- 1Department of Medical Laboratory, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2State Key Laboratory for Infectious Disease Prevention and Control, Chinese Center for Disease Control and Prevention, Beijing, China
- 3Cancer Research Institute of Wuhan, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Streptococcus sinensis was originally described as a causative agent for infective endocarditis in three Chinese patients from Hong Kong in 2002. Subsequently, several cases were reported outside Hong Kong, indicating that it is an emerging pathogen worldwide. We isolated a closely related strain in a young patient diagnosed with infective endocarditis in mainland China. In this paper, we reviewed the course of infection and provided a comprehensive comparison of its clinical characteristics with the reported cases.
Introduction
Infective endocarditis (IE) is a potentially fatal disease that occurs on the endocardial surface of the heart, usually involving the heart valves. The viridans group streptococci is second only to Staphylococcus aureus as a causative agent of IE, which presents in ~20% of cases worldwide (1). Among these streptococcal species, Streptococcus sinensis (S. sinensis) was reported as a new pathogen isolated from a 42-year-old Chinese woman in Hong Kong with mitral regurgitation due to chronic rheumatic heart disease and IE in 2002 (2). Subsequent studies by Woo et al. found another two strains screened from 302 patients with bacteremia caused by viridans streptococci over a 6-year period, and demonstrated that S. sinensis is the common ancestor of the anginosus and mitis groups of streptococci (3, 4). In 2008, using the 16S rRNA sequencing method, the same group concluded that the oral cavity is the natural reservoir of S. sinensis (5). The increasing number of cases reported outside Hong Kong indicates that the organism is an emerging pathogen that is of interest globally (6–9).
Case presentation
A 19-year-old man with no obvious incentive for intermittent joint pain in both knees and the right elbow over the previous 2 months was admitted to our hospital on December 26, 2020. At admission, the patient complained of occasional numbness and pain in both feet, difficulty squatting, and lower extremity edema. Physical examination showed a sick male, with a blood pressure of 135/85 mmHg, a temperature of 38.1°C, a pulse of 110 beats/min, and a respiratory rate of 19 breaths/min. Laboratory tests revealed the following: a white blood cell (WBC) count of 12.9 × 109/L (83.9% neutrophils), an erythrocyte sedimentation rate of 114 mm/h (normal, 0–20 mm/h), hypersensitive C-reactive protein (CRP, whole blood) of 16.5 mg/dl (normal, 0–0.8 mg/dl), hemoglobin of 87 g/L (normal, 120–160 g/L), total protein of 81 g/L (normal, 65–85 g/L), albumin of 25.9 g/L (normal, 40–55 g/L), and rheumatoid factor (RF) of 68.3 IU/ml. Urinalysis revealed a red blood cell count of 2,442/μl with heterogeneous morphology, urine protein of 2+, and a WBC count of 188/μl. Two sets of blood cultures were prepared before empirical antibiotic treatment with intravenous (i.v.) teicoplanin 1 g/day (qd) and cefoselis 2 × 1 g/day (q 12 h). Over the cardiac apex, a grade 2/6 proto-mesosystolic murmur was audible. Transthoracic echocardiography (TTE) revealed aortic valve bicuspid malformation (Type 0), flocculent hypoechoic vegetations of the aortic valve and anterior mitral valve leaflets (Figure 1A), and moderate regurgitation bundles on the left ventricular outflow tract side during diastole of the aortic valve (Figure 1B), supporting the diagnosis of IE. In all blood culture bottles, Gram-positive cocci were observed. The strain named BC1012612 was identified as S. sinensis by matrix-assisted laser desorption ionization/time of flight mass spectrometry (MALDI-TOF MS, Bruker Daltonik GmbH, Germany) with a high confidence level. A definite diagnosis of IE requires two major, one major with three minor, or five minor criteria (10). Thus, 6 days after admission, IE was diagnosed by the presence of two major and two minor criteria according to the modified Duke criteria (10).
