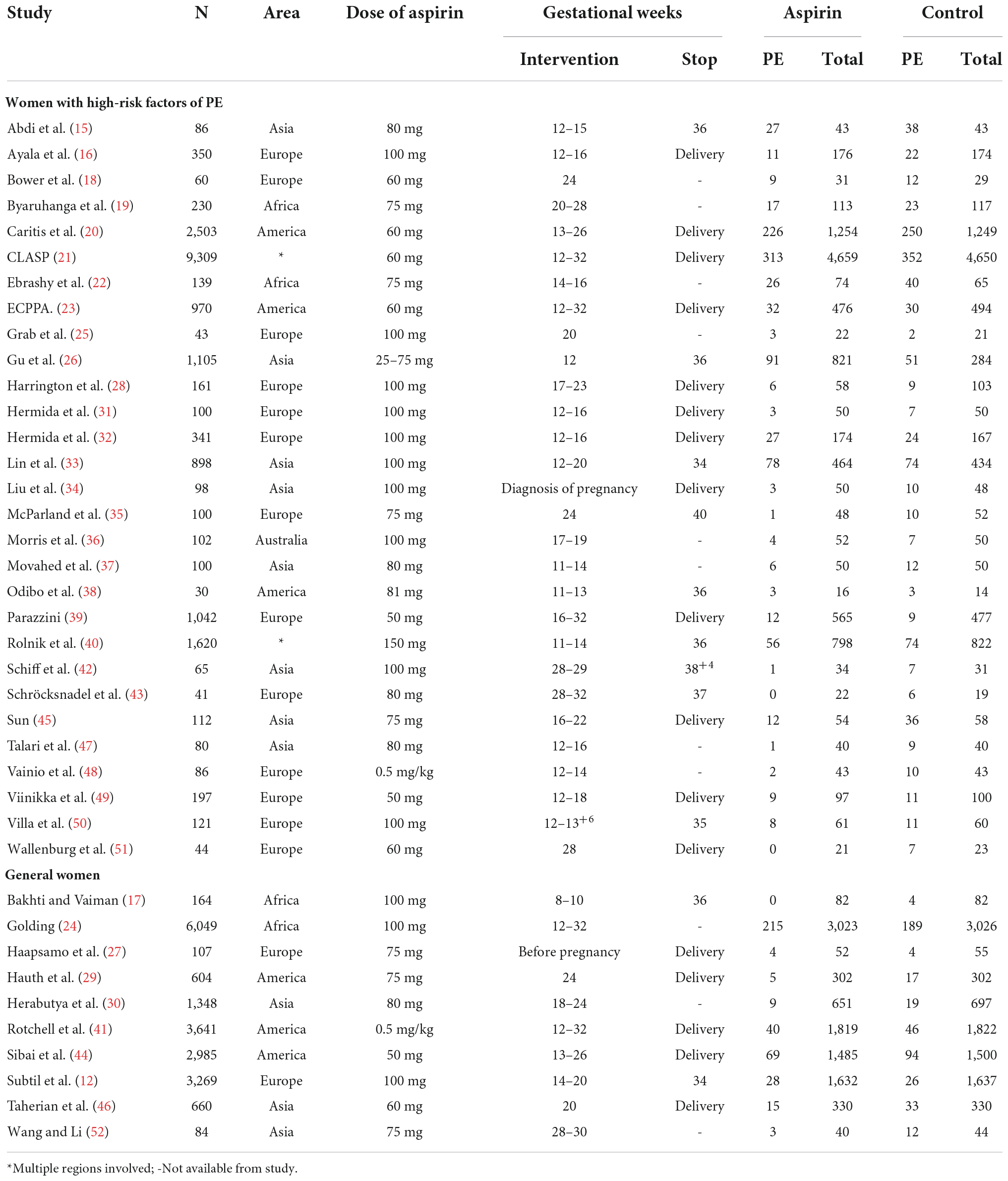
The main characteristics of the included studies are presented in Table 1. A total of 39 articles were included (12, 15–52), of which 29 studied pregnant women at high risk of PE [a total of 20,133 patients (15, 16, 18–23, 25, 26, 28, 31–40, 42, 43, 45, 47–51)] and 10 studied the general population of pregnant women (a total of 18,911 patients) (12, 17, 24, 27, 29, 30, 41, 44, 46, 52). Among those at high risk for PE, 10,366 women received aspirin prophylaxis before delivery, and 9,767 patients received a placebo or no treatment. Two studies were completed in Africa (19, 22), three in the Americas (20, 23, 38), eight in Asia (15, 26, 33, 34, 37, 42, 45, 47), one in Australia (36), 13 in Europe (16, 18, 25, 28, 31, 32, 35, 39, 43, 48–51), and two covering multiple continents (21, 40). Aspirin was used in doses ranging from 25 to 150 mg/day, and one study used a dose of 0.5 mg/kg/day (48). The initiation of aspirin intervention varied from 11 to 32 weeks of gestation in all but one study that initiated aspirin use at the time of pregnancy diagnosis (34). In the studies involving the general population, 9,416 women received aspirin prophylaxis before delivery, and 9,495 women received a placebo or no treatment. Aspirin was used in doses ranging from 60 to 100 mg/day. The initiation time of aspirin intervention varied from 12 to 32 weeks of gestation, except in one study that started aspirin before pregnancy (27). The inclusion and exclusion criteria for the included studies and their PE diagnostic criteria are presented in Supplementary material 2.
Total pooled effects
The heterogeneity of the PE high-risk women studies was I2 = 47% (P < 0.10), so we chose a random-effects model (Figure 2A). The overall pooled effect showed that aspirin was effective in preventing the onset of PE in high-risk women (RR 0.72, 95% CI 0.62–0.83). Compared with the control group, the use of aspirin reduced the incidence of PE by 28%. The funnel plot showed significant asymmetry, suggesting that there may be some publication bias (Figure 2B). Sensitivity analysis found that omitting any article had no significant effect on RR and 95% CI, and the results were relatively stable (Figure 2C). The heterogeneity of the general population group of studies was I2 = 66% (P < 0.10), so the random-effects model was applied (Figure 3A). The overall pooled effect showed that aspirin was effective in preventing the incidence of PE among women in the general population group (RR 0.70, 95% CI 0.52–0.95). Compared with the control group, the use of aspirin was associated with a 30% reduction in the incidence of PE. The funnel plot showed significant asymmetry (Figure 3B). Sensitivity analysis found that omission of the following studies: Hauth 1993 (RR 0.76, 95% CI 0.57–1.02) (29), Herabutya 1996 (RR 0.73, 95% CI 0.53–1.00) (30), Taherian 2002 (RR 0.76, 95% CI 0.56–1.03) (46), or Wang 1993 (RR 0.75, 95% CI 0.56–1.00) (52), led to significant changes in RR and 95% CI; thus, the findings for this classification were not robust (Figure 3C).
