
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 25 July 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.934598
This article is part of the Research Topic Novel and Emerging Therapies in Acute and Chronic Heart Failure View all 12 articles
 Zunjiang Li1,2
Zunjiang Li1,2 Ye Fan1,2,3
Ye Fan1,2,3 Chunxia Huang1,2
Chunxia Huang1,2 Quanle Liu1,2,3
Quanle Liu1,2,3 Manhua Huang1,2
Manhua Huang1,2 Baijian Chen1,2,3
Baijian Chen1,2,3 Zhe Peng1,2
Zhe Peng1,2 Wei Zhu1,2,3*
Wei Zhu1,2,3* Banghan Ding1,2,3*
Banghan Ding1,2,3*Objective: This study aimed to assess the adjunctive efficacy and safety of Puerarin injection (PI) on acute heart failure (AHF) based on a systematic review and meta-analysis.
Methods: Nine databases were searched from March 1990 to March 2022 to identify randomized controlled trials (RCTs) related to the adjunctive treatment of PI for AHF. The Cochrane collaboration tool was used to assess the risk of bias in the included studies. Meta-analysis and subgroup and sensitivity analyses were conducted by RevMan 5.3 software. The evidence’s certainty was evaluated by grading recommendations assessment, development, and evaluation (GRADE) methods.
Results: A total of 8 studies were included with a total of 614 patients with AHF. The meta-analysis demonstrated that adjunctive treatment with PI on AHF was superior to conventional medicine alone. It increased the total effective rate (RR = 1.38; 95% CI, 1.22–1.55; p < 0.001) and improved left ventricular ejection fraction [SMD = 0.85; 95% CI (0.62, 1.09); p < 0.001]. Regarding safety, a total of 11.9% (23/194) adverse reactions were observed in the PI group and 9.8% (19/194) adverse reactions in the control group, and there were no significant differences in the incident rate of adverse events between both groups [RR = 1.16; 95% CI (0.66–2.05); p = 0.061]. The outcomes’ evidentiary quality was assessed as “moderate.”
Conclusion: PI had an adjunctive effect on AHF combined with conventional medicine, and it seemed to be safe and more effective than the conventional medical treatment alone for improving the total clinical effective rate and left ventricular ejection fraction. But further well-designed RCTs are required to confirm the efficacy and safety of XBP in treating AHF due to the poor methodological quality of the included RCTs.
Systematic Review Registration: [https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=327636], identifier [CRD42022327636].
Acute heart failure (AHF) is a clinical complex syndrome characterized by rapid deterioration and reduction in ventricular function necessitating hospitalization (1, 2). It has a prevalence of more than 23 million worldwide, associated with significant mortality, morbidity, and healthcare expenditures (3, 4). Significant drug advances have been developed and recommended in the treatment of patients with AHF in the past decades, including diuretic drugs, positive inotropes, vasodilators, neurohormonal antagonists, mechanical circulatory support, respiratory management, etc., [2021; (3, 5)], while none of the treatments tested to date have been definitively proven to improve AHF survival (6). Regarding patients with acutely decompensated HF or HF with preserved ejection fraction, approximately 50% of HF patients with preserved ejection fraction die within 5 years (5), and up to one in six patients with acute decompensation HF die during admission or within 30 days after discharge (4). Thus a new and an alternative drugs management of AHF is still challenging and of imperative need.
Puerarin (7,4′-dihydroxy-8-C-glucosylisoflavone) is the major bioactive ingredient of the root of Radix Puerariae, which was isolated in the late 1950s (7). Puerarin injection (PI) has been widely applied for the adjunctive management of coronary heart disease treatment and its main drug delivery method is intravenous injection (8). Clinical and experimental research proved that PI combined with conventional treatment could further improve the curative unstable angina pectoris (8, 9). PI could dilate coronary artery, increase coronary blood flow, decrease heart rate, inhibit platelet aggregation, and improve microcirculation (8, 10, 11). Literatures continuously reported clinical adjunctive efficacy and safety of PI, as well as their experimental effect and mechanism in animal models on AHF, but they still lacked relevant reviews summarizing the efficacy and safety of PI in the treatment of AHF in terms of the quality of methodology and evidence.
In the present study, we aimed to clarify the efficacy and safety of PI as an adjunctive treatment for acute heart failure (AHF) based on the available evidence in clinical practice. We mainly focused on clarifying whether PI had an adjunctive effect by combined use with conventional treatment and evaluating the safety of PI regarding its combined use.
The effectiveness and safety of PI were critically assessed by a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12).
A total of 5 English databases (the MEDLINE via PubMed, the Cochrane Library, EMBASE, the Web of Science and Ovid database) and 4 Chinese databases [China Science and Technology Journal Database (VIP), Chinese Biomedical Literature Database (CBM), Wan-fang Database, China National Knowledge Infrastructure (CNKI)] were searched for identifying studies from March 1990 to March 2022.
Patients diagnosed with AHF in consistence with the AHF diagnostic criteria recognized at the time of publication of the study, regardless of age, gender, and course of the disease.