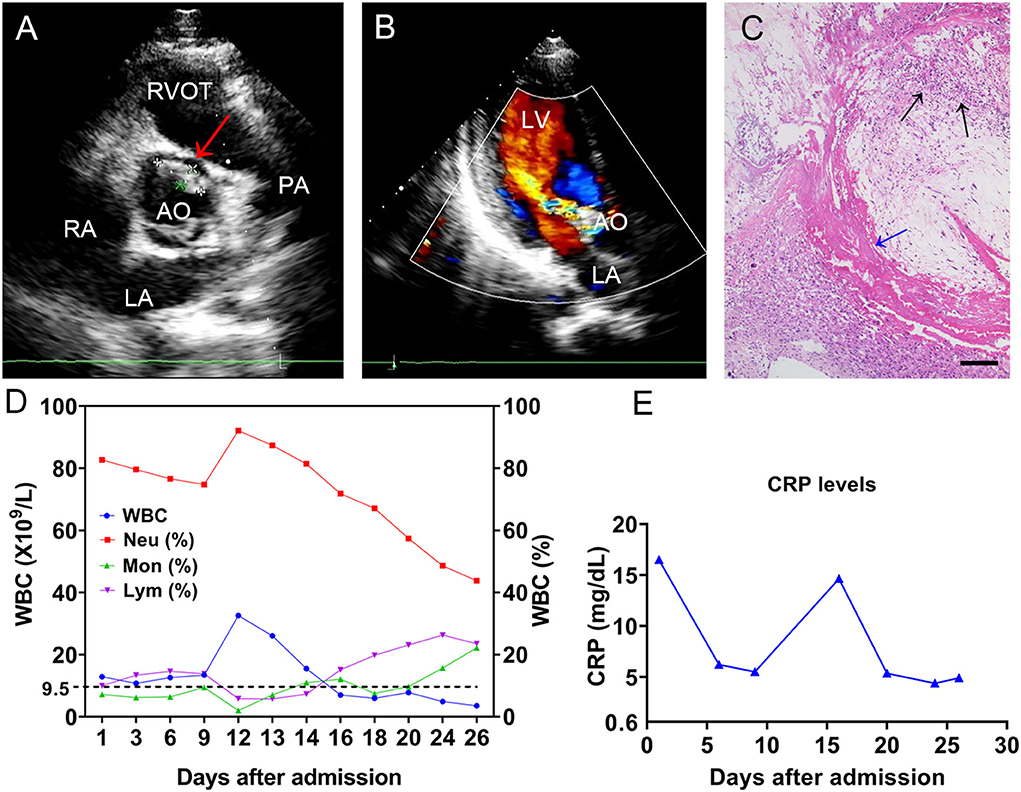
Figure 1. The TEE results of the mitral valve and WBC levels. TTE revealed (A) flocculent hypoechoic vegetations of the aortic valve (the red arrow) and (B) a moderate regurgitation bundle on the left ventricular outflow tract side during diastole of the aortic valve. (C) The histochemical analysis of the valve tissue revealed local myxoid and hyaline degeneration, missing endothelial cells, necrotic attached substances (the blue arrow), and neutrophil aggregation (the black arrows). Scale bars approximate 50 μm in length. The (D) WBC count and cell proportions and (E) CRP throughout the course. TTE, transthoracic echocardiography; LA, left atrium; LV, left ventricle; RA, right atrium; RVOT, right ventricular outflow tract; AO, aorta; PA, pulmonary artery; WBC, white blood cell; CRP, C-reactive protein.