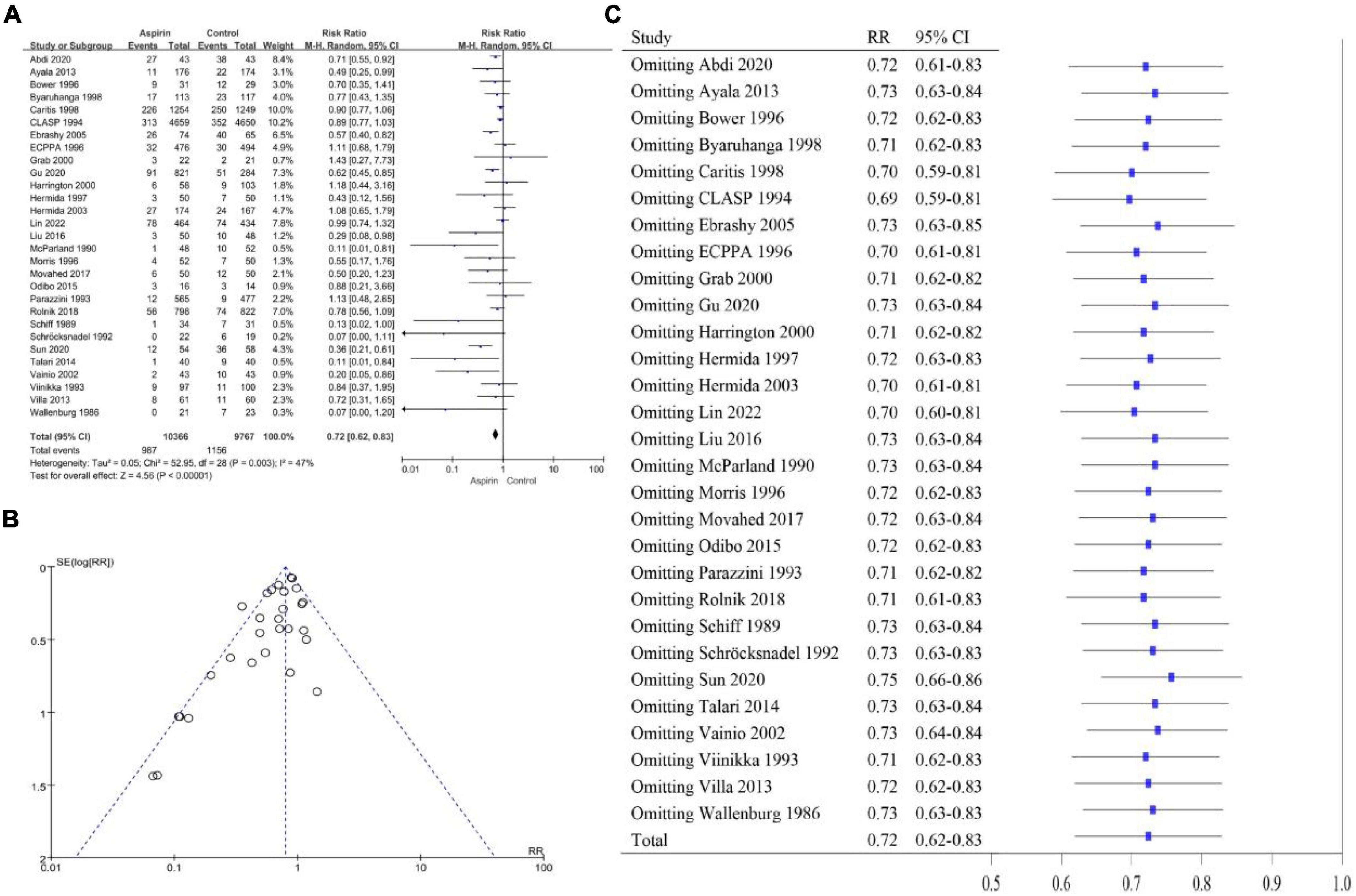
Figure 2. (A) Forest plot of studies on the effect of aspirin on the development of PE in women at high risk for PE. (B) Funnel plot of the studies on women at high risk for PE. (C) Sensitivity analysis of the included studies.
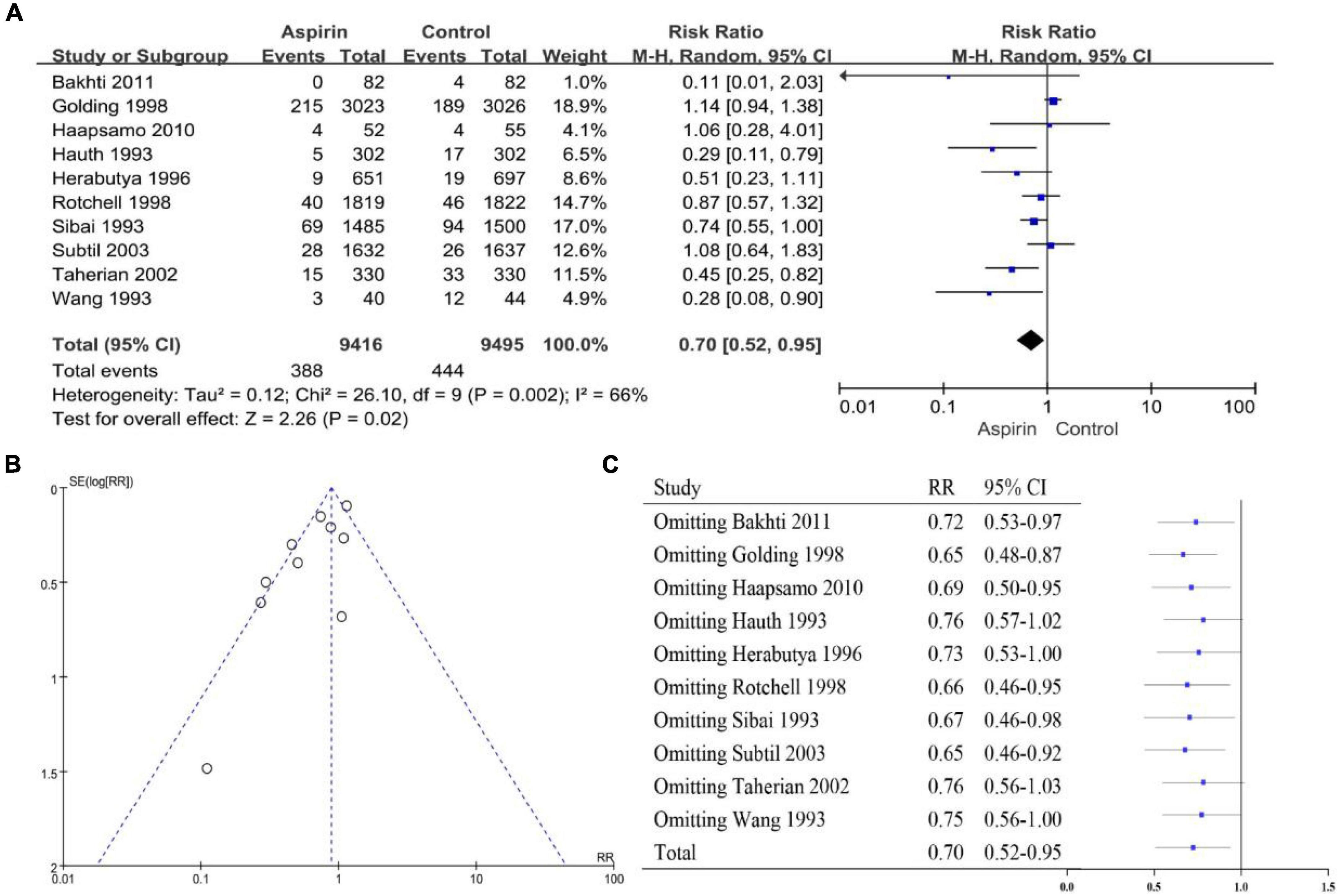
Figure 3. (A) Forest plot showing the effect of aspirin on the development of PE in the general population of women. (B) Funnel plot showing studies that involved women in the general population. (C) Sensitivity analysis of the included studies.