Control group: Conventional western medicine treatment, including diet and life regulation, diuretics, cardiotonic, oxygen inhalation, ECG monitoring, low-salt diet, restricted liquid intake etc. The treatment group was treated with PI in addition to the control group.
Primary outcomes (O): ①Total clinical effective rate; ②left ventricular ejection fraction (LVEF); secondary outcomes: ①left ventricular end-diastolic dimension (LVEDD); ②isovolumic relaxation time (IVRT); ③peak A velocity of the mitral inflow; ④peak E velocity of the mitral inflow; ⑤stroke volume SV; safety outcome: adverse events.
Randomized controlled trials (RCTs) of PI in the treatment of AHF, without limit on method and language.
①Repeated publications; ②case report; ③pure theoretical research; ④The data in the literature were wrong or incomplete.
The MeSH terms of PICOS were combined to search in [Title/Abstract] by developing our search strategies sequentially. A combination of P+I, P+I+C, P+I+C+O, and P+I+C+O+S was used to search for studies. If the number of searched studies were small, we would search as P+I. The artificially screened studies according to the included and excluded criteria and the searching strategy are detailed in Supplementary File 1.
After two review authors search out the articles, another two authors retrieved full text after screening the titles and abstracts, which meet with criteria of PICOS. Any discrepancies were handled by a discussion among all the authors. Then Kappa-coefficient analysis was performed regarding the level of agreement among the reviewers in article selection.
For data extraction, two reviewers independently identified the details for each study and presented them in a standardized form. The author’s name, published year, sample size, initial characteristics of patients, treatment detail, criteria for AHF diagnosis, outcomes and adverse reactions, etc., were extracted by two authors independently.
The quality evaluation was assessed by the risk of bias assessment tool recommended by Cochrane Handbook 5.1. Seven aspects were assessed by two review authors, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. The quality evaluation was judged as “high,” “low,” or “unclear” risk of bias. Any discrepancies were handled by consensus.
The effect size was pooled using the Review Manager Software tool (RevMan, v.5.3; The Cochrane Collaboration). A fixed-effect model was chosen for the pool that had low heterogeneity, and a random-effects model was used where there was high heterogeneity. Mean deviation (MD) or Std mean difference (SMD) and 95% confidence intervals (CI) were utilized for continuous data, and relative risk (RR) with 95% CI were calculated for dichotomous data. Subgroup analysis and sensitivity analysis were also used to investigate potential sources of heterogeneity.
Sensitivity analysis was used to explore the significant heterogeneity that existed in studies, aiming to assess whether the conclusions were robust to the decision-making process. This study conducted a sensitivity analysis to observe whether the new effect-size results and heterogeneity changed significantly after removing single studies.
The grading recommendations assessment, development, and evaluation (GRADE) technique (13) were used to assess the evidence’s certainty following the instructions of the website1. RCT evidence was initially classified as high quality, but it would be downgraded due to the risk of bias, inaccuracy, inconsistency, informality, and publication bias. The level of evidence was classified into four categories: “high,” “moderate,” “low,” and “very low.”
A total of 75 related articles were initially detected. After 25 duplicate studies were eliminated, 50 RCTs were included for further screening. Then 39 studies were excluded without matching the inclusion requirements, and 3 non-RCT studies were eliminated after reviewing the article in detail. Finally, 8 studies (14–21) with a total of 614 patients with AHF were incorporated for systematic review and meta-analysis. Kappa-coefficient analysis suggested that the level of agreement among the two reviewers in article selection had a high degree of consistency (Kappa = 0.805, Supplementary Table 1). Figure 1 depicted the literature screening process and results.
All 8 included RCTs were conducted in China between 2012 and 2019, the sample size ranged from 58 to 100, and the treatment duration varied from 7 days to 14 days, except in one study (21) which had no report on duration. All research interventions were Puerarin injection (PI) in combination with conventional western treatment, and the drug delivery methods of PI were intravenous injection in all the studies. In terms of the usage and dose of PI, 3 studies diluted 500 mg of Puerarin with 500 ml 5% glucose (14, 16, 17), 2 studies diluted 500 mg Puerarin with 250 ml 5% glucose (18, 21), and 3 studies diluted 200–400 mg with Puerarin with 500 ml of 5% glucose (15, 19, 20). Only 3 studies (14, 15, 17) reported that the AHF diagnostic criteria was inconsistent with acute heart failure diagnosis and treatment guide (2010 version) published by the Cardiovascular Disease Branch of Chinese Medical Association (22). None of the studies reported follow-up results. The basic characteristics of included RCTs are detailed in Table 1.