An antibiotic susceptibility test via the Kirby–Bauer method showed the strain was susceptible to levofloxacin (32 mm), ceftriaxone (32 mm), linezolid (31 mm), vancomycin (23 mm), cefepime (32 mm), and chloramphenicol (28 mm). In addition, the minimal inhibitory concentrations (MICs) for penicillin G (0.064 mg/L) and meropenem (0.048 mg/L) were tested via an E-test. Based on the drug susceptibility result, the antimicrobial drug was changed to ceftriaxone (2 g/day). The patient underwent mechanical aortic valve replacement and mitral valve repair with no postoperative complications on Day 6 after his diagnosis. Histochemical analysis of the valve tissue showed local myxoid and hyaline degeneration, missing endothelial cells, and a large number of necrotic attached substances, and there were many neutrophils in the valve wall at the bottom of the necrotic substances (Figure 1C). The WBC count (Figure 1D) and CRP (Figure 1E) remained at high levels post-operation, and intravenous teicoplanin (1 g/day) and meropenem (1 g/8 h) were used for treatment. Due to the occurrence of delirium and muscle spasms during anti-infective therapy, meropenem was changed to ceftriaxone. After 2 weeks of symptomatic and supportive treatment, the patient's physiological conditions and inflammatory indicators were normalized, and he was discharged with a monthly follow-up 26 days after admission. At the time of writing, the patient has remained clinically stable for more than 18 months. Additionally, we present a timeline for the case presentation (Figure 2).

Figure 2. The timeline of the case presentation. The patient was discharged with a monthly follow-up 26 days after his admission. BT, body temperature; ESR, erythrocyte sedimentation rate; BCs, blood cultures; IE, infective endocarditis; PCT, procalcitonin.
16S rRNA gene sequence analysis was conducted to classify the isolated strain BC1012612 with universal 16S rRNA primers (forward primer: 5′-AGTTTGATCMTGGCTCAG-3′, reverse primer: 5′-GGTTACCTTGTTACGACTT-3′). A total of 1,439 contiguous nucleotides were determined. The complete 16S rRNA sequence was analyzed with the Basic Local Alignment Search Tool (BLAST) at the GenBank Database (https://blast.ncbi.nlm.nih.gov). The strain BC1012612 exhibited the highest (99.93%) 16S rRNA gene sequence similarity with the type strain of S. sinensis HKU4T (GenBank accession No. AF432856). Among the 1,439 bases, there was only one base difference from strain HKU4T. Multiple alignments with sequences of the most closely related streptococci and the calculations of the levels of sequence similarity were carried out using CLUSTALW (11). A phylogenetic tree was constructed using the neighbor-joining method by using MEGA software version 11 (12). The topology of the phylogenetic tree was evaluated by using the bootstrap resampling method of Felsenstein (13) with 1,000 replicates. The phylogenetic tree showed that strain BC1012612 was clustered with strains HKU4T, HDP 2005-0155, and 11026353, and this cluster was strongly supported with a bootstrap value of 100% (Figure 3). The results of the comparative 16S rRNA gene sequence analysis demonstrated that the isolated strain BC1012612 belongs to the S. sinensis species. We have submitted the 16S rRNA sequencing results to GenBank (accession No. OM780285).