Bias assessment
We conducted a bias assessment according to the Cochrane Risk of Bias template. In the PE high-risk women group, two studies had a high risk of random sequence generation (31, 47) and six studies had an unclear risk (16, 18, 32, 36, 37, 39); three studies had a high risk of allocation concealment (33, 39, 45) and four studies had an unclear risk (15, 28, 37, 48); two studies had a high risk of performance bias (26, 45) and three studies had an unclear risk (15, 33, 37); one study had a high risk of detection bias (45) and eight studies had an unclear risk (15, 25, 26, 32, 34, 37, 38, 43); two studies had a high risk of attrition bias (36, 47) and three studies had an unclear risk (37–39); and one study had an unclear reporting bias (37) and one study had unclear other biases (37) (Figures 4A,B). Among the studies on the general population of women, one study had a high risk of random sequence generation (24) and three studies had an unclear risk (29, 30, 52); one study had a high risk of allocation concealment (47) and three studies had an unclear risk (17, 30, 52); three studies had an unclear performance bias (30, 46, 52); and six studies had an unclear detection bias (17, 27, 29, 30, 46, 52). Attrition bias was unclear in two studies (30, 52). In three studies, reporting bias was not clear (30, 46, 52). Two studies had unclear other biases (30, 52) (Figures 4C,D).
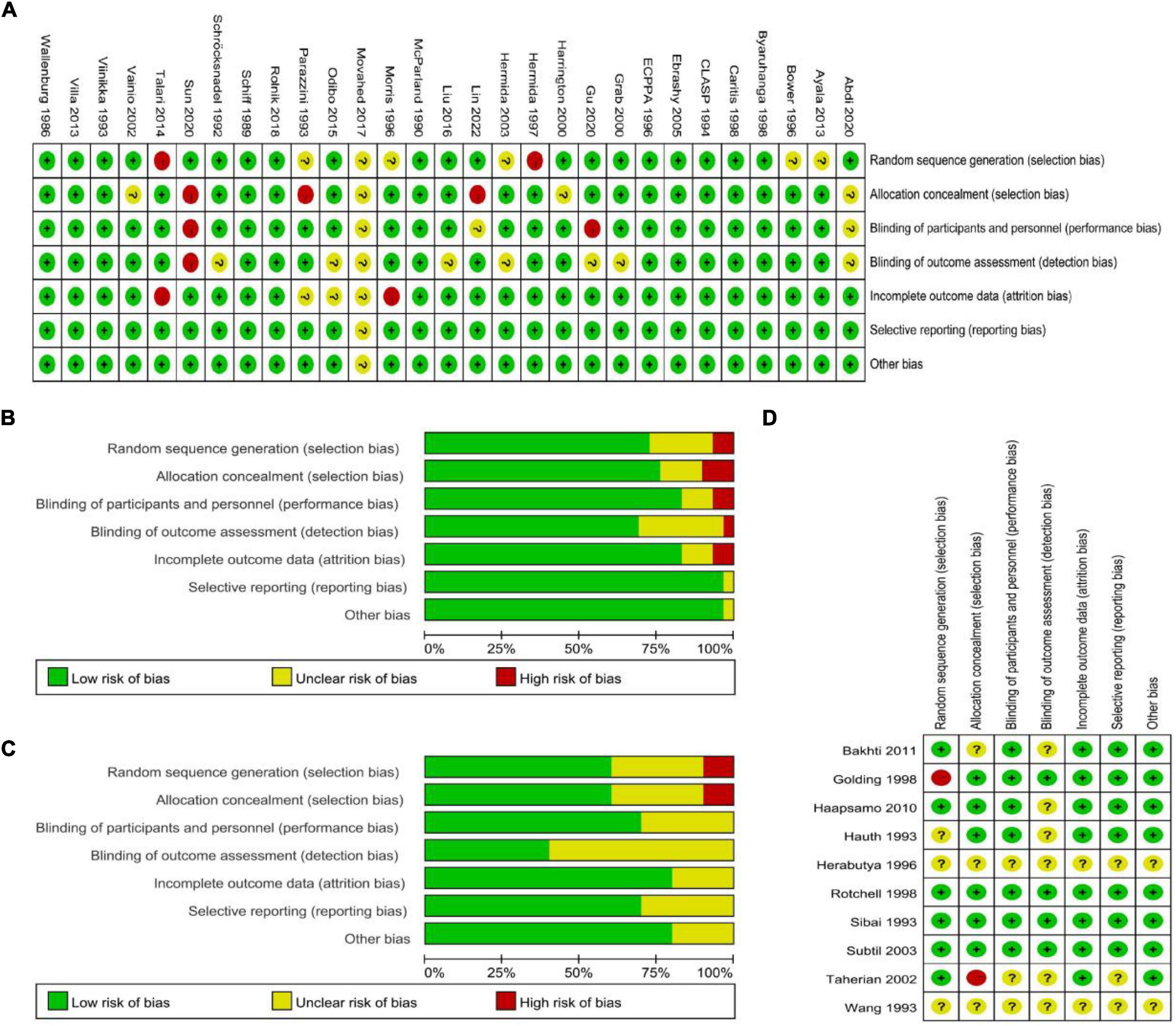
Figure 4. (A,B) Bias assessment of studies with PE high-risk women. (C,D) Assessment of bias in studies related to the general population of women.