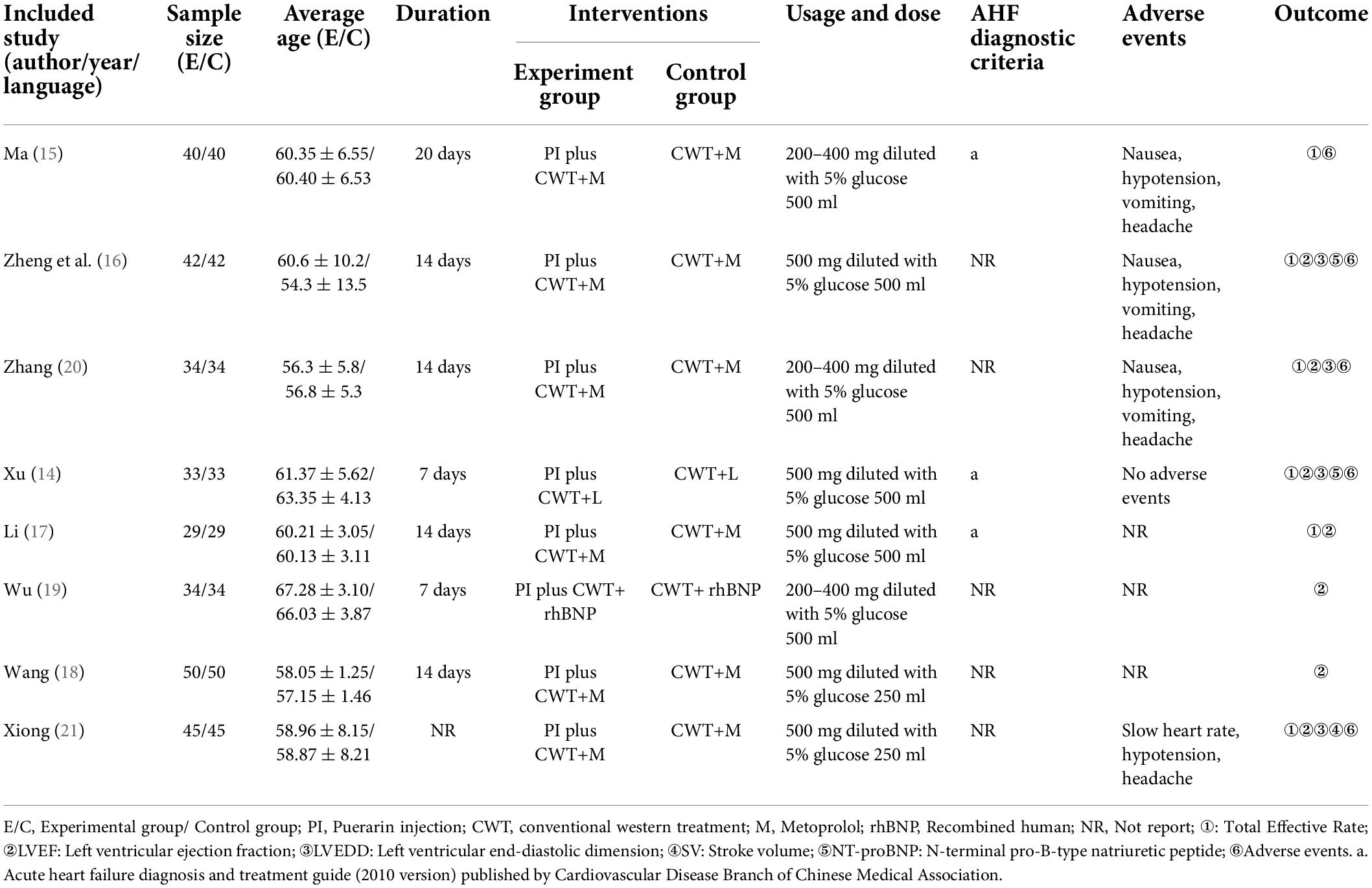
Table 1. Characteristics of included RCTs investigating the adjunctive effect of Puerarin injection (PI) on acute heart failure.
One trial (16) was rated as low risk for using random number tables to generate sequences, while the other studies (14, 15, 17–21) provided no details about the method of random sequences generation. All the included studies published complete data, and no selective outcomes were reported, so the risk of bias was considered “low.” Beyond that, no studies mentioned the information about concealing of allocation, blinding of researchers, participants, and outcome evaluators, resulting in the risk of bias regarding performance, and detection was considered “unclear.” The risk of other bias was considered “low,” since no other obvious bias was observed in all RCTs. Table 2 shows the results of the risk of bias of the included RCTs.
Six studies (14–17, 20, 21) involving 444 patients reported the total effective rate. The fixed-effects model was used for meta-analysis as there existed little heterogeneity between the studies (p = 0.83, I2 = 0%). As shown in Figure 2, the results of the meta-analysis suggested that PI combined with conventional medical treatment increased the total effective rate by comparing with conventional medicine alone (RR = 1.38; 95% CI, 1.22–1.55; p < 0.001), indicating that PI had a favorable adjunctive effect on the total effective rate of AHF. Subgroup analyses according to PI doses showed that 200–400 mg/day (RR = 1.30; 95% CI, 1.09–1.55; p = 0.003) and 500 mg/day (RR = 1.42; 95% CI, 1.22–1.67; p < 0.001) of PI combined with conventional medicines treatments both increased the total effective rate compared with conventional medicine alone.