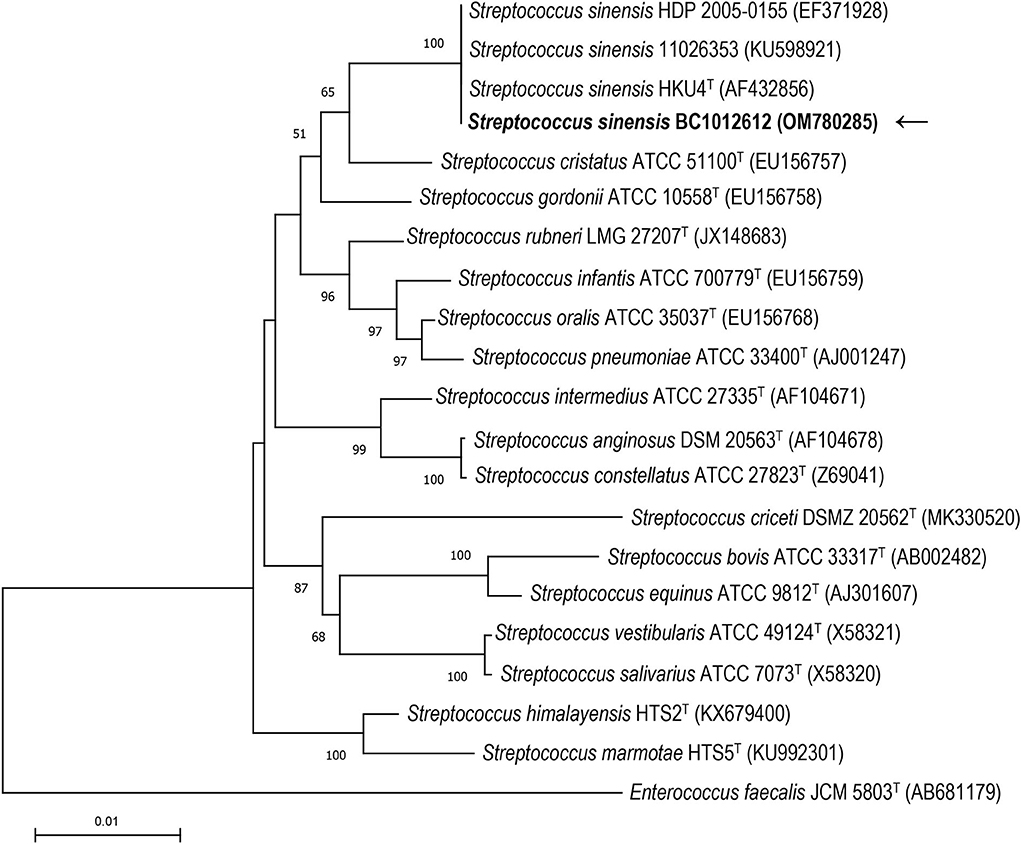
Figure 3. The phylogenetic tree based on the 16S rRNA gene sequences showing the relationship of isolated strain BC1012612 (the black arrow) and members within the genus Streptococcus. The tree was reconstructed by the neighbor-joining method, and Enterococcus faecalis JCM5803T was used as an outgroup. Bootstrap values (>50%) based on 1,000 replicates are shown at branch nodes. T, type strain.
Literature review and discussion
S. sinensis, a newly described species of viridans streptococci, was originally isolated in 2002 from blood cultures of a female patient with chronic rheumatic heart disease in Hong Kong (2). It has subsequently been reported in a few case reports outside Hong Kong, such as in Switzerland, France, Great Britain, and the Netherlands (6, 8, 9, 14–16), highlighting its importance as an emerging pathogen in the healthcare field. Continuous studies by Woo et al. revealed that the oral cavity is the natural reservoir of S. sinensis in healthy individuals, and that S. sinensis may be the common ancestor of the anginosus and mitis groups of streptococci according to clinical, phenotypic, and genotypic comparisons of these strains (4, 5). Based on phylogenomic and MALDI-TOF MS analysis, they proposed a new group called the “sinensis group,” which includes S. sinensis, Streptococcus oligofermentans (S. oligofermentans), and Streptococcus cristatus (S. cristatus), in 2014 (7). Jensen et al. reported S. oligofermentans as a later synonym of S. cristatus (17). With the development of genome sequencing technology, increasingly more details of S. sinensis have been explored.
IE caused by S. sinensis was diagnosed by blood cultures collected before antibiotic therapy. We isolated a strain of S. sinensis BC1012612 from the blood of a young patient with IE. The strain was identified by MALDI-TOF MS, and 16S rRNA sequencing was performed for further analysis. Among a total of 1,439 bases, the 16S rRNA sequence of this strain was found to have only one base difference from the type strain HKU4. A phylogenetic tree also showed a cluster within the previously reported S. sinensis strains and our strain BC1012612, suggesting that our strain is most closely related to the type strain. According to the emended taxonomy of the Mitis group of the genus Streptococcus carried out by Jensen et al., S. sinensis, together with S. cristatus, belongs to the S. cristatus clade but is distantly related to other strains and may represent a distinct taxon at the species level (17). The 16S rRNA sequencing method alone is inadequate for bacterial classification, and multidimensional analysis will be helpful for the accurate classification of the large numbers of species within genus Streptococcus.