Subgroup analysis
Dosage of aspirin
The ACOG recommends that pregnant women with any risk factors for PE should receive low-dose aspirin (81 mg/day) to prevent the development of PE (9). We performed subgroup analyses based on aspirin dose because different doses of aspirin were used in each study. The groups were divided as follows: 50, 60, 75, 80, 81, 100, and 150 mg/day. Two studies were not included in the subgroup analysis because they used 0.5 mg/kg/day (26) and 25–75 mg/day (48) of aspirin, respectively. The incidence of PE was reduced by 11 and 50% with 60 mg/day (RR 0.89, 95% CI 0.80–0.99, Figure 5B) and 75 mg/day (RR 0.50, 95% CI 0.32–0.78, Figure 5C) of aspirin, respectively. In addition, sensitivity analysis showed that the pooled results of a dosage of 60 mg/d of aspirin were not reliable (Supplementary Figure 1B). The risk of PE was not reduced when other dosages of aspirin were used (Figures 5A,D–G). The funnel plot was relatively symmetrical in all groups except for the 100 mg/d aspirin group, where it was asymmetrical (Supplementary Figure 2).
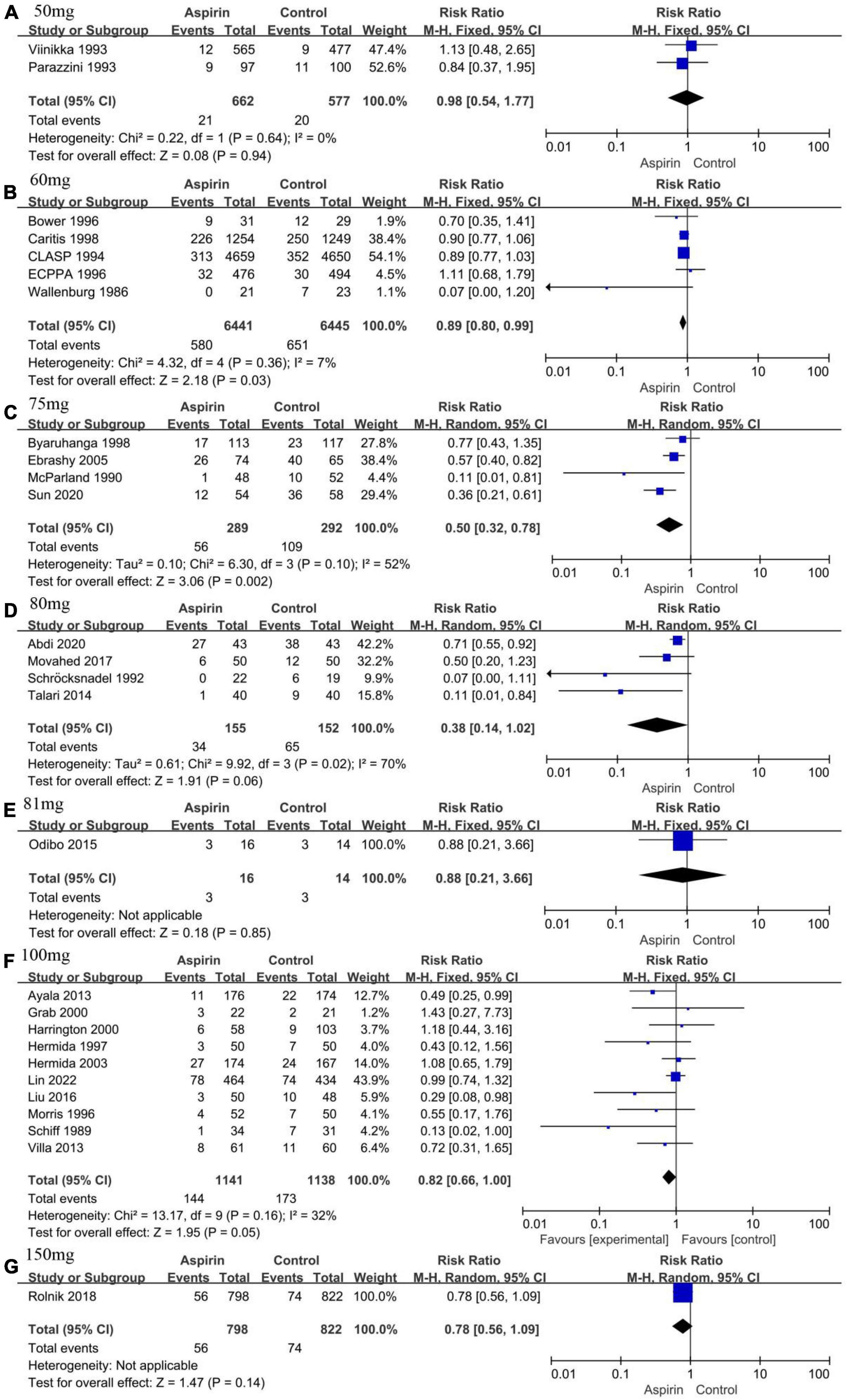
Figure 5. Forest plot showing subgroup analysis according to aspirin dose. (A) 50 mg/day. (B) 60 mg/day. (C) 75 mg/day. (D) 80 mg/day. (E) 81 mg/day. (F) 100 mg/day. (G) 150 mg/day.
Timing of initiation of aspirin interventions
The aspirin intervention recommended by the ACOG begins between 12 and 28 weeks of gestation (preferably before 16 weeks of gestation) and continues into labor (9). However, the timing of initiation of aspirin interventions in the studies was not always as recommended by the guidelines. Therefore, we performed a subgroup analysis of the timing of the initiation of aspirin intervention. All studies were divided into 12–16 weeks of gestation (15, 16, 22, 26, 31, 32, 47, 48, 50) and 17–28 weeks of gestation (18, 19, 25, 28, 35, 36, 51) according to the time of initiation of the intervention. The pooled effect of aspirin intervention at 12–16-weeks gestation showed a 38% reduction in the risk of PE (RR 0.62, 95% CI 0.53–0.74, Figure 6A), with essential symmetry on both sides of the funnel chart (Figure 6B). Omission of any of the studies did not cause the conclusion to change, and thus, this conclusion was robust (Figure 6C). The pooled effect of the aspirin intervention from 17 to 28 weeks of gestation showed a 38% reduction in the risk of PE (RR 0.62, 95% CI 0.44–0.89) (Figure 6D), with a largely symmetric funnel plot (Figure 6E). Notably, the total pooled effect size changed significantly when McParland 1990 (35) was omitted (RR 0.71, 95% CI 0.49–1.02, Figure 6F). Therefore, the intervention at 17–28 weeks of gestation was less robust or effective than the intervention at 12–16 weeks of gestation. Aspirin intervention should be started at 12–16 weeks of gestation if possible.