Figure 2. Forest plot of total effective rate (the total effective rate = effective rate + significant effective rate; invalid rate: patient’s heart function, physical signs, and clinical AHF symptoms have not been improved or even worsen; effective rate: patient’s heart function improved with 1 level, physical signs, and clinical AHF symptoms are relieved; significant effect rate: patient’s heart function improved with 2 level or more, the heart rate decreased to normal level, physical signs, and clinical AHF symptoms have disappeared).
Seven studies involving 534 patients reported the results of LVEF. The random-effects model was used for meta-analysis as there existed high heterogeneity between studies (p < 0.001, I2 = 96%). The results of the meta-analysis indicated that combining PI with a conventional medical treatment significantly improved LVEF (RR = 1.07; 95% CI, 0.87–1.27; p < 0.001, Supplementary Figure 1). Sensitivity analyses were performed by excluding studies one by one. After removing the studies reported by “(17)” (17) and “(18)” (18), heterogeneity between studies was significantly reduced to 66%. As shown in Table 1, the sample size of the study “(17)” (17) and “(18)” (18) were the largest and smallest compared with other studies respectively, which might contribute to high heterogeneity. The results showed that the LVEF of patients with AHF was still significantly improved by the combined use of PI with conventional medical treatment (SMD = 0.79; 95% CI, 0.58–1.00; p < 0.001, Figure 3), and it indicated that combined use of PI was beneficial for LVEF in patients with AHF. Subgroup analyses showed that 500 mg/day of PI combined with conventional medicines treatments also improved the LVEF compared with conventional medicine alone (SMD = 0.85; 95% CI, 0.62–1.09; p < 0.001, Figure 3). As “(15)” (15) did not report the LVEF valve, there were no sufficient studies (≥2) for subgroup analyses on the dose of 200–400 mg/day.
Three studies (16, 20, 21) involving 242 patients reported the value of LVEDD and two studies (16, 20) involving 152 patients reported the value of IVRT. The fixed-effects model was used for meta-analysis on LVEDD (p = 0.02, I2 = 75%) and IVRT (p = 1.00, I2 = 0%) as there existed low to median heterogeneity between studies. As shown in Figure 4, the results of meta-analysis indicated that combining PI with conventional medicine treatment improved the heart function, including increased LVEDD (MD = 1.67; 95% CI, 0.25–3.09; p < 0.001, Figure 4) and decreased IVRT (MD = −6.70; 95% CI, −8.26 to −5.14; p < 0.001, Figure 4) when compared with conventional medicine alone.
Two studies (16, 20) involving 152 patients reported the value of peak E, two studies (16, 20) involving 152 patients reported the value of peak A, and two studies (14, 21) involving 156 patients reported the value of SV. The fixed-effects model was used for meta-analysis on peak E (p = 0.96, I2 = 0%), Peak A (p = 1.00, I2 = 0%) and IVRT (p = 0.44, I2 = 0%) as there existed no heterogeneity between studies. As shown in Figure 5, the results of meta-analysis indicated that combining PI with conventional medicine treatment improved the left ventricular diastolic function, including increased peak E (MD = 7.55; 95% CI, 5.57–9.52; p < 0.001, Figure 5), decreased Peak A (MD = −4.40; 95% CI, −4.81 to −3.99; p < 0.001, Figure 5), and increased SV (MD = 7.99; 95% CI, 4.98–11.01; p < 0.001, Figure 5) when compared with conventional medicine alone.
Five studies (14–16, 20, 21) involving 388 patients reported adverse events. As detailed in Table 3, one study (14) reported no adverse reactions in both groups and one study (20) reported the total number of adverse effects without classification. Three studies (15, 16, 21) reported a detailed number of each kind of adverse reactions in both groups. In all, it reported a total of 11.9% (23/194) adverse reactions in the PI group and 9.8% (19/194) adverse reactions in the control group. All of the adverse reactions were modest, and no significant difference in the incident rate of adverse events was observed in both groups (RR = 1.16; 95% CI, 0.66–2.05; p = 0.061, Figure 6), indicating that adjunctive use of PI was safe as a conventional medical treatment.
We assessed publication bias on the results of total effective rate, LVEF, and adverse effect, as other results had less than three studies included. We detected that there is no publication bias on the results of total effective rate, LVEF, and adverse effect (Figure 7). But because of lacking access to the information on the clinical trial registry or study protocol, it could not rule out the potential of selectively reporting existing results. The published bias result of other results are provided in Supplementary File 2.