The extant literature on S. sinensis-related IE is presented in Table 1. Unfortunately, a small number of cases have been reported without any clinical descriptions, making it impossible for a comprehensive summary and analysis of these cases (6, 18). Among the eight listed observations, including ours, there were only two female patients, and the age of the patients ranged from 19 to 63; the patient in our case is the youngest reported yet. All the patients had acquired congenital heart disease, and six out of eight patients were found to have vegetations and varying degrees of mitral regurgitation via echocardiography. It is intriguing that at least three patients with IE caused by S. sinensis had tooth problems, which may corroborate the oral origin of this organism. S. sinensis was also identified by a multiple PCR method in the subgingival plaque of two subjects with localized severe chronic periodontitis (19). However, more evidence is needed to ascertain whether S. sinensis is present in the oral cavity of individuals living in other regions outside Hong Kong. Two patients were reported to have travailed to Hong Kong or had undergone dental procedures there, thus hinting at the geographical reservoirs for S. sinensis. In addition, studies of the stomach and gut microbiota also revealed the presence of S. sinensis from gastric mucosa and stool samples at the genetic level via 16S rRNA sequencing (20, 21), but without any isolated strains. To date, no infections other than IE caused by S. sinensis have been reported in humans, suggesting that this organism may possess specific virulence factors to invade heart valves and cause damage. The sequence analysis of the manganese-dependent superoxide dismutase gene (sodA) via a PCR assay based on degenerate primers was initially carried out by Poyart et al. to identify the genus Streptococcus to a species level (22). A high congruence of strain grouping by MALDI-TOF MS in comparison with sodA sequence analysis regarding streptococcus bovis/equinus-complex was observed by Hinse et al., demonstrating the accuracy and reliability of MALDI-TOF MS in comparison to the DNA sequence-based method (23). It is difficult to identify viridans streptococci by traditional biomedical methods, even MALDI-TOF MS; genetic characterization should be performed to distinguish strains isolated in infectious diseases and the epidemiology of each species.
All previously reported patients were treated with ampicillin or amoxicillin, or with combined therapy with gentamicin. It seems that the strain is susceptible to the majority of antibiotics tested, especially β-lactam antibiotics, so the routinely used antibiotics could achieve an antibacterial effect. However, more antibiotics were applied in this case, such as ceftriaxone and teicoplanin, mainly due to the persistently high level of inflammatory markers during anti-infective therapy. Almost all the patients received mitral valve replacement because of the severity of the preexisting mitral regurgitation, and had a good outcome. The patient in this case underwent mechanical aortic valve replacement and mitral valve repair. Surgical exploration showed scattered and small vegetations on the left ventricular surface of the anterior mitral valve without obvious valve leaf thickening, ulceration, or involvement of chordae tendineae, so mitral valve repair was performed. Several studies have shown that mitral valve repair has low mortality, fewer complications, and a better long-term prognosis than mitral valve replacement (24–26). Of these cases, only one death occurred in a 58-year-old man who refused surgery and died of multiple cerebral infarctions several days after admission, suggesting that timely treatment is of great importance in the acute phase of infection.