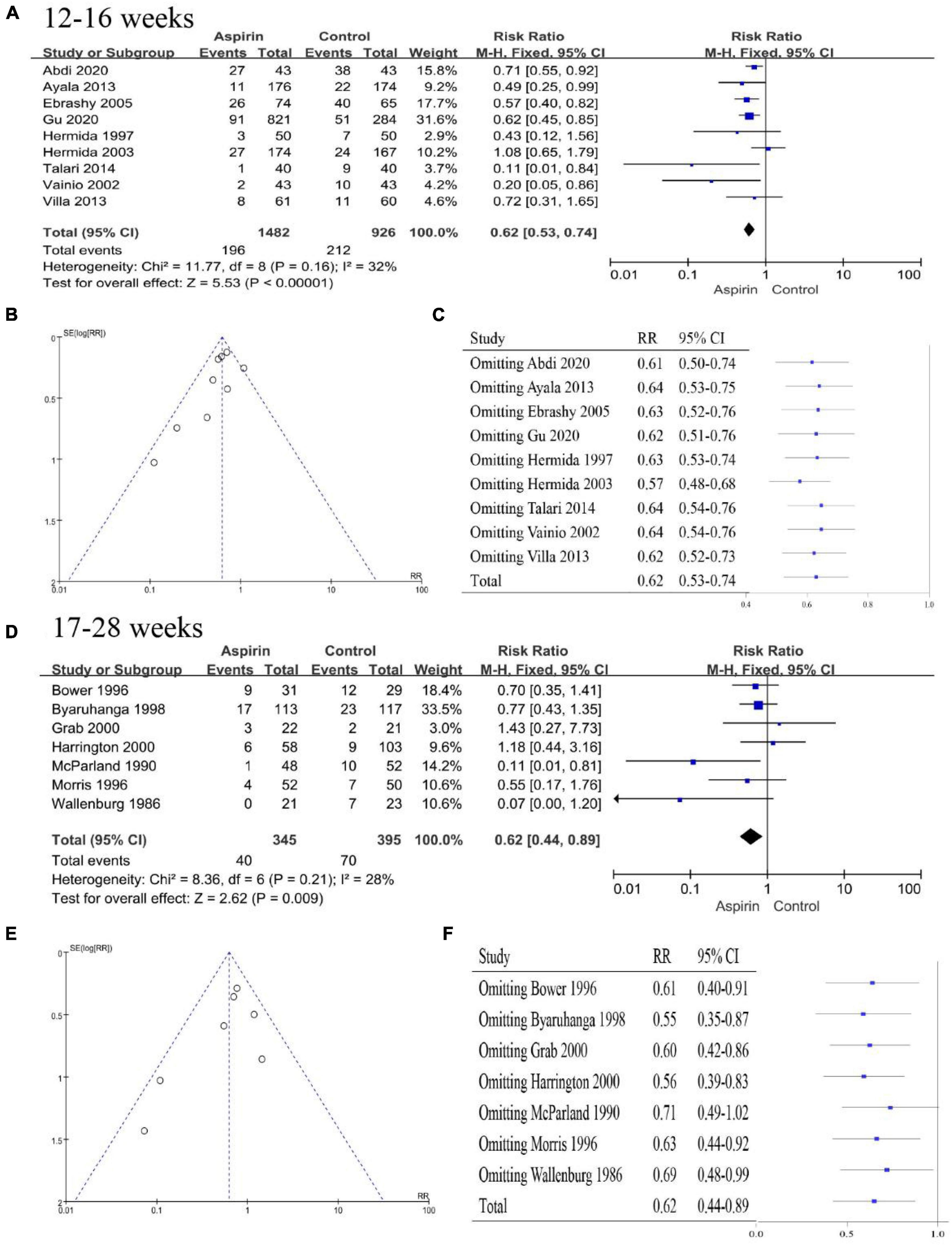
Figure 6. Subgroup analysis by time of initial aspirin intervention. (A) Forest plot showing the intervention at 12–16 weeks. (B) Funnel plot showing the intervention at 12–16 weeks. (C) Sensitivity analysis of the intervention at 12–16 weeks. (D) Forest plot for the intervention at 17–28 weeks. (E) Funnel plot showing the intervention at 17–28 weeks. (F) Sensitivity analysis of the intervention at 17–28 weeks.
Continent where the study was conducted
Considering the apparent asymmetry of the funnel plot shown in Figure 2A, I2 = 47%, P < 0.10, and Figure 2B, we conducted a subgroup analysis by continent for all the studies to explore possible sources of heterogeneity and publication bias. The studies were divided into an African group (N = 2, I2 = 0%, P = 0.39, Figure 7A), an American group (N = 3, I2 = 0%, P = 0.73, Figure 7B), an Asian group (N = 8, I2 = 66%, P < 0.10, Figure 7C), an European group (N = 13, I2 = 38%, P = 0.08, Figure 7D), and a multi-regional group (N = 2, I2 = 0%, P = 0.48, Figure 7E). Except for the Asian subgroup, which shows significant asymmetry in the funnel plot, studies conducted on all continents show no significant asymmetry (Supplementary Figure 3). In addition, the results of sensitivity analysis showed that the results were stable in all groups (Supplementary Figure 4). Therefore, we believe that the main reason for the high heterogeneity and publication bias lies in studies conducted in Asia.