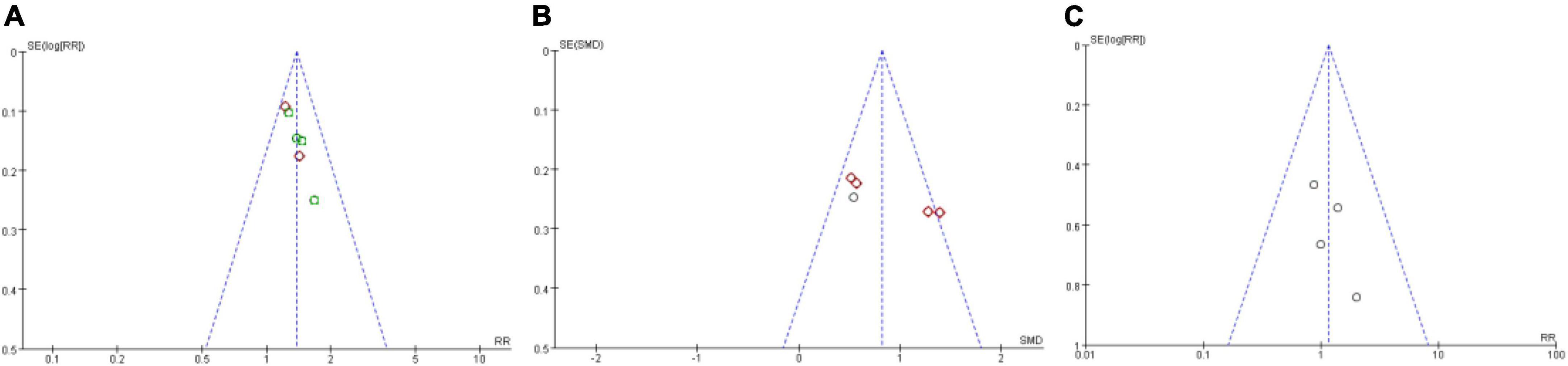
Figure 7. Funnel plot of publication bias assess. (A) Publication bias assess on total effective rate; (B) publication bias assess on LVEF; (C) Publication bias assess on adverse effect.
The certainty of evidence on meta outcomes was assessed by the grading recommendations assessment, development, and evaluation (GRADE) methods, and it showed that evidentiary quality of meta results varied from “very low” to “moderate.” The rationale for the downgrade was mainly due to small sample sizes and unclear risk of bias in the selected studies, as shown in Table 4.
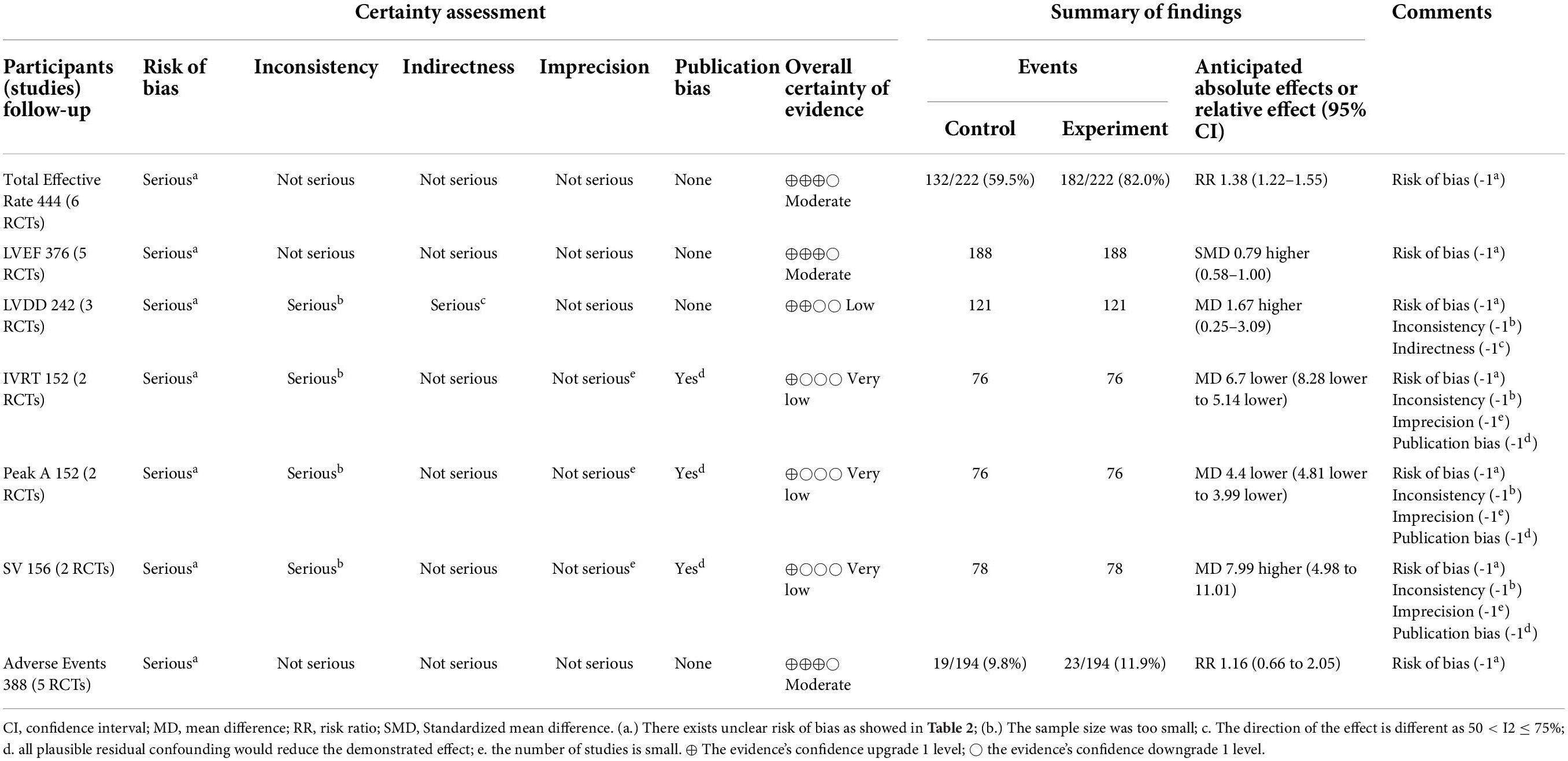
Table 4. The summary findings by the grading recommendations assessment, development, and evaluation (GRADE) methods.