Conclusion
Herein, we report the first case of IE caused by S. sinensis in mainland China. The patient in our case is the youngest ever reported, and his initial symptoms were mainly joint pain. The patient was diagnosed with IE after the isolation of S. sinensis from blood cultures and treated with drug-sensitive antibiotics as well as surgery. The early identification of S. sinensis is critical to the diagnosis and treatment of IE, but routine biochemical methods and MALDI-TOF MS are not sufficient because of the variety of species within genus Streptococcus. Certain genetic methods, such as 16S rRNA sequencing, are required for the accurate classification of those obtained strains. In addition, the patients' travel history should be taken into consideration when determining the possible geographical reservoirs for this agent, as it has been proposed to be an oral flora present in Hong Kong. Our case highlights the importance of S. sinensis as an emerging pathogen and provides a comprehensive understanding of S. sinensis-related IE.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Sciences and Technology. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Designed and conceived the experiments: ZL, HW, and YZhang. Performed the experiments: YZhang, JW, and YZhan. Analyzed the data: YZhang and RT. Wrote and reviewed the manuscript: YZhang, TQ, and ZL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Wuhan Association for Science and Technology (Project No. HB2021C15) and by one local grant from The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology to YZhang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective endocarditis: a contemporary review. Mayo Clin Proc. (2020) 95:982–97. doi: 10.1016/j.mayocp.2019.12.008
2. Woo PC, Tam DM, Leung KW, Lau SK, Teng JL, Wong MK, et al. Streptococcus sinensis Sp. nov, a novel species isolated from a patient with infective endocarditis. J Clin Microbiol. (2002) 40:805–10. doi: 10.1128/JCM.40.3.805-810.2002
3. Woo PC, Teng JL, Leung KW, Lau SK, Tse H, Wong BH, et al. Streptococcus sinensis may react with lancefield group F antiserum. J Med Microbiol. (2004) 53:1083–8. doi: 10.1099/jmm.0.45745-0
4. Woo PC, Teng JL, Lau SK, Yuen KY. Clinical, phenotypic, and genotypic evidence for Streptococcus sinensis as the common ancestor of anginosus and mitis groups of streptococci. Med Hypotheses. (2006) 66:345–51. doi: 10.1016/j.mehy.2005.03.033
5. Woo PC, Teng JL, Tsang SN, Tse CW, Lau SK, Yuen KY. The oral cavity as a natural reservoir for Streptococcus sinensis. Clin Microbiol Infect. (2008) 14:1075–9. doi: 10.1111/j.1469-0691.2008.02083.x
6. Uçkay I. Streptococcus sinensis endocarditis outside Hong Kong. Emerg Infect Dis. (2007) 13:1250–2. doi: 10.3201/eid1308.080124
7. Teng JL, Huang Y, Tse H, Chen JH, Tang Y, Lau SK, et al. Phylogenomic and maldi-Tof Ms analysis of Streptococcus sinensis Hku4t reveals a distinct phylogenetic clade in the genus Streptococcus. Genome Biol Evol. (2014) 6:2930–43. doi: 10.1093/gbe/evu232
8. Francisco AS. Lesson of the month 2: when steroids stop working–infective endocarditis, the great mimicker. Clin Med. (2019) 19:82–4. doi: 10.7861/clinmedicine.19-1-82
9. van Ommen A, Slavenburg S, Diepersloot R, de Vries Feyens CA. Fatal outcome of first case of Streptococcus sinensis in infective endocarditis in the Netherlands: a case report. Eur Heart J Case Rep. (2020) 4:1–4. doi: 10.1093/ehjcr/ytz237
10. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. (2000) 30:633–8. doi: 10.1086/313753
11. Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. (1994) 22:4673–80. doi: 10.1093/nar/22.22.4673
12. Tamura K, Stecher G, Kumar S. Mega11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. (2021) 38:3022–7. doi: 10.1093/molbev/msab120
13. Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. (1985) 39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x