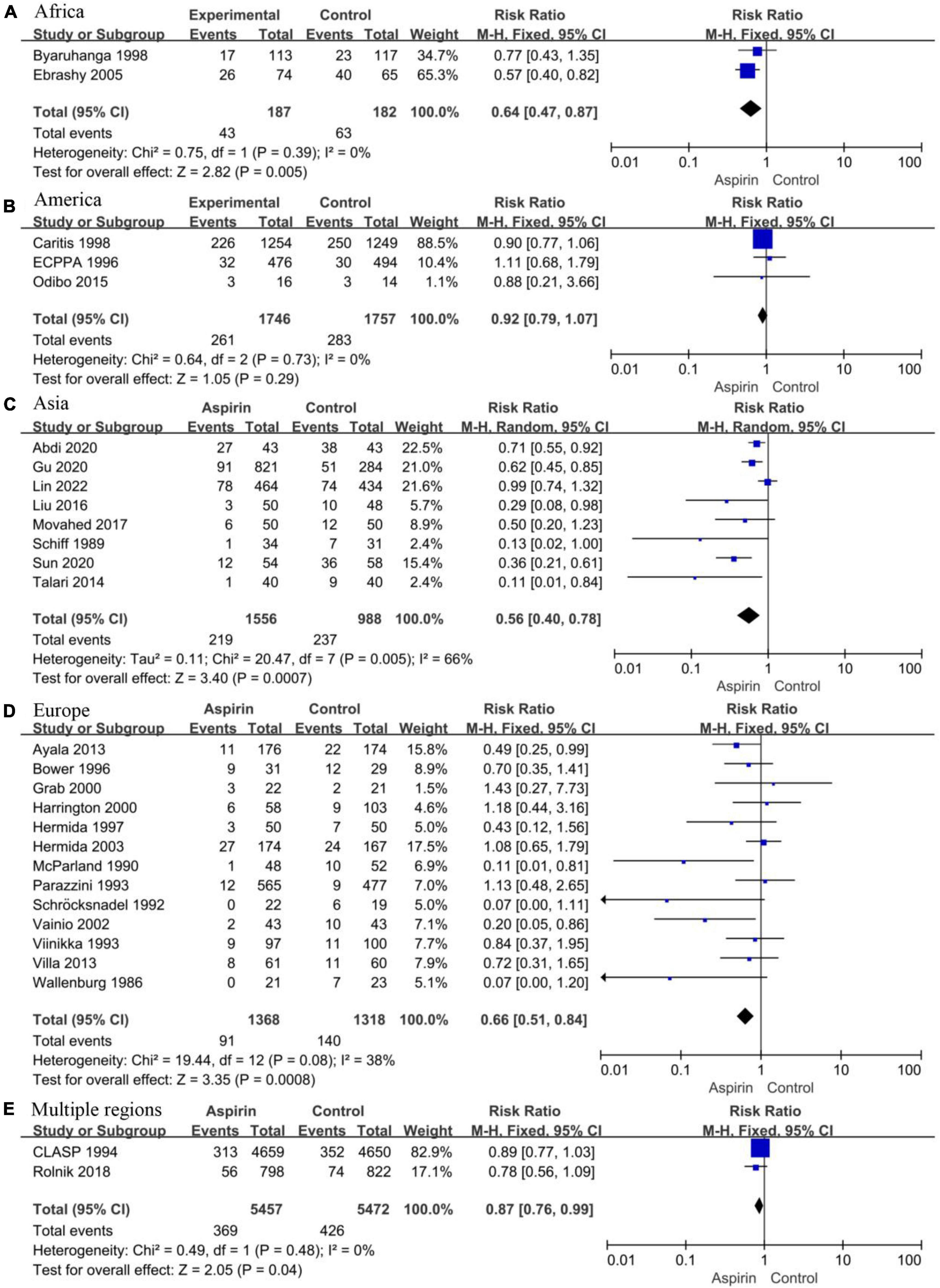
Figure 7. Forest plot showing subgroup analysis by study region. (A) Africa. (B) America. (C) Asia. (D) Europe. (E) Multiple regions.
Discussion
We analyzed some previous meta-analyses that examined the use of aspirin for the prevention of PE and identified certain issues with their research conclusions. A meta-analysis published in 2021 did not differentiate between high-risk groups and the general population of women. The study concluded that starting low-dose aspirin before 20 weeks of gestation can significantly reduce the incidence of PE (13).
Our study shows that aspirin is effective in preventing the occurrence of PE in high-risk groups. In the general population of women not screened for high-risk factors for PE, the results of sensitivity analysis showed that the effect of aspirin was unstable, and this is consistent with the results of another meta-analysis examining the protective effect of aspirin on PE in low-risk nulliparous women (RR 0.70, 95% CI 0.47–1.05, P = 0.08) (53).
Aspirin belongs to the family of non-steroidal anti-inflammatory drugs, and its analgesic, antipyretic, and anti-inflammatory effects are due to the inactivation of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), inhibiting the production of prostaglandins and thromboxane (54, 55). This reduced thromboxane can also inhibit platelet aggregation, resulting in an antithrombotic effect. There is increasing evidence that suboptimal invasion of trophoblasts leads to an imbalance of angiogenic and antiangiogenic proteins, ultimately leading to extensive inflammation and endothelial damage, increased platelet aggregation, and thrombotic events in placental infarction (56). Due to the possible mechanism of aspirin in preventing PE, we excluded the general population of women who were not screened for high-risk factors for PE in subsequent analyses and focused on the protective effect of aspirin in PE high-risk groups.
Furthermore, we conducted a subgroup analysis of the differences in the timing and dosage of aspirin intervention. The dose of aspirin recommended by the ACOG is 81 mg/day; however, the conventional aspirin dose in some countries is not 81 mg, for example, the common aspirin doses in China are 50 mg, 75 mg, and 100 mg. The use of a 75 mg/day aspirin dose significantly reduced the incidence of PE (RR 0.50, 95% CI 0.32–0.78). Concerning the timing of aspirin intervention, we recommend intervention in high-risk women at 12–16 weeks of gestation given that placenta implantation is essentially completed within 14–18 weeks of gestation (57).
Considering the significant heterogeneity and potential publication bias of the 29 included studies, we performed subgroup analyses by study region. The results showed that, except for the eight studies conducted in Asia, which had significant heterogeneity (I2 = 66%, P < 0.10), the heterogeneity of studies conducted in other regions was not significant. In the funnel diagram of each region, only Asia showed obvious asymmetry. We speculate that differences in ethnicities, regions, and countries in Asia, the interaction between internal (including genetic factors, metabolic factors, and pharmacokinetics) and external factors (including environmental, cultural differences, and dietary habits), as well as different definitions of high-risk women, etc., may contribute to the heterogeneity (58, 59).
Our study has some limitations. It was not possible to define specific risk factors for PE, for example, some studies used umbilical artery Doppler to identify high-risk groups and some studies included pregnant women diagnosed with PE in a previous pregnancy. The inability to differentiate between risk factors may affect the reliability of the conclusions. Notably, postpartum PE was not described or discussed in any of the studies, which may lead to errors in the statistics of the incidence of PE. After uniform criteria are obtained for the diagnosis of postpartum PE, studies on the prevention and treatment of postpartum PE with aspirin should be conducted.
In addition, we did not perform regression analysis when exploring the source of heterogeneity. This was because some studies included baseline characteristics of subjects who were lost to follow-up or dropped out, and this could pose unknown risks to the regression analysis. Moreover, only some of the randomized controlled studies accounted for patient compliance, and this may have potential implications for the conclusions of the studies.
Previous studies have shown that aspirin reduces the risk of complications such as preterm birth, perinatal death, and intrauterine growth retardation without increasing the risk of hemorrhage. So, we did not analyze other complications or side effects associated with aspirin use.