Traditional Chinese medicine (TCM) is proved to have an adjunctive effect in treating diseases when combined with the use of western medicine in improving the symptoms and quality of life of patients (23). Due to the side effects of western medicine therapy, TCM integration with routine western or conventional medical interventions plays a significant adjunctive role in enhancing the therapeutical effect and reducing the occurrence of adverse effects (24), thus the unique advantages of TCM have received increasing attention, but it still lacks systematic overviews to summarize the effectiveness of TCM based on the existing clinical evidence. Our systematic review and meta-analysis contained eight RCTs and revealed that PI combination therapy had an adjunctive effect in the treatment of patients with AHF, it could better increase the total effective rate, improved the heart function, and be safe for adjunctive use in treating AHF.
Our study also suggested that PI treatment in conjunction with conventional medical treatment was superior in increasing the total effective rate, improving heart function of the valve of LVEF, LVEDD, and IVRT, and improving the left ventricular diastolic function of the valve of Peak A, Peak E and stroke volume, in terms of comparing with conventional medical treatment alone. In addition, subgroup analysis was performed on the primary outcomes of total effective rate and LVEF. Interestingly, the results showed that adjunctive use of PI could both improve the LVEF value and increase the total effective rate regardless of the dose (200–400 mg/day or 500 mg/day). As previously reported, PI showed a satisfied clinical efficacy in the treatment of cardiovascular diseases that PI was more effective than using conventional western medical alone in the treatment of unstable angina pectoris (9). It is also more effective and relatively safe in the clinic for treating acute ischemic stroke and diabetic peripheral neuropathy (25, 26).
When we further explored the association between PI and favorable results in patients with AHF, it was proposed that PI had the effect of alleviating impaired heart function and inhibiting the levels of myocardial injury and inflammatory markers (27), as it was found that inflammation and heart function impaired in AHF resulted in neutral effects or worsening of clinical outcomes (28, 29). In addition, patients with AHF presented with similar congestion symptoms, which could lead to HF decompensation, which occurred owing to both fluid accumulation and redistribution, and further progress in the deterioration of AHF, thus decongestive therapy and diuretic drugs were recommended for AHF (1). PI was found that it could expand the coronary artery to promote coronary blood flow (10) and improve microcirculation to alleviate congestion symptoms (11), which might be the mechanism that PI could alleviate the AHF symptoms. Furthermore, clinical trials pointed out that a higher heart rate was a strong predictor of 1-year mortality of AHF, and reductions in coronary blood flow and myocardial oxygen consumption may be beneficial for AHF treatment (30, 31). Song et al reported that PI had the effect of decreasing heart rate and reducing myocardial oxygen consumption (32), which may also be the potential mechanism that PI had favorable results in patients with AHF.
Regarding clinical safety, a total of 9.8% (19/194) adverse reactions occurred in the control groups while 11.9% (23/194) in the PI group, including nausea, hypotension, vomiting, headache, and slow heart rate. As 5 (62.5%) studies (14–16, 20, 21) reported the adverse effects and moderate evidence for safety assessment, we preliminary put forward the argument that combination therapy of PI was safe in treating AHF. But since the record for risk of bias assessment of included RCTs was “unclear,” it implied that there is still a need for further eligible and critical clinical trials to validate the safety of PI.
The findings of meta-results were consistent with previously published research (33). To assess the credible clinical evidence of our results, evaluation of bias risk and evidence’s confidence were performed. It showed that all the included studies lack details in selection bias, blinding performance, and blinding outcome assessment (Table 2), which may result in the overstated effect of outcomes and reported bias in selected results. In addition, GRADE evaluation indicated that the confidence of the evidence was graded, which varied from very low to moderate quality for evidences (Table 4), and risk of bias, inconsistency, imprecision, and publication bias were mainly responsible for the downgrading of evidence because of the quality of included RCTs, thus larger RCTs with improved methodological quality in future are expected to further update the results of this systematic review.