14. Faibis F, Mihaila L, Perna S, Lefort JF, Demachy MC, Le Fleche-Mateos A, et al. Streptococcus sinensis: an emerging agent of infective endocarditis. J Med Microbiol. (2008) 57:528–31. doi: 10.1099/jmm.0.47528-0
15. Seta V, Teicher E, Fortineau N, Ladouceur M, Lambotte O. Infective endocarditis caused by Streptococcus sinensis. Med Mal Infect. (2015) 45:56–7. doi: 10.1016/j.medmal.2014.11.001
16. Goret J, Baudinet T, Camou F, Issa N, Gaillard P, Wirth G, et al. Identification of Streptococcus sinensis from a patient with endocarditis using maldi-Tof mass spectrometry, 16s Rdna- and soda-based phylogeny. J Microbiol Immunol Infect. (2019) 52:507–9. doi: 10.1016/j.jmii.2018.04.004
17. Jensen A, Scholz CFP, Kilian M. Re-Evaluation of the taxonomy of the mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis Subsp. dentisani comb nov, Streptococcus tigurinus as Streptococcus oralis subsp tigurinus Comb Nov, and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int J Syst Evol Microbiol. (2016) 66:4803–20. doi: 10.1099/ijsem.0.001433
18. Watt G, Pachirat O, Baggett HC, Maloney SA, Lulitanond V, Raoult D, et al. Infective endocarditis in Northeastern Thailand. Emerg Infect Dis. (2014) 20:473–6. doi: 10.3201/eid2003.131059
19. de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, et al. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol Immunol. (2006) 21:61–8. doi: 10.1111/j.1399-302X.2005.00255.x
20. Aviles-Jimenez F, Vazquez-Jimenez F, Medrano-Guzman R, Mantilla A, Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep. (2014) 4:4202. doi: 10.1038/srep04202
21. Toscano M, De Grandi R, Stronati L, De Vecchi E, Drago L. Effect of Lactobacillus rhamnosus Hn001 and Bifidobacterium longum Bb536 on the healthy gut microbiota composition at phyla and species level: a preliminary study. World J Gastroenterol. (2017) 23:2696–704. doi: 10.3748/wjg.v23.i15.2696
22. Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P. Identification of Streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J Clin Microbiol. (1998) 36:41–7. doi: 10.1128/JCM.36.1.41-47.1998
23. Hinse D, Vollmer T, Erhard M, Welker M, Moore ER, Kleesiek K, et al. Differentiation of species of the Streptococcus bovis/equinus-complex by maldi-Tof mass spectrometry in comparison to soda sequence analyses. Syst Appl Microbiol. (2011) 34:52–7. doi: 10.1016/j.syapm.2010.11.010
24. Di Tommaso E, Rapetto F, Guida GA, Zakkar M, Bruno VD. Benefits of mitral valve repair over replacement in the elderly: a systematic review and meta-analysis. J Card Surg. (2021) 36:2524–30. doi: 10.1111/jocs.15506
25. Tianyu Zhou JL, Lai H, Sun Y. Mitral valve repair and mitral valve replacement in the treatment of infective endocarditis mitral value regurgitation in the long-term curative effect comparison. Chin J Thorac Cardiovasc Surg. (2017) 33:408–12. doi: 10.3760/cma.j.issn.1001-4497.2017.07.007
Keywords: Streptococcus sinensis, infective endocarditis, emerging pathogen, 16S rRNA sequence, case report
Citation: Zhang Y, Wang J, Zhan Y, Tang R, Wang H, Qin T and Lu Z (2022) Case report: Infective endocarditis caused by Streptococcus sinensis: The first case in mainland China and literature review. Front. Cardiovasc. Med. 9:935725. doi: 10.3389/fcvm.2022.935725
Received: 04 May 2022; Accepted: 29 June 2022;
Published: 22 July 2022.
Edited by:
Maximillian A. Rogers, Moderna Therapeutics, United StatesReviewed by:
Symeon Panagiotakis, University Hospital of Heraklion, GreeceFabrice Compain, L'Institut Mutualiste Montsouris Paris, France
Copyright © 2022 Zhang, Wang, Zhan, Tang, Wang, Qin and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Qin, cWludGlhbkBpY2RjLmNu; Zhongxin Lu, bHV6aG9uZ3hpbkB6eGhvc3BpdGFsLmNvbQ==
 Yingmiao Zhang
Yingmiao Zhang Jing Wang1
Jing Wang1 Ruizhi Tang
Ruizhi Tang Hui Wang
Hui Wang Tian Qin
Tian Qin