Conclusion
Aspirin is currently widely accepted for the prevention and treatment of PE; however, relevant research conclusions are inconsistent. Multicenter randomized controlled placebo studies involving various ethnicities and regions are needed. This meta-analysis finds that aspirin is only suitable for the prevention of PE among high-risk groups of women and its effect is better when initiated at 12–16 weeks of pregnancy at the recommended dose of 75 mg/day.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
YW and XG were responsible for the study design, data extraction and analysis, and article writing. NO was responsible for the research design and direction and language polish. HD was responsible for the study design and direction. CW and HY were responsible for the research design and revision, manuscript review, and guidance. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.936560/full#supplementary-material
References
1. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. (2013) 347:f6564. doi: 10.1136/bmj.f6564
2. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. (2016) 387:999–1011. doi: 10.1016/S0140-6736(15)00070-7
3. Obstetrics and Gynecology,. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. (2019) 133:1. doi: 10.1097/AOG.0000000000003020
4. Obstetrics and Gynecology. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. (2013) 122:1122–31.
5. Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. (2022) 226:S1108–19. doi: 10.1016/j.ajog.2020.08.045
6. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. (2014) 4:97–104. doi: 10.1016/j.preghy.2014.02.001
7. Ma’ayeh M, Rood KM, Kniss D, Costantine MM. Novel interventions for the prevention of preeclampsia. Curr Hypertens Rep. (2020) 22:17.
8. Konijnenberg A, Stokkers EW, Van Der Post JA, Schaap MC, Boer K, Bleker OP, et al. Extensive platelet activation in preeclampsia compared with normal pregnancy: enhanced expression of cell adhesion molecules. Am J Obstet Gynecol. (1997) 176:461–9. doi: 10.1016/S0002-9378(97)70516-7
9. Obstetrics and Gynecology. Gestational hypertension and preeclampsia: ACOG practice bulletin summary, number 222. Obstet Gynecol. (2020) 135:1492–5. doi: 10.1097/AOG.0000000000003892
10. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. (2017) 377:613–22. doi: 10.1056/NEJMoa1704559
11. Van Doorn R, Mukhtarova N, Flyke IP, Lasarev M, Kim K, Hennekens CH, et al. Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: a systematic review and meta-analysis. PLoS One. (2021) 16:e0247782. doi: 10.1371/journal.pone.0247782
12. Subtil D, Goeusse P, Puech F, Lequien P, Biausque S, Breart G, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the essai régional aspirine mère-enfant study (Part 1). BJOG. (2003) 110:475–84. doi: 10.1016/S1470-0328(03)02996-3
13. Choi YJ, Shin S. Aspirin prophylaxis during pregnancy: a systematic review and meta-analysis. Am J Prev Med. (2021) 61:e31–45. doi: 10.1016/j.amepre.2021.01.032
14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
15. Abdi N, Rozrokh A, Alavi A, Zare S, Vafaei H, Asadi N, et al. The effect of aspirin on preeclampsia, intrauterine growth restriction and preterm delivery among healthy pregnancies with a history of preeclampsia. J Chin Med Assoc. (2020) 83:852–7. doi: 10.1097/JCMA.0000000000000400
16. Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. (2013) 30:260–79. doi: 10.3109/07420528.2012.717455
17. Bakhti A, Vaiman D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertens Res. (2011) 34:1116–20. doi: 10.1038/hr.2011.111
18. Bower SJ, Harrington KF, Schuchter K, McGirr C, Campbell S. Prediction of pre-eclampsia by abnormal uterine Doppler ultrasound and modification by aspirin. Br J Obstet Gynaecol. (1996) 103:625–9. doi: 10.1111/j.1471-0528.1996.tb09829.x
19. Byaruhanga RN, Chipato T, Rusakaniko S. A randomized controlled trial of low-dose aspirin in women at risk from pre-eclampsia. Int J Gynaecol Obstet. (1998) 60:129–35. doi: 10.1016/S0020-7292(97)00257-9
20. Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med.. (1998) 338:701–5. doi: 10.1056/NEJM199803123381101
21. Lancet. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet. (1994) 343:619–29. doi: 10.1016/S0140-6736(94)92633-6
22. Ebrashy A, Ibrahim M, Marzook A, Yousef D. Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14-16 weeks pregnancy: randomized controlled clinical trial. Croat Med J. (2005) 46:826–31.
23. British Journal of Obstetrics and Gynaecology. ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. ECPPA (Estudo Colaborativo para Prevenção da Pré-eclampsia com Aspirina) Collaborative Group. Br J Obstet Gynaecol. (1996) 103:39–47. doi: 10.1111/j.1471-0528.1996.tb09513.x
24. Golding J. A randomised trial of low dose aspirin for primiparae in pregnancy. Br J Obstet Gynaecol. (1998) 105:293–9. doi: 10.1111/j.1471-0528.1998.tb10089.x
25. Grab D, Paulus WE, Erdmann M, Terinde R, Oberhoffer R, Lang D, et al. Effects of low-dose aspirin on uterine and fetal blood flow during pregnancy: results of a randomized, placebo-controlled, double-blind trial. Ultrasound Obstet Gynecol. (2000) 15:19-27. doi: 10.1046/j.1469-0705.2000.00009.x
26. Gu W, Lin J, Hou YY, Lin N, Song MF, Zeng WJ, et al. Effects of low-dose aspirin on the prevention of preeclampsia and pregnancy outcomes: a randomized controlled trial from Shanghai, China. Eur J Obstet Gynecol Reprod Biol. (2020) 248:156–63. doi: 10.1016/j.ejogrb.2020.03.038
27. Haapsamo M, Martikainen H, Tinkanen H, Heinonen S, Nuojua-Huttunen S, Räsänen J, et al. Low-dose aspirin therapy and hypertensive pregnancy complications in unselected IVF and ICSI patients: a randomized, placebo-controlled, double-blind study. Hum Reprod. (2010) 25:2972–7. doi: 10.1093/humrep/deq286
28. Harrington K, Kurdi W, Aquilina J, England P, Campbell S. A prospective management study of slow-release aspirin in the palliation of uteroplacental insufficiency predicted by uterine artery Doppler at 20 weeks. Ultrasound Obstet Gynecol. (2000) 15:13–8. doi: 10.1046/j.1469-0705.2000.00002.x
29. Hauth JC, Goldenberg RL, Parker CR, Philips JB III, Copper RL, DuBard MB, et al. Low-dose aspirin therapy to prevent preeclampsia. Am J Obstet Gynecol. (1993) 168:1083–91. doi: 10.1016/0002-9378(93)90351-I
30. Herabutya Y, Jetsawangsri T, Saropala N. The use of low-dose aspirin to prevent preeclampsia. Int J Gynaecol Obstet. (1996) 54:177–8. doi: 10.1016/0020-7292(96)02701-4
31. Hermida RC, Ayala DE, Iglesias M, Mojón A, Silva I, Ucieda R, et al. Time-dependent effects of low-dose aspirin administration on blood pressure in pregnant women. Hypertension. (1997) 30:589–95. doi: 10.1161/01.HYP.30.3.589
32. Hermida RC, Ayala DE, Iglesias M. Administration time-dependent influence of aspirin on blood pressure in pregnant women. Hypertension. (2003) 41:651–6. doi: 10.1161/01.HYP.0000047876.63997.EE
33. Lin L, Huai J, Li B, Zhu Y, Juan J, Zhang M, et al. A randomized controlled trial of low-dose aspirin for the prevention of preeclampsia in women at high risk in China. Am J Obstet Gynecol. (2022) 226:251.e1–12. doi: 10.1016/j.ajog.2021.08.004
34. Liu FM, Zhao M, Wang M, Yang HL, Li L. Effect of regular oral intake of aspirin during pregnancy on pregnancy outcome of high-risk pregnancy-induced hypertension syndrome patients. Eur Rev Med Pharmacol Sci. (2016) 20:5013–6.