The adjunctive efficacy and safety of PI regarding curative effect among patients with AHF were for the first time systematically reviewed and evaluated in this study. At present, AHF treatment still lacks specific and effective medicine, leading to a relatively high recurrence rate, hospitalization rate, and mortality rate. We found that integrated use with PI could improve heart function, increased total effective rate, and was safe as the conventional western medication, which could be chosen by physicians when patients with AHF faced with unexpected treatment effects. The methodology of the present study was designed to a high standard according to the methodological quality of systematic reviews-2 (AMSTAR 2) by identifying relevant literature comprehensively, developing evaluation plans, and strict implementation, which could improve the accuracy and clinical applicability of the results of this study (34).
Although the results were encouraging, restrictions were unavoidably present in this study. Due to the small number and low to moderate quality of included studies, strictly designed trials according to the Consolidated Standards of Reporting Trials (CONSORT) statement also need to be further performed to verify the efficacy of PI as an adjunctive therapy for AHF. Duration included 7 days and 14 days, and the dose of PI included 500 mg/day and 200–400 mg/day, but we only did perform subgroup analysis of dose on the total effective rate due to small number of studies. Besides, the control group involves different conventional medical treatments, which potentially led to heterogeneity between the studies. Although there was no restriction on language when screening literature, the final included studies were all performed in China, which may lead to potential selection bias in the research. In addition, a few have data available for each outcome, for instance, the number of studies included in the meta-analysis of LVEDD, IVRT, Peak A, Peak E, and SV was 2-3/8 (25–37.5%), which limited the credibility of the above results. Thus, much more caution should be taken about the results until further trials in different populations and high-quality designed studies were performed to strengthen and update the results of the present meta-results.
In conclusion, PI plus CMT may be more beneficial than CMT alone for increasing the total effective rate, improve the heart function and left ventricular diastolic function. Also, it may be safe to combine PI with CMT in treating AHF. Regarding the very low to moderate evidence on the quality of meta-results, we should proceed with caution. Multi-center randomized controlled and double-blind trials are required with large sample sizes, rigorous design, and long follow-up period to confirm the efficacy and safety of PI in the future.
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
ZL and YF provided conceptualization, methodology, investigation, and writing—original draft. CH helped provide methodology, investigation, and formal analysis. QL and BC helped provide investigation, validation, data collection, and visualization. MH and ZP helped provide data collection and validation. BD and WZ provided conceptualization, funding acquisition, supervision, writing—review and editing, and project administration. All authors contributed to the article and approved the submitted version.
This study was supported by the Department of Science and Technology of Guangdong Province (No. 2021A1515012224); clinical effect evaluation of kuanxiong aerosol on patients with “chest tightness and pain” in emergency-An open, randomized, controlled study [HT 2020-0974 (KY)]; clinical study of Astragalus injection on diuretic resistance in senile patients with acute heart failure (No. 20211162); and pre-hospital care-clinical study on the treatment of acute ST-segment elevation myocardial infarction by the combination of medicine and balance needle (No. AX046DS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.934598/full#supplementary-material
1. Sinnenberg L, Givertz MM. Acute heart failure. Trends Cardiovasc Med. (2020) 30:104–12. doi: 10.1016/j.tcm.2019.03.007
2. Njoroge JN, Teerlink JR. Pathophysiology and therapeutic approaches to acute decompensated heart failure. Circ Res. (2021) 128:1468–86. doi: 10.1161/CIRCRESAHA.121.318186
3. Orso F, Fabbri G, Maggioni AP. Epidemiology of heart failure. Handb Exp Pharmacol. (2017) 243:15–33. doi: 10.1007/164_2016_74
4. Taylor CJ, Ordonez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. (2019) 364:l223. doi: 10.1136/bmj.l223
5. Berliner D, Hanselmann A, Bauersachs J. The treatment of heart failure with reduced ejection fraction. Dtsch Arztebl Int. (2020) 117:376–86. doi: 10.3238/arztebl.2020.0376
6. Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. (2019) 393:1034–44. doi: 10.1016/S0140-6736(18)31808-7
7. Zhang L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. (2019) 26:860–9. doi: 10.1080/10717544.2019.1660732
8. Gao Z, Wei B, Qian C. Puerarin injection for treatment of unstable angina pectoris: a meta-analysis and systematic review. Int J Clin Exp Med. (2015) 8:14577–94.
9. Shao H, Huang Y, Xu D, Huang S, Tong R. A systematic review and meta-analysis on the efficacy of Puerarin injection as adjunctive therapy for unstable angina pectoris. Front Cardiovasc Med. (2022) 9:763567. doi: 10.3389/fcvm.2022.763567
10. Zhang Z, Lam TN, Zuo Z. Radix puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. (2013) 53:787–811. doi: 10.1002/jcph.96
11. Wei SY, Chen Y, Xu XY. Progress on the pharmacological research of puerarin: a review. Chin J Nat Med. (2014) 12:407–14. doi: 10.1016/S1875-5364(14)60064-9
12. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
13. Qaseem A, Forciea MA, Mclean RM, Denberg TD, Barry MJ, Cooke M, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American college of physicians. Ann Intern Med. (2017) 166:818–39. doi: 10.7326/M15-1361
14. Xu F. Clinical efficacy and safety analysis of patients with puerathin injection and zuo ximen dan in patients with acute heart failure. (Translated from Chinese). J Med Theory Pract. (2017) 30:1760–1.