35. Mcparland P, Pearce JM, Chamberlain GV. Doppler ultrasound and aspirin in recognition and prevention of pregnancy-induced hypertension. Lancet. (1990) 335:1552–5. doi: 10.1016/0140-6736(90)91377-M
36. Morris JM, Fay RA, Ellwood DA, Cook CM, Devonald KJ. A randomized controlled trial of aspirin in patients with abnormal uterine artery blood flow. Obstet Gynecol. (1996) 87:74–8. doi: 10.1016/0029-7844(95)00340-1
37. Movahed F, Lalooha F, Moinodin R, Dabbaghi Ghale T, Rezaee Majd Z, Yazdi Z. The effect of aspirin in the prevention of preeclampsia in women with abnormal uterine artery doppler ultrasonography findings. J Zanjan Univ Med Sci Health Serv. (2017) 25:11–9.
38. Odibo AO, Goetzinger KR, Odibo L, Tuuli MG. Early prediction and aspirin for prevention of pre-eclampsia (EPAPP) study: a randomized controlled trial. Ultrasound Obstet Gynecol. (2015) 46:414–8. doi: 10.1002/uog.14889
39. Lancet. Low-dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy-induced hypertension. Italian study of aspirin in pregnancy. Lancet. (1993) 341:396–400. doi: 10.1016/0140-6736(93)92988-6
40. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. Obstet Gynecol Surv. (2018) 73:11–2. doi: 10.1097/01.ogx.0000528015.09400.25
41. Rotchell YE, Cruiekshank JK, Gay MP, Griffiths J, Stewart A, Farrell B, et al. Barbados low dose aspirin study in pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications. Br J Obstet Gynaecol. (1998) 105:286–92. doi: 10.1111/j.1471-0528.1998.tb10088.x
42. Schiff E, Peleg E, Goldenberg M, Rosenthal T, Ruppin E, Tamarkin M, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med. (1989) 321:351–6. doi: 10.1056/NEJM198908103210603
43. Schröcksnadel H, Sitte B, Alge A, Steckel-Berger G, Schwegel P, Pastner E, et al. Low-dose aspirin in primigravidae with positive roll-over test. Gynecol Obstet Invest. (1992) 34:146–50. doi: 10.1159/000292748
44. Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The national institute of child health and human development network of maternal-fetal medicine units. N Engl J Med. (1993) 329:1213–8. doi: 10.1056/NEJM199310213291701
45. Sun H. Preventive effect of low-dose aspirin on preeclampsia occured in preeclampsia high-risk pregnant women and its mechanism. J Jilin Univ. (2020) 46:138–43.
46. Taherian AA, Taherian A, Shirvani A. Prevention of preeclampsia with low-dose aspirin or calcium supplementation. Arch Iran Med. (2002) 5:151–6.
47. Talari H, Mesdaghinia E, Kalahroudi MA. Aspirin and preeclampsia prevention in patients with abnormal uterine artery blood flow. Iran Red Crescent Med J. (2014) 16:e17175. doi: 10.5812/ircmj.17175
48. Vainio M, Kujansuu E, Iso-Mustajärvi M, Mäenpää J. Low dose acetylsalicylic acid in prevention of pregnancy-induced hypertension and intrauterine growth retardation in women with bilateral uterine artery notches. BJOG. (2002) 109:161–7. doi: 10.1111/j.1471-0528.2002.01046.x
49. Viinikka L, Hartikainen-Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin-thromboxane balance in mother and newborn. Br J Obstet Gynaecol. (1993) 100:809–15. doi: 10.1111/j.1471-0528.1993.tb14304.x
50. Villa PM, Kajantie E, Räikkönen K, Pesonen AK, Hämäläinen E, Vainio M, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. BJOG. (2013) 120:64–74. doi: 10.1111/j.1471-0528.2012.03493.x
51. Wallenburg HC, Dekker GA, Makovitz JW, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. (1986) 1:1–3. doi: 10.1016/S0140-6736(86)91891-X
52. Wang ZH, Li WJ. Prevention of fetal growth retardation by low dose aspirin. Zhonghua fu chan ke za zhi. (1993) 28:492–5.
53. Man R, Hodgetts Morton V, Devani P, Morris RK. Aspirin for preventing adverse outcomes in low risk nulliparous women with singleton pregnancies: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2021) 262:105–12. doi: 10.1016/j.ejogrb.2021.05.017
54. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. (2003) 110:255–8. doi: 10.1016/S0049-3848(03)00379-7
55. Tóth L, Muszbek L, Komáromi I. Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by QM/MM calculations. J Mol Graph Model. (2013) 40:99–109. doi: 10.1016/j.jmgm.2012.12.013
56. Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P. Aspirin for prevention of preeclampsia. Drugs. (2017) 77:1819–31. doi: 10.1007/s40265-017-0823-0
57. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. (1986) 93:1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x
58. Huang SM, Temple R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin Pharmacol Ther. (2008) 84:287–94. doi: 10.1038/clpt.2008.144
Keywords: pregnancy, aspirin, preeclampsia, meta-analysis, systematic review
Citation: Wang Y, Guo X, Obore N, Ding H, Wu C and Yu H (2022) Aspirin for the prevention of preeclampsia: A systematic review and meta-analysis of randomized controlled studies. Front. Cardiovasc. Med. 9:936560. doi: 10.3389/fcvm.2022.936560
Received: 05 May 2022; Accepted: 12 October 2022;
Published: 09 November 2022.
Edited by:
Amanda Henry, University of New South Wales, AustraliaReviewed by:
Xin Zhou, Tianjin Medical University General Hospital, ChinaDonna Ann Santillan, The University of Iowa, United States
Copyright © 2022 Wang, Guo, Obore, Ding, Wu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengqian Wu, 1468480384@qq.com; Hong Yu, yuhong650325@sina.com
†These authors have contributed equally to this work
 Yixiao Wang
Yixiao Wang Xiaojun Guo
Xiaojun Guo Nathan Obore1
Nathan Obore1 Hongjuan Ding
Hongjuan Ding Chengqian Wu
Chengqian Wu