15. Ma Y. Clinical efficacy observation of puerase injection and medelolol combined with the treatment of acute heart failure. (translated from Chinese). Chin J Modern Drug Appl. (2018) 12:93–4.
16. Zheng Z, Hu J, Zhang X. Clinical efficacy of puerasee injection combined with metolol’s treatment of acute heart failure. (Translated from Chinese). China Biochem Drug Magazine. (2012) 33:852–4.
17. Li Q. Joint application of puerarin injection and methalore in the treatment of acute heart failure patients. (translated from Chinese). J Contemp Med Forum. (2014) 12:189–90.
18. Wang H. Observation on the effect of puerase injection combined with metolol’s treatment of acute heart failure (translated from Chinese). Cardiovasc Dis J Integr Tradit Chin Western Med. (2015) 3:22+24.
19. Wu S. Observation the effect of reorganized human sodium peptide combined with puerasein injection to treat acute heart failure. (Translated from Chinese). Dietary Health Magazine. (2019) 6:61–2. doi: 10.3969/j.issn.2095-8439.2019.06.074
20. Zhang Y. The clinical efficacy of puerase injection combined with metolol to treat acute heart failure. (Translated from Chinese). Shenzhen J Integr Tradit Chin Western Med. (2016) 26:46–7.
21. Xiong J. The efficacy of puerase injection combined with metolol’s treatment of acute heart failure. (Translated from Chinese). J Clin Med Pract. (2016) 20:122–3.
23. Zhao Z, Li Y, Zhou L, Zhou X, Xie B, Zhang W, et al. Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine. (2021) 85:153308. doi: 10.1016/j.phymed.2020.153308
24. Huang J, Tang X, Ye F, He J, Kong X. Clinical therapeutic effects of aspirin in combination with fufang danshen Diwan, a traditional Chinese medicine formula, on coronary heart disease: a systematic review and meta-analysis. Cell Physiol Biochem. (2016) 39:1955–63. doi: 10.1159/000447892
25. Zheng QH, Li XL, Mei ZG, Xiong L, Mei QX, Wang JF, et al. Efficacy and safety of puerarin injection in curing acute ischemic stroke: a meta-analysis of randomized controlled trials. Medicine (Baltimore). (2017) 96:e5803. doi: 10.1097/MD.0000000000005803
26. Xie B, Wang Q, Zhou C, Wu J, Xu D. Efficacy and safety of the injection of the traditional chinese medicine puerarin for the treatment of diabetic peripheral neuropathy: a systematic review and meta-analysis of 53 randomized controlled trials. Evid Based Complement Alternat Med. (2018) 2018:2834650. doi: 10.1155/2018/2834650
27. Li X, Yuan T, Chen D, Chen Y, Sun S, Wang D, et al. Cardioprotective effects of puerarin-V on isoproterenol-induced myocardial infarction mice is associated with regulation of PPAR-Upsilon/NF-kappaB pathway. Molecules. (2018) 23:3322. doi: 10.3390/molecules23123322
28. Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, et al. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. (2017) 14:238–50. doi: 10.1038/nrcardio.2016.203
29. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. (2020) 17:269–85. doi: 10.1038/s41569-019-0315-x
30. Sepehrvand N, Ezekowitz JA. Oxygen therapy in patients with acute heart failure: friend or foe? JACC Heart Fail. (2016) 4:783–90. doi: 10.1016/j.jchf.2016.03.026
31. Ancion A, Tridetti J, Nguyen TM, Oury C, Lancellotti P. Serial heart rate measurement and mortality after acute heart failure. ESC Heart Fail. (2020) 7:103–6. doi: 10.1002/ehf2.12530
32. Song XP, Chen PP, Chai XS. Effects of puerarin on blood pressure and plasma renin activity in spontaneously hypertensive rats. Zhongguo Yao Li Xue Bao. (1988) 9:55–8.
33. Lian B, Xu J, Guo Z, Liu M, Deng C, Liao L. Clinical efficacy on Gegensu injection combined with western medicine for curing heart failure. (Translated from Chinese). Chin J Exp Tradit Med Formulae. (2016) 22:189–94.
Keywords: acute heart failure, Puerarin injection, meta-analysis, systematic review, traditional Chinese medicine
Citation: Li Z, Fan Y, Huang C, Liu Q, Huang M, Chen B, Peng Z, Zhu W and Ding B (2022) Efficacy and safety of Puerarin injection on acute heart failure: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:934598. doi: 10.3389/fcvm.2022.934598
Received: 02 May 2022; Accepted: 01 July 2022;
Published: 25 July 2022.
Edited by:
John Skoularigis, University Hospital of Larissa, GreeceReviewed by:
Dimitrios E. Magouliotis, University Hospital of Larissa, GreeceCopyright © 2022 Li, Fan, Huang, Liu, Huang, Chen, Peng, Zhu and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhu, MTM4MjYyNjAxMTJAMTYzLmNvbQ==; Banghan Ding, MTM2ODIyMzgyMjVAMTM5LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.